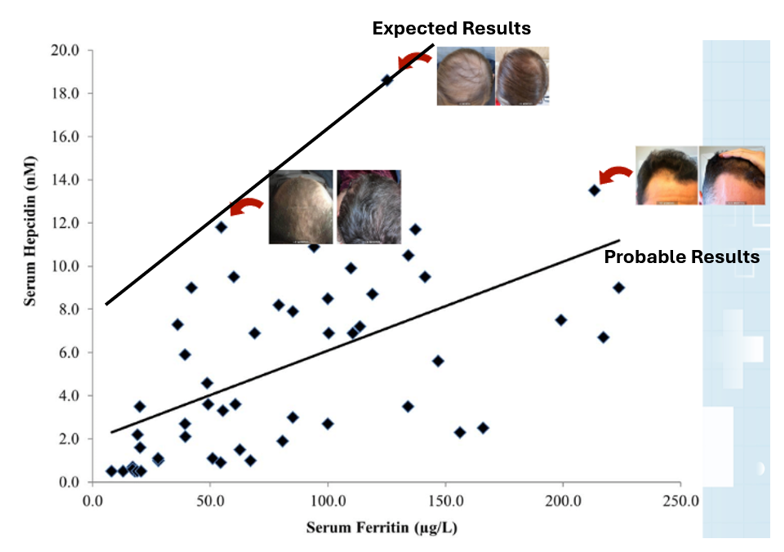
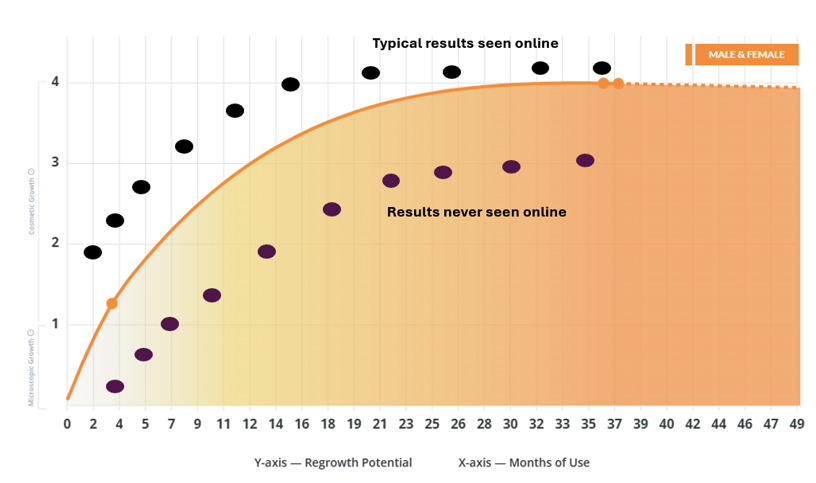
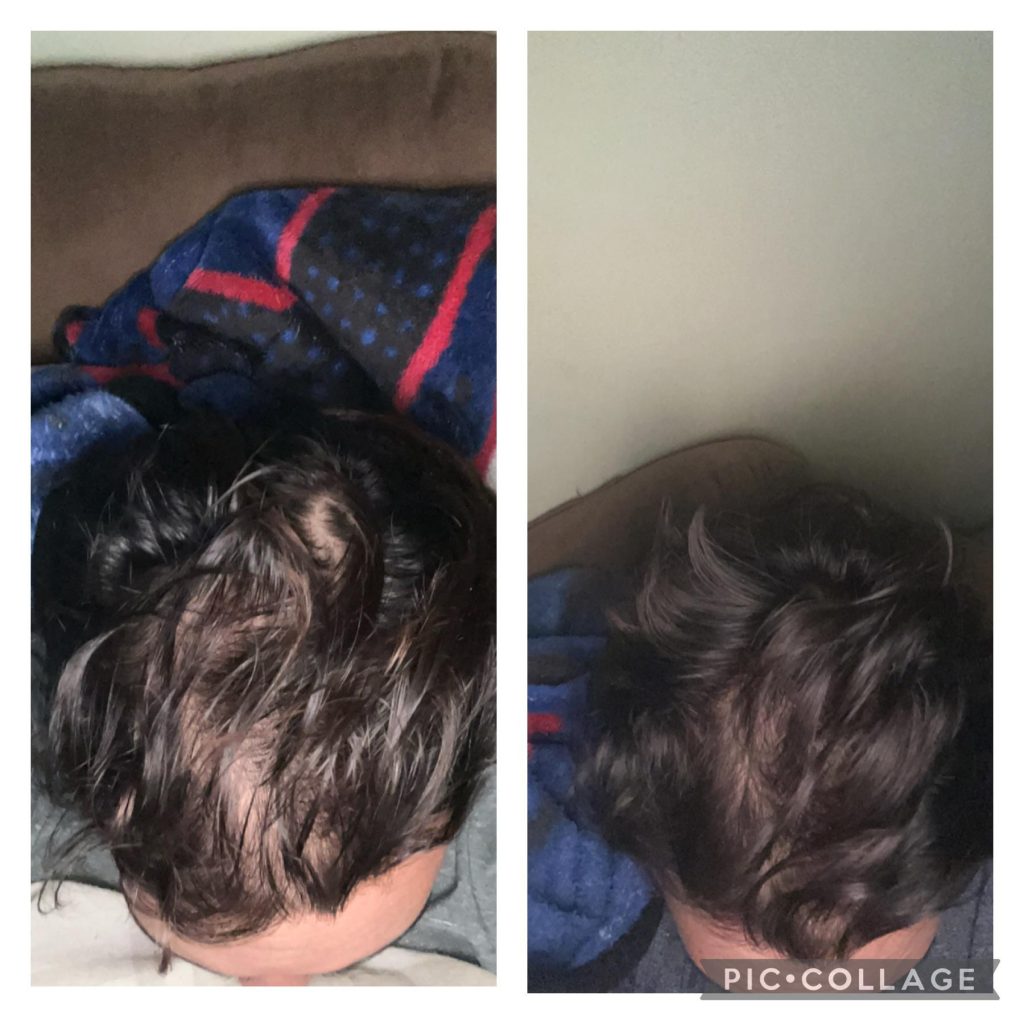
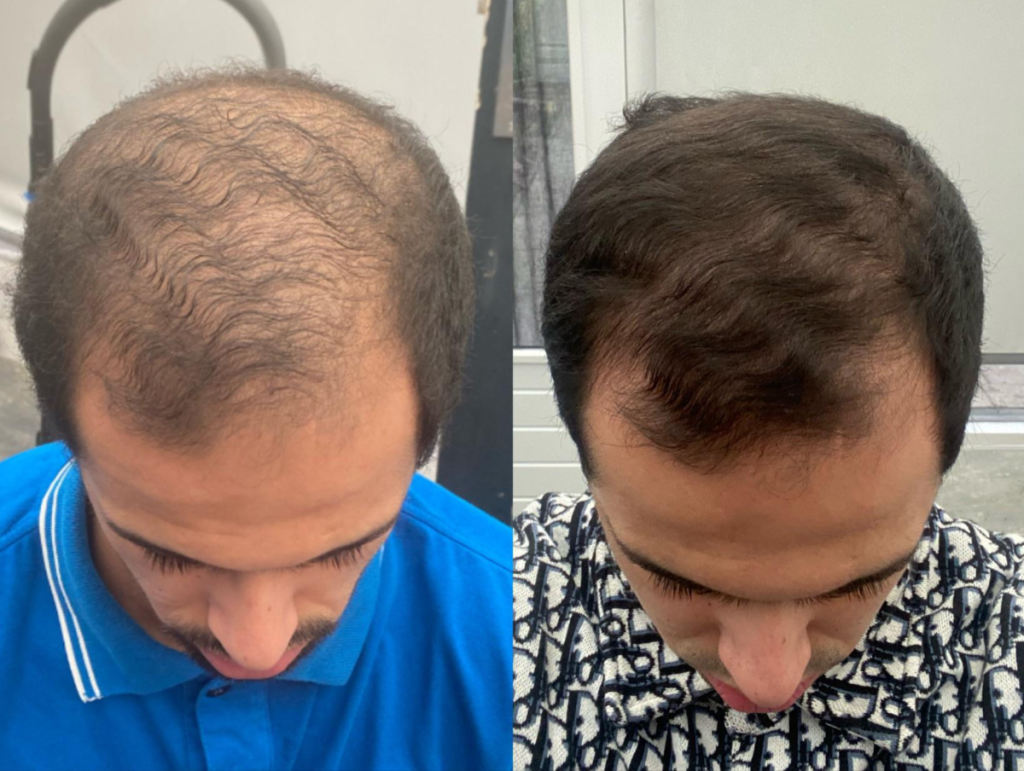
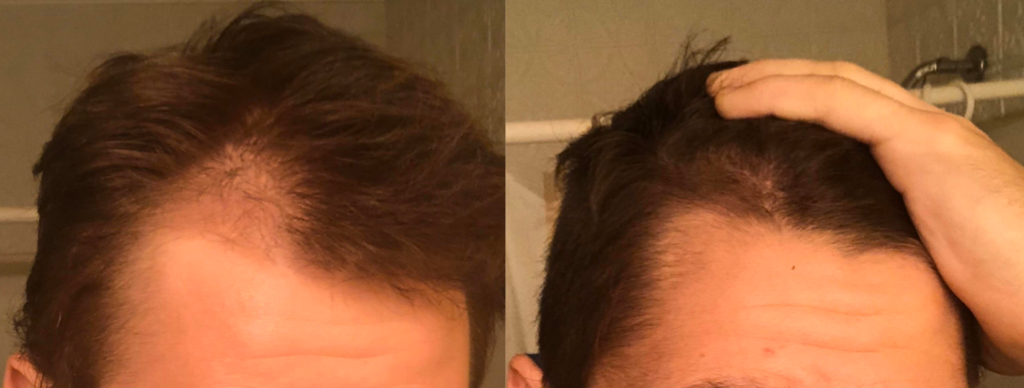
- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
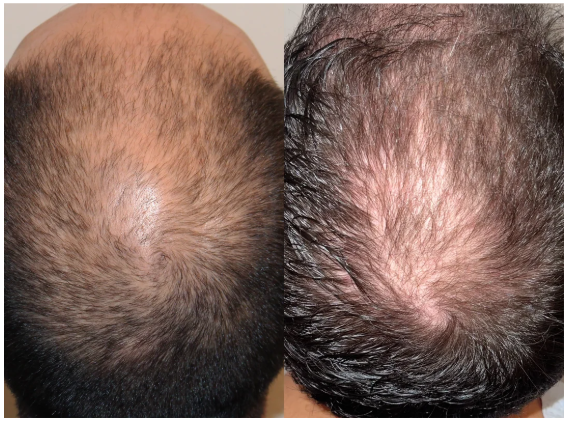
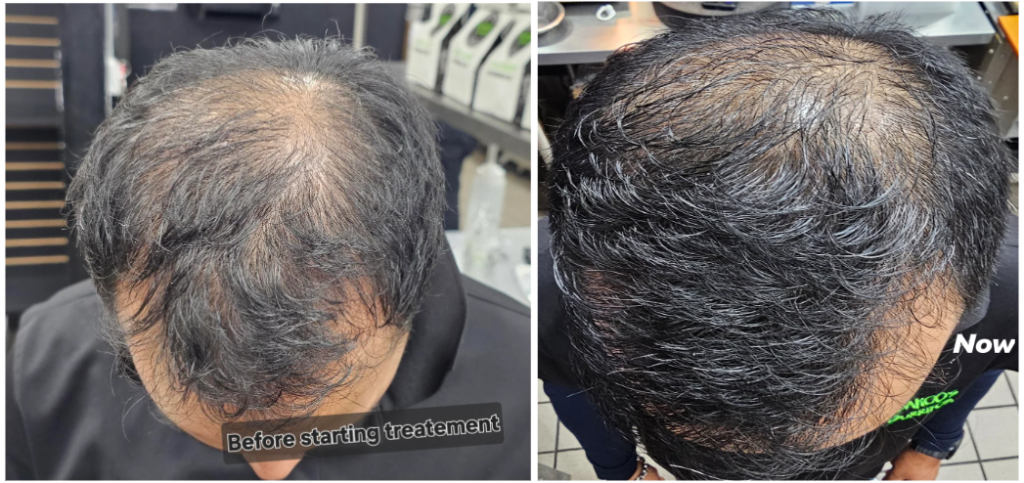
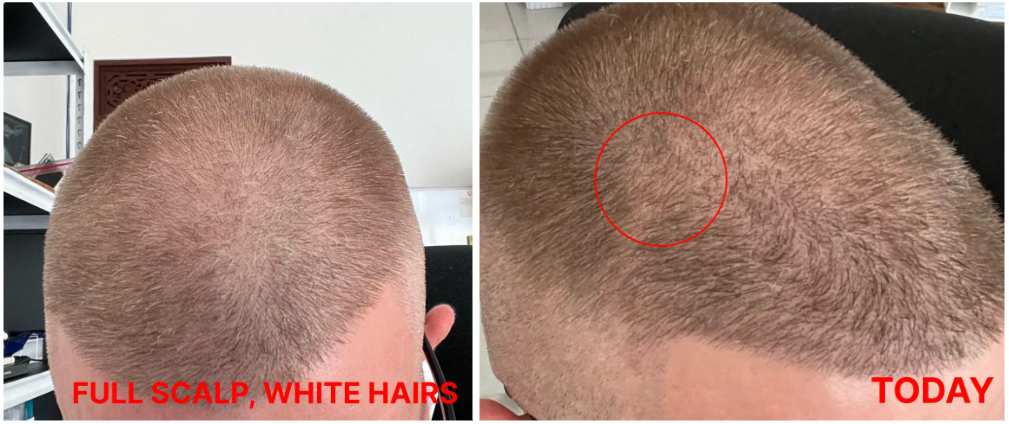
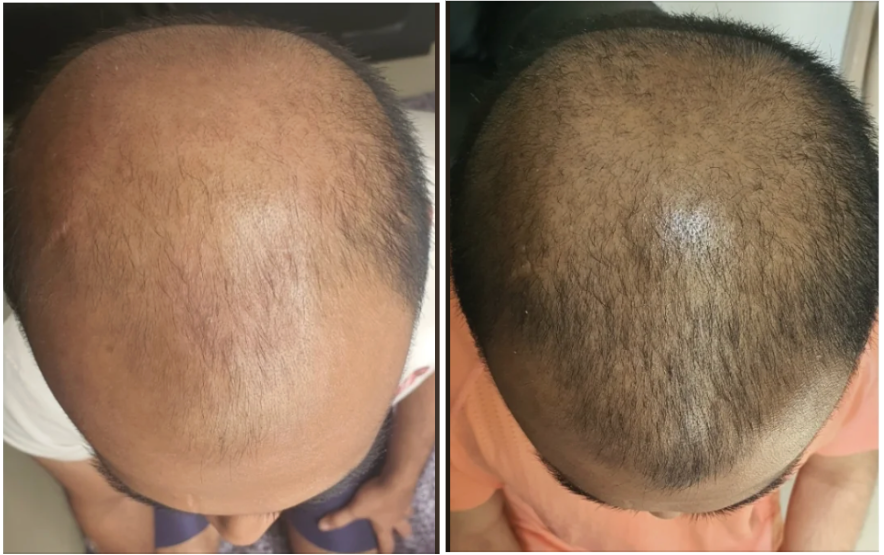
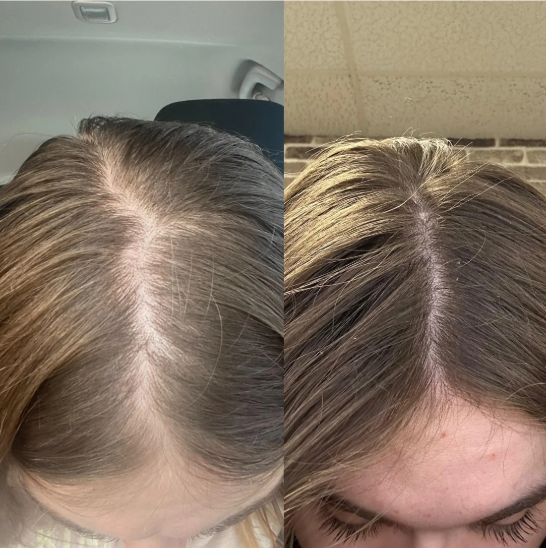
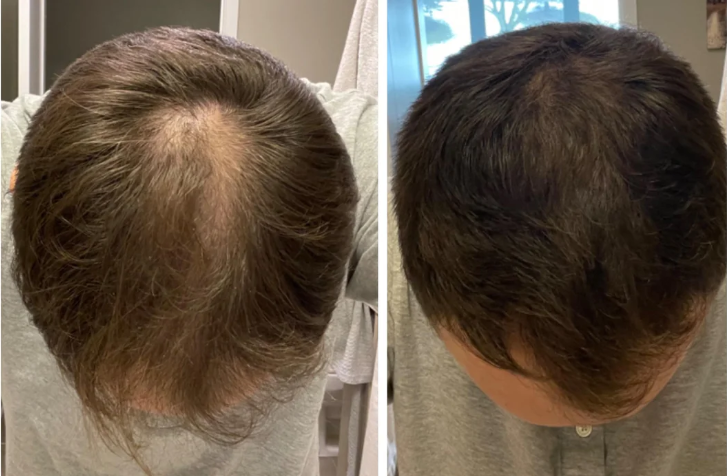
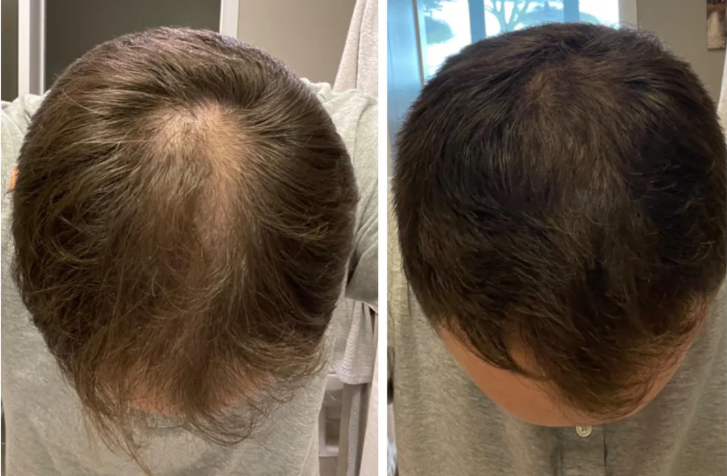
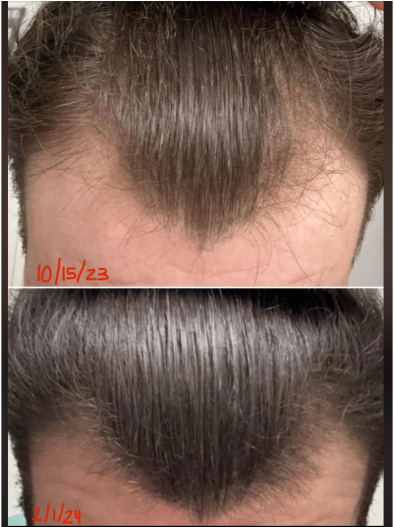
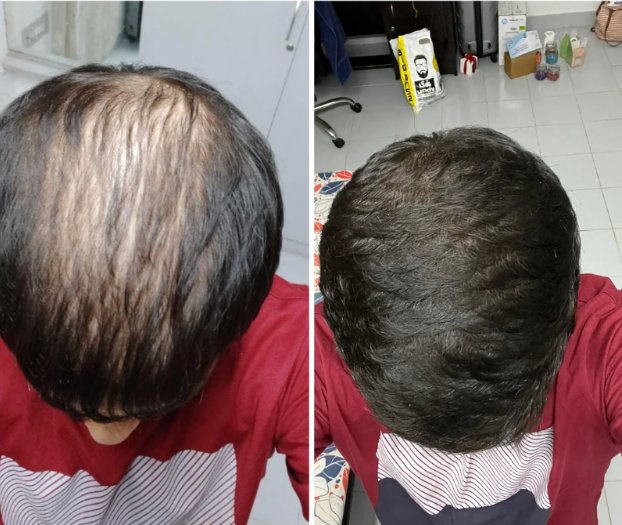
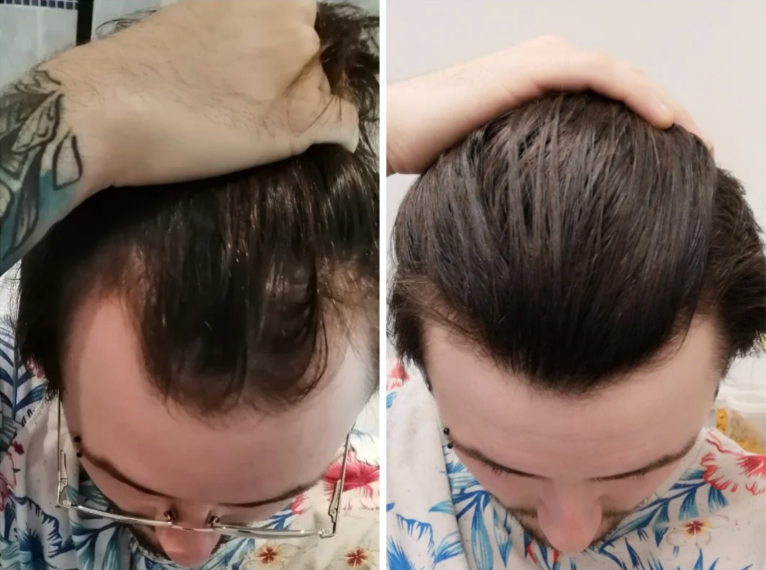
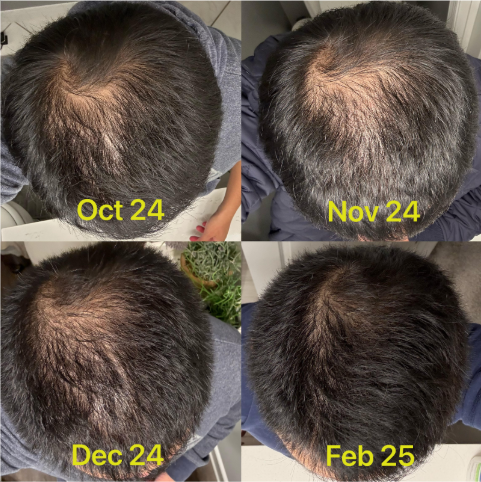
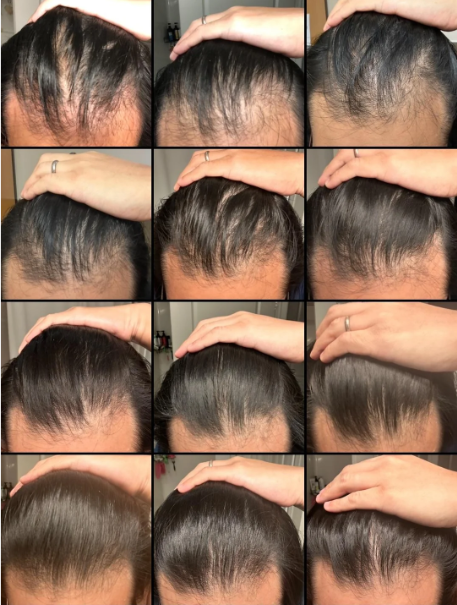
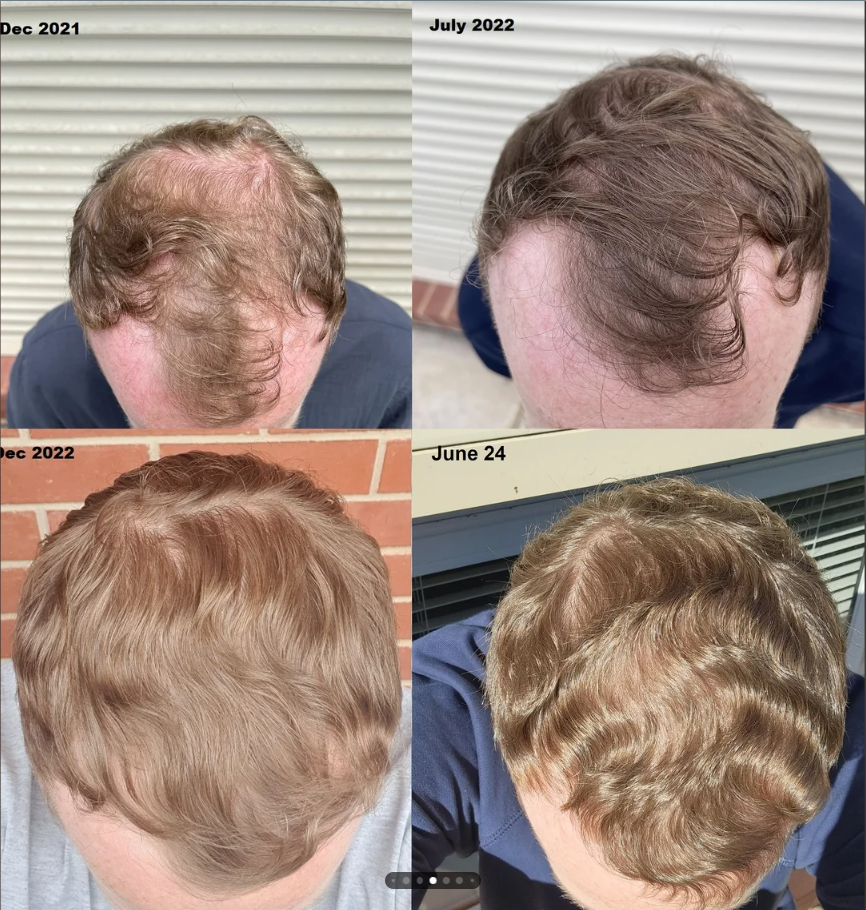
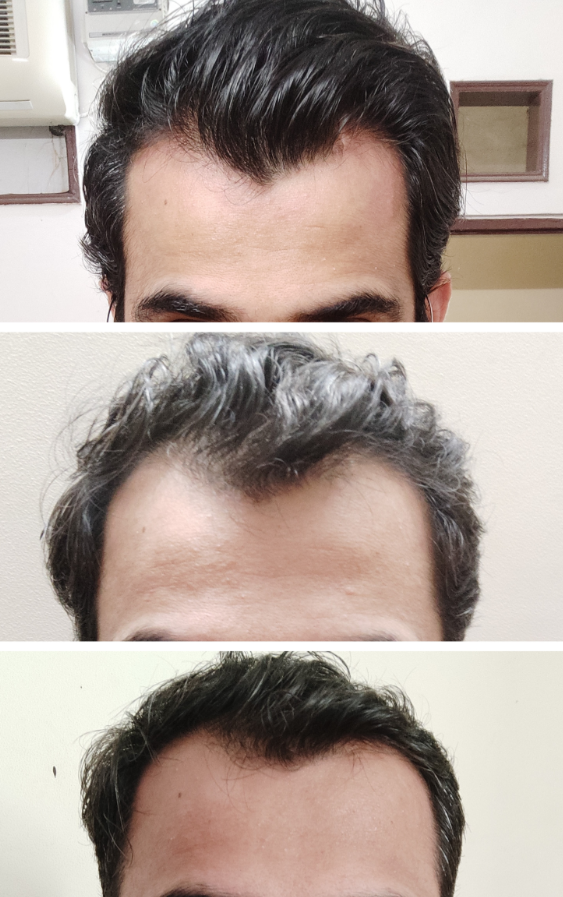
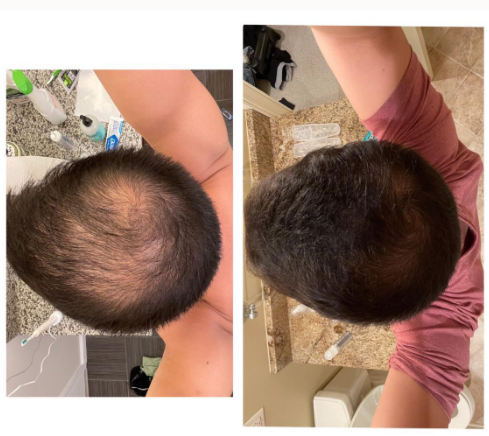
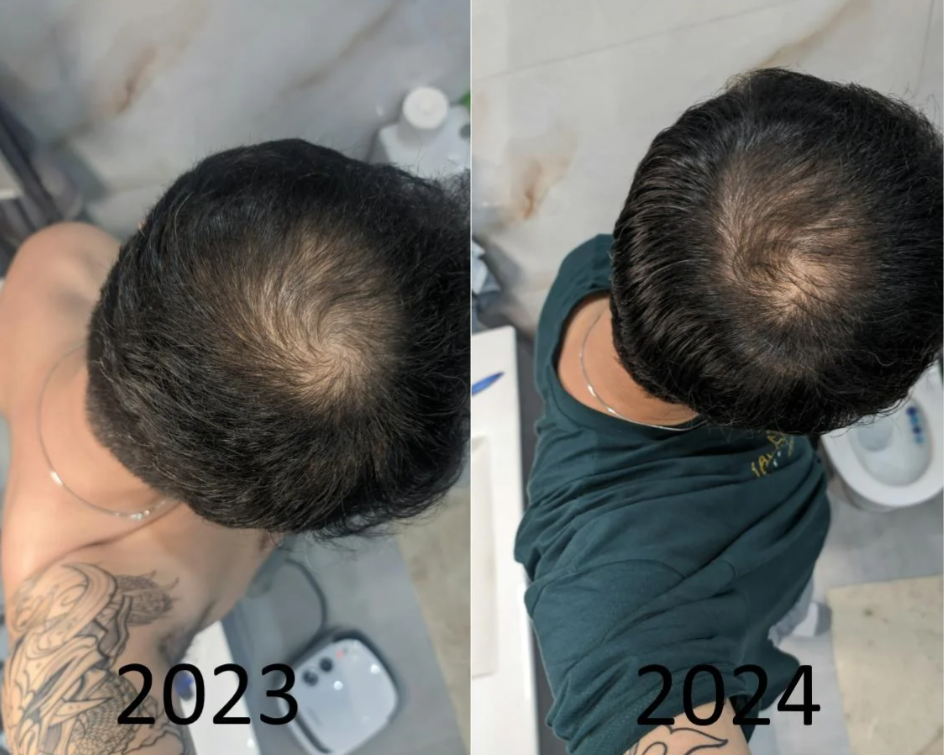
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
Articles
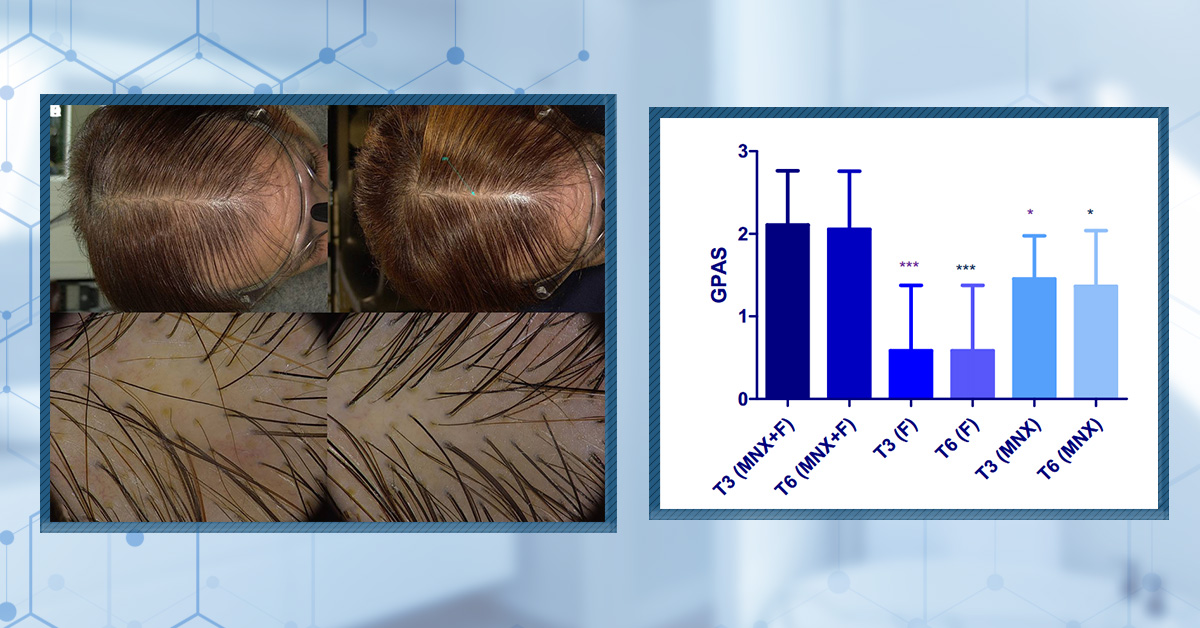
Minoxidil is a widely used medication for treating androgenic alopecia (AGA) in both men and women. Originally developed as a treatment for high blood pressure, minoxidil was found to improve hair growth outcomes as a side effect, making its topical form one of only two (the other being finasteride) FDA-approved treatments for AGA.
Minoxidil is primarily available in two forms: topical and oral. The topical form, which includes both solutions and foams, is more commonly used and can be purchased over the counter. Oral minoxidil, however, is prescription only and is typically given at doses of 2.5 mg or 5 mg for hair loss treatment.
While minoxidil is generally considered safe, it’s important to understand its potential side effects and who might be more at risk from them. In this article, we will examine the side effects of topical and oral minoxidil and discuss how you can adjust your treatment regimen to mitigate these.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

How Does Minoxidil Work?
Minoxidil was originally developed as an antihypertensive medication. However, when treated participants started experiencing hypertrichosis (excessive hair growth), studies were conducted to find out if it could improve hair regrowth outcomes in humans (spoiler alert – it could!).[1]Bryan, J. (2011). How minoxidil was transformed from an antihypertensive to hair-loss drug. The Pharmaceutical Journal. Available at: … Continue reading
Minoxidil’s mechanism of action is not fully understood, but it is thought to work through two main mechanisms:
- Vasodilatory effects: It activates vascular endothelial growth factor (VEGF), increasing blood flow, oxygen, and growth factor delivery to the hair follicle.[2]Alhayaza, G., Hakami, A., AlMarzouk, L.H., Al Qurashi, A.A., Alghamdi, G., Alharithy, R. (2023). Topical minoxidil reported hair discoloration: a cross-sectional study. Dermatology Reports. 16(1). … Continue reading
- Potassium channel activation: This prolongs the growth (anagen) phase and shortens the resting (telogen) phase.
Research also shows that minoxidil can act on androgenic receptors, suppressing the expression of the androgen receptor and CYP17A1 and boosting the activity of CYP19A1. This decreases the formation and binding of dihydrotestosterone and enhances the production of estradiol, which may also benefit those with AGA.[3]Shen, Y., Zhu, Y., Zhang, L., Sun, J., Xie, B., Zhang, H., Song, X. (2023). New Target for Minoxidil in the Treatment of Androgenetic Alopecia. Drug Design, Development and Therapy. 17. 2537-2547. … Continue reading
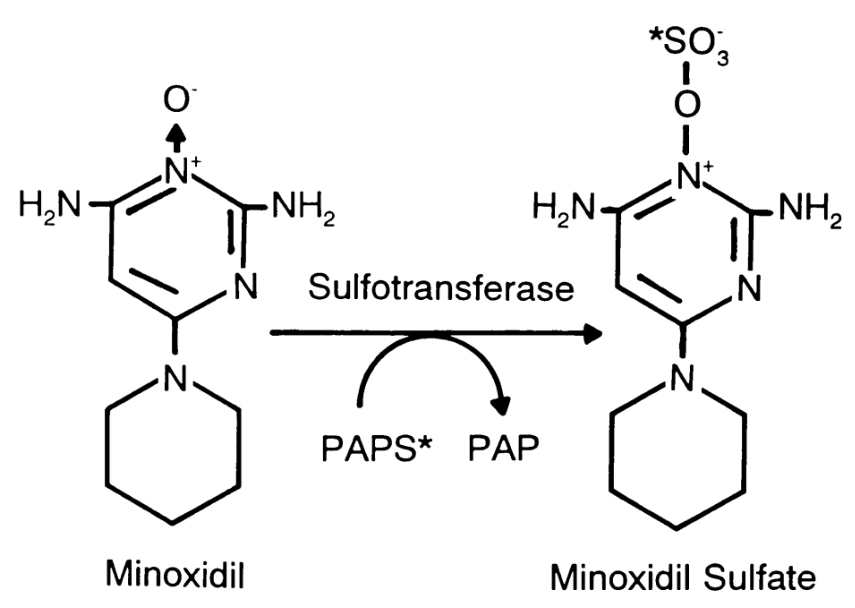
How is Minoxidil Activated in the Body?
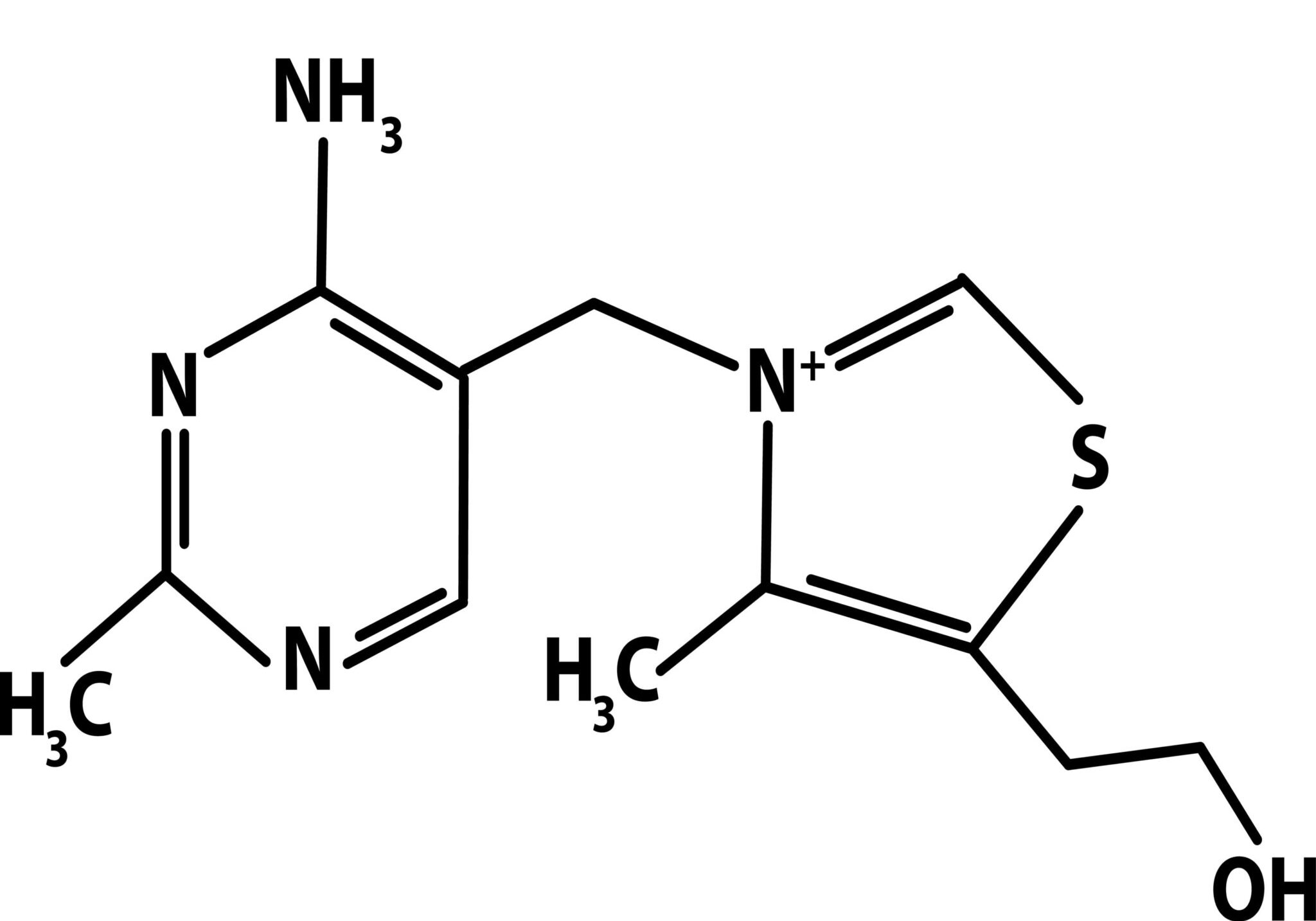
Minoxidil needs to be converted into its active form, minoxidil sulfate, by sulfotransferase enzymes before it can effectively stimulate hair growth.[4]Dhurat, R., Daruwalla, S., Pai, S., Kovacevic, M., McCoy, J., Shapiro, J., Sinclair, R., Vano-Galvan, S., Goren, A. (2021). SULT1A1 (Minoxidil Sulfotransferase) enzyme booster significantly improves … Continue reading This conversion primarily occurs in the scalp for topical minoxidil and in the liver for oral minoxidil.
Figure 1: The conversion of minoxidil to minoxidil sulfate by sulfotransferase.[5]Anderson, R.J., Kudlacek, P.E., Clemens, D.L. (1998). Sulfation of minoxidil by multiple human cytosolic sulfotransferases. Chemico-Biological Interactions. 109. 53-67. Available at: … Continue reading
However, enzyme activity varies significantly among individuals, meaning that some people naturally produce higher levels of sulfotransferase, allowing for better activation and increased effectiveness of the drug, while others have lower enzyme activity, which can limit its impact. You can read our article on how your genetics can influence sulfotransferase activity here.
Topical Minoxidil Vs. Oral Minoxidil: Activation Pathways
Topical minoxidil and oral minoxidil differ in application and effectiveness. Topical minoxidil is applied directly to the scalp, while oral minoxidil is taken as a pill.
The efficacy of these two treatment routes differs because of their distinct metabolic pathways.
Topical Minoxidil
Topical minoxidil is applied directly to the scalp, presenting a challenge for drug activation. The drug requires conversion to its active form, minoxidil sulfate, by sulfotransferase enzymes in the scalp. However, research has shown that 40-60% of people may lack sufficient enzyme levels to effectively activate the medication. This enzymatic variability means that for many users, topical minoxidil may not be 100% effective.
Around 1.4% of topical minoxidil is systemically absorbed, further limiting minoxidil efficacy. To counteract this limitation, researchers have explored combining minoxidil with treatments like microneedling or retinoic acid. It was found that with these combinations, minoxidil efficacy can significantly increase.[6]Lama, S.B.C., Pérez-González, L.A., Kosoglu, M.A., Dennis, R., Ortega-Quijano, D. (2024). Physical Treatments and Therapies for Androgenetic Alopecia. Journal of Clinical Medicine. 13(15). 4534. … Continue reading
Oral Minoxidil
Oral minoxidil, however, is metabolized in the liver, where sulfotransferase enzymes are abundant. The activated minoxidil sulfate then enters the bloodstream, where it reaches the hair follicle. Studies consistently demonstrate higher response rates for oral minoxidil compared to topical.[7]Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: … Continue reading However, because oral minoxidil involves systemic absorption, it comes with a broader potential for side effects.
What Are the Side Effects of Topical Minoxidil?
Common Side Effects
While topical minoxidil typically has a great safety profile, there are some side effects that people can experience.
Common side effects include:
- Scalp Irritation and Contact Dermatitis: Redness, itching, flaking, and burning sensations.
These are the most frequently reported side effects from minoxidil. One retrospective study found that 6.4% of men reported mainly irritant and allergic reactions to minoxidil.[8]Shadi, Z. (2023). Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy (Heidelb). 13(5). 1157-1169. Available at: … Continue reading These effects are typically due to an allergic reaction to propylene glycol rather than the minoxidil itself.[9]Lessmann, H., Schnuch, A., Geier, J., Uter, W. (2005). Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 53(5). 247-259. Available at: … Continue reading
To mitigate the negative skin effects of topical minoxidil, you can do several things. Starting with a lower concentration, such as 2% instead of 5%, can reduce the risk of irritation while still providing benefits. Additionally, reducing application frequency from twice to once daily can limit exposure. Switching to a foam-based minoxidil product can be particularly effective, as these formulations typically don’t contain propylene glycol, which is often responsible for uncomfortable side effects like irritation, redness, and scalp burning. Furthermore, incorporating a moisturizer into your scalp care routine can help keep the skin hydrated and comfortable, further alleviating potential irritation.
- Shedding Phase: Temporary increase in hair loss when starting treatment.
One study reported that 55% of topical minoxidil users experience minoxidil shedding.[10]Ghonemy, S., Bessar, H., Alarawi, A. (2019). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic … Continue reading This is considered a normal side effect and usually indicates that the treatment is working. The shedding phase typically begins 2 to 4 weeks after starting treatment and subsides within 6 to 8 weeks as the hair cycle normalizes. After a few months of continuous use, most users should start seeing visible new hair growth.[11]Kaiser, M., Abdin, R., Gaumond, S.I., Issa, T. N., Jiminez, J.J. (2023). Treatment of androgenetic alopecia: current guidance and unmet needs. Clinical Cosmetic and Investigational Dermatology. 16. … Continue reading
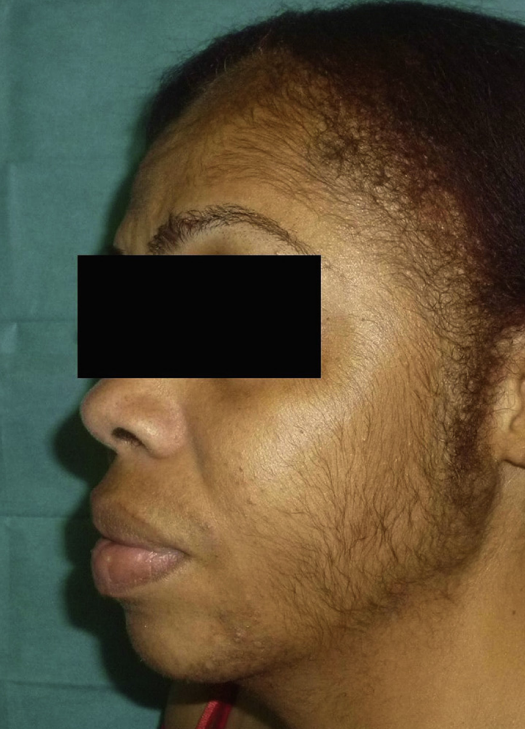
- Facial Hypertrichosis: Unwanted excess facial hair.
Women, especially those over 50 or with pre-existing facial hair, seem to be at higher risk of developing hypertrichosis from topical minoxidil use, and as with other side effects, it is more common when using the 5% than the 2% concentration.[12]Dawber, R.P.R, Rundegren, J. (2003). Hypertrichosis in females applying minoxidil topical solution and in normal controls. Journal of the European Academy of Dermatology and Venereology. 17(3). … Continue reading
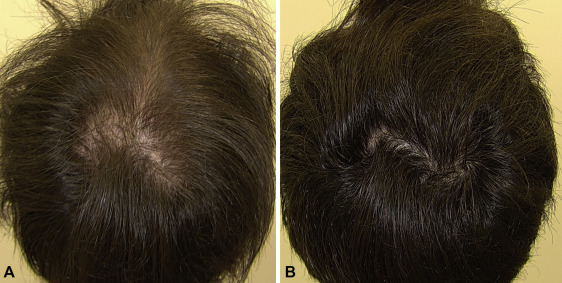
Figure 2: A 42 year old woman with generalized hypertrichosis after using 5% topical minoxidil for two weeks.[13]Gargallo V, Gutierrez C, Vanaclocha F, Guerra-Tapia A. Hipertricosis generalizada secundaria a minoxidil tópico. Actas Dermosifiliogr. 2015;106:599–600. Available at: … Continue reading
There are several theories for why hypertrichosis occurs in people using topical minoxidil.
- Product application: The product wasn’t applied carefully enough or was not completely dried before bed, leading to product transfer from the pillow to the face. However, this doesn’t account for hypertrichosis seen away from the site of application and is unlikely to cause the effects seen in case studies.
- Percutaneous absorption: Although topical minoxidil is primarily intended for local effects, a small amount can be absorbed systemically through the skin. This can lead to circulating minoxidil reaching hair follicles in distant areas of the body.
- Follicular hypersensitivity: Some people may have hair follicles that are exceptionally responsive to minoxidil. In these cases, even minimal systemic exposure might be sufficient to stimulate hair growth in distant follicles. This hypersensitivity appears to occur randomly, depending on the person.
- Dose-dependent response: Higher concentrations are more likely to cause hypertrichosis.
While hypertrichosis can occur, it is generally reversible once minoxidil treatment is stopped and typically resolves within 3-4 months.
There are a number of ways that hypertrichosis can be avoided or treated once it occurs.
Starting with a lower concentration can reduce the risk, especially for women and those with a history of excess facial hair. Careful application to the scalp only, allowing proper drying time, and adhering to the recommended dosage can also minimize systemic absorption and unintended spread.
Switching to a foam version may help those experiencing side effects. If you are experiencing mild hypertrichosis and don’t want to stop using minoxidil, you could use hair removal methods while continuing treatment.
For more severe cases, spironolactone (~25 mg daily) ando/or low-dose bicalutamide (~10 mg daily) have shown promise in managing minoxidil-induced hypertrichosis. However, these medications should be used under medical supervision.[14]Darendeliler, F., Bas, F., Balaban, S., Bundak, R., Demirkol, D., Saka, N., Gunoz, H. (1996). Spironolactone therapy in hypertrichosis. European Journal of Endocrinology. 135(5).604-608. Available … Continue reading,[15]Moussa, A., Kazmi, A., Bakhari, L., Sinclair, R.D. (2022). Bicalutamide improves minoxidil-induced hypertrichosis in female pattern hair loss: a retrospective review of 35 patients. Journal of the … Continue reading
- Headaches
Some people also experience headaches after using topical minoxidil. One study found that in users applying 2% minoxidil solution, 0.6% reported headaches, compared to 3% of participants using 5% minoxidil solution.[16]Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading
Some people may simply experience headaches due to the smell of the product they are using, in which case, switching to an alcohol-free or unscented alternative may help. Others may be particularly sensitive to minoxidil and its vasodilatory effects, which might contribute to headaches. In this case, switching to a lower concentration or consulting with a healthcare provider might be preferable.
Some of our members have also switched to nanoxidil, an analogue of minoxidil that may offer a better safety profile (you can read more about the research quality of nanoxidil here), and found that their headaches resolved.
The above side effects are more often seen in the 5% than 2% solutions and are typically considered to be non-serious.[17]Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of … Continue reading
Some of the rarer side effects include:
- Water Retention
While less common than with oral minoxidil, topical minoxidil application can lead to water retention. Some topical minoxidil users have reported under-eye bags, which may be due to increased water retention near the application areas. There are also anecdotal reports of “puffy face” from topical minoxidil application, suggesting localized fluid retention.[18]Gungor, S., Kocaturk, E., Topal, I.O. (2015). Frontal Edema Due to Topical Application of %5 Minoxidil Solution Following Mesotherapy Injections. International Journal of Trichology. 7(2). 86-87. … Continue reading
Anecdotally, there have also been reports of swollen feet and weight gain from using topical minoxidil; however, we couldn’t find these reports reflected in peer-reviewed literature. These effects may also stop with continued use of the drug, so some people keep an eye on the symptoms and wait to see if they go away.
However, you can try reducing the concentration or frequency of usage if the symptoms continue longer than you are comfortable with. Furthermore, excessive salt intake can exacerbate symptoms of edema. Limiting salt intake may help you resolve the edema without having to stop using minoxidil.[19]Patel, P., Nessel, T.A., Kumar, D. (2023). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (Accessed: … Continue reading
- Cardiovascular Issues
Some people also experience cardiovascular side effects. One case study found that applying large amounts of 2% topical minoxidil led to hypotension (low blood pressure) and feelings of faintness.[20]Ponomareva, M.A., Romanova, M.A., Shapshnikova, A.A., Piavchenko, G.A. (2024). Topical Minoxidil Overdose in a Young Man with Androgenetic Alopecia: A Case Report. Cureus. 16(6). E62382. Available … Continue reading Another case also documented low blood pressure and fainting after applying 12.5% topical minoxidil daily.[21]Dubrey, S.W., vanGriethuysen, J., Edwards, C.M.B. A hairy fall: syncope resulting from topical application of minoxidil. BMJ Case Reports. 1-2. Available at: https://doi.org/10.1136/bcr-2015-210945
Other people may experience heart palpitations, feelings of the heart beating rapidly or “skipping a beat”. While this is rare, one study found that 3.5% of women developed heart palpitations or a rapid heart rate after usage of 2% topical minoxidil solution compared to 1.8% who were using a 5% foam.[22]Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the … Continue reading
Therefore, we would recommend seeking medical advice and potentially finding a new treatment for hair loss if you experience these side effects.
So we’ve covered the side effects of topical minoxidil, but what about oral?
What Are the Side Effects of Oral Minoxidil?
Oral minoxidil side effects are similar to those of topical minoxidil, and both forms exhibit dose-dependent effects. However, oral administration leads to significantly greater systemic exposure to the drug than topical application. As a result, oral minoxidil typically produces more pronounced hair regrowth and more pronounced systemic side effects. It should be noted that use of oral minoxidil to improve hair regrowth is an off-label use of the drug and it is not FDA-approved for this indication.
Common Side Effects
- Hair Shedding
Like with topical minoxidil, you may experience increased hair shedding when you start taking minoxidil. This is considered to be a normal part of the hair cycle remodeling process, typically beginning two to four weeks after starting treatment and subsiding within six to eight weeks as the hair cycle normalizes.
- Hypertrichosis
While rare with topical minoxidil use, one of the most frequently reported side effects of oral minoxidil is hypertrichosis, which involves excessive hair growth on various parts of the body. This effect is dose-dependent, with some studies showing that increasing the dosage of oral minoxidil by just 1 mg daily is associated with a 17.6% increased risk of hypertrichosis.[23]Gupta, A.K., Hall, D.C., Talukder, M., Bamimore, M.A. (2022). There is a Positive Dose-Dependent Association between Low-Dose Oral Minoxidil and Its Efficacy for Androgenetic Alopecia: Findings from … Continue reading
Hypertrichosis frequently occurs on the face (sideburns, temples, upper lip, and chin), with rarer cases of generalized hypertrichosis occurring over the whole body.[24]Desai, D.D., Nohria, A., Brinks, A., Needle, C., Shapiro, J., Lo Sicco, K.I. (2024). Minoxidil-induced hypertrichosis: Pathophysiology, clinical implications, and therapeutic strategies. JAAD … Continue reading
- Fluid Retention
Fluid retention is another common side effect of oral minoxidil, occurring in about 1.3% of patients.[25]Trueb, R.M., Caballero-Uribe, N., Luu, N.N.C., Dmitriev, A. (2022). Serious complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 30. 97-98. Available at: … Continue reading This can manifest as mild swelling in the face, hands, or feet. In some cases, it may lead to rapid weight gain.
This weight gain can be significant and sudden, with some patients experiencing up to 20 pounds of weight gain (as water weight) in as little as one month.[26]Patel.K., Omar, J. (2023). Low dose oral minoxidil causing peripheral edema and rapid weight gain. Journal of General Internal Medicine. 38(Suppl 3). S592. Available at: … Continue reading
Fluid retention can manifest in several ways:
- Peripheral edema: Swelling in the extremities, particularly in the legs, ankles, and feet.
- Facial swelling: Puffiness or swelling in the face.
- Overall weight gain: A sudden increase in body weight, often 5 pounds or more.
This rapid retention can be concerning for patients and may lead to additional health risks if left unmanaged. Excess fluid in the body can potentially lead to congestive heart failure if not properly addressed.
To mitigate these side effects, there are a number of options:
- Use of diuretics: Doctors can prescribe a diuretic (water pill) alongside minoxidil to help counteract fluid retention. This is typically a low-dose loop diuretic.
- Sodium restriction: Limiting sodium intake can help reduce fluid retention. Patients are often not advised about this when starting minoxidil, which can exacerbate the problem.
- Dose adjustment: If fluid retention becomes severe, temporarily discontinuing minoxidil or significantly reducing the dose may be necessary. This allows the edema to resolve naturally before restarting at a lower dose.
- Regular monitoring: Daily weight monitoring can help identify fluid retention early, making it easier to manage.
- Gradual titration: Slowly increasing the dose of minoxidil over time, rather than starting with 5 mg, can help minimize side effects.
If these symptoms don’t resolve when trying these strategies, then it’s recommended to visit your doctor and potentially stop taking minoxidil.
- Lightheadedness, Tachycardia (high heart rate), Headache and Insomnia
Oral minoxidil can cause several cardiovascular and neurological side effects, including lightheadedness, tachycardia, headache, and insomnia.[27]Trueb, R.M., Caballero-Uribe, N., Luu, N.N.C., Dmitriev, A. Serious complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 30. 97-98. Available at: … Continue reading These effects are generally dose-dependent and more common at higher doses.
Lightheadedness has been reported to occur in 1.7% of patients, tachycardia in 0.9%, headache in 0.4%, and insomnia in 0.2% of patients. These can all be symptoms of decreased blood pressure due to minoxidil’s vasodilatory properties.
Headaches and insomnia are reported in around 0.4% and 0.2% of patients, respectively, using low-dose oral minoxidil. While the exact mechanism of these side effects is not clear, it is thought that it could be related to the vasodilatory effects of the drug.
What Are The Cardiovascular Concerns of Oral Minoxidil Usage?
The severity and frequency of cardiovascular side effects are closely tied to the dosage of oral minoxidil. A meta-regression analysis found a positive dose-dependent correlation between low-dose oral minoxidil and the risk of cardiovascular adverse events.
Low Dose (0.25-1.25 mg/day)
At lower doses, oral minoxidil is generally well-tolerated. Women typically start with doses ≤ 1 mg, which minimizes the risk of significant side effects. Lower doses are considered to be a safer starting point for most patients, and even very low doses (0.25 mg/day) have shown efficacy in some studies.[28]Ramírez-Marín, H.A., Tosti, A. (2022). Role of oral minoxidil in patterned hair loss. Indian Dermatology Online Journal. 13(6). 729-733. Available at: https://doi.org/10.4103/idoj.idoj_246_22 If you are experiencing side effects at higher doses, you can reduce your dose at home using a pill cutter.
Moderate Dose (2.5-5 mg/day)
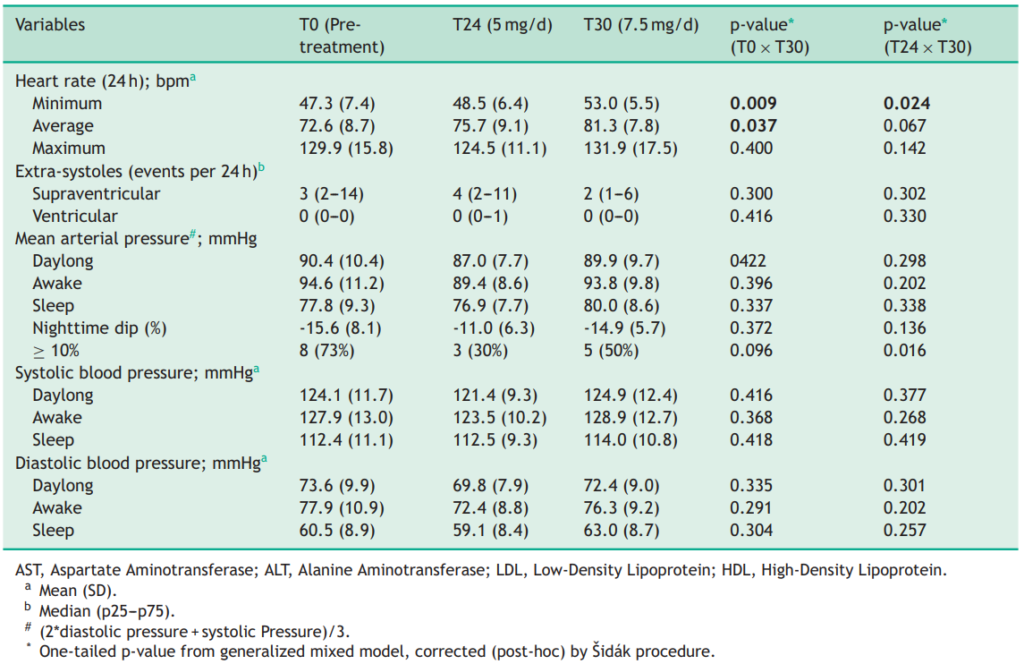
Men may be prescribed up to 5 mg of minoxidil daily, which can increase the likelihood of side effects such as dizziness and fluid retention. A recent study examined the effects of 7.5 mg/day oral minoxidil in patients with normal blood pressure and AGA.[29]Sanabria, B.D., Perdomo, Y.C., Miot, H.A., Ramos, P.M. (2024). Oral minoxidil 7.5 mg for hair loss increases heart rate with no change in blood pressure in 24 h ambulatory blood pressure monitoring. … Continue reading The results showed a mild increase in heart rate but no significant changes in blood pressure, suggesting that doses slightly higher than the typical 5 mg can be tolerated. However, this should only be considered under medical supervision.
Figure 3: Heart rate and blood pressure monitoring of 11 adult males with AGA after 24 weeks (T24) of treatment with 5 mg/day of oral minoxidil and after 6 weeks (T30) of treatment with 7.5 mg/day of oral minoxidil.[30]Sanabria, B.D., Perdomo, Y.C., Miot, H.A., Ramos, P.M. (2024). Oral minoxidil 7.5 mg for hair loss increases heart rate with no change in blood pressure in 24 h ambulatory blood pressure monitoring. … Continue reading
High Dose (10 mg/day and above)
Doses above 10 mg daily are associated with a higher risk of serious cardiac events and are typically not recommended for hair loss treatment. The hypotensive effect of oral minoxidil becomes more significant at these higher doses and is often prescribed alongside beta blockers and diuretics to manage the side effects.
How Can I Mitigate Oral Minoxidil Side Effects?
We have covered a number of ways to mitigate oral minoxidil side effects, but there are some further ways that you can adjust the use of minoxidil to reduce your risk.
Split-Dosing
Splitting the daily dosage of oral minoxidil into two administrations, one in the morning and one in the evening, can potentially optimize its efficacy while minimizing side effects. This approach is based on the pharmacokinetics of oral minoxidil, which has a relatively short half-life of approximately 3-4 hours.[31]Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., … Continue reading
By dividing the total daily dose, you can maintain more consistent blood levels of minoxidil throughout the day, potentially leading to more stable hair growth stimulation. For example, if 5 mg is prescribed daily, taking 2.5 mg in the morning and 2.5 mg in the evening may be more beneficial than a single 5 mg dose.
Sublingual Minoxidil
Some recommend using sublingual minoxidil as an alternative to traditional oral minoxidil. Sublingual administration involves a tablet that dissolves under the tongue. One 2021 randomized, double-blind, placebo-controlled phase 1b clinical trial investigated this delivery method.[32]Bokhari, L., Jones, L.N., Sinclair, R.D. (2021). Sublingual minoxidil for the treatment of male and female pattern hair loss: a randomized, double-blind, placebo-controlled, phase 1B clinical trial. … Continue reading The study tested daily doses of 0.45 mg to 4.05 mg of sublingual minoxidil.
This method offers several advantages:
- Bypasses first-pass metabolism in the liver.
- It allows for direct entry into the bloodstream, which can travel inactivated to the scalp and be activated by sulfotransferase at the site.
- Reduces systemic side effects.
- Improves bioavailability.
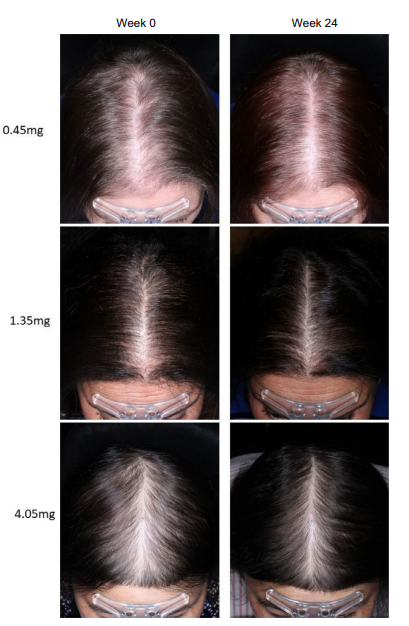
Key findings from the study included a dose-dependent improvement in hair parameters, with reduced side effects compared to oral minoxidil and no significant effect on blood pressure. In the blood, peak serum concentrations of minoxidil were only 10% of those seen with typical oral minoxidil.
Figure 4: Effect of different doses of sub-lingual minoxidil on hair regrowth outcomes after 24 weeks.[33]Bokhari, L., Jones, L.N., Sinclair, R.D. (2021). Sublingual minoxidil for the treatment of male and female pattern hair loss: a randomized, double-blind, placebo-controlled, phase 1B clinical trial. … Continue reading
At the 24-week follow-up, approximately 45% of patients in the 0.45 mg sublingual minoxidil group experienced improvements in frontal hair density, and 55% showed vertex improvement. Higher doses (4.05 mg) led to further results, with nearly 67% of patients experiencing improvements in both frontal and vertex hair density.
Sublingual minoxidil appears to be particularly beneficial for people concerned about the side effects of oral minoxidil. The medication was undetectable in plasma after 24 hours, and the mean peak minoxidil plasma concentration was significantly below the threshold associated with changes in blood pressure.
While the results are promising, it should be noted that this is the only study using sublingual minoxidil for AGA, and further studies with larger patient numbers are needed.
Switch To Topical Minoxidil
There is a chance that none of these options will work out for you, so you can try to switch to topical minoxidil. The side effects are more manageable for topical treatments, meaning that you can increase the dose and try to pair them with other treatments like microneedling or retinoic acid to further improve hair growth outcomes.
Can I Use Minoxidil If I Am Planning a Family?
Medical professionals generally advise against using both topical and oral minoxidil for both men and women when planning a family and for women during pregnancy and while breastfeeding.
While there is limited data on human pregnancies, animal studies have shown potential risks, including evidence of increased fetal resorption at high doses, one case report of fetal malformation associated with topical minoxidil use, and neonatal hypertrichosis reported following exposure during pregnancy.[34]Drugs. (2023). Minoxidil pregnancy and breastfeeding warnings. Drugs.com. Available at: https://www.drugs.com/pregnancy/minoxidil.html (Accessed: February 2025),[35]Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth defects research. Part A, Clinical and … Continue reading
There is a significant lack of well-controlled studies on minoxidil use during pregnancy and lactation. However, given the animal studies, medical professionals typically recommend avoiding minoxidil use when planning pregnancy, during pregnancy, and while breastfeeding, using adequate contraception if taking minoxidil, and discontinuing minoxidil use before attempting to conceive.[36]National Institute of Health and Care Excellence. (2021). Topical minoxidil. NICE. Available at: … Continue reading
Does Minoxidil Affect Male Fertility?
Current research suggests that minoxidil has minimal to no direct impact on male fertility. However, some research has linked minoxidil with oxidative stress and morphological changes to the testicles, which could indicate a potential negative impact.[37]Santana, F.F.V., Lozi, A.A., Goncalves, R.V., Silva, J.D., Matta, S.L.P.D. (2023). Comparative effects of finasteride and minoxidil on the male reproductive organs: A systematic review of in vitro … Continue reading
If you’re thinking about trying minoxidil and you are trying to conceive or have a pregnant partner, then it is advisable to talk to a medical professional before starting any treatment.
What if I’m Still Experiencing Side Effects?
Fortunately, minoxidil is just one of several treatment options available. If you’ve tried all of them and still experience side effects, you might consider exploring alternative therapies.
We have a wealth of information available so you can weigh your options and find out exactly how each treatment works and what your regrowth roadmap might look like. If you have any questions, reach out in the dedicated discussion thread below.
Final Thoughts
While minoxidil remains one of the most widely used treatments for AGA, both topical and oral formulations present unique challenges. The choice of which to use should be weighed carefully against the side effects, varying from mild scalp irritation to more significant cardiovascular effects. Ultimately, while the research supports minoxidil’s efficacy, it is not the only option out there, and if it isn’t working for you, then it is important to find the right one.
References[+]
References ↑1 Bryan, J. (2011). How minoxidil was transformed from an antihypertensive to hair-loss drug. The Pharmaceutical Journal. Available at: https://pharmaceutical-journal.com/article/news/how-minoxidil-was-transformed-from-an-antihypertensive-to-hair-loss-drug#:~:text=DAMN%2DO%20was%20effective%20in,seen%20in%20canine%20toxicity%20studies.&text=Despite%20the%20adverse%20effects%2C%20demand,week%20limit%20on%20treatment%20duration.&text=Owing%20to%20the%20drug’s%20effectiveness,of%20hypertrichosis%20began%20to%20emerge. (Accessed: February 2025) ↑2 Alhayaza, G., Hakami, A., AlMarzouk, L.H., Al Qurashi, A.A., Alghamdi, G., Alharithy, R. (2023). Topical minoxidil reported hair discoloration: a cross-sectional study. Dermatology Reports. 16(1). 9745. Available at: https://doi.org/10.4081/dr.2023.9745 ↑3 Shen, Y., Zhu, Y., Zhang, L., Sun, J., Xie, B., Zhang, H., Song, X. (2023). New Target for Minoxidil in the Treatment of Androgenetic Alopecia. Drug Design, Development and Therapy. 17. 2537-2547. Available at: https://doi.org/10.2147/DDDT.S427612 ↑4 Dhurat, R., Daruwalla, S., Pai, S., Kovacevic, M., McCoy, J., Shapiro, J., Sinclair, R., Vano-Galvan, S., Goren, A. (2021). SULT1A1 (Minoxidil Sulfotransferase) enzyme booster significantly improves response to topical minoxidil for hair growth. 21(1). 343-346. Available at: https://doi.org/10.1111/jocd.14299 ↑5 Anderson, R.J., Kudlacek, P.E., Clemens, D.L. (1998). Sulfation of minoxidil by multiple human cytosolic sulfotransferases. Chemico-Biological Interactions. 109. 53-67. Available at: https://doi.org/10.1016/S0009-2797(97)00120-8 ↑6 Lama, S.B.C., Pérez-González, L.A., Kosoglu, M.A., Dennis, R., Ortega-Quijano, D. (2024). Physical Treatments and Therapies for Androgenetic Alopecia. Journal of Clinical Medicine. 13(15). 4534. Available at: https://doi.org/10.3390/jcm13154534 ↑7 Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: https://doi.org/10.1080/09546634.2021.1945527 ↑8 Shadi, Z. (2023). Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy (Heidelb). 13(5). 1157-1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑9 Lessmann, H., Schnuch, A., Geier, J., Uter, W. (2005). Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 53(5). 247-259. Available at: https://doi.org/10.1111/j.0105-1873.2005.00693.x. ↑10 Ghonemy, S., Bessar, H., Alarawi, A. (2019). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. Journal of Dermatological Treatment. 32(2). 236-241. Available at: https://doi.org/10.1080/09546634.2019.1654070 ↑11 Kaiser, M., Abdin, R., Gaumond, S.I., Issa, T. N., Jiminez, J.J. (2023). Treatment of androgenetic alopecia: current guidance and unmet needs. Clinical Cosmetic and Investigational Dermatology. 16. 1387-1406. Available at: https://doi.org/10.2147/CCID.S385861 ↑12 Dawber, R.P.R, Rundegren, J. (2003). Hypertrichosis in females applying minoxidil topical solution and in normal controls. Journal of the European Academy of Dermatology and Venereology. 17(3). 271-275. Available at: https://doi.org/10.1046/j.1468-3083.2003.00621.x. ↑13 Gargallo V, Gutierrez C, Vanaclocha F, Guerra-Tapia A. Hipertricosis generalizada secundaria a minoxidil tópico. Actas Dermosifiliogr. 2015;106:599–600. Available at: https://doi.org/10.1016/j.adengl.2015.06.019 ↑14 Darendeliler, F., Bas, F., Balaban, S., Bundak, R., Demirkol, D., Saka, N., Gunoz, H. (1996). Spironolactone therapy in hypertrichosis. European Journal of Endocrinology. 135(5).604-608. Available at: https://doi.org/10.1530/eje.01350604 ↑15 Moussa, A., Kazmi, A., Bakhari, L., Sinclair, R.D. (2022). Bicalutamide improves minoxidil-induced hypertrichosis in female pattern hair loss: a retrospective review of 35 patients. Journal of the American Acadamy of Dermatology. 87(2). 488-490. Available at: https://doi.org/10.1016/j.jaad.2021.10.048 ↑16 Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑17 Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of Cosmetic Dermatology. 20. 3759-3781. Available at: https://doi.org/10.1111/jocd.14537 ↑18 Gungor, S., Kocaturk, E., Topal, I.O. (2015). Frontal Edema Due to Topical Application of %5 Minoxidil Solution Following Mesotherapy Injections. International Journal of Trichology. 7(2). 86-87. Available at: https://doi.org/10.4103/0974-7753.160124 ↑19 Patel, P., Nessel, T.A., Kumar, D. (2023). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (Accessed: February 2025) ↑20 Ponomareva, M.A., Romanova, M.A., Shapshnikova, A.A., Piavchenko, G.A. (2024). Topical Minoxidil Overdose in a Young Man with Androgenetic Alopecia: A Case Report. Cureus. 16(6). E62382. Available at: https://doi.org/10.7759/cureus.62382 ↑21 Dubrey, S.W., vanGriethuysen, J., Edwards, C.M.B. A hairy fall: syncope resulting from topical application of minoxidil. BMJ Case Reports. 1-2. Available at: https://doi.org/10.1136/bcr-2015-210945 ↑22 Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. Journal of the American Academy of Dermatology. 65(6). 1126-1134. Available at: https://doi.org/10.1016/j.jaas.2010.09.724 ↑23 Gupta, A.K., Hall, D.C., Talukder, M., Bamimore, M.A. (2022). There is a Positive Dose-Dependent Association between Low-Dose Oral Minoxidil and Its Efficacy for Androgenetic Alopecia: Findings from a Systematic Review with Meta-Regression Analyses. Skin Appendage Disorders. 8(5). 355-361. Available at: https://doi.org/10.1159/000525137 ↑24 Desai, D.D., Nohria, A., Brinks, A., Needle, C., Shapiro, J., Lo Sicco, K.I. (2024). Minoxidil-induced hypertrichosis: Pathophysiology, clinical implications, and therapeutic strategies. JAAD Reviews. 2. 41-49. Available at: https://doi.org/10.1016/j.jdrv.2024.08.002 ↑25 Trueb, R.M., Caballero-Uribe, N., Luu, N.N.C., Dmitriev, A. (2022). Serious complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 30. 97-98. Available at: https://doi.org/10.1016/j.jdcr.2022.09.035 ↑26 Patel.K., Omar, J. (2023). Low dose oral minoxidil causing peripheral edema and rapid weight gain. Journal of General Internal Medicine. 38(Suppl 3). S592. Available at: https://scholarlycommons.henryford.com/internalmedicine_mtgabstracts/162/ (Accessed: February 2024 ↑27 Trueb, R.M., Caballero-Uribe, N., Luu, N.N.C., Dmitriev, A. Serious complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 30. 97-98. Available at: https://doi.org/10.1016/j.jdcr.2022.09.035 ↑28 Ramírez-Marín, H.A., Tosti, A. (2022). Role of oral minoxidil in patterned hair loss. Indian Dermatology Online Journal. 13(6). 729-733. Available at: https://doi.org/10.4103/idoj.idoj_246_22 ↑29 Sanabria, B.D., Perdomo, Y.C., Miot, H.A., Ramos, P.M. (2024). Oral minoxidil 7.5 mg for hair loss increases heart rate with no change in blood pressure in 24 h ambulatory blood pressure monitoring. Anais Brasileiros de Dermatologia. 99(5). 734-736. Available at: https://doi.org/10.1016/j.abd.2023.08.016 ↑30 Sanabria, B.D., Perdomo, Y.C., Miot, H.A., Ramos, P.M. (2024). Oral minoxidil 7.5 mg for hair loss increases heart rate with no change in blood pressure in 24 h ambulatory blood pressure monitoring. Anais Brasileiros de Dermatologia. 99(5). 734-736. Available at: https://doi.org/10.1016/j.abd.2023.08.016 ↑31 Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., Salas-Callo, C.I., Vincenzi, C., Yin, L., Lo-Sicco, K., Waskiel-Burnat, A., Starace, M., Zamorano, J.L., Jaen-Olasolo, P., Piraccini, B.M., Rudnicka, L., Shapiro, J., Tosti, A., Sinclair, R., Bhoyrul, B. (2021). Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. Journal of the American Academy of Dermatology. 84(6). 1644-1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑32 Bokhari, L., Jones, L.N., Sinclair, R.D. (2021). Sublingual minoxidil for the treatment of male and female pattern hair loss: a randomized, double-blind, placebo-controlled, phase 1B clinical trial. Journal of the European Academy of Dermatology and Venereology. (36)1. E62-e66. Available at: https://doi.org/10.1111/jdv.17623 ↑33 Bokhari, L., Jones, L.N., Sinclair, R.D. (2021). Sublingual minoxidil for the treatment of male and female pattern hair loss: a randomized, double-blind, placebo-controlled, phase 1B clinical trial. Journal of the European Academy of Dermatology and Venereology. (36)1. E62-e66. Available at: https://doi.org/10.1111/jdv.17623 ↑34 Drugs. (2023). Minoxidil pregnancy and breastfeeding warnings. Drugs.com. Available at: https://www.drugs.com/pregnancy/minoxidil.html (Accessed: February 2025) ↑35 Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth defects research. Part A, Clinical and molecular teratology. 67(12). 997-1001. Available at: https://doi.org/10.1002/bdra.10095 ↑36 National Institute of Health and Care Excellence. (2021). Topical minoxidil. NICE. Available at: https://cks.nice.org.uk/topics/female-pattern-hair-loss-female-androgenetic-alopecia/prescribing-information/topical-minoxidil/ (Accessed: February 2025) ↑37 Santana, F.F.V., Lozi, A.A., Goncalves, R.V., Silva, J.D., Matta, S.L.P.D. (2023). Comparative effects of finasteride and minoxidil on the male reproductive organs: A systematic review of in vitro and in vivo evidence. Toxicology and Applied Pharmacology. 478. 11670. Available at: https://doi.org/10.1016/j.taap.2023.116710. Research continues to show that oral minoxidil is an effective off-label treatment for men with pattern hair loss. The general rule-of-thumb: the bigger the dose, the better hair regrowth. But there’s a catch…
Higher dosages of oral minoxidil come at a risk of higher risk of side effects: excessive body hair growth, limb swelling, low blood pressure, and even heart palpitations.
Knowing this, is there a “best dose” for oral minoxidil (in mg) for men with pattern hair loss? More specifically, which dose of oral minoxidil maximizes our chances for hair regrowth and minimizes our risks of adverse events?
This Quick Win uncovers the latest research. The short answer: studies show that 2.5mg daily seems to be a tolerable, effective dose for most men with pattern hair loss. But the right dose for you will depend on (1) your severity of hair loss, and (2) your tolerance with side effects (not all of them are bad).
Note: Quick Wins are short articles focused on answering one question about hair loss. Given their specificity, these articles are written in a more scientific tone. If you’re new to hair loss education, start with these articles.
Interested in Oral Minoxidil?
Low-dose oral minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Oral minoxidil: research at a glance
To date, there have been fewer than 10 studies published on oral minoxidil for androgenic alopecia (AGA). Doses studied range from 0.25mg to 5.0mg daily, and study durations (at least the ones we evaluated) range from 24-52 weeks.
Across studies, there is a clear trend: the higher the dose of oral minoxidil, the better the hair regrowth. But this relationship doesn’t tell the whole story – as these higher dosages seem to confer with higher reports of side effects.
So, here’s what you should know before starting any daily dose of oral minoxidil.
#1: Oral minoxidil dosages from 0.25mg to 5mg improve pattern hair loss
The three most recent (and most robust) studies on oral minoxidil all varied dosing by 0.25mg, 2.5mg, and 5.0mg daily. All of them showed benefit – with higher dosages demonstrating visual improvements. Just see these photos of a male who took 5mg of oral minoxidil daily for 3 months.[1]Jimenez-Cauhe, Juan et al. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. Journal of the American Academy of Dermatology, Volume 81, Issue 2, 648 – 649
Male with AGA: improvement with 5mg oral minoxidil over 3 months
So, let’s organize the findings of these three studies by dosage. Then, let’s evaluate their results in terms of:
- Response rates (i.e., the percent of people who experienced an improvement), and…
- Side effects (i.e., unintended effects of the drug)
Do we see any trends in data? Specifically, is there a “sweet spot” where most men can maximize their chances of hair regrowth from oral minoxidil while minimizing their risk of serious side effects?
Yes.
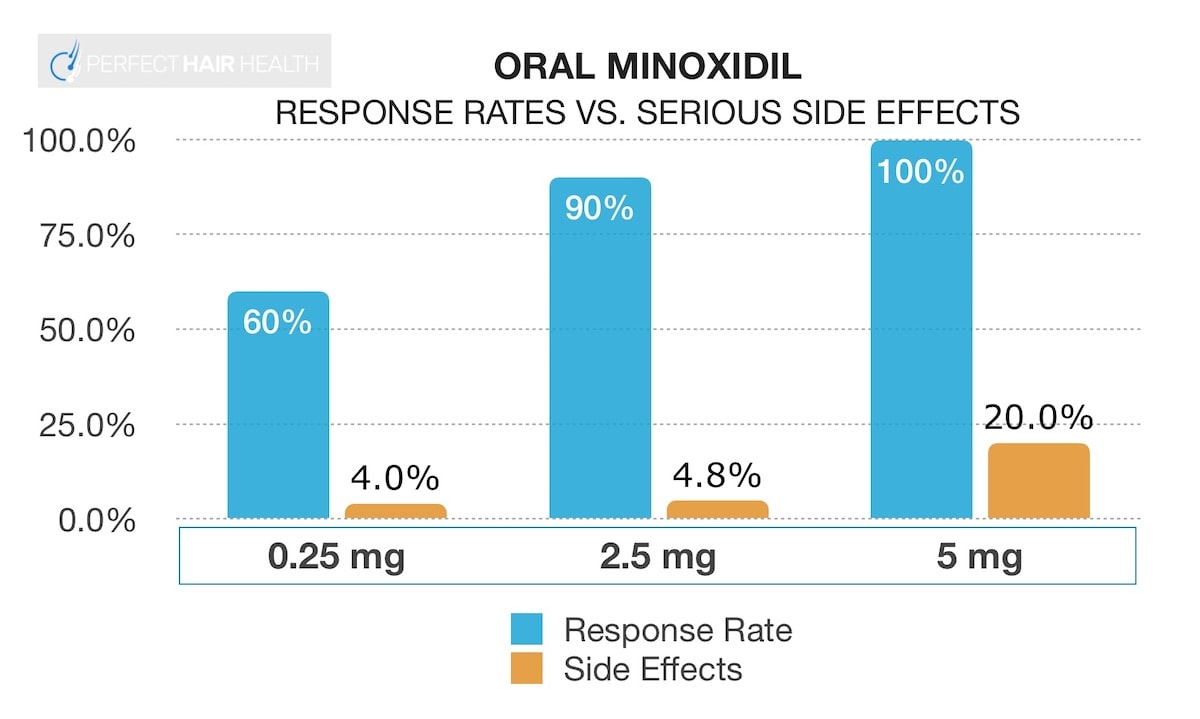
Oral minoxidil for AGA: study results by dosage
See each study’s summaries.
Daily Dose (Duration)
Response Rate
Side Effects
0.25 mg
(6+ months)[2]Pirmez, Rodrigo et al. Very-low-dose oral minoxidil in male androgenetic alopecia: A study with quantitative trichoscopic documentation. Journal of the American Academy of Dermatology, Volume 82, … Continue reading60%
90%:
increased beard growth (52%), increased body hair (20%), hair shedding (16%), pedal edema (4%)2.5 mg to 5 mg
(6-12 months)[3]Jimenez-Cauhe, Juan et al. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. Journal of the American Academy of Dermatology, Volume 81, Issue 2, 648 – 64990%
29.3%:
increased body hair (24.3%), lower limb edema (4.8%), hair shedding (2.4%)5 mg
(6 months)[4]Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Journal of the American Academy of Dermatology, Volume 72, Issue 5, AB113100%
100%:
increased body hair growth (93%), pedal edema (10%), heart / EKG alterations (10%)At first glance, the risk of side effects across even small dosages seems ridiculously high (90%+).
However, not all of these side effects are bad.
For instance, of the side effects reported in these studies, the overwhelming majority of them constituted increased body/facial hair growth. Most men aren’t going to care about this. In fact, man men might even prefer more body or facial hair.
Secondly, increased hair shedding was generally only reported at the beginning of each study. This is because, when starting minoxidil (topical or oral), the drug can “kickstart” a new anagen (growth) phase of hairs affected by androgenic alopecia (AGA). This can lead us to shed any hairs that were already primed to fall out soon anyway – specifically, catagen or telogen hairs – thereby giving the illusion of thinner hair in the first 1-2 months of treatment. However, as these hairs grow back, they’re usually much thicker, and thereby improve hair density. Long-story short: in most cases, hair shedding from minoxidil isn’t long-lived.
So, for this exercise, let’s discount increased body/facial hair growth and increased hair shedding as temporary and/or non-problematic side effects. Instead, let’s re-run our analysis and only consider serious side effects – edema, EKG alterations, etc.
Within this context, are all dosages of oral minoxidil as scary?
No. In fact, it seems like there’s a “sweet spot” for oral minoxidil where we can maximize hair regrowth while minimizing our risk of bad side effects: at 2.5mg daily.
Keep in mind the following chart is based on preliminary data on low-dose oral minoxidil, and that this article reflects the clinical studies available on oral minoxidil for androgenic alopecia as of 2020. As better-designed studies are published, these numbers will evolve:
Moreover, it’s really only at dosages of 5mg that we see an appreciable increase in concerning side effects – namely, edema (water retention / swelling) and cardiac alterations (i.e., lower heart rates).
So, 2.5 mg daily of oral minoxidil might be the “sweet spot” for most male pattern hair loss sufferers.
#2: Oral minoxidil’s efficacy may vary depending on hair loss severity
Most clinical trials on androgenic alopecia will select study participants who all have similar severities of hair loss. This is known as standardization. And for most trials, investigators usually prefer men who have medium-severity androgenic alopecia (i.e., Norwood 3-4). This is usually because men with Norwood 3-4 level hair loss (1) are representative of the population of hair loss sufferers who may later opt for this treatment, and (2) have enough hair follicle miniaturization and hair loss to effectively evaluate cosmetic improvements to hair thinning.
Having said that, most studies on oral minoxidil aren’t standardized to Norwood 3-4 participants. So, it’s a bit disingenuous to make comparisons across studies for response rates and side effects. In other words, please take our above analysis with a grain of salt.
With that said, with different age and/or hair loss severity across studies, we can get more granular data on who tends to respond well to oral minoxidil.
Based on the above studies (and others we looked into for our analysis), the trend aligned with intuition: if you don’t have severe hair loss, you can get away with lower dosages of oral minoxidil. If you do have severe hair loss, you’ll need a higher dose.
In other words:
- If you have mild male pattern baldness, 0.25mg daily might work fine as a starting point
- A more moderate case of hair loss might require a dose of 1-2.5mg daily
- If you have severe male pattern baldness, you’re probably going to need closer to 5mg daily
- If other treatments haven’t worked for you (resistant hair loss), you may also need a higher daily dose (2.5-5mg) or combination treatments with finasteride
Then again, higher doses come with more side effects. This is where dosing gets hyper-specific.
#3: If you’re going to try oral minoxidil, you’ll need to work with a doctor to figure out your unique risk profile and the right dose
Oral minoxidil is an antihypertensive drug (lowers blood pressure). It can also cause fluid retention. Therefore, if your health history indicates problems surrounding low blood pressure, fainting spells, or edema (swelling), you may be at a higher risk of complications from taking the drug.
Moreover, oral minoxidil can stimulate hair growth everywhere… not just on the scalp. For some men, this may be a bonus. For others, it might be a drawback. If this is a drawback for you, then it’s worth noting that reports of increased body / facial hair even occurred at lower dosages (0.25 mg) of oral minoxidil. So, if you’re concerned about this, maybe oral minoxidil isn’t right for you.
Long-tory short: for the safest and most effective use of oral minoxidil, discuss your medical history and preferences with your doctor. Then convince him or her to prescribe you oral minoxidil.
Note: a dermatologist specializing in hair loss is much more likely to write you a prescription. So, if you don’t want to waste any time, make a list of dermatologists in your area, call them to see if they’re open to prescribing oral minoxidil, and then only visit the ones who prescribe the drug.
#4: Like topical minoxidil, oral minoxidil likely works better alongside other treatments
When it comes to treating pattern hair loss, combination treatments tend to almost always outperform mono-treatments.
In some cases, combination therapies allow us to use the lowest dose of a drug possible without sacrificing results. Some studies suggest this is the case for women with pattern hair loss who take 0.25mg of oral minoxidil + 25mg of spironolactone: they minimize the risk of side effects of either drug while getting hair regrowth that often exceeds that of high dosages of either drug.
In other cases, combination therapies can actually enhance the efficacy of drugs. This tends to be true of men taking topical minoxidil, and who then add in once-weekly microneedling, thereby making topical minoxidil 400% more effective (according to some investigation groups). [5]English RS Jr, Ruiz S, DoAmaral P. Microneedling and Its Use in Hair Loss Disorders: A Systematic Review. Dermatol Ther (Heidelb). 2022 Jan;12(1):41-60. doi: 10.1007/s13555-021-00653-2. Epub 2021 Dec … Continue reading
While there aren’t many studies that exhaustively explore this relationship for oral minoxidil, the odds are that this medication also works better as a combination therapy. So, if you’re going to commit to oral minoxidil, consider stacking it with other therapies.
Again, research here is limited, but there are a host of things you can try in combination with oral minoxidil that might increase results.
- DHT reducers (finasteride, saw palmetto, etc.)
- Microneedling
- Massage
- Ketoconazole shampoo
- Low-level laser therapy (LLLT)
- Platelet-rich plasma therapy (PRP)
…and more.
The bottom line
When it comes to oral minoxidil, the best daily dosage for men with pattern hair loss may vary depending on(1) your tolerance for certain side effects, and (2) your severity of hair loss. Consider these recommendations a mere starting point until more research emerges:
- Men with mild AGA, men at a higher risk of complication, or men who don’t want extra hair growth on their body/face: 0.25-1mg.
- Men with moderate AGA: 1-2.5mg.
- Men with resistant AGA (you’ve tried other treatments and they haven’t worked) or severe AGA: 2.5-5mg.
Although these guidelines are a rough ballpark, chances are you fit into one of these categories and, with the help of a doctor, can find the best oral minoxidil dosage for you.
Questions? Comments? Please reach out in the comments section.
References[+]
References ↑1, ↑3 Jimenez-Cauhe, Juan et al. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. Journal of the American Academy of Dermatology, Volume 81, Issue 2, 648 – 649 ↑2 Pirmez, Rodrigo et al. Very-low-dose oral minoxidil in male androgenetic alopecia: A study with quantitative trichoscopic documentation. Journal of the American Academy of Dermatology, Volume 82, Issue 1, e21 – e22 ↑4 Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Journal of the American Academy of Dermatology, Volume 72, Issue 5, AB113 ↑5 English RS Jr, Ruiz S, DoAmaral P. Microneedling and Its Use in Hair Loss Disorders: A Systematic Review. Dermatol Ther (Heidelb). 2022 Jan;12(1):41-60. doi: 10.1007/s13555-021-00653-2. Epub 2021 Dec 1. PMID: 34854067; PMCID: PMC8776974. Vitamin B1 – also known as thiamin (or thiamine) – is part of the B-vitamin complex. Marketers claim that vitamin B1 can help support healthy hair growth, reduce hair shedding, and even prevent hair loss. Then again, marketers also make the same claims about B-vitamins like biotin, niacin, and vitamin B12. And typically, the claims are just plain wrong.
So, is vitamin B1 any different? In this article, we’ll dive into the evidence (and answers).
First, we’ll uncover why some people that vitamin B1 helps support hair growth. Then, we’ll dive into the evidence on the vitamin B1 / thiamin-hair loss connection. Finally, we’ll dive into evidence that might implicate vitamin B1 as an accelerator of hair loss… and steps to take if you suspect you’re deficient.
By the end, you’ll have a better idea of whether vitamin B1 is a worthy investment for your hair, or just another marketing gimmick. If you have any questions or comments, please post them below!
All-Natural Hair Supplement
The top natural ingredients for hair growth, all in one supplement.
Take the next step in your hair growth journey with a world-class natural supplement. Ingredients, doses, & concentrations built by science.

What is Vitamin B1?
Vitamin B1 (also thiamin or thiamine), is one of the many members of the B vitamin family.
Vitamin B1/thiamine
Researchers first identified this essential micronutrient through the study of beri beri, a serious disease of the nervous system that was common in South East Asia prior to the 1900s.
Unlike most diseases in that time, beri beri was much more common among wealthy citizens than it was among poorer citizens. Upon further investigation, researchers found the reason for the perplexing discrepancy was actually attributed to the differences in rice consumption.
While poorer individuals tended to consume brown rice, richer individuals tended to consume milled white rice — devoid of the husks, bran, and germ.
Through experimentation with this brown rice, a Polish biochemist, Casmir Funk, was able to isolate the compound that prevented beri beri. This compound? He termed it thiamine, meaning sulfur-containing amine — what we now know as vitamin B1.
Fast forward to today: we now know that vitamin B1 plays a crucial role in mitochondrial health, metabolism of macronutrients, and energy production — processes that are essential for the normal functioning of almost every cell in the body (1).
But, beyond the prevention of serious neurological conditions, does vitamin B1 have any benefit to our hair?
Let’s explore the evidence.
Vitamin B1: might it help support hair growth?
There’s no doubt that vitamin B1 is critical for important processes, like (1):
- The production of ATP, the energy molecule that fuels cellular activity
- The synthesis of amino acids
- The creation of NADPH, a co-factor necessary for steroid hormone production, fatty acid synthesis, and more.
And similar to other B-complex vitamins, vitamin B1’s role in these processes often form the basis of the claim that vitamin B1 can influence hair loss. After all, if you can’t produce amino acids — the raw material our hair is actually made of — how can you grow hair?
But, is this actually true? Are there any other ways that B1 might influence our hair? And what does this mean in the context of diet in the developed world?
Let’s explore the evidence.
Does a thiamine / vitamin B1 deficiency cause hair loss?
Maybe. But this isn’t the right question to ask. Rather, we need to ask this question in two parts:
- Does a vitamin B1 deficiency cause hair loss?
- Does a vitamin B1 deficiency within realistic parameters cause hair loss?
Why would we do this? Because at the extremes, almost anything causes hair loss. For instance, a “water deficiency” can cause hair loss. If we don’t drink water, we die. If we’re dead, we can’t grow hair. But that doesn’t mean that drinking water will regrow our hair. It also doesn’t mean we should warn people that hair loss is a side effect of a water deficiency.
The truth is that these types of logic leaps are what marketers use to claim that deficiencies in selenium, vitamin E, and iodine can all cause hair loss. Yes, this is true – but only if our scope of deficiency includes the endpoints: malnourished poverty-stricken children, people with genetic disorders who can’t absorb these nutrients, and people with certain chronic conditions that make nutrient assimilation nearly impossible.
All this is to say that we should ask if a thiamine deficiency, in the absolutes, causes hair loss. But the better question is: does a thiamine / vitamin B1 deficiency within realistic parameters cause hair loss, too?
Let’s take these one-by-one.
1. Does a thiamine deficiency, at the extremes, cause hair loss?
Maybe (in rodent models).
This 1968 study (2) sought to determine what happens in mice fed a diet that rapidly induces a thiamine deficiency. After two and a half weeks, some mice began to experience rapid weight loss, followed by abnormal hair shedding. Soon thereafter, neurological function began to decline. After four weeks, the mice were confused and could barely walk – symptoms similar to those seen in humans with beri beri.
However, it was unclear if the hair loss was caused by the vitamin B1 deficiency or the rapid weight loss.
2. Does a thiamine deficiency, within realistic parameters, cause hair loss?
Probably not.
For starters, people with beri beri rarely reported hair shedding (even despite their rapid weight loss). Moreover, when we expand our scope to human studies, we haven’t found any hard evidence that causally links a vitamin B1 deficiency to hair loss.
We could close the case right there, and say the article is done. At the same time, the absence of evidence doesn’t always imply evidence of absence.
For instance, there’s always the possibility that vitamin B1 might exacerbate certain chronic conditions linked to hair loss, or certain disease states associated with shedding disorders.
In fact, we could assert that since a vitamin B1 deficiency can lead to rapid neurological decline and thereby weight loss, and because rapid weight loss can trigger excessive (but temporary) hair shedding, then vitamin B1 deficiencies might be indirectly related to hair loss. The deficiency causes the weight loss; the weight loss causes the hair loss.
But again, if you’re so deficient in vitamin B1 that you start losing weight, you’ve got bigger things to worry about than your hair (like rapid impending neurological deterioration).
So, do we see any other circumstances where vitamin B1 is indirectly linked to hair shedding or hair loss?
Potentially. We can find them by look at the role of vitamin B1 in the body, and then comparing this to how different types of hair loss actually develop.
Vitamin B1 deficiency may exacerbate autoimmunity
At present time, there have been several animal studies conducted to investigate a link between vitamin B1 and autoimmunity. In general, these studies have suggested that vitamin B1 deficiencies may exacerbate autoimmunity in certain autoimmune disorders – specifically, multiple sclerosis (3, 4). It stands to reason that improving vitamin B1 deficiency may also improve these autoimmune conditions.
So, how could these effects translate to hair loss?
There are several forms of hair loss that seem to be mediated by autoimmune processes. These include alopecia areata as well as some forms of scarring alopecia.
If thiamine deficiencies happened to exacerbate the autoimmune processes involved in these hair loss disorders, it’s possible that restoring thiamine levels could improve these conditions.
Again, there’s no evidence that vitamin B1 deficiency is related to these hair-related autoimmune conditions. And while all autoimmune conditions involve autoimmune processes, not all autoimmune conditions develop in the same way. Thus, we can’t necessarily extrapolate the results from the animal studies, which primarily looked at multiple sclerosis, to the autoimmune conditions that lead to hair loss.
There’s also another point to consider: the effects of certain compounds reflected in animal studies are also traditionally very difficult to extrapolate to humans. Take our rodent study from earlier: thiamin-deficient rodents developed weight loss, neurological decline, and hair loss; whereas humans with beri beri – a sign of a thiamin deficiency – typically only develop weight loss and neurological decline.
That leaves us with one last piece of evidence to consider when it comes to linking autoimmune hair loss to vitamin B1: a case series on three human patients with autoimmune thyroid conditions (5).
Vitamin B1, autoimmune thyroid disorders, and hair shedding: a possible connection?
Autoimmune conditions that affect the thyroid – like Graves’ disease and Hashimoto’s thyroiditis – can have a domino effect on hair growth which is, in part, controlled by thyroid hormones. When these hormones get too high (i.e., Graves’ disease) or too low (i.e., Hashimoto’s thyroiditis), it can cause telogen effluvium – a form of diffuse hair shedding.
Interestingly, vitamin B1 might have relevance here, especially in the context of Hashimoto’s thyroiditis.
In one case series, doctors administered vitamin B1 to three patients with Hashimoto’s thyroiditis. They hypothesized that vitamin B1 could help relieve one of the hallmark symptoms of low thyroid hormone: fatigue.
Amazingly, that’s exactly what vitamin B1 did. In just a few hours to a few days, administration of B1 drastically improved the patients’ fatigue.
But, this wasn’t because of a subsequent improvement in the underlying autoimmune condition.
Instead, the authors hypothesized that the autoimmune processes involved in Hashimoto’s thyroiditis may have resulted in a vitamin B1 deficiency, subsequently leading to a reduction in energy production and, thus, fatigue.
In other words, the vitamin B1 deficiency likely isn’t a contributor to the development of Hashimoto’s thyroiditis. Instead, vitamin B1 deficiency, in this case, is a consequence of the condition.
As such, we can’t expect vitamin B1 to actually improve Hashimoto’s thyroiditis or the hair loss that occurs as a result. Instead, vitamin B1 can only improve symptoms related to a vitamin B1 deficiency that may occur alongside the condition.
So, we’ve established that vitamin B1 may or may not improve autoimmune forms of hair loss. We’ve also ruled out vitamin B1 as a means to improve Hashimoto’s thyroiditis and the hair shedding that ensues as a result.
But, are there any other pathways by which B1 might influence hair loss? Maybe… and that leads us to point number two.
Vitamin B1 deficiency may impair glutathione synthesis
Glutathione is a sulfur-containing compound with powerful antioxidant activity. Unlike antioxidants we consume in our diet (like polyphenols in green tea, berries, and other health-promoting foods), glutathione is manufactured by our own cells from amino acids like cysteine, glycine, and glutamic acid. This process requires NADPH, which requires vitamin B1 (along with various other B vitamins) (6).
So, how does this relate to hair loss?
Glutathione deficiency is associated with many conditions including diabetes, cardiovascular disease, as well as autoimmune diseases (7). Some studies also show low glutathione is associated with androgenic alopecia (AGA) (8).
As an anti-inflammatory agent, it’s possible that glutathione deficiency could exacerbate the microinflammation in AGA follicles — a process that drives the hair loss observed in AGA (7, 9).
In this context, it’s possible that through a possible increase in glutathione, vitamin B1 could reduce inflammation in AGA and, thus, improve AGA.
But there’s a difference between possible and plausible. Yes, if we stretch our imagination, it’s possible that a vitamin B1 deficiency might decrease glutathione production, and that if enough of this occurs in balding hair follicle sites, this decrease might exacerbate inflammation in AGA.
Possible, yes. But plausible?
Probably not.
While B1 deficiency seems to be related to low glutathione levels, vitamin B1 deficiency is extremely rare amongst most of the population. So, in this context, vitamin B1 is probably not something that most AGA patients need to worry about.
In these cases, glutathione deficiency is more likely to be related to co-morbidities seen in AGA: conditions like diabetes, obesity, and cardiovascular disease (all of which we know can deplete glutathione) (10).
So, while vitamin B1 deficiency could, technically, lead to or exacerbate a glutathione deficiency, it’s safe to say this is probably not the case for most AGA patients with low glutathione.
A quick recap
So far, we’ve established that B1 is an essential micronutrient. We’ve also established that in its complete absence, it can cause hair loss in rodents. Aside from that, it doesn’t appear that a vitamin B1 deficiency is a major driver of hair loss in humans.
The reason for this is three-fold:
- Vitamin B1 deficiency is extremely uncommon in the general hair loss population.
- In the rare cases where vitamin B1 levels are depleted and associated with hair loss, improving vitamin B1 levels only improves symptoms associated with vitamin B1 depletion. In other words, vitamin B1 improves nervous system abnormalities like fatigue but not the underlying conditions that lead to hair loss.
- When looking at the relationship between vitamin B1, glutathione, and AGA, it’s unlikely that vitamin B1 deficiency contributes to low glutathione levels that may exacerbate hair loss. Rather, low glutathione levels observed in some patients appear to be a consequence of some common co-morbidities associated with AGA.
So, that leads us to this conclusion: in the overwhelming majority of cases, vitamin B1 is not likely to confer any benefit in hair loss.
This leaves us with one last question worth asking before we close the books on the vitamin B1-hair health connection…
Could increasing vitamin B1 levels beyond normal levels have any negative effect on hair loss?
Maybe, maybe not. Let’s look at the research.
Why vitamin B1 might be bad for hair: the NADPH-DHT connection
Vitamin B1 is an essential cofactor in the production of the molecule, NADPH.
NADPH – or nicotinamide adenine dinucleotide phosphate – is a cofactor for enzymatic reactions. In other words, it’s a molecule that helps kickstart processes in the body. Earlier we established that NADPH was crucial for glutathione production. But that’s not all that NADPH does. NADPH is also essential for the production of steroid hormones.
Specifically, NADPH is a key molecule of the enzymatic process that converts testosterone into dihydrotestosterone, or DHT. Without NADPH, the enzyme that performs this conversion cannot function (11).
So, what does this mean for hair?
If you’ve done any research into androgenic alopecia (AGA), you probably already know that DHT is a significant contributor to the development of pattern hair loss (12). As such, any increase in DHT conversion could potentially worsen or speed up the balding process.
But, this would require NADPH to increase beyond what’s considered “physiological” — or what’s considered normal. So, does vitamin B1 do this?
Probably not. But we just don’t know.
What we do know is that, oftentimes, enzymes that produce molecules like NADPH have negative feedback mechanisms in place. This means that when their end-products increase, the body automatically reduces the activity of the enzymes that produce them. The net product is no increase in production.
However, this isn’t always the case. In some cases, these negative feedback loops are dysfunctional.
So, it’s possible that vitamin B1 doesn’t increase NADPH beyond what’s considered normal. As such, it’s also possible that vitamin B1 has no impact on DHT levels. At the same time, it’s also possible that it could.
In either case, the solution is the same: leverage diet to ensure vitamin B1 sufficiency and address a vitamin B1 deficiency if it’s present — but don’t go overboard.
If we’re deficient in B1, what should we do?
It’s important to note that an overwhelming majority of individuals likely aren’t deficient in vitamin B1. But, that doesn’t mean it’s impossible. Of the small pool of individuals B1 deficiency seems to affect in the modern world, risk factors appear to be:
- Hashimoto’s thyroiditis (5)
- Gastric bypass surgery (13)
- Anorexia (13)
- Severe crash dieting (13)
- Marked impairments in nutrient absorption, as in severe inflammatory bowel disease (13)
- Alcoholism (13)
- Septic shock (14)
But, again, it’s important to underscore that, even in these cases, we shouldn’t expect vitamin B1 to regrow our hair. This is because a vitamin B1 insufficiency is highly likely to present alongside other contributors to hair loss – like low thyroid hormone, zinc deficiency, iron deficiency, and severe calorie deficit. So, an improvement in B1 levels alone isn’t going to override these other factors. If anything, the thiamine deficiency-hair loss connection is more association than it is causation.
In any case, maintaining sufficient vitamin B1 levels is essential for overall health. So, you should aim to hit the recommended daily intake (RDI) everyday.
The good news is that you’re probably already doing this without even thinking about it – especially with all of the B-complex fortified foods out there. And if you find yourself falling into any of the risk categories, it’s not hard to find a supplement containing thiamine; nearly every multivitamin includes it as an ingredient.
At the same time, there could be a small minority of B1 deficiencies that go undetected, as was the case with the case series of Hashimoto’s thyroiditis patients. So, if you find yourself with fatigue that isn’t responding to standard treatment for Hashimoto’s thyroiditis, it may be worth discussing the possibility of vitamin B1 supplementation with your doctor.
Summary
Vitamin B1 is an essential vitamin. It ensures our body can effectively produce amino acids, glutathione, and other cofactors that are crucial for cellular function.
Deficiencies in vitamin B1 have been linked to weight loss, neurological decline, and hair loss in rodents. In humans, the evidence points more so toward neurological decline and weight loss than it does hair shedding. Having said that, a vitamin B1 deficiency might indirectly exacerbate hair loss through:
- Rapid weight loss
- Autoimmunity
- Glutathione impairment
But the bottom line is this: if your thiamine / vitamin B1 levels are low enough to associate with hair loss, you’ve got bigger problems than hair… like mental debilitation, neurological deterioration, and impending death.
Needless to say, 99.9% of us probably don’t need to be supplementing with vitamin B1 as a hair loss preventive. In fact, given vitamin B1’s relationship to NADPH production, and NADPH’s relationship to DHT, we could make a similar logic-leap argument that too much vitamin B1 may exacerbate hair loss.
This leaves us with one firm conclusion: maintain sufficient vitamin B1 levels by consuming the recommended daily intake. In the developed world, nearly every single diet will do this for you… provided you don’t have any of the hallmarks that increase your risk of a B1 deficiency (i.e., alcoholism, gastric bypass surgery, anorexia, etc.).
In the rare case that you are deficient, work with a doctor to address why your levels might be decreased in the first place and, if needed, to increase your levels with supplementation.
Have any questions about Vitamin B1 and hair health? Please leave them below in the comments!
References
- Davis, R. E., & Icke, G. C. (1983). Clinical chemistry of thiamin. Advances in clinical chemistry, 23, 93–140. https://doi.org/10.1016/s0065-2423(08)60399-6
- McCandless, D. W., Schenker, S., & Cook, M. (1968). Encephalopathy of thiamine deficieny: studies of intracerebral mechanisms. The Journal of clinical investigation, 47(10), 2268–2280. https://doi.org/10.1172/JCI105912
- Ji, Z., Fan, Z., Zhang, Y., Yu, R., Yang, H., Zhou, C., Luo, J., & Ke, Z. J. (2014). Thiamine deficiency promotes T cell infiltration in experimental autoimmune encephalomyelitis: the involvement of CCL2. Journal of immunology (Baltimore, Md. : 1950), 193(5), 2157–2167. https://doi.org/10.4049/jimmunol.1302702
- Natalie Nemazannikova, Kathleen Mikkelsen, Lily Stojanovska, Gregory L. Blatch and Vasso Apostolopoulos*, “Is there a Link between Vitamin B and Multiple Sclerosis?”, Medicinal Chemistry (2018) 14: 170. https://doi.org/10.2174/1573406413666170906123857
- Costantini, A., & Pala, M. I. (2014). Thiamine and Hashimoto's thyroiditis: a report of three cases. Journal of alternative and complementary medicine (New York, N.Y.), 20(3), 208–211. https://doi.org/10.1089/acm.2012.0612
- Martel JL, Kerndt CC, Franklin DS. Vitamin B1 (Thiamine) [Updated 2020 May 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482360/
- Ballatori, N., Krance, S. M., Notenboom, S., Shi, S., Tieu, K., & Hammond, C. L. (2009). Glutathione dysregulation and the etiology and progression of human diseases. Biological chemistry, 390(3), 191–214. https://doi.org/10.1515/BC.2009.033
- Prie, B. E., Iosif, L., Tivig, I., Stoian, I., & Giurcaneanu, C. (2016). Oxidative stress in androgenetic alopecia. Journal of medicine and life, 9(1), 79–83.
- Mahé, Y. F., Michelet, J. F., Billoni, N., Jarrousse, F., Buan, B., Commo, S., Saint-Léger, D., & Bernard, B. A. (2000). Androgenetic alopecia and microinflammation. International journal of dermatology, 39(8), 576–584. https://doi.org/10.1046/j.1365-4362.2000.00612.x
- Su, L. H., & Chen, T. H. (2010). Association of androgenetic alopecia with metabolic syndrome in men: a community-based survey. The British journal of dermatology, 163(2), 371–377. https://doi.org/10.1111/j.1365-2133.2010.09816.x
- Azzouni, F., Godoy, A., Li, Y., & Mohler, J. (2012). The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Advances in urology, 2012, 530121. https://doi.org/10.1155/2012/530121
- Ustuner E. T. (2013). Cause of androgenic alopecia: crux of the matter. Plastic and reconstructive surgery. Global open, 1(7), e64. https://doi.org/10.1097/GOX.0000000000000005
- Wiley KD, Gupta M. Vitamin B1 Thiamine Deficiency. [Updated 2020 Jun 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537204/
- Moskowitz, A., & Donnino, M. W. (2020). Thiamine (vitamin B1) in septic shock: a targeted therapy. Journal of thoracic disease, 12(Suppl 1), S78–S83. https://doi.org/10.21037/jtd.2019.12.82
Subscription services have gained popularity in recent years for all types of products, from home cleaning supplies to underwear, razors, and even toothpaste. These services make the most sense for products used consistently, saving the consumer time and money. Hair regrowth treatments certainly meet the criteria. For the most part, they only work as long as you keep using them. So, it was only a matter of time before startups put men’s hair care on autopay.
Of the telemedicine providers focusing on men’s health, Hims, Keeps and Roman have risen to the top of this competitive market. But which one is best? We offer an objective side-by-side comparison of these 3 hair loss subscription companies. In the process, you’ll learn about the following:
- About the Products Sold
- What Differentiates the Providers
- Why Choose One Over The Other
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

About the Products
Hims, Keeps, and Roman all offer the same hair regrowth products, namely Finasteride and Minoxidil. These are the only two medications approved by the FDA to treat androgenic alopecia (AGA), or male pattern baldness.
Finasteride
Finasteride, also known by the brand name Propecia, is one of the most well studied, and most powerful, drug for AGA. The daily pill stops the progression of hair loss in 80-90% of men and, on average, leads to a 10% increase in hair count over two years.[1]https://www.sciencedirect.com/science/article/pii/S0022202X15529357
Oral finasteride is an FDA-approved treatment for AGA that inhibits the enzyme type II 5-alpha reductase, and thereby reduces dihydrotestosterone (DHT) levels in the body. While it’s effective in slowing, stopping, and partially reversing AGA’s progression, some men can experience side effects associated with the systemic reduction in DHT levels.
Topical Finasteride aims to avoid such side effects by localizing the drug’s effects to the scalp. Research on topical finasteride is still in its infancy, but the drug does appear to be effective for lowering DHT in the scalp. Real world results vary widely in part because method of delivery, dilution ratios and carrier agents (non-active ingredients) used to deliver the drug, also vary widely.
Minoxidil
Minoxidil, also known by the brand name Rogain, is the other FDA-approved drug for the treatment of AGA. Although available orally, it’s most popular as a topical. Over half of those who try topical minoxidil respond to the drug within 3-6 months. Of those, research reports hair count increases of up to 12% over 48 weeks.[2]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6691938/
Among the downsides of Minoxidil is that the treatment’s effectiveness does wane over time. When that happens, those who quit tend to lose any and all gains they had from the drug, ending up back where they started before ever having used it.
Having Trouble Separating Follicle Facts from Fiction?
One reason why Minoxidil’s effectiveness might taper is that it doesn’t address hair follicle miniaturization. So, while it does increase hair counts by kicking hair back into the growth cycle, hair continues to thin over time.
When combined with additional therapies, however, most if not all of these downsides can be mitigated. Minoxidil may work better when combined with scalp exfoliators such as retinol or retinoic acid. Research also finds minoxidil is up to four times more effective when combined with microneedling.[3]http://www.ijtrichology.com/article.asp?issn=0974-7753;year=2013;volume=5;issue=1;spage=6;epage=11;aulast=Dhurat
The effectiveness of Minoxidil may be best when used with Finasteride. These two treatment options can be combined.
- Minoxidil Response rate: 30-40% alone; 70+% if combined with microneedling and/or finasteride
- Minoxidil Regrowth rate: ~10% alone; 25-40% if combined with microneedling and/or finasteride
Both Finasteride and Minoxidil require ongoing, daily application for results maintenance. Hence, the appeal of subscription services. So let’s take a closer look at the three biggest players offering Finasteride and Minoxidil online.
Hims vs Keeps vs Roman
Hims, Keeps and Roman are telemedicine providers who, after a phone or video consultation, provide prescriptions for hair loss treatments. Products are shipped discreetly to the buyer’s home, with savings available for ongoing, subscription-based delivery.
Hims
Hims brands themselves as ‘all about personal wellness.’ The company offers products not only for hair loss, but also for men’s sexual health. They sell both prescription-based and over-the-counter solutions.
Roman
Roman is the men’s health wing of Ro.co, a consumer healthcare company with a mission to make healthcare accessible and convenient. The digital healthcare provider wants to make it easier for more men to seek preventative care. Of these 3 companies, they treat the widest range of men’s health conditions.
Keeps
Keeps focuses only on treating hair loss, versus general men’s health. Founded in 2018, the company aims to ‘help more men keep more hair.’ In addition to selling prescription treatments and hair care products, Keeps will link clients to hair restoration surgeons.
Each of the above companies sells generic Finasteride and Minoxidil, but the products differ slightly between sites. Below is a closer look at what’s on offer.
Subscription-Based Hair Loss Products
Hims Products
Of the 3 providers, Hims sells the greatest variety of products and hair treatment packages. The Hair Power Pack includes a combination of oral finasteride and 5% Minoxidil, plus Biotin gummies and a thickening shampoo.
Hims is also the only of the three companies to offer a topical Finasteride. The topical combines 0.3% finasteride with 6% Minoxidil. Active ingredients are diluted in alcohol (ethanol), propylene glycol and citric acid and delivered via spray bottle.
Propylene glycol as a carrier does lead to skin irritation in up to 6% of users, although this is considered a very mild side effect.[4]https://pubchem.ncbi.nlm.nih.gov/compound/Propylene-glycol
Keeps Products
Keeps does not offer a topical finasteride, but does include options for either foam or serum-based Minoxidil just as Hims does. They are also the only provider to sell a 2% ketoconazole shampoo. Alongside finasteride and minoxidil, ketoconazole is considered one of the “big three” treatment options for pattern hair loss.
Despite its popularity, however, ketoconazole is not actually FDA-approved for pattern hair loss, nor is it as well-researched as minoxidil or finasteride. However, the low-cost, low-effort treatment (used 2-3 times per week) seems to carry little (if any) risk of side effects.
Roman Products
Roman is the biggest generalist of the companies we’re comparing here. Hair loss is not necessarily their primary focus and they have the least number of hair-related products on offer. Roman offers oral finasteride, a Propecia generic made by Ascend in India, as well as a solution-based Minoxidil made by Pure Source, LLC. They do not offer a foam-based Minoxidil as Hims and Keeps do.
The Subscription & Cost
Topical minoxidil is an over-the-counter product and can be purchased directly without a telemedicine consult. Finasteride, whether oral or topical, and 2% ketoconazole do, however, require a prescription. How this process works differs slightly between Roman, Hims and Keeps.
The Hims Consultation Process
Hims begins with a free online consultation that includes a few personal questions, and offers quick access to a licensed medical provider, in all 50 states. Subscribed medications are then filled at a licensed pharmacy, and shipped to you directly.
Shipping info and billing frequency??
The Keeps Process
Keeps also begins with a free online consultation. After answering a few questions online, Keeps requires an upload of photos, which are then reviewed by a licensed provider. Treatments are shipped every 3, 6 or 12 months. Continued on-demand access to a live medical professional is available via their app for $10/month.
The Roman Process
Like the rest, Roman’s process begins with a free online visit. Depending on the state, a phone or video chat with a doctor or nurse practitioner may be required. Prescriptions are filled via the Ro Pharmacy Network, and shipped directly in discreet packaging. Roman offers free, unlimited follow-ups if needed. Prescriptions are available in monthly or quarterly auto-shipments.
Cost Comparison
Pros and Cons
Whether Roman, Hims, or Keeps is best is a personal decision that depends on the treatment sought, any extra over-the-counter shampoos, vitamins or other add-ons desired, and what state the patient lives in.
Hims Pros & Cons
Of the three companies in our comparison, Hims offers the most treatment options and is the only one to offer topical finasteride. Topical finasteride is also available from the following, lesser known companies:
- Happy Head: 0.25% Finasteride + 8% Minoxidil 8% + 0.01% Retinoic Acid + 1% Hydrocortisone. The formula is customizable and available for $79+/month
- Strut: Strut offers a customizable gel or solution-based topical with the following options, Finasteride 0.1-0.25%, Minoxidil 0.0%-7.5%, Tretinoin 0.0%-0.0125%, available starting at $59/month
On the other hand, Hims also ranks as the most expensive for either Finasteride or Minoxidil.
Keeps Pros & Cons
Keeps is the only provider on our list to offer Ketoconazole shampoo. So for those interested in trying the ‘Big 3’ protocol for hair loss, Keeps is a good bet. It’s also the least expensive provider of Finasteride and Minoxidil.
The main drawback of Keeps is that it’s not yet available in every state. They can, however, fill prescriptions in every state. So if you have a prescription from another doctor, Keeps may be able to fill it for a lower price.
Roman Pros & Cons
Of these 3 digital medicine providers, Roman is doing its best to position itself as a one-stop-shop for all things health-related. Contact with a licensed healthcare professional is available in every state, and you can go to Roman for nearly all things health-related.
The downside is that Roman is not focused on hair loss prevention nor hair regrowth alone, and they offer the most limited treatments options out of the 3 on our list.
Summary
Roman, Hims, and Keeps are all telemedicine providers who have risen to the top of the competitive men’s wellness market in part due to their focus on hair loss prevention and treatment.
Each company focuses primarily on oral finasteride and topical (OTC) minoxidil and will prescribe the former after a brief online consultation.
Subscription services and products vary. Which is best may depend on the state the patient lives in, and exactly which treatments are being sought.
References[+]
References ↑1 https://www.sciencedirect.com/science/article/pii/S0022202X15529357 ↑2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6691938/ ↑3 http://www.ijtrichology.com/article.asp?issn=0974-7753;year=2013;volume=5;issue=1;spage=6;epage=11;aulast=Dhurat ↑4 https://pubchem.ncbi.nlm.nih.gov/compound/Propylene-glycol If you’re experiencing hair loss, and you’re searching for a solution to help regrow hair, you’ve likely read several articles that reference different parts of the scalp. But what would you say if you were asked where on your scalp you’re losing hair? And how well do you understand the various parts of the scalp that lay beneath the skin, as well as their roles in hair loss?
In this article, we’ll give a quick introduction to the scalp. After that, we’ll take a tour of the scalp, starting with the different regions on the surface; then moving beneath the skin through the various layers of scalp tissue; then exploring the bones that lay beneath the tissue; then discussing the arteries, veins, nerves, and lymph vessels than run through those layers of tissue; and then examining the muscles that pull on the scalp. After touring the different areas of the scalp, we’ll discuss some ways that scalp mechanics may impact hair growth. Finally, we’ll look at some clinical evidence that has opened up the possibility of new treatments for androgenic alopecia.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Introduction to the Scalp
The scalp consists of layers of skin and subcutaneous tissue that cover the human cranium. It extends from the supraorbital foramina (i.e. two openings in the bone just above the eye sockets and just beneath the eyebrows) to the superior nuchal line (i.e. one of four curved ridges on the exterior surface of the occipital bone, which lies at the back of the head). It is bordered on the front by the face and on the sides and back by the neck.
The scalp acts as a physical barrier to protect the cranium against physical trauma and potential pathogens. It is also the area where human hair typically grows in order to 1) aid in heat conservation, and 2) play a role in aesthetic appearance and sexual signaling.
A Tour of Your Head: Regions of the Scalp
If someone were to ask you where on your scalp you’re losing hair, would you know how to answer them? If you’re unsure how to answer that question, check out the descriptions below.
The surface of your head has seven main areas, or regions, which are described below:
- Temples: The temples are located on either side of the head, behind the eye and between the forehead and ear. Each of the two temples is a latch where four skull bones – the frontal, parietal, temporal, and sphenoid bones – fuse together. Male pattern baldness typically starts at the temples, then continues to recede into the scalp, resulting in an M-shaped hairline.
- Frontal Region: If you were to draw an imaginary vertical line through your head, with the line positioned just in front of your ears, that line would demarcate the border between the frontal and mid-scalp regions. The part of the scalp that lies in front of that line is the frontal region. Also called the forelock, this region includes the hairline and the hair above the temples. If you’re losing hair due to male pattern baldness, this is often one of the first places you can expect to see hair loss.
- Mid-Scalp Region: If you were to draw two imaginary vertical lines through your head, with the first line positioned just in front of your ears and the second line positioned just behind them, the relatively flat area between those two lines would be the mid-scalp region. Unless you’re at an advanced stage of male pattern baldness, you probably won’t experience much hair loss in the mid-scalp region.
- Vertex Transition Zone: Also called the vertex transition point, this area is located between the mid-scalp region and the vertex. The vertex transition zone is the area where the scalp goes from a horizontal to a vertical plane posteriorly. For this reason, it is often referred to as the posterior hairline. Although this area is technically part of the vertex (see next bullet), it is often listed as a separate region.
- Vertex (Crown): Also called the crown, the vertex is the highest point on your scalp (the word “vertex” literally means “highest point”), which is located towards the back of your head. On non-balding heads, the vertex is typically identified by a swirl pattern of hair. In addition to losing hair around the temples and in the frontal region, many folks see noticeable hair loss on the crowns of their heads.
- Sides (Temporal Regions): Also known as the temporal regions (because they sit over the temporal bones and muscles), these are the areas on either side of the head that are positioned just above and behind the ears. Because the sides of the head have a different subcutaneous anatomy than the rest of the scalp, they are sometimes treated as separate from the scalp. However, the temporal regions generally grow hair, so we’ve chosen to include them in this tour of the scalp. Hair loss is much less common in this region.
- Back (Occipital Region): Also known as the occipital region (because it sits over the occipital bone and muscle), this area covers the back of the head, underneath the crown. As with the sides of the head, hair loss is much less common in this region.
Digging into the Anatomy: Layers of the Scalp
While the previous section provides a good overview of the various areas on the surface of the scalp, there is a lot more going on beneath the surface.
The scalp consists of the following five layers:Tajran J, Gosman AA. Anatomy, Head and Neck, Scalp. [Updated 2021 Jul 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.
- Skin: The first layer of the scalp is the skin, which acts as a protective barrier to the rest of the scalp, helps prevent the loss of moisture, and helps regulate body temperature, among other things. This layer is thick and contains numerous hair follicles and sebaceous glands (i.e. small, oil-producing glands).
- Dense Connective Tissue: The second layer is the dense connective tissue layer, which connects the skin to the galea aponeurotica (described below). In addition to connective tissue, this layer contains arteries, veins, nerves, and lymphatic vessels. Hair follicles can also extend from the skin down into this layer.
- Galea Aponeurotica: Also known as the epicranial aponeurosis, the galea aponeurotica is a layer of dense, fibrous connective tissue that runs along the top of the cranium, connecting to the frontalis muscle at the front of the head and the occipitalis muscle at the back of the head.
The first three layers of the scalp are firmly attached to each other and move as a unified structure.
- Loose Areolar Connective Tissue: This layer forms a flexible connection to both the galea aponeurotica and the pericranium (described below), allowing the top three layers of the scalp to slide over the lower pericranium. The loose areolar connective tissue also contains numerous blood vessels.
- Pericranium: Composed of dense connective tissue, the pericranium is the deepest layer of the scalp, which tightly adheres to the calvaria (i.e. the bones that form the top of the skull). It also contains the vascular supply that’s essential to supporting the calvaria.
The Scalp’s Foundation: The Calvaria and Other Cranial Bones
As noted above, the pericranium is firmly attached to the calvaria bones, which form the top of the human skull. In addition to the calvaria, there are some other cranial bones that play a role in scalp function. We discuss these bones below.
Minonzio, Claudio. (2019). A step towards aberration corrections for transcranial ultrasound – Estimation of skull thickness and speed of sound.
The neurocranium, which is pictured in the image above, consists of the upper part of the skull that surrounds the cranial cavity and contains the brain. There are eight bones that make up the neurocranium:
- The frontal bone forms a skeletal roof above the eye sockets and nasal cavity, and forms the main part of the forehead.
- The left and right parietal bones sit behind the frontal bone. These bones form the top and sides of the cranium.
- The occipital bone forms the back and base of the skull. It has a large opening, called the foramen magnum, where the spinal cord passes into the skull.
- The left and right temporal bones help form the sides and base of the skull, where they protect the temporal lobe of the brain and surround the ear canal.
- The sphenoid bone is situated in the middle of the skull towards the front. It helps form the base and sides of the skull, and has a shape that resembles a butterfly.
- The ethmoid bone is a cube-shaped bone located in the center of the skull between the eyes. It helps form the walls of the eye sockets, as well as the roof, sides, and interior of the nasal cavity.
Although these are eight individual bones, they are joined together by various fibrous sutures.
The neurocranium can be further divided into an upper and lower part:
- The upper part, known as the calvaria, consists of the flat bones of the skull that form a dome-like roof. This includes the frontal bone, parietal bones, and occipital bone.
- The lower part, known as the cranial base, includes the lower parts of the frontal bone, the sphenoid bone, ethmoid bone, temporal bones, and the lower parts of the occipital bone.
Because the calvaria makes up the top part of the skull, these bones are more significant when discussing hair growth/loss. However, the muscles that attach to the temporal and sphenoid bones may also play a role in hair loss (more on that in a bit).
The Vertical Layer: Arteries, Veins, Nerves, and Lymphatic Vessels
We previously discussed the five layers of the scalp. There are also a number of arteries, veins, nerves, and lymphatic vessels that run through those layers. We discuss each of these below.
Arteries of the Scalp
The common carotid arteries, a pair of arteries on either side of the neck, provide the main supply of blood to the scalp. While still in the neck, each of the common carotid arteries splits into an internal and external carotid artery. Each of these branches supplies blood to different areas of the scalp.
Internal Carotid Artery
Wikipedia contributors. Ophthalmic artery. Wikipedia, The Free Encyclopedia. January 5, 2022, 11:55 UTC.
The internal carotid artery (on either side of the skull) gives rise to an ophthalmic artery, which further branches into a supratrochlear artery and a supraorbital artery. Both the supratrochlear and supraorbital arteries rise through the supraorbital foramen (i.e. an opening in the bone just above the eye socket and just beneath the eyebrow) and connect with their counterparts on the opposite side of the skull, as well as with the superficial temporal artery (also on either side of the skull), which provides most of the blood supply to the front of the scalp.
External Carotid Artery
Hacking, C., Bell, D. Scalp. Reference article, Radiopaedia.org. (accessed on 12 May 2022)
The external carotid artery (on either side of the skull) branches into the following arteries:
- The superficial temporal artery passes over the back of the cheekbone, where it splits again into a frontal and parietal branch. The frontal branch runs across the temple, supplying blood to the frontal and temporal regions of the scalp. The parietal branch continues over the top of the skull, supplying blood to the parietal region of the scalp.
- The posterior auricular artery splits away from the external carotid artery just above/behind the jaw and just below the ear. The posterior auricular artery then passes underneath the ear and travels to the deep tissues that converge between the mastoid process (i.e. the protruding, cone-shaped bone found just below and behind the ears on either side of the head) and the cartilage of the ear. This artery supplies blood to the parts of the scalp located above and behind the ears.
- The occipital artery splits from the external carotid artery at the back of the neck, passing under the ear and in front of the mastoid process. This artery supplies blood to the back of the scalp.
Veins of the Scalp
The venous drainage of the scalp can be split into superficial and deep components.
Superficial Veins
The superficial veins follow their respective arteries. The supraorbital and supratrochlear veins (on either side of the skull) drain blood from the top of the scalp through the front of the face, while the superficial temporal, occipital, and posterior auricular veins (also on either side of the skull) drain blood from the scalp through the back of the head. Also, just like its arterial counterparts, the superficial temporal vein has a frontal and parietal branch.Tajran J, Gosman AA. Anatomy, Head and Neck, Scalp. 2021 Jul 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 31855392.
Deep Veins
The deeper regions of the scalp drain into the pterygoid venous plexus (on either side of the skull), which is a large plexus (i.e. a bundle of intersecting blood vessels, nerves, or lymphatic vessels) of veins situated between the temporalis (i.e. thin, fan-shaped muscles on either side of the skull) and lateral pterygoid (i.e. one of the muscles in the jaw that helps us chew) muscles. From there, the venous blood passes into the maxillary vein (on either side of the skull), then into the retromandibular vein (on either side of the skull), and then into the external jugular vein (on either side of the skull), which is the venous counterpart to the external carotid artery.Rivard AB, Kortz MW, Burns B. Anatomy, Head and Neck, Internal Jugular Vein. 2021 Jul 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 30020630.
The Connection Between Superficial and Deep Veins
It’s also worth noting that there is a drainage connection between the superficial and deep venous systems, whereby parietal emissary veins located in the loose areolar connective tissue layer of the scalp connect to diploic veins that penetrate the skull, eventually flowing into the dural venous sinuses (e.g. superior sagittal sinus) that drain venous blood from inside the cranial cavity.Germann AM, Jamal Z, Al Khalili Y. Anatomy, Head and Neck, Scalp Veins. 2021 Dec 15. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 31082005.
Nerves of the Scalp
The front and sides of the scalp are innervated by trigeminal nerves, while the back of the scalp is innervated by cervical nerves.
Trigeminal Nerves
Wikipedia contributors. Trigeminal nerve. Wikipedia, The Free Encyclopedia. March 31, 2022, 01:23 UTC.
There are two trigeminal nerves – one on each side of the skull. Each trigeminal nerve has three major branches:
- The ophthalmic nerve branches into the frontal nerve, which eventually splits into the following two branches:
- The supratrochlear nerve innervates the front, middle section of the forehead.
- The supraorbital nerve innervates a large portion of the scalp including the top and sides of the forehead, as well as the vertex.
- The maxillary nerve branches into the zygomaticotemporal nerve, which innervates the temple.
- The mandibular nerve branches into the auriculotemporal nerve, which innervates the skin in front of and above the ear.
Cervical Nerves
Cervical nerves emerge from the spinal cord to innervate the rest of the body. There are four cervical nerves that innervate the scalp:Roesch ZK, Tadi P. Anatomy, Head and Neck, Neck. 2021 Jul 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 31194453.
- The lesser occipital nerve (on either side of the skull) arises from the anterior ramus (i.e. front-facing branch) of the second cervical nerve and innervates the skin above and behind the ear.
- The greater occipital nerve (on either side of the skull) arises from the posterior ramus (i.e. rear-facing branch) of the second cervical nerve and innervates the skin at the upper back of the scalp.
- The great auricular nerve (on either side of the skull) arises from the anterior rami (i.e. front-facing branches) of the second and third cervical nerves and innervates the skin below and behind the ear.
- The occipital nerve (on either side of the skull) arises from the posterior ramus (i.e. rear-facing branch) of the third cervical nerve and innervates the skin at the lower back of the scalp.
The Scalp’s Lymphatic Vessels
The lymphatic vessels of the scalp are located in the dense connective tissue layer and follow the venous drainage. In general, the front portions of the scalp drain lymphatic fluid through the parotid nodes (i.e. lymph nodes found in front of and just below each ear), which continue to drain through the submandibular lymph nodes (i.e. lymph nodes located just beneath the jaw bone) and deep cervical lymph nodes (i.e. lymph nodes located in the neck).Rivard AB, Kortz MW, Burns B. Anatomy, Head and Neck, Internal Jugular Vein. 2021 Jul 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–.
The rear portions of the scalp drain lymphatic fluid through the posterior auricular lymph nodes (i.e. a small group of lymph nodes located just beneath the ear) and occipital lymph nodes (i.e. lymph nodes located at the back of the head, near the occipital bone). The posterior auricular lymph nodes drain the area of the scalp located directly behind the ear and flow into the occipital lymph nodes, while the occipital lymph nodes drain lymphatic fluid from the remaining regions in the back of the scalp.
Muscles that Act on the Scalp
There are a handful of muscles that act on the scalp.
The largest and most important muscle in the scalp is the occipitofrontalis muscle. The occipitofrontalis muscle is actually two sections of muscle, known as bellies, that connect to the front and back of the galea aponeurotica. The frontalis belly connects to the galea aponeurotica at the front of the head and inserts into the superior orbicularis oculi (i.e. the muscle that closes the eyelids) near the eyebrow. The occipitalis belly connects to the galea aponeurotica at the back of the head and attaches to the superior nuchal line (i.e. one of four curved ridges on the exterior surface of the occipital bone) and mastoid processes (i.e. the protruding, cone-shaped bones found just below and behind the ears on either side of the head) at the bottom of the scalp. These muscles, or bellies, work in conjunction to pull the scalp back and elevate the eyebrows (the contraction of the frontalis belly creates wrinkles on the forehead).Roesch ZK, Tadi P. Anatomy, Head and Neck, Neck. 2021 Jul 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 31194453.
The auricular muscles are a group of muscles of the auricle (i.e. the visible portion of the ear). Although there are nine total auricular muscles on either side of the skull, six of those muscles are intrinsic muscles located deep within the skull, while the other three are extrinsic muscles that are located more superficially. It is the three extrinsic muscles that we’re concerned with for this article.
- The auricularis superior, the largest of the three extrinsic auricular muscles, is a thin, fan-shaped muscle located above the ear. It helps to elevate the ear, attaching to the galea aponeurotica at the top and a tendon just above the ear at the bottom.
- The auricularis anterior, the smallest of the three extrinsic auricular muscles, is a thin, fan-shaped muscle located in front of the ear. It helps draw the ear upward and forward, attaching to the lateral edge of the galea aponeurotica at one end and cartilage at the top, front of the ear at the other.
- The auricularis posterior is a muscle located behind the ear. It helps pull the ear backward, attaching to the mastoid process (i.e. the protruding, cone-shaped bone found just below and behind the ears on either side of the head) at one end and the lower part of the cranial surface at the back of the ear at the other end.
Although the auricular muscles play a minor role in fixing the position of the ear, they are considered vestigial in humans. Nevertheless, these muscles may play a role in restricting blood flow to the scalp (more on this in a bit).
The temporoparietalis muscles are a pair of muscles located just above and in front of the auricularis superior muscles on either side of the head. These muscles lie over both the temporal and occipital bones, connecting to the fascia (i.e. a thin sheath of fibrous tissue enclosing a muscle or other organ) just above the ear on one end and the galea aponeurotica on the other. The temporoparietalis muscles help to fix the galea aponeurotic and elevate the ears.
The temporalis muscles are broad, fan-shaped muscles located on either side of the head. These muscles cover most of the temporal bones and help produce movements of the mandible, or jawbone, when chewing. Although these muscles are more associated with the mandible than they are the scalp, the contraction of these muscles may restrict blood flow to the scalp, which could have an effect on hair growth/loss (more on this below).
Ways Scalp Mechanics May Impact Hair Growth
Now that you have a better understanding of scalp anatomy, we can examine ways that scalp mechanics may impact hair growth. In particular, the galea aponeurotica and the surrounding musculature may play a role in restricting blood flow (and oxygen) to the scalp.
We know from previous studies that balding scalps tend to have 2.6 x less subcutaneous blood supply compared to non-badling controls. Moreover, several blood-pressure-lowering medications – such as minoxidil, diaoxide, and pinacidil – have been shown to improve androgenic alopecia – one of the world’s most common hair loss disorders. These findings implicate reduced blood supply as a potential contributor to pattern hair loss. And while it’s still debated just how much the reductions to blood flow are a cause or consequence of androgenic alopecia, there may be a therapeutic benefit to improving blood, oxygen, and nutrient levels to balding hair follicles.
Below, we examine the impact of scalp musculature on arterial branches, as well as the impact of the galea aponeurotica and surrounding musculature on microcirculation (i.e. capillaries in the scalp).
The Impact of Musculature on Arterial Blood Flow
Dermatologists report that roughly 80% of men with androgenic alopecia tend to have “tight” scalps, suggesting that the muscles around the perimeter of the scalp have involuntarily contracted and are pinching the arterial branches, causing a reduction in blood supply. This is also supported by research measuring scalp hardness in balding versus non-balding men across a variety of scalp regions.[1]https://www.researchgate.net/publication/338624064_Androgenetic_alopecia_is_associated_with_increased_scalp_hardness
So, do the muscles surrounding the scalp perimeter and anchored to the galea aponeurotica have anything to do with these phenomena? Some evidence suggests yes.
As noted previously, the supraorbital and supratrochlear arteries are two of the three arterial branches that supply the majority of blood to the frontal region of the scalp. These arteries pass through the cranial bone at the eyebrows, run underneath the frontalis muscle, then pierce through the frontalis muscle before running upward toward the hair line. When the frontalis muscle flexes, this flexing can compress the supraorbital and supratrochlear arteries, restricting blood flow to the scalp.[2]https://academic.oup.com/asj/article-abstract/41/11/NP1599/6206455
In addition, the deep temporal artery resides between the cranium and the temporalis muscles. When these muscles contract, they can compress the deep temporal artery against the skull and constrict blood supply.
Moreover, some branches of the auricularis anterior and posterior arteries weave between the auricular muscles and their supporting tendons. It is possible that the contraction of the auricular muscles may restrict blood flow in the same manner.
Finally, while many of the scalp’s arteries reside in the fascia that overlies the scalp muscles, the contraction of the muscles underlying these arterial branches can still affect them. We know this because studies have shown that when the temporalis muscle contracts, the artery overlying it (the superficial temporal artery) constricts by 50%, thus leading to a ~75% decrease in blood supply in that arterial branch.[3]https://n.neurology.org/content/11/11/935
But that’s not the whole story.
The Impact of the Galea Aponeurotica on Microcirculation
Despite the possible restriction of arterial blood flow described in the previous section, it is generally believed that reductions to blood flow in androgenic alopecia are mainly due to the loss/restriction of the microcapillary networks (i.e., the small blood vessels supporting the hair follicles themselves), rather than reductions from the carotid arterial branches supporting those microcapillary networks.
The microcapillary networks may constrict or compress as a result of mechanical stretch across the galea aponeurotica (mediated by the contraction of the scalp’s perimeter muscles). Because the top of the scalp is a fixed area, stretching of the galea aponeurotica would generate compression of the underlying microcapillary networks supplying hair follicles.
How Relevant Is Blood Flow to Androgenic Alopecia?
We see hypoxia (i.e. insufficient supply of oxygen) as a trigger of hair loss in mouse models, but is it relevant to humans with androgenic alopecia? Unfortunately, we still don’t know (since there are also reductions to blood supply resulting from the hair follicle miniaturization that follows each re-entry into the anagen stage of the hair cycle).
Interestingly, there used to be a surgery called “scalp reduction,” where surgeons would place a balloon underneath the galea aponeurotica, slowly inflate it over a series of weeks, and then give patients a surgery to “remove” bald regions and pull the hair-bearing stretched skin over to create the appearance of a fuller head of hair. In the 1990s, these surgeries were quietly abandoned after surgeons started voicing concerns of accelerated hair loss in patients following the procedure. Was this accelerated hair loss caused by skin tension? Did this tension lead to microcapillary compression? We still don’t know the answers, but the questions certainly are interesting.
Clinical Evidence Suggests Possible New Hair Loss Treatment Targets
Despite the lack of certainty regarding the impact of blood flow (and oxygen) on androgenic alopecia, the results from a number of Botox studies show that when the muscles surrounding the scalp are forcibly relaxed in patients with androgenic alopecia, hair growth/loss improves.
Botulinum toxin, which is commonly referred to by the brand name Botox, is an injectable neuro modifier that’s used as a therapeutic treatment for many clinical and cosmetic concerns. Specifically, Botox is used to help relax muscles and reduce certain inflammatory signaling proteins.
Over the last decade, there have been five clinical studies published on the hair-promoting effects of Botox on men with androgenic alopecia. Four of those studies tested intramuscular Botox injections (i.e. injections directly into the scalp’s perimeter muscles), while the fifth study tested intradermal Botox injections (i.e. injections directly into balding regions of the scalp). We examine the results from both types of studies below.
The Results of Studies Testing Intramuscular Botox Injections
Across the four studies testing intramuscular Botox injections, 75-80% of participants responded favorably (i.e. hair growth) with an average hair count increase of 18-21% after 6 to 10 months. Injections into the scalp perimeter muscles were done once every 4-6 months, with results becoming cosmetically significant after the second round of injections.
In addition to testing the use of Botox injections on their own, one of the four studies also examined using Botox injections alongside oral finasteride (i.e. the active ingredient in Propecia). This combined therapeutic approach led to improved response rates and hair count increases that average nearly 35%.
Researchers suspect that two mechanisms contributed to these results:
- First, intramuscular Botox injections might relax involuntarily contracted muscles around the perimeter of the scalp. As noted previously, arterial branches run through those perimeter muscles in order to supply the scalp with blood. When perimeter muscles are contracted, they may pinch those arteries, restricting the supply of blood to the scalp. Researchers theorize that injecting those perimeter muscles with Botox helps to unclamp the pinched arterial branches, increasing the supply of blood (and oxygen).
- Second, by relaxing those perimeter muscles, researchers also speculate that Botox injections might help reduce tension across the top of the scalp, and in doing so, potentially reduce tension-mediated inflammation and/or lower dihydrotestosterone (DHT) in the scalp.
Despite researcher’s suspicions, it’s important to note that these suggested mechanisms are only speculative at this point. We will need more data in order to corroborate these suspicions.
The Results of the Study Testing Intradermal Botox Injections
In the one study that tested intradermal Botox injections, 60-80% of participants responded favorably (i.e. hair growth) with an average hair count increase of 5% after 6 months. Injections into balding regions were done every four weeks.
Although this study’s response rate is similar to the intramuscular studies above, the hair count increases were much lower. It’s worth noting that these results might improve with higher-dose injections. This study used just 30 units of Botox, spread across the entire scalp. When another investigator increased the amount of Botox injected from 30 units to 100 units, and also doubled the frequency of injections, they observed more significant hair growth.[4]https://www.dovepress.com/getfile.php?fileID=73853
While conducting the study of intradermal Botox injections, researchers also conducted a cell culture on human hair follicles. They found that Botox appears to decrease the expression of transforming growth factor beta 1 (TGFB-1), a signaling protein that acts as a negative regulator of the hair cycle. TGFB-1 also appears to be intimately tied to hair follicle miniaturization, causing researchers to speculate that intradermal Botox injections might be down regulating TGFB-1 in human hair follicle sites, and in doing so, allowing for hair loss improvements.
It’s important to note that the reduction of TGFB-1 happens in a dose- and time-dependent manner. Given this fact, it makes sense that increasing both the volume and frequency of intradermal injections evokes a more robust improvement in hair count.
As with the intramuscular studies above, it’s important to note that the suspected mechanism (i.e. the down regulating of TGFB-1) identified in the intradermal study is only speculative. In order to corroborate this suspicion, more data will need to be collected.
How Reliable Are the Findings from the Botox Studies?
Although the results from the Botox studies look promising, the amount of evidence on Botox as a treatment for androgenic alopecia is still rather small. And while several research groups have found consistent results, these studies tend to score lower on the hierarchy of evidence. So, we should interpret those results with caution.
There are at least three significant issues with the Botox studies:
- The Botox Studies Had a Small Number of Participants: To date, fewer than 200 participants have tested Botox to treat androgenic alopecia (in a clinical, peer-reviewed setting). For comparison, thousands of participants have tested the use of finasteride for treating androgenic alopecia.
- None of the Botox Studies Used a Control Group: At this point, no Botox studies have used control groups (i.e. patients who receive a placebo treatment). Using placebo groups is important because they help researchers control for hair count improvements (and losses) due to seasonal hair shedding.
- None of the Botox Studies Assessed Hair Diameter: While the Botox studies assessed hair count, they did not examine changes to hair diameter. Androgenic alopecia progresses through the process of hair follicle miniaturization, and we can effectively stop androgenic alopecia by reversing this process. Because we don’t know whether Botox has an effect on hair follicle miniaturization, we also don’t know whether Botox is an effective long-term solution for androgenic alopecia.
In addition to the above three issues, it’s also worth noting that 1) there are currently no established best practices for the use of Botox to treat androgenic alopecia, and 2) Botox treatment can be rather expensive, with intramuscular injections costing $2,400 to $4,000 per year and intradermal injections running $4,800 to $12,000 per year.
This isn’t to say we should dismiss the evidence in favor of using Botox to treat androgenic alopecia. At the same time, it’s important not to jump to any conclusions regarding the efficacy of using Botox versus other treatment options.
Final Thoughts
In this article, we’ve shown how the scalp has a rather complex anatomy – including seven regions on the surface of the scalp; five tissue layers; several bones that the scalp attaches to; a number of arteries, veins, nerves, and lymphatic vessels running through the scalp; and a collection of muscles that pull on the scalp. We’ve also discussed ways that scalp mechanics may impact hair growth, and reviewed five studies that suggest Botox injections may improve hair loss by relaxing the muscles around the perimeter of the scalp.
Although the findings from Botox studies are still preliminary, they have opened up the possibility of new treatment targets for androgenic alopecia. Any relevant treatment targets would need to relax the muscles around the scalp so that 1) arterial branches can deliver an adequate supply of blood (and oxygen) to the scalp, and 2) tension in the galea aponeurotica doesn’t restrict blood flow in the microcapillary networks that feed hair follicles.
In addition to the results from the Botox studies, there’s mechanistic research to suggest that skin tension in balding regions (generated from the contraction of muscles around the scalp) might have something to do with the arrival of dihydrotestosterone (DHT) and the balding process itself.[5]https://www.sciencedirect.com/science/article/pii/S0306987717310411
Finally, it’s worth noting that Botox injections are not the only way to relax the muscles around the scalp. Scalp massages may offer a viable, less-expensive method for treating androgenic alopecia that targets the same mechanisms as Botox injections.
References[+]
References ↑1 https://www.researchgate.net/publication/338624064_Androgenetic_alopecia_is_associated_with_increased_scalp_hardness ↑2 https://academic.oup.com/asj/article-abstract/41/11/NP1599/6206455 ↑3 https://n.neurology.org/content/11/11/935 ↑4 https://www.dovepress.com/getfile.php?fileID=73853 ↑5 https://www.sciencedirect.com/science/article/pii/S0306987717310411 Recent years have witnessed a surge of interest in topical dutasteride for hair loss, especially among those who have either not tolerated or seen insufficient results with conventional oral therapies. This growing popularity is fueled by multiple online anecdotes and user testimonials that describe marked improvements in hair density, shedding reduction, and scalp health.
However, these topical dutasteride before and after photos typically show the very extremes of success or failure, which can give a misleading impression of what average outcomes truly are. This tends to create an environment where typical results are overshadowed by outliers, making it harder to gauge what most people should realistically expect.
Where does topical dutasteride truly fit in the landscape of hair loss treatment? How can one reliably distinguish between what’s achievable and what’s most likely? What’s the best way to interpret anecdotal stories versus the outcomes most users may expect? And, crucially, what kind of regrowth pattern could unfold after years on topical therapy?
This article tackles these questions head-on. Clear expectations will be set for topical dutasteride use, including practical guidance on interpreting online results, examples of standout before-and-after transformations, and insights into what the average treatment journey actually looks like.
We will also introduce a framework for evaluating topical therapies, helping readers compare dutasteride’s regrowth potential, evidence strength, and long-term safety against other hair loss options.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Topical Dutasteride?
Topical dutasteride is a compounded medication for androgenic alopecia (AGA) that works as a dual 5-alpha reductase inhibitor, reducing dihydrotestosterone (DHT) locally at the scalp. The main distinction from oral dutasteride is that topical delivery is intended to minimize systemic absorption, though some absorption into the bloodstream still occurs, and complete elimination of systemic exposure is not guaranteed. DHT suppression with topical forms is less studied compared to oral; oral dutasteride can lower systemic DHT by 90% or more, but topical formulations do not consistently reach those levels in serum.
How Topical Dutasteride Works
Dutasteride inhibits both type 1 and type II 5-alpha reductase, the enzymes responsible for converting testosterone to DHT.[1]Frye, S.V. (2006). Discovery and clinical development of dutasteride, a potent dual 5alpha-reductase inhibitor. Current topics in medicinal chemistry. 6(5). 405-421. Available at: … Continue reading By inhibiting these enzymes, topical dutasteride targets DHT production where hair loss is occurring at the scalp, rather than throughout the body.
Studies show that topical application leads to significantly lower systemic absorption compared with oral forms, which should result in fewer side effects. Absorption data from Franz cell and skin retention studies indicate that only a small fraction of the topically applied dose reaches the circulation.[2]Noor, N.M., Sheikh, K., Somavarapu, S., Taylor, K.M.G. (2017). Preparation and characterization of dutasteride-loaded nanostructured lipid carriers coated with stearic acid-chitosan oligomer for … Continue reading
DHT reduction with topical dutasteride is robust in scalp tissue but generally milder in serum compared to oral therapy.
Common compounded strengths vary widely: for example, Ulo offers concentrations ranging from 0.02% to 0.2%.
No topical dutasteride product is FDA-approved; all are compounded by pharmacies for individual patients based on prescriptions, with dosing customized to tolerance and response.
We could find three studies that report significant local efficacy:
Study 1: Nada et al., 2018 (Prospective Randomized; 30 Men; 24 Weeks)
- Design/groups: Compared minoxidil plus topical dutasteride (0.02%) versus minoxidil alone.[3]Nada, E.A., El-Dawla, R.E., El Maged, W.M.A., Emagd, A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). Available at: … Continue reading
- Outcomes: The dutasteride group showed a significant increase in hair density, hair shaft width, and terminal to vellus hair ratio relative to minoxidil-only controls. Patient-reported satisfaction was higher for the topical dutasteride cohort.
- Systemic/Serum: A reduction in serum DHT was observed in the topical dutasteride group, confirming some degree of systemic impact but still substantially less than typical oral dosing.
Study 2: Obeid et al, 2024 (Double-Blind RCT; 24 men, 6 women; 16 Weeks)
- Design/groups: Compared minoxidil plus topical dutasteride (0.01%) to minoxidil alone.[4]Obeid, M.N.A., Fattah, N.S.A., Elfangary, M.M., Husseni, R.M.A. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 … Continue reading
- Outcomes: The combination group achieved superior overall improvements in hair thickness and density compared to minoxidil monotherapy. Patient satisfaction and photographic evidence suggested enhanced regrowth when dutasteride was added.
- Systemic/Serum: Systemic DHT or hormone changes were not evaluated or reported in this study.
Study 3: Panuganti et al., 2025 (Phase II Double-Blind; 135 Men; 24 Weeks)
- Design/Groups: Compared three concentrations of topical dutasteride (0.01%, 0.02%, 0.05% w/v), oral finasteride (1 mg), and placebo.[5]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate … Continue reading
- Outcomes: Dose-dependent improvements in target area hair count (TAHC) were observed. Dutasteride 0.05% was superior at 24 weeks to both topical finasteride and placebo. Mean TAHC increase: 0.015 (12.78), 0.02% (20.03), 0.05% (34.30) vs oral finasteride (12.57). More patients reached global photographic assessment thresholds in the 0.05% group.
- Systemic/Serum: Topical dutasteride produced only modest changes in serum testosterone and DHT compared to moderate suppression seen with oral finasteride. The highest topical strength exhibited a better pharmacokinetic profile (lower systemic absorption) than oral finasteride.
These data provide evidence that topical dutasteride can offer clinically relevant improvement in hair quantity and quality, while keeping systemic risk modest and tolerability high in well-compounded, patient-tailored regimens.
Why Expectation Setting Matters
Setting realistic expectations is important for anyone considering topical dutasteride or other emerging therapies for AGA. Online testimonials and dramatic before-and-after photos can mislead, often emphasizing rare “hyper responders” or downplaying variables like lighting and styling.
In truth, most users experience gradual, modest improvements, not dramatic transformations, and disappointment is common when hopes are too high. Conversely, setting expectations too low may discourage trials of potentially worthwhile treatments that could deliver meaningful benefits.
For better outcomes with topical dutasteride:
- Expect gradual change rather than remarkable regrowth.
- Rely on professional advice and published clinical data, not social media images for guidance.
Remember: staying grounded in evidence and expert recommendations will help identify therapies truly worth pursuing on the hair regrowth journey.
Principle #1: What’s Possible ≠ What’s Probable
When looking at treatment outcomes, it’s essential to distinguish between what can happen and what usually happens. Look at the scatterplot below: individual data points are scattered widely, showing what’s possible for one person, while the trendline running through the middle represents the average, or what’s probable.
Figure 1: A graph showing possible and probable results. The data points show possible results, and the trendline shows probable results.[6]Badenhorst CE, Dawson B, Goodman C, Sim M, Cox GR, Gore CJ, Tjalsma H, Swinkels DW, Peeling P. Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur J Appl Physiol. 2014 … Continue reading
Online forums tend to showcase the most eye-catching success stories with topical dutasteride, cases where dramatic regrowth happens.
Figure 2: Anecdotal results are often way more dramatic than the typical.
These anecdotes, while genuine, sit far above the trendline. The problem comes when patients see only these high points and assume the same results are likely for themselves. When their own progress looks slower or less dramatic, they may quit early or wrongly conclude that the treatment doesn’t work.
Figure 3: Expected results are often far more dramatic than probable results.
The key is avoiding unrealistic benchmarks based on outliers. Our Regrowth Potential framework is designed to ground expectations in the probable but not the possible. For most patients, topical dutasteride leads to stabilization plus modest regrowth, with rare cases of strong “hyper responses” that shouldn’t be seen as the norm.
Most people using topical dutasteride experience stabilization of hair loss first, with the earliest cosmetic regrowth occurring at around 3 months. Typical regrowth usually brings hair growth back to where it was between 1-3 years ago, rather than to pre-hair loss density.
Several factors influence where someone falls on the response spectrum with topical dutasteride. Starting treatment earlier generally leads to better outcomes, as newer thinning areas respond more readily while long-standing bald spots are far less likely to improve. The specific therapy or combination of treatments used also matters, since some approaches are more effective depending on the stage or pattern of hair loss. Consistency is critical; missed applications or stopping too soon can quickly lower results below the expected average.
Principle #2: Poorer Evidence = Greater Variability
Predictability depends heavily on evidence quality. Treatments with a large body of research product outcomes that cluster tightly around the average, while those with fewer studies show more variability between patients.
Figure 4: Bell curves are a great way to show the typical outcomes depending on evidence. For finasteride, a narrow bell curve shows predictable outcomes. For topical dutasteride, a wide, shallow curve represents a much wider spread of outcomes.
Finasteride is a good comparison point: with dozens of randomised controlled trials, we can be confident that most users fall close to the mean response, represented by a narrow bell curve. Topical dutasteride, however, is newer and much less studied. While emerging data and user reports suggest potential benefits, even in some cases matching or exceeding oral outcomes, the evidence base is still limited.
In short, the more research that exists, the stronger our confidence in expected results. For topical dutasteride, the smaller body of evidence means higher variability. Some users may beat the average, but others will fall below it. Recognizing this can help you temper expectations and make better-informed decisions.
Evidence quality is the rubric we can use to determine whether the results will be predictable or variable.
10 Topical Dutasteride User Transformations
Now that we’ve covered the mechanisms of topical dutasteride while setting reasonable expectations, let’s look at what the results might actually look like in practice. Below, we’ve curated 10 Reddit case studies of users who saw clear improvement with topical dutasteride.
The examples below help see what’s possible and do not represent what every user should expect. They are purely anecdotal and not independently verified by us or any qualified third party.
User Profile #1: 14–15 Months on Topical Dutasteride 0.1% (once weekly; early 1%)
Source: u/inferismetal via r/tressless.
This user started topical dutasteride monotherapy, initially at 1% for the first 3 weeks, then at 0.1%, which he typically applied once weekly at 2 mL. He also used a low-level laser therapy (LLLT) device 3x/week in 15-minute sessions.
He reported noticeable thickening at month 3 and significant visible improvement with complete recovery of the crown by month 4.5. He also reported that results were maintained at months 14-15 and that gains were stabilized as of a later post. He observed that shedding had reduced to 5 hairs/day from the peak of 100/day. He did not report any systemic side effects on topical dutasteride 0.1%.
User Profile #2: 6 Months on Topical Dutasteride 2%
Source: u/Asleep_Ad_8009 via r/tressless.
This user chose topical dutasteride 2% twice per week for 6 months, increasing the frequency to daily application on the hairline only. He previously tried topical minoxidil and weekly microneedling but considered himself a non-responder.
He reported a heavy shedding phase starting from month 2 and lasting for 1-2 months. At the 6-month mark, he reported that shedding is still above pre-treatment levels but is lower than the peak shedding phase. He also reported no regrowth at month 6 and stated that his hair was in worse condition than at baseline. He reported no side effects overall from topical dutasteride.
User Profile #3: 8 Months on Topical Dutasteride 0.1% Monotherapy
Source: u/colter108 via r/tressless.
This male chose to use topical dutasteride monoetherapy at the 0.1% concentration with an application frequency of once weekly at months 0-4 and twice weekly at months 5-8.
At the 8-month mark, the photos showed subtle improvement with the user perceiving a slightly improved appearance. The user stated that his alopecia was aggressive and that a twice-weekly application may still be insufficient. He reported no side effects when asked.
User Profile #4: 4 Months on Topical Dutasteride 0.1% (with Minoxidil/Tret Mix in Vehicle)
Source: u/DaBoa70 via r/tressless
This user started applying topical dutasteride 0.1% every other day, eventually switching to once daily. His compounded lotion also contained minoxidil, plus tretinoin, melatonin, silicon, and biotin. He used a separate AM minoxidil and tretinoin when at home, in addition to once-weekly derma rolling sessions.
After 4 months on the topical dutasteride-based regimen, he reported the emergence of some baby hairs and some definite regrowth. He exhibited a noticeably better hairline compared to the pre-minoxidil baseline. He reported no side effects.
User Profile #5: 2 Months on Topical Dutasteride (w/ Minoxidil)
Source: u/Methibosheth via r/tressless.
This user applied both a topical dutasteride 0.5% solution and topical minoxidil twice daily over the course of 2 months. The user stated that his shedding had dropped noticeably after adding dutasteride to his minoxidil regimen. He reported no side effects with topical dutasteride and acknowledged that twice-daily application at 0.5% may be overkill.
It should be noted that it is really quite difficult to see improvements in cases where users take photos with different lighting and at different distances. If you are tracking your progress, try to keep as many variables the same as possible so that you can give yourself as accurate a comparison as possible.
User Profile #6: 6 Months on Topical Dutasteride 0.05% + Dutasteride Mesotherapy 0.01%
Source: u/Zealousideal-Ice4996 via r/tressless.
This user presented with diffuse thinning and was previously on finasteride with no side effects. He is motivated and willing to use medical interventions to achieve recovery. He applied topical dutasteride 0.05% twice weekly and dutasteride mesotherapy 0.01% once weekly via a 0.5 mm dermapen for a total duration of 6 months.
At the 6-month follow-up, he showed visible thickening, although he acknowledged that the results have not been dramatic. He stated in replies that he attained enough stabilization to later proceed with a crown transplant. The user reported no side effects.
User Profile #7: Male (53), 10 Weeks on Topical Dutasteride 0.3% + Minoxidil 8% (twice daily)
Source: u/Ok-Carrot-9987 via r/tressless.
This 53-year-old male applied a topical solution of 0.3% dutasteride and 8% minoxidil twice daily over 10 weeks. He later reduced the frequency to once daily and added intermittent microneedling, rosemary oil, and onion juice for gray hairs.
He noted a shedding phase starting in week 2, with visible thickening and density improvement by week 10, when he posted the before/after photos. In a later update, the user affirmed his continued improvement with reduced application frequency (once daily) and maintenance of results. He reported no side effects and expressed satisfaction with the early response.
User Profile #8: 4 Months on Topical Dutasteride + Topical Minoxidil
Source: u/Weird_Year2254 via r/tressless.
This Redditor’s intervention of choice was topical dutasteride (Anagenica) once daily, topical minoxidil, and dermastamp once weekly. At the 4-month mark, he reported a 90% reduction in hair shedding, the emergence of baby hairs at the crown and temples, and early vellus activation at the crown, which remained slower to respond.
He reported no side effects to date and did not mention any scalp irritation despite weekly stamping sessions. Notably, he did not state the exact dutasteride concentration in the topical solution, nor whether his dutasteride and minoxidil were contained in separate bottles or were pre-mixed in a single solution.
User Profile #9: Post-Hair Transplant Patient, 5.5 Months on Topical Dutasteride (Avodart capsules mixed into topical Minoxidil)
Source: u/grandtheftpixel via r/tressless.
This user began using a self-prepared solution by crushing Avodart (dutasteride) capsules and mixing them into a minoxidil PG/alcohol bottle, which he applied twice daily for 5.5 months. He did not report the dutasteride concentration in his homemade solution. He observed significant shedding and thinning, especially at the vulnerable right-sided native zone. After the first month, he also observed many thin, white vellus hairs appearing on top. He reported no systemic side effects.
User Profile #10: Male (NW7) with 18 Months Total Therapy Prior to Full-Dose Oral Dut
Source: u/Oxi_Dat_Ion via r/tressless.
This man used topical dutasteride for an unspecified period after 12+ months on minoxidil, before escalating to oral dutasteride 0.5 mg every two weeks, then to twice daily. Additionally, he underwent microneedling monthly at 0.75 mm and used ketoconazole shampoo 1 to 2 times weekly.
The patient showed visible improvement despite starting therapy from an advanced stage of hair loss (NW7). He reported no significant side effects, but has decreased his microneedling frequency due to scalp soreness.
Since he did not specify the duration of his topical dutasteride use and given that he has used several other formulations at varying doses over the 18 months, it is not possible to reliably estimate the degree to which topical dutasteride is responsible for the regrowth.
Probable Regrowth Timeline for Topical Dutasteride
We have laid out a likely timeline for topical dutasteride, but this is based on three studies we could find using it.
Months 0-3: Early cosmetic improvements
- Most users won’t see regrowth yet.
- Shedding may occur in the first few weeks (especially if combined with microneedling).
- Early pharmacokinetic studies show scalp DHT reduction with minimal systemic absorption at appropriate doses.
Months 3-6: First cosmetic improvements
- This is the case for both low (0.025%) and higher concentrations (0.1-0.3%) of topical dutasteride.
- Clinical studies found measurable increases in hair density when topical dutasteride was paired with microneedling.
- Improvements are usually more visible in the crown and mid-scalp than at long-standing bald spots.
Months 6-9: Noticeable regrowth in responders
- Hair density and thickness continue to improve.
- Studies reported ~10-15% increases in hair accounts and 15-20% increases in density, especially with microneedling.
- Cosmetic changes (less scalp show-through, fuller appearance) often emerge in this window.
- Data is limited after ~7 months.
Months 9+
- Likely that many responders achieve their most visible results by 9-12 months.
- Long-term clinical data are sparse; by 36 months, most hair gains will have occurred.
So, what about all the success stories we see online? This is typically called survivorship bias.
Figure 5: Survivorship bias skews perception of hair loss treatments. Online accounts often highlight extreme outcomes, either exceptional success or very poor results, while the typical, average outcomes experienced by most remain underrepresented.
Combining Treatments
If you want to increase the effects of topical dutasteride, combining it with additional therapies can have a beneficial effect.
One study compared topical minoxidil 5% alone vs. in combination with topical dutasteride, showing both approaches to be effective and safe for AGA in men and women.[7]Obeid, M.N.A., Fattah, N.S.A., Elfangary, M.M., Husseni, R.M.A. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 … Continue reading
Another trial evaluated “minoxidil-dutasteride tattooing”, a combined delivery, which led to notable clinical improvement in hair growth for patients with AGA.[8]Ragi, S.D., Ghanian, S., Rogers, N., Peterson, D.M., Johnson, L.S., Wambier, C.G. (2023). Evaluation of hair regrowth after minoxidil and dutasteride tattooing in men with androgenetic alopecia. JAAD … Continue reading
Microneedling is increasingly used to enhance drug penetration for hair loss therapies. Clinical protocols recommend microneedling (often with 1.5-2.5 mm rollers)before topical dutasteride application for improved efficacy, with studies confirming significant increases in hair density and shaft thickness.[9]Sanchez-Meza.E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-Y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
Recent reviews discuss rotational therapy for partial responders, switching between topical and oral 5ɑ-reductase inhibitors to individualize regimens and maximize hair regrowth while managing side effects.[10]Mysore, V., Kumaresan, M., Dashore, S., Venkatram, A. (2023). Combination and Rotational Therapy in Androgenetic Alopecia. Journal of Cutaneous and Aesthetic Surgery. 16(2). 71-80. Available at: … Continue reading
How Can I Maintain Realistic Expectations?
To keep realistic expectations with topical dutasteride, it’s important to remember that most users will see noticeable improvement and stabilization, though individual results can vary. Realistic does not mean pessimistic; most people benefit, but baseline expectations should be guided by typical clinical results rather than extreme cases.
Topical dutasteride may “rewind” hair loss by several months to a couple of years, but full restoration to pre-hair loss density is uncommon. The most likely outcome is gradual improvement in hair density and a slowing of hair loss, rather than dramatic, multi-year regrowth or complete reversal.
Practical Takeaways
- Topical dutasteride is promising, but still considered to be experimental. Early clinical studies show significant efficacy, especially in comparison to finasteride, but more long-term research is needed to confirm its effectiveness and safety in larger populations.
- Systemic side effects appear reduced compared to oral use, but topical dutasteride is not entirely risk-free. Phase II trials report little to no local irritation and only modest changes in serum hormone levels with topical formulations, suggesting an improved systemic safety profile, but ongoing monitoring is recommended.
- Topical dutasteride is best used as part of a multi-therapy regimen. Combination approaches (for example, with minoxidil or microneedling) tend to enhance results and individualize treatment, maximizing regrowth while managing side effects.
Final Thoughts
When evaluating topical dutasteride, it’s essential to apply the framework of Regrowth Potential and Evidence Quality. While initial research and clinical experience suggest topical dutasteride is promising, the clinical data are lacking. Setting realistic expectations is important; focusing on typical results rather than extreme anecdotes helps prevent disappointment and treatment abandonment, encouraging patients to make informed decisions based on both scientific evidence and personal goals.
References[+]
References ↑1 Frye, S.V. (2006). Discovery and clinical development of dutasteride, a potent dual 5alpha-reductase inhibitor. Current topics in medicinal chemistry. 6(5). 405-421. Available at: https://doi.org/10.2174/156802606776743101 ↑2 Noor, N.M., Sheikh, K., Somavarapu, S., Taylor, K.M.G. (2017). Preparation and characterization of dutasteride-loaded nanostructured lipid carriers coated with stearic acid-chitosan oligomer for topical delivery. European journal of pharmaceutics and biopharmaceutics. 117. 372-384. Available at: https://doi.org/10.1016/j.ejpb.2017.04.012 ↑3 Nada, E.A., El-Dawla, R.E., El Maged, W.M.A., Emagd, A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). Available at: https://smj.journals.ekb.eg/article_42083_24b61cbba4be9982db23c318414034c0.pdf ↑4, ↑7 Obeid, M.N.A., Fattah, N.S.A., Elfangary, M.M., Husseni, R.M.A. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 mg) in Treatment of Androgenetic Alopecia. QJM: An International Journal of Medicine. 117(2). Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑5 Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects with Androgenetic Alopecia. Cureus. 17(8). E89309. Available at: https://doi.org/10.7759/cureus.89309 ↑6 Badenhorst CE, Dawson B, Goodman C, Sim M, Cox GR, Gore CJ, Tjalsma H, Swinkels DW, Peeling P. Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur J Appl Physiol. 2014 May;114(5):951-9. doi: 10.1007/s00421-014-2829-6. Epub 2014 Feb 1. PMID: 24487960. ↑8 Ragi, S.D., Ghanian, S., Rogers, N., Peterson, D.M., Johnson, L.S., Wambier, C.G. (2023). Evaluation of hair regrowth after minoxidil and dutasteride tattooing in men with androgenetic alopecia. JAAD International. 12. 103-104. Available at: PMID 37404245 ↑9 Sanchez-Meza.E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-Y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. Journal of the European Academy of Dermatology and Venereology. 36(10). E806-e808. Available at: https://doi.org/10.1111/jdv.18285 ↑10 Mysore, V., Kumaresan, M., Dashore, S., Venkatram, A. (2023). Combination and Rotational Therapy in Androgenetic Alopecia. Journal of Cutaneous and Aesthetic Surgery. 16(2). 71-80. Available at: https://doi.org/10.4103/JCAS.JCAS_212_22 Topical minoxidil has been a cornerstone of androgenic alopecia (AGA) treatment since its FDA approval in the 1980s. With millions of users worldwide, it stands as one of the most widely adopted therapies for hair loss, supported by decades of clinical evidence, and an enormous body of real-world anecdotes, reviews, and before-and-after photos.
However, results with minoxidil can be highly variable, and this variability can be traced in part to biological differences, particularly in the activity of the sulfotransferase enzyme (SULT1A1), which is responsible for converting minoxidil into its active form. Not everyone sulfates minoxidil effectively, meaning that while some achieve meaningful regrowth, others see little to no improvement.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Adding to this challenge is the influence of online topical minoxidil before and after photos (like you can see below), which can create inflated expectations compared to the more modest, probable outcomes seen in practice.
Source: u/United_Speaker3381 via r/tressless.
This article will break through that noise to answer a critical question: when it comes to minoxidil, what is actually the most probable result, not just possible?
First, let’s take a look at what topical minoxidil is.
Refresher: What is Topical Minoxidil?
Topical minoxidil was the first therapy ever approved by the FDA for the treatment of AGA, making it a landmark in the medical management of pattern hair loss.[1]Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786 Introduced in the 1980s, it has since become one of the most widely available and researched treatments across the globe. Its primary action is as a potassium channel opener, which helps to stimulate blood flow, prolong the hair’s growth phase (anagen), and increase follicle size, mechanisms that together can support thicker, denser hair regrowth over time.[2]Messenger, A.G., Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 150(2). 186-194. Available at: https://doi.org/10.1111/j.1365-2133.2004.05785.x
Minoxidil is known as a “pro-drug”, meaning that it needs to be activated within the body to exert its effect. The enzyme responsible for this is called sulfotransferase or, more specifically, SULT1A1. The level of sulfotransferase activity differs depending on the individual, which means that the effectiveness of treatment also differs depending on who you are.[3]Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a … Continue reading
Formulations typically come in 2% and 5% strengths. The 2% solution was originally FDA-approved for women, while the 5% concentration was approved for men; however, both are now commonly used off-label across genders. Beyond these standard strengths, compounding pharmacies have made higher concentrations available for patients seeking more individualized treatment approaches, with some online telehealth providers (such as Ulo) offering customized formulations. Standard dose topical minoxidil is available over-the-counter in most countries, ensuring accessibility without the need for a prescription.
The Evidence
Topical minoxidil’s robust efficacy for hair regrowth is supported by dozens of high-quality randomized controlled trials (RCTs) and meta-analyses across men and women, consistently demonstrating average increases in hair count of around 10-20% over 6-12 months.
We have collated many of these studies into a table, which you can look at here. However, we have summarised some of the most recent topical minoxidil studies.
Liang et al. (2022)
- Prospective, evaluator-blinded RCT with 120 women with female pattern hair loss (FPHL) assigned to three groups: 5% topical minoxidil alone; 5% minoxidil + oral spironolactone (80-100 mg); and 5% minoxidil and microneedling every 2 weeks.[4]Liang, X., Chang, Y., Wu, H., Liu, Y., Zhao, J., Wang, L., Zhuo, F. (2022). Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female … Continue reading
- Duration: 24 weeks; outcomes measured included dermascope, ultrasound biomicroscopy, physician, and patient assessments.
- Effective rate at week 2:
- 55.27% (topical minoxidil)
- 86.49% (topical minoxidil and oral spironolactone)
- 95% (topical minoxidil and microneedling)
- Combo therapies (Groups 2&3) outperformed topical minoxidil alone for hair density, shaft diameter, scalp/thickness, shedding, and quality of life; Group 3 showed the greatest benefit.
- Adverse effects were reported across all groups; more menstrual disorders were reported in the spironolactone group. Main limitations: small sample, exclusion of severe cases, possible bias from unblinded treatments.
Hassan et al. (2022)
- Comparative study in 60 women with FPHL: 5% minoxidil topical foam and oral vitamin D3, minoxidil foam only, or vitamin D3 for 12 weeks (plus 15 controls for vitamin D comparison).[5]Hassan, G.F.R., Sadoma, M.E.T., Elbatsh, M.M., Ibrahim, Z.A. (2022). Treatment with oral vitamin D alone, topical minoxidil, or combination of both in patients with female pattern hair loss: A … Continue reading
- Primary outcomes: reduction in thin hair count and Ludwig stage improvement via clinical and dermoscopic evaluation.
- Best results from combination therapy (minoxidil foam and vitamin D3), followed by minoxidil foam alone; vitamin D3 monotherapy had minimal effect.
- Vitamin D3 appeared to enhance minoxidil efficacy, though the sample size was small, and adverse events were not reported.
Bao et al. (2022)
- RCT with 71 men, split into: 5% topical minoxidil twice daily, electrodynamic microneedling every 3 weeks, or topical minoxidil and microneedling combined.[6]Bao, L., Zong, H., Fang, S., Zheng, L., Li, Y. (2019). Randomized trial of electrodynamic microneedling combined with 5% minoxidil topical solution for treating androgenetic alopecia in Chinese males … Continue reading
- Follow-up: 24 weeks, with an additional 6-month observation period.
- All treatments increased non-vellus hair density; the combined group had the greatest improvements in hair density and diameter.
- The combination group received additional scalp massages (potential confounder); 12 participants experienced adverse reactions (dermatitis, dandruff, lymph node enlargement), none severe.
Singh et al. (2020)
- Double-blind, placebo-controlled RCT in 80 men: Groups received either 5% topical minoxidil, 5% topical minoxidil and PRP injections, placebo, or PRP alone.[7]Singh, S.K., Kumar, V., Rai, T. (2020). Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: A randomized, double-blind placebo control … Continue reading
- 12 weeks’ treatment with 2-month follow-up; outcome: changes in total, terminal, vellus, and non-vellus hair density, pigmentation, and global assessments.
- Highest hair density and satisfaction with topical minoxidil and PRP; both monotherapies are better than placebo, but the combo was most effective.
- Limitations: short-term follow-up, some subjective outcomes; low adverse event rate (mostly mild, e.g., scalp pruritus, facial hair growth).
Overall, when you look across all of the data, consistent benefits are seen with topical minoxidil usage. However, these results are variable. The key reason for this is differences in sulfotransferase activity. As we mentioned before, minoxidil is a pro-drug, and it needs to be enzymatically activated to exert its effect. We will go into more detail about this below.
What’s Possible ≠ What’s Probable
When evaluating treatment outcomes, especially online minoxidil before and after photos, it’s important to separate possibility from probability. The scattered data points below show the full range of what might occur for individuals, while the central trendline reflects the average outcome (i.e., what typically happens).
Figure 1: A graph showing possible and probable results. The data points show possible results, and the trendline shows probable results.[8]Badenhorst CE, Dawson B, Goodman C, Sim M, Cox GR, Gore CJ, Tjalsma H, Swinkels DW, Peeling P. Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur J Appl Physiol. 2014 … Continue reading
Online anecdotes often showcase what we would call “hyper-responders” – people who experience dramatic results. These people may also have higher levels of sulfotransferase activity.
Figure 2: Anecdotal results are often way more dramatic than the typical.
These stories, while real, represent outcomes that are well above average. The issue arises when patients focus only on these peak results and expect the same for themselves. If their own progress seems slower or less dramatic, they may stop prematurely or mistakenly believe the treatment is ineffective.
Figure 3: Expected results are often far more dramatic than probable results.
Dramatic results are often showcased more than modest outcomes because of survivorship bias. This bias occurs when only the most striking successes are noticed or shared, while the many average or less impressive results go unreported.
Figure 4: Survivorship bias skews perception of hair loss treatments. Online accounts often highlight extreme outcomes, either exceptional success or very poor results, while the typical, average outcomes experienced by most remain underrepresented.
Why Results Are Variable: The SULT1A1 Enzyme
Minoxidil must be converted into minoxidil sulfate by the sulfotransferase enzyme SULT1A1, which is present in the outer root sheath of hair follicles, to become pharmacologically active and stimulate hair growth.
Several peer-reviewed studies confirm that the level of SULT1A1 activity in scalp follicles is a key determinant of how well a patient responds to minoxidil therapy.[9]Goren, A., Castano, J.A., McCoy, J., Bermudez, F., Lotti, T. (2014). Novem enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatologic Therapy. 27(3). 171-173. … Continue reading Enhancing SULT1A1 activity, such as by using enzyme-boosting adjuvants, has been shown in recent trials to convert a majority of prior non-responders into responders, further confirming the mechanistic role of this enzyme.[10]Chandrashekar, B.S., Chandu, M., Shenoy, C., Chander, A., Roopa, M.S. (2024). SULT1A1 enzyme booster to amplify topical minoxidil response in androgenic alopecia: a single-center prospective study. … Continue reading
This enzyme-based variability explains why two people can apply minoxidil in the same way but see very different outcomes, and why more than half of users may see only limited or no benefit despite consistent application.
Fixing the Enzymatic Problem
A number of evidence-based strategies exist to improve minoxidil response. These include microneeding, retinoic acid co-application, formulation changes, consistency, and combination therapy.
Microneedling
Multiple systematic reviews and clinical trials confirm microneedling significantly improves minoxidil efficacy. A 2023 meta-analysis showed that combining the two led to a significantly greater increase in hair count compared to minoxidil alone, regardless of needle depth or device used. Hair count improvements are notable after 12 to 24 weeks of combined treatment.[11]Ahmed, K.M.A., Kozaa, Y.A., Abuawwad, M.T., Al-Najdawi, A.I., Mahmoud, Y.W., Ahmed, A.M., Taha, M.J.J., Fadhli, T., Giannopoulou, A. (2025). Evaluating the efficacy and safety of combined … Continue reading
You can read more about how microneedling improves minoxidil efficacy here.
Retinoic Acid (Tretinoin)
Topical tretinoin has been shown in peer-reviewed studies to upregulate follicular SULT1A1, thereby enhancing the conversion of minoxidil to its active form. In a 2019 study, tretinoin not only improved clinical response in difficult cases but also increased scalp SULT1A1 activity, supporting its role as a mechanistic booster.[12]Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvan, S., Chen, G., Tan, Y., Kovacevic, M. (2019). Tretinoin enhances minoxidil response in androgenetic alopecia … Continue reading
You can see the benefits of adding retinoic acid in our Compare Treatments graph below.
Figure 5: Combining topical minoxidil with retinoic acid significantly boosts regrowth potential.
Learn more about retinoic acid and its effects on sulfotransferase here.
Formulation Adjustments (Foam vs. Solution 2% vs. 5%)
Clinical research shows that 5% minoxidil is more effective than 2% for both men and women. Foam is as effective as a solution but better tolerated, with reduced risk of contact dermatitis due to the absence of propylene glycol. Some evidence suggests liquid formulations might yield slightly higher absorption, but both forms are clinically comparable.[13]Purnak, T., Senel, E., Sahin, C. (2011). Liquid formulation of minoxidil versus its foam formulation. Indian Journal of Dermatology. 56(4). 462. Available at: https://doi.org/10.4103/0019-5154.84714
Consistency and Adherence
Missed doses can reduce topical minoxidil efficacy. Some clinical guidance suggests that missing minoxidil applications even once per week can diminish results, and consistent, daily use is necessary to achieve and maintain hair regrowth.[14]Shadi, Z. (2023). Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy. 13(5). 1157-1169. Available at: … Continue reading
Combination Therapy
Combining minoxidil with finasteride/dutasteride, PRP, low-level laser therapy (LLLT), or, in resistant cases, oral minoxidil, provides superior results versus monotherapy. Meta-analyses and randomized trials consistently find greater improvement in hair density, count, and patient satisfaction when using combination regimens.[15]Xia, Y., Chen, H., Chen, Y., Chen, Z. (2025). Relative efficacy of minoxidil in combination with other treatments for androgenic alopecia: a network meta-analysis based on randomized controlled … Continue reading
So, what do online topical minoxidil before and after photos look like?
Online Topical Minoxidil Successes
Now that we have discussed how topical minoxidil works, potential regimens, and likely timelines, here are 10 reports published by Reddit users with before and after photos.
Disclaimer: The stories below are unverified anecdotes. Consequently, they illustrate individual outcomes and should not be construed as clinical proof or medical advice.
Case 1: Male (age unknown), 6 months on topical minoxidil monotherapy (5% once daily)
Source: u/Dual270x via r/tressless.
This individual applied 5% topical minoxidil once daily for six months. They chose topical minoxidil out of a general preference for avoiding oral medications. From the before-and-after photos, diffuse thinning can be observed from the center of the crown, and the protocol yielded a noticeable increase in crown density by month six. No side effects or early shedding have been reported.
Case 2: Female (age unknown), about 5 months on topical minoxidil monotherapy (5% once nightly)
Source: u/maddogyr via r/FemaleHairLoss.
This user applied 5% topical minoxidil once nightly for about 5 months, using a liquid solution that she purchased over the counter after a telemedicine consult. She stated that she had AGA and did not note any other treatment. Based on both her comments and photos, she experienced marked improvement in hair density and coverage, stating that minoxidil “saved her hair” and that she wishes she had started earlier. She did not describe any notable early shedding, but noted dry scalp and some dandruff as potential side effects.
Case 3: Male (age unknown), 60 days on topical minoxidil monotherapy (5% foam nightly)
This Redditor applied 5% minoxidil foam once nightly before bed for two months without any concurrent treatment. He reports the typical amount applied daily as “one cap-full”, rinsed out the next morning. The user has Crohn’s disease with prior nutrient absorption issues, and chose to avoid ifnasteride due to side-effect concerns. He had about one year of noticeable thinning before starting. Upon starting minoxidil, shedding increased for the first two weeks, then eased. The first measurable improvement was visible by day 30, with clear density gains at day 60. The crown region improved the fastest, while hairline changes were slower.Source: u/[deleted] via r/tressless.
Case 4: Female (age unknown), 1 year 5 months on topical minoxidil foam (5% once daily)
Source: u/Hyper-Fang via r/FemaleHairLoss.
This user had AGA with prior telogen effluvium, reporting diffuse thinning along the part and temples and a reportedly high daily shedding. She began therapy with Rogaine Men’s Foam 5% once nightly, supported by Ketoconazole 2% shampoo once weekly. At 2 months of treatment, “peach fuzz”/baby hairs were visible at the hairline and temples. At 6 months, there was a noticeable density across the top with new hairs evident but still short. After 1 year and 5 months of therapy, she notes ongoing thickening and widespread new hair growth. She also reported that her shower shed decreased from ~70 hairs to ~10-15, with visual density improving at the part and temples. She reported a mild scalp itch at the start and subtle peach-fuzz on the upper lip and chin as side effects.
Case 5: Male (age unknown), 2.5 months on topical minoxidil (5% liquid 1 mL twice daily)
Source: u/pcybits via r/tressless.
This male user, likely with AGA, reported recession at the hairline and thinning at the crown after 2.5 months of using a Rogaine-style 5% liquid at 2 mL/day, without any concurrent DHT blocker. He experienced an early shedding phase ~1 month into treatment, which stabilized with continued use. He exhibited visible thickening at the hairline and crown by 2.5 months of therapy. He did not report any systemic or local side effects and plans to continue minoxidil while considering adding weekly microneedling and/or topical finasteride if progression resumes.
Other Redditors have used combination treatments to further improve their hair regrowth. As you can see below, some of the results look dramatic.
Case 6: Male (age unknown), 3 months on topical combo (minoxidil 5% and finasteride 0.1% once nightly)
Source: u/United_Speaker3381 via r/tressless.
This user had thinning at the hairline and vertex areas of the scalp and began treatment to improve hair density and maintenance. He applied a single compounded solution (Novegrow) containing minoxidil 5% and finasteride 0.1%, once nightly, for three months before reporting on progress. Additionally, he dermarolled his scalp with a 0.5 mm needle twice weekly and supplemented with daily biotin and a multivitamin. He reported mild shedding in the first two weeks of therapy, and by month 3, he noted visible thickening and improved coverage. He did not report any side effects and added that the once-daily schedule was practical and helped with adherence.
Case 7: Male (25), 11 months on combination therapy (finasteride 1 mg daily and topical minoxidil foam 5% once daily)
Source: u/Jordan_cadagan via r/tressless.
This Redditor exhibited typical AGA with frontal recession and diffuse vertex thinning. His core regimen is oral finasteride 1 mg daily and minoxidil 5% foam (Kirkland) once daily. He noted he took regular ice/cold baths, ensured an increased water intake, engaged in exercise, avoided junk food, and did not smoke. He saw noticeable thickening from around 6 months after several shedding cycles. By month 11, he experienced substantial cosmetic improvement, with some commenters even comparing the results to transplant-level density. He did not report any adverse effects from finasteride or minoxidil, and subjectively felt less “brain fog” after starting finasteride.
Case 8: Male (37), 4 months on topical minoxidil 5% and topical finasteride 0.1% (1 mL nightly)
Source: u/Far-Instance268 via r/tressless.
This male had crown-predominant thinning with a stable hairline at baseline. His core regimen is a single combined solution containing minoxidil 5% and finasteride 0.1%, applied to the crown only. He did not report any microneedling or supplementation. He reported that shedding began around week 3 and was “heavy” for approximately 4 weeks. The shedding tapered and largely stopped at the 2.5-month mark. By month 4, he achieved visible crown regrowth and thickening compared to the baseline, despite the application being limited to the crown. He perceived an increase in overall density and did not report any side effects.
Case 9: Male (34), about 12 months on topical finasteride 0.3% and minoxidil 6% (twice daily)
Source: u/iTsundere via r/tressless.
This male reported on his progress after using a combination of topical finasteride 0.3% and minoxidil 6% treatment twice daily for nearly one year. He first experienced hair thinning and hairline recession beginning in his late 20s. Alongside the topical treatments, he also applied a dermastamp at approximately 1.5 mm every 10 days. He reported significant shedding during the first two months of treatment along with mild chest tenderness in the first month, which resolved after temporarily halving the topical dose. He reported month-on-month thickening, with clear improvement by months 7-8 and robust density by the one-year mark.
Case 10: Male (22), 90 days on finasteride 1 mg and minoxidil 5% twice daily plus dermarolling
Source: u/M3ga_Shniz via r/tressless.
This user with early temple recession began a combination therapy of finasteride 1 mg oral daily, minoxidil 5% foam twice daily, and dermaoll 1.5 mm weekly. He experienced noticeable shedding at weeks 3-6 of therapy, and at the 90-day progress mark, he reported visible filling in of the temple and general thickening of the hair. He did not report any side effects and noted that pushing through the initial shedding phase was crucial to his progress thus far.
Probable Regrowth Timeline
Based on the available evidence, we have mapped out a likely regrowth timeline (although some may experience results faster or later).
Months 0-3: Initial shedding
- Temporary shedding may occur as follicles shift into a new growth phase.
- This usually resolves within the first 4-8 weeks.
Months 3-6: Early cosmetic improvements
- Early visible improvements, such as reduced shedding and appearance of fine new hairs, can be expected.
- Thicker, darker regrowth starts to become apparent for most users during this period.
Months 6-12: Peak cosmetic changes
- Cosmetic changes peak, with stronger hair, increased density, and the greatest coverage observed.
- This is the period of maximum regrowth for most individuals.
Months 12+: Plateau, maintenance, & slight regression
- Further gains plateau, with 5-year studies showing hair counts remaining above baseline –– but with some loss in “peak” results from year one. Continued daily use is needed to maintain improvements, as stopping treatment leads to a gradual reversal within a few months.
Safety and Side Effects
Local side effects are relatively common with topical minoxidil, especially with the liquid formulations. The principal culprits are propylene glycol and ethanol, which frequently cause redness, itching, dryness, and a dandruff-like scaling of the scalp.[16]Makhlouf, A., Elnawawy, T. (2023). Hair regrowth boosting via minoxidil cubosomes: Formulation development, in vivo hair regrowth evaluation, histopathological examination and confocal laser … Continue reading Patients with sensitive skin are particularly prone to these adverse reactions and may experience contact dermatitis, which can make long-term use uncomfortable.
The foam formulation is generally better tolerated because it lacks propylene glycol. Most clinical trials and user surveys report fewer cases of scalp irritation, itching, or flaking with foam compared to the liquid version.[17]Purnak, T., Senel, E., Sahin, C. (2011). Liquid formulation of minoxidil versus its foam formulation. Indian Journal of Dermatology. 56(4). 462. Available at: https://doi.org/10.4103/0019-5154.84714
While systemic absorption of topical minoxidil is rare, some users may experience unwanted side effects such as dizziness and the growth of facial or body hair away from the application site. These effects are more likely if the medication is used excessively or applied to broken or abraded skin, which can increase absorption. We do not recommend using topical products if you have an inflammatory scalp condition – deal with that problem first, and then take a look at topical hair regrowth products.
Overall, when used as directed, topical minoxidil has a strong safety profile, with most side effects limited to mild local irritation.
Practical Takeaways
- Topical minoxidil is effective and well-established, but clinical results vary between individuals.
- Most patients achieve stabilization of hair loss with modest regrowth, though complete restoration is uncommon.
- Response depends partly on scalp sulfotransferase enzyme activity, which explains why some non-responders see little or no improvement.
- Best results come with consistent, long-term use and may be enhanced by adjuncts such as microneedling or topical retinoids to improve absorption and efficacy.
Final Thoughts
When evaluating minoxidil, it is important to recognize it as the most widely accessible and evidence-backed treatment for androgenetic alopecia. While outcomes can vary, most users see stabilization and modest regrowth rather than dramatic reversal. Success depends on realistic expectations, consistent use, and optimized regimens that may include adjunctive therapies. Applying a framework that emphasizes the likely trendline over anecdotal extremes can help you stay committed, avoid discouragement, and make informed decisions grounded in evidence and personal treatment goals.
References[+]
References ↑1 Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786 ↑2 Messenger, A.G., Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 150(2). 186-194. Available at: https://doi.org/10.1111/j.1365-2133.2004.05785.x ↑3 Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. Advances in Dermatology and Allergology. 39(3). 472-478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑4 Liang, X., Chang, Y., Wu, H., Liu, Y., Zhao, J., Wang, L., Zhuo, F. (2022). Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A Prospective, Single-Center, Parallel-Group, Evaluator Blinded, Randomized Trial. Frontiers in Medicine. 11(9). 905140. Available at: https://doi.org/10.3389/fmed.2022.905140 ↑5 Hassan, G.F.R., Sadoma, M.E.T., Elbatsh, M.M., Ibrahim, Z.A. (2022). Treatment with oral vitamin D alone, topical minoxidil, or combination of both in patients with female pattern hair loss: A comparative clinical and dermoscopic study. Journal of Cosmetic Dermatology. 21(9). 3917-3924. Available at: https://doi.org/10.1111/jocd.14743 ↑6 Bao, L., Zong, H., Fang, S., Zheng, L., Li, Y. (2019). Randomized trial of electrodynamic microneedling combined with 5% minoxidil topical solution for treating androgenetic alopecia in Chinese males and molecular mechanistic study of the involvement of the Wnt/ꞵ-catening signaling pathway. Journal of Dermatological Treatment. 33(1). 483-493. Available at: https://doi.org/10.1080/09546634.2020.1770162 ↑7 Singh, S.K., Kumar, V., Rai, T. (2020). Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: A randomized, double-blind placebo control trial. Indian Journal of Dermatology, Venereology, and Leprology. 86(2). 150-157. Available at: https://doi.org/10.4103/ijdvl.IJDVL_589_18 ↑8 Badenhorst CE, Dawson B, Goodman C, Sim M, Cox GR, Gore CJ, Tjalsma H, Swinkels DW, Peeling P. Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur J Appl Physiol. 2014 May;114(5):951-9. doi: 10.1007/s00421-014-2829-6. Epub 2014 Feb 1. PMID: 24487960. ↑9 Goren, A., Castano, J.A., McCoy, J., Bermudez, F., Lotti, T. (2014). Novem enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatologic Therapy. 27(3). 171-173. Available at: https://doi.org/10.1111/dth.12111 ↑10 Chandrashekar, B.S., Chandu, M., Shenoy, C., Chander, A., Roopa, M.S. (2024). SULT1A1 enzyme booster to amplify topical minoxidil response in androgenic alopecia: a single-center prospective study. International Journal of Research in Medical Sciences. 12(11). Available at: https://doi.org/10.18203/2320-6012.ijrms20243362 ↑11 Ahmed, K.M.A., Kozaa, Y.A., Abuawwad, M.T., Al-Najdawi, A.I., Mahmoud, Y.W., Ahmed, A.M., Taha, M.J.J., Fadhli, T., Giannopoulou, A. (2025). Evaluating the efficacy and safety of combined microneedling therapy versus topical Minoxidil in androgenetic alopecia: a systematic review and meta-analysis. Archives of Dermatological Research. 317(1). 528. Available at: https://doi.org/10.1007/s00403-025-04032-1 ↑12 Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvan, S., Chen, G., Tan, Y., Kovacevic, M. (2019). Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatologic Therapy. 32(3). E12915. Available at: https://doi.org/10.1111/dth.12915 ↑13, ↑17 Purnak, T., Senel, E., Sahin, C. (2011). Liquid formulation of minoxidil versus its foam formulation. Indian Journal of Dermatology. 56(4). 462. Available at: https://doi.org/10.4103/0019-5154.84714 ↑14 Shadi, Z. (2023). Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy. 13(5). 1157-1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑15 Xia, Y., Chen, H., Chen, Y., Chen, Z. (2025). Relative efficacy of minoxidil in combination with other treatments for androgenic alopecia: a network meta-analysis based on randomized controlled trials. Frontiers in Medicine. 12. Available at: https://doi.org/10.3389/fmed.2025.1638496 ↑16 Makhlouf, A., Elnawawy, T. (2023). Hair regrowth boosting via minoxidil cubosomes: Formulation development, in vivo hair regrowth evaluation, histopathological examination and confocal laser microscopy imaging. International Journal of Pharmacology. 634. 122665. Available at: https://doi.org/10.1016/j.ijpharm.2023.122665 Oral finasteride has long been the mainstay for androgenic alopecia (AGA), but many patients and clinicians have turned to dutasteride, a more potent 5ɑ-reductase inhibitor, in pursuit of superior hair growth.
Oral dutasteride (0.5 mg daily) can reduce dihydrotestosterone (DHT) by 90% or more (compared to ~70% with finasteride), and clinical studies show it grows more hair than finasteride. In fact, a 24-week trial found that oral dutasteride 0.5 mg significantly outperformed finasteride 1 mg in increasing hair counts.[1]Shanshanwal, S.J.S., Dhurat, R.S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled … Continue reading
However, dutasteride’s very potency raises concerns: will it also increase side effects like decreased libido or hormonal disturbances? Interestingly, research so far suggests that dutasteride has a similar tolerability profile to finasteride with no clear increase in sexual side effects despite its stronger DHT suppression.[2]Almudimeegh, A., Almutairi, H., AlTassan, F., AlQuraishi, Y., Nagshabandi, K.N. (2024). Comparison between dutasteride and finasteride in hair regrowth and reversal of miniaturization in male and … Continue reading
Even so, oral dutasteride’s reputation (it’s approved for prostate enlargement and used off-label for hair loss) makes some men uneasy, especially those who experienced side effects on finasteride. This has led to rising interest in topical dutasteride formulations. By delivering dutasteride directly to the scalp, the goal is to concentrate its DHT-blocking action where it’s needed (hair follicles) while limiting how much enters the bloodstream. The questions we need to answer are:
- Does topical dutasteride work?
- Does it match or even exceed the efficacy of oral treatments?
- Can it maintain these benefits with a more favorable side effect profile?
Ulo offers dutasteride options that range from low to high dose dutasteride – allowing you to be flexible in your treatment choices.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Oral vs. Topical Dutasteride
Early evidence indicates that topical dutasteride can indeed improve hair growth, in some cases achieving results comparable to oral therapy but with lower systemic DHT reduction.
Study One
One study tested dutasteride solutions of 0.01%, 0.02%, and 0.05% against a placebo (and an active control) over 24 weeks. All dutasteride groups showed significant increases in hair count versus placebo, and the 0.05% topical solution actually showed more efficacy than oral finasteride (1 mg/day) in promoting regrowth.[3]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammed, J., Rao, S., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the … Continue reading
Figure 1: Representative images of hair growth in male-pattern androgenetic alopecia after treatment with 0.01% dutasteride topical solution at 12 week (b) and 24 week (c) vs baseline (a); 0.02% dutasteride topical solution at 12 week (e) and 24 week (f) vs baseline (d); 0.05% dutasteride topical solution at 12 week (h) and 24 week (i) vs baseline (g); oral finasteride 1 mg tablets at week 12 (k) and week 24 (l) vs baseline (j); placebo at week 12 (n) and week 24 (o) vs baseline (m).[4]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammed, J., Rao, S., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the … Continue reading Image obtained in line with the PMC Copyright License.
Study Two
A recent randomized controlled study compared oral dutasteride (0.5 mg daily) to topical dutasteride (0.02% administered via monthly microneedling), both combined with daily topical minoxidil 5%. The trial found that all groups (including minoxidil alone) saw improvement, but the topical dutasteride via microneedling group demonstrated results comparable to the oral dutasteride group in terms of increased hair density and width, with the added advantage of fewer systemic side effects.[5]Obeid, M.N.A., Fatteh, N.S.A., Elfangary, M.M., Husseni, R.M.A. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 … Continue reading
Patient satisfaction and physician assessment favored the microneedling + topical dutasteride group, suggesting that this method may offer similar efficacy to systemic dutasteride, but with reduced risk of systemic adverse effects. The study included both men and women with AGA.
How Does Dutasteride Work?
Dutasteride is a potent inhibitor of the enzyme 5ɑ-reductase, which exists in multiple isoforms in the body. Unlike finasteride (which selectively targets the type II isoenzyme), dutasteride blocks both type I and type II 5ɑ-reductase.[6]Botto, H., Lan, O., Poulain, J-E., Comenducci, A. (2005). Effect of dutasteride on reduction of plasma DHT following finasteride therapy in patients with benign prostatic hyperplasia. Progrés en … Continue reading
Type II is abundant in hair follicles and the prostate, while Type I is found in skin, sebaceous glands, and liver. By inhibiting both, dutasteride more completely prevents the conversion of testosterone to DHT.
At a standard oral dose of 0.5 mg/day, dutasteride can drive serum and scalp DHT levels down by ~90% or more.[7]Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology. (5-6). 833-843. Available at: … Continue reading This is a more extensive suppression than finasteride achieves at typical doses. This dramatic drop in DHT removes the androgenic stimulus that causes susceptible hair follicles to shrink and enter shorter growth phases, thereby slowing hair loss and allowing follicles to recover over time.
Topical dutasteride aims to harness this same mechanism locally. When applied on the scalp, dutasteride penetrates into the skin and hair follicle, binding to 5ɑ-reductase enzymes in the dermis and around the follicle bulb. Inhibiting local DHT production creates a scalp environment more conducive to hair growth.
Pharmacokinetic studies of dutasteride demonstrate parallel linear and nonlinear elimination, with nonlinear (saturable) pathways dominating at low doses. These pathways quickly become saturated, so even small concentrations can lead to substantial enzyme inhibition. As drug concentrations rise and saturate the target enzyme, higher doses yield diminishing increases in effect.[8]Gisleskog, P.O., Hermann, D., Hammarlund-Udenaes, M., Karlsson, M.O. (1999). The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. … Continue reading
Therefore, topical dutasteride at 0.01-0.05% may achieve a large fraction of the DHT reduction that an oral dose would, as long as it reaches the target tissue. This is why even leakage of a small dose into circulation can suppress serum DHT measurably.
Another factor is dutasteride’s long half-life, about 4-5 weeks.[9]Azzouni, F., Godoy, A., Li, Y., Mohler, J. (2011). The 5 Alpha-Reductase Isozyme Family: A Review of Basic Biology and Their Role in Human Diseases. Advances in Urology. 2012(530121). 1-18. Available … Continue reading This prolonged half-life means that any dutasteride absorbed systemically will accumulate with continued use. From a mechanism standpoint, this is a double-edged sword:
- It ensures continuous enzyme inhibition (dutasteride remains bound to 5ɑ-reductase).
- But it also means systemic exposure is amplified over time if absorption isn’t minimized.
What the Science Says
Research on topical dutasteride is not as extensive as that on topical finasteride, but a growing number of trials and case series shed light on its efficacy. Below, we summarize key findings across different concentrations and use cases:
Study Concentration & Vehicle Hair Growth-Outcomes Systemic/Serum Findings Nada et al, 2018, prospective randomized study, 30 men, 24 weeks. Group 1: Minoxidil + topical dutasteride (0.02%). Group 2: Minoxidil only.
Group 1 showed a significant increase in hair density, width, and terminal/vellus ratio compared to Group 2. Patient assessment showed higher satisfaction in Group 1 than in Group 2. Reduction in serum DHT observed. Sanchez-Meta et al, 2022, double-blind RCT, 34 men, 16 weeks. Group 1: Minoxidil and topical dutasteride (0.01%). Group 2: Minoxidil only.
Minoxidil and dutasteride produced a superior overall change in hair thickness and density compared to minoxidil alone. Not evaluated. Panuganti et al, 2025, phase II randomized, double-blind study, 135 men, 24 weeks. Group 1: Dutasteride 0.01% w/v topical solution. Group 2: Dutasteride 0.02% w/v. Group 3: Dutasteride 0.05% w/v. Group 4: Oral finasteride 1 mg. Group 5: Placebo. Dose-dependent increase in target area hair count (TAHC) vs placebo. Dutasteride 0.05% superior to finasteride at week 24. Mean TAHC increase: 0.01% (12.78), 0.02% (20.03), 0.05% (34.30) vs finasteride (12.57). More patients achieved investigator global photography assessment ≥+2 with 0.05% vs finasteride. Dutasteride caused modest changes in serum testosterone/DHT, while finasteride caused moderate changes. 0.05% dutasteride showed a better pharmacokinetic profile with reduced systemic absorption compared to oral finasteride. Overall, while there is a lack of peer-reviewed scientific literature, it does support the idea that topical dutasteride is an effective treatment for AGA. Where topical dutasteride does shine is in offering these benefits with reduced systemic involvement, making it a potential option for those who need dutasteride’s effects but are wary of its systemic effects.
Why Formulation and Dose Matter
Not all topical dutasteride products are created equal. Two main factors determine how well topical dutasteride works (and how “safe” it is systemically): formulation (the vehicle and additives) and dosage regimen (concentration and frequency).
- Vehicle and Penetration: Dutasteride is a large lipophilic molecule with poor water solubility.[10]Kim, N.A., Choi, D.H., Kim, J.Y., Kim, K.H., Lim, D.G., Lee, E., Park, E-S., Jeong, S.H. (2014). Investigation of polymeric excipients for dutasteride solid dispersion and its physicochemical … Continue reading This makes formulation challenging; you need solvents or carriers that keep it stable and help it traverse the skin barrier. Newer vehicles like lipid carriers or cyclodextrin inclusion complexes might improve scalp targeting while limiting diffusion into blood.[11]Kim, M-S., Ha, E-S., Choo, G-H., Baek, I-H. (2015). Preparation and in vivo evaluation of a dutasteride-loaded solid-supersaturable self-microemulsifying drug delivery system. International Journal … Continue reading,[12]Min, M-H., Park, J-H., Choi, M-R., Hur, J-H., Ahn, B-N., Kim, D-D. (2018). Formulation of a film-coated dutasteride tablet bioequivalent to a soft gelatin capsule (Avodart): Effect of γ-cyclodextrin … Continue reading
- Applied Dose vs. Concentration: Users often focus on the percentage (e.g., 0.1% vs 0.02%), but the total amount of drug applied per session is just as important. For example, using 2 mL of a 0.1% solution (which contains 2 mg dutasteride) will deliver four times the dutasteride to your scalp as 1 mL of the same solution. For example, one study using a 0.25% topical used at 4 sprays (around 400 μL) caused a much larger drop in serum DHT than the same solution used at 1-2 sprays (100-200 μL).[13]Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology. 240(5-6). 833-843. Available at: … Continue reading
- Furthermore, volume matters: a seemingly “low” concentration could cause systemic effects if applied in large quantities. Conversely, high concentrations used sparingly might localize better.
- Dosing Frequency: Dutasteride’s long half-life means you may not need daily application to maintain its effect on the scalp. Ultimately, the goal is to find a regimen that keeps follicles saturated with dutasteride whilst avoiding leakage into the bloodstream.
Minimizing Systemic Absorption
As we have mentioned above, one of the primary motivations for using topical dutasteride is to limit the risk of side effects that come with systemic DHT suppression. There are several strategies you can use to achieve this.
Vehicle & Penetration Enhancers
As discussed, formulation plays a huge role. Nanoemulsion-based gels and nanoemulgels significantly increase skin permeation and deposition of dutasteride compared to conventional formulations, with optimized nanoemulsions showing up to 1.5-fold enhancement in permeation.[14]Ali, M.S., Alam, M.S., Alam, N., Siddiqui, M.R. (2014). Preparation, Characterization and Stability Study of Dutasteride-Loaded Nanoemulsion for Treatment of Benign Prostatic Hypertrophy. Iranian … Continue reading If systemic absorption is a concern, you could opt for a slightly less efficient formulation.
Time On Scalp
The longer the product stays on your scalp, the more the drug can penetrate, increasing efficacy (and potentially systemic absorption). Washing your hair too soon can rinse away some of the medication, potentially reducing efficacy. Many protocols recommend allowing at least 4 hours of contact time (and preferably 6-8 hours) before washing off a topical.
Application Frequency
Frequency can be adjusted to manage systemic load. Because dutasteride binds 5ɑ-reductase for so long, daily application might not be necessary for full benefit. If you find that daily use lowers your serum DHT more than desired, you could try every other day or even twice-weekly dosing.
Scalp Condition and Individual Variation
Your skin’s permeability is unique to you..Differences in skin thickness can affect drug permeability (and therefore efficacy).[15]Noor, N.M., Abudl-Aziz, A., Sheikh, K., Somavarapu, S., Taylor, K.M.G. (2020). In vitro Performance of Dutasteride-Nanostructured Lipid Carriers Coated with Lauric Acid-Chitosan Oligomer for Dermal … Continue reading However, you should be mindful that if you have a scalp condition (like seborrheic dermatitis, etc), you should avoid using any topical hair loss treatments until it has resolved.
Application Area
The larger the area of scalp you treat, the more drug can be absorbed. Treating just the crown vs. the entire scalp could proportionally change systemic exposure. If you have diffuse thinning and apply broadly, you might consider using a lower concentration to compensate for the larger area.
To minimize systemic absorption without compromising results, a practical approach can be:
- Use the lowest effective concentration.
- Use the smallest effective volume.
- Use the least frequent schedule.
This will often require trial and error as you find out what works for you. If you notice side effects like decreased libido, brain fog, or breast tenderness, these could be signs of systemic absorption. In such cases, dial back the frequency or concentration.
How to Maximize Gains and Minimize Risk
If you decide to use topical dutasteride, here are strategies to get the best hair growth results while keeping your risk low:
- Establish a Baseline (Lab Tests): Before starting, it’s wise to get bloodwork for your hormone levels – especially serum DHT and testosterone. This baseline will tell you where you started. Some people also get a full hormone panel (if advised to do so by their doctor). You can then re-test after 4-6 weeks to monitor any significant changes.
- Standardize Your Application Routine: Consistency is key for results. Apply the treatment to a dry scalp. If using a dropper or spray, try to target thinning areas evenly and rub it in gently. Pick a time of day you can stick with. Many prefer nighttime so it can absorb overnight; just be cautious about transferring to pillows (and thereby to partners). If you apply it in the morning, ensure it dries before any activity that might cause sweat (sweating could increase absorption or cause the product).
- Add Proven Adjuncts (like minoxidil or microneedling): To maximize regrowth, consider combining therapies. Minoxidil can synergize with dutasteride by stimulating follicles directly, while dutasteride tackles the hormonal aspect. As seen above, studies show that dutasteride and minoxidil yield better density than either alone. Microneedling is another option. Weekly or monthly sessions of dermarolling have been shown to boost topical drug delivery and induce growth factors.[16]Ahmed, K.M.A., Kozaa, Y.A., Abuawwad, M.T., Al-Najdawi, A.I., Mahmoud, Y.W., Ahmed, A.M., Taha, M.J.J., Fadhli, T., Giannopoulou, A. (2025). Evaluating the efficacy and safety of combined … Continue reading
- Be Patient and Track Progress: Hair growth is a slow process. Do not expect major changes in the first weeks. In fact, you might experience some shedding initially. This can happen with any effective treatment and usually subsides. Give the treatment at least 6 months to a year to judge its effect. To accurately gauge progress, use objective tracking, such as standardized photos, hair counting, or a dermascope.
- Lifestyle and Supplements: While on treatment, avoid introducing supplements that might complicate treatment. For instance, creatine may increase DHT, and quercetin may decrease it.[17]van der Merwe, J., Brooke, N.E., Myburgh, K.H. (2009). Three weeks of creatine monohydrate supplementation affects dihydrotestosterone to testosterone ratio in college-aged rugby players. Clinical … Continue reading,[18]Ma, Z., Nguyen, T.H., Huynh, T.H., Do, P.T., Huynh, H. (2004). Reduction of rat prostate weight by combined quercetin-finasteride treatment is associated with cell cycle deregulation. Journal of … Continue reading
- Monitor Side Effects: If you notice any difference, then keep a note and let your healthcare provider know. You may be able to adjust the dosage to maintain hair gains and reduce side effects.
Combination Therapies: Enhancing Outcomes
Combination therapy can amplify the benefits of topical dutasteride. We’ve summarized a few of these below:
Dutasteride and Fractional CO2 Laser
One study was conducted in which 30 male AGA patients were randomized to either fractional CO2 laser plus topical dutasteride (0.002%) applied after each session.[19]Galal, S.A., Ali, M.S., HafizHala, H.S.A. (2025). Comparative study between fractional CO2 laser alone versus fractional CO2 laser combined with topical dutasteride in treatment of male androgenic … Continue reading Each group received 3 sessions, one month apart. The combination group had significantly superior increases in terminal hair count and reductions in vellus hair and hair diameter diversity versus laser alone. Higher patient satisfaction was also reported in the combination group.
Figure 1: Combination treatment results: (A) Clinical photos before treatment (B) showing moderate improvement after treatment. (C) dermoscopic photos before treatment, counting vellus hair (16.8%), terminal hair (83.2%), and calculating hair diameter diversity (52.1%) per field. (D) dermoscopy after treatment with fractional CO2 laser combined with topical dutasteride; vellus hair (13.5%), terminal hair (86.5%), and hair diameter diversity (86.5%) per field. Vellus hair; blue arrow, terminal hair; black arrow, single pilosebaceous unit; green arrow, and yellow dot; yellow arrow.[20]Galal, S.A., Ali, M.S., HafizHala, H.S.A. (2025). Comparative study between fractional CO2 laser alone versus fractional CO2 laser combined with topical dutasteride in treatment of male androgenic … Continue reading Image obtained in line with the Creative Commons License.
Triple Topical Therapy: Dutasteride, Finasteride, and Minoxidil
Fifteen male AGA patients, including both atopic and non-atopic subjects, used a topical compound containing finasteride, dutasteride, and minoxidil (“NuH Hair”), with optional additions of oral finasteride, topical minoxidil foam, and ketoconazole shampoo.[21]Rafi, A.W., Katz, R.M. (2011). Pilot Study of 15 Patients Receiving a New Treatment Regimen for Androgenic Alopecia: The Effects of Atopy on AGA. ISRN Dermatology. 2011(241953). Available at: … Continue reading
All patients experienced significant new hair growth. Among those using all four components, significant regrowth was seen as early as 30 days, with “major” growth in three patients by day 30 and in all by day 60. In those using only the triple topical, significant regrowth was observed in all by 3 months, despite some using it less frequently than recommended. No systemic or local adverse effects were reported.
Topical Dutasteride + Microneedling
A 20-week, randomized, double-blind, placebo-controlled trial of microneedling plus topical 0.01% dutasteride solution (MNSD) vs. microneedling plus saline. The combination group demonstrated significant improvement in hair growth and density compared to the control. No systemic androgen suppression was detected, indicating a favorable safety profile.[22]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
The overarching theme of combinations like the above is synergy. By attacking hair loss from multiple angles, you often get faster, fuller, and more sustained regrowth.
Who Should (or Shouldn’t) Use Topical Dutasteride
Good Candidates
- Men with AGA who need a stronger option.
- Those who experienced side effects from oral finasteride/dutasteride.
Bad Candidates
- Anyone trying to conceive, or with infants, toddlers, or pregnant partners.
- People with non-AGA hair loss.
- Those with severe persistent side effects from 5ɑ-reductase inhibitors.
- People who aren’t able to commit to a consistent routine.
Final Verdict
So, does topical dutasteride work? In short, yes. Clinical trials and real-world use show that topical dutasteride can significantly improve hair growth and reduce shedding in men with androgenetic alopecia. When formulated correctly, it offers comparable scalp DHT suppression and regrowth potential to oral finasteride, and in some cases, it even approaches the efficacy of oral dutasteride, but with lower systemic exposure and fewer side effects.
The key is proper use. Results depend heavily on the right concentration, vehicle, dosage, and frequency. A well-balanced regimen, often guided by a medical provider, offers the best chance of success.
References[+]
References ↑1 Shanshanwal, S.J.S., Dhurat, R.S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study. 83(1). 47-54. Available at: https://doi.org/10.4103/0378-6323.188652 ↑2 Almudimeegh, A., Almutairi, H., AlTassan, F., AlQuraishi, Y., Nagshabandi, K.N. (2024). Comparison between dutasteride and finasteride in hair regrowth and reversal of miniaturization in male and female androgenetic alopecia: a systematic review. Dermatology Reports. 16(4). 9909. Available at: https://doi.org/10.4081/dr.2024.9909 ↑3 Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammed, J., Rao, S., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects with Androgenetic Alopecia. Cureus. 17(8). E89309. Available at: https://doi.org/10.7759/cureus.89309 ↑4 Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammed, J., Rao, S., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects with Androgenetic Alopecia. Cureus. 17(8). E89309. Available at: https://doi.org/10.7759/cureus.89309 ↑5 Obeid, M.N.A., Fatteh, N.S.A., Elfangary, M.M., Husseni, R.M.A. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 mg) in Treatment of Androgenetic Alopecia. An International Journal of Medicine. 117(2). Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑6 Botto, H., Lan, O., Poulain, J-E., Comenducci, A. (2005). Effect of dutasteride on reduction of plasma DHT following finasteride therapy in patients with benign prostatic hyperplasia. Progrés en Urologie. 15(6). 1090-1095. Available at: PMID: 16429658 ↑7 Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology. (5-6). 833-843. Available at: https://doi.org/10.1159/000541395 ↑8 Gisleskog, P.O., Hermann, D., Hammarlund-Udenaes, M., Karlsson, M.O. (1999). The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. British Journal of Clinical Pharmacology. 47(1). 53-58. Available at: https://doi.org/10.1046/j.1365-2125.1999.00843.x ↑9 Azzouni, F., Godoy, A., Li, Y., Mohler, J. (2011). The 5 Alpha-Reductase Isozyme Family: A Review of Basic Biology and Their Role in Human Diseases. Advances in Urology. 2012(530121). 1-18. Available at: https://doi.org/10.1155/2012/530121 ↑10 Kim, N.A., Choi, D.H., Kim, J.Y., Kim, K.H., Lim, D.G., Lee, E., Park, E-S., Jeong, S.H. (2014). Investigation of polymeric excipients for dutasteride solid dispersion and its physicochemical characterization. Archives of Pharmacal Research. 37(2). 214-224. Available at: https://doi.org/10.1007/s12272-013-0180-9 ↑11 Kim, M-S., Ha, E-S., Choo, G-H., Baek, I-H. (2015). Preparation and in vivo evaluation of a dutasteride-loaded solid-supersaturable self-microemulsifying drug delivery system. International Journal of Molecular Sciences. 16(5). 10821-10833. Available at: https://doi.org/10.3390/ijms160510821 ↑12 Min, M-H., Park, J-H., Choi, M-R., Hur, J-H., Ahn, B-N., Kim, D-D. (2018). Formulation of a film-coated dutasteride tablet bioequivalent to a soft gelatin capsule (Avodart): Effect of γ-cyclodextrin and solubilizers. Asian Journal of Pharmaceutical Sciences. 14(3). 313-320. Available at: https://doi.org/10.1016/j.alps.2018.08.007 ↑13 Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology. 240(5-6). 833-843. Available at: https://doi.org/10.1159/000541395 ↑14 Ali, M.S., Alam, M.S., Alam, N., Siddiqui, M.R. (2014). Preparation, Characterization and Stability Study of Dutasteride-Loaded Nanoemulsion for Treatment of Benign Prostatic Hypertrophy. Iranian Journal of Pharmaceutical Research. 13(4). 1125-1140. Available at: PMID: 25587300 ↑15 Noor, N.M., Abudl-Aziz, A., Sheikh, K., Somavarapu, S., Taylor, K.M.G. (2020). In vitro Performance of Dutasteride-Nanostructured Lipid Carriers Coated with Lauric Acid-Chitosan Oligomer for Dermal Delivery. Pharmaceutics. 12(10). 994. Available at: https://doi.org/10.3390/pharmaceutics12100994 ↑16 Ahmed, K.M.A., Kozaa, Y.A., Abuawwad, M.T., Al-Najdawi, A.I., Mahmoud, Y.W., Ahmed, A.M., Taha, M.J.J., Fadhli, T., Giannopoulou, A. (2025). Evaluating the efficacy and safety of combined microneedling therapy versus topical Minoxidil in androgenetic alopecia: a systematic review and meta-analysis. Archives of Dermatological Research. 317(1). 528. Available at: https://doi.org/10.1007/s00403-025-04032-1 ↑17 van der Merwe, J., Brooke, N.E., Myburgh, K.H. (2009). Three weeks of creatine monohydrate supplementation affects dihydrotestosterone to testosterone ratio in college-aged rugby players. Clinical Journal of Sports Medicine. 19(5). 399-404. Available at: https://doi.org/10.1097/JSM.0b013e3181b8b52f. ↑18 Ma, Z., Nguyen, T.H., Huynh, T.H., Do, P.T., Huynh, H. (2004). Reduction of rat prostate weight by combined quercetin-finasteride treatment is associated with cell cycle deregulation. Journal of Endocrinology. 181(3). 493-507. Available at: https://doi.org/10.1677/joe.0.1810493 ↑19 Galal, S.A., Ali, M.S., HafizHala, H.S.A. (2025). Comparative study between fractional CO2 laser alone versus fractional CO2 laser combined with topical dutasteride in treatment of male androgenic alopecia. 40(1). 16. Available at: https://doi.org/10.1007/s10103-024-04269-8 ↑20 Galal, S.A., Ali, M.S., HafizHala, H.S.A. (2025). Comparative study between fractional CO2 laser alone versus fractional CO2 laser combined with topical dutasteride in treatment of male androgenic alopecia. 40(1). 16. Available at: https://doi.org/10.1007/s10103-024-04269-8 ↑21 Rafi, A.W., Katz, R.M. (2011). Pilot Study of 15 Patients Receiving a New Treatment Regimen for Androgenic Alopecia: The Effects of Atopy on AGA. ISRN Dermatology. 2011(241953). Available at: https://doi.org/10.5402/2011/241953 ↑22 Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. JEADV. 36(10). Available at: https://doi.org/10.1111/jdv.18285 Topical finasteride is gaining traction as a lower-risk alternative to oral finasteride, especially for those concerned about systemic side effects. In the U.S., providers like Ulo and Strut stand out for customization and lab-tested purity. Canadian options include Xyon Health and Essential Clinic, while the UK and EU offer licensed sprays and private compounding through clinics like Hair Repair and Sons. No matter your location, choose sources with strong medical oversight and clear quality standards to ensure you’re getting a safe, effective treatment.
Top Places to Buy Topical Finasteride
- #1 Choice for Quality and Growth: Ulo
- Top Runner-Up in the USA: HappyHead
- Top Pick in Canada: Xyon Health
- Top Runner-Up in Canada: Jupiter
- Top Pick in the UK: Hair Repair Clinic
- Top Runner-Up in the UK: UsMen
Topical finasteride is a well-known 5ɑ-reductase inhibitor used off-label as a localized treatment for androgenic alopecia (AGA). Finasteride in oral form is an FDA-approved medication for AGA, but its topical formulation is not officially approved in the US. However, compounding pharmacists and telehealth services do prepare it for off-label use.
The goal of using finasteride topically is to deliver the drug to hair follicles in the scalp while minimizing systemic absorption, thereby reducing the risk of side effects commonly associated with the oral pill. Below, we’ll discuss how topical finasteride works and why the source of your topical finasteride matters. Then we’ll explore where to buy topical finasteride in the US and around the world.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Mechanism of action
Finasteride works by inhibiting the enzyme 5ɑ-reductase (specifically the type II isoenzyme) that converts testosterone into dihydrotestosterone (DHT). DHT is the androgen hormone that binds to hair follicles and causes them to miniaturize in genetically susceptible individuals, leading to thinning hair and eventual hair loss. By blocking DHT production, especially in the scalp, finasteride helps halt this miniaturization process, thereby maintaining or increasing hair density.
Oral finasteride (1 mg daily) is a proven treatment that significantly increases hair counts compared to placebo in men with AGA.[1]Gupta, K.A., Venkataraman, M., Talukdar, M., Bamimore, M.A. (2021). Finasteride for hair loss: a review. Journal of Dermatological Treatment. 1938-1946. Available at: … Continue reading It’s this effect that topical formulations also aim to achieve, but with a more localized effect.
Advantages of Topical Finasteride
The hope of the topical finasteride formulation is localized delivery of the drug to the scalp, which can minimize systemic exposure. By applying finasteride directly to the balding areas, high drug concentrations can be achieved in the skin and hair follicles where it’s needed, while ideally limiting how much finasteride enters the bloodstream. This targeted approach means the scalp DHT can be lowered substantially (similar to oral finasteride’s effect on scalp DHT) without as large a reduction in blood DHT levels.[2]Zhou, C., Bin, Y., Huiming, Z., Rushan, X., Ningning, D., Qinping, Y., Chunlei, Z., Guogiang, Z., Aihua, W., Wei, L., Shuxia, Y., Qingchun, D., Yangfeng, D., Liming, W., Lunfei, L., Danyang, J., … Continue reading In other words, topical finasteride can maintain the same inhibitory effect on scalp DHT with significantly less impact on systemic DHT levels.
While research is ongoing, the suspected outcome of this is that at the right doses, topical finasteride usage will lead to lower side effects than oral. Oral finasteride is well-tolerated by most, but a minority of users experience side effects like reduced libido, erectile dysfunction, or other hormone-related issues. Research shows that these side effects are less common with topical finasteride.[3]Gupta, A.K., Talukder, M. (2022). Topical finasteride for male and female pattern hair loss: Is it a safe and effective alternative? Journal of Cosmetic Dermatology. 21(5). 1841-1848. Available at: … Continue reading
Clinical trials of a 0.25% finasteride topical spray in men showed significant hair regrowth versus placebo, with good safety and tolerability; no serious adverse effects were reported, and only mild localized side effects (like slight scalp irritation) occurred.[4]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray … Continue reading
Other studies similarly report that topical finasteride users experience far fewer sexual side effect reports than oral users, suggesting that keeping finasteride localized to the scalp can indeed mitigate many unwanted systemic effects.[5]Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated with Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study with Data from … Continue reading Moreover, by delivering the drug via the skin, it’s possible to use a small total dose of finasteride (since it isn’t undergoing first-phase metabolism in the liver), yet still achieve a therapeutic effect in the hair follicle.
Why Sourcing Matters
Since topical finasteride lacks FDA approval as a commercial hair loss treatment, it relies on compounding pharmacies and specialized telehealth providers for preparation. This creates substantial variation in formulation quality and standards across different sources.
The compounding process introduces multiple variables that can significantly impact the final product. Different providers may use varying preparation techniques, carrier systems, and active ingredient concentrations, resulting in inconsistent efficacy and safety profiles between products.[6]American Hair Loss Association. (no date). Hair Loss Treatments Online: What to Know About Compounded Medications. Available at: … Continue reading Without the stringent regulatory framework that governs mass-produced pharmaceuticals, these compounded formulations face less oversight regarding quality control and manufacturing standards.
This regulatory gap creates particular concerns around dosing accuracy. Compounded topical finasteride products may contain active ingredient levels that differ from their labeled concentrations, potentially leading to subtherapeutic underdosing or potentially harmful overdosing. The lack of standardized manufacturing processes and quality assurance protocols means patients cannot be certain they are receiving the intended dose or purity level.
These factors underscore why careful selection of telehealth providers and compounding sources becomes essential when considering topical finasteride treatment, as the wide variation in preparation methods and quality standards can directly influence both treatment outcomes and patient safety.
Price and Purity Considerations
The cost of topical finasteride can vary depending on where you get it and what’s included. In general, because these are custom prescriptions, they tend to be more expensive than generic oral finasteride. Prices can fluctuate based on local pharmacy compounding costs, the inclusion of additional active ingredients, and how the product is sold (subscription model vs. one-time purchase).
Ingredient purity represents another consideration, as the quality of raw finasteride used in compounding can fluctuate significantly based on the supplier and sourcing practices. This introduces concerns about both therapeutic effectiveness and the risk of contamination or impurities in the final product. The inconsistency in raw material quality means that patients may receive formulations with compromised potency or unknown adulterants that could affect safety profiles.
These quality variations highlight the importance of thoroughly evaluating both the provider and their compounding standards when selecting a topical finasteride treatment. Patients and healthcare providers should prioritize sources that demonstrate transparency in their sourcing practices, manufacturing processes, and quality control measures to ensure they receive a safe and effective compounded topical finasteride product.
Now, let’s explore where you can get topical finasteride in various regions (US, Canada, UK/EU, and elsewhere).
Where To Buy Topical Finasteride
United States Providers
In the US, there is no FDA-approved topical finasteride product on the market. Therefore, all topical finasteride is obtained via prescription compounding. The US has a thriving telehealth industry for hair loss, so many patients get topical finasteride by consulting an online healthcare provider who can prescribe a compounded solution shipped to them.
Ulo – #1 Choice for Quality & Growth
Ulo stands out in the US telehealth market for its high degree of flexibility and rigorous standards:
- Evidence-First Formulations:
- Offered in both low-dose (0.005%) and full-strength (0.2%) topical finasteride, accurately compounded to mirror the proven effects of oral finasteride on scalp DHT.
- Every batch undergoes independent GC/MS and HPLC testing with lab reports published online so you know exactly what’s in your bottle.
You can read more about our product lab testing results here.
- Unrivaled Personalization:
- Add up to five enhancers: 7% minoxidil, 0.01% tretinoin, 0.2% caffeine, 0.01% melatonin, and 1% cetirizine, to tailor your regimen.
- Partner physicians collaborate with you to adjust concentrations and ingredients over time, based on your response and tolerance.
- Treatment regimens can be tailored for once- or twice-daily application, with thoughtfully designed dropper-marked bottles to facilitate accurate dosing.
- Consumer Safety Built-In:
- No propylene glycol, no corticosteroids, and no ”trending” additives that lack robust safety data (e.g., no latanoprost or copper peptides).
- Proprietary, low-irritation vehicle ensures deep follicle penetration without the need to mask side effects with steroids.
- Transparent Quality & Pricing:
- Third-party lab reports accompany every shipment for total transparency.
- Ulo’s pricing starts at $0.82 per ml (or $0.61 per ml with the discount) for the low-dose finasteride-only formulation, increasing with dose and other ingredients.
- Ongoing Medical Support:
- Unlimited access to Ulo’s network of licensed physicians via a secure portal, ask questions, report progress, and get real-time guidance anytime.
- Clear timelines set “possibility vs. probability”, so you know when to expect regrowth, when it might plateau, and how to safely escalate your protocol.
Ulo was built by advocates who have spent over a decade exposing the hair loss industry’s worst practices and decided to solve them.
Pros
- Connects patients directly with licensed providers, offering discreet prescriptions and continuous support throughout their hair restoration journey.
- The customized topical treatments blend finasteride and minoxidil, addressing the underlying drivers of hair loss to deliver more noticeable regrowth.
- Additional ingredients, such as cetirizine, melatonin, caffeine, and retinoic acid, are included to further improve regrowth outcomes.
Cons
- Twice daily usage can be inconvenient.
HappyHead – #1 Runner Up in the USA
HappyHead is a US-based telehealth company specializing in hair growth solutions with a strong focus on medical-grade, customizable topical treatments. HappyHead offers prescription Topical Finasteride, either as monotherapy or in combination with minoxidil and/or retinoic acid, compounded for each patient after an online consultation with a licensed physician.
Some versions include minoxidil (up to 8%) and tretinoin, aiming to optimize results based on patient needs and tolerance. HappyHead’s platform emphasizes medical guidance, ongoing support, and flexible subscriptions, shipping directly to consumers in most US states.
Pros
- Offers personalized prescriptions through its telehealth platform, ensuring patients receive discreet access to treatment from licensed providers.
- Its compounded topical solution has the option to combine finasteride with minoxidil and retinoic acid.
- Flexible formulation strengths enable tailoring to patient needs, which can be particularly beneficial for individuals sensitive to the side effects of oral finasteride.
Cons
- Twice-daily application is the standard recommendation, which may feel less convenient compared to simpler once-daily regimens.
- Higher concentrations of finasteride may lead to an increased risk of systemic side effects.
- No dosing flexibility.
Strut Health
Strut Health is an online compounding pharmacy that provides custom formulas for hair loss. Strut allows some customization: their Finasteride Hair Loss Formula can include finasteride 0.25%, minoxidil 0-7.5%, tretinoin 0-0.0125%, fluocinolone 0.01%, and biotin. Corticosteroids are often used to offset the potential irritation that comes with topical use; however, this comes at the risk of long-term skin thinning for customers.
We conducted third-party testing on their custom formula and found that it met the required levels of finasteride, minoxidil, and tretinoin.
Pros
- Strut Health offers tailored hair loss treatments and ongoing support from licensed providers.
- The compounded topical formula combines finasteride with proven agents like minoxidil and tretinoin, both targeting DHT and promoting follicular activity.
- Custom blends may also include additional ingredients that can strengthen hair, reduce inflammation, and further adapt the treatment to specific requirements.
Cons
- Strut Health includes corticosteroids in its formulation to help with the potential irritation of topical use. However, this comes at the risk of long-term skin thinning for customers.
- Not the cheapest option, costing $1.97 per serving.
Hims
Hims & Hers is a large digital health platform focused on men’s and women’s health. They offer a Topical Finasteride & Minoxidil Spray for hair loss, featuring finasteride 0.1% and minoxidil 6%. A dual-action, pill-free formula is available by prescription after an online consultation. The spray is designed to minimize systemic absorption and side effects compared to oral finasteride, and it’s marketed for its convenient application and personalized telehealth experience.
While we haven’t done third-party testing on their topical formulation, we did on their oral finasteride counterpart – and it met the claimed level of finasteride.
Pros
- Combines finasteride and minoxidil into a single, easy-to-use topical spray, offering a convenient alternative to oral pills.
- Once daily usage is recommended, making the regimen less time-consuming than some alternatives requiring multiple applications daily.
Cons
- Some users may find sprays less suitable than dropper or foam-based applications, especially if precise targeting of thinning areas is preferred.
- The formulation’s alcohol base could lead to scalp dryness or irritation for sensitive individuals.
Ro (formerly Roman)
Ro is a well-known direct-to-consumer telehealth provider. Their Roman Hair Solution Rx is a 3-in-1 prescription topical spray: finasteride 0.3%, minoxidil 6%, and tretinoin 0.025%. Ro emphasizes convenience with free follow-ups and no required video visits. Customization is not as extensive as with some compounding pharmacies.
Similar to Hims, we haven’t done third-party testing on their topical formulation, but we did on their oral finasteride product – and it met the claimed level of finasteride.
Pros
- Roman Hair Solution Rx blends finasteride, minoxidil, and tretinoin into one topical formula.
- The solution offers a once-daily, convenient spray application.
Cons
- No customization offered
- No dosing flexibility
- Some users may experience mild scalp irritation, redness, or dryness due to alcohol-based solutions
Keeps
Keeps is a telehealth brand specializing exclusively in men’s hair loss treatments. Their Topical Finasteride & Minoxidil Gel delivers 0.25% finasteride and 5% minoxidil in a single application. Keeps focuses on ease of use, competitive pricing, and online medical evaluations, shipping treatments directly to most US states.
As with the above, we haven’t done third-party testing on their topical formulation, but we did on their oral finasteride product – and it met the claimed level of finasteride.
Pros
- Offers a topical gel combining finasteride and minoxidil to target two AGA routes.
- Gel formula designed to minimize systemic absorption.
Cons
- No customization available.
- No dosing flexibility available.
- Most expensive option (price per serving)
Provider Customization Add-Ons Dosing Flexibility Approx. Price/Serving Purity Testing Ulo Yes Minoxidil 7%, Cetirizine 1%, Tretinoin 0.01%, Melatonin 0.01%, and Caffeine 0.2% Yes, 0.005% or 0.2% finasteride $0.61 3rd-party lab reports. HappyHead No, the doctor customizes the product for you Finasteride (0.3%), Minoxidil (8%), Retinoic Acid (0.001%), Hydrocortisone (1%) No $1.98 3rd-party lab reports of another product were mixed, with some ingredients not meeting the claimed dose. Strut Yes Finasteride 0.25%, Minoxidil 0-7.5%, Tretinoin 0-0.0125%, Fluocinolone 0.01%, and Biotin. Yes $1.97 3rd-party lab reports of topical finasteride were good. Hims No Finasteride and Minoxidil No $0.65 No 3rd party testing for their topical product, but their oral finasteride product passed lab testing. Ro No Finasteride 0.3%, Minoxidil 6%, and Tretinoin 0.025% No $0.65 No 3rd party testing done for topical finasteride Keeps No Finasteride and Minoxidil No $2 No 3rd party testing for their topical product, but their oral finasteride product passed lab testing. Canada Providers
Xyon Health – Top Pick in Canada
Xyon Health is a telehealth brand offering topical hair treatments, including dutasteride and finasteride. For their finasteride options, you can either buy finasteride alone or combined with minoxidil. The platform requires online consultations with specialist physicians and delivers medications in discreet packaging across Canada.
Pros
- Xyon Health uses its patented SiloxysSystem gel technology to deliver its treatment directly to the scalp.
- Offers 92% less systemic absorption than oral finasteride.
- Alcohol-free formula.
- Have a choice of finasteride alone or in combination with minoxidil.
- Convenient once-daily administration.
Cons
- Not the cheapest option ($327 per order for a 3-month supply).
Jupiter – Top Runner-Up in the USA
Jupiter is a telehealth platform offering personalized topical hair loss treatments. Through Jupiter, customers can access a compounded topical that may include finasteride as well as up to four other active ingredients, depending on their needs, including minoxidil, tretinoin, panthenol, and caffeine.
Unfortunately, they don’t advertise their concentrations or prices, mentioning that you can only view specific medication pricing and options after completing an online assessment. Jupiter provides free shipping Canada-wide, however.
Pros
- Personalized hair loss treatment, which includes finasteride, minoxidil, tretinoin, panthenol, or caffeine.
- Convenient once-daily treatments.
Cons
- A lack of freely available data, including whether dosing flexibility is possible and the price per serving.
Essential Clinic
Essential Clinic is a Canadian telehealth platform that specializes in men’s health issues like hair loss and erectile dysfunction. They offer Topical Finasteride + Minoxidil Liposomal Gel that combines finasteride with minoxidil in a liposomal delivery system designed to enhance scalp penetration while minimizing systemic absorption. The platform provides online consultations with licensed Canadian doctors and nurse practitioners.
Pros
- Treatment is paired with ongoing access to licensed Canadian physicians for follow-up and dose adjustments, plus fast, confidential home delivery of medications.
- The topical finasteride treatment is combined with minoxidil for greater efficacy.
Cons
- The most expensive option that we are able to see prices for.
- No customization.
- No dosing flexibility.
Beyoung Health
Beyoung Health is another Canadian telehealth clinic (based in Ontario) that offers treatments for men’s health, including hair loss. They prescribe compounded topical finasteride as part of their hair loss program. Beyoung offers finasteride alone or in combination with minoxidil for hair loss therapy.
Beyoung’s approach is relatively patient-tailored. The doses that they offer are 0.25% finasteride + 5% minoxidil, 0.1% finasteride + 7% minoxidil foam, or finasteride 0.1% + minoxidil 10%.
Pros
- Offers customizable finasteride doses combined with minoxidil.
- Dosing flexibility.
Cons
- A lack of freely available data, including the price per serving.
Jack Health
Jack Health is a prominent Canadian telehealth platform that offers personalized hair loss treatments, including topical finasteride formulations. Their hair loss program features a product called “Hello Hair”. This program provides custom topical ingredients that may include topical finasteride to assist with hair regrowth.
Pros
- Dosing flexibility offered.
Cons
- A lack of freely available data, including the price per serving.
- No available customization on the site – it is conducted after consultation.
Provider Customization Add-Ons Dosing Flexibility Approx. Price/Serving Purity Testing Xyon Health Yes Minoxidil 6% Yes, Finasteride 0.25% (with 5% minoxidil) or 2.5% (finasteride alone) $1.21 No Essential Clinic No Finasteride 2.5% and Minoxidil 5% No $1.61 No Jupiter Yes Minoxidil, Tretinoin, Panthenol, and Caffeine. Unknown Unknown None Beyoung Health Yes Minoxidil (different strengths) Yes Finasteride 0.25% (+5% minoxidil) or 0.1% (+7/10% minoxidil) Unknown None Jack Health No N/A Yes Unknown None If you don’t want to buy topical finasteride from a telehealth company, a few dermatology clinics can also prescribe and compound topical finasteride. If you prefer an in-person route, you could ask your dermatologist about a topical finasteride prescription to be made at a local compounding pharmacy. The downside is that compounding costs in Canada can be high, and many dermatologists are still more comfortable with oral finasteride.
United Kingdom & Europe
In the UK and EU, the situation is a bit different because, unlike the US and Canada, there has been a topical finasteride formulation approved in some markets. In 2021, a finasteride 0.25% topical solution received approval in certain European countries.[7]Zhou, C., Bin, Y., Huiming, Z., Rushan, X., Ningning, D., Qinping, Y., Chunlei, Z., Guogiang, Z., Aihua, W., Wei, L., Shuxia, Y., Qingchun, D., Yangfeng, D., Liming, W., Lunfei, L., Danyang, J., … Continue reading
Hair Repair Clinic – Top Pick in the UK
Hair Repair Clinic is a UK-based specialist compounding pharmacy offering Finasol, their custom-formulated topical finasteride solution. They use TrichoSol, a proprietary alcohol-light delivery system made from natural mineral salts that’s free of propylene glycol to minimize scalp irritation. Available in concentrations ranging from 0.025% to 0.1% finasteride, with custom strengths available upon request.
Pros
- Products custom-made in the UK by compounding pharmacies, with same-day approval for most orders.
- Uses the TrichoSol base for improved delivery and minimised local irritation.
- Offers customization and dosing flexibility.
- Doesn’t use propylene glycol.
- Convenient once-daily application.
Cons
- Prescription required and needs to be paid for privately; will not be reimbursed by the NHS.
UsMen – Top Runner-Up in the UK
UsMen is a UK telehealth platform specializing in men’s health that offers Topical Finasteride 0.1% and Minoxidil 5% spray. They emphasize competitive pricing and do not require subscriptions. To purchase their prescription products, you must complete a consultation.
Pros
- Cheapest UK offering that we found ($0.51 per serving).
- Topical finasteride is offered combined with minoxidil.
- Users report easy ordering, low costs, flexible purchase options, and rapid home delivery.
Cons
- No customization or dosing flexibility available.
Dr Fox
Dr Fox is an established UK online pharmacy that offers Dr. Fox Hair Growth Spray containing finasteride 0.3% and minoxidil 5%. They provide a dual-action topical spray designed to combine the DHT-blocking effects of finasteride with the growth-stimulating properties of minoxidil.
Pros
- Good pricing: $0.58 per serving.
- Same day approval and discreet UK home delivery.
- The product contains finasteride, minoxidil, and tretinoin for comprehensive treatment.
Cons
- No customization options.
- No dosing flexibility.
Sons
Sons is a major UK telehealth platform offering a Topical Finasteride & Minoxidil Spray, combining finasteride with minoxidil and tretinoin. They claim to be the only UK licence holder for both finasteride and minoxidil treatments.
Pros
- Convenient, once daily usage.
- Their finasteride product is combined with minoxidil.
Cons
- A lack of freely available data, including whether dosing flexibility is possible and the price per serving.
- No add-ons available.
Manual
Manual is another leading UK men’s health platform offering an all-in-one spray containing topical finasteride, 10% minoxidil, and 5% azelaic acid. This triple-therapy approach is designed to address multiple aspects of hair loss. Manual provides comprehensive medical support throughout treatment.
Pros
- The “All-in-One Spray” combines prescription-strength topical finasteride, high-dose minoxidil, and azelaic acid into a single formula for male AGA.
- Convenient once-daily application.
- Base formulation designed for efficient absorption.
Cons
- Women and those with non-androgenic hair loss are excluded, with use restricted to adult men who pass clinical screening.
- No customization and no dosing flexibility available.
- A lack of freely available data, including the price per serving.
Hims UK
Hims UK operates as the British arm of the US company, offering topical finasteride and minoxidil combinations. They provide subscription-based services with online consultations and ongoing medical support. Unfortunately, without going through the consultation, we cannot find out the dosage and price options.
Pros
- Convenient once-daily treatment
- Combination of finasteride and minoxidil.
- Ongoing medical support available.
Cons
- A lack of freely available data, including customization, dosing flexibility, and price per serving.
Hair Medics UK
This London-based hair clinic also offers compounded topical formulas for sale. They advertise a topical finasteride (0.1%) and dutasteride (0.1%) combination solution (for men only) for £69.99 ($94.72). They also offer a minoxidil (5%) and finasteride (0.25%) combination solution for the same price.
Pros
- Offer a customizable topical finasteride solution that can be combined with either dutasteride or minoxidil.
- Dosing flexibility from 0.1-0.25%.
Cons
- The most expensive option that we have found per serving.
Provider Customization Add-Ons Dosing Flexibility Approx. Price/Serving Purity Testing Hair Repair Clinic Yes Minoxidil 0.025%,0.05%, and 0.1% $0.81 No Dr Fox No None No $0.58 No UsMen No None No $0.51 No Sons No None No Unknown No Manual No None No Unknown No Hims UK Unknown Unknown Unknown Unknown No Hair Medics UK Yes Minoxidil, and Dutasteride 0.1% and 0.25% $2.71 None If you choose this path, keep in mind that you’re essentially experimenting on yourself with a product of unknown quality. Whenever possible, opt for a reputable telehealth provider or licensed pharmacy. This way, you’ll have professional medical supervision and a much greater assurance that your medication actually contains the right amount of finasteride.
Final Thoughts
Topical finasteride offers a compelling alternative to oral finasteride for individuals seeking effective hair loss treatment with potentially fewer systemic side effects. While the medication is not FDA-approved in topical form in the US, reputable telehealth and compounding options are available throughout the US, Canada, and parts of Europe. Providers like Ulo, Strut, Happy Head, Xyon, and others have stepped in to fill this gap by offering prescription-based, customized topical finasteride, often with additional active ingredients like minoxidil or tretinoin to enhance results.
When choosing a provider, look beyond marketing claims. Consider customization options, pricing transparency, dosing flexibility, and, crucially, whether third-party testing verifies what’s actually in the bottle. A cheap, poorly compounded product may do more harm than good, and skipping medical oversight could leave you exposed to unnecessary risks.
If you’re serious about topical finasteride, make sure you’re sourcing it from a trusted telehealth platform or compounding pharmacy that provides medical consultation and prioritizes formulation quality. Your treatment’s success and your peace of mind depend on it.
References[+]
References ↑1 Gupta, K.A., Venkataraman, M., Talukdar, M., Bamimore, M.A. (2021). Finasteride for hair loss: a review. Journal of Dermatological Treatment. 1938-1946. Available at: https://doi.org/10.1080/09546634.2021.1959506 ↑2, ↑7 Zhou, C., Bin, Y., Huiming, Z., Rushan, X., Ningning, D., Qinping, Y., Chunlei, Z., Guogiang, Z., Aihua, W., Wei, L., Shuxia, Y., Qingchun, D., Yangfeng, D., Liming, W., Lunfei, L., Danyang, J., Hanjie, Z., Jianzhong, Z. (2025). Efficacy and safety of topical finasteride spray solution in the treatment of Chinese men with androgenetic alopecia: A phase III multicenter, randomized, double-blind, placebo-controlled study. Chinese Medical Journal. 10. 1097. Available at: https://doi.org/10.1097/CM9.0000000000003495 ↑3 Gupta, A.K., Talukder, M. (2022). Topical finasteride for male and female pattern hair loss: Is it a safe and effective alternative? Journal of Cosmetic Dermatology. 21(5). 1841-1848. Available at: https://doi.org/10.1111/jocd.14895 ↑4 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. JEADV. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑5 Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated with Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study with Data from the United States Food and Drug Administration Adverse Event Reporting System. International Journal of Dermatology. Available at: https://doi.org/10.1111/ijd.17957 ↑6 American Hair Loss Association. (no date). Hair Loss Treatments Online: What to Know About Compounded Medications. Available at: https://www.americanhairloss.org/hair-loss-treatments-online-what-to-know-about-compounded-medications-from-telemedicine-providers/#:~:text=One%20of%20the%20primary%20concerns,in%20outcomes%20and%20side%20effects. (Accessed: July 2025) When it comes to hair loss treatments, oral dutasteride often emerges as the most powerful option, consistently outperforming FDA-approved alternatives like finasteride in clinical studies. Across the internet, personal stories echo this sentiment: discussion boards and Reddit threads feature dramatic recoveries, with some users claiming transformations from advanced thinning to a full, healthy head of hair.
Source: u/Throwaway_no_hair via r/tressless.
(You can read more about this one below.)
However, online spaces tend to spotlight the extremes, either remarkable success stories or reports of failure, creating a distorted picture of what typical results look like.
So, where does dutasteride truly stand? How do we distinguish between what’s possible and what’s probable? How can you weigh anecdotal experiences against the outcomes you’re most likely to see? And importantly, what might your pattern of regrowth look like after 1, 2, 3, or even 10 years of therapy?
This article addresses these questions directly. We’ll set clear expectations for oral dutasteride, explore how to interpret results shared online, highlight some of the most dramatic dutasteride before and after photos, and offer guidance on what an average treatment journey may look like.
Finally, we’ll introduce a framework you can use to evaluate and compare all hair loss therapies, helping you see dutasteride ranks in terms of regrowth potential, evidence quality, and long-term safety.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Quick Refresher on Androgenic Alopecia (AGA)
Androgenic alopecia (AGA) is the most common form of hair loss affecting both men and women, marked by progressive thinning and miniaturization of hair follicles. This is primarily thought to be a result of genetic predisposition and the influence of androgens, especially dihydrotestosterone (DHT).[1]Chen, S., Xie, X., Zhang, G., Zhang, Y. (2022). Comorbidities in Androgenetic Alopecia: A Comprehensive Review. Dermatology and Therapy. 12(10). 2233-2247. Available at: … Continue reading
There is a huge choice of treatment options for AGA, with FDA-approved therapies such as topical minoxidil and oral finasteride. Many other options are either used off-label or under investigation, including low-level laser therapy, platelet-rich plasma injections, hair transplant surgery, herbal compounds, and emerging agents like prostaglandin analogs and caffeine-based solutions.[2]Bajoria, P.S., Dave, P.A., Rohit, R.K., Tibrewal, C., Modi, N.S., Gandhi, S.K., Patel, P. (2023). Comparing Current Therapeutic Modalities of Androgenic Alopecia: A Literature Review of Clinical … Continue reading This diversity reflects both the complexity of the disease and a significant unmet need in achieving consistent, satisfactory results for patients.
Despite existing for decades, oral dutasteride has recently surged in popularity as an off-label AGA treatment. Like finasteride, it inhibits the conversion of testosterone to DHT. But it blocks both type I and II 5-alpha-reductase enzymes more effectively, potentially offering a more potent effect.
Dutasteride 101
Dutasteride blocks both type I and type II isoforms of the 5ɑ-reductase enzyme. This enzyme converts testosterone to DHT, a key hormone driving male pattern hair loss and prostate growth. By inhibiting both isoforms, dutasteride causes a near-complete suppression of DHT, reducing its levels in blood by up to 98%, which is higher than finasteride’s reduction of around 65-70%.[3]Clark, R.V., Hermann, D.J., Cunningham, G.R., Wilson, T.H., Morrill, B.B., Hobbs, S. (2004). Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual … Continue reading,[4]Nickel, J.C. (2004). Comparison of Clinical Trials with Finasteride and Dutasteride. Reviews in Urology. 6(Suppl 9). S31-S39. Available at: PMID: 16985923
The standard and most studied dose is 0.5 mg daily. This is the FDA-approved dose for benign prostatic hyperplasia and the most common dose used off-label for hair loss. Lower doses like 0.2 mg daily have been used in one clinical trial, though 0.5 mg remains the norm.[5]Escamilla-Cruz, M., Magana, M., Escandon-Perez, Bello-Chavolla, O.Y. (2023). Use of 5-Alpha Reductase Inhibitors in Dermatology: A Narrative Review. Dermatology and Therapy. 13(8). 1721-1731. … Continue reading,[6]Lee, S., Kim, J.E., Lew, B-L., Huh, C.H., Kim, J., Kwon, O., Kim, B.M., Lee, Y.W., Lee, Y., Park, J., Kim, S., Kim, D.Y., Choi, G.S., Kang, S. (2025). Efficacy and Safety of Low-Dose 0.2 mg … Continue reading
Why “Expectation Setting” Matters
Realistic expectation setting is fundamentally important for anyone starting their AGA treatment journey, particularly with treatments that do not have a large amount of evidence.
Some people may expect dramatic hair regrowth, often influenced by online testimonials or dutasteride before and after photos showing “hyper responders”.
Others may experience disappointment, frustration, and a decline in self-esteem when their own results are modest or average.
On the other hand, those with overly low expectations may forgo potentially beneficial treatments altogether, missing out on improvements that could positively impact their quality of life.
Online before-and after photos and personal anecdotes present another challenge. They are not standardized, can be misleading, and often feature individuals who have responded exceptionally well to a product, rather than the majority who see more moderate effects. These images rarely account for factors like lighting, hair styling, photo angles, or even digital enhancement, all of which can distort perceived efficacy.
To help guide your treatment selections & expectations, here are two key principles that, once understood, will make all the difference in how you approach your hair growth journey.
Principle #1: What’s Possible ≠ What’s Probable
Consider the following chart.
Figure 1: A graph showing possible and probable results. The data points show possible results, and the trendline shows probable results.[7]Badenhorst CE, Dawson B, Goodman C, Sim M, Cox GR, Gore CJ, Tjalsma H, Swinkels DW, Peeling P. Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur J Appl Physiol. 2014 … Continue reading
Note the individual datapoints and how scattered they appear. Also note the trendline, which represents the average of all individual datapoints shown in the chart.
The individual datapoints represent what’s possible for any one individual. The trendline represents what’s probable.
Figure 2: Anecdotal results are often way more dramatic than the typical.
Resultantly, people see these dutasteride before and after success stories, and think, “This is all I see. And so I’ll probably get the same regrowth, too.” This shifts their expectations for the drug far above the trendline:
Figure 3: Therefore, some people expect results that are far higher than what they are likely to get.
Then they try dutasteride and either quit too early to see regrowth (more on that soon)… or they don’t get the same results, and determine (often erroneously) that the drug just isn’t working for them.
The solution: don’t set expectations by what’s possible. Set expectations by what’s probable. Understand the trendlines for oral dutasteride: when results will typically first start to show, when results will peak and/or plateau, and what results will look like 5+ years into the future.
You can do this by using a standardized rubric we created called Regrowth Potential.
Regrowth Potential
As mentioned above, Principle 1 shows us that results tend to scatter across a spectrum: some datapoints high, some low, with a trendline running through the middle. The problem is that online anecdotes usually highlight only the high datapoints, while clinical trial data can feel abstract and hard to translate into lived experience.
Regrowth Potential is our way of standardizing this landscape. It’s designed to cut through anecdotal extremes and align patients with the trendline, what most people can realistically expect.
So what does the average look like?
Most people see stabilization first, regrowth second, and a slowing of hair loss, with partial reversal layered on top.
Gains usually peak within 6-12 months, then plateau.[8]Nestor, S, M., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of … Continue reading
Typical regrowth brings hair back to where it was 1-3 years earlier, not to pre-hair-loss density.
And what influences where you fall on that spectrum?
- How long you’ve been losing hair: Earlier intervention = higher trendline. Long-standing bald spots rarely respond.[9]Feldman, P.R., Gentile, P., Piwko, C., Motswaledi, H.M., Gorun, S., Pesachov, J., Markel, M., Silver, M., Brenkel, M., Feldman, O.J., Kamen, C.L., Uleryk, E., Guevara-Aguirre, J., Fiebig, K.M. … Continue reading
- Which therapy you use: Some are more effective at certain stages or patterns.
- How consistent you are: Skipped doses or stopping early push results below the trendline.
Most patients should expect stabilization and modest regrowth. A small minority will experience “hyper-responsiveness,” but these rare outcomes should not set the benchmark.
Principle #2: Poorly-Studied Treatments = More Variability In Results
When fewer studies exist on a treatment, expectations for hair regrowth become harder to pin down. With limited data, some patients may get spectacular results, while others see very little. But, without enough trials, it’s nearly impossible to know how common each outcome really is.
Take finasteride and oral dutasteride as examples. Finasteride has been tested in dozens of randomized controlled trials, which means we can say with high confidence what the average regrowth looks like and where most patients will land. Oral dutasteride, by contrast, has fewer high-quality studies.
That doesn’t mean dutasteride is ineffective. In fact, the regrowth gains by dutasteride have often outperformed finasteride users. But it does mean the range of possible outcomes is wider, with more unpredictability between “great responders” and “minimal responders”.
When it comes to understanding expectations for androgenic alopecia treatments, the normal distribution model helps clarify why individual results can be so different, even when clinical averages sound reassuring. Treatment outcomes typically form a bell-shaped curve rather than a single value, meaning people experience a range of responses.
- Clinical averages, which are often cited in studies, represent the peak of this curve, not the experience of every patient. Most individuals see results somewhere near this average, but plenty fall above or below it.
- Wide curve (high variability, low evidence): When evidence for a treatment is limited or inconsistent, like with newer therapies, the curve is broad. Some may see fantastic regrowth, others very little, and outcomes are harder to predict.
- Narrow curve (predictable outcomes, high evidence): For well-researched medications, the curve is tight. Most people’s results cluster close to the average, giving new patients more confidence in knowing what to expect.
Importantly, average never means “everyone”. Treatment outcomes often show a spread, so while clinical trials help set realistic expectations, individual journeys may differ depending on genetics, age, stage of hair loss, and treatment adherence.
Figure 4: Bell curves are a great way to show the typical outcomes depending on evidence. For finasteride, a narrow bell curve shows predictable outcomes. For oral dutasteride, a wide, shallow curve represents a much wider spread of outcomes.
This variability also explains why anecdotes online can feel so misleading. When people share their results in forums, they’re often the outliers, the best or worst cases. On a scatterplot of individual datapoints, these stories sit at the edges. With dutasteride, the scatter is typically above the trendline, meaning a Reddit “success story” may be far removed from what most users should expect.
Figure 5: Individual anecdotes (black dots) often sit at the edges of possible outcomes, but they don’t always represent what’s probable (orange line). What you don’t see online are the results that can occur below what is probable.
The rubric we can use to get a sense of whether our results will be predictable or variable is Evidence Quality.
Evidence Quality
Evidence quality answers the question “How reliable is the evidence that this intervention will actually help regrow my hair?” The strength of this evidence is determined not only by the number of studies available but also by their design, especially RCTs, which typically provide the most trustworthy evidence.
The concept of confidence in treatment outcomes can be likened to a game show in which money is hidden behind two doors. Behind door number 1, the amount of money behind it could be anywhere between $0 and $100. You might walk away with nothing, a little, or a lot, and there’s no way to know for sure, because there just isn’t enough evidence to narrow down the possibilities.
With door number 2, the money behind it ranges tightly between $49 and $51. Because so many high-quality studies exist, you can be confident that your outcome will almost certainly fall within this narrow window, making it much easier to know what to expect.
More evidence means a smaller range and greater predictability; less evidence means more uncertainty and a wider range of possible outcomes.
Relating this back to oral dutasteride, the treatment has fewer published randomized controlled trials compared to finasteride. This means less outcome certainty, so patients considering dutasteride face a wider bell curve of potential results (as shown above), from exceptional regrowth to only minor improvement.
To summarize:
- More and better studies = tighter confidence around the true average results
- Fewer studies = more uncertainty, a wider range of possible outcomes, and less ability to predict what will happen for any individual.
Understanding the evidence quality of a treatment empowers patients to interpret clinical claims and anecdotal stories with a critical eye, helping them choose interventions with the best established track record and manage expectations where data is still emerging.
Anecdotal Successes
In the world of hair loss drugs, Redditors and hair loss forums tend to overshare the best of what’s “possible”.
Let’s have a look at 10 real-life anecdotal reports shared by Reddit users.
It goes without saying that the experiences related below, including any before-and-after photos, are anonymously submitted by users and strictly represent their personal experiences. They are not considered medical evidence, nor should they be relied upon as medical advice.
It should also be noted that some of these individuals used other treatments alongside dutasteride to assist in their hair regrowth journey. With combination treatments, it is not easy to see what’s causing the hair growth, so we need to be even more cautious about the success seen.
Case 1: 14-month course of oral dutasteride 0.5 mg daily
Source: u/PriorAd8136 via r/tressless
This 25-year-old male reported aggressive hair thinning between ages 19–21, which he reportedly stabilized with dutasteride monotherapy at 0.5mg/day. He had previously tried finasteride for about a year but did not report any visible regrowth. The patient described significant regrowth of previously thin hairs, with increased hair density compared to treatment start. He noted that there were no side effects throughout treatment. Notably, he mentioned that finasteride provided stabilization only, while dutasteride produced visible regrowth after one year of use. Other users also highlighted the greater potency and longer half-life of dutasteride compared to finasteride.
Case 2: 10-month course on dutasteride 0.5 mg and oral minoxidil 0.625 mg daily
Source: u/hereforhairtips via r/FemaleHairLoss.
A female individual (age unspecified) with diffuse thinning, especially around the parting, reported on her experience using dutasteride 0.5mg/day, along with oral minoxidil 0.625mg/day (which was scaled back from an initial dose of 1.25mg). Further into the treatment, she added topical minoxidil to her treatment stack. At the 10-month mark, she reported a significant increase in hair density compared to baseline, with notable thickening at the part line, as confirmed by the user-submitted photos. The user was delighted with her results and intends to decrease her dutasteride use to a maintenance dose eventually.
Case 3: 10-month course on dutasteride 0.5 mg and oral minoxidil 5 mg daily
Source: u/ZoneFuzzy2966 via r/tressless.
This report was from a 26-year-old male with clearly noticeable balding by age 20 and a family history of male pattern baldness, including his father and both grandfathers. The user’s protocol included oral dutasteride 0.5mg and oral minoxidil 5mg daily. The user noted heavy shedding in the first two months of treatment, with the first noticeable signs of improvement appearing at months 2-3, before achieving a successful outcome by month 10, when they reported marked hair thickening and a renewed sense of confidence and self-esteem. The user did not report any side effects from dutasteride, but linked minoxidil use to a mild increase in arm hair. It’s worth noting that the user’s physician had recommended he switch from his initial finasteride therapy to dutasteride due to their “aggressive” alopecia.
Case 4: 3.5 months on oral dutasteride 0.5 mg and minoxidil 5 mg daily
Source: u/n70m via r/tressless.
A 22-year-old male shared his experience using oral dutasteride (0.5mg/daily), oral minoxidil (5mg/daily), plus dermarolling (1.5mm/weekly) for 3.5 months. The user achieved significant increases in hair density and regrowth, a self-reported improvement in self-esteem, and no reported side effects, namely as to his libido and sexual function. He noted his consistent use and starting relatively early in the hair loss process as reasons for his favorable outcome. Additionally, he highlighted that he did not titrate up to his final doses but started the full protocol from day one.
Case 5: 6 months on dutasteride 0.5 mg (ed 1st month – 3x/wk) + microneedling 1.0 mm weekly
Source: u/fozzzy5 via r/tressless.
This male (23 y/o) used dutasteride at the dose of 0.5mg daily for the first month, then reduced to 0.5mg thrice weekly, along with microneedling 1.0mm once weekly, over the course of six months. The apparent results include hairline and temple regrowth, with the hair improving in thickness and health overall. The user did not report any side effects beyond bleeding associated with microneedling, noting that he began to notice changes within a month. In explaining his results, he also believed himself to be highly responsive to the treatment.
Case 6: 2+ years on dutasteride 0.5 mg daily and sublingual minoxidil 0.9 mg – 2.7 mg daily
Source: u/Throwaway_no_hair via r/tressless.
In this report, a 31-year-old male shared his results on a protocol of oral dutasteride (0.5mg daily) and sublingual minoxidil (gradually increased from 0.9mg to 2.7mg daily). While he started treatment with oral finasteride, he switched to dutasteride and sublingual minoxidil at the advice of his dermatologist. The users reported significant improvement after 3–4 months, with darker hair color and thickening of hair at the crown and mid-scalp. He added that most improvements came within 12 months, noting that he did experience any shedding with minimal side effects.
Case 7: 2 months on dutasteride 0.5 mg daily and oral minoxidil 2.5 mg daily
Source: u/BunnySlosh via r/tressless.
A 31-year-old male detailed his experience taking dutasteride 0.5mg daily alongside oral minoxidil following a series of unsuccessful treatments. He initially began with finasteride 1mg, which slowed hair loss but did not affect major regrowth. He then tried minoxidil and microneedling, before restarting finasteride and topical minoxidil alongside dermarolling. Upon switching to dutasteride, he noted major improvement in just two months, including achieving thicker hair and the disappearance of bald spots. The user reported no major side effects and noted supplementing his protocol with a daily multivitamin (Becadexamin) and a collagen supplement to support skin and hair.
Case 8: 3.5 months on dutasteride 0.5 mg daily and topical minoxidil 2x daily
Source: u/LongjumpingAd717 via r/tressless.
A 24-year-old male reported significant improvement in hair density and coverage following 3.5 months of oral dutasteride 0.5mg/day and topical minoxidil (Rogaine) applied twice daily. The Redditor reported hair shedding, especially around the hairline, at month one. In the second half of the treatment period (months 2-3.5), however, he experienced accelerated hair regrowth with the appearance of a full head of hair. He was prescribed dutasteride directly (no prior finasteride use) and reported no major side effects as of the 3.5-month mark.
Case 9: 1 year on dutasteride 0.5 mg daily and 10 years on topical minoxidil
Source: u/External-Bad-9075 via r/tressless.
This user (age 31) used daily dutasteride (Avodart 0.5mg) for one year in addition to ten years of consistent topical minoxidil use. He had previously used finasteride for two years with satisfactory results, stating that he could have achieved more regrowth by combining minoxidil with a DHT blocker. Following his switch from finasteride to dutasteride, he noted improvements in hair strength, libido increase, and clearer thinking. Months 3-8 were marked by shedding and brittle, weaker hair, with the patient considering switching back to finasteride. But after month 8, he saw thicker strands and mild regrowth. He also noted longer hair cycles, which now allow him to better maintain hair length. The user planned to continue with treatment and reevaluate at the two-year mark.
Case 10: 8 months on dutasteride 0.5 mg and 10 months on oral minoxidil 2.5 mg
Source: u/Fast_Tomatillo_3840 via r/tressless.
This male user (29 y/o) undertook a protocol of oral dutasteride 0.5mg/day and oral minoxidil 0.5mg/day concurrently for eight months, with strong improvements in hair regrowth and thickness. He reported shedding at months 1-3 followed by regrowth starting at the 3-month mark. He observed increased hair density in the crown and temples from months 3 to 8, and noted major progress by month 10, with the crown nearly full, despite a persistent “M-shaped” hairline. The user reported no major side effects and plans to undergo a hair transplant, notwithstanding the favorable outcome.
But these anecdotes are often far above the trendline, over “average” expectations.
Probable Regrowth Timeline for Oral Dutasteride
We have laid out a likely timeline for oral dutasteride treatment. However, this is based on four studies that we could find using oral dutasteride for hair loss, including one where it was used for frontal fibrosing alopecia.[10]Tsunemi, Y., Irisawa, R., Yoshiie, H., Brotherton, B., Ito, H., Tsuboi, R., Kawashima, M., Manyak, M. (2016). Long-term safety and efficacy of dutasteride in the treatment of male patients with … Continue reading,[11]Pindado-Ortega, C., Saceda-Corralo, D., Moreno-Arrones, O., Rodrigues-Barata, A.R., Hermosa-Gelbard, A., Jaen-Olasolo, P., Vano-Galvan, S. (2020). Effectiveness of Dutasteride in a Large Series of … Continue reading,[12]Vano Galvan, S., Saceda-Corralo, D., Morena-Arrones, O.M., Rodrigues-Barata, R., Morales, C., Gil-Redondo, R., Bernardez-Guerra. C., Hermosa-Gelbard, A., Jaen-Olasolo, P. (2019). Effectiveness and … Continue reading,[13]Harcha, W.G., Martinez, J.B., Tsai, T-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., Chetty, D. (2014). A randomized, active and placebo-controlled study of the efficacy … Continue reading
Month 0-3: No visible changes:
- Most studies note minimal to no observable regrowth in the first 3 months as dutasteride begins to reduce DHT levels, but hair follicles have not yet responded morphologically.
Month 3-6: Early results seen:
- Clinical improvements in hair thickness and reduced shedding are often first noted from around month 3 onward.
- Quantitative hair count changes become statistically significant compared with placebo after 12 weeks; global photographic assessments begin to reflect mild positive change (~22-31% of subjects with slight/moderate increase at week 12).
Month 6-9: First cosmetic improvements:
- By 6-9 months, most patients exhibit visible improvement in hair density and coverage, with the peak initial response typically building upon earlier thickening.
- In a Japanese study, mean hair count increased significantly at 26 weeks and continued to improve at 52 weeks (68-87 hairs in a 2.54cm area).
- Panel assessments at month 6 indicated ~75-85% of patients had some improvement in global photographs.
Months 9-15: Peak response period:
- Maximum regrowth and cosmetic benefits are typically observed within the first 12-15 months, based on clinical results.
- “Marked improvement” rates ranged from 23-25% at 12 months with daily dosing in large cohorts. Median improvement in photographic rating scores and increases in terminal hair count continue through this period.
- Sustained effects shown to persist up to 1 year in an open-label extension study.
Months 15-24: Maintenance phase:
- Most patients maintain results with continued therapy, improvements plateau or slightly decline, but remain superior to baseline and other treatments.
- Longer-term users (mean follow-up up to 17 months) show stabilization and ongoing maintenance with only rare further large gains.
- Clinical improvement was seen in studies where doses ranged 3-7 capsules/week, with higher doses correlating to better maintenance and slightly higher efficacy.
Months 24-26: Possible plateau, limited data:
- Efficacy plateaus; possible mild regression or fluctuation, but proper adherence maintains gains for most patients.
- Real-world data of up to at least 24 months confirm sustainability in appropriately selected patients, especially at higher doses.
- No long-term studies exceed 36 months, so late-stage trends remain speculative.
But what about all the success stories we see online, users on Reddit or other forums who have experienced dramatic hair regrowth?
Well, this difference usually stems from a phenomenon called survivorship bias.
What is Survivorship Bias?
- Survivorship bias happens when the accounts you see are not representative of all experiences but are disproportionately skewed toward the most remarkable, positive cases.[14]Rao, T. (2024). Understanding Survivorship Bias: Implications for Research and Decision-Making. Journal of Emerging Technologies and Innovative Research, 11(6). JETIR2406276
- People with extreme positive results are much more likely to post about their experiences, eager to share their success.
- In contrast, those who experience average or mediocre outcomes (which is the majority) rarely feel motivated to post.
- As a result, the outcomes you typically see online are samples from the tails of the bell curve: exceptional or very poor cases, not the “center” or average results.
Clinical Trial vs. Online Reports
- Clinical trials measure average results across a large, diverse population, using objective metrics.
- Online reports are anecdotal and self-selected. Because impressive transformations are inspiring, these are far more likely to be posted and shared.
- This dynamic can mislead readers into believing that dramatic regrowth is almost guaranteed, when in reality it is just one possibility among many, and not the most probable outcome.
Combining Treatments: Narrowing the Curve
While oral dutasteride is one of the most effective monotherapies for AGA, if you want to increase your odds of good results, combining it with additional therapies can have a dramatic impact.
Studies have shown that adding minoxidil to dutasteride can further improve regrowth outcomes, leveraging the vasodilatory and follicle-activating effects of minoxidil, alongside DHT suppression from dutasteride.[15]Obeid, M.N.A., Fattah, N.S. A., Elfangary, M.M., Al Husseni, R.M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral … Continue reading
Furthermore, adding low-dose dutasteride to ongoing finasteride therapy in patients with suboptimal response resulted in a dramatic increase in hair density, suggesting that combined therapy can be beneficial for those not fully responding to finasteride alone.[16]Boyapati, A., Sinclair, R. (2013). Combination therapy with finasteride and low-dose dutasteride in the treatment of androgenetic alopecia. Australasian Journal of Dermatology. 54(1). 49-51. … Continue reading
Going back to the bell curves, more modalities = less variability. By targeting different mechanisms (like minoxidil) or by targeting more types of 5-alpha-reductase (like in the finasteride study), outcomes become less dependent on any single pathway. This leads to more predictable, less variable results across a wider patient group.[17]Mysore, V., Kumaresan, M., Dashore, S., Venkatram, A. (2023). Combination and rotational therapy in androgenetic alopecia. Journal of Cutaneous and Aesthetic Surgery. 16(2). 71-80. Available at: … Continue reading
How Can I Maintain “Realistic Expectations”?
To keep realistic expectations on oral dutasteride for hair regrowth, it’s critical to understand both the typical timelines and the nature of outcome variability:
What does “realistic” mean?
- Realistic does not mean pessimistic: Most users will benefit, but responses vary between individuals. A realistic expectation is based on clinical averages, not the best or worst case.
- Don’t expect to be “cured” of your hair regrowth. Most treatments “rewind the clock” a certain amount. Oral dutasteride may reverse hair loss by 6-24 months on average. However, dramatic multi-year regrowth is much less common, possible but not probable for most users.
- Your most probable result is noticeable improvement and stabilization, rather than full reversal of all past hair loss.
Practical Takeaways
- Be patient: Oral dutasteride takes time – most users don’t see meaningful change until at least 6 months in, with optimal results often peaking around 12-15 months.
- Track your own progress: Use consistent photos or, ideally, hair counts in a fixed area to monitor changes. Comparing to internet success stories often skews expectations and reflects outliers rather than the norm.
- Define success on your own terms: For some, success means stopping hair loss. For others, it’s regaining density. Know what you’re aiming for and adjust expectations accordingly.
- Consider combination treatments: Pairing dutasteride with therapies like minoxidil or microneedling may improve outcomes and reduce variability, especially if you’re not seeing early improvements.
- Use clinical data to ground your expectations: Clinical trials give you an average expectation, not a guarantee. Use online anecdotes as inspiration, not prediction.
Final Thoughts
Your expectations for any hair loss treatment, even oral dutasteride, should rest on two key pillars: regrowth potential x evidence quality.
Together, they shape your position on the “bell curve” of likely outcomes.
- If you’re early in your hair loss journey, consistently take the medication, and possibly combine it with other therapies, you’re more likely to end up closer to the favorable side of the curve.
- If your hair loss is advanced or you’ve tried many options before, you might fall closer to the average or lower end.
Either way, understanding that most people land somewhere near the middle of the curve, not at the extremes, helps you avoid frustration and stay committed.
Setting expectations isn’t about limiting hope or being overly pessimistic; it’s about anchoring your treatment outcomes to reality, so you can make better, more sustainable decisions about your treatment journey.
References[+]
References ↑1 Chen, S., Xie, X., Zhang, G., Zhang, Y. (2022). Comorbidities in Androgenetic Alopecia: A Comprehensive Review. Dermatology and Therapy. 12(10). 2233-2247. Available at: https://doi.org/10.1007/s13555-022-00799-7 ↑2 Bajoria, P.S., Dave, P.A., Rohit, R.K., Tibrewal, C., Modi, N.S., Gandhi, S.K., Patel, P. (2023). Comparing Current Therapeutic Modalities of Androgenic Alopecia: A Literature Review of Clinical Trials. Cureus. 15(7). E42768. Available at: https://doi.org/10.7759/cureus.42768 ↑3 Clark, R.V., Hermann, D.J., Cunningham, G.R., Wilson, T.H., Morrill, B.B., Hobbs, S. (2004). Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5ɑ-Reductase Inhibitor. JCEM 89(5) 2179-2184. Available at: https://doi.org/10.1210/jc.2003.030330 ↑4 Nickel, J.C. (2004). Comparison of Clinical Trials with Finasteride and Dutasteride. Reviews in Urology. 6(Suppl 9). S31-S39. Available at: PMID: 16985923 ↑5 Escamilla-Cruz, M., Magana, M., Escandon-Perez, Bello-Chavolla, O.Y. (2023). Use of 5-Alpha Reductase Inhibitors in Dermatology: A Narrative Review. Dermatology and Therapy. 13(8). 1721-1731. Available at: https://doi.org/10.1007/s13555-023-00974-4 ↑6 Lee, S., Kim, J.E., Lew, B-L., Huh, C.H., Kim, J., Kwon, O., Kim, B.M., Lee, Y.W., Lee, Y., Park, J., Kim, S., Kim, D.Y., Choi, G.S., Kang, S. (2025). Efficacy and Safety of Low-Dose 0.2 mg Dutasteride for Male Androgenic Alopecia: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Phase III Clinical Trial. Annals of Dermatology. 37(4). Available at: https://doi.org/10.5021/ad.25.048 ↑7 Badenhorst CE, Dawson B, Goodman C, Sim M, Cox GR, Gore CJ, Tjalsma H, Swinkels DW, Peeling P. Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur J Appl Physiol. 2014 May;114(5):951-9. doi: 10.1007/s00421-014-2829-6. Epub 2014 Feb 1. PMID: 24487960. ↑8 Nestor, S, M., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of Cosmetic Dermatology. 20(12). 3759-3781. Available at: https://doi.org/10.1111/jocd.14537 ↑9 Feldman, P.R., Gentile, P., Piwko, C., Motswaledi, H.M., Gorun, S., Pesachov, J., Markel, M., Silver, M., Brenkel, M., Feldman, O.J., Kamen, C.L., Uleryk, E., Guevara-Aguirre, J., Fiebig, K.M. (2023). Hair regrowth treatment efficacy and resistance in androgenetic alopecia: A systematic review and continuous Bayesian network meta-analysis. Frontiers in Medicine. 23(9). Available at: https://doi.org/10.3389/fmed.2022.998623 ↑10 Tsunemi, Y., Irisawa, R., Yoshiie, H., Brotherton, B., Ito, H., Tsuboi, R., Kawashima, M., Manyak, M. (2016). Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. The Journal of Dermatology. 1-8. Available at: https://doi.org/10.1111/1346-8138.13310 ↑11 Pindado-Ortega, C., Saceda-Corralo, D., Moreno-Arrones, O., Rodrigues-Barata, A.R., Hermosa-Gelbard, A., Jaen-Olasolo, P., Vano-Galvan, S. (2020). Effectiveness of Dutasteride in a Large Series of Patients with Frontal-Fibrosing Alopecia in Real Clinical Practice. JAAD. Available at: https://doi.org/10.1016/j.jaad.2020.09.093 ↑12 Vano Galvan, S., Saceda-Corralo, D., Morena-Arrones, O.M., Rodrigues-Barata, R., Morales, C., Gil-Redondo, R., Bernardez-Guerra. C., Hermosa-Gelbard, A., Jaen-Olasolo, P. (2019). Effectiveness and safety of oral dutasteride for male androgenetic alopecia in real clinical practice: A descriptive monocentric study. Dermatologic Therapy. 33. E13182. Available at: https://doi.org/10.1111/dth.13182 ↑13 Harcha, W.G., Martinez, J.B., Tsai, T-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., Chetty, D. (2014). A randomized, active and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. Journal of the American Academy of Dermatology. 70(3). 489-498. E3. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑14 Rao, T. (2024). Understanding Survivorship Bias: Implications for Research and Decision-Making. Journal of Emerging Technologies and Innovative Research, 11(6). JETIR2406276 ↑15 Obeid, M.N.A., Fattah, N.S. A., Elfangary, M.M., Al Husseni, R.M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in treatment of androgenetic alopecia. QJM: An International Journal of Medicine. 117(2). Available at: https://doi/org/10.1093/qjmed/hcae175.207 ↑16 Boyapati, A., Sinclair, R. (2013). Combination therapy with finasteride and low-dose dutasteride in the treatment of androgenetic alopecia. Australasian Journal of Dermatology. 54(1). 49-51. Available at: https://doi.org/10.1111/j.1440-0960.2012.00909 ↑17 Mysore, V., Kumaresan, M., Dashore, S., Venkatram, A. (2023). Combination and rotational therapy in androgenetic alopecia. Journal of Cutaneous and Aesthetic Surgery. 16(2). 71-80. Available at: https://doi.org/10.4103/JCAS.JCAS_212_22 Top Places to Buy Topical Dutasteride
- #1 Choice for Quality and Growth: Ulo
- Top Runner-Up in the USA: Happy Head
- Top Pick in Canada: Xyon Health
- Top Runner-Up in Canada: Jupiter
- Top Pick in the UK: Hair Repair Clinic
- Top Runner-Up in the UK: Hair Medics UK
Topical dutasteride is a potent 5ɑ-reductase inhibitor, and while dutasteride is normally taken orally to treat benign prostatic hyperplasia, its off-label topical use is being explored for localised hair regrowth therapies.[1]Andrade, J.F.M., Verbinnen, A., Bakst, A., Cunha-Filho, M., Gelfuso, G.M., Tais Gratieri. (2024). Topical dutasteride for androgenic alopecia: current state and prospects. Therapeutic Delivery. … Continue reading
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Mechanism of Action: 5ɑ-Reductase Inhibition
- Dutasteride inhibits both type I and type II isoenzymes of 5ɑ-reductase, the enzyme responsible for converting testosterone into dihydrotestosterone (DHT).
- DHT is a key driver of hair follicle miniaturization in AGA.
- By blocking this conversion, dutasteride effectively reduces scalp and systemic DHT levels, which can help maintain or restore hair density.
- Dutasteride is significantly more potent than finasteride, another 5ɑ-reductase inhibitor, in reducing DHT and has shown superior efficacy in clinical studies on hair loss.[2]Gupta, A.K., Venkataraman, M., Talukder, M., Bamimore, M.A. (2022). Relative Efficacy of Minoxidil and the 5-ɑ-reductase Inhibitors in Androgenetic Alopecia Treatment of Male Patients A Network … Continue reading
Advantages of Topical Formulations
Topical application of dutasteride is being explored as a way to localize treatment to the scalp and potentially reduce systemic exposure. The hope is that by delivering the medication directly to hair follicles, topicals may achieve meaningful concentrations where they are needed while lowering the risk of systemic side effects sometimes reported with oral therapy. However, while early studies suggest this may be the case, large-scale clinical trials directly comparing topical and oral dutasteride are still lacking.
By delivering the medication directly to the hair follicles, topical dutasteride achieves high local drug concentrations where it is needed most, potentially improving effectiveness with a lower overall dose.[3]Alam, M., Mishra, A., Yadav, K.S., Pradhan, D., Kar, B., Ghosh, G., Rath, G., Rai, V.K. (2024). Development and Evaluation of Dutasteride Nanoemulgel for the Topical Delivery Against Androgenic … Continue reading Importantly, emerging data support a lower incidence of adverse systemic reactions in patients using topical formulations, all while delivering meaningful improvements in hair regrowth.[4]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Hertz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
Why Sourcing Matters
When it comes to topical dutasteride, sourcing is crucial due to the significant variability in compounding and formulation standards. Since topical dutasteride is not commercially available as an approved product for hair loss, it is typically prepared by compounding pharmacies or specialty providers.
This means that compounding methods, choice of solvents, and the concentration of the active ingredient can differ widely, leading to variability in both efficacy and safety.[5]American Hair Loss Association. (no date). Hair Loss Treatments Online: What to Know About Compounded Medications. Available at: … Continue reading Moreover, without the regulatory oversight afforded to mass-manufactured drugs, compounded topical dutasteride products are more prone to inaccuracies in labeled potency, which could result in under- or overdosing.
Price and Purity
The cost and purity of compounded topical dutasteride are also highly variable. Pricing can fluctuate based on local regulations, pharmacy standards, inclusion of additional actives, and the source of individual ingredients.
Purity is also a significant concern; the quality of the active pharmaceutical ingredient used in compounding may vary depending on the source, raising uncertainty about both efficacy and the potential presence of contaminants. This variability makes it essential for patients and healthcare providers to carefully consider the source and standards behind any compounded topical dutasteride product.
So, let’s take a look at where you can get topical dutasteride in the US and around the world, and what the benefits (and potential drawbacks) of each one are.
Where To Buy Topical Dutasteride
United States Providers
When considering topical dutasteride in the US, your choice of provider can impact the quality, customization, and transparency you receive. With no FDA-approved commercial topical dutasteride products, most options are compounded by specialized pharmacies or telehealth companies. These providers differ in terms of formula customization, purity verification, dosing flexibility, and pricing, making it essential to understand your options before starting therapy.
Ulo
Ulo stands out in the US telehealth market for its high degree of flexibility and rigorous standards:
- Evidence-First Formulations:
-
- Every product’s ingredient concentrations are based on published studies relative to a user’s goals: localization, max hair gains, and more.
- Independent Laboratory Testing. Third-party reports for GC/MS and HPLC testing are publicly posted, verifying that what’s on the label is what’s in the bottle.
You can read more about our product lab testing results here.
- Ulo offers both low-dose and full-strength topical dutasteride (0.02% & 0.2%), enabling a range of options for localization and results.
- Unrivaled Personalization:
-
-
- Customize your formula by adding 7% minoxidil 0.01% tretinoin, 0.2% caffeine, 0.01% melatonin, and 1% cetirizine.
- Treatment regimens can be tailored for once- or twice-daily application, with thoughtfully designed dropper-marked bottles to facilitate accurate dosing.
- Partner physicians work with you one-on-one, making real-time adjustments to dosing and ingredients based on your response and tolerability.
-
- Consumer Safety Built-In
-
-
- No propylene glycol, no corticosteroids, and no “trending” off-label additives (e.g., latanoprost, copper peptides) that carry unproven risks.
- Formulations are crafted to maximize follicle prevention while minimizing scalp irritation, so there’s no need to mask side effects with steroids.
-
- Transparent Quality & Pricing
-
-
- Third-party lab reports accompany every batch.
- Ulo’s pricing starts at $1.15 per ml (or $0.86 per ml with the discount) for the low-dose dutasteride-only formulation, increasing with dose and other ingredients.
-
- Ongoing Medical Support
-
- Unlimited access to Ulo’s network of licensed physicians via a secure portal, ask questions, report progress, and get tailored advice anytime.
- Realistic timelines and expectation setting: know when to expect regrowth, potential plateaus, and strategies to boost results.
Ulo represents a true evolution in telehealth haircare, built by advocates who’ve spent over a decade exposing industry-wide bad practices. At Ulo, you get formulations that have been vetted inside and out, with the power to tweak every detail to your needs, backed by experts who prioritize your safety and success.
Pros
- Cheaper per ml than other options.
- Highly customizable formulas with options for minoxidil, tretinoin, caffeine, melatonin, and cetirizine.
- Dosing adjustments available.
- No unnecessary additions (no corticosteroids, propylene glycol, or trending unproven ingredients.
- Direct access to licensed physicians for real-time support.
Cons
- If prescribed to use twice daily, it may be inconvenient.
Only a few other online US telehealth providers provide topical dutasteride:
Happy Head
Happy Head is a teledermatology service offering personalized prescription solutions for hair loss. They offer topical formulas that may include dutasteride, which can be combined with minoxidil or finasteride, depending on the specific product chosen. Happy Head’s standard plans are typically ~$89 per month, depending on the complexity of ingredients.
They also emphasize potency (advertising a 10-in-1 custom mix that includes 0.25% dutasteride) and convenience, though such “mega” formulations may increase the risk of side effects.
Pros
- Flexible custom formulas available, with high minoxidil concentrations (up to 8%), with the option to include a large number of additional ingredients.
- Fast shipping and responsive customer service.
Cons
- Twice-daily application can be less convenient.
- The most expensive option.
- Formulated with propylene glycol, which can cause skin dryness.
Strut Health
Strut Health is an online compounding pharmacy that provides custom dutasteride formulas. Strut allows some customization: their “Strut 5-in-1” topical can include 0.1% dutasteride, minoxidil (up to 7.5%), tretinoin (up to 0.0125%), fluocinolone 0.01% (a corticosteroid), and biotin in either a solution or gel base. Unfortunately, the addition of corticosteroids to offset potential irritation comes at the risk of long-term skin thinning for customers.
Pros
- Fully compounded formulas with strong customization can adjust dutasteride strength, add/remove ingredients, and choose a base (no alcohol or propylene glycol)
- Option to combine dutasteride and minoxidil.
- Streamlined telehealth process
Cons
- Additions of corticosteroids come with the risk of long-term skin thinning.
Below is an overview table of these providers
Provider Customization Add-Ons Dosing Flexibility Approx. Price/mL Purity Testing Ulo Yes Minoxidil, Tretinoin, Cetirizine, Melatonin, and Caffeine Yes (can choose 0.02 – 0.2%) Starts at $1.15 per mL 3rd-party lab reports. Strut Yes Minoxidil 0-7.5%, Tretinoin 0-0.0125%, Fluocinolone 0.01%, Biotin No 0.1% offered only $2.30 per mL 3rd-party lab reports of topical finasteride were good. HappyHead No, the doctor customizes the product for you Minoxidil 8%, Cetirizine 1%, Latanoprost 0.005%, Dutasteride 0.25%, Finasteride 0.25%, Melatonin 1%, Caffeine 0.2%, Tretinoin 0.001%, Vitamin D3 1,000 IU/mL
Biotin 0.2%
No 0.25% offered only $2.97 per mL 3rd-party lab reports of another product were mixed, with some ingredients not meeting the claimed dose. Note: Mainstream men’s telehealth brands, such as Hims and Keeps, do not currently offer topical dutasteride; instead, they typically focus on finasteride.
When choosing a provider, consider that formulation differences can impact both efficacy and safety. Ulo’s approach of using lower concentrations for localized effect contrasts with some competitors selling “mega-dosed” topicals (e.g., extremely high finasteride or dutasteride percentages) that may “leak” systemically and negate the benefit of a topical.
Also, be aware of whether a product includes corticosteroids to reduce scalp irritation. While this can make usage more comfortable, chronic steroid use on the scalp can lead to skin thinning over time.[6]Mir-Bonage, J.F., Piquero-Casals, J., Prudkin, L., Delgado, J., Martinez, J.S., Briones, V.G-P. (2024). Use of Topical Corticosteroids in the Treatment of Noninfectious Inflammatory Dermatoses of the … Continue reading
Canada Providers
In Canada, dutasteride (oral or topical) is a prescription medication; however, telehealth and pharmacy services are available, similar to those found in the US.
Xyon Health
Xyon Health is a telehealth brand based in Canada (although it is also available in the US). It contains the highest concentration of dutasteride yet – 2% – alongside its proprietary SiloxysSystem slow-release gel base, which is claimed to reduce systemic absorption.
Xyon does not offer any customization options, focusing solely on monotherapy dutasteride treatments (although their finasteride treatment can be combined with minoxidil). The product is typically shipped in a 3-month supply for about $387 per shipment.
Pros
- Proprietary slow-release gel base is stated to reduce systemic absorption.
- Highest known dutasteride concentration (2%) for those seeking potent therapy
- Simple no-additive formulas.
Cons
- No customization beyond the base solution.
- Expensive cost.
Jupiter
Jupiter is a telehealth platform offering personalized topical hair loss treatments. Through Jupitor, customers can access a compounded topical that may include dutasteride as well as up to four other active ingredients, depending on their needs, including minoxidil, tretinoin, panthenol, and caffeine.
Unfortunately, they don’t advertise their concentrations or prices, mentioning that you can only view specific medication pricing and options after completing an online assessment. Jupiter provides free shipping Canada-wide, however.
Pros
- Personalized compound formulas with multiple additive options (minoxidil, tretinoin, panthenol, caffeine).
- Free Canada-wide shipping and online physician consultation.
Cons
- Lack of transparency on formula concentrations and price until after assessment.
- Dosing flexibility unclear.
Beyoung Health
Beyoung Health is another Canadian telehealth clinic (based in Ontario) that offers treatments for men’s health, including hair loss. They prescribe compounded topical dutasteride as part of their hair loss program. Beyoung offers finasteride or dutasteride, along with minoxidil, either alone or in combination, for hair loss therapy. In practice, this means a Beyoung patient can get a topical solution with dutasteride as the active ingredient, or a combination of dutasteride and minoxidil if appropriate.
Beyoung’s approach is relatively patient-tailored. They don’t list fixed strengths, but they do mention that the price depends on formulation and concentration, typically ranging from $50 to $150 per month. Beyoung also charges a $25 fee for their online medical questionnaire consultation.
Pros
- Can customize both dutasteride and minoxidil concentrations.
- Patient-centered pharmacy with tailored dosing and options.
Cons
- Pricing and product specifics are only revealed after a medical assessment.
Provider Customization Add-Ons Dosing Flexibility Approx. Price/mL Purity Testing Xyon Health No None No 2% $4.30 None Jupiter Yes Minoxidil, Tretinoin, Panthenol, and Caffeine. Unknown Unknown None Beyoung Health Yes Minoxidil Yes, but unknown Unknown None Overall, Canadian residents have growing access to topical dutasteride through these services. Just ensure any provider is operating legally with Canadian-licensed healthcare professionals. As dutasteride is not officially approved for hair loss in Canada (similar to the US, it is used off-label), you won’t find it at regular pharmacies without a prescription and through the compounding route.
United Kingdom
In the UK, dutasteride is prescription-only and is not officially authorized for hair loss (oral dutasteride isn’t licensed for AGA in the UK). Nevertheless, if you are looking for where to buy topical dutasteride, doctors can prescribe it off-label, and a few online compounding services and clinics offer topical dutasteride for patients.
Many online providers, such as Manual, Numan, and Sons – all well-known UK telehealth brands – typically do not list topical dutasteride among their available hair loss treatments. However, some online clinics do offer topical dutasteride compounding services.
Hair Repair Clinic
Via their compounding arm (Compounding Labs Ltd), Hair Repair Clinic offers Dutasol, topical dutasteride solutions in a proprietary low-alcohol vehicle called TrichoSol. Customers can choose a strength of 0.025%, 0.05%, or 0.1% dutasteride. You can also add minoxidil to this formula (called Dutasol-M), which combines dutasteride with 5% or higher minoxidil.
A free online prescription (assessment) is included with the purchase, and the product is made fresh to order in the UK. Pricing is £70 ($94.81) for a 3-month supply of dutasteride-only treatment. This price increases if you add extras, such as high-strength minoxidil. Shipping within the UK costs £5.50, and they also ship to some EU countries.
Pros
- Multiple vehicle and dosing choices with the option to add minoxidil for further regrowth.
- Transparent formulation, made to order in the UK.
- Free online prescription.
Cons
- Not covered by the NHS
Hair Medics UK
This London-based hair clinic also offers compounded topical formulas for sale. They advertise a topical finasteride (0.1%) and dutasteride (0.1%) combination solution (for men only) for £69.99 ($94.72). They also offer a dutasteride-alone solution (0.1%) for £59.99 ($81.19).
Pros
- Offers dutasteride only and dutasteride-finasteride combination solutions.
Cons
- Limited customization compared to options in other regions.
Provider Customization Add-Ons Dosing Flexibility Approx. Price/mL Purity Testing Hair Repair Clinic Yes Minoxidil Yes 0.025%, 0.05%, 0.01% £1.40 ($1.90) None Hair Medics UK Yes Finasteride No 0.1% £2.00 ($2.71) None Topical dutasteride is available in the UK, but primarily through private telehealth/clinic channels. Expect to pay out of pocket, as these are private prescriptions.
International Options
A few companies openly market topical dutasteride worldwide without a prescription, operating in a gray area of enforcement. They are able to do this by exploiting international legal ambiguities, inconsistent enforcement, and gaps between national regulatory systems, which enable the global shipping of their products that might otherwise be restricted in the customer’s country. These practices largely persist due to a lack of harmonized global enforcement and prioritization of more serious pharmaceutical offenses.[7]Feder, J. (2004). Legal Issues Related to Prescription Drug Sales on the Internet. CRS Report for Congress. Available at: … Continue reading
MinoxidilMax
MinoxidilMax offers a product called Duderma, which is a 0.1% dutasteride topical (1 mg/mL) in a 60 mL dropper bottle. It’s available with or without propylene glycol, and they also sell combinations with minoxidil or finasteride. Notably, MinoxidilMax requires no prescription; you can simply add it to your cart and purchase it directly from their site.
The cost is also relatively low compared to other options (approximately $40 for one bottle). It is essential to note that these are unregulated products, and you are essentially relying on the vendor’s word regarding the contents of the bottle. At PHH, we have tested Duderma and it has passed laboratory testing. However, we have found issues with other MinoxidilMax products, like the Dualgen-15 No PG Plus.
Pros
- Wide variety of compounded products (dutasteride alone or with minoxidil/finasteride).
- Low cost per milliliter.
Cons
- No prescription required raises regulatory/legal concerns.
- Quality control consistency is variable; some products tested below label potency
HemiaCosmetics
HemiaCosmetics is a Europe-based company that claims to operate out of Serbia. They sell Dutagen, a topical dutasteride solution at 0.1% (1 mg/mL) in 50 mL bottles. Like MinoxidilMax, no prescription is required to buy products from this company. Prices are also in a similar range (~$50).
Users have reported mixed experiences with HemiaCosmetics products; some experienced increased shedding or no results, raising suspicions that the product may not contain the promised actives in the right concentration.[8]u/motorcuadam (2023). Hemiacosmetics: Are they legit? Reddit. Available at: … Continue reading In short, there is a trust deficit with such grey-market sellers.
Pros
- Good inventory and pricing for topical dutasteride delivered to the EU/UK.
- Option to customize by enquiry.
Cons
- Mixed user feedback with concerns about formulation efficacy and potential shedding.
Provider Customization Add-Ons Dosing Flexibility Approx. Price/mL Purity Testing MinoxidilMax Yes Minoxidil, Finasteride No 0.1% $0.33 3rd-party lab reports. Hemia Cosmetics Yes (but only available upon enquiry) No No 0.1% $1.01 None Important: We do not endorse buying prescription-strength medications from unregulated sources. While it may be tempting due to lower costs or the absence of a doctor’s consultation, you are taking on risks in terms of product purity and legality.
If you go this route, be aware that you’re essentially conducting an experiment on yourself with a substance of uncertain quality. Whenever possible, use a legitimate telehealth service or pharmacy; you’ll have medical oversight and a far better assurance that the bottle contains real dutasteride in the correct dosage.
Final Thoughts
Topical dutasteride presents a promising option for individuals who have not achieved sufficient success with topical finasteride or who wish to maximize DHT reduction at the scalp while potentially minimizing systemic effects. Several telehealth options are available if you are looking for where to buy topical dutasteride, but it’s crucial to choose a reputable source. In the U.S. and Canada, specialized telehealth companies like Ulo, Happy Head, Xyon, Strut, and others can prescribe and deliver custom-compounded solutions to your home.
In the UK and Europe, a few compounding pharmacies (often linked with hair clinics) will work with you via online consultations to provide topical dutasteride legally. When comparing options, pay attention to factors like formula customization (do you want minoxidil or other growth boosters included?), convenience, and proven quality. Providers such as Ulo have set a high bar by offering third-party testing and personalized care, whereas some others might prioritize a one-size-fits-all high-dose approach or include unnecessary additives.
Lastly, be wary of any vendor that seems too good to be true (for instance, selling dutasteride without a prescription or at suspiciously cheap prices). Saving a bit of money is not worth the risk of applying a mystery substance to your scalp. Whenever possible, stick with licensed telehealth and pharmacy services; you’ll ensure you’re getting authentic dutasteride in the proper concentration, and you’ll have medical guidance on how to use it safely and effectively. Your hair (and health) will be better off for it!
References[+]
References ↑1 Andrade, J.F.M., Verbinnen, A., Bakst, A., Cunha-Filho, M., Gelfuso, G.M., Tais Gratieri. (2024). Topical dutasteride for androgenic alopecia: current state and prospects. Therapeutic Delivery. 16(3). 271-283. Available at: https://doi.org/10.1080/20415990.2024.2437973 ↑2 Gupta, A.K., Venkataraman, M., Talukder, M., Bamimore, M.A. (2022). Relative Efficacy of Minoxidil and the 5-ɑ-reductase Inhibitors in Androgenetic Alopecia Treatment of Male Patients A Network Meta-analysis. JAMA Dermatology. 158(3). 266-274. Available at: https://doi.org/10.1001/jamadermatol.2021.5743 ↑3 Alam, M., Mishra, A., Yadav, K.S., Pradhan, D., Kar, B., Ghosh, G., Rath, G., Rai, V.K. (2024). Development and Evaluation of Dutasteride Nanoemulgel for the Topical Delivery Against Androgenic Alopecia. Pharmaceutical Nanotechnology. 12(5). 459-470. Available at: https://doi.org/10.2174/0122117385269151231031161411 ↑4 Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Hertz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized, placebo-controlled study. JEADV. 36(10). 806-808. Available at: https://doi.org/10.1111/jdv.18285 ↑5 American Hair Loss Association. (no date). Hair Loss Treatments Online: What to Know About Compounded Medications. Available at: https://www.americanhairloss.org/hair-loss-treatments-online-what-to-know-about-compounded-medications-from-telemedicine-providers/#:~:text=One%20of%20the%20primary%20concerns,in%20outcomes%20and%20side%20effects. (Accessed: July 2025) ↑6 Mir-Bonage, J.F., Piquero-Casals, J., Prudkin, L., Delgado, J., Martinez, J.S., Briones, V.G-P. (2024). Use of Topical Corticosteroids in the Treatment of Noninfectious Inflammatory Dermatoses of the Scalp: A Survey of Practicing Dermatologists and Dermatology Residents Using Delphi Methodology. Clinical, Cosmetic and Investigational Dermatology. 17. 671-681. Available at: https://doi.org/10.2147/CCID.S448016 ↑7 Feder, J. (2004). Legal Issues Related to Prescription Drug Sales on the Internet. CRS Report for Congress. Available at: https://www.everycrsreport.com/files/20050524_RS21711_b88764b8707fe38c86bc35ca438e383c1b84ef69.pdf (Accessed: July 2025) ↑8 u/motorcuadam (2023). Hemiacosmetics: Are they legit? Reddit. Available at: http://reddit.com/r/Hairloss/comments/12i4dlm/hemiacosmetics_are_they_legit/#:~:text=TL%3BDR%3A%20I%20bought%20hair%20products,side%20effects%20using%20their%20products (Accessed: July 2025) Topical minoxidil is one of the most widely used treatments for hair loss, known for its accessibility, relatively low risk profile, and ability to slow or partially reverse androgenetic alopecia in both men and women. First developed as a blood pressure medication, it gained FDA approval in the 1980s after researchers observed its unexpected side effect, stimulating hair growth when applied to the scalp.
In this article, we examine the mechanisms of action, formulation differences, dosing strategies, long-term efficacy, side effects, and methods to enhance results through combination therapies, such as microneedling or retinoids. Whether you’re just starting your hair restoration journey or reassessing your regimen, this guide provides an evidence-based foundation to help you make informed decisions.
Key Takeaways
- What is it? Topical minoxidil is an over-the-counter, FDA-approved liquid or foam. It is usually supplied as 2% or 5% as standard (although it can be offered at higher concentrations). It acts locally and is converted to minoxidil sulfate by scalp SULT1A1.
- Clinical Data. Topical minoxidil has been demonstrated in multiple randomized controlled trials to increase hair count, prolong the anagen phase, and enhance patient satisfaction in the short term, particularly at a 5% concentration. However, long-term data reveal diminishing efficacy over time, with many users reporting waning results or plateauing benefits after 12–18 months, particularly when used without adjunctive therapies like microneedling or anti-androgens.
- Safety. Topical minoxidil is generally safe, with a low incidence of systemic side effects due to minimal absorption. The most common adverse reactions are local, including scalp irritation, itching, and flaking, which are primarily attributed to the presence of propylene glycol in liquid formulations. More severe side effects like hypertrichosis or cardiovascular symptoms are rare and typically linked to misuse or high-sensitivity individuals. It is highly toxic to cats and should be handled with care by pet owners.
- Evidence Quality. Topical minoxidil scored 97/100 for evidence quality by our metrics.
- Best Practices. Apply topical minoxidil to a dry scalp once or twice daily, using 1 mL of solution or half a capful of foam per session. Gently massage to enhance penetration and avoid washing the scalp for at least two hours after application. Foam formulations are often better tolerated due to their lack of propylene glycol. For enhanced results, consider combining this treatment with microneedling, topical retinoids to boost SULT1A1 activity, or anti-androgenic therapies to counteract ongoing miniaturization.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Topical Minoxidil?
Topical minoxidil formulations were originally developed in the early 1980s after an interesting side effect was discovered when it was used as an oral hypertensive agent. This side effect was hypertrichosis, or increased hair growth. The U.S. FDA approved topical minoxidil for male pattern hair loss in 1988 and for female pattern hair loss in 1991.[1]Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading
Minoxidil is a pro-drug: it requires enzymatic activation to exert its pharmacological effect on hair follicles. The key enzyme involved in this is sulfotransferase SULT1A1, which is highly expressed in the outer root sheath (ORS) of hair follicles.[2]Bacqueville, D., Jacques, C., Duprat, L., Jamin, E.L., Guiraud, B., Perdu, E., Bessou-Touya, S., Zalko, D., Duplan, H. (2017). Characterization of xenobiotic metabolizing enzymes of a reconstructed … Continue reading The level of SULT1A1 activity in the hair follicle correlates strongly with clinical response to topical minoxidil; individuals with low SULT1A1 activity are less likely to benefit from treatment.[3]Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a … Continue reading
Topical minoxidil typically comes in two main formulations:
- Solution: The original and most widely used form. Some use carrier agents like propylene glycol to improve solubility and scalp penetration, but it is a common cause of local irritation (itching, redness, flaking).[4]Epstein, G.K., Epstein, J., Cohen, J. (2019). Hair Loss in Men and Women: Medical and Surgical Therapies. 2(1). 161-176. Available at: https://doi.org/10.1016/j.yacs.2019.02.006
- Foam: Developed to address PG sensitivity. The foam formulation is propylene glycol-free, reducing the risk of irritation and allergic reactions. It is easier to apply, dries quickly, and is FDA-approved at 5% strength.[5]Lupatini, R., Sidhu, R., Patel, H., Bichar, K. (2021). Stability Evaluation of Minoxidil in FOAMIL Foam Base with Bracketing Study Design. International Journal of Pharmaceutical Compounding. 25(3) … Continue reading
Some options also offer propylene glycol-free solutions and gels. Some newer products use water-based or liposomal vehicles to cater to users with sensitive skin or allergies.
Why Might I Consider Topical Minoxidil Over Oral?
Many people opt for topical minoxidil over oral formulations due to key differences in safety, accessibility, and side effect profiles.
Over-the-Counter Access & fewer Barriers
Topical minoxidil is available over the counter, making it easy to obtain and start treatment promptly. Oral minoxidil, however, requires a prescription and is typically used off-label for hair loss, which can delay access and introduce hurdles within the healthcare system.
Minimal Systemic Exposure
Applying minoxidil directly to the scalp results in minimal absorption into the bloodstream. This localized delivery reduces the risk of systemic side effects that are more common with oral minoxidil, which circulates throughout the body.[6]Ashique, S., Sandhu, N.K., Haque, S.N., Koley, K. (2020). A Systemic Review on Topical Marketed Formulations, Natural Products, and Oral Supplements to Prevent Androgenic Alopecia: A Review. Natural … Continue reading
Lower Risk of Hypertrichosis, Edema, and Cardiac Events
Oral minoxidil demonstrates a high incidence of hypertrichosis, whereas in topical minoxidil, it is usually localized and linked to misuse.[7]Jiminez-Cauhe, J., Sicco, K.I.L., Shapiro, J., Hermosa-Gelbard, A., Burgos-Blasco, P., Melian-Olivera, A., Ortega-Quijano, D., Pindado-Ortega, C., Buendia-Castano, D., Asz-Sigall, D., Vano-Galvan, S. … Continue reading
Similarly, edema (fluid retention/swelling) and cardiac side effects (such as tachycardia, pericardial effusion, and exacerbation of angina) are rare with topical use but do have the potential to cause issues for those with preexisting cardiovascular, renal, or hepatic conditions.[8]do Nascimento, I.J.B., Harries, M., Rocha, V.B., Thompson, J.Y., Wong, C.H., Varkaneh, H.K., Guimaraes, N.S., Arantes, A. J. R., Marcolini, M.S. (2020). Effect of Oral Minoxidil for Alopecia: … Continue reading
How Does Topical Minoxidil Work?
Topical minoxidil stimulates hair growth through a variety of interconnected biological mechanisms, many of which have been elucidated in peer-reviewed research.
Local Vasodilation and VEGF Up-Regulation
Minoxidil acts as a potent vasodilator by opening potassium channels in vascular smooth muscle, leading to increased blood flow around hair follicles. This enhanced microcirculation delivers more oxygen and nutrients to the follicular environment, creating conditions favorable for hair growth.
Additionally, minoxidil upregulates the expression of vascular endothelial growth factor (VEGF) in dermal papilla cells. VEGF is a key mediator of angiogenesis, supporting the development and maintenance of the follicular blood supply necessary for robust hair growth.[9]Zeltzer, A.A., Keren, A., Paus, R., Gilhar, A. (2024). Topical minoxidil rejuvenates hair follicles from men with androgenetic alopecia in vivo. Acta Dermato Venereologica. 104(24213). Available at: … Continue reading
Anagen Induction and Telogen Shortening
One of the hallmark effects of topical minoxidil is its ability to induce the anagen (growth) phase of the hair cycle and shorten the telogen (resting) phase.[10]Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study … Continue reading By prompting resting hair follicles to re-enter the growth phase more rapidly, minoxidil effectively “resets” the hair cycle, leading to increased hair density and thickness over time. This is a central reason for its clinical efficacy in treating AGA and other hair loss disorders.
Wnt/ꞵ-Catenin Activation in Dermal Papilla
Recent studies have shown that minoxidil activates the Wnt/ꞵ-catenin signaling pathway within dermal papilla cells. This pathway is essential for hair follicle development, regeneration, and maintenance. Activation of ꞵ-catenin dermal papilla cells prolongs the anagen phase and supports the proliferation and differentiation of follicular cells, further enhancing hair growth.[11]Kwack, M.H., Kang, B.M., Kim, M.K., Kim, J.C., Sung, Y.K. (2011). Minoxidil activates ꞵ-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. … Continue reading
Prostaglandin Modulation (PGE2)
Minoxidil has been found to increase the production of prostaglandin E2 (PGE2) by activating prostaglandin synthase-1 (PGHS-1) in dermal papilla fibroblasts. Elevated PGE2 levels are associated with hair growth promotion, possibly by providing cytoprotective effects and modulating local inflammation within the follicle environment.[12]Michelet, J.F., Commo, S., Billoni, N., Mahe, Y.F., Bernard, B.A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating … Continue reading This prostaglandin modulation may also counteract the inhibitory effects of certain nonsteroidal anti-inflammatory drugs (NSAIDs).
Penetration Factors: Vehicle, Skin Barrier, and Enzymes
The effectiveness of topical minoxidil depends significantly on its ability to penetrate the scalp and reach the hair follicle. Only about 1.4% of applied minoxidil is typically absorbed through intact skin.[13]Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: … Continue reading
The formulation’s vehicle (e.g., alcohol-based solution, foam, or novel delivery systems like cetosomes), the integrity of the skin barrier (stratum corneum), and the presence of activating enzymes (notably sulfotransferase SULT1A1 in the outer root sheath) all influence absorption and efficacy.[14]Sattur, S. Talathi, A., Shetty, G., Arsiwala, S., Pereira, R., Dhoot, D. (2023). Comparative Clinical Study Evaluating the Efficacy and Safety of Topical 5% Cetosomal Minoxidil and Topical 5% … Continue reading
Dosing and Application Logistics
Once vs Twice Daily – What the Evidence Shows
Clinical studies have demonstrated that once-daily application of 5% topical minoxidil achieves hair count improvements comparable to twice-daily use of the 2% solution in men with AGA.[15]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading In long-term studies, men who switched from twice daily to once-daily minoxidil maintained most of their hair gains, though there was a slightly greater mean loss in those on the once-daily regimen compared to those who remained on twice-daily dosing.[16]Olsen, E.A., DeLong, E.R., Weiner, M.S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. Journal of the American Academy of Dermatology. 16(3 Pt 2). … Continue reading
Importantly, using 5% minoxidil twice daily can provide an incremental benefit, approximately 10-15% greater hair regrowth, over once-daily 5% application or twice-daily 2% solution, though this comes with a higher risk of local irritation.[17]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
For women with FPHL, randomized clinical trials have shown that once-daily application of 5% minoxidil foam is non-inferior to twice-daily use of the 2% minoxidil solution in terms of hair regrowth and target area hair counts.[18]Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the … Continue reading Both regimens lead to similar improvements, but the once-daily 5% foam offers a significant compliance advantage, as it is easier to incorporate into daily routines and is associated with fewer reports of scalp irritation.[19]Ramos, P.M., Melo, D.F., Radwanski, H., de Almeida, R.F.C., Miot, H.A. (2023). Female-pattern hair loss: therapeutic update. Anais Brasileiros de Dermatologica. 98(4). 506-519. Available at: … Continue reading This practical benefit makes once-daily 5% foam a preferred initial therapy for many women.
Choosing a Topical Minoxidil Concentration
Selecting the right minoxidil concentration depends on your goals, tolerance, and clinical context.
2% Minoxidil
- Profile: Traditionally labeled for women, especially those with sensitive scalps or mild hair loss.
- Efficacy: Offers modest improvement in hair counts and density; superior to the placebo but less effective than higher strengths.[20]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
- Tolerability: Minimal irritation and side effects; preferred for those prone to dermatitis or with a low threshold for adverse reactions.
- Best for: Mild AGA, sensitive skin, or those who prioritize safety over maximal regrowth.
5% Minoxidil
- Profile: The most widely studied and FDA-approved concentration for both men and women.
- Efficacy: Consistently outperforms 2% for both hair counts and patient satisfaction; considered the best balance between response rate and tolerability.[21]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
- Tolerability: Slightly higher risk of local irritation (itching, redness, flaking) than 2%, but generally well-tolerated, especially in foam (propylene glycol-free) formulations.
- Best for: Most patients with AGA seeking robust, evidence-based results.
7% Minoxidil
- Profile: Custom-compounded, not commercially available or FDA-approved; sometimes used for those with low follicular sulfotransferase activity (“enzyme low-responders”).
- Efficacy: May offer incremental benefit for select non-responders to 5%, though supporting data are limited and mixed.[22]Singh, S., Patil, A., Kianfar, N., Waskiel-Burnat, A., Rudnicka, L., Sinclair, R., Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit? … Continue reading
- Tolerability: Higher risk of contact dermatitis due to increased propylene glycol and alcohol content; requires careful monitoring for irritation.
- Best for: Patients who do not respond to 5% and have confirmed low enzyme activity, under clinical supervision.
10-15% Minoxidil
- Profile: Compounded, not FDA-approved; considered a last-line topical option before transitioning to oral minoxidil.
- Efficacy: Evidence is conflicting; some studies suggest no added benefit over 5%, while others report modest gains in select cases. Side effects increase substantially with higher concentrations.[23]Vasantha, K.L. (2025). Efficiency and Safety of 10% Minoxidil in the Treatment of Alopecia Areata: A Randomised Controlled Trial. Journal of Population Therapeutics and Clinical Pharmacology. 32(4). … Continue reading,[24]Ghonemy, S., Alarawi, A., Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic … Continue reading
- Risks: Marked increase in scalp irritation, dermatitis, and cost. Formulations typically require more propylene glycol and alcohol for solubility, compounding the risk of adverse reactions.
- Best for: Refractory cases under specialist care, when all lower strengths have failed, and before considering oral therapy.
Vehicle and Formulation Tweaks
There are a number of options you can take in terms of formulation or additional treatments to improve the efficacy of topical minoxidil.
Foam vs. Liquid: Propylene Glycol, Irritation, and Drying Time
Liquid minoxidil formulations can contain propylene glycol, which improves drug solubility and skin penetration.[25]Grice, J.E., Ciotti, S., Weiner, N., Lockwood, P., Cross, S.E., Roberts, M.S. (2010). Relative uptake of minoxidil into appendages and stratum corneum and permeation through human skin in vitro. … Continue reading However, propylene glycol is a frequent cause of scalp irritation; itching, redness, and flaking are common complaints, especially among users with sensitive skin.[26]Patel, K., Palmer, A., Nixon, R. (2023). Allergic contact dermatitis from propylene glycol: A case series from Australia. Contact Dermatitis. 89(2). 79-84. Available at: … Continue reading
Foam minoxidil was specifically developed to avoid propylene glycol usage and minimize irritation. As a result, foam is generally much better tolerated, especially for individuals prone to dermatitis or scalp discomfort.[27]Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of … Continue reading Additionally, the foam dries faster than the traditional liquid, making it more convenient for daily use.
Efficacy: Most comparative studies and clinical experience suggest the 5% foam and 2% liquid are equivalent in hair regrowth when used appropriately; the choice often comes down to scalp tolerance and application preference.[28]Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once-daily versus 2% minoxidil solution twice daily in the … Continue reading
Propylene Glycol Free, Fast-Drying, Glycerin-Based Recipes.
For patients unable to tolerate propylene glycol or those seeking even less greasy, faster-drying options, newer propylene glycol-free formulations have been developed. These often use other solvents or humectants, with in vitro and clinical studies showing similar efficacy to products containing propylene glycol.[29]Barbareschi, M., Vescovi, V., Starace, M., Piraccini, B.M., Milani, M. (2020). Propylene glycol free 5% minoxidil lotion formulation: cosmetic acceptability, local tolerability, clinical efficacy and … Continue reading
Addition of Retinol/Tretinoin (0.01%-0.025%) to Up-Regulate SULT1A1
As mentioned above, SULT1A1 is the key enzyme in the hair follicle that activates minoxidil by converting it to minoxidil sulfate, its effective form. Retinoids, notably tretinoin (0.01-0.025%), can be added to topical regimens to upregulate SULT1A1 in the outer root sheath, enhancing the local activation of minoxidil.[30]Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvin, S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in … Continue reading
The mechanism appears to involve both increased skin permeability and direct modulation of SULT1A1 gene expression, making this combination particularly useful for patients with known or suspected low enzyme activity.
Topical Minoxidil Efficacy
When assessing the benefits of topical minoxidil, it’s critical to consider how response rates, regrowth rates, and especially months of use influence real-world outcomes for individuals with AGA. While many early studies highlight minoxidil’s promise, longer-term data reveal a more nuanced reality.
Short-Term Response
Within 3-6 months, minoxidil demonstrates high response rates. Surveys and clinical studies consistently show that roughly 60% of men perceive a meaningful response (slowing, stopping, or reversal of hair loss) in this window (as per self-assessment and investigator evaluations).[31]Asilian, A., Farmani, A., Saber, M. (2023). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: A randomized controlled trial. … Continue reading
Long-Term Response
After one year or longer, response rates drop dramatically. Multiple studies report rates below 30%, and even lower in five-year follow-up populations, with only about 20-30% of users satisfied with the results.[32]Olsen, E.A., Weiner, M.S., Amara, I.A., DeLong, E.R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. Journal of American Academy of Dermatology. 22(4). … Continue reading
Real-world data indicate discontinuation rates of 86–95% by the one-year mark. The leading reason cited by users for stopping? “Low effect,” or disappointment with the cosmetic improvement offered by the drug.[33]Shadi, Z. (2023) Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy. 13(5). 1157-1169. Available at: … Continue reading
Regrowth Rate: Short-Lived Gains
In one pivotal trial, men using 5% minoxidil twice daily saw a nearly 60% increase in hair “weight” (total mass of regrown/thickened hair) at 6 months. By 96 weeks, gains had diminished to just 25% above the baseline, a significant drop from the initial response.[34]Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study … Continue reading
Over 48 weeks, users displayed a 12% rise in hair count compared with 3% for placebo (statistically significant). Still, these increases in hair number did not always correspond to better visible scalp coverage when judged by experts, indicating that raw hair counts may overstate clinical benefit.
Why Does Efficacy Diminish With Time?
Lack of DHT Targeting and Ongoing Miniaturization
Minoxidil’s primary action is to stimulate hair follicles into a growth (anagen) phase; however, it does not block the effects of DHT, the androgen responsible for follicle miniaturization in genetic hair loss.[35][Updated 2023 Feb 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482378/ Accessed: July 2025 Therefore, hair shafts may still become thinner despite greater numbers, diminishing real-world cosmetic impact.
Fibrosis: The Hidden Limitation
Emerging evidence points to fibrosis, the gradual formation of restrictive, scar-like tissue around miniaturizing follicles, as a rate-limiting factor in AGA.[36]Trueb, R.M., Dias, M.F.R.G., Rezende, H.D. (2021). Comment on Follicular Inflammation and Fibrosis in Pattern Hair Loss. Skin Appendage Disorders. 7(2). 159-160. Available at: … Continue reading Minoxidil does not directly address this fibrotic process, so even if follicles are nudged back into growth, their ability to produce robust hair continues to decline as surrounding tissue stiffens and shrinks.
The Limits of Hair Count as a Metric
Statistical increases in hair count do not guarantee clinical satisfaction. Studies repeatedly show that even impressive numeric gains in hair counts (or surrogate measures, such as hair mass index) are not always matched by noticeable differences in scalp coverage or user satisfaction.[37]Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study … Continue reading Patient dissatisfaction and discontinuation remain high in the absence of true visual improvement, underscoring the practical limits of relying on surrogate endpoints.
Combining Minoxidil with Microneedling
Recent clinical trials have shown that adding microneedling to minoxidil treatment can significantly enhance both response and regrowth rates. One landmark study reported a nearly fourfold greater increase in hair count (approximately a 40% gain) compared to minoxidil alone at 12 weeks, with participants experiencing actual visible improvements in density.[38]Ahmed, K.M.A., Kozaa, Y.A., Abuawwad, M.T., Al-Najdawi, A.I.A., Mahmoud, Y.W., Ahmed, A.M., Taha, M.J.J., Fadhli, T., Giannopoulou, A. (2025). Evaluating the efficacy and safety of combined … Continue reading
A six-month trial showed an 85% effective rate and a significant increase in hair count for the combination approach.[39]Zhang, Y., Sheng, Y., Zeng, Y., Hu, R., Zhao, J., Wang, W., Yang, Q. (2022). Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair … Continue reading
Figure 1: A 35-year-old female patient with 5 years of FPHL in group 2 (combination treatment) at baseline and endline.[40]Zhang, Y., Sheng, Y., Zeng, Y., Hu, R., Zhao, J., Wang, W., Yang, Q. (2022). Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair … Continue reading
Why Does Microneedling Work So Well?
- Improved drug absorption: Microneedling transiently disrupts the scalp’s barrier, allowing minoxidil to penetrate more effectively.
- Enzyme upregulation: The procedure may increase the local activity of sulfotransferase enzymes needed for minoxidil activation, potentially overcoming a key bottleneck for some non-responders.[41]Sharma, A., Surve, R., Dhurat, R., Sinclair, R., Tan, T., Zou, Y., Ramos, M.P., Wambier, C., Vernier, I., Kovacevic, M., Goren, A. (2020). Microneedling improves minoxidil response in androgenetic … Continue reading
- Counteracting fibrosis: Early research suggests that microneedling may help remodel fibrotic tissue, making the scalp environment more conducive to robust hair regrowth.
Non-Responder Pathway (SULT1A1-Guided)
For people who don’t respond adequately to topical minoxidil, several strategies can help overcome this resistance and optimize hair growth.
Increase Topical Minoxidil Concentration (7-10%)
Raising the concentration of topical minoxidil beyond 5%, to compounded strengths such as 7% or 10%, is a common clinical approach for patients identified as poor responders. The rationale is that higher drug levels may compensate, at least partially, for suboptimal enzymatic conversion of minoxidil to its active sulfate form in the outer root sheath.
Add Topical Retinoic Acid (0.01-0.05%)
Topical retinoic acid (such as tretinoin) can be combined with minoxidil to upregulate SULT1A1 activity, thereby enhancing the local activation of minoxidil in the hair follicle. Peer-reviewed research indicates that applying topical tretinoin upregulates follicle sulfotransferase enzymes, thereby enhancing the response to minoxidil.[42]Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvan, S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in … Continue reading
Incorporate Weekly Microneedling (0.5-1.5 mm)
Microneedling is an increasingly popular adjunctive treatment for hair loss, particularly in individuals who do not respond to minoxidil. Weekly sessions using devices with needles 0.5–1.5 mm in length create controlled micro-injuries in the scalp, stimulating growth factors, activating dermal papilla cell signaling (such as Wnt/β-catenin), and boosting SULT1A1 enzyme expression.[43]Dhurat, R., Sukesh, M.S., Avhad, G., Dandale, A., Pal, A., Pund, P. (2013). A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study. International … Continue reading,[44]Kumar, K.M., Inamadar, A.C., Palit, A. (2018). A Randomized Controlled, Single-Observer Blinded Study to Determine the Efficacy of Topical Minoxidil plus Microneedling versus Topical Minoxidil Alone … Continue reading,[45]Zhang, Y., Sheng, Y., Zeng, Y., Hu, R., Zhao, J., Wang, W., Yang, Q. (2022). Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair … Continue reading
Switch to or Add Low-Dose Oral Minoxidil
Low-dose oral minoxidil, typically 0.25–5 mg daily, offers systemic activation, as minoxidil is converted to its active sulfate in the liver (where SULT1A1 levels are abundant).[46]Ramirez-Marin, H.A., Tosti, A. (2022). Role of Oral Minoxidil in Patterned Hair Loss. Indian Dermatology Online Journal. 13(6). 729-733. Available at: https://doi.org/10.4103/idoj.idoj_246_22 For individuals whose follicular SULT1A1 is insufficient for topical efficacy, oral minoxidil can bypass the need for high local enzyme activity. Recent comprehensive reviews and clinical experience suggest that low-dose oral minoxidil is effective for hair regrowth in male and female pattern hair loss, with a favorable safety profile when started at low dosages.[47]Gupta, A.K., Talukder, M., Shemer, A., Piraccini, B.M., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: … Continue reading
Explore Compounded Minoxidil Sulfate (Direct Active Form)
Compounding pharmacies can prepare topical formulations containing minoxidil sulfate, the direct, active metabolite, bypassing the need for SULT1A1 conversion. Though theoretically attractive for SULT1A1 nonresponders, minoxidil sulfate is chemically less stable than parent minoxidil and is also more expensive.[48]Chemical Book. (no date). Minoxidil Sulphate. ChemBook. Available at: https://www.chemicalbook.com/Price/Minoxidil-sulphate.htm Accessed: July 2025,[49]Shan, Y., Xu, C., Guo, Y., Wen, L., Zhou, S., Fang, L., Xu, J., Zhen, H. (2025). Liposomes enhance the hair follicle delivery of minoxidil sulfate with improved treatment of androgenetic alopecia. … Continue reading Its shelf-life, risk of degradation, and limited availability currently constrain its widespread clinical use. Efficacy and safety data are lacking beyond case reports and experimental settings, and this approach remains investigational.
What To Expect Time-Line Wise
Understanding the typical milestones and changes during minoxidil treatment helps set realistic expectations, avoids premature discontinuation, and guides regimen adjustments for optimal hair regrowth. Here’s a week-by-week and month-by-month breakdown based on clinical evidence and peer-reviewed research.
Weeks 4-6: The Shedding Spike (“Telogen Synch”)
What happens: Many users notice an initial increase in hair shedding after starting minoxidil, usually peaking around weeks 4–6.
Why: This effect, known as telogen effluvium, occurs as minoxidil accelerates the transition of hair follicles from the resting (telogen) to the active (anagen) growth phase. Older, miniaturized hairs are shed to make way for new ones.
What to do: This shedding is temporary and generally indicates that the treatment is starting to take effect. Stopping minoxidil because of early shedding is unnecessary and may forfeit later gains.
Month 3: Early Vellus Thickening and the First Cosmetic Hints
What happens: Fine, soft “vellus” hairs begin to thicken. With combination treatments (such as minoxidil plus microneedling), these changes may be more pronounced and start to become cosmetically noticeable.
Why: Follicles kickstart new anagen cycles, and some can already be seen transitioning to more pigmented, visible hairs.[50]Rafi, A.W., Katz, R.M. (2011). Pilot study of 15 patients receiving a new treatment regimen for androgenic alopecia: the effects of atopy on AGA. ISRN Dermatology. 11. 241953. Available at: … Continue reading
Expectations: While cosmetic gains are modest at this stage using monotherapy, up to 60–74% of users report improvement in density and coverage when asked at 3–4 months.[51]Rundegren, J. (2004). Rapid onset of action of minoxidil 5% topical solution in a 4-month German observational study on both patients and physicians. Journal of the American Academy of Dermatology. … Continue reading
Months 6 – 8: Peak Visible Gains (Most Noticeable Results) and Plateau
What happens: The majority of the visible thickening and coverage improvements emerge by 6-8 months. Around this time, many people also see gains plateau and further visible regrowth slows considerably
Clinical evidence: At this point, studies show peak increases in terminal hair count and density (e.g., a 12–15% rise for standard 5% twice-daily solutions in men).[52]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
What to do: Regimen reevaluation is appropriate here; ensure ongoing compliance, assess satisfaction, and consider any adjuncts or boosters if progress is suboptimal. If additional improvement is desired, this is the window to explore adjunctive approaches, such as adding microneedling, retinoids, or anti-androgen therapies, to enhance or sustain growth.
Month 9 +: Gradual Waning Without Additional Support
What happens: Many users see diminishing improvements after 9 months; some may even experience slow retreating benefits despite continued use.
Why: Minoxidil alone does not address the underlying drivers of androgenetic alopecia, namely, DHT-mediated follicle miniaturization and progressive perifollicular fibrosis. Without combining minoxidil with anti-DHT agents or regenerative wounding therapies, natural progression often limits sustained gains.
Long-term evidence: Only ~30% of users report being satisfied after several years; discontinuation is common, with the lack of a visible effect being the primary reason.[53]Shadi, Z. (2023) Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy. 13(5). 1157-1169. Available at: … Continue reading
Safety and Side Effects
Dermatitis (2-6%): Mainly Propylene Glycol
- Incidence: Irritant or allergic contact dermatitis occurs in ~2-6% of users.
- Primary Cause: Most cases are induced by propylene glycol, a key solvent in liquid minoxidil solutions, rather than by minoxidil itself.[54]Jadeed, H.B., Almudimeegh, A.M., Alomran, S.A., Alshathry, A.H. (2021). A Case of Contact Allergic Dermatitis to Topical Minoxidil. Cureus. 13(1). E12510. Available at: … Continue reading
- Symptoms: Itching, redness, scaling, and sometimes localized swelling.
- Management: Switching to propylene glycol-free foam formulations or compounded solutions using alternative solvents (like glycerol or butylene glycol) can resolve symptoms for sensitive users. Those with confirmed allergy to minoxidil itself should avoid all forms.
Scalp Dryness and Flaking
- Presentation: Dryness and flaking of the scalp are common, primarily due to the alcohol and propylene glycol content in the formulation.
- Management:
- Use gentle, sulfate-free shampoos.
- Prefer shorter contact times and allow the product to dry completely.
- Consider propylene glycol or alcohol-free versions when persistent dryness occurs.
Facial Hypertrichosis
- Incidence: Mild, reversible facial hair growth (hypertrichosis) may develop, especially with high doses or accidental application beyond the intended area, such as the scalp.
- Course: Usually appears within 2-5 months of starting minoxidil and typically resolves within one to five months after stopping or reducing use.
- Mechanism: Thought to result from inadvertent transfer or high follicular sensitivity, not systemic toxicity.[55]Chellini, P.R., Pirmez, R., Raso, P., Sodre, C.T. (2015). Generalized hypertrichosis induced by topical minoxidil in an adult woman. International Journal of Trichology. 7(4). 182-183. Available at: … Continue reading
Toxicity to cats (prevalence: low if you’re careful; high if you’re reckless)
- Cats lack the enzyme required to metabolize minoxidil, much like dogs lack the enzymes required to quickly metabolize substances within chocolate. Resultantly, minoxidil is highly toxic to cats.[56]DeClementi, C., Bailey, K.L., Goldstein, S.C., Orser, M.S. (2004). Suspected toxicosis after topical administration of minoxidil in 2 cats. Veterinary Emergency & Critical Care. 14(4). 287-292. … Continue reading From 2001 to 2014, there were four reported feline deaths from minoxidil exposure.
- This doesn’t mean that if you have cats, you can’t use minoxidil. It simply means that you need to take extra precautions during storage, application, and drying, and ensure your cats don’t come into contact with the drug (even through their fur).
Heart palpitations (prevalence: very low)
- There is some systemic absorption from the use of topical minoxidil. As such, a small percentage of users have reported blood pressure-related effects ranging from dizziness to heart palpitations.[57]Leenen, F.H., Smith, D.L., Unger, W.P. (1988). Topical minoxidil: cardiac effects in bald man. British Journal of Clinical Pharmacology. 26(4). 481-485. Available at: … Continue reading
Stopping Topical Minoxidil
Discontinuing topical minoxidil is a significant decision, as virtually all clinical evidence and real-world experience indicate that hair gains made during treatment will be lost, usually within 3 to 12 months. This process is driven by the underlying physiology of follicles and the progressive nature of AGA.
The Physiology Behind Minoxidil Withdrawal
- Follicular Cycling: When minoxidil is stopped, hair follicles revert to their pre-treatment anagen (growth phase) timing within approximately three months. This leads to a synchronized shedding phase and a temporary drop in hair density, often falling below pre-treatment baseline before returning to the genetic trajectory over the subsequent months.
- Rebound Shedding: After ceasing minoxidil, the majority of users experience significant shedding, which can sometimes be more aggressive than before they began treatment. Typically, this shedding lasts for 3–6 months, but can persist for up to a year in some cases. The extent of the shed varies from person to person, but those who respond best to the drug are usually most affected, simply because they maintain or regrow more hair than they lose.
Evidence in Clinical Studies
One 96-week study on both 2% and 5% topical minoxidil found that after treatment was halted, hair counts and weight fell below both baseline and placebo levels within 3 months.[58]Price, V.H., Menefee, E., Strauss, P.C. (1999). Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. … Continue reading By six months post-withdrawal, most subjects’ hair counts rebounded to baseline, indicating the temporary and reversible effects of minoxidil therapy in AGA.
Figure 2: Comparison of mean percentage change in interval weight per square centimeter for 4 treatment groups. Vertical line at 96 weeks marks cessation of treatment.[59]Price, V.H., Menefee, E., Strauss, P.C. (1999). Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. … Continue reading
Tapering Off: Protocol to Blunt Rebound Shedding
Abruptly ceasing minoxidil use (“cold turkey”) tends to induce more severe and acute shedding. To mitigate this shock to the hair cycle and scalp, expert opinion and clinical guidelines recommend a gradual taper, similar to protocols used for oral minoxidil or other hair loss medications.
A 6-month Tapering Schedule could look like this.
Weeks Protocol Description 1-2 Alternate once-daily and twice-daily applications 3-4 Three days once-daily, one day twice-daily 5-6 Once daily, Monday-Friday, twice on weekends 7-9 Once daily 10-12 Once daily on weekdays, off weekends 13-15 Once daily, Monday-Thursday, off Friday-Sunday 16-18 Apply every other day 19 Every third day 20 Every fourth day 21 Every fifth day 22 Every sixth day 23 Once weekly, then discontinue Bridging Strategies During the Transition
No protocol can eliminate post-minoxidil shedding, but supporting the scalp with additional therapies may enhance retention and cushion the transition.
- Microneedling: Weekly sessions (with or without ongoing minoxidil) can upregulate growth factors and SULT1A1, thereby enhancing follicle health, and have been shown to help some users retain density for several months after discontinuing minoxidil.
- Anti-Androgens: Initiating topical or oral anti-androgens (e.g., finasteride, spironolactone) before and during withdrawal can help offset DHT-driven miniaturization—a process that is not alleviated by minoxidil alone.
- Low-Level Laser Therapy (LLLT): LLLT devices supply scalp stimulation and may decrease shedding when minoxidil is reduced or stopped.
- Alternative Topicals: Some transitioners try diluted rosemary oil (2–5%) or similar topicals for additional support, though strong clinical evidence for these alternatives is limited.
You can read our article on what happens when you quit taking minoxidil here.
Best Practices
- Apply only to a dry scalp: Before treatment, ensure both hair and scalp are completely dry. This maximizes absorption and efficacy.
- Dosage: For solution, use exactly 1 mL (measure with the supplied dropper); for foam, use half a capful per application.
- Massage in: Gently massage the product into thinning areas for approximately 30 seconds to improve penetration.
- Wait before washing: Allow at least 2 hours after application before shampooing or rinsing your scalp. This ensures adequate drug absorption.
Who Is an Ideal Candidate for Topical Minoxidil?
You’re a good candidate if you are:
- An adult man or woman with AGA: This is the main, FDA-approved indication for topical minoxidil. It has the strongest evidence in these groups.
- Able to commit to at least 6 months of consistent use: Appreciable improvements are most common with twice-daily application and become noticeable after 3–6 months. Stopping before this period makes it hard to judge the benefit.
- Ready for ongoing, indefinite use: All gains from minoxidil are lost within 3–6 months of discontinuation, so lifelong adherence is necessary for persistent effects.
- Prepared for manageable, mostly mild side effects: Most users tolerate minoxidil well, but 2–6% experience skin irritation, mainly from propylene glycol in liquid formulas. Switching to propylene glycol-free foams or other vehicle-adjusted products usually solves these issues.
- Willing to pair with additional therapies (optional, but recommended): Combining minoxidil with weekly microneedling or oral/topical finasteride increases efficacy by two- to four-fold, with up to 70%+ response rates and greater visual gains. Microneedling notably enhances regrowth and can prolong the effectiveness of minoxidil.
- Financially able to sustain $15–$30/month long-term: Over the years of use, the accumulated cost is a practical consideration.
- Comfortable following a simple scalp-care routine: Best practices include applying the product to a dry scalp, massaging for at least 30 seconds, allowing it to absorb for 2 hours, and treating any scalp inflammation first with anti-inflammatory shampoos (like ketoconazole or zinc pyrithione).
Who May Want to Reconsider?
- Catowners unable to limit pet exposure: Cats are highly sensitive to minoxidil and can suffer fatal toxicity from even minute exposure. Strict precautions are required to keep pets safe.
- People with severe or persistent skin sensitivity/allergy: If unable to tolerate any formulation or if allergic to both minoxidil and carrier ingredients, other options may be preferable.
- Individuals seeking a permanent or “cure-all” solution: Minoxidil does not address androgenic miniaturization (DHT effect) or scalp fibrosis. On its own, efficacy usually plateaus or wanes, making it mostly a maintenance or adjunct therapy.
- Those seeking visible, dramatic regrowth from monotherapy alone: Statistical hair count increases may not always correspond to better scalp coverage or visually satisfying restoration.
FAQs
Will minoxidil lower my libido? No, minoxidil is not known to lower libido, as it does not affect hormones and has shown no significant link to sexual side effects in clinical studies or post-marketing data. For more information, refer to the following resources: Minoxidil: Getting Started (Interactive Guide).
Can I dilute topical minoxidil without losing its efficacy? You can dilute topical minoxidil or mix it with other topicals, but doing so may reduce its efficacy if it alters absorption, concentration, or stability, so it’s best to either separate applications (morning vs. evening) or use professionally compounded combinations to ensure reliable results. For more information, watch this video.
Can I use topical minoxidil alongside other topicals? Yes, you can use topical minoxidil alongside other topicals, but to avoid interference with absorption, it’s best to either apply them at different times of day (e.g., morning vs. evening) or combine them into a single formulation, ideally through a compounding pharmacy or by carefully preparing them at home following best practices.
How long should I leave minoxidil on my scalp? You should leave minoxidil on your scalp for at least one hour, but ideally for four hours or more, to ensure optimal absorption, especially if you’re applying it only once a day. Most of the drug is absorbed within the first few hours, so extending contact time helps maximize its effectiveness.
Does minoxidil accelerate skin aging? Minoxidil is unlikely to accelerate skin aging; reported changes in facial skin quality are more likely due to mild allergic reactions to propylene glycol or temporary water retention, both of which are typically reversible and manageable with formulation changes or improved application practices.
Final Thoughts
Topical minoxidil remains the cornerstone over‑the‑counter therapy for androgenetic alopecia, valued for its solid safety record, ease of access, and decades of clinical data supporting meaningful, if often modest, improvements in hair density and retention. For many men and women, especially those early in their hair-loss journey or those averse to systemic medications, it offers a practical first line of defense that can be further amplified with adjuncts such as microneedling, retinoids, or anti-androgens.
Yet its benefits are not limitless: results plateau without complementary treatments, lifelong use is required to maintain gains, and irritation or simple user fatigue remain common reasons for discontinuation. Approached realistically, committing to at least six months of consistent, correctly dosed application, monitoring scalp health, and integrating synergistic therapies where appropriate, topical minoxidil can serve as a reliable, evidence‑based pillar in a comprehensive hair‑restoration plan.
References[+]
References ↑1 Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑2 Bacqueville, D., Jacques, C., Duprat, L., Jamin, E.L., Guiraud, B., Perdu, E., Bessou-Touya, S., Zalko, D., Duplan, H. (2017). Characterization of xenobiotic metabolizing enzymes of a reconstructed human epidermal model from adult hair follicles. Toxicology and Applied Pharmacology. 15(329). 190-201. Available at: https://doi.org/10.1016/j.taap.2017.05.040 ↑3 Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. Advances in Dermatology and Allergology. 39(3). 472-478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑4 Epstein, G.K., Epstein, J., Cohen, J. (2019). Hair Loss in Men and Women: Medical and Surgical Therapies. 2(1). 161-176. Available at: https://doi.org/10.1016/j.yacs.2019.02.006 ↑5 Lupatini, R., Sidhu, R., Patel, H., Bichar, K. (2021). Stability Evaluation of Minoxidil in FOAMIL Foam Base with Bracketing Study Design. International Journal of Pharmaceutical Compounding. 25(3) 236-240. Available at: PMID: 34125714 ↑6 Ashique, S., Sandhu, N.K., Haque, S.N., Koley, K. (2020). A Systemic Review on Topical Marketed Formulations, Natural Products, and Oral Supplements to Prevent Androgenic Alopecia: A Review. Natural Products and Bioprospecting. 10(6). 345-365. Available at: https://doi.org/10.1007/s13659-020-00267-9 ↑7 Jiminez-Cauhe, J., Sicco, K.I.L., Shapiro, J., Hermosa-Gelbard, A., Burgos-Blasco, P., Melian-Olivera, A., Ortega-Quijano, D., Pindado-Ortega, C., Buendia-Castano, D., Asz-Sigall, D., Vano-Galvan, S. (2025). Characterization and Management of Adverse Events of Low-Dose Oral Minoxidil Treatment for Alopecia: A Narrative Review. Journal of Clinical Medicine. 14(6). 1805. Available at: https://doi.org/10.3390/jcm14061805 ↑8 do Nascimento, I.J.B., Harries, M., Rocha, V.B., Thompson, J.Y., Wong, C.H., Varkaneh, H.K., Guimaraes, N.S., Arantes, A. J. R., Marcolini, M.S. (2020). Effect of Oral Minoxidil for Alopecia: Systematic Review. International Journal of Trichology. 12(4). 147-155. Available at: https://doi.org/10.4103/ijt.ijt_19_20 ↑9 Zeltzer, A.A., Keren, A., Paus, R., Gilhar, A. (2024). Topical minoxidil rejuvenates hair follicles from men with androgenetic alopecia in vivo. Acta Dermato Venereologica. 104(24213). Available at: https://doi.org/10.2340/actadv.v104.24213 ↑10 Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study protocol. Skin Research and Technology. 26(4). 542-557. Available at: https://doi.org/10.1111/srt.12827 ↑11 Kwack, M.H., Kang, B.M., Kim, M.K., Kim, J.C., Sung, Y.K. (2011). Minoxidil activates ꞵ-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. Journal of Dermatological Science. 154-159. Available at: https://doi.org/10.1016/j.jdermsci.2011.01.013 ↑12 Michelet, J.F., Commo, S., Billoni, N., Mahe, Y.F., Bernard, B.A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. Journal of Investigative Dermatology. 108(2). 205-209. Available at: https://doi.org/10.1111/1523-1747.ep12334249 ↑13 Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: https://doi.org/10.1080/09546634.2021.1945527 ↑14 Sattur, S. Talathi, A., Shetty, G., Arsiwala, S., Pereira, R., Dhoot, D. (2023). Comparative Clinical Study Evaluating the Efficacy and Safety of Topical 5% Cetosomal Minoxidil and Topical 5% Alcohol-Based Minoxidil Solutions for the Treatment of Androgenetic Alopecia in Indian Men. Cureus. 15(10). E46568. Available at: https://doi.org/10.7759/cureus.46568 ↑15, ↑17, ↑20, ↑21, ↑52 Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. Journal of the American Academy of Dermatology. 47(3). 377-385. Available at: https://doi.org/10.1067/mjd.2002.124088 ↑16 Olsen, E.A., DeLong, E.R., Weiner, M.S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. Journal of the American Academy of Dermatology. 16(3 Pt 2). 688-695. Available at: https://doi.org/10.1016/s0190=9622(87)70089-9 ↑18 Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. Journal of the American Academy of Dermatology. 65(6). 1126-1134. Available at: https://doi.org/10.1016/j.jaad.2010.09.724 ↑19 Ramos, P.M., Melo, D.F., Radwanski, H., de Almeida, R.F.C., Miot, H.A. (2023). Female-pattern hair loss: therapeutic update. Anais Brasileiros de Dermatologica. 98(4). 506-519. Available at: https://doi.org/10.1016/j.abd.2022.09.006 ↑22 Singh, S., Patil, A., Kianfar, N., Waskiel-Burnat, A., Rudnicka, L., Sinclair, R., Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit? Clinical and Experimental Dermatology. 47(11). 1951-1955. Available at: https://doi.org/10.1111/ced.15338 ↑23 Vasantha, K.L. (2025). Efficiency and Safety of 10% Minoxidil in the Treatment of Alopecia Areata: A Randomised Controlled Trial. Journal of Population Therapeutics and Clinical Pharmacology. 32(4). 724-730. Available at: https://doi.org/10.53555/56ggpq88 ↑24 Ghonemy, S., Alarawi, A., Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. Journal of Dermatological Treatment. 32(2). 236-241. Available at: https://doi.org/10.1080/09546634.2019.1654070 ↑25 Grice, J.E., Ciotti, S., Weiner, N., Lockwood, P., Cross, S.E., Roberts, M.S. (2010). Relative uptake of minoxidil into appendages and stratum corneum and permeation through human skin in vitro. Journal of Pharmaceutical Science. 99(2). 712-718. Available at: https://doi.org/10.1002/jps.21856 ↑26 Patel, K., Palmer, A., Nixon, R. (2023). Allergic contact dermatitis from propylene glycol: A case series from Australia. Contact Dermatitis. 89(2). 79-84. Available at: https://doi.org/10.1111/cod.14325 ↑27 Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of Cosmetic Dermatology. 20(12). 3759-3781. Available at: https://doi.org/10.1111/jocd.14537 ↑28 Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once-daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. Journal of the American Academy of Dermatology. 65(6). 1126-1134. Available at: https://doi.org/10.1016/j.jaad.2010.09.724 ↑29 Barbareschi, M., Vescovi, V., Starace, M., Piraccini, B.M., Milani, M. (2020). Propylene glycol free 5% minoxidil lotion formulation: cosmetic acceptability, local tolerability, clinical efficacy and in-vitro skin absorption evaluations. Edizioni Minerva Medica. 155(3). 341-345. Available at: https://doi.org/10.23736/S0392-0488.20.06554-2 ↑30 Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvin, S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatologic Therapy. 32(3). 12915. Available at: https://doi.org/10.1111/dth.12915 ↑31 Asilian, A., Farmani, A., Saber, M. (2023). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: A randomized controlled trial. Journal of Cosmetic Dermatology. 23(3). 949-957. Available at: https://doi.org/10.1111/jocd.16086 ↑32 Olsen, E.A., Weiner, M.S., Amara, I.A., DeLong, E.R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. Journal of American Academy of Dermatology. 22(4). 643-646. Available at: https://doi.org/10.1016/0190-9622(90)70089-z ↑33, ↑53 Shadi, Z. (2023) Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy. 13(5). 1157-1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑34, ↑37 Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study protocol. Skin Research & Technology. 26(4). 542-557. Available at: https://doi.org/10.1111/srt.12827 ↑35 [Updated 2023 Feb 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482378/ Accessed: July 2025 ↑36 Trueb, R.M., Dias, M.F.R.G., Rezende, H.D. (2021). Comment on Follicular Inflammation and Fibrosis in Pattern Hair Loss. Skin Appendage Disorders. 7(2). 159-160. Available at: https://doi.org/10.1159/000513089 ↑38 Ahmed, K.M.A., Kozaa, Y.A., Abuawwad, M.T., Al-Najdawi, A.I.A., Mahmoud, Y.W., Ahmed, A.M., Taha, M.J.J., Fadhli, T., Giannopoulou, A. (2025). Evaluating the efficacy and safety of combined microneedling therapy versus topical Minoxidil in androgenetic alopecia: a systematic review and meta-analysis. Archives of Dermatological Research. 317(1). 528. Available at: https://doi.org/10.1007/s00403-025-04032-1 ↑39, ↑40, ↑45 Zhang, Y., Sheng, Y., Zeng, Y., Hu, R., Zhao, J., Wang, W., Yang, Q. (2022). Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair loss in a Chinese population. Journal of Cosmetic Dermatology. 21(12). 6985-6991. Available at: https://doi.org/10.1111/jocd.15424 ↑41 Sharma, A., Surve, R., Dhurat, R., Sinclair, R., Tan, T., Zou, Y., Ramos, M.P., Wambier, C., Vernier, I., Kovacevic, M., Goren, A. (2020). Microneedling improves minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Journal of Biological Regulators and Homeostatic Agents. 34(2). 659-661. Available at: https://doi.org/10.23812/19-385-L-51 ↑42 Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvan, S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatologic Therapy. 32(3). E12915. Available at: https://doi.org/10.1111/dth.12915 ↑43 Dhurat, R., Sukesh, M.S., Avhad, G., Dandale, A., Pal, A., Pund, P. (2013). A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study. International Journal of Trichology. 5(1). 6-11. Available at: https://doi.org/10.4103/0974-7753.114700 ↑44 Kumar, K.M., Inamadar, A.C., Palit, A. (2018). A Randomized Controlled, Single-Observer Blinded Study to Determine the Efficacy of Topical Minoxidil plus Microneedling versus Topical Minoxidil Alone in the Treatment of Androgenetic Alopecia. Journal of Cutaneous and Aesthetic Surgery. 11(4). 211-216. Available at: https://doi/org/10.4103/JCAS.JCAS_130_17 ↑46 Ramirez-Marin, H.A., Tosti, A. (2022). Role of Oral Minoxidil in Patterned Hair Loss. Indian Dermatology Online Journal. 13(6). 729-733. Available at: https://doi.org/10.4103/idoj.idoj_246_22 ↑47 Gupta, A.K., Talukder, M., Shemer, A., Piraccini, B.M., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: https://doi.org/10.1159/000531890 ↑48 Chemical Book. (no date). Minoxidil Sulphate. ChemBook. Available at: https://www.chemicalbook.com/Price/Minoxidil-sulphate.htm Accessed: July 2025 ↑49 Shan, Y., Xu, C., Guo, Y., Wen, L., Zhou, S., Fang, L., Xu, J., Zhen, H. (2025). Liposomes enhance the hair follicle delivery of minoxidil sulfate with improved treatment of androgenetic alopecia. 677. 125642. Available at: https://doi.org/10.1016/j.ijpharm.2025.125642 ↑50 Rafi, A.W., Katz, R.M. (2011). Pilot study of 15 patients receiving a new treatment regimen for androgenic alopecia: the effects of atopy on AGA. ISRN Dermatology. 11. 241953. Available at: https://doi.org/10.5402/2011/241953 ↑51 Rundegren, J. (2004). Rapid onset of action of minoxidil 5% topical solution in a 4-month German observational study on both patients and physicians. Journal of the American Academy of Dermatology. 50(3). 91. Available at https://doi.org/10.1016/j.jaad.2003.10.290 ↑54 Jadeed, H.B., Almudimeegh, A.M., Alomran, S.A., Alshathry, A.H. (2021). A Case of Contact Allergic Dermatitis to Topical Minoxidil. Cureus. 13(1). E12510. Available at: https://doi.org/10.7759/cureus.12510 ↑55 Chellini, P.R., Pirmez, R., Raso, P., Sodre, C.T. (2015). Generalized hypertrichosis induced by topical minoxidil in an adult woman. International Journal of Trichology. 7(4). 182-183. Available at: https://doi.org/10.4103/0974-7753.171587 ↑56 DeClementi, C., Bailey, K.L., Goldstein, S.C., Orser, M.S. (2004). Suspected toxicosis after topical administration of minoxidil in 2 cats. Veterinary Emergency & Critical Care. 14(4). 287-292. Available at: https://doi.org/10.1111/j.1476-4431.2004.04014.x ↑57 Leenen, F.H., Smith, D.L., Unger, W.P. (1988). Topical minoxidil: cardiac effects in bald man. British Journal of Clinical Pharmacology. 26(4). 481-485. Available at: https://doi.org/10.1111/j.1365-2125.1988.tb03410.x ↑58, ↑59 Price, V.H., Menefee, E., Strauss, P.C. (1999). Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. Journal of the American Academy of Dermatology. 41(5). 717-721. https://doi.org/10.1016/S0190-9622(99)70006-X IngredientsPharmaceuticalsNatural RemediesCompany ReviewsBy Sarah King, PhDDec 4, 2025Topical Finasteride Side Effects: What to Know
Topical finasteride is marketed as a “safer, localized” alternative to the oral pill, but how true is that? In this article, we break down what the science actually shows about systemic absorption, sexual and mood-related side effects, real-world user reports, and why responses vary so widely. Learn...By Sarah King, PhDDec 3, 2025Low-Dose Topical Dutasteride: Better Than Oral Finasteride? (New Study & Photos)
A new clinical trial claims that low-dose topical dutasteride outperforms oral finasteride for hair regrowth, a result so surprising that it contradicts nearly everything we’ve seen in 5+ years of real-world member data. In this deep dive, we break down what we previously knew about topical dutaster...By Perfect Hair Health TeamNov 29, 2025Topical Finasteride: The Best Dosage for Maximizing Regrowth and Minimizing Side Effects
Since earning FDA approval in 1997, Finasteride has been one of the most widely used hair loss drugs. To avoid potential sexual side effects, many opt for topical finasteride in hopes of isolating the drug’s DHT-reducing effects on the scalp. However, at certain doses, topical finasteride can enter ...By Michael Williams, PhDNov 27, 2025Blueprint Haircare Stack: Product Review
Bryan Johnson’s Blueprint Haircare Stack promises hair longevity rooted in science, but how much does that translate into real-world results? In this review, we unpack the ingredients, scrutinize the evidence, compare it to proven alternatives, and ask whether the Haircare Stack offers meaningful be...By Michael Williams, PhDNov 25, 2025Natural vs. Pharmaceutical DHT Blockers: Ranked
Natural and pharmaceutical DHT blockers both aim to slow or reverse hair loss, but their strength, safety, and side effects vary widely. This article ranks the most common DHT inhibitors from weakest to strongest, comparing how natural compounds, such as pumpkin seed oil and EGCG, stack up against p...By Perfect Hair Health TeamNov 17, 2025Oral Dutasteride for Hair Loss: Results, Side Effects, Dosing & How It Compares to Finasteride
Dutasteride is one of the most powerful treatments for male pattern hair loss, offering faster and greater regrowth than finasteride with comparable or even lower rates of sexual side effects. This comprehensive guide explains how dutasteride works, how it compares to finasteride in clinical trials,...By Perfect Hair Health TeamNov 17, 2025Topical & Mesotherapy Dutasteride for Hair Loss: Evidence, Dosing, Safety
Dutasteride isn’t just oral anymore; emerging research shows that topical and mesotherapy dutasteride can significantly improve androgenic alopecia while keeping most of the action localized to the scalp. This guide breaks down the science, realistic regrowth expectations, dosing ranges, and how the...By Sarah King, PhDNov 13, 2025Does Topical Finasteride Work?
Topical finasteride is quickly emerging as a compelling alternative to oral finasteride for treating androgenic alopecia, promising similar hair regrowth with significantly fewer systemic side effects. But does topical finasteride work? In this article, we break down how topical finasteride works, h...By Michael Williams, PhDNov 12, 2025Women: Is Topical Minoxidil Safe While Trying to Conceive?
Topical minoxidil is the most widely used treatment for female hair loss, but is it safe when trying to conceive? Here’s what current research says about fertility, pregnancy, and reproductive risks.
By Michael Williams, PhDNov 12, 2025Men: Is Topical Minoxidil Safe While Trying to Conceive?
Many men use topical minoxidil to combat hair loss, but is it safe to continue while trying to conceive? Curious how the science stacks up and what experts actually recommend? Explore our evidence-based review to learn the facts about topical minoxidil and male fertility.... - Mission Statement
 Scroll Down
Scroll Down