- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
OS-01 Hair Review: Does It Live Up to the Hype?
-
Stretching The Truth: 3 Misrepresented Claims From Hair Loss Studies
-
Minoxidil Shedding – What to Expect & When it Stops
-
Does Minoxidil Cause Skin Aging?
-
Thermus Thermophilus Extract Does Not Increase Hair Density By 96.88%, Despite Dermatology Times’ Claims.
-
Does Retinoic Acid (Tretinoin) Improve Hair Growth From Minoxidil?
-
Topical Cetirizine: An Anti-Histamine That Regrows Hair? (New Evidence)
-
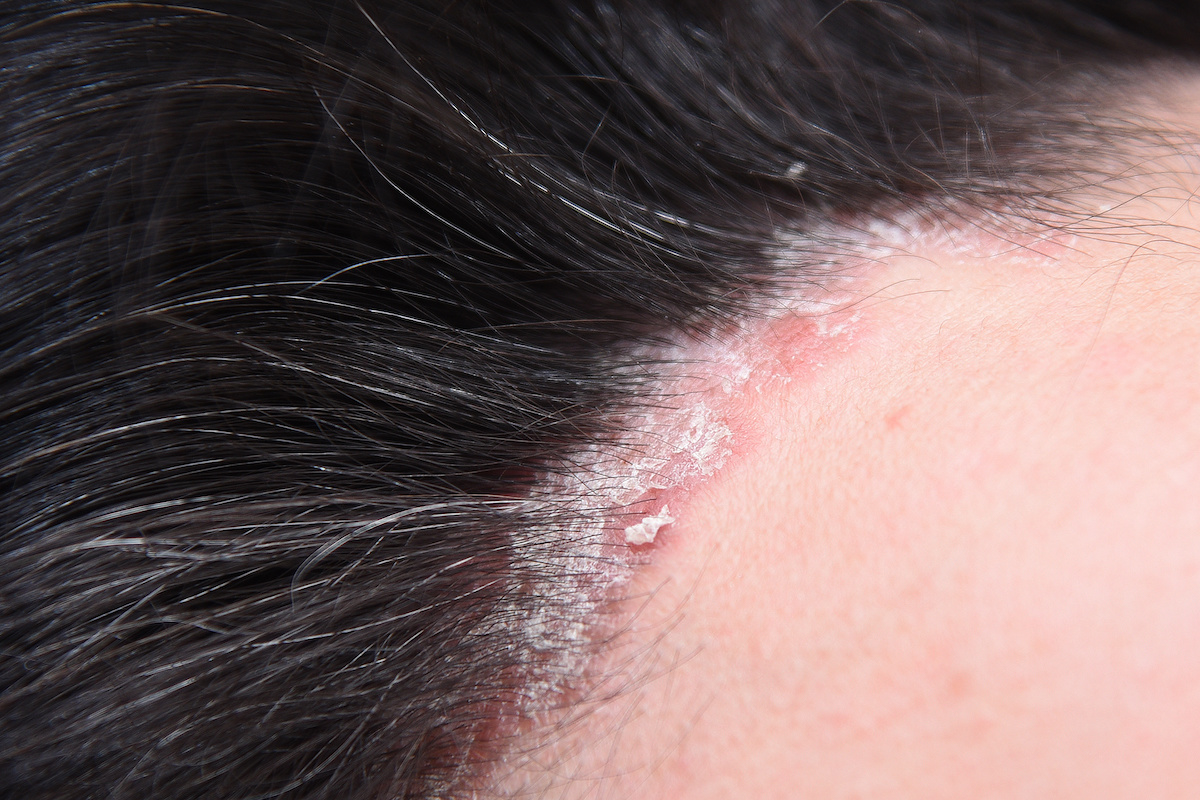
Scalp Psoriasis: Symptoms, Causes, and Effects on Hair Loss
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
TreatmentsKetoconazole Shampoo
Evidence35%RegrowthViabilityTopical Ketoconazole
Evidence25%RegrowthViability-
Shampoo
-
Topical
-

Evidence Quality
35%
Regrowth Potential
Long-Term Viability
Written by Perfect Hair Health TeamMedically Reviewed by Rob EnglishFirst Published Oct 4, 2024Last Updated Oct 28, 2024Key Information
Free Resources
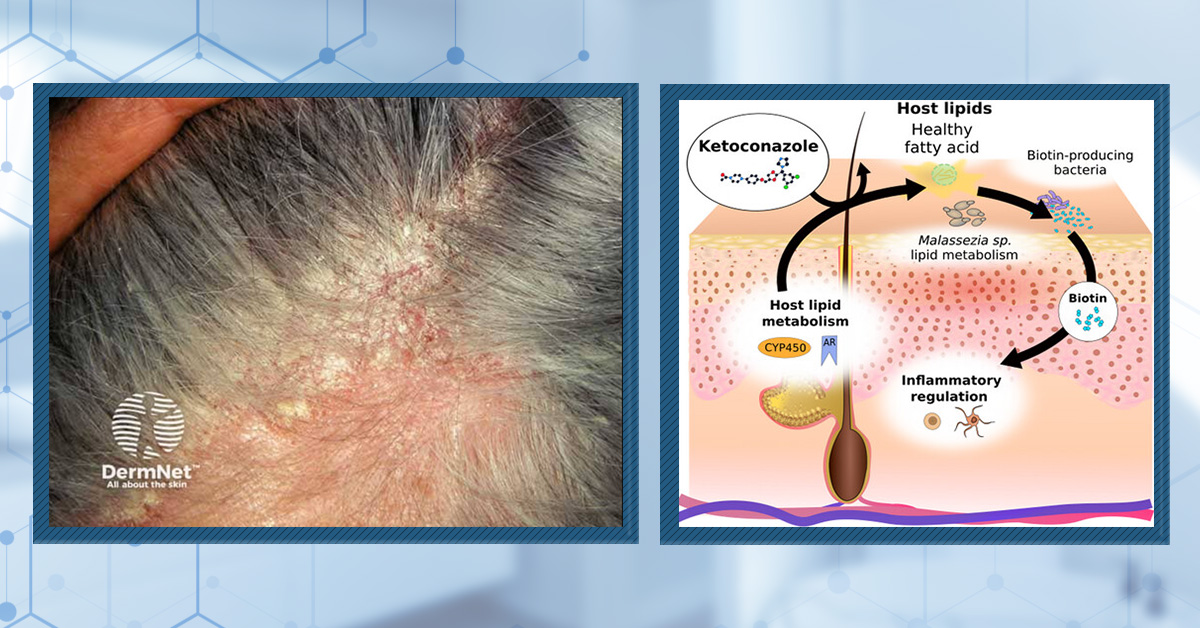
KEY INFORMATIONWhat Is Ketoconazole?
Ketoconazole is drug originally intended to treat fungal infections, but later reformulated as a shampoo to treat androgenic alopecia and telogen effluvium associated with dandruff, dermatitis, and excessive scalp oiliness.
How Does Ketoconazole Work?
Ketoconazole helps reduce pathogenic microorganisms in the scalp skin, which in turn helps reduce inflammation. When used as a topical and/or shampoo, it also may have mild antiandrogenic properties. Both of these mechanisms might explain its hair growth-promoting effects.
What You Should Know
For those who have androgenic alopecia alongside excessive dandruff, dermatitis, scalp itch, and/or scalp oiliness, 2% ketoconazole shampoo may act as a low-cost, low-effort, moderately-helpful intervention to improve hair counts.
-
Evidence Quality
25%
Regrowth Potential
Long-Term Viability
Written by Perfect Hair Health TeamMedically Reviewed by Rob EnglishFirst Published Oct 4, 2024Last Updated Oct 28, 2024Key Information
Free Resources
KEY INFORMATIONWhat Is Ketoconazole?
Ketoconazole is drug originally intended to treat fungal infections, but later reformulated as a shampoo to treat androgenic alopecia and telogen effluvium associated with dandruff, dermatitis, and excessive scalp oiliness.
How Does Ketoconazole Work?
Ketoconazole helps reduce pathogenic microorganisms in the scalp skin, which in turn helps reduce inflammation. When used as a topical and/or shampoo, it also may have mild antiandrogenic properties. Both of these mechanisms might explain its hair growth-promoting effects.
What You Should Know
For those who have androgenic alopecia alongside excessive dandruff, dermatitis, scalp itch, and/or scalp oiliness, 2% ketoconazole shampoo may act as a low-cost, low-effort, moderately-helpful intervention to improve hair counts.
FREE RESOURCESArticles
Explore the science behind hair loss and hair growth. Our in-depth articles cover topics ranging from natural remedies to pharmaceuticals to breakthroughs in hair loss science. Want to request an article topic? Contact us.
By Sarah King, PhDMar 25, 2025Scalp Psoriasis: Symptoms, Causes, and Effects on Hair Loss
Scalp psoriasis goes beyond surface-level flaking; it’s a chronic autoimmune condition driven by genetic and immune system factors. From thick plaques and itching to potential hair loss, it can seriously impact daily life. Curious about what causes it, how to tell it apart from similar conditions, a...By Sarah King, PhDFeb 25, 2025Seborrheic Dermatitis: Symptoms, Treatments, & Its Impact On Hair Loss
Seborrheic dermatitis is a common cause of dandruff, but what’s really happening to your scalp when it flares up? From excess sebum and yeast overgrowth to genetic factors, this condition has multiple triggers. Wondering what treatments work best and how to manage symptoms long-term? Check out our i...By Sarah King, PhDApr 19, 2024Why Am I Shedding After Starting Ketoconazole?
Some people experience increased hair shedding after using ketoconazole shampoos or topicals. This can be due to several factors: treatment-induced hair shedding, a drug reaction, seasonal hair shedding, changes to other aspects of your hair growth regimen, ketoconazole overuse, scalp drying, & ...By Sarah King, PhDApr 11, 2024Does Ketoconazole Work for Women?
Several clinical studies support the effectiveness of ketoconazole, an anti-fungal treatment, in treating androgenic alopecia and telogen effluvium. However, the evidence for ketoconazole in women is limited. One study found that daily ketoconazole use can facilitate hair growth in women with patter...By Perfect Hair Health TeamApr 11, 2024I Am Experiencing Side Effects From Ketoconazole. What Should I Do?
Ketoconazole shampoo, a anti-fungal medication, is widely used to treat dandruff and seborrheic dermatitis. It is also sometimes used as an off-label treatment for androgenic alopecia and telogen effluvium. Some users experience side effects such as scalp itchiness & dryness. But with a bit of t...By Perfect Hair Health TeamApr 6, 2022The 6 Best DHT Blockers For Men
Which DHT blockers work? Which ones put hair loss sufferers at a greater risk of side effects? What does the actual evidence say versus marketing hype for the latest DHT-reducing supplements, topicals, and drugs? The answers may surprise you. In this article, the Perfect Health Team evaluates 6 DHT ...By Perfect Hair Health TeamMar 25, 2022Nizoral for Hair Loss – Does it Work?
Ketoconazole, better known as Nizoral®, is often regarded as an effective hair growth shampoo. But does this antifungal, dandruff-reducing shampoo live up to its billing as a key member of the Big 3 of hair loss treatments? In this article, we’ll analyze the evidence to determine the effect of...By Perfect Hair Health TeamJan 7, 20201% Or 2% Ketoconazole Shampoo For Hair Loss: Does It Matter?
While 1% ketoconazole is sold as an ingredient in hair loss shampoos worldwide, only 2% ketoconazole is clinically shown to improve pattern hair loss. Unfortunately, 2% ketoconazole requires a prescription. But is the 2% formulation really worth the the hassle of seeing your doctor for a prescriptio...FREE RESOURCESArticles
Explore the science behind hair loss and hair growth. Our in-depth articles cover topics ranging from natural remedies to pharmaceuticals to breakthroughs in hair loss science. Want to request an article topic? Contact us.
By Sarah King, PhDMar 25, 2025Scalp Psoriasis: Symptoms, Causes, and Effects on Hair Loss
Scalp psoriasis goes beyond surface-level flaking; it’s a chronic autoimmune condition driven by genetic and immune system factors. From thick plaques and itching to potential hair loss, it can seriously impact daily life. Curious about what causes it, how to tell it apart from similar conditions, a...By Sarah King, PhDFeb 25, 2025Seborrheic Dermatitis: Symptoms, Treatments, & Its Impact On Hair Loss
Seborrheic dermatitis is a common cause of dandruff, but what’s really happening to your scalp when it flares up? From excess sebum and yeast overgrowth to genetic factors, this condition has multiple triggers. Wondering what treatments work best and how to manage symptoms long-term? Check out our i...By Perfect Hair Health TeamApr 6, 2022The 6 Best DHT Blockers For Men
Which DHT blockers work? Which ones put hair loss sufferers at a greater risk of side effects? What does the actual evidence say versus marketing hype for the latest DHT-reducing supplements, topicals, and drugs? The answers may surprise you. In this article, the Perfect Health Team evaluates 6 DHT ...-
FREE RESOURCES
Research Tables
Want the latest research on Ketoconazole Shampoo? Every quarter, our research team conducts a literature search on Ketoconazole Shampoo to keep you up-to-date on new studies. See our search criteria & research tables below – including a summary of key findings from every single study.
Last updated: October 2024
Results
Search Criteria
Parameter
Inclusion Criteria
Exclusion Criteria
Patients Patients of any age with hair loss. Patients with no hair loss disorder. Intervention Ketoconazole shampoo as a standalone or adjunct therapy. A study that doesn’t contain ketoconazole shampoo either as a standalone or adjunct therapy. Comparator Placebo and/or other therapies or baseline, or in the observational studies - none. Outcomes Primary Endpoints of phototrichogram, investigator, and/or patient assessments. Any study not designed to adequately test for the standalone or additive effect of ketoconazole shampoo. Study Design Prospective, observational, retrospective, and case series studies. Literature reviews, non-human subjects, or ongoing clinical trials. Search Terms
Search Terms:
shampoo hair loss alopecia ketoconazole-
Abbreviations:
-
RCTRandomized Controlled Trial
-
AGAAndrogenic Aloepcia
-
KTZKetoconazole
-
PTOPiroctone Olamine
-
ZPTZinc Pyrrithione
-
PIPilary Index
Summarized Studies
Study Participants Design Treatment Results Key Takeaway Adverse Effects Evidence Quality Authors (year) Sex Hair Loss Type Design Dose Usage Duration Endpoints Hair Growth Assessments Summary Limitations Adverse Effects Jadad Score Rafi & Katz.(2011) n = 15 (M) AGA
Prospective pilot study 2% KTZ shampoo. 8 used NuH Hair + finasteride + minoxidil + KTZ, 5 used only NuH Hair, 1 used NuH Hair + finasteride + KTZ, 1 used NuH Hair + KTZ. KTZ shampoo used 2-3 times a week 9 months Average time for hair regrowth. 30 days for hair regrowth with NuH Hair + finasteride + minoxidil + ketoconazole.
30 days for hair regrowth with NuH Hair + finasteride + ketoconazole.
60 days for hair regrowth with NuH Hair + ketoconazole.
90 days for hair regrowth with NuH Hair alone.Ketoconazole works well as a combination therapy with finasteride and minoxidil and can improve AGA in men with atopic and/or seborrheic dermatitis. Small sample size, lack of control, short follow-up, ketoconazole was not used alone. No significant adverse effects were reported. 1Khandpur et al. (2002) n = 100 (M) AGA
Open, randomized, parallel-group study. Finasteride 1 mg
Finasteride 1 mg + Minoxidil 2%
Minoxidil 2%
Finasteride 1 mg + 2% KTZ shampooOnce daily
Once daily
Once daily
Once daily
12 months Patient self-assessment and physicians’ assessment. Finasteride + minoxidil and finasteride + KTZ reap the best improvement scores for both self- and physician-assessments.
However, finasteride + minoxidil slightly outperformed finasteride + ketoconazole.2% KTZ works well as a combination therapy with finasteride. Open-label design, no control, and subjective outcome measures. Finasteride 1 patient - loss of libido after 3 months but continued without further decline.
Minoxidil 1 patient - low blood pressure and increased heart rate.2Pierard-Franchimont et al. (2002) n = 150 (M) Mild-to-
moderate dandruff, telogen effluvium related to vertexAGA
RCT 1% KTZ shampoo
1% PTO shampoo
1% ZPT shampoo
2-3 times weekly 6 months Hair shedding, hair density, percentage of anagen hairs, mean proximal hair shaft diameter, and sebum excretion rates. Pruritis and dandruff cleared rapidly in all 3 shampoo groups.
Hair density remained unchanged in all 3 shampoo groups.
Hair shedding decreased by 17.3% in the KTZ group, 16.5% in the PTO group, and 10.1% in the ZPT group.
Anagen hair ratio increased by 4.9% in the KTZ group, 7.9% in the PTO group, and 6.8% in the ZPT group.
Sebum excretion rate decreased by 4.8% in the KTZ group, 2.9% in the PTO group, and 5.5% in the ZPT group.1% KTZ shampoo 2-3 times weekly lessened hair shedding by ~20%, increased hair shaft thickness by ~5%, and decreased sebum output by ~5%. No placebo, short follow-up. None reported 2Pierard-Franchimont et al. (1998) n = 61 (M)
n = 39 with AGA
n = 22 without AGAAGA
Comparative, observational study. 2% KTZ shampoo
Normal Shampoo
2-4 times weekly 21 months AGA pilary index (PI): defined as the percentage of hairs in anagen x average diameter of the hair shafts 1.5 cm from the hair bulb The PI of non-AGA controls remained unchanged regardless of the type of shampoo used (KTZ vs. normal shampoo).
The PI of only AGA subjects with unmedicated shampoo showed a slow linear decrease over time, while the ketoconazole group showed a progressive increase evident after 6 months.In men without AGA, KTZ doesn’t improve hair counts or hair shaft diameter.
In men with AGA, KTZ improves both anagen hair counts and overall hair shaft diameter.Small sample size None reported 0 -
-
FREE RESOURCES
Research Tables
Want the latest research on Topical Ketoconazole? Every quarter, our research team conducts a literature search on Topical Ketoconazole to keep you up-to-date on new studies. See our search criteria & research tables below – including a summary of key findings from every single study.
Last updated: October 2024
Results
Search Criteria
Parameter
Inclusion Criteria
Exclusion Criteria
Patients Patients of any age with hair loss. Patients with no hair loss disorder. Intervention Topical ketoconazole as a standalone or adjunct therapy. A study that doesn’t contain ketoconazole either as a standalone or adjunct therapy. Comparator Placebo and/or other therapies or baseline, or in the observational studies - none. Outcomes Primary Endpoints of phototrichogram, investigator, and/or patient assessments. Any study not designed to adequately test for the standalone or additive effect of topical ketoconazole. Study Design Prospective, observational, retrospective, and case series studies. Literature reviews, non-human subjects, or ongoing clinical trials. Search Terms
Search Terms:
topical hair loss alopeci ketoconazole-
Abbreviations:
-
RCTRandomized Controlled Trial
-
AGAAndrogenic Aloepcia
-
KTZKetoconazole
-
PTOPiroctone Olamine
-
ZPTZinc Pyrrithione
-
ARAndrogen Receptors
-
FPHLFemale Pattern Hair Loss
-
MXTTopical Minoxidil
Summarized Studies
Study Participants Design Treatment Results Key Takeaway Adverse Effects Evidence Quality Authors (year) Sex Hair Loss Type Design Dose Usage Duration Endpoints Hair Growth Assessments Summary Limitations Adverse Effects Jadad Score El-Garf, Mohie, and Salah (2019) n = 40
Group A = 20
Group B = 20
FPHL
RCT Group A: 2% Minoxidil
Group B: 2% KTZ
1 mL daily 6 months Hair growth (clinical and trichoscopic) and patient satisfaction. Hair Growth:
Group A: Significant improvement in hair growth at months 4 and 6 compared to the baseline.
Group B: Hair growth is delayed compared to minoxidil, and it is seen only in month 6 of treatment. A notable improvement was observed in reducing brown peripilar signs (related to perifollicular inflammation).
Ludwig Score:
Group A: Significant improvement in severity seen by months 4 and 6.
Group B: Significant improvement by the 6th month.
Patient satisfaction:
No significant difference between the two groups. 75% of patients in both groups reported being satisfied. 20% in each group were very satisfied.
KTZ demonstrated significant hair growth but with a delayed response compared to minoxidil. KTZ had fewer side effects, making it a potentially safer alternative for FPHL. Small sample size, short duration, no placebo, no long-term follow-up. Group A: 55% reported side effects, including dermatitis and facial hypertrichosis
Group B: 10% reported minor cases of dermatitis.
3Inui and Itami (2007) n = 6 (M) AGA
Open-label 2% KTZ lotion Almost every day during or immediately after hair washing with an unmedicated shampoo. Up to 12 months Hair regrowth, consistency of hair regrowth, and mechanism of action. 2 of 6 participants showed significant hair regrowth on the vertex area after 6 and 10 months of using KTZ.
1 of 6 patients showed mild improvement in vertex hair density after one year of consistent use.
3 of 6 participants did not show any noticeable hair regrowth during the study.
Laboratory tests indicated the KTZ suppressed AR activity in cell assays.
KTZ might promote hair growth through an anti-androgenic mechanism. Small sample size, lack of control, no quantitative measures. None reported. 1 -
- Mission Statement
 Scroll Down
Scroll Down