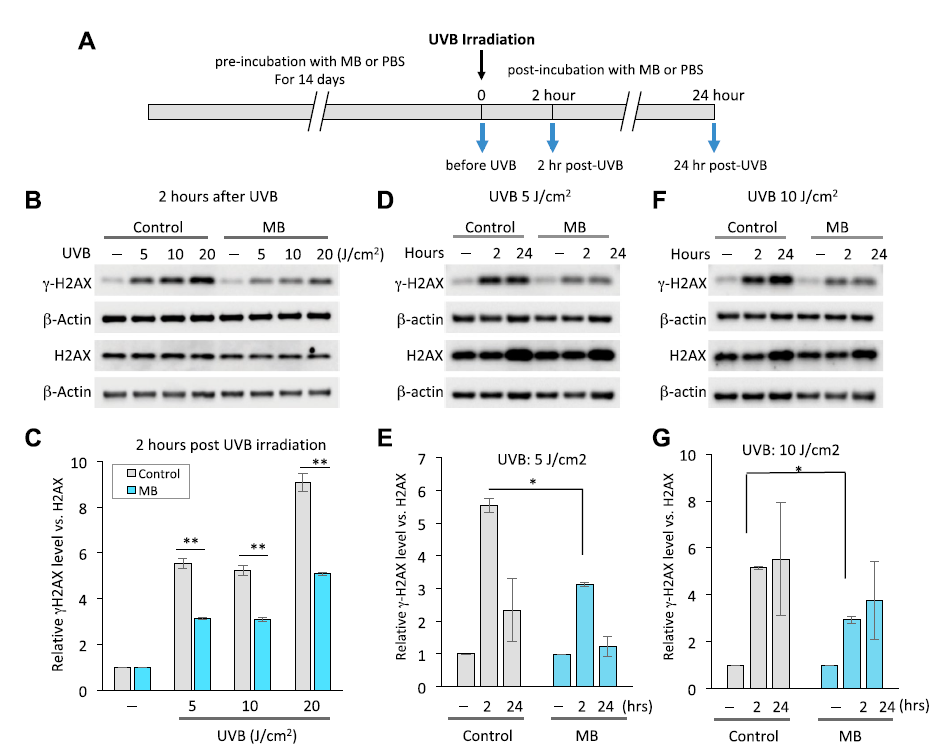
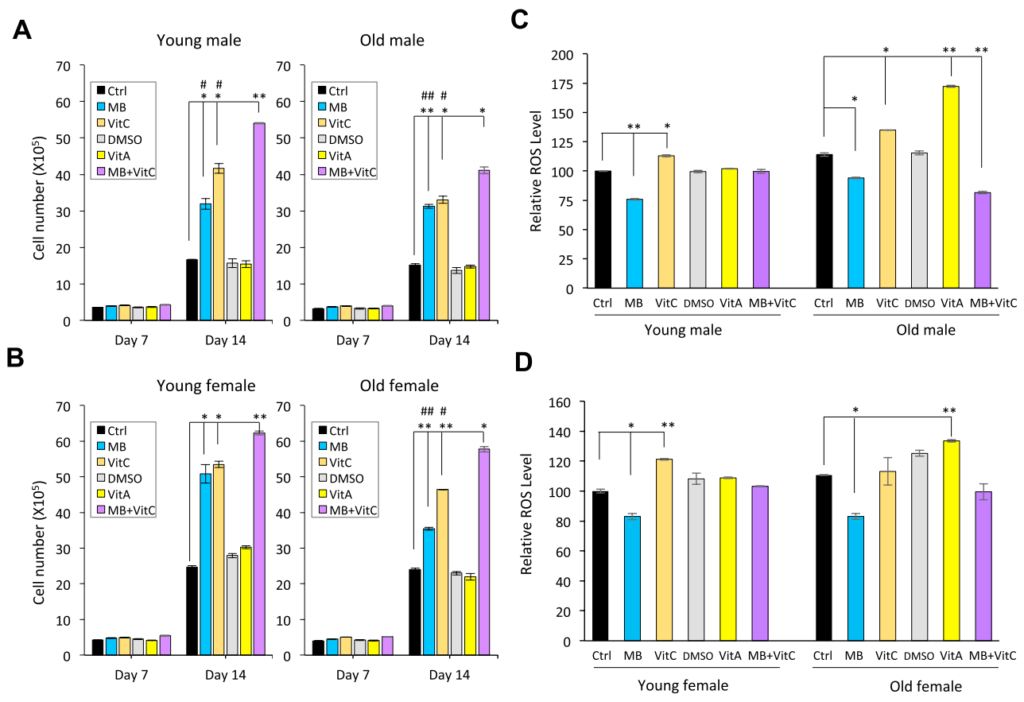
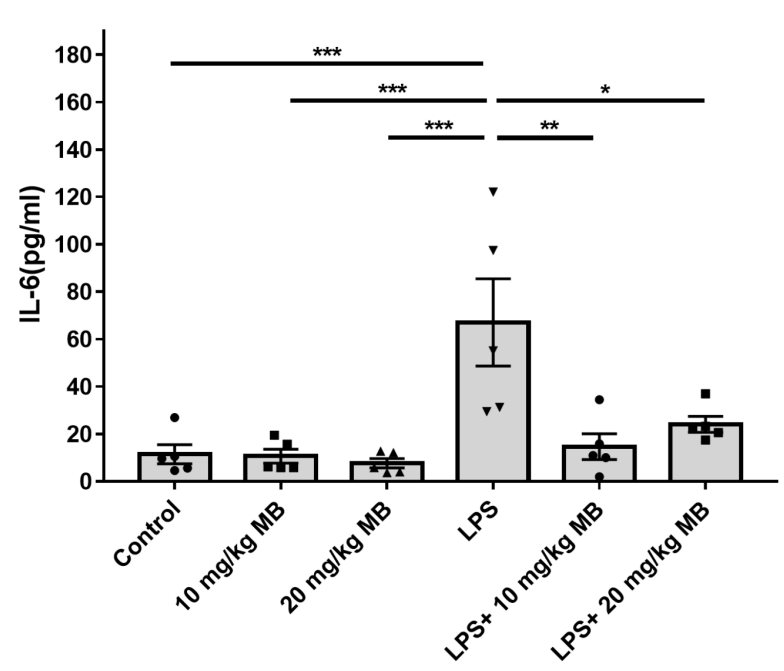
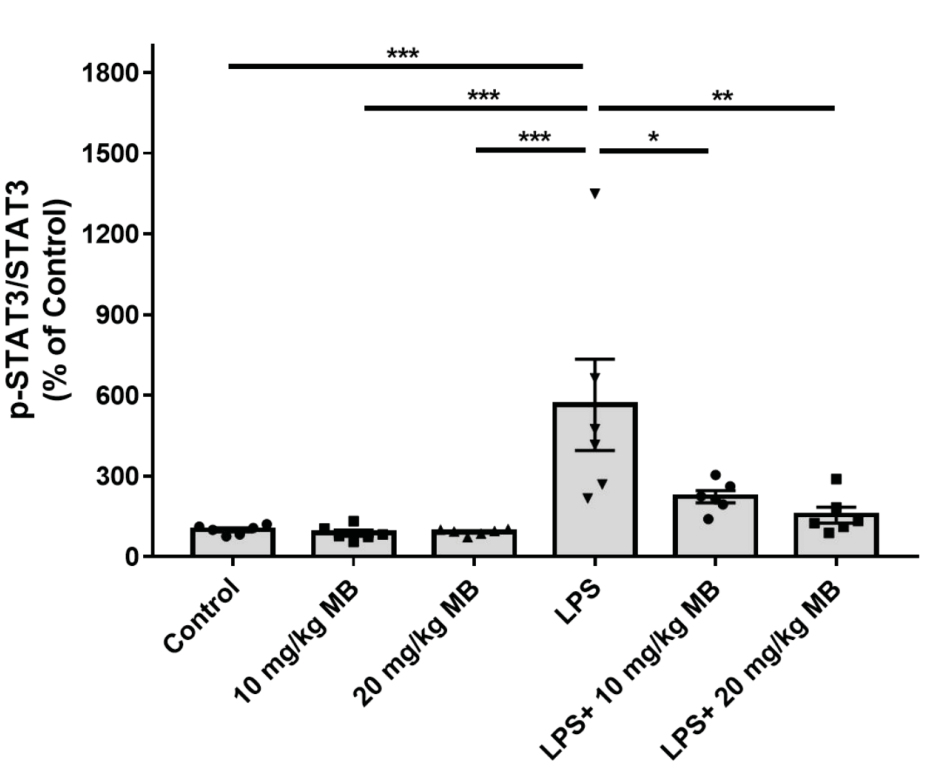
- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
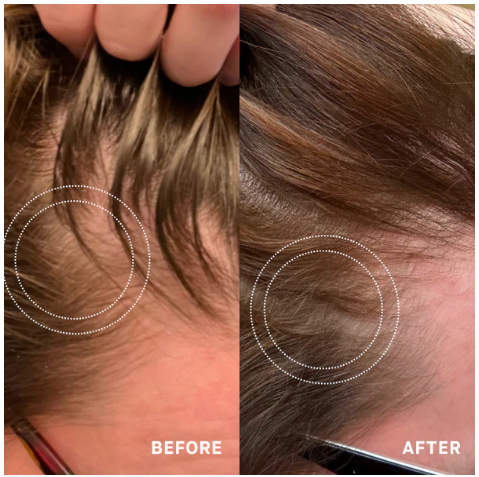
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
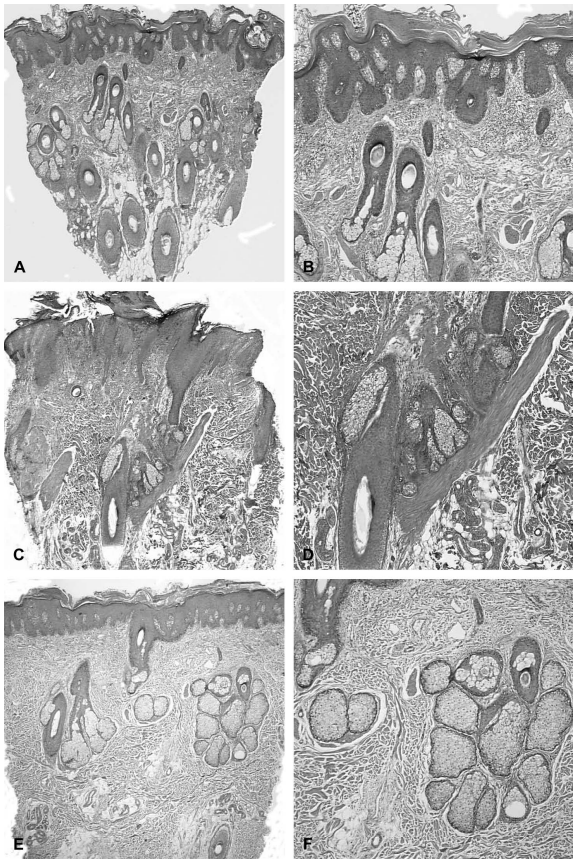
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
Articles
Oral minoxidil is an increasingly popular off-label treatment for hair loss, offering a convenient alternative to topical solutions for both men and women. Originally developed as a blood pressure medication, it was discovered to promote hair growth, sometimes dramatically, by enhancing blood flow and stimulating key pathways involved in hair growth.
In this article, we break down what oral minoxidil is, how it works, what the research reveals across different types of hair loss, potential side effects, and best practices for safe and effective use.
Key Takeaways:
- What is it? Oral minoxidil is a prescription vasodilator originally used to treat high blood pressure. At lower doses (0.25–5 mg daily), it’s increasingly prescribed off-label to treat hair loss, especially in people who don’t respond well to topical minoxidil. It promotes regrowth by enhancing blood flow, shortening the hair’s resting phase, and activating key follicular growth pathways.
- Clinical Data. Studies have shown that oral minoxidil is effective for multiple types of hair loss, including androgenic alopecia (AGA), chronic telogen effluvium, and even permanent chemotherapy-induced alopecia. For AGA, it offers comparable or better results than 5% topical minoxidil, particularly at doses of 2.5–5 mg in men. In women, low-dose therapy (0.25–1.25 mg) is also effective, often combined with spironolactone for added benefit.
- Safety. At low doses, oral minoxidil is generally well-tolerated. The most common side effects include excess body hair, mild fluid retention, and lightheadedness. Rarely, serious cardiac issues like pericardial effusion may occur, especially in individuals with underlying health conditions. Most side effects are dose-dependent and reversible with adjustment or discontinuation.
- Evidence Quality. Oral minoxidil scored 63/100 for evidence quality by our metrics.
- Best Practices. Start with a low dose (2.5 mg for men, 0.25–1.25 mg for women) and titrate upward only if needed. Splitting doses between morning and night may improve tolerability. Sublingual minoxidil is a promising alternative to reduce systemic exposure. Combine with microneedling or topical agents if the results plateau. Always consult a healthcare provider, especially if you have concerns related to cardiovascular, renal, or hepatic health.
Interested in Oral Minoxidil?
Low-dose oral minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Oral Minoxidil?
Oral minoxidil is a medication originally developed in the 1970s as an oral hypertensive agent, designed to lower high blood pressure by acting as a potent vasodilator. During its use for hypertension, clinicians observed a notable side effect: increased hair growth, or hypertrichosis.[1]Patel, P., Nessel, T.A., Kumar, D. (2023). Minoxidil. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/book/NBK482378/ Accessed: … Continue reading
This unexpected effect led to the development of topical minoxidil for hair loss, which was approved by the FDA in 1988 for male patients and in 1992 for female patients.[2]Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of … Continue reading Oral minoxidil is also used as an off-label treatment for both men and women.
Why Might I Consider Oral Over Topical Minoxidil?
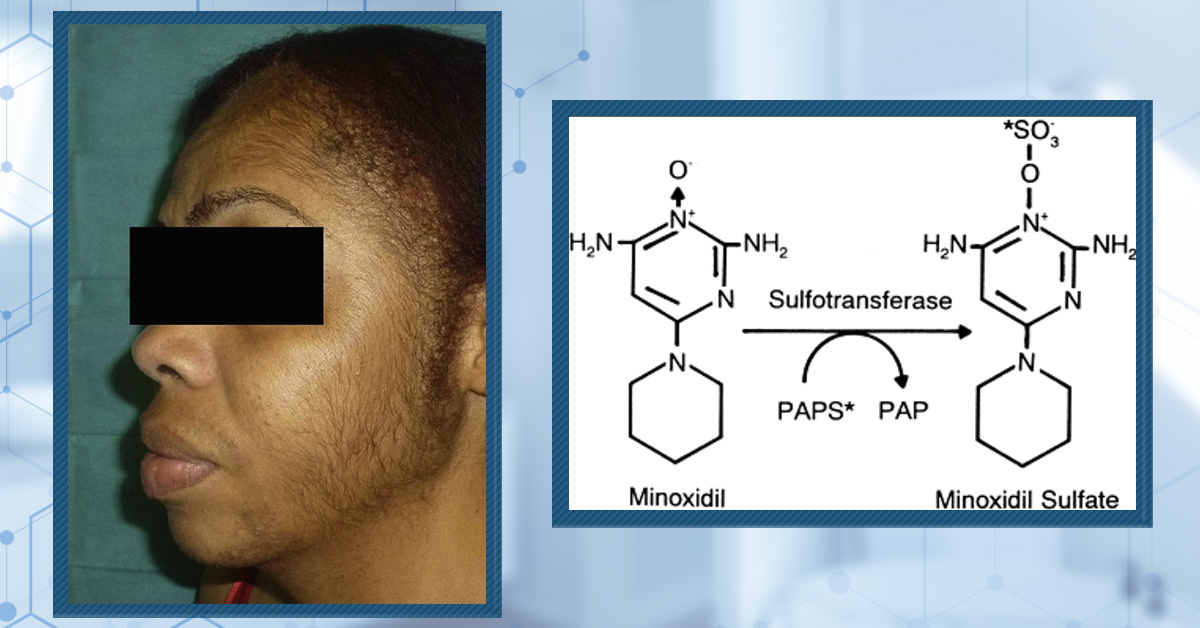
Many people consider oral minoxidil over topical due to fundamental differences in how each form is activated and delivered to hair follicles. Topical minoxidil is a pro-drug that requires conversion to its active form, minoxidil sulfate, by the sulfotransferase enzyme (specifically SULT1A1) located in the outer root sheath of scalp hair follicles.[3]Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a … Continue reading
However, the activity of this enzyme varies significantly between individuals. For example, in one study on 120 patients, 40.8% exhibited low levels of sulfotransferase activity in the scalp.[4]Chitalia, J., Dhurat, R., Goren, A., McCoy, J., Kovacevic, M., Situm, M., Naccarato, T., Lotti, T. (2018). Characterization of follicular minoxidil sulfotransferase activity in a cohort of pattern … Continue reading
Additionally, topical minoxidil faces a “penetration problem” where only about 1.4% of the drug applied is actually absorbed into the scalp skin under normal conditions.[5]Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading
Oral minoxidil largely bypasses these limitations. When taken by mouth, minoxidil is absorbed through the gastrointestinal tract and converted to its active form in the liver, where sulfotransferase activity is abundant. This ensures that nearly all ingested minoxidil is activated and delivered systemically, reaching hair follicles throughout the scalp and body, regardless of individual differences in scalp enzyme activity. As a result, oral minoxidil can be effective even for those who are non-responders to topical therapy due to low scalp sulfotransferase activity or poor drug penetration.[6]Beach, R.A. (2018). Case series of oral minoxidil for androgenetic and traction alopecia: Tolerability & the five C’s of oral therapy. Dermatologic Therapy. 31(6). E12707. Available at: … Continue reading
How Does Oral Minoxidil Work?
Oral minoxidil promotes hair growth through several interrelated mechanisms at the cellular and molecular levels.
Potassium Channel Activation and Vasodilation
Minoxidil is converted in the liver to its active form, minoxidil sulfate (first phase metabolism), which opens adenosine triphosphate (ATP)-sensitive potassium channels in vascular smooth muscle and hair follicle cells.[7]Gupta, A.K., Talukder, M., Shemar, A., Priaccini, B.A., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: … Continue reading
This action triggers a process known as membrane hyperpolarization, resulting in vasodilation and increased blood flow to the scalp. This increased microcirculation delivers more oxygen, nutrients, and growth factors to hair follicles, creating a favorable environment for hair growth.
Stimulation of the Hair Growth Cycle
Minoxidil shortens the telogen (resting) phase and induces early entry of hair follicles into the anagen (growth) phase.[8]Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair loss disorders. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading It also prolongs the duration of anagen, leading to longer and potentially thicker hair.
Activation of Wnt/ꞵ-Catenin Signaling
Minoxidil has been shown to activate the Wnt/ꞵ-Catenin pathway in dermal papilla cells. This pathway is a critical signaling route for hair follicle regeneration and maintenance of the anagen phase.[9]Kwack, M.H., Kang, B.M., Kim, M.K., Kim, J.C., Sung, Y.K. (2011). Minoxidil activates ꞵ-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. … Continue reading It also promotes the growth and specialization of hair follicle stem cells, supporting follicle growth.[10]Wang, X., Liu, Y., He, J., Wang, J., Chen, X., Yang, R. (2022). Regulation of signaling pathways in hair follicle stem cells. Burns Trauma. 10. 1-19. Available at: … Continue reading
Direct Effects on Dermal Papilla Cells
Minoxidil stimulates the proliferation and survival of dermal papilla cells (DPCs), which are essential for hair follicle health.[11]Kang, J-I., Choi, K.Y., Han, S-C., Nam, H., Lee, G., Kang, J-H., Koh, Y.S., Hyun, J.W., Yoo, E.S., Kang, H.K. (2022). 5-Bromo-3,4-dihydroxybenzaldehyde Promotes Hair Growth through Activation of … Continue reading
It also activates the extracellular signal-regulated kinase (ERK) and protein kinase B signaling pathways, increases the ratio of anti-apoptotic to pro-apoptotic proteins (Bcl-2/Bax), and prevents cell death, thereby prolonging the anagen phase.[12]Jan, J.H., Kwon, O.S., Chung, J.H., Cho, K.H., Eun, H.C., Kim, K.H. (2004). Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. Journal of … Continue reading
Minoxidil also increases the expression of key growth factors, such as vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1), which further support follicle nourishment and angiogenesis.[13]Nagai, N., Iwai, Y., Sakamoto, A., Otake, H., Oaku, Y., Abe, A., Nagahama, T. (2019). Drug Delivery System Based on Minoxidil Nanoparticles Promotes Hair Growth in C57BL/6 Mice. International Journal … Continue reading
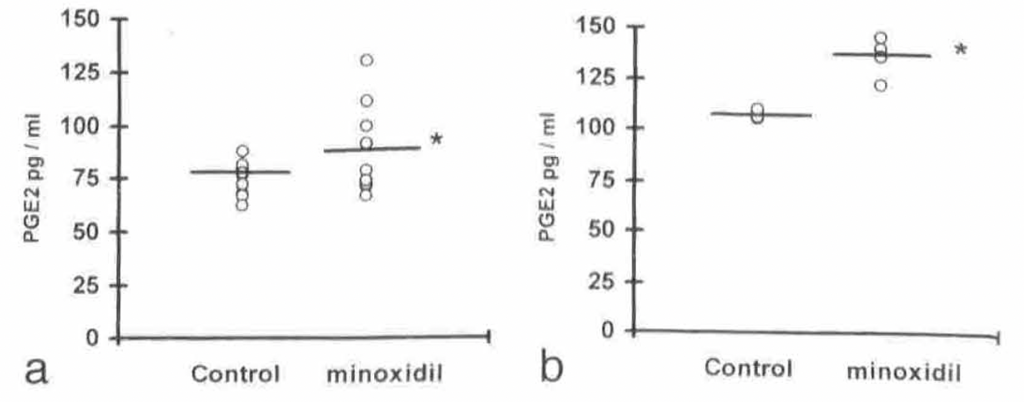
Anti-Inflammatory and Anti-Fibrotic Properties
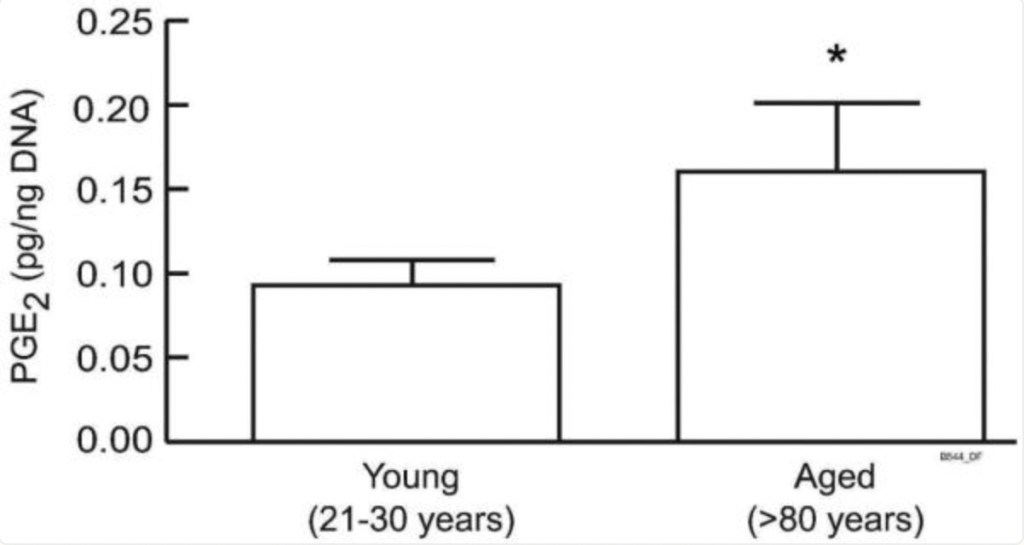
Minoxidil exhibits anti-inflammatory effects by modulating the production of prostaglandins and other inflammatory mediators.[14]Shin, D.W. (2022). The physiological and pharmacological roles of prostaglandins in hair growth. The Korean Journal of Physiology and Pharmacology. 26(6). 405-415. Available at: … Continue reading [15]Majewski, M., Gardas, K., Waskiel-Burnat, A., Ordak, M., Rudnicka, L. (2024). The Role of Minoxidil in Treatment of Alopecia Areata: A Systematic Review and Meta-Analysis. Journal of Clinical … Continue reading It also inhibits collagen synthesis, which could reduce fibrosis around hair follicles, maintaining a healthy microenvironment for hair growth.[16]Fechine, C.O.C., Valente, N.Y.S., Romiti, R. (2022). Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. Anais Brasileiros de Dermatologia. … Continue reading
How Effective Is Oral Minoxidil?
Let’s break this down into the types of hair loss that minoxidil can assist with.
Pattern Hair Loss/Androgenic Alopecia (Females)
Oral minoxidil has been found to be as effective as 5% topical minoxidil at treating female pattern hair loss. The study found no significant difference between topical and oral minoxidil 1 mg daily in terms of efficacy for female pattern hair loss (FPHL).[17]Ramos, M.P., Sinclair, R.D., Kasprzak, M., Miot, H.A. (2020). Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss. Journal of the American Academy … Continue reading There were, however, differences in the occurrence of adverse events. While scalp itching occurred only in the topical group, only users of oral minoxidil experienced excess hair growth outside of the scalp.
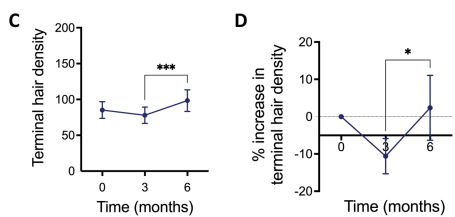
Another study combined low-dose minoxidil (0.25 mg) and 25 mg spironolactone to treat FPHL.[18]Sinclair, R.D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. International Journal of Dermatology. 57(1). 104-109. … Continue reading The results of the study demonstrated that the combined therapy:
- Decreased hair shedding as early as 3 months into the treatment.
- Increased hair density after 6 months.
However, the authors didn’t report any objective measurements of hair density, either at baseline or at the end of the study period. This makes it impossible to effectively compare the results of the previously mentioned study with those of this study.
So, we can’t effectively assess the difference in efficacy of spironolactone and low-dose oral minoxidil combination therapy or oral minoxidil monotherapy. However, the authors of this study do note that they believe the combination has an added benefit for FPHL.
Pattern Hair Loss/Androgenic Alopecia (Males)
Of all forms of hair loss, AGA may derive the most benefit from minoxidil (whether oral or topical). This is because one of the main mechanisms of action directly addresses one of the pathological facets of AGA: reduced blood flow.
This is evidence in one study on men with AGA.[19]Lueangarun, S., Panchaprateep, R., Tempark, T., Noppakun, N. (2015). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Journal of the American … Continue reading In particular, it demonstrates a 100% response rate to a daily dose of 5 mg oral minoxidil. Even more impressively, subjects achieved an average 19% increase in hair count after 24 weeks.
The risk profile also appears to be favorable, with only 10% of participants reporting leg swelling. Ten percent of participants also reported a more significant side effect: an alteration in electrocardiogram (EKG) results.
These risks could theoretically be mitigated by lowering the dosage; however, one would need to consider the potential for reduced benefit. To better understand this, we’ll have to look at another study.
In line with the above results, this retrospective study showed a 90% response rate at 6-12 months for men taking 2.5-5.0 mg of oral minoxidil daily.[20]Jiminiez-Couche, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Fernandez-Nieto, D., Vano-Galvan, S. (2019). Effectiveness and safety of low-dose oral … Continue reading Perhaps even more impressive is the fact that 60% of these men had tried other treatments for AGA, but had previously quit due to side effects or lack of efficacy.
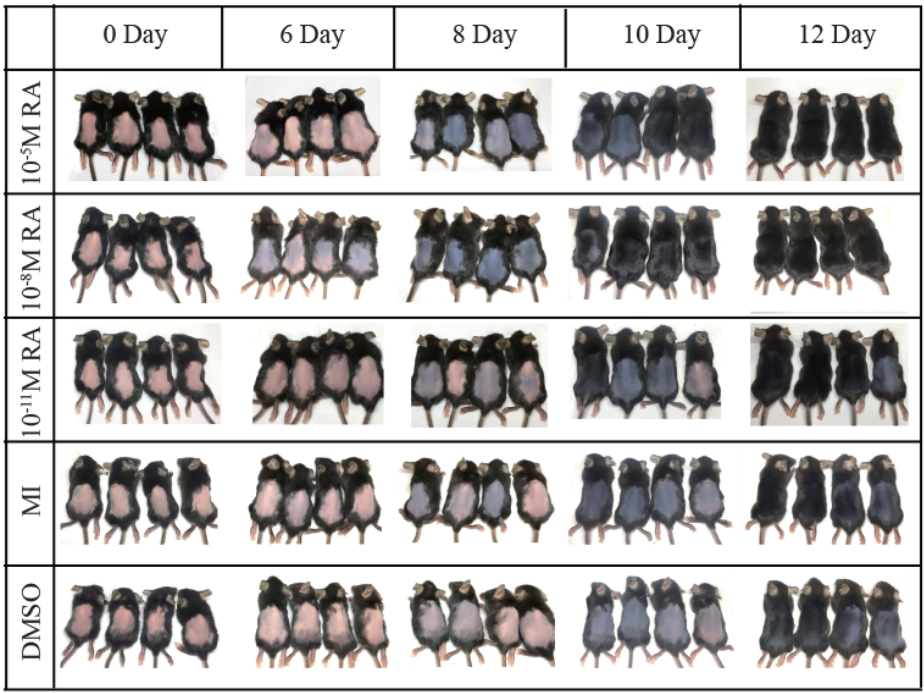
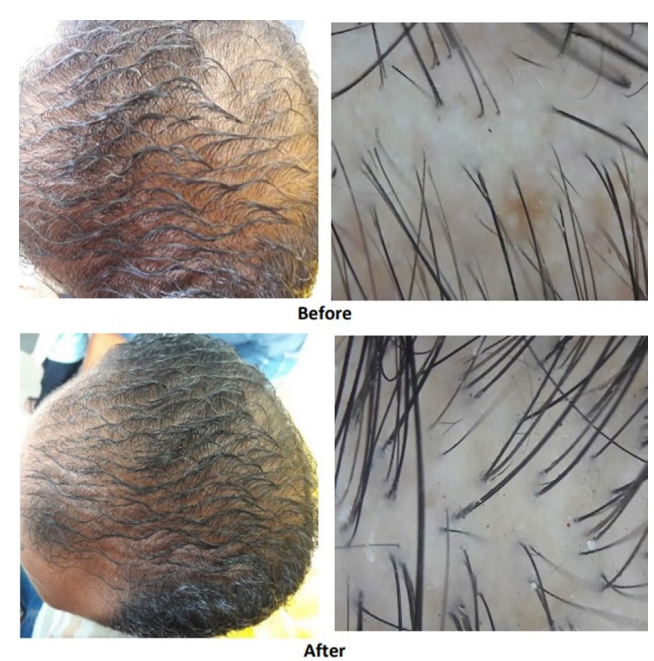
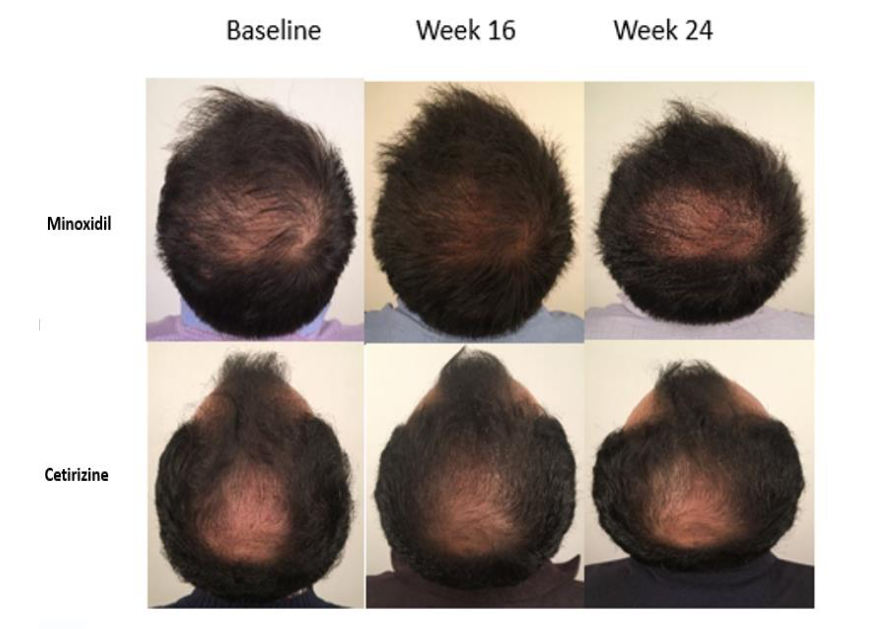
Figure 1: Male with AGA: Improvement with 5 mg oral minoxidil over 3 months.[21]Jiminiez-Couche, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Fernandez-Nieto, D., Vano-Galvan, S. (2019). Effectiveness and safety of low-dose oral … Continue reading
In line with the trend of “decreasing dosage, decreasing efficacy”, one study using 0.25 mg of oral minoxidil found a 60% response rate in male AGA patients.[22]Pirmez, R., Salas-Callo, C-I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: A study with quantitative trichoscopic documentation. Journal of the American Academy of Dermatology. … Continue reading That’s on the low side for oral minoxidil overall, but on the high side when compared to topical minoxidil.
It’s also worth noting that there’s a key difference between this study and the one showing a 100% response rate at a 5mg daily dose. The 5mg study only included men with mild to moderate AGA; this study included both mild to moderate and severe AGA patients.
The mild to moderate group comprised 40% of study subjects, with the remaining 60% in the severe category. This suggests that low doses, such as 0.25mg, may be effective in severe AGA cases, at least in slowing and/or stopping their progression.
Figure 2: Pretreatment and post-treatment frontal region images of a 32-year-old man.[23]Pirmez, R., Salas-Callo, C-I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: A study with quantitative trichoscopic documentation. Journal of the American Academy of Dermatology. … Continue reading
Notably, this study recorded leg swelling in only 4% of the group and found no difference in arterial pressure, suggesting that the risk of side effects reduces with decreasing dose. If these results were indeed confounded by the addition of severe AGA patients, these findings may indicate that 0.25 mg has a similar efficacy to 5 mg with a better risk profile.
All in all, it’s challenging to draw conclusions because the study designs differ significantly. In the future, it would be interesting to see how different groups with the same grade of AGA respond to 0.25 mg of minoxidil versus 5 mg of minoxidil. This could help us construct realistic treatment regimens that are both effective and minimize the risk of side effects.
Telogen Effluvium
Chronic telogen effluvium (CTE) is a common condition characterized by excessive hair shedding. Because one of minoxidil’s proposed mechanisms of action is its ability to shorten the “resting” telogen phase (where a lot of follicles stay in CTE, as opposed to the anagen growing phase), researchers have hypothesized that oral minoxidil may be a helpful treatment for the condition.
These hypothetical notions were explored in a recent 2017 study.[24]Perera, E., Sinclair, R. (2017). Treatment of chronic telogen effluvium with oral minoxidil: A retrospective study. F1000 Research. 6. 1650. Available at: … Continue reading This particular study was retrospective and looked at individuals with CTE who had been prescribed oral minoxidil (dosages ranging from 0.25 mg to 2.5 mg, with the most common being 1 mg) in-clinic.
They found that most patients experienced a decrease in hair loss after 6 months, with all patients showing an improvement either at the 6 or 12-month mark. This does not make minoxidil the be-all and end-all for CTE. The oral minoxidil treatment wasn’t compared against a placebo or any other potential treatments (including combination therapies) for the condition, so we can only conclude that topical minoxidil is one potential treatment for CTE.
We also need to acknowledge that most CTE cases may have an underlying cause that, when resolved, could improve hair shedding. In these cases, addressing the root cause of CTE, whenever possible, is far more effective.
Alopecia Areata
Upon investigation of topical minoxidil’s benefits for alopecia areata (AA), an autoimmune form of hair loss, researchers found that topical minoxidil, on its own, really doesn’t do much for alopecia areata (AA) patients.[25]Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading However, interestingly, oral minoxidil at a dose of 5 mg daily appears to be effective.
Although oral minoxidil for AA hasn’t been well-studied, one study reported that 18% of AA subjects taking oral minoxidil showed an improved cosmetic response after ~35 weeks of use.
Considering the side effects of oral minoxidil are increased at a dose of 5 mg, the 18% response rate doesn’t seem too promising. But there may be one combination therapy that proves to be more effective.
Another study found that adding oral minoxidil to a current alopecia areata treatment may enhance outcomes without increasing the current dosage.
Tofacitinib is an immunomodulator drug currently used to treat alopecia areata. Although the drug is usually effective for most individuals at a 10 mg daily dosage, some users may need to double the dose to see hair growth.[26]Wambier, C.G., Craiglow, B.G., King, B.A. (2021). Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. Journal of the American Academy of Dermatology. 85(3). 743-745. … Continue reading
While this usually resolves treatment resistance, increasing the dose may also increase the risk associated with strong immunomodulatory effects, as well as the cost. So, there’s a real need for safe combination therapies to improve treatment outcomes without increasing the tofacitinib dose.
Oral minoxidil may be able to do just this. One study demonstrated that daily addition of oral minoxidil (2.5 mg for women and 5 mg, split into 2.5 mg doses, for men) to doses of tofacitinib ranging from 10 mg to 20 mg daily resulted in:
- 67% of patients achieved 75% or greater hair regrowth, compared to another study’s results, which demonstrated that 20% of patients achieved cosmetically acceptable regrowth on oral minoxidil alone.
- The remaining 33% of patients experienced hair regrowth, with a range of 11% to 74%.
Chemotherapy-Induced Hair Loss
Permanent chemotherapy-induced alopecia (PCIA), a hair-shedding disorder in which hair lost to chemotherapy does not grow back 6 months after the end of chemotherapy, is considered to be generally irreversible.
One case study challenges this.[27]Yang, X., Thai, K-E. (2016). Treatment of permanent chemotherapy-induced alopecia with low-dose oral minoxidil. The Australasian Journal of Dermatology. 57(4). E120-e132. Available at: … Continue reading After one year of 1 mg daily oral minoxidil treatment, a woman with PCIA achieved considerable growth. This is pretty amazing, considering no consistently effective treatment has been found for PCIA patients, yet. While future studies are still needed to confirm these findings, these results are certainly promising.
Ultra-Low and Ultra-High Doses of Minoxidil
Recent Brazilian studies have been published showing efficacy from both low and high doses of minoxidil.
Low-Dose
One 2025 Brazilian double-blind, randomized clinical trial was conducted on 100 men aged 25-55 years with Norwood-Hamilton stage 3V to 5V AGA. The participants were randomized 1:1 to receive either 2.5 mg or 5 mg oral minoxidil daily for 24 weeks.[28]Varma, D. (2025). Oral Minoxidil 2.5 mg vs 5 mg: Similar Efficacy for Androgenetic Alopecia in Men. Medscape. Available at: … Continue reading,[29]Fonseca, L.P.C., Miot, H.A., Chaves, C.R.P., Ramos, P.M. (2025). Oral minoxidil 2.5 mg versus 5 mg for male androgenetic alopecia: A double-blind randomized clinical trial. Journal of the American … Continue reading
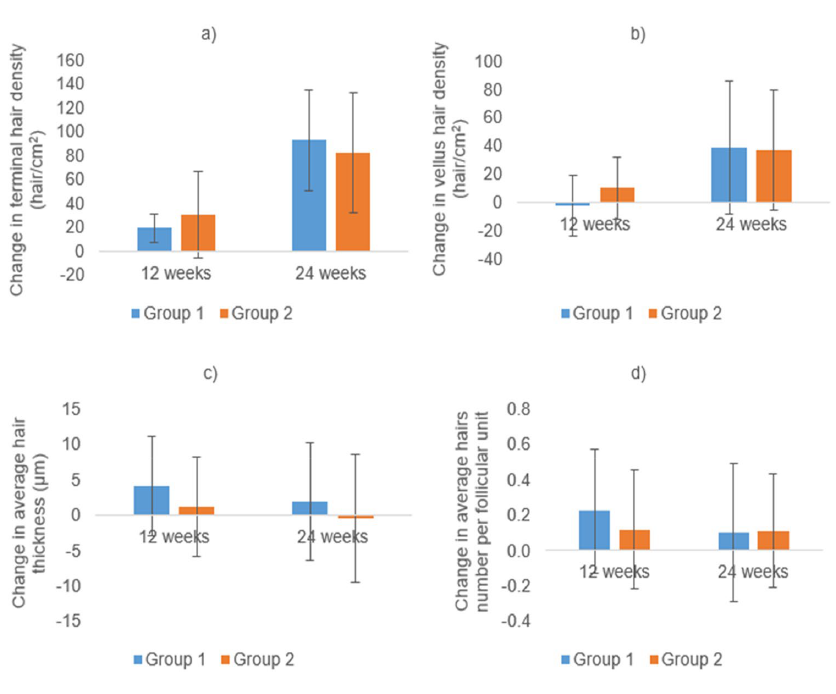
The primary outcome was the change in non-vellus hair density at the vertex. Secondary outcomes were total hair density changes, global photographic assessment, and adverse events.
At 24 weeks, there was no significant difference in non‑vellus hair density between the 2.5‑mg and 5‑mg groups (mean difference = 0.9 hairs/cm²; P = .403). Total hair density was likewise comparable (mean difference = 3.6 hairs/cm²; P = .078). Dermatologists assessing photographs in a blinded manner reported similar clinical improvement rates (64% with 2.5 mg vs 62% with 5 mg; P = .386), although self‑reported improvement was higher with 5 mg (92% vs 84%; P = .009). Adverse events, notably pedal edema and dizziness, were more common in the 5‑mg group (P = .024), while heart rate and both systolic and diastolic blood pressure remained similar between groups.
The study authors concluded that oral minoxidil at 2.5 mg/day provided comparable efficacy to the 5 mg/day dose in treating male AGA, while offering a better safety profile. They further noted that these results support 2.5 mg/day as an appropriate starting dose in clinical settings.
High-Dose
A 2025 retrospective multicenter study in Brazil and Spain evaluated high-dose oral minoxidil (>5 mg/day) for AGA in 57 men. Most received 10 mg nightly, often alongside finasteride or dutasteride, after at least one year on low-dose minoxidil (≤5 mg/day).[30]Moreno-Arrones, O.M., Hermosa-Gelbard, A., Saceda-Corralo, D., Jiminez-Cauhe, J., Ortega-Quijano, D., Pirmez, R., Galvan, S. (2025). High-dose Oral Minoxidil for the Treatment of Androgenetic … Continue reading
After 9-12 months, 45.6% improved hair density by 10-30%, 17.5% improved by >50%, and 17.5% improved by <10%, while 7% saw no change.
Adverse effects occurred in 24.6%, mainly hypertrichosis (17.5%) and tachycardia (3.5%), with hypertensive patients tolerating treatment well under blood-pressure monitoring.
The authors concluded that high-dose oral minoxidil may benefit selected men with AGA refractory to standard therapy, but variability in response and increased side effects highlight the need for gradual dose escalation and further research in larger, more diverse populations.
Based on this evidence, we think that starting off with a low dose can be effective (and potentially safer) than the standard oral minoxidil dose. However, if you are struggling to see benefits, there is efficacy data for high doses. It would be interesting for a study to compare how much more effective ultra-high doses of minoxidil are for hair regrowth than 5 mg (for example). This would help us to determine whether it is worth the potential increased side effects.
Furthermore, those who decide to go the low-dose oral minoxidil route may find it beneficial to add spironolactone to their routine.
Is Oral Minoxidil Safe?
Oral minoxidil has gained attention as an effective treatment for hair loss, but questions about its safety persist, particularly in comparison to the topical form. Here’s what the evidence shows.
Cardiovascular Risks: Myocardial and Pericardial Effusion
There are documented cases where people taking oral minoxidil developed pericardial effusion (fluid around the heart) and even pericarditis, sometimes at low doses used for hair loss.[31]Dlova, N.C., Jacobs, T., Singh, S. (2022). Pericardial, pleural effusion and anasarca: A rare complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 11(28). 94-96. Available at: … Continue reading These events are rare but have prompted caution within the medical community.
Severe cardiovascular complications like pericardial effusion have been recorded the high doses (10-40 mg) used for hypertension, with rates around 3% in these populations.[32]Bentivegna, K., Zhou, A.E., Adalsteinsson, J.A., Sloan, B. (2022). Letter in reply: Pericarditis and peripheral edema in a healthy man on low-dose oral minoxidil therapy. JAAD Case Reports. 20(29). … Continue reading However, it should be noted that this was reported in the 1980s in patients with severe hypertension, with no recent data available.
However, at the lower doses used for hair loss (0.25-5 mg), these complications are much less common and appear to be idiosyncratic (based on the individual response) rather than dose-dependent.[33]Gupta, A.K., Bamimore, M.A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., Talukder, M. (2024). Low-Dose Oral Minoxidil and Associated Adverse Events: Analyses of the FDA Adverse Event … Continue reading
Large observational studies and clinical experience with thousands of patients using low-dose oral minoxidil have not shown a significant increase in life-threatening cardiac events.[34]Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., … Continue reading Most side effects are mild and reversible with dose adjustments or discontinuation of the medication.[35]Bloch, D.L., Carlos, R.M.D. (2025). Side Effects’ Frequency Assessment of Low Dose Oral Minoxidil in Male Androgenetic Alopecia Patients. Skin Appendage Disorders. 11(1). 14-18. Available at: … Continue reading
Autopsy reports from patients who took extremely high doses (e.g., 60 mg) have not consistently shown pericardial or myocardial effusion, suggesting the risk is not universal or inevitable, even at high exposures.[36]Sobota, J.T. (1989). Review of cardiovascular findings in humans treated with minoxidil. Toxicologic pathology. 17(1 Pt 2). 193-202. Available at: https://doi.org/10.1177/019262338901700115
Subclinical and EKG Changes
At high doses, minoxidil can cause changes in the heart’s electrical activity, and this has been observed in up to 60-90% of patients.[37]Hall, D., Charocopos, F., Froer, K.L., Rudolph, W. (1979). ECG changes during long-term minoxidil therapy for severe hypertension. Archives of Internal Medicine. 139(70. 790-794. Available at: PMID: … Continue reading These changes are typically subclinical, meaning they do not cause symptoms or progress to life-threatening arrhythmias, and often resolve with continued use or a reduction in the dose.
In studies of low-dose minoxidil for hair loss, only minor, asymptomatic EKG changes have been observed, with no evidence of clinically significant heart rhythm disturbances in otherwise healthy individuals.[38]Jiminiez-Cauhe, J., Pirmez, R., Muller-Ramos, P., Melo, D.F., Ortega-Quijano, D., Moreno-Arrones, O.M., Saceda-Corralo, D., Gil-Redondo, R., Hermosa-Gelbard, A., Dias-Sanabria, B., Restom, D., … Continue reading
Real-World Safety: Observational and Clinical Data
The most common side effects at hair loss doses are hypertrichosis, mild ankle swelling, lightheadedness, and occasional palpitations.[39]Bloch, D.L., Carlos, R.M.D. (2025). Side Effects’ Frequency Assessment of Low Dose Oral Minoxidil in Male Androgenetic Alopecia Patients. Skin Appendage Disorders. 11(1). 14-18. Available at: … Continue reading Serious cardiac events are exceedingly rare. Most patients who experience side effects can continue treatment with dose adjustments. Only a small fraction discontinue due to adverse effects.
This favorable safety profile is not only a function of the lower dose but may also reflect the generally healthier status of this population compared to those prescribed high-dose minoxidil for hypertension. Most studies examining minoxidil for hair growth routinely exclude individuals with significant underlying disease.
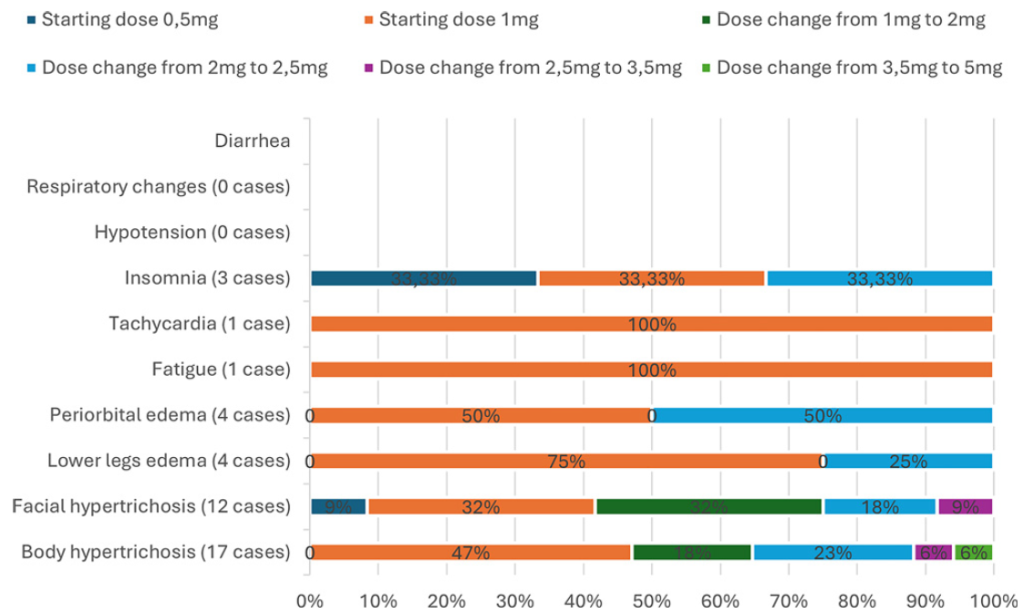
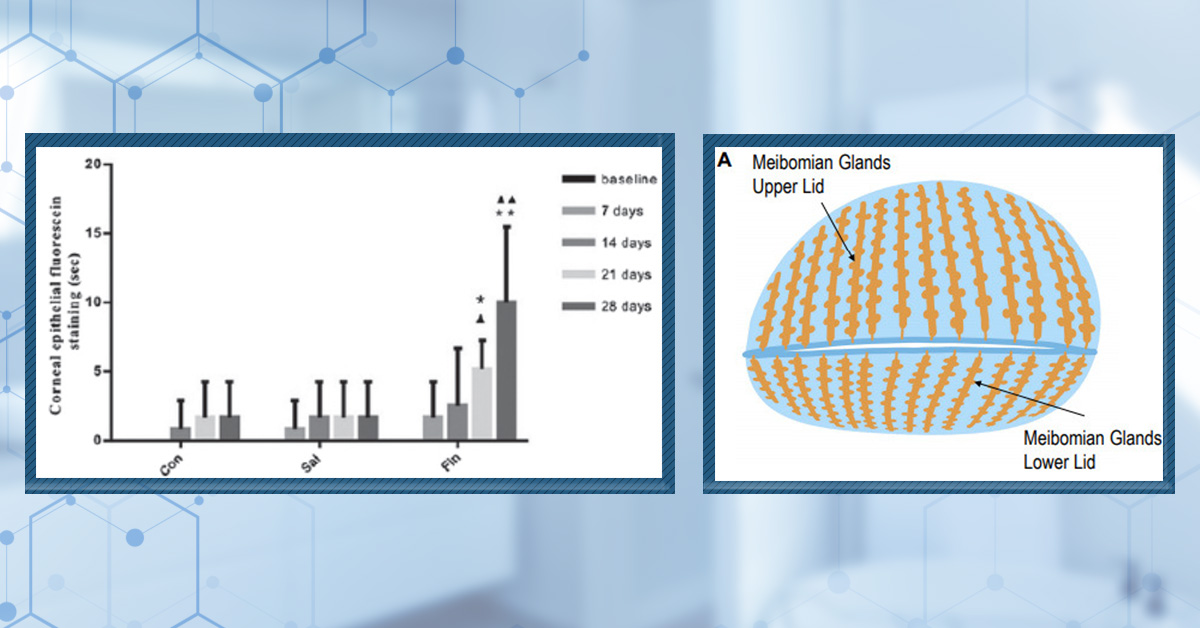
Figure 3: Frequency of side effects using low-dose oral minoxidil according to dose.[40]Bloch, D.L., Carlos, R.M.D. (2025). Side Effects’ Frequency Assessment of Low Dose Oral Minoxidil in Male Androgenetic Alopecia Patients. Skin Appendage Disorders. 11(1). 14-18. Available at: … Continue reading
Experts recommend that patients, especially those with pre-existing heart, kidney, or liver conditions, be monitored by a healthcare provider when starting oral minoxidil.[41]Gupta, A.K., Bamimore, M.A., Haber, R., Williams, G., Piguet, V., Talukder, M. (2024). The Role of Patient- and Drug-Related Factors in Oral Minoxidil and Pericardial Effusion: Analyses of Data from … Continue reading
How Can I Make Oral Minoxidil Safer?
Here are a number of strategies you can use to help minimize side effects while maintaining results:
- Titrate Your Dose
Begin with the lowest effective dose and increase gradually if needed. This approach helps your body adjust and can reduce the risk of side effects, such as swelling, dizziness, or heart palpitations.
Based on the published evidence, we believe that the best balance between efficacy and safety is 2.5 mg daily for men and 0.25-1.25 mg daily for women.
- Split the Dose: Morning & Evening
Oral minoxidil has a short half-life (about 3 hours). Taking your daily dose all at once can cause higher peak blood levels, increasing the risk of side effects. Taking half in the morning and half in the evening can help keep blood levels steadier and may reduce side effects.
- Consider Sublingual Administration
Taking minoxidil sublingually (letting it dissolve under your tongue) allows the drug to enter your bloodstream directly, bypassing the liver’s first-pass metabolism. Sublingual minoxidil becomes active when it reaches tissues with the right enzymes, such as those in the scalp, potentially enhancing its effectiveness. Studies suggest that sublingual minoxidil can lead to lower peak blood concentrations and fewer systemic side effects, while still promoting hair growth.[42]Guo, R-X., Zhao, Y-K., Hu, K-J., Hia, K.M., Shi, W., Yi, Y-X., Gong, H-Y., Wang, J-B., Gao, Y. (2025). Research progress in the treatment of non-scarring alopecia: mechanism and treatment. Frontiers … Continue reading
- Switch To Topical Minoxidil
If oral minoxidil isn’t working for you because of the side effects, consider switching to the topical formulation. And, if you’re able to tolerate a 2% or 5% formulation well, you could consider adding microneedling or retinoic acid to boost your gains.
To find out more information about the potential side effects and how to avoid them, watch our video: Oral Minoxidil: How to Eliminate Side Effects.
Is Oral Minoxidil Better Than Topical Minoxidil?
Deciding between oral and topical minoxidil involves weighing efficacy, convenience, safety, and individual response.
Efficacy: Which Works Better?
At standard strengths, both 5% topical minoxidil and low-dose oral minoxidil (1-5 mg) show similar efficacy in clinical trials for total area hair count (TAHC).[43]Fazal, F., Malik, B.H., Malik, H.M., Sabir, B., Mustafa, H., Ahmed, M., Abid, A., Adil, M.L., Shafi, U., Saad, M. (2025). Can oral minoxidil be the game changer in androgenetic alopecia? A … Continue reading However, oral minoxidil often leads to slightly thicker, more robust regrowth for some users, especially those with diffuse thinning.
When topical minoxidil is combined with enhancers, such as retinoic acid, at concentrations of 7-8% (or higher), the results can surpass those seen with oral minoxidil. Our members consistently report better outcomes with these combinations, even compared to 5 mg oral minoxidil.
Advantages of Oral Minoxidil
- More convenient: Oral minoxidil is taken as a pill once or twice daily, while topical minoxidil requires 1-2 daily applications, which can be inconvenient and affect adherence.
- No product residue: Oral minoxidil eliminates the sticky residue and styling issues associated with topical solutions.
- Cost-effective: Oral minoxidil is generally about half the price of topical formulations.
- Bypasses scalp enzyme variance: Oral minoxidil is activated in the liver, sidestepping the variability in scalp sulfotransferase enzyme activity that limits topical efficacy for some users.[44]Goren, A., Shapiro, J., Roberts, J., McCoy, J., Desai, N., Zarrab, X., Pietrzak, A., Lotti, T. (2014). Clinical utility and validity of minoxidil response testing in androgenetic alopecia. … Continue reading
- Less scalp irritation: Oral minoxidil avoids the dryness, irritation, and brittleness sometimes caused by topical applications.
- May improve beard density: Some users report increased facial hair growth, which can be a benefit or drawback, depending on personal preference.[45]Pirmez, R., Salas-Callo, C-I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: a study with quantitative trichoscopic documentation. Journal of the American Academy of Dermatology. … Continue reading
- Better for brittle hair: Those with fragile or chemically treated hair may prefer oral minoxidil to avoid further brittleness.
Drawbacks of Oral Minoxidil
- Unwanted body hair: Oral minoxidil can cause hair growth on other parts of the body, which is especially concerning for women.
- Systemic side effects: Risks include fluid retention, rare cardiovascular effects, headaches, and skin rashes. These are uncommon at hair loss doses (0.25-5 mg), but still possible.
- Prescription required: Unlike topical minoxidil, oral formulations require a prescription from a doctor.
Advantages of Topical Minoxidil
- Fewer systemic effects: Topical minoxidil acts locally, so there’s less risk of systemic side effects.
- Easier access: Available over-the-counter without a prescription.
- Customizable formulations: Various strengths and enhancers (like retinoic acid) can be tailored to individual needs.
Drawbacks of Topical Minoxidil
- Variable response: Efficacy depends on scalp enzyme activity, which varies between individuals.
- Scalp irritation: Some may experience irritation, dryness, or increased hair brittleness.[46]Shadi, Z. (2023). Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatological Therapy (Heidelb). 13(5). 1157-1169. Available at: … Continue reading
- Residue and Compliance: Daily application can be messy and inconvenient, which can impact adherence.
Special Considerations: Scalp Inflammation
Underlying scalp inflammation (like seborrheic dermatitis) can reduce the effectiveness of topical minoxidil.[47]Mahe, Y.F., Cheniti, A., Tacheau, C., Antonelli, R., Planard-Luong, L., de Bernard, S., Buffat, L., Barbarat, P., Kanoun-Copy, L. (2021). Low-Level Light Therapy Downregulates Scalp Inflammatory … Continue reading Addressing inflammation is crucial before starting or modifying any hair loss treatment.
The bottom line:
Oral minoxidil is more convenient, cost-effective, and bypasses scalp enzyme limitations, making it a strong option, especially for those who don’t respond to topical or want to avoid scalp irritation.
High-strength topical minoxidil, when combined with treatments such as retinoic acid and microneedling, often delivers the best results but comes with more hassle, a higher cost, and a greater risk of scalp irritation.
Standard topical minoxidil remains a solid, accessible first-line option, especially for those who prefer to avoid systemic medications.
Ultimately, the best choice depends on your hair loss pattern, your response to treatment, your tolerance for side effects, and your lifestyle.
Can You Take Them Together?
Yes, it is possible to use oral and topical minoxidil together; however, this approach should only be considered under the supervision of a healthcare provider. Combining both forms may provide a more robust treatment for hair loss.
Some dermatologists suggest that using both can enhance efficacy, especially for individuals who have not achieved optimal results with one form alone. However, using both simultaneously increases the risk of side effects. Therefore, the decision to combine oral and topical minoxidil should be made with professional guidance to ensure proper dosing and monitoring for adverse effects.
Who are the Best Candidates for Oral Minoxidil?
According to the research we just outlined, you’re a good candidate for oral minoxidil if:
- You’re a male with AGA, TE, or permanent chemotherapy-induced alopecia who doesn’t respond to topical minoxidil and/or finds it difficult to comply with the treatment protocol.
- You’re a female with FPHL, TE, or permanent chemotherapy-induced alopecia who doesn’t respond to topical minoxidil and/or finds it difficult to comply with the treatment protocol, and you’re okay with a chance of increased hair growth in other places of the body.
- You have a healthy liver and normal to high blood pressure. If you are somewhat hypertensive, oral minoxidil may have the joint benefit of hair growth and antihypertensive effects.
- You’re a female who’s already taking spironolactone for FPHL and wants to see better results, or has seen a plateau with spironolactone alone.
- You’re an AA patient resistant to traditional immunomodulatory treatment.
- You suffer from a brittle hair disorder or hair loss and brittle hair and want to avoid exacerbating brittleness with topical minoxidil.
- You can obtain a prescription.
On the other hand, you’re not a good candidate for oral minoxidil if…
- You’re a female who’s not comfortable with the idea of increased hair growth outside of the scalp.
- You regularly drink alcohol, which can exacerbate the antihypertensive effects of minoxidil.
- You have or have had kidney, cardiovascular, or liver issues.
- You’re pregnant or planning on becoming pregnant.
- You’re currently taking another vasodilating/antihypertensive drug or a muscle relaxer like tizanidine. Taking two vasodilating drugs at once can increase the risk of side effects associated with low blood pressure.
What Are The Best Practices for Taking Oral Minoxidil?
When taking oral minoxidil for hair loss, it’s best to start with the lowest effective dose, typically 2.5 mg daily for men and 0.25–1.25 mg daily for women. The dose should be increased gradually only if needed, under the supervision of a healthcare provider. Splitting the total daily dose into morning and evening halves can help maintain steady blood levels and reduce the risk of side effects, such as palpitations, swelling, or dizziness.
Sublingual administration (allowing the tablet to dissolve under your tongue) is an alternative that may further minimize systemic side effects by bypassing the liver’s first-pass metabolism, potentially making the medication more tolerable for individuals who are sensitive to it. Throughout treatment, monitor for common side effects, such as unwanted hair growth (hypertrichosis), ankle swelling, or lightheadedness, and promptly report any serious symptoms, including chest pain or significant changes in heart rate.
Regular check-ins with your healthcare provider are essential, especially if you have underlying heart, kidney, or liver issues or take other medications that affect blood pressure. If side effects occur, consider reducing the dose, splitting it further, switching to a sublingual or topical minoxidil formulation, or discontinuing use as advised by your healthcare provider.
Avoid combining oral minoxidil with other vasodilators or muscle relaxants without consulting a healthcare professional. Address any scalp inflammation before starting or adjusting therapy to maximize results and safety.
Final Thoughts
Oral minoxidil has emerged as a compelling option for individuals seeking effective hair loss treatment, offering robust regrowth potential and convenience for both men and women who may not respond to or tolerate topical formulations. While research supports its efficacy across a range of hair loss conditions, including androgenic alopecia and chronic telogen effluvium, it is not without risks, most notably rare but serious cardiovascular side effects, as well as more common issues such as unwanted hair growth and mild edema. For many, oral minoxidil represents a powerful, evidence-based tool in the fight against hair loss, but its use should always be informed, individualized, and guided by medical expertise to ensure both safety and satisfaction. If you are a good candidate and proceed thoughtfully, oral minoxidil can play a pivotal role in your hair restoration journey.
References[+]
References ↑1 Patel, P., Nessel, T.A., Kumar, D. (2023). Minoxidil. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/book/NBK482378/ Accessed: July 2025 ↑2 Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of Cosmetic Dermatology. 20(12). 3759-3781. Available at: https://doi.org/10.1111/jocd.14537 ↑3 Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. Advances in Dermatology and Allergology. 39(3). 472-478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑4 Chitalia, J., Dhurat, R., Goren, A., McCoy, J., Kovacevic, M., Situm, M., Naccarato, T., Lotti, T. (2018). Characterization of follicular minoxidil sulfotransferase activity in a cohort of pattern hair loss patients from the Indian Subcontinent. Dermatologic Therapy. 31(6). E12688. Available at: https://doi.org/10.1111/dth.12688 ↑5, ↑25 Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑6 Beach, R.A. (2018). Case series of oral minoxidil for androgenetic and traction alopecia: Tolerability & the five C’s of oral therapy. Dermatologic Therapy. 31(6). E12707. Available at: https://doi.org/10.1111/dth.12707 ↑7 Gupta, A.K., Talukder, M., Shemar, A., Priaccini, B.A., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: https://doi.org/10.115//000531890 ↑8 Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair loss disorders. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑9 Kwack, M.H., Kang, B.M., Kim, M.K., Kim, J.C., Sung, Y.K. (2011). Minoxidil activates ꞵ-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. Journal of Dermatological Science. 62(3). 154-159. Available at: https://doi.org/10.1016/j.dermsci.2011.01.013 ↑10 Wang, X., Liu, Y., He, J., Wang, J., Chen, X., Yang, R. (2022). Regulation of signaling pathways in hair follicle stem cells. Burns Trauma. 10. 1-19. Available at: https://doi.org/10.1093/burnst/tkac022 ↑11 Kang, J-I., Choi, K.Y., Han, S-C., Nam, H., Lee, G., Kang, J-H., Koh, Y.S., Hyun, J.W., Yoo, E.S., Kang, H.K. (2022). 5-Bromo-3,4-dihydroxybenzaldehyde Promotes Hair Growth through Activation of Wnt/β-Catenin and Autophagy Pathways and Inhibition of TGF-β Pathways in Dermal Papilla Cells. Molecules. 27(7). 2176. Available at: https://doi.org/10.3390/molecules27072176 ↑12 Jan, J.H., Kwon, O.S., Chung, J.H., Cho, K.H., Eun, H.C., Kim, K.H. (2004). Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. Journal of Dermatological Science. 34(2). 91-98. Available at: https://doi.org/10.1016/j.dermsci.2004.01.002 ↑13 Nagai, N., Iwai, Y., Sakamoto, A., Otake, H., Oaku, Y., Abe, A., Nagahama, T. (2019). Drug Delivery System Based on Minoxidil Nanoparticles Promotes Hair Growth in C57BL/6 Mice. International Journal of Nanomedicine. 1(14). 7921-7931. Available at: https://doi.org/10.2147/IJN.S225496 ↑14 Shin, D.W. (2022). The physiological and pharmacological roles of prostaglandins in hair growth. The Korean Journal of Physiology and Pharmacology. 26(6). 405-415. Available at: https://doi.org/10.4196/kjpp.2022.26.6.405 ↑15 Majewski, M., Gardas, K., Waskiel-Burnat, A., Ordak, M., Rudnicka, L. (2024). The Role of Minoxidil in Treatment of Alopecia Areata: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 13(24). 7712. Available at: https://doi.org/10.3390/jcm13247712 ↑16 Fechine, C.O.C., Valente, N.Y.S., Romiti, R. (2022). Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. Anais Brasileiros de Dermatologia. 97(3). 348-357. Available at: https://doi.org/10.1016/j.abd.2021.08.008 ↑17 Ramos, M.P., Sinclair, R.D., Kasprzak, M., Miot, H.A. (2020). Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss. Journal of the American Academy of Dermatology. 82(1). 252-253. Available at: https://doi.org/10.1016/j.jaad.2019.08.060 ↑18 Sinclair, R.D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. International Journal of Dermatology. 57(1). 104-109. Available at: https://doi.org/10.1111/ijd.13838 ↑19 Lueangarun, S., Panchaprateep, R., Tempark, T., Noppakun, N. (2015). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Journal of the American Academy of Dermatology. 72(5). AB113. Available at: https://doi.org/10.1016/j.jaad.2015.02.466 ↑20, ↑21 Jiminiez-Couche, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Fernandez-Nieto, D., Vano-Galvan, S. (2019). Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. 81(2). 648-649. Available at: https://doi.org/10.1016/j.jaad.2019.04.054 ↑22, ↑23 Pirmez, R., Salas-Callo, C-I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: A study with quantitative trichoscopic documentation. Journal of the American Academy of Dermatology. 82(1). E21-e22. Available at: https://doi.org/10.1016/j.jaad.2019.08.084 ↑24 Perera, E., Sinclair, R. (2017). Treatment of chronic telogen effluvium with oral minoxidil: A retrospective study. F1000 Research. 6. 1650. Available at: https://doi.org/10.12688/f1000research.11775.1 ↑26 Wambier, C.G., Craiglow, B.G., King, B.A. (2021). Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. Journal of the American Academy of Dermatology. 85(3). 743-745. Available at: https://doi.org/10.1016/j.jaad.2019.08.080 ↑27 Yang, X., Thai, K-E. (2016). Treatment of permanent chemotherapy-induced alopecia with low-dose oral minoxidil. The Australasian Journal of Dermatology. 57(4). E120-e132. Available at: https://doi.org/10.1111/ajd.12350 ↑28 Varma, D. (2025). Oral Minoxidil 2.5 mg vs 5 mg: Similar Efficacy for Androgenetic Alopecia in Men. Medscape. Available at: https://www.medscape.com/viewarticle/oral-minoxidil-2-5-mg-vs-5-mg-similar-efficacy-androgenetic-2025a1000ox7?reg=1 Accessed: October 2025 ↑29 Fonseca, L.P.C., Miot, H.A., Chaves, C.R.P., Ramos, P.M. (2025). Oral minoxidil 2.5 mg versus 5 mg for male androgenetic alopecia: A double-blind randomized clinical trial. Journal of the American Academy of Dermatology. S0190-9622(25)02819-1 Available at: https://doi.org/10.1016/j.jaad.2025.09.031 ↑30 Moreno-Arrones, O.M., Hermosa-Gelbard, A., Saceda-Corralo, D., Jiminez-Cauhe, J., Ortega-Quijano, D., Pirmez, R., Galvan, S. (2025). High-dose Oral Minoxidil for the Treatment of Androgenetic Alopecia. Actas Dermo-Sifiliograficas. 116. T769 – T772. Available at: https://doi.org/10.1016/j.ad.2025.05.018 ↑31 Dlova, N.C., Jacobs, T., Singh, S. (2022). Pericardial, pleural effusion and anasarca: A rare complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 11(28). 94-96. Available at: https://doi.org/10.1016/j.jdcr.2022.07.044 ↑32 Bentivegna, K., Zhou, A.E., Adalsteinsson, J.A., Sloan, B. (2022). Letter in reply: Pericarditis and peripheral edema in a healthy man on low-dose oral minoxidil therapy. JAAD Case Reports. 20(29). 110-111. Available at: https://doi.org/10.1016/j.jdcr.2022.08.057 ↑33 Gupta, A.K., Bamimore, M.A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., Talukder, M. (2024). Low-Dose Oral Minoxidil and Associated Adverse Events: Analyses of the FDA Adverse Event Reporting System (FAERS) With a Focus on Pericardial Effusions. Journal of Cosmetic Dermatology. 24(1). E16574. Available at: https://doi.org/10.1111/jocd.16574 ↑34 Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., Salas-Callo, C.I., Vincenzi, C., Yin, L., Lo-Sicco, K., Piraccini, B.M., Rudnicka, L., Shapiro, J., Tosti, A., Sinclair, R., Bhoyrul, B. (2021). Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. Journal of the American Academy of Dermatology. 84(6). 1644-1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑35, ↑39, ↑40 Bloch, D.L., Carlos, R.M.D. (2025). Side Effects’ Frequency Assessment of Low Dose Oral Minoxidil in Male Androgenetic Alopecia Patients. Skin Appendage Disorders. 11(1). 14-18. Available at: https://doi.org/10.1159/000539969 ↑36 Sobota, J.T. (1989). Review of cardiovascular findings in humans treated with minoxidil. Toxicologic pathology. 17(1 Pt 2). 193-202. Available at: https://doi.org/10.1177/019262338901700115 ↑37 Hall, D., Charocopos, F., Froer, K.L., Rudolph, W. (1979). ECG changes during long-term minoxidil therapy for severe hypertension. Archives of Internal Medicine. 139(70. 790-794. Available at: PMID: 36861 ↑38 Jiminiez-Cauhe, J., Pirmez, R., Muller-Ramos, P., Melo, D.F., Ortega-Quijano, D., Moreno-Arrones, O.M., Saceda-Corralo, D., Gil-Redondo, R., Hermosa-Gelbard, A., Dias-Sanabria, B., Restom, D., Porrino-Bustamante, M.L., Pindado-Ortega, C., Berna-Rico, E., Fernandez-Nieto, D., Ramos, M., Jaen-Olasolo, P., Vano-Galvan, S. (2024). Safety of Low-Dosr Oral Minoxidil in Patients With Hypertension and Arrhythmia: A Multicenter Study of 264 Patients. Actas Dermo-Sifiliograficas. 115(1). 28-35. Available at: https://doi.org/10.1016/j.ad.2023.07.019 ↑41 Gupta, A.K., Bamimore, M.A., Haber, R., Williams, G., Piguet, V., Talukder, M. (2024). The Role of Patient- and Drug-Related Factors in Oral Minoxidil and Pericardial Effusion: Analyses of Data from the United States Food and Drug Administration Adverse Event Reporting System. Journal of Cosmetic Dermatology. 24(2). E16732. Available at: https://doi.org/10.1111/jocd.16732 ↑42 Guo, R-X., Zhao, Y-K., Hu, K-J., Hia, K.M., Shi, W., Yi, Y-X., Gong, H-Y., Wang, J-B., Gao, Y. (2025). Research progress in the treatment of non-scarring alopecia: mechanism and treatment. Frontiers in Pharmacology. 26(1544068). Available at: https://doi.org/10.3389/fphar.2025.1544068 ↑43 Fazal, F., Malik, B.H., Malik, H.M., Sabir, B., Mustafa, H., Ahmed, M., Abid, A., Adil, M.L., Shafi, U., Saad, M. (2025). Can oral minoxidil be the game changer in androgenetic alopecia? A comprehensive review and meta-analysis comparing topical and oral minoxidil for treating androgenetic alopecia. Skin Health and Disease. 5(2). 95-101. Available at: https://doi.org/10.1093/skinhd/vzaf009 ↑44 Goren, A., Shapiro, J., Roberts, J., McCoy, J., Desai, N., Zarrab, X., Pietrzak, A., Lotti, T. (2014). Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatologic Therapy. Available at: https://doi.org/10.1111/dth.12164 ↑45 Pirmez, R., Salas-Callo, C-I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: a study with quantitative trichoscopic documentation. Journal of the American Academy of Dermatology. 82(1). E21-e22. Available at: https://doi.org/10.1016/j.jaad.2019.08.084 ↑46 Shadi, Z. (2023). Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatological Therapy (Heidelb). 13(5). 1157-1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑47 Mahe, Y.F., Cheniti, A., Tacheau, C., Antonelli, R., Planard-Luong, L., de Bernard, S., Buffat, L., Barbarat, P., Kanoun-Copy, L. (2021). Low-Level Light Therapy Downregulates Scalp Inflammatory Biomarkers in Men with Androgenetic Alopecia and Boost Minoxidil 2% to Bring a Sustainable Hair Regrowth Activity. Lasers in Surgery & Medicine. 53(9). Available at: https://doi.org/10.1002/lsm.23398 We’re thrilled to announce the arrival of Ulo: a haircare brand that offers best-in-class hair growth products and focuses on solving the biggest problems plaguing the hair loss industry.
Ulo’s core commitment to hair loss sufferers is its prioritization of evidence, personalization, and consumer safety. And there’s a reason we’re so confident in Ulo’s ability to deliver: we co founded the brand.
In fact, the Perfect Hair Health team was involved in every aspect of Ulo’s product development: its selection of manufacturers, ingredients, formulations, concentrations, & more. Now the product formulations for which we’ve been advocating for 10+ years are finally offered, all through a single provider.
Whether it’s over-the-counter serums or prescription products, Ulo represents the next evolution in telehealth haircare: one that puts the consumer first, sets realistic expectations, and supports customers at every step on their journey to better hair.
Below, we’ll detail our journey to launching Ulo, the problems Ulo solves, its core offerings, and why we currently consider Ulo to be a revolutionary step forward for consumers.
For those interested in this level of product personalization and support, Ulo is also offering 15% off all their products, forever. Any purchase made through the links in this article will result in a 15% discount applied to all product purchases, including all renewals. This is our thank you to you, and your opportunity to lock in a permanently discounted price on all products.
Why Ulo?
For 11 years, Perfect Hair Health has acted solely as a consumer advocacy resource for men and women fighting hair loss.
During this time, we never once endorsed a single hair growth product: no pill, supplement, topical, shampoo, or device. Instead, we focused entirely on educating hair loss sufferers on how to properly read studies, select treatments, and maximize their chances for hair regrowth.
For just a few examples of this commitment, see the following content pieces:
- What Causes Hair Loss? (Interactive Guide)
- Understanding Hair Loss Disorders (Interactive Guide)
- The Hair Loss Industry Is Broken: Evidence Quality Masterclass (Video)
- Nutrafol: The Problem With Their Clinical Studies (Company Investigation)
- Our Peer-Reviewed Studies (Publications)
- Independent Third-Party Lab Testing Results (Product Investigations)
But, as the hair loss industry continued to evolve, so too did its bad practices – particularly the sale of dangerous, improperly formulated products with ingredients that were “trending” on social media, but that lacked adequate efficacy and safety data.
For years, our organization called attention to these concerns – creating free evidence-based articles, videos, & interactive guides detailing these exact problems & how to protect yourself. And yet this entire time, as the hair loss industry expanded, we watched these problems only grow worse. We’ll detail several of them later in this article.
Two years ago, Perfect Hair Health was offered a once-in-a-lifetime opportunity to become more than just a voice of advocacy. We were approached with an offer to go beyond education and to start solving the very problems that have plagued this industry for years.
This meant an opportunity to create products that could finally serve as the antidote to every bad practice currently employed by other haircare brands at scale.
Initially, we were skeptical. We’ve seen firsthand how other brands deprioritize consumer safety in the name of “business decisions,” like with brands that include corticosteroids in their Rx topicals to offset irritation and increase recurring purchases… but at the expense of the risk of long-term, permanent skin thinning for their patients.
If we were going to become involved in a brand, we would need full control over the entire production process for hair loss. We wanted the sole ability to fire compounding pharmacies that failed to meet purity standards. We wanted final say in every aspect of product development: the ingredients, concentrations, formulations, & more. Without this level of control, we would never be able to prioritize what’s right for the consumer over what’s right for business.
We were pleasantly surprised to find 100% alignment with those offering a partnership. With just how easy it is to “cut corners” in the hair loss industry, we can’t describe how refreshing it was to connect with others who actually wanted to do right by the consumer.
After much reflection, we decided to step off the sidelines as critics and instead, start building a brand that walked the walk and started solving the hair loss industry’s biggest problems.
That brand is Ulo: a telehealth company dedicated exclusively to hair growth, and founded on three key pillars: evidence, personalization, and consumer safety.
What Problems Need Solving In The Hair Loss Industry?
Over the years, we’ve watched haircare brands adopt a set of bad practices that seemed uniquely targeted toward their bottom line, rather than consumer interests.
We’ll detail just a few of these below, along with how Ulo solves these problems and sets the bar higher.
Problem #1: Mega-Dosed Topicals
Other telehealth brands are selling mega-dose versions of topical finasteride & topical dutasteride – letting people think it’s staying on the scalp, when in reality, the dose is 60x too high, so it leaks into the blood & causes hormonal changes to the same extent as oral finasteride.
Videos here and here. FDA warning here.
Ulo’s Solution: Low-Dose Topical Finasteride & Low-Dose Topical Dutasteride
Ulo offers two kinds of topical finasteride & topical dutasteride: low-dose and full-strength.
For those truly interested in localization, the lower-dose topicals are far more appropriate than the mega-dosed topicals sold by other telehealth brands. And even more encouragingly, Ulo offers full customization of either topical – with the ability to add in ingredients like 7% minoxidil, 0.01% retinoic acid (tretinoin), 0.2% caffeine, 0.01% melatonin, and 1% cetirizine – so prospective users can ensure better control over every ingredient, pending a prescription:
Ulo’s Topical Finasteride
- Low-Dose Topical Finasteride (Optional: Add Enhancers)
- Full-Strength Topical Finasteride (Optional: Add Enhancers)
Ulo’s Topical Dutasteride
- Low-Dose Topical Dutasteride (Optional: Add Enhancers)
- Full-Strength Topical Dutasteride (Optional: Add Enhancers)
Problem #2: Corticosteroids In Rx Topicals (Permanent Skin Thinning)
Brands are adding dangerous ingredients to their Rx topicals –– like corticosteroids. They do this because their serums often contain propylene glycol, which helps with ingredient penetration but also causes skin irritation. Yet rather than fix their formulations, brands offset the irritation with corticosteroids (hydrocortisone, fluocinolone, etc.). This works in the short-term, and very likely, causes permanent, irreversible skin thinning after years of uninterrupted use.
Article here. Video here. Study here.
Ulo’s Solution: No Corticosteroids, No Propylene Glycol, & Less-Irritating Formulations
Ulo doesn’t use propylene glycol in any of its topicals. Instead, its partner pharmacies have spent significant time developing & iterating base formulations containing water, alcohol, and glycol-like compounds – formulations that apply easily, dry quickly, allow for ample ingredient penetration into the hair follicles, and above all, are less irritating.
The end-result: Ulo’s topicals don’t need to add corticosteroids to mask irritation – because they were formulated properly from the start.
Note: some of Ulo’s optional ingredients – like retinoic acid (tretinoin) – can enhance the activation and penetration of minoxidil, but may also cause mild irritation in a subset of individuals. For those who discover they cannot tolerate retinoic acid (tretinoin), Ulo’s physician partners can easily remove this ingredient for them. This is a much better, much more sustainable alternative than simply masking irritation with a corticosteroid, especially given the long-term risks of skin irritation.
Ulo’s topicals can be found here, with a guide to help you select your offering here.
Problem #3: Selling Dangerous, “Trending” Ingredients
Other telehealth brands are offering “trending” drugs with long-term safety risks, like latanoprost. Originally developed to treat glaucoma, latanoprost is now widely added to Rx topicals for hair growth. The basis? Two small clinical studies, the longest of which ran for 9 months. The problem? The doses sold by telehealth brands are, at times, thousands of times higher than what was used to treat glaucoma. They absolutely go systemic. And there’s a permanent side effect that occurs in up to 20% of glaucoma patients using latanoprost – one that typically isn’t noticeable until after 12 months of use: the blackening of your irises, an effect that often persists even after quitting the medication. No studies exist evaluating this risk for topical latanoprost, and also, no telehealth brand seems to care.
Ulo’s Solution: No “Trending” Ingredients That Risk Your Safety. Ever.
Unless an off-label ingredient offers notable hair gains through mechanisms distinct from other Ulo ingredients – and unless that ingredient has a respectable safety profile – Ulo will not include it as an offering to its product line.
That means no Carbon 60. No copper peptides. No latanoprost. No bimatoprost.
Ulo focuses on getting you results without compromising your safety. While the telehealth brand does offer off-label ingredients to certain users pending a prescription, such as dutasteride, spironolactone, melatonin, caffeine, and cetirizine, it only does so if safety & efficacy thresholds are met to justify their inclusion for added hair gains.
This is distinct from many other companies out there who simply attempt to capture your interest in ingredients “trending” on social media, but without enough data to determine whether these ingredients are safe, effective, and right for you.
You can see a sample of Ulo’s ingredient selections & concentrations here.
Problem #4: Compounding Dutasteride Without Emulsifiers
Other telehealth brands are compounding drugs that can’t be compounded, like dutasteride. To differentiate from the competition, telehealth brands often compound drugs like dutasteride & minoxidil into a single capsule, claiming bigger hair gains than finasteride and the convenience of a single daily pill.
The truth? When these companies “compound” dutasteride, they turn it from a soft gel into a powder, and inadvertently, they destroy its bioavailability, rendering dutasteride less effective than finasteride & causing major hair loss for customers who unknowingly switch to the 2-in-1 pills.
We have the lab tests to prove it. Post here. Video here.
Ulo’s Solution: No Compounded Dutasteride. Just The Real Thing.
Rather than offer compounded dutasteride, Ulo offers dutasteride as a soft gel – and in a formulation that has undergone bioequivalence testing, such that the efficacy should match that of the studies showing oral dutasteride outperformed oral finasteride for hair gains.
This is a distinction unrivaled by many other brands. Rather than sell you convenience in a 2-in-1 pill, Ulo sells you what works.
Better yet, Ulo still offers both oral dutasteride and oral minoxidil to those who want to multi-target their hair loss. They are just delivered as separate pills, in separate formulations, to maximize your potential for hair growth.
You can see Ulo’s oral Rx offerings here.
Beyond Ulo’s Products: Personal Support, Every Step Of The Way, Through Ulo’s Partner Physicians
As part of Ulo’s commitment to consumers, all subscriptions to their Rx product lines come with unlimited access to partner physicians – one of whom you’ll personally partner with to support your hair growth journey.
What does this look like? A completely different experience compared to other brands:
-
- Real customization: dosing & ingredient adjustments made by our partner physicians depending on your goals & real-time experiences with the products.
- Realistic expectations: separate “possibility” from “probability”. Our network physicians rely on clinical data to set realistic responses to your hair loss medications, including guides for when regrowth starts, when it might plateau, and what to do next to escalate your current protocol.
- Unrivaled personal support: the ability to contact a doctor, any time, through Ulo’s portal. Someone in your corner who not only assumes care for you, but also wants to help you achieve the best possible outcomes.
We hope you feel the difference. This piece is something we see other brands “promise”, but rarely deliver. It’s time to change that.
15% Off All Ulo Orders, Forever
Ulo is a part of the Perfect Hair Health ecosystem, and as a thank you for being a part of our community, all orders made through this article will automatically receive 15% off the purchase price, forever. That includes all future renewals, so long as the orders are made through these links.
We hope you enjoy everything Ulo has to offer. We certainly love the product line and are thrilled that we finally have the chance to offer products in the exact formulations for which we’ve advocated over the past 10+ years.
We wish you the best with your hair health journey, and we look forward to supporting you every step of the way.
OS-01 is a topical scalp serum developed by OneSkin, a biotech company based in San Francisco. Marketed as both an anti-aging skin treatment and a hair regrowth product, OS-01 aims to address hair thinning by targeting cellular senescence in the scalp. The serum has gained attention through social media and interviews with the company’s CEO, Carolina Reis Oliveira. In this article, we will examine what OS-01 is, explore the science of cellular senescence and its role in hair loss, and assess the evidence behind OS-01’s claims as a potential treatment for androgenetic alopecia.
Key Takeaways:
- Branding. OS-01 has positioned itself as a biotech-backed anti-aging and hair regrowth serum from OneSkin, a company known for its focus on longevity science. The branding strongly emphasizes scientific credibility and a “rooted in science” approach.
- Unique Selling Point. OS-01 is a peptide that specifically targets cellular senescence in the scalp, aiming to rejuvenate the hair follicle microenvironment by modulating the senescence-associated secretory phenotype (SASP).
- Clinical Support. OneSkin reports a 6-month third-party clinical study showing improvements in hair density, thickness, and hair cycling when used with daily dermarolling. However, these results have not been published in peer-reviewed journals and rely on company-provided summaries.
- Concerns. Key concerns include the lack of independently published human data, reliance on dermarolling for efficacy, marketing claims that oversimplify complex skin and hair biology, and typical limitations seen in brand-led before-and-after photos.
- Evidence Quality. The OS-01 serum scored 31/100 for evidence quality by our metrics.
- Recommendations. We recommend that OS-01 consider publishing the results of their 6-month pilot study and, ideally, conduct a larger, registered, placebo-controlled clinical trial.
All-Natural Hair Topical
The top natural ingredients for hair growth, all in one serum.
Take the next step in your hair growth journey with a world-class natural serum. Ingredients, doses, & concentrations built by science.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What Is OS-01?
OS-01 for hair is a topical scalp serum developed by OneSkin, a biotech company based in San Francisco, as both an anti-aging skin product and a hair regrowth product. The product is claimed to address hair thinning and loss by targeting the biological process of cellular senescence in the scalp.[1]OneSkin. (no date). Rooted in Science: The Clinical Evidence Supporting OS-01 Hair. Available at: … Continue reading
The product is sold in 1.7 fl oz bottles, available in 1-, 3-, or 6-month supplies, and comes with a dermaroller for $69, $207, or $424.
OS-01 for hair serum bottles.
The product contains a number of ingredients, of which you can see the whole list here:
“Water, Glycerin, 1,2-Hexanediol, Butylene Glycol, Hydroxyacetophenone, Panthenol, Inulin, Helianthus Annuus (Sunflower) Sprout Extract, Cellulose Gum, Alpha-Glucan Oligosaccharide, Tetrasodium Glutamate Diacetate, Propanediol, Morus Nigra Leaf Extract, Arginine, Acetyl Tyrosine, Rehmannia Chinensis Root Extract, Pentylene Glycol, Sodium PCA, Erythritol, Chondrus Crispus, PEG-12 Dimethicone, Oryza Sativa (Rice) Bran Water, Decapeptide-52*, Calcium Pantothenate, Zinc Gluconate, Sodium Benzoate, Niacinamide, Ornithine HCL, Caprylyl Glycol, Polyquaternium-11, Citrulline, Hydrolyzed Soy Protein, Xanthan Gum, Glucosamine HCL, Disodium Succinate, Fisetin, Raspberry Ketone, Sodium Benzoate, Citric Acid, Potassium Sorbate, Arctium Majus Root Extract, Panax Ginseng Root Extract, Biotin. *OS-01 Peptide.”
Several familiar ingredients are present here, including niacinamide, calcium pantothenate, zinc gluconate, raspberry ketone, ginseng, fisetin, and biotin.
- Niacinamide: Research shows that it may help protect hair follicle cells from oxidative stres, reduce the expression of DKK-1 (a protein that promotes hair follicle regression), and prolong the anagen (growth) phase of hair follicles.[2]Choi, Y-H., Shin, J.Y., Kim, J., Kang, N-G., Lee, S. (2021). Niacinamide down-regulates the expression of DKK-1 and protects cells from oxidative stress in cultured human dermal papilla cells. … Continue reading Clinical studies have demonstrated increased hair fullness and thickness in some participants using niacinamide derivatives, though it is thought that it doesn’t actually increase hair density.[3]Draelos, Z.D., Jacobson, E.L., Kim, H., Kim, M., Jacobson, M.K. (2005). A pilot study evaluating the efficacy of topically applied niacin derivatives for treatment of female pattern alopecia. Journal … Continue reading
- Calcium Pantothenate: This ingredient is commonly found in hair care products and supplements. Some studies suggest it may help improve hair thickness and reduce hair loss, especially when combined with zinc. For example, a clinical trial in women found that co-administration of zinc sulfate and calcium pantothenate alone was less pronounced, and more research is needed.[4]Siavash, M., Tavakoli, F., Mokhtari, F. (2017). Comparing the effects of zinc sulfate, calcium pantothenate, their combination and minoxidil solution regimens on controlling hair loss in women: a … Continue reading
- Raspberry Ketone: This has been studied in small human trials and animal models. Topical application promoted hair growth in about 50% of humans with alopecia, possibly by increasing dermal IGF-1 production through sensory neuron activation.[5]Harada, N., Okajima, K., Narimatsu, N., Kurihara, H., Nakagata, N. (2008). Effect of topical application of raspberry ketone on dermal production of insulin-like growth factor-I in mice and on hair … Continue reading However, the evidence base remains limited, and larger studies are necessary to confirm these effects.
- Fisetin: Fisetin is a polyphenol that has shown promise in preclinical studies. It may promote hair growth by increasing telomerase reverse transcriptase (TERT) expression, activating hair follicle stem cells, and facilitating the transition from a resting to a growth phase.[6]Kubo, C., Ogawa, M., Uehara, N., Katakura, Y. (2020). Fisetin promotes hair growth by augmenting TERT expression. Frontiers in Cell and Developmental Biology. 8(566617). Available at … Continue reading Animal studies suggest that fisetin may induce hair growth; however, human data are currently lacking.
- Biotin: This ingredient is widely marketed for hair health, but strong evidence for its effectiveness in preventing hair loss or promoting hair growth in people without a deficiency is lacking. Biotin supplementation can help restore hair growth in cases of true biotin deficiency, but such deficiencies are rare. Most studies do not support its use for hair loss in otherwise healthy individuals, though it remains a popular ingredient.[7]Yelich, A., Jenkins, H., Holt, S., Miller, R. (2024). Biotin for Hair Loss: Teasing Out the Evidence. Journal of Clinical and Aesthetic Dermatology. 17(8). 56-61. Available at: … Continue reading
However, the one we will be focusing on in this article is Decapeptide-52, also known as the OS-01 Peptide.
There has been a lot of hype on social media about this product, including a particularly interesting YouTube video featuring Dave Asprey, who talks with Carolina Reis Oliveira, the CEO and Co-founder of OneSkin.[8]Asprey. D. (2025). Hair Growth Expert: Scientists Discover a Secret Peptide that Reverses Balding | Caroline Oliveria. YouTube. Available at: https://www.youtube.com/watch?v=FSY1x40N2ys Accessed: … Continue reading Carolina holds degrees in stem cell biology and tissue engineering, as well as a doctorate in immunology, which bodes well for the scientific integrity of the product.
However, in the first ~15 seconds of the above video, Carolina makes some interesting statements:
- “The scalp of the skin basically has a similar structure of the skin in your face…”
- “Your hair follicles are basically embedded in the epidermal layer…”
- “…a lot of the things that we see happening in the skin of our face also happen in our scalp”
Now let’s break down what is wrong with these statements:
- “The scalp of the skin basically has a similar structure of the skin in your face…”
This statement is partially accurate. While the scalp and facial skin share the same basic layers (epidermis, dermis, etc.), there are significant structural and functional differences:
- When measuring total skin thickness in both men and women, scalp skin was found to be among the thickest.[9]Oitulu, P., Tekecik, M., Taflioglu, T., Kilinc, F., Ince, B. (2022). Measurement of Epidermis, Dermis, and Total Skin Thicknesses from Six Different Face Regions. Selcuk Medical Journal. 38(4). … Continue reading
- The scalp contains a much higher density of hair follicles and sebaceous glands than facial skin.[10]Gao, J., Liu, C., Zhang, S., Teacher, M.P., Bouabbache, S., Pouradier, F., Pangard. (2018). Revisiting, in vivo, the hair greasing process by the Sebuprint method. Skin Research and Technology. … Continue reading
- The scalp is more prone to certain inflammatory and microbiome-related disorders due to its unique structure and physiology.[11]Xu, Z., Wang, Z., Yuan, C., Liu, X., Yang, F., Wang, T., Wang, J., Manabe, K., Qin, O., Wang, X., Zhang, Y., Zhang, M. (2016). Dandruff is associated with the conjoined interactions between host and … Continue reading
So, while the basic structure is similar, the differences are clinically and biologically significant.
- “Your hair follicles are basically embedded in the epidermal layer…”
This is factually incorrect. Hair follicles are not embedded in the epidermal layer; they are skin appendages that extend deep into the dermis, with the bulb even reaching the hypodermis (subcutaneous tissue).[12]Oh, J.W., Kloepper, J., Langan, E.A., Kim, Y., Yeo, J., Kim, M.J., Hsi, T.C., Rose, C., Yoon, G.S., Lee, S.J., Seykora, J., Kim, J.C., Sung, Y.K., Kim, M., Paus, R., Plikus, M.V. (2016). A guide to … Continue reading
Figure 1: In the anagen (growth phase) of the hair follicle cycle, the bulb is typically located deep in the adipose layer (in the hypodermis).[13]Oh, J.W., Kloepper, J., Langan, E.A., Kim, Y., Yeo, J., Kim, M.J., Hsi, T.C., Rose, C., Yoon, G.S., Lee, S.J., Seykora, J., Kim, J.C., Sung, Y.K., Kim, M., Paus, R., Plikus, M.V. (2016). A guide to … Continue reading
- “…a lot of the things that we see happening in the skin of our face also happen in our scalp”
This is generally true, but it oversimplifies the differences. Many skin conditions (like psoriasis, eczema, and seborrheic dermatitis) can occur on both the scalp and face. However, the scalp’s unique anatomy, thicker skin, increased hair follicles, more sebaceous glands, and its coverage by hair, means it is prone to specific issues (like dandruff and increased oiliness) that are less common or present differently on facial skin. So, while there is overlap, the statement ignores important distinctions in disease prevalence and presentation.
These statements reinforce what we have been discussing in other articles – that you cannot blindly trust what people say. We should always adopt a scientific approach to the information we read online and attempt to find the scientific basis for their claims.
With this in mind, we will explore the science of the product, examine what senescence is, and explore the data that suggests OS-01 can enhance hair growth.
What is Senescence and How is It Involved in Hair Cycling and Loss?
Before we get into how OS-01 works, it is essential to understand what senescence is, when it can be beneficial for hair growth, and when it can be detrimental.
Cellular senescence can be thought of as a “pause button for cells”. When cells are stressed or damaged due to factors such as DNA damage, aging, or excessive environmental stress, they cease to divide permanently. But instead of dying like in programmed cell death (apoptosis) they stay active, sending out signals to their neighboring cells, and beyond.
Key features of these cells include:[14]Nakanishi, M. (2025). Cellular senescence as a source of chronic microinflammation that promotes the aging process. Proceedings of the Japan Academy, Ser.B, Physical and Biological Sciences. 101(4). … Continue reading
- A permanent stop in cell division: They’re kept in check by guardian pathways (p54/p2 and p16/Rb) that make sure they never divide again.
- Shape changes: They get bigger, flatter, and may look like “fried eggs” under the microscope.
- Special markers: Scientists detect them using a blue stain (called senescence-associated β-galactosidase (SA-β-gal)) that shows they have more lysosomes – cell “recycling bins”.
- Secretory profile: They secrete a cocktail of chemicals, cytokines, growth factors, and enzymes, known as the senescence-associated secretory phenotype (SASP). This cocktail affects the surrounding tissue, sometimes beneficially, sometimes harmfully.
In recent years, senescence has become a trendy buzzword in the beauty and longevity industries, often used to market products as anti-aging breakthroughs. While genuine scientific research shows that senescent cells play a real role in aging and tissue decline, many companies oversimplify or exaggerate this science to promote creams, serums, or supplements with vague claims of “targeting senescence”. In reality, effectively targeting senescent cells is complex and context-dependent, and not every product touted as a senescence solution is supported by robust clinical evidence. As a result, the term is sometimes misappropriated more as a marketing hook than a rigorously validated mechanism.
Furthermore, while senescence was once thought of as a detrimental mechanism, it can actually be beneficial for both overall health and hair health.
Helpful Senescence: Encouraging Hair Growth
As mentioned above, some senescent cells can actually benefit hair growth:[15]Wang, X., Ramos, R., Phan, A.Q., Yamaga, K., Flesher, J.L., Jiang, S., et al. (2023). Signalling by senescent melanocytes hyperactivates hair growth. Nature. 618(7966). 808-817. Available at: … Continue reading
- Osteopontin signal: Senescent pigment cells (melanocytes) in skin moles produce a molecule called osteopontin. This acts like a motivational speaker for nearby hair cells by binding to CD44 receptors, waking them up.
- Stem cell activation: This process prompts hair follicles to exit their resting phase and re-enter growth mode, much like flipping the switch from winter to spring.
- Proven boost: Injecting osteopontin or turning up its production in lab animals triggered hair growth; blocking it canceled the effect.
Harmful Senescence: Fueling Hair Loss
Recent studies show that accumulation of senescent cells can play a role in both androgenic alopecia (AGA) and age-related hair thinning.
As we age, more cells in the hair follicle’s support structures, like dermal papilla cells (DPCs) and hair follicle stem cells (HFSCs), become senescent.[16]Shin, W., Rosin, N.L., Sparks, H., Sinha, S., Rahmani, W., Sharma, N., Workentine, M., Abbasi, S., Labit, E., Stratton, J.A., Biernaskie, J. (2020). Dysfunction of Hair Follicle Mesenchymal … Continue reading These cells lose their ability to renew themselves and support healthy hair growth, resulting in weaker follicles that gradually shrink over time. This process, known as hair follicle miniaturization, is why aging hair tends to become thinner and sparser.
Age-related hair thinning features a gradual loss of follicle stem/progenitor cell function, increased senescent cell burden, and mitochondrial dysfunction.[17]Shin, W., Rosin, N.L., Sparks, H., Sinha, S., Rahmani, W., Sharma, N., Workentine, M., Abbasi, S., Labit, E., Stratton, J.A., Biernaskie, J. (2020). Dysfunction of Hair Follicle Mesenchymal … Continue reading This leads to both HFSC and DPC dysfunction.
Preclinical studies (studies done in cells or animals) have shown that antioxidants, senolytics, and interventions that reduce DPC/HFSC senescence or SASP can delay or ameliorate hair loss.[18]Deng, Y., Wang, M., He, Y., Liu, F., Chen, L., Xiong, X. (2023). Cellular senescence: Ageing and Androgenetic Alopecia. Dermatology. 239(4). 533-541. Available at: https://doi.org/10.1159/000530681 However, clinical translation remains limited. Furthermore, some evidence suggests that senescence may be a consequence, rather than a sole cause, of follicle dysfunction, meaning that treating just the senescence aspect alone may not be beneficial for hair regrowth.[19]Mirmirani, P., Karnik, P. (2010). Comparative Gene Expression Profiling of Senescent and Androgenetic Alopecia Using Microarray Analysis. Aging Hair. 67-76. Available at: … Continue reading
How Does the OS-01 Peptide Work?
According to OneSkin, OS-01 for hair targets senescent cells in the hair follicle to improve hair regrowth. One study frequently referenced on their website was conducted using human skin models.[20]Zonari, A., Brace, L.E., Al-Katib, K., Porto, W.F., Foyt, D., Guiang, M., Cruz, E.A.O., Marshall, B., Gentz, M., Guimaraes, G.R., Franco, O.L., Oliveira, C.R., Boroni, M., Carvalho, J.L. (2023). … Continue reading In this study, OS-01 (referred to as pep14) was identified and characterized for its senomorphic activity. As a senomorphic, the OS-01 peptide reduces the burden of cellular senescence by suppressing the harmful secretions associated with the senescence-associated secretory phenotype (SASP) and by preventing pre-senescent cells from progressing to full senescence. Importantly, it does not kill or eliminate existing senescent cells.
OneSkin also seems to have conducted a laboratory study using outer root sheath keratinocytes (ORSKs).[21]OneSkin. (no date). Discover the science behind every claim. Available at: https://www.oneskin.co/pages/claims?_ab=0&_fd=0&_sc=1 Accessed: June 2025
Interestingly, we had some difficulty determining the source of this information. It’s present on their shop page for OS-01 HAIR, but they cite 6 studies that don’t contain any information about this study. We then went to their Claims section and found the information below – again, however, there was no reference.
We went to read some of the blogs where this information was also stated; the citation, however, led us back to the Claims section. The only conclusion we can draw is that OneSkin conducted an internal study that has not yet been published. Therefore, we should take all results with a pinch of salt.
OneSkin treated the cells with corticotropin-releasing hormone (CRH) (to induce senescence) alone or in combination with OS-01. According to the website, after 72 hours, cells treated with OS-01 + CRH showed significantly lower CDKN1A (p21) levels compared to those treated with CRH alone.
Unfortunately, they don’t have any further data or information about this, so we can only go with what is written here.
Can OS-01 Benefit Patients with Androgenetic Alopecia?
OneSkin has also conducted a 6-month independent third-party clinical study in which 30 participants (23 women and 7 men) applied OS-01 HAIR twice daily and dermarolled their scalps once daily.[22]OneSkin. (no date). Discover the science behind every claim. Available at: https://www.oneskin.co/pages/claims?_ab=0&_fd=0&_sc=1 Accessed: June 2025 Again, this data is unpublished in any peer-reviewed journal and so we are just having to go from what is on the site.
The overall results showed:
Category Details Hair Density 86.67% showed 39.62% avg increase after 6 months Hair Density 70% showed 9.97% avg increase after 3 months Hair Density Double-blind, 30 participants, twice daily + dermarolling Hair Density (Men) 85.71% men showed 34.86% avg increase after 6 months (7 men) Hair Thickness 83.33% showed 42.58% avg increase after 6 months Hair Thickness Significant increase between 3 and 6 months Hair Thickness (Men) 100% men showed 36.68% avg increase after 6 months; 85.71% men 28.42% after 3 months Hair Cycle 73.33% showed 42.39% avg increase in anagen hairs after 6 months Hair Cycle Significant increase between 3 and 6 months Hair Cycle (Men) 85.71% men showed 20.19% avg increase at 3 months, 35.42% at 6 months Scalp Microbiome Supported scalp microbiome: increased M. globosa, improved ratio, better bacterial diversity Consumer Perception (Clinical) – 3 Months 80% saw hair improvement, 76.67% healthier hair, 70% faster growth, 73.33% fuller hair, 70% nourished scalp Consumer Perception (Clinical) – 6 Months 70% faster growth, 76.67% healthier hair, 73.33% less shedding, 83.33% nourished hair, 70% thicker, 73.33% stronger, 70% better texture Consumer Perception (Brand-led) – Immediate 89.47% could style hair, 85% lightweight Consumer Perception (Brand-led) – 2 Months 80.95% more hydrated scalp Consumer Perception (Brand-led) – 3 Months 81.82% new hair growth, 72.73% denser hair Sensitive Skin Dermatologically tested on 55 volunteers; no reactions Lab Data – Senescence OS-01 peptide reduces cellular senescence (lab data) Lab Data – Senescence (Stress-induced) Significant reduction in CRH-induced senescence (*p<0.05) Lab Data – Inflammation Significant reduction in IL-6 inflammation marker (*p<0.05) We see some issues immediately jumping out about the clinical trial OneSkin has conducted:
- OneSkin do not explicitly mention whether the participants have any type of hair loss.
- The study included no placebo or treatment group – this means we can’t find out if hair cycle seasonality played any part in the participants’ hair growth.
- The treatment was combined with microneedling. Microneedling has been shown to potentially improve terminal hair counts by itself (we covered one here) so how can we separate out the effects of the peptide vs. the microneedling?
- In a similar vein, the product itself contains a number of other ingredients (with varying evidence showing improvement on hair regrowth) – so how can we separate out the effects of these compared to the peptide?
- While OneSkin do tell us how the hair counts were done, there was no disclosure of how terminal hairs were defined and starting hair zone parameters for the participants. For example in the niostem study, the researchers clearly outlined how they categorized terminal/vellus hair density (vellus (< 40 μm) and terminal (> 40 μm) hairs). A number of other studies however also don’t define terminal hairs (one that comes to mind is the CBD oil study) – so while it does happen, it is not good practice.
- Certain phrasings appear to be potentially misleading:
- From the Claims section: “After 6 months, 83.33% of participants experienced a 42.58% average increase in hair thickness (sum of hair widths). (****p < 0.0001)”. The statistic refers only to the subset (83.33%) of participants who experienced an increase, not the entire study population. This means that 16.67% of participants did not experience an increase, but their results are not included in the average, potentially inflating the reported effect. The phrasing also raises the question of whether participants with no improvement or negative outcomes were excluded from the calculation. If so, and if those excluded participants had poor or negative results, the true average increase would be lower than reported.
- This is not the only claim that does this and so we have to wonder what is happening with the other participants?
- From the Claims section: “Participants saw a significant increase in hair thickness (sum of hair widths) between 3 and 6 months (**p < 0.01)”. Because we don’t have any graphs or data for this, we have to wonder…do we have a Niostem problem all over again? Niostem published their clinical data in February 2025 and claimed that “Terminal hair density improved significantly over time…” but this is not compared to the baseline – it’s compared to the three month time point, where there was actually a decrease in terminal hair density from the baseline. You can read our article about this here. Unfortunately we do not have any further information from OneSkin so we can’t know exactly what is happening.
- From the Claims section: “After 6 months, 83.33% of participants experienced a 42.58% average increase in hair thickness (sum of hair widths). (****p < 0.0001)”. The statistic refers only to the subset (83.33%) of participants who experienced an increase, not the entire study population. This means that 16.67% of participants did not experience an increase, but their results are not included in the average, potentially inflating the reported effect. The phrasing also raises the question of whether participants with no improvement or negative outcomes were excluded from the calculation. If so, and if those excluded participants had poor or negative results, the true average increase would be lower than reported.
You can read or watch some of our content that talk about these subjects here:
Deceptive But Legal: 3 Ways Marketers Cheat Hair Loss Studies
The Hair Loss Industry Is Broken | Evidence Quality Masterclass
Understanding Evidence Quality | How Hair Loss Companies Cheat Clinical Trials
Oneskin also has some before-and-after photos on their website, allowing us to see the progress people have experienced. However, they do fall for the usual pitfalls that we have mentioned in other articles, including different lighting, angles, and manipulating the hair so that it appears different in the before-and-after images (see below).
Figure 2: Before-and after image of a person showing their hair regrowth. However, they are scraping their hair back in the before which makes their regrowth look more dramatic than it probably is.
According to OneSkin, several positive results have been observed in their clinical trial; however, like many of these companies, the information has only been published on their website, and we have no way of verifying the data.
Let’s break down the biological plausibility of OS-01 HAIR, based on the mechanism OneSkin claims it has and current scientific understanding:
- Targeting Cellular Senescence
There is solid evidence that senescent cells accumulate in the hair follicle microenvironment with age and stress, disrupting the stem cell niche and impairing normal cycling. Studies show that hair follicle cells lose inductive capacity partly due to senescence and SASP-driven inflammation.[23]Pappalardo, A., Kim, J.Y., Abaci, H.E., Christiano, A.M. (2024). Restoration of hair follicle inductive properties by depletion of senescent cells. Aging Cell. 24(1). E14353. Available at: … Continue reading So, reducing senescence or its effects could, in theory, rejuvenate follicle activity.
- OS-01 Peptide Mechanism
As mentioned above, OS-01 has been shown to reduce markers of cellular senescence in skin cells in vitro (and has been claimed to do the same in ORSKs). While these are promising cell culture results, translating that to sustained, clinically meaningful effects in human follicles in vivo is the critical step.
- Delivery and Context
Topical peptides face challenges: skin penetration, stability, and reaching target cells in viable concentrations.[24]Pintea, A., Manea, A., Pintea, C., Vlad, R.A., Birsan, M., Antonoaea, P., Redai, E.M., Ciurba, A. (2025). Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. … Continue reading Dermarolling (used in the clinical study) does enhance delivery through microchannels, aligning with other evidence that microneedling can improve topical drug uptake for hair growth.[25]Zhang, S., Qiu, Y., Gao, Y. (2014). Enhanced delivery of hydrophilic peptides in vitro by transdermal microneedle pretreatment. Acta Pharmaceutica Sinica B. 4(1). 100-104. Available at: … Continue reading
- Supporting Evidence
The company’s small clinical trial shows statistically significant improvements in hair density, thickness, and anagen hairs, aligning with the proposed mechanism. However, the studies are short-term, small, and unpublished in peer-reviewed journals. Larger, independent trials would strengthen credibility.
How Does OS-01 Compare to Rapamycin?
Rapamycin is a well-studied compound that has garnered significant attention in aging research due to its ability to slow cellular senescence, promote autophagy, and extend lifespan in multiple animal models. Rapamycin’s unique mechanism, which targets the mechanistic target of rapamycin (mTOR pathway), a central regulator of cell growth and aging, makes it a gold standard for interventions aimed at reducing age-related cellular dysfunction and tissue decline. So, let’s see how OS-01 stands up to it.
Both OS-01 and rapamycin aim to mitigate cellular senescence but use distinct mechanisms and applications.
Aspect OS-01 Rapamycin Primary target Senescence-associated secretory phenotype (SASP). mTOR pathway. Senotherapeutic Class Senomorphic (modulates SASP, prevents progression of pre-senescent cells). Dual senomorphic/senolytic- inhibits mTOR and promotes autophagy (a process where a cell breaks down and recycles its own components). Key Action Reduces SASP markers, including IL-6, CXCL1, and CXCL8, in skin cells. Inhibits mTORC1, enhances autophagy, and clears senescent cells.[26]Selvarani, R., Mohammed, S., Richardson, A. (2020). Effect of rapamycin on aging and age-related diseases – past and future. GeroScience. 43(3). 1135-1158. Available at: … Continue reading Delivery Topical serum applied with dermarolling for enhanced penetration. Systemic (oral/injected) or localized formulations. Efficacy in Hair and Senescence
OS-01
- Lab Studies: Reduced CRH-induced senescence in outer root sheath keratinocytes (ORSKs) by lowering p21 levels.
- Clinical Claims: In a 6-month trial (30 participants), reported 39.6% average hair density and 42.6% thickness improvement with dermarolling.
- Limitations: Small sample size, unpublished peer-reviewed data, and reliance on self-reported metrics.
Rapamycin
- Preclinical Evidence: Reduces senescence in cardiac progenitor cells and hair follicles by suppressing mTOR and promoting autophagy.
- Hair Growth: Extends the anagen phase and increases follicle size and density in animal models.[27]Suzuki, T., Cheret, J., Scala, F.D., Akhundlu, A., Gherardini, J., Demetrius, D.L., O’Sullivan, J.D.B., Epstein, G.K., Bauman, A.J., Demetriades, C., Paus, R. (2023). mTORC1 activity negatively … Continue reading
- Human Data: Limited direct hair studies, but show systemic anti-aging benefits (e.g., improved vascular function, cognitive decline reversal).
Safety and Practical Considerations
Factor OS-01 Rapamycin Side Effects Minimal (skin dryness and irritation).[28]Zonari, A., Brace, L.E., Harder, N.H.O., Harker, C., Oliveira, C.R., Boroni, M., Carvalho, J.L. (2024). Double-blind, vehicle-controlled clinical investigation of peptide OS-01 for skin rejuvenation. … Continue reading Immunosuppression and metabolic disruptions. Application Twice daily topical use and dermarolling. Requires systemic dosing or specialized delivery. Evidence Strength Early-stage, company-led studies. Robust preclinical and some clinical data. Key Differences
- Approach: OS-01 focuses on localized SASP modulation, while rapamycin systemically targets mTOR to influence multiple aging pathways.
- Accessibility: OS-01 is available as a consumer product, whereas rapamycin is primarily used off-label or in research settings.
- Scope: Rapamycin has broader anti-aging applications beyond hair (e.g., cardiovascular, cognitive), while OS-01 targets scalp senescence and hair metrics.
In summary, OS-01 may offer a targeted, low-risk option for hair senescence; however, the data is preliminary and unpublished. Rapamycin, however, offers systemic or targeted action to provide broader anti-aging benefits but may have higher complexity and a more severe risk profile.
Is OS-01 Safe?
As mentioned above, in the clinical trial in which OS-01 was used for skin aging, skin dryness and irritation was observed in two participants.
Beyond this, OneSkin mentioned that the peptide has been tested in in vitro toxicity and irritation tests, genotoxicity testing, and a repeated insult patch test (RIPT), and its effect on cancer cells has also been evaluated, with no negative effects observed.[29]OneSkin. (no date). How Do We Know the OS-01 Peptide is Safe? Available at: https://www.oneskin.co/blogs/reference-lab/how-os-01-peptide-safety Accessed: June 2025,[30]Zonari, A., Brace, L.E., Alencar-Silva, T., Porto, W.F., Foyt, D., Guiang, M., Cruz, E.A.O., Franco, O.L., Oliveira, C.R., Boroni, M., Carvalho, J.L. (2022). In vitro and in vivo toxicity assessment … Continue reading
However, it is worth noting that there have been no long-term human studies to evaluate any other potential effects.
Is OS-01 for Me?
OS-01 for hair may be worth a try for you:
- You want to add another product to your routine (it can be used alongside topical minoxidil, etc.)
- You do not suffer from inflammatory scalp conditions, which might mean you can’t use a dermaroller.
- Are happy using a dermaroller every day to facilitate penetration of the peptide into the scalp.
Final Thoughts
While OS-01 shows promise as an innovative approach to addressing hair thinning through targeting cellular senescence, the current evidence is still early and largely company-reported. Laboratory and small-scale clinical data suggest that it may help improve hair density and thickness, particularly when combined with dermarolling; however, these results need to be confirmed by larger, independent studies. There is no definitive proof yet that OS-01 can effectively reverse androgenic alopecia in the long term. If you are interested in trying OS-01, it may be reasonable to use it in conjunction with established treatments like minoxidil; however, it’s best to manage expectations and continue to follow emerging research as more data becomes available.
References[+]
References ↑1 OneSkin. (no date). Rooted in Science: The Clinical Evidence Supporting OS-01 Hair. Available at: https://www.oneskin.co/blogs/reference-lab/rooted-in-science-the-clinical-evidence-supporting-os-01-hair Accessed: June 2025 ↑2 Choi, Y-H., Shin, J.Y., Kim, J., Kang, N-G., Lee, S. (2021). Niacinamide down-regulates the expression of DKK-1 and protects cells from oxidative stress in cultured human dermal papilla cells. Clinical, Cosmetic and Investigational Dermatology. 14. 1519-1528. Available at: https://doi.org/10.2147/CCID.S334145 ↑3 Draelos, Z.D., Jacobson, E.L., Kim, H., Kim, M., Jacobson, M.K. (2005). A pilot study evaluating the efficacy of topically applied niacin derivatives for treatment of female pattern alopecia. Journal of Cosmetic Dermatology. 4(4). 258-261. Available at: https://doi.org/10.1111/j.1473-2165.2005.00201.x ↑4 Siavash, M., Tavakoli, F., Mokhtari, F. (2017). Comparing the effects of zinc sulfate, calcium pantothenate, their combination and minoxidil solution regimens on controlling hair loss in women: a randomized controlled trial. Journal of Research in Pharmacy Practice. 6(2). 89-93. Available at: https://doi.org/10.4103/jrpp.JRPP_17_17 ↑5 Harada, N., Okajima, K., Narimatsu, N., Kurihara, H., Nakagata, N. (2008). Effect of topical application of raspberry ketone on dermal production of insulin-like growth factor-I in mice and on hair growth and skin elasticity in humans. Growth Hormone & IGF Research. 18(4). 335-344. Available at: https://doi.org/10.1016/j.ghir.2008.01.005 ↑6 Kubo, C., Ogawa, M., Uehara, N., Katakura, Y. (2020). Fisetin promotes hair growth by augmenting TERT expression. Frontiers in Cell and Developmental Biology. 8(566617). Available at https://doi.org/10.3389/fcell.2020.566617 ↑7 Yelich, A., Jenkins, H., Holt, S., Miller, R. (2024). Biotin for Hair Loss: Teasing Out the Evidence. Journal of Clinical and Aesthetic Dermatology. 17(8). 56-61. Available at: https://jcadonline.com/biotin-for-hair-loss-evidence/ Accessed: June 2025 ↑8 Asprey. D. (2025). Hair Growth Expert: Scientists Discover a Secret Peptide that Reverses Balding | Caroline Oliveria. YouTube. Available at: https://www.youtube.com/watch?v=FSY1x40N2ys Accessed: June 2025 ↑9 Oitulu, P., Tekecik, M., Taflioglu, T., Kilinc, F., Ince, B. (2022). Measurement of Epidermis, Dermis, and Total Skin Thicknesses from Six Different Face Regions. Selcuk Medical Journal. 38(4). 210-215. Available at: https://doi.10.30733/std.2022.01572 ↑10 Gao, J., Liu, C., Zhang, S., Teacher, M.P., Bouabbache, S., Pouradier, F., Pangard. (2018). Revisiting, in vivo, the hair greasing process by the Sebuprint method. Skin Research and Technology. 25(1). 79-87. Available at: https://doi.org/10.1111/srt.12613 ↑11 Xu, Z., Wang, Z., Yuan, C., Liu, X., Yang, F., Wang, T., Wang, J., Manabe, K., Qin, O., Wang, X., Zhang, Y., Zhang, M. (2016). Dandruff is associated with the conjoined interactions between host and microorganisms. Scientific Reports. 6(24877). Available at: https://doi.org/10.1038/srep24877 ↑12 Oh, J.W., Kloepper, J., Langan, E.A., Kim, Y., Yeo, J., Kim, M.J., Hsi, T.C., Rose, C., Yoon, G.S., Lee, S.J., Seykora, J., Kim, J.C., Sung, Y.K., Kim, M., Paus, R., Plikus, M.V. (2016). A guide to studying human hair follicle cycling in vivo. Journal of Investigative Dermatology. 136(1). 34-44 Available at: https://doi.org/10.1038/JID.2015.354 ↑13 Oh, J.W., Kloepper, J., Langan, E.A., Kim, Y., Yeo, J., Kim, M.J., Hsi, T.C., Rose, C., Yoon, G.S., Lee, S.J., Seykora, J., Kim, J.C., Sung, Y.K., Kim, M., Paus, R., Plikus, M.V. (2016). A guide to studying human hair follicle cycling in vivo. Journal of Investigative Dermatology. 136(1). 34-44 Available at: https://doi.org/10.1038/JID.2015.354 ↑14 Nakanishi, M. (2025). Cellular senescence as a source of chronic microinflammation that promotes the aging process. Proceedings of the Japan Academy, Ser.B, Physical and Biological Sciences. 101(4). 224-237. Available at: https://doi.org/10.2183/pjab.101.014. ↑15 Wang, X., Ramos, R., Phan, A.Q., Yamaga, K., Flesher, J.L., Jiang, S., et al. (2023). Signalling by senescent melanocytes hyperactivates hair growth. Nature. 618(7966). 808-817. Available at: https://doi.org/10.1038/s41586-023-06172-8 ↑16, ↑17 Shin, W., Rosin, N.L., Sparks, H., Sinha, S., Rahmani, W., Sharma, N., Workentine, M., Abbasi, S., Labit, E., Stratton, J.A., Biernaskie, J. (2020). Dysfunction of Hair Follicle Mesenchymal Progenitors Contributed to Age-Associated Hair Loss. Developmental Cell. 53(2). 185-198. Available at: https://doi.org/10.1016/j.devcel.20203.03.019 ↑18 Deng, Y., Wang, M., He, Y., Liu, F., Chen, L., Xiong, X. (2023). Cellular senescence: Ageing and Androgenetic Alopecia. Dermatology. 239(4). 533-541. Available at: https://doi.org/10.1159/000530681 ↑19 Mirmirani, P., Karnik, P. (2010). Comparative Gene Expression Profiling of Senescent and Androgenetic Alopecia Using Microarray Analysis. Aging Hair. 67-76. Available at: https://doi.org/10.1007/978-3-642-02636-2_8 ↑20 Zonari, A., Brace, L.E., Al-Katib, K., Porto, W.F., Foyt, D., Guiang, M., Cruz, E.A.O., Marshall, B., Gentz, M., Guimaraes, G.R., Franco, O.L., Oliveira, C.R., Boroni, M., Carvalho, J.L. (2023). Senotherapeutic peptide treatment reduces biological age and senescence burden in human skin models. Npj aging. 9(10). 1-15. Available at: https://doi.org/10.1038/s41514-023-00109-1 ↑21, ↑22 OneSkin. (no date). Discover the science behind every claim. Available at: https://www.oneskin.co/pages/claims?_ab=0&_fd=0&_sc=1 Accessed: June 2025 ↑23 Pappalardo, A., Kim, J.Y., Abaci, H.E., Christiano, A.M. (2024). Restoration of hair follicle inductive properties by depletion of senescent cells. Aging Cell. 24(1). E14353. Available at: https://doi.org/10.1111/acel.14353 ↑24 Pintea, A., Manea, A., Pintea, C., Vlad, R.A., Birsan, M., Antonoaea, P., Redai, E.M., Ciurba, A. (2025). Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. Biomolecules. 15(1). 88. Available at: https://doi.org/10.3390/biom15010088 ↑25 Zhang, S., Qiu, Y., Gao, Y. (2014). Enhanced delivery of hydrophilic peptides in vitro by transdermal microneedle pretreatment. Acta Pharmaceutica Sinica B. 4(1). 100-104. Available at: https://doi.org/10.1016/j.apsb.2013.12.011 ↑26 Selvarani, R., Mohammed, S., Richardson, A. (2020). Effect of rapamycin on aging and age-related diseases – past and future. GeroScience. 43(3). 1135-1158. Available at: https://doi.org/10.1007/s11357-020-00274-1 ↑27 Suzuki, T., Cheret, J., Scala, F.D., Akhundlu, A., Gherardini, J., Demetrius, D.L., O’Sullivan, J.D.B., Epstein, G.K., Bauman, A.J., Demetriades, C., Paus, R. (2023). mTORC1 activity negatively regulates human hair follicle growth and pigmentation. EMBO Reports. 24(7). E56574. Available at: https://doi.org/10.15252/embr.202256574 ↑28 Zonari, A., Brace, L.E., Harder, N.H.O., Harker, C., Oliveira, C.R., Boroni, M., Carvalho, J.L. (2024). Double-blind, vehicle-controlled clinical investigation of peptide OS-01 for skin rejuvenation. Journal of Cosmetic Dermatology. 23(6). 2135-2144. Available at: https://doi.org/10.1111/jocd.16242 ↑29 OneSkin. (no date). How Do We Know the OS-01 Peptide is Safe? Available at: https://www.oneskin.co/blogs/reference-lab/how-os-01-peptide-safety Accessed: June 2025 ↑30 Zonari, A., Brace, L.E., Alencar-Silva, T., Porto, W.F., Foyt, D., Guiang, M., Cruz, E.A.O., Franco, O.L., Oliveira, C.R., Boroni, M., Carvalho, J.L. (2022). In vitro and in vivo toxicity assessment of the senotherapeutic Peptide 14. Toxicology Reports. 9. 1632-1638. Available at: https://doi.org/10.1016/j.toxrep.2022.07.018 In dermatology, clear and accurate reporting of research findings isn’t just important; it’s essential. Yet, data misinterpretation and misrepresentation remain a persistent issue, often slipping through in abstracts, press releases, and articles from otherwise trusted sources. These distortions can have real consequences, affecting how products are marketed, how clinicians make decisions, and the public’s understanding of the science.
In this article, we break down recent examples from hair growth research to illustrate exactly how scientific data can be misinterpreted, why it matters more than ever in our 24-hour news cycle culture, and what that means for companies that prefer to share their data rather than relying on press releases.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Thermos Thermophilus Extract (TTFE) and Hair Density
A striking example of data misinterpretation appeared in a recent Dermatology Times article, which claimed that Thermus Thermophilus Ferment Extract (TTFE) increased hair density by 96.88%.[1]Bosslett, M. (2025). Thermos Thermophilus Fermentation Extract Can Treat Androgenic Alopecia. Dermatology Times. Available at: … Continue reading At first glance, this figure suggests a near doubling of hair density, an extraordinary claim that would be making waves in cosmetic dermatology.
Screenshot of the interpretation of the data from the Dermatology Times article.
However, a closer examination of the original peer-reviewed study reveals a critical nuance: the figure refers not to the magnitude of increase in hair density, but to the proportion of participants who experienced any increase in hair density. In other words, nearly all subjects showed some improvement, but the actual percentage increase in hair density was much smaller.
This distinction is vital. Reporting the statistic as a 96.88% increase in hair density grossly inflates the effect size and misleads readers about the product’s efficacy. Read more about what we thought about this here.
Niostem Device Pilot Study
Another recent example involves the Niostem device. The device’s pilot study, published in the Journal of Cosmetic Dermatology, reported increases in total hair density (12% at 3 months, 19.3% at 6 months) and hair shaft thickness (8.8% over 6 months).[2]Jellard, S., Moore, S., Chacon-Martinez, C.A. (2025). Novel Electrotrichogenic Device Promotes Hair Growth in Men with Androgenetic Alopecia: A Pilot Study. Journal of Cosmetic Dermatology. 24. … Continue reading
While these results are promising, the study’s abstract contains a potentially misleading statement, claiming: “terminal hair density improved significantly over time”.
Niostem’s description in their abstract of participants’ terminal hair growth
However, the data showed that terminal hair density initially decreased at 3 months compared to baseline before increasing at 6 months. The significant increase is relative to the 3-month point, not the baseline, which would be the more relevant comparison for assessing treatment efficacy (and for which there was no statistically significant change).
Actual Niostem terminal hair growth data. Significant growth at 6 months is only compared to 3 month data where terminal hair counts decreased.[3]Jellard, S., Moore, S., Chacon-Martinez, C.A. (2025). Novel Electrotrichogenic Device Promotes Hair Growth in Men with Androgenetic Alopecia: A Pilot Study. Journal of Cosmetic Dermatology. 24. … Continue reading
This subtle but important spin in the abstract can create an overly optimistic impression of the device’s performance. You can read more in our article here.
CB-03-01 Phase II Study and Press Release Limitations
The second Phase II study of CB-03-01, a topical antiandrogen for female pattern hair loss, illustrates the pitfalls of relying on press releases rather than peer-reviewed publications. The press release summarized the main findings from a 293-participant study, but omitted critical details such as how CB-03-01 compared to the 2% minoxidil group.[4]Cassiopea Spa, (2021). Cassiopea SpA Announces Topline Results of Phase II Proof of Concept Trial of Clascoterone Solution for the Treatment of Androgenetic Alopecia in Females. Available at: … Continue reading
Part of the Breezula press release in which it was mentioned that treatment with clascoterone was compared with 2% minoxidil treatment or a vehicle control. The comparison with 2% minoxidil was not mentioned anywhere in the results.[5]Cassiopea Spa, (2021). Cassiopea SpA Announces Topline Results of Phase II Proof of Concept Trial of Clascoterone Solution for the Treatment of Androgenetic Alopecia in Females. Available at: … Continue reading
Without all the data, readers can’t fully assess the study’s methodology, statistical analysis, or properly contextualize the results.
Should we trust the Kintor Pharma Press Releases?
When you see mistakes, misrepresentations, or only partial displays of results like these, it casts a shadow across companies like Kintor Pharmaceuticals, which have disseminated their efficacy and safety data primarily through press releases, investor announcements, and conference abstracts, rather than peer-reviewed journal articles. This lack of transparency makes it difficult for independent experts to critically appraise the robustness and clinical relevance of the findings.
For instance, Kintor’s Phase II trials report increases in target area hair count of approximately 10 to 22 hairs per cm2 compared to baseline or placebo after 24 weeks.[6]Kintor Pharma. (2023). Kintor Pharma Announces Successful Completion of Phase II Clinical Trial of KX-826 for Treatment of Androgenetic Alopecia in the US. Available at: … Continue reading While these numbers appear promising, without full access to raw data, confidence intervals, or responder analyses, it is impossible to determine how consistent or meaningful these improvements are across the treated population. Moreover, the clinical significance of such hair count increases, whether they translate into visibly noticeable hair regrowth or improved patient satisfaction, is unclear without detailed patient-reported outcomes or photographic evidence.
Kintor’s decision to market KX-826-containing products as cosmetics rather than drugs in some regions further complicates trust in their claims. Cosmetics do not require FDA approval or demonstration of efficacy, and safety requirements are less stringent. This positioning allows products to be sold despite incomplete evidence of clinical benefit, potentially exposing consumers to unproven treatments.
Why Misrepresentation Happens and Its Consequences
Misrepresentation or “spin” in scientific communication can be intentional or unintentional. Authors and publishers may emphasize positive findings to attract attention, secure funding, or support marketing goals. Press releases often simplify complex results to appeal to broader audiences, sometimes glossing over limitations or nuances.[7]PR Newswire. (no date). How Healthcare Companies are Using Press Releases. Available at: https://www.prnewswire.com/resources/articles/healthcare-company-press-releases/ (Accessed: June 2025)
Done wrong, data can be misrepresented or misinterpreted, leading to:
- Mislead clinicians and potential consumers
- Dissemination of misinformation through social media and other internet sources
- Confusion and skepticism in scientific and medical fields
- Loss of trust from the consumer
The Role of Peer Review and Scientific Literacy
Peer review serves as a critical quality control mechanism, ensuring that research is rigorously evaluated by experts before it is published.[8]Taylor & Francis. (no date). Understanding the peer review process. Available at: https://authorservices.taylorandfrancis.com/publishing-your-research/peer-review/ (Accessed: June 2025) It helps prevent unwarranted claims and promotes transparent reporting of methods and results. However, peer review is not foolproof, and errors or spin can still appear in abstracts or articles.
Peer review is only one part of the equation, however. Scientific literacy, especially source literacy, remains essential for anyone navigating research claims, whoever you are. This means not just reading headlines or abstracts, but digging into the methods, understanding what is being measured, and questioning whether the reported outcomes are truly meaningful in a real-world context.
In today’s environment, where information can be amplified and distorted through social media and marketing, the responsibility for critical evaluation doesn’t just fall on scientists or peer reviewers; it’s shared by all of us who read, share, and act on scientific news.
Final Thoughts
The examples from TTFE, Niostem, and CB-03-01 illustrate how easily data can be misrepresented, whether intentionally or not. While press releases can be useful for quick updates, they are no substitute for full, peer-reviewed publications that allow for independent scrutiny. As the boundaries between scientific communication, marketing, and media continue to blur, the risk of misinterpretation and the consequences for patient care, consumer trust, and scientific progress only increase.
Therefore, when it comes to companies that only publish their data through press releases, we would advise approaching with caution. It is good to have an open mind, but try not to blindly trust everything you see.
If you’d like a deeper dive into these topics, visit these videos here and here.
References[+]
References ↑1 Bosslett, M. (2025). Thermos Thermophilus Fermentation Extract Can Treat Androgenic Alopecia. Dermatology Times. Available at: https://www.dermatologytimes.com/view/thermus-thermophilus-fermentation-extract-can-treat-androgenic-alopecia (Accessed: May 2025) ↑2, ↑3 Jellard, S., Moore, S., Chacon-Martinez, C.A. (2025). Novel Electrotrichogenic Device Promotes Hair Growth in Men with Androgenetic Alopecia: A Pilot Study. Journal of Cosmetic Dermatology. 24. E70302. 1-8. Available at: https://doi.org/10.1111/jocd.70202 ↑4 Cassiopea Spa, (2021). Cassiopea SpA Announces Topline Results of Phase II Proof of Concept Trial of Clascoterone Solution for the Treatment of Androgenetic Alopecia in Females. Available at: https://www.old.cassiopea.com/wp-content/uploads/2021/09/210910_Cassiopea_Media-Release_Clascoterone-Solution-Phase-2-Female-AGA-Results_EN-FINAL.pdf (Accessed: June 2025) ↑5 Cassiopea Spa, (2021). Cassiopea SpA Announces Topline Results of Phase II Proof of Concept Trial of Clascoterone Solution for the Treatment of Androgenetic Alopecia in Females. Available at: https://www.old.cassiopea.com/wp-content/uploads/2021/09/210910_Cassiopea_Media-Release_Clascoterone-Solution-Phase-2-Female-AGA-Results_EN-FINAL.pdf (Accessed: June 2025) ↑6 Kintor Pharma. (2023). Kintor Pharma Announces Successful Completion of Phase II Clinical Trial of KX-826 for Treatment of Androgenetic Alopecia in the US. Available at: https://en.kintor.com.cn/news_details/1803365133011234816.html#:~:text=The%20results%20showed%20that%3A&text=The%20TAHC%20of%20the%200.5,significant%20(P%3D0.0088). (Accessed: June 2025) ↑7 PR Newswire. (no date). How Healthcare Companies are Using Press Releases. Available at: https://www.prnewswire.com/resources/articles/healthcare-company-press-releases/ (Accessed: June 2025) ↑8 Taylor & Francis. (no date). Understanding the peer review process. Available at: https://authorservices.taylorandfrancis.com/publishing-your-research/peer-review/ (Accessed: June 2025) What is Minoxidil?
Minoxidil was originally developed in the 1970s as an oral medication for the treatment of hypertension (high blood pressure). This was due to its key function as a vasodilator, widening the blood vessels and, in turn, increasing blood flow and reducing blood pressure. However, an unintended side effect quickly became apparent in nearly all use cases: excessive hair growth.
Seeking to take advantage of this, minoxidil was re-formulated as a topical solution for the treatment of hair loss. After showing great efficacy in clinical trials of patients with androgenetic alopecia (AGA), topical minoxidil [5%] received FDA approval for the treatment of male pattern hair loss in 1988, with a lower dose of topical minoxidil [2%] later being approved for the treatment of female pattern hair loss. And, while it is not technically an FDA-approved treatment, oral minoxidil is also used off-label to treat hair loss.[1]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug design, development and therapy. 2777-2786. Available at: … Continue reading
Given that this application of minoxidil was first identified as an unintended side effect, it is unsurprising that the use of both topical and oral minoxidil can lead to other unwanted effects. In a previous article, we discussed the most common and widely recognized side effects of minoxidil use. Here, however, we will explore a potential side effect that has been reported by some minoxidil users: accelerated skin aging.
Is it possible that minoxidil can accelerate skin aging? In this article, we’ll review the current landscape of research and dive into the anecdotes, the biological mechanisms, alternative explanations, and where we currently stand on this topic.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Skin Aging?
Skin aging is a multifactorial biological process that is mediated by intrinsic (genetically determined) and extrinsic (environmental) factors. Natural, intrinsic aging occurs over a long time, with a gradual decrease in skin structure and function leading to the formation of fine lines, wrinkles, thinning, dryness, and reduced elasticity. This is compounded by the extrinsic factors, such as ultraviolet radiation and pollution, which can cause wrinkles that are more coarse, more severe loss of elasticity, and pigmentation disorders.[2]Shin, S. H., Lee, Y. H., Rho, N. K., & Park, K. Y. (2023). Skin aging from mechanisms to interventions: focusing on dermal aging. Frontiers in physiology. 14. 1195272. Available at: … Continue reading
There are several key mechanisms that underlie these factors, including:
- Oxidative stress: the accumulation of reactive oxygen species leads to oxidative stress, which subsequently causes damage to the DNA, proteins, and lipids that are found in the skin.[3]Rinnerthaler, M., Bischof, J., Streubel, M. K., Trost, A., & Richter, K. (2015). Oxidative stress in aging human skin. Biomolecules. 5(2). 545-589. Available at: … Continue reading
- Cellular senescence: aged cells of the skin enter a permanent state of arrest whereby they no longer divide, impairing the ability of the skin to repair and regenerate. Moreover, senescent cells release harmful factors (senescence-associated secretory phenotype) that can disrupt the homeostasis of the skin and promote inflammation.[4]Chin, T., Lee, X. E., Ng, P. Y., Lee, Y., & Dreesen, O. (2023). The role of cellular senescence in skin aging and age-related skin pathologies. Frontiers in physiology. 14. 1297637. Available at: … Continue reading
- Altered collagen homeostasis: with age, collagen production (primarily type I collagen) decreases in the skin. This compromises the structural integrity of the skin, reducing its elasticity and firmness and leading to the appearance of wrinkles.[5]He, X., Gao, X., & Xie, W. (2023). Research progress in skin aging, metabolism, and related products. International journal of molecular sciences. 24(21). 15930. Available at: … Continue reading
As stated, intrinsic factors and genetics means that these mechanisms will naturally cause skin health to deteriorate as we get older. However, we also know that certain environmental factors can accelerate the aging process by promoting the activity of the described mechanisms. It is therefore possible that a therapy that acts upon these mechanisms could influence the aging process.
Is There a Link Between Minoxidil and Skin Aging?
On reddit, some people using topical minoxidil have reported what sounds like accelerated aging: that their under-eye bags worsened and that their facial skin seemed drier and less smooth than before. Others have documented these changes with before-and-after photos taken just 1-2 months after starting the topical medication. Similar anecdotes have been reported by some users of oral minoxidil. This has led to claims that minoxidil use may accelerate skin aging.
But do these anecdotes make sense biologically? And are these reports truly suggestive of faster skin aging, or is something else going on?
Let’s take a look at what the scientific evidence says.
Could Minoxidil Accelerate Skin Aging?
There is a lack of understanding regarding the specific underlying mechanism by which minoxidil promotes hair growth. Indeed, several different mechanisms have been suggested, and it is believed that more than one may be responsible for mediating the effects of minoxidil. In the context of aging skin, one of these mechanisms is particularly interesting: the minoxidil-induced increase in prostaglandin E2 (PGE2) production.
Back in 1997, researchers discovered that minoxidil significantly increased the production of PGE2 in murine fibroblast cells in vitro. Moreover, they showed that minoxidil also increased the production of PGE2 in human dermal papilla fibroblasts, cells that sit within the base of the hair follicle and play an important role in maintaining hair health.[6]Michelet, J. F., Commo, S., Billoni, N., Mahé, Y. F., & Bernard, B. A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair … Continue reading Later, in mice, it was also demonstrated that the topical application of PGE2 exhibited some, albeit limited, ability to promote hair growth. [7]Sasaki, S., Hozumi, Y., & Kondo, S. (2005). Influence of prostaglandin F2α and its analogues on hair regrowth and follicular melanogenesis in a murine model. Experimental dermatology. 14(5). … Continue reading
Figure 1: Minoxidil increases the production of PGE2 in vitro. Treatment with minoxidil increased PGE2 levels in cultured human dermal papilla fibroblasts (a) and murine fibroblasts (b).[8]Michelet, J. F., Commo, S., Billoni, N., Mahé, Y. F., & Bernard, B. A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair … Continue reading
So PGE2 may be part of a mechanism that minoxidil uses to promote hair growth, but what does that have to do with skin aging?
One study found that PGE2 levels in the skin increase with age, with fibroblasts being the main source of increased production. When the same researchers cultured fibroblasts with PGE2 in vitro, they also demonstrated that PGE2 treatment causes a reduction in the expression of type I procollagen.[9]Li, Y., Lei, D., Swindell, W.R., Xia, W., Weng, S., Fu, J., Worthen, C.A., Okubo, T., Johnston, A., Gudjonsson, J.E. and Voorhees, J.J. 2015. Age-associated increase in skin fibroblast–derived … Continue reading
Figure 2: PGE2 levels in the skin. Aged populations exhibit increased levels of PGE2 within their skin that younger populations.[10]Li, Y., Lei, D., Swindell, W.R., Xia, W., Weng, S., Fu, J., Worthen, C.A., Okubo, T., Johnston, A., Gudjonsson, J.E. and Voorhees, J.J. 2015. Age-associated increase in skin fibroblast–derived … Continue reading
Additional studies have provided further evidence that PGE2 impairs collagen homeostasis within the skin. In cultured human dermal fibroblasts, it was shown that treatment with PGE2 significantly decreased collagen type I levels, which may have been driven by the significant increase in matrix metallopeptidase 1 (MMP1) levels.[11]Shim, J. H. (2019). Prostaglandin E2 induces skin aging via E-prostanoid 1 in normal human dermal fibroblasts. International Journal of Molecular Sciences, 20(22), 5555. Available at: … Continue reading Thus, there exists a wealth of evidence which suggests that PGE2 plays a role in the aging of skin by impairing collagen homeostasis.
Does Minoxidil Really Accelerate Skin Aging?
Given that minoxidil has been shown to increase PGE2, and that PGE2 is associated with aging skin, does this mean that minoxidil use leads to accelerated aging of the skin?
Not necessarily.
Firstly, and most crucially, there are currently no studies that have shown a direct link between the use of minoxidil and accelerated skin aging. Until such research is conducted, it is not possible to draw an accurate conclusion.
Now, in general, with safety concerns — it’s usually important to emphasize that the absence of evidence does not mean evidence against a concept. For instance, just because we don’t have a randomized, controlled clinical study determining the likelihood of death from jumping out of an airplane without a parachute, the risk here is inherently obvious: we don’t need a study to prove death is likely. We can just look at surrogate data on max velocity, the heights of falls, and the human capacity to endure such a violent impact. And then we can presume death is the most likely outcome.
So, can we use surrogate data on minoxidil — such as these in vitro studies — and then can we associate these to skin thinning, pair those with the anecdotal reports from Reddit, and presume that topical minoxidil is accelerating skin aging in some people, regardless of what the clinical studies say?
You could. But given that this side effect wasn’t picked up in any of the large, randomized, controlled clinical trials on minoxidil prior to these internet posts, our opinion is that we should exercise extreme caution in coming to this conclusion. Why? Because beyond the absence of clinical data on minoxidil doing this in well-designed studies, there are also two side effects of minoxidil application that might be fully-to-blame for why some think minoxidil use accelerates skin aging. And encouragingly, these side effects aren’t permanent:
- Water retention
- Allergic dermatitis (skin dryness)
We’ll take these one-by-one.
Minoxidil & water retention
Minoxidil is able to function as a vasodilator by opening potassium channels, thus relaxing the vascular smooth muscle and lowering blood pressure. However, this modification of potassium channels also increases sodium reabsorption by the kidneys which, in turn, leads to greater retention of water by the body.[12]Sica, D. A. (2004). Minoxidil: an underused vasodilator for resistant or severe hypertension. The Journal of Clinical Hypertension. 6(5). 283-287. Available at: … Continue reading
Water retention can lead to edema (swelling) and, although this is typically seen in peripheral regions such as the feet, use of oral minoxidil (even at low doses) has been found to cause facial edema in up to 1% of use cases.[13]Sanabria, B., de Nardo Vanzela, T., Miot, H. A., & Ramos, P. M. (2021). Adverse effects of low-dose oral minoxidil for androgenetic alopecia in 435 patients. Journal of the American Academy of … Continue reading Indeed, a recent study demonstrated a dose-dependent relationship between oral minoxidil and the incidence of edema.[14]Salas, J., Esse, I., Kincaid, C. M., Birda, A., Choe, S., & Mesinkovska, N. A. (2025). Characterizing low-dose oral minoxidil-induced peripheral edema in alopecia patients. Journal of the … Continue reading Some users of topical minoxidil have also reported swelling around the face after use, particularly in the region surrounding their eyes.
A consequence of facial edema is a more plump appearance of the face and potentially sagging of the skin, which can also accentuate under-eye bags and make it appear as though someone is less rested than they actually are. These symptoms mimic those of aging skin and they can make it seem like minoxidil is accelerating the aging process. However, the same studies that observed the cases of facial edema also noted that the swelling typically resolved within two weeks of stopping treatment, so it is unlikely that these effects are truly reflective of accelerated skin aging.
Minoxidil & skin dryness (allergic dermatitis)
Another side effect of minoxidil use, particularly when it is applied topically, is allergic dermatitis. At the site of application, symptoms of allergic dermatitis such as itching, redness, and scaling, were observed in 5.7% of topical minoxidil [5%] users.[15]Friedman, E. S., Friedman, P. M., Cohen, D. E., & Washenik, K. (2002). Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. Journal of the American Academy of … Continue reading
It was initially believed that the majority of allergic dermatitis incidences were caused by the carrier, not by the minoxidil. A 2002 study used patch testing to demonstrate that 81.8% of patients reacted to propylene glycol, which is a solvent that is used to improve the delivery of minoxidil. This was compared to just 36.4% of patients being allergic to minoxidil itself, and an even lower 9.1% showing a reaction to the alternative carrier butylene glycol.[17]Friedman, E. S., Friedman, P. M., Cohen, D. E., & Washenik, K. (2002). Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. Journal of the American Academy of … Continue reading However, more recent studies have started to challenge this theory. By reviewing 21 studies that investigated allergic dermatitis from minoxidil use, a 2025 study found that minoxidil is the predominant allergen, followed by propylene glycol.[18]Junge, A., Radonjic-Hoesli, S., Bossart, S., Simon, D., De Viragh, P., Hunger, R.E., Heidemeyer, K. and Jafari, S.M.S., 2025. Contact Dermatitis Caused by Topical Minoxidil: Allergy or Just … Continue readingFigure 3: Allergic dermatitis in a minoxidil user. Patch testing with minoxidil [1%] in isopropanol revealed a positive allergic contact reaction.[16]Friedman, E. S., Friedman, P. M., Cohen, D. E., & Washenik, K. (2002). Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. Journal of the American Academy of … Continue reading
Whether the allergen is minoxidil, propylene glycol, or another ingredient, it is clear that topical minoxidil has the potential to cause allergic dermatitis. Of course, the relevance of this is that the symptoms associated with allergic dermatitis may mimic signs of aging skin, once again leading users to believe that minoxidil is causing accelerated skin aging. However, as observed with water retention, it has been shown that the symptoms of contact dermatitis stop after ceasing minoxidil use.[19]Pasricha, J. S., Nanda, A., & Bajaj, N. (1991). Contact dermatitis due to minoxidil. Indian Journal of Dermatology, Venereology and Leprology. 57. 235. Available at: … Continue reading
Together, these effects are probably more likely to explain the skin quality-topical minoxidil connection than true accelerated skin aging. Otherwise, the results would sustain even after quitting the medication, but there is no scientific or anecdotal evidence of this.
What Can Be Done To Prevent These Effects?
How can one avoid potential skin-related minoxidil side effects? There are several options at your disposal:
- If you are getting allergic dermatitis, it may be useful to determine which ingredient(s) in the minoxidil are driving that reaction. There are many alternative carrier ingredients available for topical minoxidil, and switching to a different brand of minoxidil (which uses different ingredients) may prevent these symptoms from emerging.
- Switching to oral minoxidil may be another option for anyone struggling with allergic dermatitis from topical minoxidil. To date, there is no scientific evidence that the systemic effects of oral minoxidil can cause allergic dermatitis. However, if you do make the switch, be aware that oral minoxidil is linked to increased water retention in a small number of people.
- Allow topical minoxidil to dry fully before going to bed. Some people apply topical minoxidil and then immediately go to bed – thereby pressing their heads against their pillow before letting the topical minoxidil fully dry. If this is you, you might be creating a situation whereby any undried topical minoxidil can spread to the pillow and, therefore, the face (when you move around throughout the night). This increased spread may lead to increased risk of allergic dermatitis and water retention in areas of the face where topical minoxidil is not applied, nor is supposed to reach. So, let the topical minoxidil fully dry and wait at least 20-30 minutes after an application before putting your head down at night.
Final Thoughts
There is some anecdotal evidence which suggests that the use of minoxidil causes accelerated aging of the skin. Although minoxidil does drive increased production of PGE2, which is associated with reduced collagen production in the skin, there is no direct scientific evidence that has proven a link between the use of minoxidil and skin aging. Furthermore, two side effects of minoxidil use – increased water retention and allergic dermatitis – produce symptoms that mimic those of aging skin. Therefore, it is possible that temporary side effects of minoxidil use, the effects of which reverse after stopping treatment, are simply being mistaken for accelerated skin aging. Based on this, it is unlikely (though not impossible) that there is a link between minoxidil and skin aging.
References[+]
References ↑1 Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug design, development and therapy. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑2 Shin, S. H., Lee, Y. H., Rho, N. K., & Park, K. Y. (2023). Skin aging from mechanisms to interventions: focusing on dermal aging. Frontiers in physiology. 14. 1195272. Available at: https://doi.org/10.3389/fphys.2023.1195272 ↑3 Rinnerthaler, M., Bischof, J., Streubel, M. K., Trost, A., & Richter, K. (2015). Oxidative stress in aging human skin. Biomolecules. 5(2). 545-589. Available at: https://doi.org/10.3390/biom5020545 ↑4 Chin, T., Lee, X. E., Ng, P. Y., Lee, Y., & Dreesen, O. (2023). The role of cellular senescence in skin aging and age-related skin pathologies. Frontiers in physiology. 14. 1297637. Available at: https://doi.org/10.3389/fphys.2023.1297637 ↑5 He, X., Gao, X., & Xie, W. (2023). Research progress in skin aging, metabolism, and related products. International journal of molecular sciences. 24(21). 15930. Available at: https://doi.org/10.3390/ijms242115930 ↑6 Michelet, J. F., Commo, S., Billoni, N., Mahé, Y. F., & Bernard, B. A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. Journal of investigative dermatology. 108(2). 205-209. Available at: https://doi.org/10.1111/1523-1747.ep12334249 ↑7 Sasaki, S., Hozumi, Y., & Kondo, S. (2005). Influence of prostaglandin F2α and its analogues on hair regrowth and follicular melanogenesis in a murine model. Experimental dermatology. 14(5). 323-328. Available at: https://doi.org/10.1111/j.0906-6705.2005.00270.x ↑8 Michelet, J. F., Commo, S., Billoni, N., Mahé, Y. F., & Bernard, B. A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. Journal of investigative dermatology. 108(2). 205-209. Available at: https://doi.org/10.1111/1523-1747.ep12334249 ↑9 Li, Y., Lei, D., Swindell, W.R., Xia, W., Weng, S., Fu, J., Worthen, C.A., Okubo, T., Johnston, A., Gudjonsson, J.E. and Voorhees, J.J. 2015. Age-associated increase in skin fibroblast–derived prostaglandin E2 contributes to reduced collagen levels in elderly human skin. Journal of Investigative Dermatology. 135(9). 2181-2188. Available at: https://doi.org/10.1038/jid.2015.157 ↑10 Li, Y., Lei, D., Swindell, W.R., Xia, W., Weng, S., Fu, J., Worthen, C.A., Okubo, T., Johnston, A., Gudjonsson, J.E. and Voorhees, J.J. 2015. Age-associated increase in skin fibroblast–derived prostaglandin E2 contributes to reduced collagen levels in elderly human skin. Journal of Investigative Dermatology. 135(9). 2181-2188. Available at: https://doi.org/10.1038/jid.2015.157 ↑11 Shim, J. H. (2019). Prostaglandin E2 induces skin aging via E-prostanoid 1 in normal human dermal fibroblasts. International Journal of Molecular Sciences, 20(22), 5555. Available at: https://doi.org/10.3390/ijms20225555 ↑12 Sica, D. A. (2004). Minoxidil: an underused vasodilator for resistant or severe hypertension. The Journal of Clinical Hypertension. 6(5). 283-287. Available at: https://doi.org/10.1111/j.1524-6175.2004.03585.x ↑13 Sanabria, B., de Nardo Vanzela, T., Miot, H. A., & Ramos, P. M. (2021). Adverse effects of low-dose oral minoxidil for androgenetic alopecia in 435 patients. Journal of the American Academy of Dermatology. 84(4). 1175-1178. Available at: https://doi.org/10.1016/j.jaad.2020.11.035 ↑14 Salas, J., Esse, I., Kincaid, C. M., Birda, A., Choe, S., & Mesinkovska, N. A. (2025). Characterizing low-dose oral minoxidil-induced peripheral edema in alopecia patients. Journal of the American Academy of Dermatology. 92(3). 632-634. Available at: https://doi.org/10.1016/j.jaad.2024.09.078 ↑15, ↑17 Friedman, E. S., Friedman, P. M., Cohen, D. E., & Washenik, K. (2002). Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. Journal of the American Academy of Dermatology. 46(2). 309-312. Available at: https://doi.org/10.1067/mjd.2002.119104 ↑16 Friedman, E. S., Friedman, P. M., Cohen, D. E., & Washenik, K. (2002). Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. Journal of the American Academy of Dermatology. 46(2). 309-312. Available at: https://doi.org/10.1067/mjd.2002.119104 ↑18 Junge, A., Radonjic-Hoesli, S., Bossart, S., Simon, D., De Viragh, P., Hunger, R.E., Heidemeyer, K. and Jafari, S.M.S., 2025. Contact Dermatitis Caused by Topical Minoxidil: Allergy or Just Irritation. Acta Dermato-Venereologica. 105. 42401. Available at: https://doi.org/10.2340/actadv.v105.42401 ↑19 Pasricha, J. S., Nanda, A., & Bajaj, N. (1991). Contact dermatitis due to minoxidil. Indian Journal of Dermatology, Venereology and Leprology. 57. 235. Available at: https://ijdvl.com/contact-dermatitis-due-to-minoxidil/ (Accessed: 16 May 2025) Retinoic acid (RA) is the biologically active form of Vitamin A, playing an important role in regulating cell growth, differentiation, and tissue maintenance in the body.[1]Peehl, D.M., Wong, S.T., Stamey, T.A. (1993). Vitamin A regulates proliferation and differentiation of human prostatic epithelial cells. Prostate. 23(1). 69-78. Available at: … Continue reading Its signaling pathway is essential not only for embryonic development but also for the maintenance of healthy skin and hair.[2]VanBuren, C.A., Everts, H.B. (2022). Vitamin A in Skin and Hair: An Update. Nutrients. 14(14). 2952. Available at: https://doi.org/10.3390/nu14142952
Interest in using retinoids (RA and its derivatives, such as tretinoin and retinol) as hair loss treatments stems from their potential to regulate the hair growth cycle and potentially prolong the anagen (growth) phase of hair follicles. In this article, we will explore what RA is and its derivatives, as well as how they can impact the hair follicle. We will also evaluate the available efficacy and safety data.
Key Takeaways
- Drug. Retinoic acid (RA), especially in the form of tretinoin, is a vitamin A derivative that regulates hair follicle stem cell activation through Wnt/β-catenin signaling. It is available in various topical formulations and is often used in combination with minoxidil to enhance hair regrowth in androgenetic alopecia (AGA).
- Clinical Data. RA/tretinoin shows moderate standalone efficacy in promoting hair regrowth, particularly in early-stage AGA, but results improve significantly when used with minoxidil. Clinical trials from 1986 to 2024 have demonstrated enhanced hair density, thickness, and stem cell activity with combination therapy. RA also improves sulfotransferase enzyme activity, boosting minoxidil’s effectiveness, and has shown positive results in alopecia areata when combined with pimecrolimus.
- Safety. Generally well-tolerated at concentrations ≤0.025%, but may cause local irritation (redness, itching, dryness). Adverse effects are dose-dependent and typically self-limiting. Systemic side effects are rare with topical use but retinoids should be avoided during pregnancy and in individuals with photosensitivity or scalp inflammation.
- Evidence Quality. Retinoic Acid scored 34/100 for evidence quality by our metrics.
- Best Practices.
- Use in combination with topical minoxidil for optimal results, especially in early AGA.
- Apply tretinoin in low concentrations (≤0.025%) once daily to minimize irritation.
- Consider tretinoin if you’re a minoxidil non-responder or wish to reduce application frequency.
- Avoid if pregnant, photosensitive, or prone to dermatitis.
- Always consult a dermatologist before starting treatment.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Retinoic Acid?
RA is a biologically active metabolite of vitamin A (all-trans-retinol) and serves as a potent regulator of cell differentiation and proliferation. It plays essential roles in embryonic development, immune function, male fertility, and the regulation of bone growth and development.[3]de Mendoca Oliveira, L., Teixera, F.M.E., Sato, M.N. (2018). Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediators of Inflammation. 9(3067126). Available at: … Continue reading
Figure 1: Structures of the most common natural retinoids.[4]Ross, S.A., McCaffery, P.J., Drager, U.C., de Luca, L.M. Retinoids in Embryonal Development. Physiological Reviews. 80(3). 1021-1054. Available at: https://doi.org/10.1152/physrev.2000.80.3.1021
RA is produced in the body through a two-step oxidation process: retinol (vitamin A) is first reversibly oxidized to retinaldehyde, and then retinaldehyde is irreversibly oxidized to retinoic acid.[5]Kedishvili, N.Y. (2017). Retinoic Acid Synthesis and Degradation. Subcellular Biochemistry. 81. 127-161. Available at: https://doi.org/10.1007/978-94-024-0945-1_5 This process is catalyzed by retinol dehydrogenases and retinaldehyde dehydrogenases (RALDH1, RALDH2, RALDH3). RA cannot be converted back to retinol, making its synthesis a tightly regulated process.
Structurally, RA is a polyunsaturated carboxylic acid with a long conjugated hydrocarbon chain and a terminal carboxyl group.[6]Sani, B.P., Hill, D.L. (1990). [3] Structural characteristics of synthetic retinoids, Methods in Enzymology, Academic Press, 189. 43-50. Available at: https://doi.org/10.1016/0076-6879(90)89274-L The most prevalent naturally occurring form is all-trans-retinoic acid (ATRA), but other isomers such as 13-cis-retinoic acid (isotretinoin) and 9-cis-retinoic acid (alitretinoin) also exist in lower concentrations.[7]Bayeva, N., Coll, E., Piskareva, O. (2021). Differentiating Neuroblastoma: A Systematic Review of the Retinoic Acid, Its Derivatives, and Synergistic Interactions. Journal of Personalized Medicine. … Continue reading
RA is part of a broader class of compounds called retinoids, which includes both natural and synthetic derivatives of vitamin A.[8]Bushue, N., Wan, Y-J, Y. (2010). Retinoid pathway and cancer therapeutics. Advanced Drug Delivery Reviews. 62(13). 1285-1298. Available at: https://doi.org/10.1016/’j.addr.2010.07.003
Retinoids are categorized into generations based on their structure:[9]Baldwin, H., Webster, G., Gold, L.S., Callender, V., Cook-Bolden, F.E., Guenin, E. (2021). 50 Years of Topical Retinoids for Acne: Evolution of Treatment. American Journal of Clinical Dermatology. … Continue reading
- First Generation: Naturally occurring compounds and their isomers, including retinol, retinal, tretinoin (ATRA), isotretinoin, and alitretinoin.
- Second Generation: Synthetic analogs such as etretinate and acitretin, primarily used for severe psoriasis.
- Third Generation: Polyaromatic retinoids, such as adapalene, bexarotene, and tazarotene, are designed to achieve greater receptor sensitivity and reduced side effects.
- Fourth Generation: Newer compounds with enhanced selectivity, such as trifarotene.
Other commonly used derivatives include retinyl esters (e.g., retinyl palmitate), retinol, retinaldehyde, and synthetic esters like hydroxypinacolone retinate.
How Does Retinoic Acid Work?
RA acts primarily by binding to nuclear receptors in cells, specifically retinoic acid receptors (RARs) and retinoid X receptors (RXRs).[10]Jin, Q., Huo, C., Yang, W., Jin, K., Cai, S., Zheng, Y., Huang, B., Wei, L., Zhang, M., Han, Y., Zhang, X., Liu, Y., Wang, X. (2022). Regulation of Tyrosinase Gene Expression by Retinoic Acid Pathway … Continue reading Once activated, these receptors function as transcription factors, directly regulating the expression of genes involved in cell proliferation, differentiation, and programmed cell death, also known as apoptosis. This ability to modulate gene expression is what gives RA its wide range of biological effects, from skin remodeling to influence over hair follicle dynamics.
What is the Impact of Retinoic Acid on Hair Follicle Biology?
Retinoic acid (RA) plays a powerful but finely tuned role in hair follicle biology. It affects how hair grows by turning certain genes on or off, acting through specialized receptors found in hair follicle cells, oil glands, and nearby skin tissue.
Too little or too much RA can disrupt the hair cycle. That’s why the body tightly regulates how much RA is made and broken down inside the follicle.
How Is Retinoic Acid Synthesized in the Hair Follicle?
RA is produced in two steps:
The enzyme SDR16C5 converts vitamin A (retinol) into an intermediate form called retinaldehyde.[11]Wu, L., Belyaeva, O.V., Adams, M.K., Klyuyeva, A.V., Lee, S-A., Goggans, K.R., Kesterson, R.A., Popov, K.M., Kedishbili, N.Y. (2019). Mice lacking the epidermal retinol dehydrogenases SDR16C5 and … Continue reading This mostly occurs in the hair follicle bulge, a crucial area where stem cells reside, and in the dermal papilla, which plays a key role in controlling hair growth. SDR16C5 activity peaks during the growth phase (anagen) and helps stimulate both stem cell activity and hair matrix cell development.
In mice, the absence of SDR16C5 and its related enzyme, SDR16C6, results in an 80% decrease in RA levels.[12]Foitzik, K., Spexard, T., Nakamura, M., Halsner, U., Paus, R. (2005). Towards Dissecting the Pathogenesis of Retinoid-Induced Hair Loss: All-Trans Retinoic Acid Induces Premature Hair Follicle … Continue reading
This causes hair follicles to exit their resting phase (telogen) prematurely, leading to abnormal cycling and lower levels of key stem cell markers, such as Lgr5 and Sox9.[13]Goggans, K.R., Belyaeva, O.V., Klyuyeva, A.V., Studdard, J., Slay, A., Newman, R.B., Christine, VanBuren, A., Everts, H.B., Kedishvili, N.Y. (2024). Epidermal retinol dehydrogenases cyclically … Continue reading
Another enzyme, ALDH1A2, completes the process by converting retinaldehyde into the active form of retinoic acid. ALDH1A2 is most active in the outer root sheath of the follicle and especially in the bulge during the early growth and regression phases.[14]Everts, H.B., King Jr, L.E., Sundberg, J.P., Ong, D.E. (2004). Hair cycle-specific immunolocalization of retinoic acid synthesizing enzymes ALDH1A2 and ALDH1A3 indicates complex regulation. Journal … Continue reading One of its key roles is to lower levels of BMP4, a protein that keeps stem cells in a resting state. By reducing BMP4, ALDH1A2 helps “awaken” stem cells and initiate new hair growth.[15]Suo, L., VanBuren, C., Hovland, E.D., Kedishvili, N.Y., Sundberg, J.P., Everts, H.B. (2021). Dietary Vitamin A Impacts Refractory Telogen. Frontiers in Cell and Developmental Biology. 9. Available … Continue reading When this enzyme is missing, hair follicles take longer to enter the growth phase, and their circadian rhythm becomes disrupted.
How is Retinoic Acid Broken Down?
While synthesizing RA is important, too much can be harmful. That’s where CYP26B1 comes in. CYP26B1 is an enzyme that breaks down RA into inactive forms. During the growth phase, it is found in the dermal papilla and pre-cortex; during rest, it shifts to the bulge to prevent RA levels from building up excessively.[16]Okano, J., Levy, C., Lichti, U., Sun, H-W., Yuspa, S.H., Sakai, Y., Morasso, M.I. (2012). Cutaneous retinoic acid levels determine hair follicle development and downgrowth. Journal of Biological … Continue reading
This careful back-and-forth between making and breaking down RA shows how tightly the hair follicle controls its environment. Low, well-regulated levels of RA can support stem cell activity and help promote healthy hair growth. However, high or unbalanced levels can actually prompt follicles to regress or block growth altogether.
To put it plainly: Your hair follicles need just the right amount of retinoic acid. When the balance is right, it may help stimulate hair growth, but when it’s off, it can slow or even reverse it.
Importantly, these regulatory mechanisms are not just theoretical; they appear to be directly involved in common hair disorders, such as androgenic alopecia (AGA).
In vivo/In vivo Evidence
Recent research has demonstrated that retinoic acid signaling is suppressed in miniaturized hair follicles from AGA patients which coincides with impaired hair follicle stem cell (HFSC) activation.[17]Wen, L., Fan, Z., Huang, W., Miao, Y., Zhang, J., Liu, B., Zhu, D., Dai, D., Zhang, J., Le, D., Zhang, Y., Qu, Q., Hu, Z., Chen, R. (2024). Retinoic acid drives hair follicle stem cell activation via … Continue reading
Using transcriptomic, in vivo (mouse), ex vivo (human hair follicle organ culture), in vitro (HFSC culture), and clinical approaches, the authors showed that retinoic acid supplementation restored HFSC function by activating Wnt7a/7b and downstream ꞵ-catenin signaling. This promotes HFSC proliferation, differentiation into progenitor cells, and entry into the anagen phase, resulting in improved hair follicle growth (Figure 2), accelerated hair regrowth in mice (Figure 3), and improved hair density and diameter in AGA patients, particularly in early treatment (more on that below).
Figure 2: Effect of different concentrations of retinoic acid (RA) on hair follicle growth. MI = minoxidil. The 10-12 M concentration of RA showed a significant increase in hair follicle regrowth compared to the control (DMSO). Both RA and MI showed significant increases in anagen hair follicles compared to DMSO. Compared with the DMSO group, *p<0.05, **p<0.01, ***p<0.001.[18]Wen, L., Fan, Z., Huang, W., Miao, Y., Zhang, J., Liu, B., Zhu, D., Dai, D., Zhang, J., Le, D., Zhang, Y., Qu, Q., Hu, Z., Chen, R. (2024). Retinoic acid drives hair follicle stem cell activation via … Continue reading
Figure 3: Effect of different concentrations of topical RA on mouse hair growth over 12 days. The 10-5 M and 10-10 M appeared to show the fastest and highest levels of hair regrowth.[19]Wen, L., Fan, Z., Huang, W., Miao, Y., Zhang, J., Liu, B., Zhu, D., Dai, D., Zhang, J., Le, D., Zhang, Y., Qu, Q., Hu, Z., Chen, R. (2024). Retinoic acid drives hair follicle stem cell activation via … Continue reading
Mechanistically, blocking either retinoic acid receptors or Wnt signaling reduced these effects, confirming that retinoic acid acts via Wnt/ꞵ-catenin to drive HFSC activation and hair regeneration.
Is Retinoic Acid Clinically Effective at Treating Hair Loss?
Retinoic acid and its derivative, tretinoin, have been investigated in clinical trials for treating both androgenetic alopecia (AGA) and alopecia areata (AA). Studies have shown potential synergistic effects with minoxidil, including improved enzyme activity and increased hair regrowth rates, although efficacy typically varies by condition and formulation.
Study One -1986 (AGA)
This study was an open-label trial of 56 participants with AGA treated with tretinoin alone or in combination with 0.5% minoxidil for 1 year.[20]Bazzano, G.S., Terezakis, N., Galen, W. (1986). Topical tretinoin for hair growth promotion. Journal of the American Academy of Dermatology. 15.880-883. Available at: … Continue reading Twelve participants received 0.025% topical tretinoin, 36 participants received a combination of 0.025% tretinoin and 0.5% minoxidil, 3 participants received 0.5% minoxidil alone, and 5 participants received a topical placebo.
Hair growth was evaluated through photographs and hair counts in a defined 1-inch diameter target area on the scalp. The duration of participation averaged 8-10 months, with some subjects following the protocol up to 18 months. Participants were categorized as:
- Good responders (Group A): ≥46% increase in terminal hairs.
- Moderate responders (Group B): 21–45% increase.
- Non-responders (Group C): <20% increase.
Placebo and Minoxidil Alone:
Placebo goup: No participants showed hair growth
Minoxidil alone: No participants showed terminal hair regrowth; one participant showed vellus hair growth.
Tretinoin Alone:
12 participants: 58% (7 participants) responded positively, 16% (2 participants) shows a “good response”, 42% (5 participants) had a “moderate” response, and 42% had no response.
However, one woman appeared to be highly responsive to treatment with a 1100% increase in hair counts after 18 months of tretinoin-only treatment.
Combination of Tretinoin and Minoxidil:
36 participants: 66% (24 participants) responded positively, 44% (16 participants) had a “good” response, 22% (8 participants) had a “moderate” response, and 33% (12 participants) had no response.
Responses were observed within 4 to 18 months, with photographic documentation showing visible regrowth in both men and women.
Ultimately, this study showed that tretinoin alone moderately improved hair regrowth, but combining the treatment with minoxidil was more effective, with two-thirds of participants showing terminal hair regrowth.
Study Two – 2007 (AGA)
31 male participants aged 28-45 were recruited for this 18-week study, which compared the efficacy and safety of a combined solution of 5% minoxidil and 0.01% tretinoin applied once daily versus conventional 5% minoxidil applied twice daily for male AGA.[21]Shin, H.S., Won, C.H., Lee, S.H., Kwon, O.S., Kim, K.H., Eun, H.C. (2007). Efficacy of 5% minoxidil versus combined 5% minoxidil and 0.01% tretinoin for male pattern hair loss. American Journal of … Continue reading
Five parameters were evaluated: total hair count, non-vellus hair count, anagen hair ratio, linear hair growth rate, and mean hair diameter. Subjective global assessments were also conducted by patients and investigators at 9 and 18 weeks.
Both groups showed significant increases in total hair count and non-vellus hair count after 18 weeks. Mean hair diameter increased significantly in the minoxidil-only group. However, there were no significant changes in anagen hair ratio or linear hair growth rate in either group. Furthermore, there were no statistically significant differences between groups in any hair growth parameter after 18 weeks.
No significant differences were observed between the two groups in patient-reported improvement or satisfaction scores at any time point. Furthermore, the investigator’s global assessment also showed no significant differences between groups.
Figure 4: Effect of tretinoin and minoxidil on hair growth outcomes.[22]Shin, H.S., Won, C.H., Lee, S.H., Kwon, O.S., Kim, K.H., Eun, H.C. (2007). Efficacy of 5% minoxidil versus combined 5% minoxidil and 0.01% tretinoin for male pattern hair loss. American Journal of … Continue reading
Safety outcomes showed that mild adverse effects (itching, irritation) were observed in 5/15 patients in the combined group and 4/14 in the minoxidil-only group, but these were all self-limiting.
The conclusion? The once-daily application of combined 5% minoxidil and 0.01% tretinoin was as effective and safe as twice-daily 5% minoxidil for treating AGA in men. This means it could potentially be a more convenient alternative to twice-daily minoxidil.
Study Three – 2019 (AGA)
Another study found that combining tretinoin with minoxidil could increase follicular sulfotransferase activity and therefore responsiveness to minoxidil.[23]Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvan S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in … Continue reading
Sulfotransferase (SULT1A1) is the enzyme responsible for converting minoxidil, a prodrug, into its active metabolite minoxidil sulfate within hair follicles.[24]Dhurat, R., Daruwalla, S., Paj, S., Kovacevic, M., McCoy, J., Shapiro, J., Sinclair, R., Vano-Galvan, S., Goren, A. (2022). SULT1A1 (Minoxidil Sulfotransferase) enzyme booster significantly improves … Continue reading This sulfation reaction is critical because minoxidil sulfate directly stimulates hair growth.
Twenty participants (10 male and 10 female) with AGA were recruited for this study, which determined the effect of topical tretinoin on follicular sulfotransferase. Non-responders to minoxidil often exhibit follicular SULT1A1 activity <0.4 OD 405 nm. In this trial, pretreatment SULT1A1 levels in participants who were minoxidil non-responders averaged 0.28 OD 405 nm.
Treatment with topical tretinoin (0.1%) increased SULT1A1 activity by 1.8-fold in 75% of subjects after 5 days, raising levels to around 0.51 OD 405 nm. This shift moved 43% of non-responders above the 0.4 OD cutoff, enabling sufficient minoxidil activation.
Study Four – 2024 (AGA)
The most recent trial was conducted in 2024, with 60 total participants with AGA.[25]Wen, L., Fan, Z., Huang, W., Miao, Y., Zhang, J., Liu, B., Zhu, D., Dai, D., Zhang, J., Le, D., Zhang, Y., Qu, Q., Hu, Z., Chen, R. (2024). Retinoic acid drives hair follicle stem cell activation via … Continue reading 30 participants received a 0.025% topical tretinoin cream, while 30 received 5% minoxidil solution alone. Each treatment was applied twice daily to the bald scalp.
Hair growth was measured using photography and dermoscopy assessments at 1, 3, and 6-month follow-ups.
The participants treated with the topical tretinoin cream showed a significant improvement in mean hair count, density, and diameter after the first month compared to the Baseline. A significant increase was also observed in hair count, density, and terminal hair ratio in the tretinoin group compared to the minoxidil-treated group.
Figure 5: Effect of topical tretinoin on (a + b) photographic evidence of hair growth, and (c) hair count, hair diameter, and terminal hair ratio.[26]Wen, L., Fan, Z., Huang, W., Miao, Y., Zhang, J., Liu, B., Zhu, D., Dai, D., Zhang, J., Le, D., Zhang, Y., Qu, Q., Hu, Z., Chen, R. (2024). Retinoic acid drives hair follicle stem cell activation via … Continue reading
Study 5 – 2014 (AA)
A randomized controlled trial was conducted with 80 participants with alopecia areata aged 18-25 years, with one to four lesions on the scalp, and lesion diameter of 1.5 to 6 cm.[27]Jalai, M.H.A., Mobasher, P., Rabbani, R., Jazi, G.A. (2014). Comparing the Efficiency of Elidel Cream and Elidel Accompanied with Tretinoin Cream in Treatment of Alopecia Areata. Journal of Skin Stem … Continue reading
Participants were treated with either 1% Elidel (pimecrolimus cream) (40 participants) or Elidel + 0.05% tretinoin (40 participants) twice daily for three days. Treatment response was categorized as complete cure, relative cure, no change, or aggravation of lesions.
Elidel Group:
- Complete cure: 8 patients (20%)
- Relative cure: 14 (35%)
- No change: 10 (25%)
- Aggravation: 8 (20%)
Eliden and Tretinoin Group:
- Complete cure: 18 patients (45%)
- Relative cure: 12 (30%)
- No change: 8 (20%)
- Aggravation: 2 (5%)
The combination therapy group had significantly higher rates of complete cure and lower rates of disease aggravation. However, adverse effects (mainly redness and burning) were more common in the combination group (45%), leading to dose reduction or discontinuation in some cases, but no serious complications were reported.
Unfortunately, the study included no images to show what the initial hair loss severity was and whether the improvements were actually noticeable/
Overall, the clinical evidence suggests that retinoic acid/tretinoin may be most effective as part of a combination therapy, and that patient selection, dosing, and timing play crucial roles in treatment success.
Another point that we can learn from these studies is that tretinoin appears to enhance the effectiveness of minoxidil (and potentially Eliden). This could be due to several mechanisms:
- Enhanced Penetration: Tretinoin may increase skin permeability, allowing better minoxidil absorption.
- Boosts Sulfotransferase Enzymes: Tretinoin upregulates SULT1A1, the enzyme responsible for converting minoxidil into its active form (minoxidil sulfate).
- Stimulates Stem Cell Activity: Both agents independently promote anagen phase entry and follicle regeneration via different pathways (minoxidil via potassium channels, RA via Wnt signaling).
Together, they work on complementary pathways, which helps explain why multiple trials (1986, 2007, 2019, 2024) found better hair growth outcomes with combined use.
How Safe is Retinoic Acid/Tretinoin?
While generally safe, retinoic acid and tretinoin can cause local irritation, especially when used topically on the scalp:
- Common Side Effects: Redness, itching, dryness, and burning sensations.[28]Yoham AL, Casadesus D. Tretinoin. [Updated 2023 Mar 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: … Continue reading
- Dose-Dependent: Higher concentrations (e.g., 0.1%) are more likely to cause irritation.[29]Griffiths, C.E., Kang, S., Ellis, C.N., Kim, K.J., Finkel, L.J., Ortiz-Ferrer, L.C., White, G.M., Hamilton, T.A., Voorhees, J.J. (1995). Two concentrations of topical tretinoin (retinoic acid) cause … Continue reading
- Mitigation: Reducing application frequency or using lower strengths can improve tolerability. Some studies reported treatment discontinuation due to discomfort.
- Systemic Risks: Rare with topical use, but oral retinoids like isotretinoin carry higher risks and should not be used for hair loss without medical supervision.
In clinical trials, adverse events were mild and reversible, making tretinoin a relatively low-risk addition to hair loss regimens, especially in concentrations ≤0.025%.
Is Retinoic Acid/Tretinoin for Me?
Retinoic acid or tretinoin may be right for you if:
- You’ve had limited success with minoxidil alone, especially if you’re a non-responder.
- You’re seeking enhanced regrowth or wish to reduce the frequency of minoxidil application (e.g., once-daily combo vs. twice-daily minoxidil).
- You’re in the early stages of hair loss, where interventions tend to be more effective.
Caution is warranted if:
- You are hypersensitive to the drug, drug class, or drug formulation.
- If you are prone to sunburn.
- You have photosensitivity issues.
- You have eczema, seborrheic dermatitis, or other scalp inflammation problems.
- You are pregnant and in the first trimester (retinoids are dangerous for the fetus if they become systemic).
Always consult a dermatologist before starting any retinoid-based hair regimen.
Final Thoughts
Retinoic acid and tretinoin offer a scientifically grounded, mechanistically rich approach to hair loss treatment, particularly for androgenetic alopecia. Although not a magic bullet, they can:
- Enhance minoxidil efficacy
- Support hair follicle stem cell activation
- Offer a new avenue for patients who have plateaued on existing treatments.
That said, results are highly individual, and the treatment must be carefully tailored, balancing dose, frequency, and formulation to optimize results while minimizing irritation. Therefore, we don’t recommend using it by itself.
For now, tretinoin appears best used in combination, especially for those seeking to maximize outcomes from minoxidil therapy.
References[+]
References ↑1 Peehl, D.M., Wong, S.T., Stamey, T.A. (1993). Vitamin A regulates proliferation and differentiation of human prostatic epithelial cells. Prostate. 23(1). 69-78. Available at: https://doi.org/10.1002/pros.2990230107 ↑2 VanBuren, C.A., Everts, H.B. (2022). Vitamin A in Skin and Hair: An Update. Nutrients. 14(14). 2952. Available at: https://doi.org/10.3390/nu14142952 ↑3 de Mendoca Oliveira, L., Teixera, F.M.E., Sato, M.N. (2018). Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediators of Inflammation. 9(3067126). Available at: https://doi.org/10.1155/2018/3067126 ↑4 Ross, S.A., McCaffery, P.J., Drager, U.C., de Luca, L.M. Retinoids in Embryonal Development. Physiological Reviews. 80(3). 1021-1054. Available at: https://doi.org/10.1152/physrev.2000.80.3.1021 ↑5 Kedishvili, N.Y. (2017). Retinoic Acid Synthesis and Degradation. Subcellular Biochemistry. 81. 127-161. Available at: https://doi.org/10.1007/978-94-024-0945-1_5 ↑6 Sani, B.P., Hill, D.L. (1990). [3] Structural characteristics of synthetic retinoids, Methods in Enzymology, Academic Press, 189. 43-50. Available at: https://doi.org/10.1016/0076-6879(90)89274-L ↑7 Bayeva, N., Coll, E., Piskareva, O. (2021). Differentiating Neuroblastoma: A Systematic Review of the Retinoic Acid, Its Derivatives, and Synergistic Interactions. Journal of Personalized Medicine. 11(3). 211. Available at: https://doi.org/10.3390/jpm11030211 ↑8 Bushue, N., Wan, Y-J, Y. (2010). Retinoid pathway and cancer therapeutics. Advanced Drug Delivery Reviews. 62(13). 1285-1298. Available at: https://doi.org/10.1016/’j.addr.2010.07.003 ↑9 Baldwin, H., Webster, G., Gold, L.S., Callender, V., Cook-Bolden, F.E., Guenin, E. (2021). 50 Years of Topical Retinoids for Acne: Evolution of Treatment. American Journal of Clinical Dermatology. 22. 315-327. Available at: https://doi.org/10.1007/s40257-021-00594-8 ↑10 Jin, Q., Huo, C., Yang, W., Jin, K., Cai, S., Zheng, Y., Huang, B., Wei, L., Zhang, M., Han, Y., Zhang, X., Liu, Y., Wang, X. (2022). Regulation of Tyrosinase Gene Expression by Retinoic Acid Pathway in the Pacific Oyster Crassostrea gigas. International Journal of Molecular Sciences. 23(21). 12840. Available at: https://doi.org/10.3390/ijms232112840 ↑11 Wu, L., Belyaeva, O.V., Adams, M.K., Klyuyeva, A.V., Lee, S-A., Goggans, K.R., Kesterson, R.A., Popov, K.M., Kedishbili, N.Y. (2019). Mice lacking the epidermal retinol dehydrogenases SDR16C5 and SDR16C display accelerated hair growth and enlarged meibomian glands. Journal of Biological Chemistry. 294(45). 17060-17074. Available at: https://doi.org/10.1074/jbc.RA119.010835 ↑12 Foitzik, K., Spexard, T., Nakamura, M., Halsner, U., Paus, R. (2005). Towards Dissecting the Pathogenesis of Retinoid-Induced Hair Loss: All-Trans Retinoic Acid Induces Premature Hair Follicle Regression (Catagen) by Upregulation of Transforming Growth Factor-ꞵ2 in the Dermal Papilla. Journal of Investigative Pathology. 124(6). 1119-1126. Available at: https://doi.org/10.1111/j.0022-202X.2005.23686 ↑13 Goggans, K.R., Belyaeva, O.V., Klyuyeva, A.V., Studdard, J., Slay, A., Newman, R.B., Christine, VanBuren, A., Everts, H.B., Kedishvili, N.Y. (2024). Epidermal retinol dehydrogenases cyclically regulate stem cell markers and clock genes and influence hair composition. Nature Communications Biology. 7(435). Available at: https://doi.org/10.1038/s42003-024-06160-2 ↑14 Everts, H.B., King Jr, L.E., Sundberg, J.P., Ong, D.E. (2004). Hair cycle-specific immunolocalization of retinoic acid synthesizing enzymes ALDH1A2 and ALDH1A3 indicates complex regulation. Journal of Investigative Dermatology. 123(2). 258-263. Available at: https://doi.org/10.1111/j.0022-202X.2004.23223.x. ↑15 Suo, L., VanBuren, C., Hovland, E.D., Kedishvili, N.Y., Sundberg, J.P., Everts, H.B. (2021). Dietary Vitamin A Impacts Refractory Telogen. Frontiers in Cell and Developmental Biology. 9. Available at: https://doi.org/10.3389/fcell.2021.571474 ↑16 Okano, J., Levy, C., Lichti, U., Sun, H-W., Yuspa, S.H., Sakai, Y., Morasso, M.I. (2012). Cutaneous retinoic acid levels determine hair follicle development and downgrowth. Journal of Biological Chemistry. 287(47). 39304-39315. Available at: https://doi.org/10.1074/jbc.M112.397273. ↑17, ↑18, ↑19 Wen, L., Fan, Z., Huang, W., Miao, Y., Zhang, J., Liu, B., Zhu, D., Dai, D., Zhang, J., Le, D., Zhang, Y., Qu, Q., Hu, Z., Chen, R. (2024). Retinoic acid drives hair follicle stem cell activation via Wnt/ꞵ-catenin signalling in androgenetic alopecia. Journal of the European Academy of Dermatology and Venereology. 39(1). 189-201. Available at: https://doi.org/10.1111/jdv.20000 ↑20 Bazzano, G.S., Terezakis, N., Galen, W. (1986). Topical tretinoin for hair growth promotion. Journal of the American Academy of Dermatology. 15.880-883. Available at: https://doi.org/10.1016/s0190-9622(86)80024-x ↑21 Shin, H.S., Won, C.H., Lee, S.H., Kwon, O.S., Kim, K.H., Eun, H.C. (2007). Efficacy of 5% minoxidil versus combined 5% minoxidil and 0.01% tretinoin for male pattern hair loss. American Journal of Clinical Dermatology. 8(5). 285-290. Available at: https://doi.org/10.2165/00128071-200708050-00003 ↑22 Shin, H.S., Won, C.H., Lee, S.H., Kwon, O.S., Kim, K.H., Eun, H.C. (2007). Efficacy of 5% minoxidil versus combined 5% minoxidil and 0.01% tretinoin for male pattern hair loss. American Journal of Clinical Dermatology. 8(5). 285-290. Available at: https://doi.org/10.2165/00128071-200708050-00003 ↑23 Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvan S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatological Therapy. 32(3). E12915. Available at: https://doi.org/10.1111/dth.12915 ↑24 Dhurat, R., Daruwalla, S., Paj, S., Kovacevic, M., McCoy, J., Shapiro, J., Sinclair, R., Vano-Galvan, S., Goren, A. (2022). SULT1A1 (Minoxidil Sulfotransferase) enzyme booster significantly improves response to topical minoxidil for hair regrowth. Journal of Cosmetic Dermatology. 21(1). 343-346. Available at: https://doi.org/10.1111/jocd.14299 ↑25 Wen, L., Fan, Z., Huang, W., Miao, Y., Zhang, J., Liu, B., Zhu, D., Dai, D., Zhang, J., Le, D., Zhang, Y., Qu, Q., Hu, Z., Chen, R. (2024). Retinoic acid drives hair follicle stem cell activation via Wnt/ꞵ-catenin signalling in androgenetic alopecia. Journal of the European Academy of Dermatology and Venereology. 39(1). Available at: https://doi.org/10.1111/jdv.20000 ↑26 Wen, L., Fan, Z., Huang, W., Miao, Y., Zhang, J., Liu, B., Zhu, D., Dai, D., Zhang, J., Le, D., Zhang, Y., Qu, Q., Hu, Z., Chen, R. (2024). Retinoic acid drives hair follicle stem cell activation via Wnt/ꞵ-catenin signalling in androgenetic alopecia. Journal of the European Academy of Dermatology and Venereology. 39(1). Available at: https://doi.org/10.1111/jdv.20000 ↑27 Jalai, M.H.A., Mobasher, P., Rabbani, R., Jazi, G.A. (2014). Comparing the Efficiency of Elidel Cream and Elidel Accompanied with Tretinoin Cream in Treatment of Alopecia Areata. Journal of Skin Stem Cells. 1(2).e20108. Available at: https://doi.org/10.17795/jssc20108 ↑28 Yoham AL, Casadesus D. Tretinoin. [Updated 2023 Mar 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557478/ (Accessed: May 2025 ↑29 Griffiths, C.E., Kang, S., Ellis, C.N., Kim, K.J., Finkel, L.J., Ortiz-Ferrer, L.C., White, G.M., Hamilton, T.A., Voorhees, J.J. (1995). Two concentrations of topical tretinoin (retinoic acid) cause similar improvement of photoaging but different degrees of irritation. A double-blind, vehicle-controlled comparison of 0.1% and 0.025% tretinoin creams. Archives of dermatology. 131(9). 1037-1044. Available at: https://doi.org/10.1001/archderm.1995.01690210067011 Cetirizine is a second-generation antihistamine that is approved by the FDA for the treatment of allergies. It works by blocking the H1 receptor and preventing the function of histamine, the biological compound that is responsible for causing allergic symptoms.[1]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008
In this article, we will explore the way in which cetirizine has been proven to work and look at some additional functions that may influence hair health. We will also look to see if there are any clinical studies that show cetirizine can promote hair growth, as well as any safety data that indicates whether it is safe to use.
Key Takeaways
- What is it? Cetirizine is a second-generation antihistamine that is approved for use as an oral medication to treat allergies. It primarily works by binding the H1 receptor, preventing histamine from binding and causing allergic symptoms. Studies have also shown that cetirizine has anti-inflammatory effects, inhibiting the migration of inflammatory mediators. Moreover, it has been shown to reduce PGD2 production, a molecule with strong links to hair loss.
- Clinical Data. In clinical studies, the topical application of cetirizine has been shown to increase hair density, hair thickness, hair regrowth, and the proportion of hairs in the anagen (growing) phase. This indicates that topical cetirizine may promote hair growth, but there are also several limitations that are discussed in detail below.
- Safety. Oral cetirizine is FDA-approved and has been shown to cause limited side effects, none of which are considered serious. No conclusive safety data for topical cetirizine exists, but it is likely to be safe based on the safety profile of oral cetirizine. That said, people who are breastfeeding or have kidney failure may not be able to use cetirizine.
- Evidence Quality. Cetirizine scored 57/100 for evidence quality by our metrics.
- Best Practices. Cetirizine is approved for use at the following dosages: 10mg within a 24-hour period in adults, 5mg twice daily in children aged 6-11 years, 2.5mg twice daily in children aged 2-5 years. No recommended dosages exist for the use of topical cetirizine, but 1% cetirizine formulations applied at 1-2 mL daily have shown benefit in several clinical studies.
Interested in Topical Cetirizine?
Support your hair with full-strength topical cetirizine, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Cetirizine?
Cetirizine is a second-generation antihistamine that is widely used for its anti-allergic properties, which makes it particularly effective for treating the symptoms of seasonal allergic rhinitis (otherwise known as hayfever) and chronic idiopathic urticaria (hives).[2]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008 Such is the importance of cetirizine that it is included in the World Health Organizations List of Essential Medicines.[3]World Health Organization. (2023). WHO Model List of Essential Medicines – 23rd list, 2023. WHO/MHP/HPS/EML/2023.02. Available at: … Continue reading
Origin and Structure
Cetirizine, a second-generation antihistamine, is derived from the metabolism of hydroxyzine, a first-generation antihistamine. A common feature of second-generation antihistamines, including cetirizine, is limited crossing of the blood-brain barrier and the rapid efflux of any molecules that do enter the brain.[4]Pagliara, A., Testa, B., Carrupt, P.A., Jolliet, P., Morin, C., Morin, D., Urien, S., Tillement, J.P. and Rihoux, J.P. (1998). Molecular properties and pharmacokinetic behavior of cetirizine, a … Continue reading This reduced uptake limits the effects on the central nervous system, thus lowering the potential for side effects such as drowsiness which were common in the first-generation.[5]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008
How Does Cetirizine Work?
When used for treating the symptoms of allergies, cetirizine is delivered orally as a liquid or tablet. Cetirizine has been used to treat allergies since the 1980s and its mechanism of action, as well as its therapeutic effects, are very well documented. We’ll start by summarizing these effects, before taking a closer look at more recent research that has explored the potential benefits of topical cetirizine in the treatment AGA.
H1-Receptor Antagonism
Cetirizine primarily functions as a potent and selective antagonist of the histamine H1 receptors. Hypersensitive reactions, which manifest as allergies, are initiated when allergens trigger the release of histamine from cells of the immune system. The released histamine binds to H1 receptors, which is the mechanism that is responsible for producing the classic symptoms of allergies.[6]Ghosh, S., Debnath, I., Bhunia, S., Hazra, S., Nandi, S., & Mandal, S. K. (2025). A Review on Mechanism of Histamine Mediated Allergic Reactions: Therapeutic Role, Safety, and Clinical Efficacy … Continue reading Cetirizine blocks this mechanism by binding to the H1 receptors and blocking the histamine, preventing it from activating the allergic response.[7]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008
Anti-inflammatory Properties
Beyond its role as an antihistamine, cetirizine exhibits significant anti-inflammatory effects that contribute to its therapeutic value. This is particularly beneficial because allergic reactions are characterized by inflammation and the infiltration of immune cells into the different tissues of the body.[8]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008
In an animal study, rats were orally administered cetirizine (900µg/kg; equivalent to the standard human dose of 10mg/kg) or a control substance. Acute inflammation was then induced by injecting the pro-inflammatory substance carrageenin into the paw. After 3 hours, the volume of edema (swelling) in the paw was quantified and compared between the treated and untreated rats. This revealed that there was significantly less swelling (-41.93%) in the cetirizine-treated rats compared to the untreated rats.[9]Vardhamane, S. H., Santoshkumar, J., & Vardhamane, S. H. (2013). Anti-inflammatory activity of H1 receptor antagonist Cetirizine in animal models. Journal of Evolution of Medical and Dental … Continue reading
The same authors also tested cetirizine in rats with chronic inflammation. Once again, rats were provided with orally administered cetirizine (900µg/kg; equivalent to the standard human dose of 10mg/kg) or a control substance. Inflammation was induced by inserting rexin pellets underneath skin and the rats monitored for 7 days, receiving a daily dose of cetirizine. After 7 days, the granuloma (area of tissue indicative of inflammation) was excised from the rats and weighed. They showed that treatment with cetirizine reduced the granuloma weight (-50.05%), indicating that cetirizine inhibited inflammation.[10]Vardhamane, S. H., Santoshkumar, J., & Vardhamane, S. H. (2013). Anti-inflammatory activity of H1 receptor antagonist Cetirizine in animal models. Journal of Evolution of Medical and Dental … Continue reading
There are also several clinical trials that have shown its anti-inflammatory properties. In one such study, a total of 12 patients faced an allergen-specific conjunctival challenge, where a known allergen was dropped into the eye. These patients were randomly assigned into one of two groups – 10 mg cetirizine or placebo, both twice daily. Although the number of inflammatory cells increased following the allergen challenge, their levels were still significantly lower in the cetirizine group than the untreated controls.[11]Ciprandi, G., Buscaglia, S., Pesce, G., Passalacqua, G., Rihoux, J. P., Bagnasco, M., & Canonica, G. W. (1995). Cetirizine reduces inflammatory cell recruitment and ICAM-1 (or CD54) expression on … Continue reading
A double-blinded, placebo-controlled study was also conducted in 14 patients with atopic dermatitis (eczema) and 16 healthy subjects. Participants either received cetirizine (10mg in the morning, 20mg in the evening) or the placebo, and they consumed their respective product every day for 3 days. They took blood samples after Day 2 and then tested the ability of monocytes, key cells of the immune system, to migrate towards a chemical stimulus. The migration of these cells forms a key part of their ability to induce inflammation, so a reduction in the ability to migrate would suggest a reduced inflammatory response. Their results showed that treatment with cetirizine reduced or even abolished ex vivo monocyte migration in both the healthy subjects and the patients with atopic dermatitis.[12]Jinquan, T., Reimert, C. M., Deleuran, B., Zachariae, C., Simonsen, C., Thestrup-Pedersen, K., & May, R. (1995). Cetirizine inhibits the in vitro and ex vivo chemotactic response of T lymphocytes … Continue reading
Therefore, the literature strongly suggests that cetirizine exhibits anti-inflammatory properties.
Mast Cell Stabilization
Studies also suggest that some of the effects of cetirizine may be mediated by its regulation of mast cells. These white blood cells are found throughout the body and they form part of the immune response, particularly the allergic response. This is because they contain inflammatory mediators (including histamine). When mast cells are stimulated by an allergen, they degranulate and release their inflammatory compounds, driving inflammation and the classic signs of allergy.[13]Amin, K. (2012). The role of mast cells in allergic inflammation. Respiratory medicine. 106(1). 9-14. Available at: https://doi.org/10.1016/j.rmed.2011.09.007
The effect of cetirizine on mast cells has been demonstrated in vitro. Mast cells were isolated from rats and then treated with increasing concentrations of cetirizine, before exocytosis (degranulation) was induced using a chemical compound. The authors observed a significant decrease in degranulation at a cetirizine concentration of 100µM, while the highest concentration of 1mM induced near-total suppression of degranulation (Figure 1). Furthermore, cetirizine was also shown to significantly outperform a first-generation antihistamine (diphenhydramine) when comparing the relative reduction of degranulation.[14]Fujimura, R., Asada, A., Aizawa, M., & Kazama, I. (2022). Cetirizine more potently exerts mast cell-stabilizing property than diphenhydramine. Drug Discoveries & Therapeutics. 16(5). 245-250. … Continue reading Thus, this study provides evidence that cetirizine may exert its anti-allergic and anti-inflammatory effects via the mediation of mast cell activity.
Figure 1: Cetirizine reduces rat mast cell degranulation in vitro. (A) Microscopic images showing the effects of cetirizine on the mast cells, with visibly reduced degranulation. (B) Cetirizine at 100µM significantly reduces degranulation, while 1mM of cetirizine causes almost total suppression.[15]Fujimura, R., Asada, A., Aizawa, M., & Kazama, I. (2022). Cetirizine more potently exerts mast cell-stabilizing property than diphenhydramine. Drug Discoveries & Therapeutics. 16(5). 245-250. … Continue reading
Histamine and Hair Loss: Why It Matters
What is Histamine?
Histamine is a biologically active compound that is derived from the amino acid histidine. As previously noted, histamine is stored in the mast cells that are found throughout the body, but it can also be found in basophils (another type of white blood cell). Histamine may also be found in the neurons of the brain, but the white blood cells are its primary store.[16]Carthy, E., & Ellender, T. (2021). Histamine, neuroinflammation and neurodevelopment: a review. Frontiers in neuroscience. 15. 680214. Available at: https://doi.org/10.3389/fnins.2021.680214
Histamine primarily functions by binding to the H1, H2, H3, and H4 receptors that are found in the brain, blood vessels, heart, neurons, and many other tissues throughout the body. Depending on the receptor it binds to, histamine can exert effects that include initiating inflammation, changing the blood flow, or making the blood vessels more permeable which facilitates the leakage of fluid into surrounding tissues (causing swelling).[17]Branco, A. C. C. C., Yoshikawa, F. S. Y., Pietrobon, A. J., & Sato, M. N. (2018). Role of histamine in modulating the immune response and inflammation. Mediators of inflammation. 2018(1). … Continue reading
This wide range of functions means that histamine can exert influence over many bodily processes, so it is not surprising that histamine has been linked to hair loss.
Histamine’s Role in Hair Biology
Although the evidence is rather limited, there are a number of studies that have suggested histamine could be contributing to hair loss.
Scalp biopsies from patients with telogen effluvium (TE; 10 patients), alopecia areata (AA; 7 patients), androgenetic alopecia (AGA or ANDRO; 9 patients) were compared to those of healthy volunteers (8 patients). They stained each of these biopsies to count the number of mast cells and determine if there was a difference between conditions. Sure enough, they found that the TE biopsies contained a significantly greater number of mast cell granules (indicated by Giemsa staining) and a greater abundance of the enzyme tryptase (Figure 2).[18]Grace, S. A., Sutton, A. M., Abraham, N., Armbrecht, E. S., & Vidal, C. I. (2017). Presence of mast cells and mast cell degranulation in scalp biopsies of telogen effluvium. International journal … Continue reading
These results are indicative of an increased number of mast cells and greater mast cell activation in the scalp of people with TE. It has been suggested by this and a number of other studies that mast cells play an important role in hair loss, involved in a system that is triggered by stress and characterized by a switch to a pro-inflammatory phenotype.[19]Arck, P. C., Handjiski, B., Hagen, E., Joachim, R., Klapp, B. F., & Paus, R. (2001). Indications for a brain‐hair follicle axis: inhibition of keratinocyte proliferation and up‐regulation of … Continue reading
Figure 2: Table showing the mast cell count (indicated by the Giemsa stain) and tryptase abundance in the scalp biopsies of patients with telogen effluvium, alopecia areata, androgenetic alopecia, and healthy controls.[20]Grace, S. A., Sutton, A. M., Abraham, N., Armbrecht, E. S., & Vidal, C. I. (2017). Presence of mast cells and mast cell degranulation in scalp biopsies of telogen effluvium. International journal … Continue reading
A separate study investigated scalp biopsies from 55 patients with AA and quantified the levels of immune cells and inflammatory mediators, comparing the results to healthy controls. They showed increased CD4+ T cells, CD8+ T cells, and mast cells in the deep perifollicular and deep perivascular areas of the scalp (Figure 3). They also observed increased expression of nerve growth factor, which is potentially indicative of inflammation within the scalp.[21]Zhang, X., Zhao, Y., Ye, Y., Li, S., Qi, S., Yang, Y., Cao, H., Yang, J. and Zhang, X. (2015). Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia … Continue reading
This provides further evidence that suggests mast cells, and therefore histamine, may play a role in hair loss. Indeed, histamine release from mast cells can trigger the release of T cells, so it is possible that the increase in T cells was driven by increased histamine.[22]Jutel, M., Watanabe, T., Klunker, S., Akdis, M., Thomet, O.A., Malolepszy, J., Zak-Nejmark, T., Koga, R., Kobayashi, T., Blaser, K. and Akdis, C.A. (2001). Histamine regulates T-cell and antibody … Continue reading
Figure 3: The abundance of CD4+ T cells, CD8+ T cells, and mast cells in the scalp of patients with AA. Control samples were compared to AA. *P < 0.05, **P < 0.01, ***P < 0.001.[23]Zhang, X., Zhao, Y., Ye, Y., Li, S., Qi, S., Yang, Y., Cao, H., Yang, J. and Zhang, X. (2015). Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia … Continue reading
There is also evidence that mast cells may contribute to the extracellular matrix remodelling that is seen in patients with AGA. Biopsies were taken from the balding (vertex) and non-balding (occipital) areas of 10 males with AGA, as well as biopsies from the same regions of 5 healthy control subjects. Using tryptase quantification, they showed that active mast cell counts were almost 2-fold greater in the scalps of AGA patients relative to the healthy controls (Figure 4). Furthermore, they showed an increase in the number of collagen bundles and elastic fibres within the balding regions of the scalp, and a positive correlation between the number of active mast cells and the number of elastic fibres.[24]Won, C.H., Kwon, O.S., Kim, Y.K., Kang, Y.J., Kim, B.J., Choi, C.W., Eun, H.C. and Cho, K.H. (2008). Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Archives of … Continue reading
It should be noted that the small study size is a limiting factor. However, the results suggest that mast cells may contribute to the remodelling that is observed in AGA. This is supported by studies that have linked tryptase to fibroblast proliferation, collagen production, and elastin production.
Figure 4: Tryptase-positive mast cells in the vertex and occipital regions of the scalp. (A) Microscopic images of the scalp, including the tryptase-positive mast cells. (B) Quantification of the tryptase-positive mast cells in the vertex and occipital regions.[25]Won, C.H., Kwon, O.S., Kim, Y.K., Kang, Y.J., Kim, B.J., Choi, C.W., Eun, H.C. and Cho, K.H. (2008). Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Archives of … Continue reading
It is possible that this recruitment and activation of mast cells (and, potentially, histamine) is caused by changes to the skin microbiome. A study was conducted on hairless mice to compare mast cell activation against control mice with hair. They found that the number of activated mast cells was significantly greater in the hairless mice. Additionally, the patchy sections of hair had fewer mast cells than the hairless sections, but more than the control mice (with a full coat of hair). This difference was associated with a greater amount of gram-positive bacteria on the skin of the hairless mice. Interestingly, when the TLR2 receptor (that recognizes the bacteria) was deleted in hairless mice, the number of mast cells reduced.[26]Wu, C. C., Kim, J. N., Wang, Z., Chang, Y. L., Zengler, K., & Di Nardo, A. (2019). Mast cell recruitment is modulated by the hairless skin microbiome. Journal of Allergy and Clinical Immunology. … Continue reading
Collectively, these studies suggest that mast cell activation may contribute to hair loss. As an effector of mast cell activity, this might suggest that histamine is also contributing to hair loss. Therefore, this raises the question of whether antihistamines could be used to prevent hair loss.
How Does Cetirizine Have an Impact on Hair Follicles?
To answer the following question, we checked the literature for studies that have investigated the use of antihistamines in hair loss treatment. Despite the limited research, cetirizine has been tested in a number of different studies and generated some interesting results. Let’s start by looking at the mechanistic effects of cetirizine that suggest it could influence hair health.
A double-blind, placebo-controlled crossover study was conducted in 10 people with ragweed allergies. The patients were given oral cetirizine (20mg) or placebo once a day, for two days, before their skin was exposed to the allergen. The exposed portion of the skin was then biopsied for testing. Surprisingly, although erythema (reddening) was reduced, cetirizine did not inhibit the release of histamine. However, the authors found that cetirizine treatment did significantly reduce the production of prostaglandin D2 (PGD2), and there was also a significant reduction in the migration of inflammatory cells white blood cells (eosinophils and neutrophils) to the exposed site.[27]Charlesworth, E. N., Kagey-Sobotka, A., Norman, P. S., & Lichtenstein, L. M. (1989). Effect of cetirizine on mast cell-mediator release and cellular traffic during the cutaneous late-phase … Continue reading
These results are interesting when it comes to hair health. PGD2 has a previously documented role in promoting hair loss, particularly in those with AGA. PGD2 is thought to block key receptors within hair follicles and prevent hairs from entering the anagen (growth phase). This consequently prevents hair growth and contributes to hair follicle miniaturization. Furthermore, suppression of PGD2 has been shown to have beneficial effects, suggesting that it may be a therapeutic target.[28]Shin, D. W. (2022). The physiological and pharmacological roles of prostaglandins in hair growth. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological … Continue reading
However, it should be noted that recent studies using PGD2 inhibitors are yet to show improvement in hair loss outcomes. You can read more about this in our GPR44 article.
Studies have also shown that inflammation plays a role in driving hair loss. Numerous studies have shown a significant inflammatory response in the scalp of people suffering with hair loss, driven by infiltration of inflammatory mediators (including mast cells) into the hair follicles. This then disrupts the hair cycle and impairs hair growth.[29]Peyravian, N., Deo, S., Daunert, S., & Jimenez, J. J. (2020). The inflammatory aspect of male and female pattern hair loss. Journal of inflammation research. 879-881. Available at: … Continue reading
Therefore, it is plausible that cetirizine could be beneficial for hair loss.
Clinical Evidence
Thankfully, a number of clinical studies have been conducted to test the efficacy of cetirizine as a hair loss treatment.
One study was conducted on 60 males with AGA between the ages of 22 and 55. The study was double-blinded and the participants were randomized into the placebo or cetirizine group. Those in the cetirizine group were required to apply 1mL of a topical lotion (1% cetirizine) to their scalp every day, while the placebo group followed the same procedure with their respective product. At the beginning and end of the study, assessments of hair regrowth were performed by a dermatologist, photographs were assessed by dermatologists to provide a Hamilton-Norwood classification, and subjective assessments were provided by the participants in the form of questionnaires. The change in each parameter relative to the start of the study was then compared between groups.[30]Zaky, M. S., Abo Khodeir, H., Ahmed, H. A., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: a case‐controlled study. … Continue reading
Dermatological assessment determined hair regrowth to be significantly greater in the cetirizine group, with 43.3% of participants exhibiting hair regrowth compared to 0% of the placebo group. Similarly, photographic assessment determined that 43.3% of the cetirizine group exhibited improvements in Hamilton-Norwood classification, compared to 0% of the placebo group, which was also a significant difference. Finally, self-assessment by questionnaire revealed significantly greater satisfaction in the cetirizine group, with 43.3% stating that their hair growth had shown an improvement compared to 0% of the placebo group.[31]Zaky, M. S., Abo Khodeir, H., Ahmed, H. A., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: a case‐controlled study. … Continue reading
Figure 5: Photographic and dermoscopic comparison of the hair and scalp before and after 6 months of treatment with topical cetirizine (1%).[32]Zaky, M. S., Abo Khodeir, H., Ahmed, H. A., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: a case‐controlled study. … Continue reading
In another study, 40 males between the ages of 18 and 49 were recruited to compare topical cetirizine (1%) and minoxidil (5%), and FDA approved therapy for hair loss. Participants in this single-blind study were randomized into one of the two groups and then applied 1mL of solution per day to the balding area of their head. After 16 weeks, every participant was switched to a placebo product for a further 8 weeks.[33]Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a … Continue reading
Using Trichoscan to conduct objective assessments of the hair, they found that both cetirizine and minoxidil caused a significant increase in total hair density and vellus hair density after 16 weeks, with minoxidil outperforming cetirizine. The percentage of hair in anagen phase increased in both groups after 16 weeks, then subsequently diminished after placebo use. Assessments completed by physicians suggested that a comparable percentage of participants in each group showed improvement in hair density, although 17% of the cetirizine group were determined to have exhibited a reduction compared to 0% of the minoxidil group. Furthermore, the participants in the minoxidil group reported greater satisfaction with their treatment.[34]Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a … Continue reading
Figure 6: Scalp photographs of people with AGA after 16 weeks of treatment with topical minoxidil or cetirizine, followed by 8 weeks of a placebo.[35]Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a … Continue reading
A third study recruited 85 males and females between the ages of 20 and 65 with AGA. 67 participants were placed in the treatment group and used 1mL of topical cetirizine (1%) daily for 6 months, with 18 participants using a placebo product for the same length of time. Using macrophotographs and Trichoscan, they found that total hair density and terminal hair density were both significantly increased in the cetirizine treatment group.[36]Rossi, A., Campo, D., Fortuna, M.C., Garelli, V., Pranteda, G., De Vita, G., Sorriso-Valvo, L., Di Nunno, D. and Carlesimo, M., 2018. A preliminary study on topical cetirizine in the therapeutic … Continue reading
Figure 7: Global photography of people with AGA before (top) and after (bottom) treatment with topical cetirizine (1%) for 6 months.[37]Rossi, A., Campo, D., Fortuna, M.C., Garelli, V., Pranteda, G., De Vita, G., Sorriso-Valvo, L., Di Nunno, D. and Carlesimo, M., 2018. A preliminary study on topical cetirizine in the therapeutic … Continue reading
One last study recruited 60 female patients, with AGA, between the ages of 20 and 50. The study was double-blinded and randomized. Both groups applied 1mL topical minoxidil (5%) every morning for 6 months; one of these groups also applied 1mL topical cetirizine (1%) in the evening, while the other group applied a placebo. Phototrichscopy was used to show that both groups exhibited a significant increase in frontal and vertex terminal and vellus hair density. Interestingly, the minoxidil+cetirizine group also showed a significant increase in hair thickness and number of hairs per follicular unit, which was not present in the minoxidil only group. However, it should be noted that change relative to baseline in both groups was comparable across all parameters. Participants in the minoxidil+cetirizine group also scored better in the self-assessment of new hairs, hair growth, bald areas, and satisfaction with the treatment.[38]Bassiouny, E. A., El-Samanoudy, S. I., Abbassi, M. M., Nada, H. R., & Farid, S. F. (2023). Comparison between topical cetirizine with minoxidil versus topical placebo with minoxidil in female … Continue reading
Figure 8: Changes in hair-related parameters relative to baseline in participants that took minoxidil (5%) and cetirizine (1%) in combination (Group 1) and participants that took minoxidil (5%) alone (Group 2).[39]Bassiouny, E. A., El-Samanoudy, S. I., Abbassi, M. M., Nada, H. R., & Farid, S. F. (2023). Comparison between topical cetirizine with minoxidil versus topical placebo with minoxidil in female … Continue reading
Collectively, these studies provide evidence that suggests topical cetirizine may be beneficial in the treatment of hair loss. However, there are limitations with the studies that have been described. For one, it would be good to assess the efficacy of cetirizine over a longer period of time (more than 6 months), to determine whether its beneficial effects improve further, plateau, or even reverse. Larger studies would be highly beneficial, particularly with the inclusion of more females. All of these studies were also limited to the inclusion of AGA patients which, while useful, means that the efficacy of cetirizine in treating other hair loss conditions remains unknown. Some of the studies also lack placebo controls, which means definitive conclusions on the effectiveness of cetirizine cannot be drawn from their results. That said, the results are promising, and seemingly suggest that cetirizine may provide some beneficial effects.
Safety and Side Effects
Oral cetirizine has received FDA approval for treating allergies and is considered safe at the recommended dose (10mg within a 24-hour period in adults, 5mg twice daily in children aged 6-11 years, 2.5mg twice daily in children aged 2-5 years).[40]NICE. (No date). Cetirizine hydrochloride. National Institute for Health and Care Excellence. Available at: https://bnf.nice.org.uk/drugs/cetirizine-hydrochloride/ (Accessed: 29 April 2025)
Common side effects include feeling of sleepiness and tiredness, as well as headaches, dizziness, dry mouth, diarrhea, sore throat, and sneezing. However, it has been reported that small amounts of cetirizine can get into breast milk, so those who are breastfeeding should use cetirizine with caution. Additionally, people with kidney failure may be unable to use cetirizine.[41]NHS. (2025). Side effects of cetirizine. National Health Service. Available at: https://www.nhs.uk/medicines/cetirizine/side-effects-of-cetirizine/ (Accessed: 29 April 2025)
In the three clinical studies that used topical cetirizine (1%) alone, no notable adverse events or side effects were reported. In the study that used minoxidil (5%) together with cetirizine (1%), itching, dry hair, initial hair loss, and dandruff were reported. However, these are known side effects of minoxidil, and there were no differences in the number of adverse events between the two groups. Although there is no conclusive safety data related to topical cetirizine, it is safe to use (up to the recommended dosage) as an oral therapeutic. Given that oral therapeutics are more likely to trigger systemic effects, this suggests that topical cetirizine may be safe for use, though some side effects may occur in relation to its application on the skin. However, please note that there is no recommended dosage for topical cetirizine.
Is Cetirizine For Me?
You may want to experiment with topical cetirizine if:
- You are happy to use a product with limited evidence of efficacy.
- You are happy to use a product with no evidence of efficacy beyond 6 months of use.
- You have not seen beneficial effects from treatment with minoxidil and would like to try something else.
- You want to try a combined treatment with other products like minoxidil.
Final Thoughts
Cetirizine is a second-generation antihistamine that has been FDA approved (as an oral medication) for the treatment of allergies since the 1990s. It primarily works by binding the H1 receptor and preventing histamine from binding there, the molecule that is responsible for allergic symptoms. Alongside its well documented function, oral cetirizine has been shown to both reduce inflammation and inhibit the production of PGD2, the activity of which is strongly linked to hair loss. Several clinical studies have been conducted in AGA patients who used topical cetirizine as a treatment, with the results showing improvements in hair growth, hair density, and hair thickness. Furthermore, no side effects were associated with the topical use of cetirizine, and the oral version of the therapeutic is considered safe for use. Although there is a lack of large, long-term studies that have investigated topical cetirizine, the results suggest that it may provide help to improve hair loss.
References[+]
References ↑1, ↑2, ↑5, ↑7, ↑8 Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008 ↑3 World Health Organization. (2023). WHO Model List of Essential Medicines – 23rd list, 2023. WHO/MHP/HPS/EML/2023.02. Available at: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02 (Accessed: 24 April 2025) ↑4 Pagliara, A., Testa, B., Carrupt, P.A., Jolliet, P., Morin, C., Morin, D., Urien, S., Tillement, J.P. and Rihoux, J.P. (1998). Molecular properties and pharmacokinetic behavior of cetirizine, a zwitterionic H1-receptor antagonist. Journal of medicinal chemistry. 41(6). 853-863. Available at: https://doi.org/10.1021/jm9704311 ↑6 Ghosh, S., Debnath, I., Bhunia, S., Hazra, S., Nandi, S., & Mandal, S. K. (2025). A Review on Mechanism of Histamine Mediated Allergic Reactions: Therapeutic Role, Safety, and Clinical Efficacy of Cetirizine in Modern Allergy and Other Diseases Management. Biomedical and Pharmacology Journal. 18(1). 411-429. Available at: https://dx.doi.org/10.13005/bpj/3097 ↑9, ↑10 Vardhamane, S. H., Santoshkumar, J., & Vardhamane, S. H. (2013). Anti-inflammatory activity of H1 receptor antagonist Cetirizine in animal models. Journal of Evolution of Medical and Dental Sciences. 2(13). 2050-2060. Available at: https://doi.org/10.14260/JEMDS/499 ↑11 Ciprandi, G., Buscaglia, S., Pesce, G., Passalacqua, G., Rihoux, J. P., Bagnasco, M., & Canonica, G. W. (1995). Cetirizine reduces inflammatory cell recruitment and ICAM-1 (or CD54) expression on conjunctival epithelium in both early-and late-phase reactions after allergen-specific challenge. Journal of allergy and clinical immunology. 95(2). 612-621. Available at: https://doi.org/10.1016/S0091-6749(95)70324-1 ↑12 Jinquan, T., Reimert, C. M., Deleuran, B., Zachariae, C., Simonsen, C., Thestrup-Pedersen, K., & May, R. (1995). Cetirizine inhibits the in vitro and ex vivo chemotactic response of T lymphocytes and monocytes. Journal of allergy and clinical immunology. 95(5). 979-986. Available at: https://doi.org/10.1016/S0091-6749(95)70098-6 ↑13 Amin, K. (2012). The role of mast cells in allergic inflammation. Respiratory medicine. 106(1). 9-14. Available at: https://doi.org/10.1016/j.rmed.2011.09.007 ↑14, ↑15 Fujimura, R., Asada, A., Aizawa, M., & Kazama, I. (2022). Cetirizine more potently exerts mast cell-stabilizing property than diphenhydramine. Drug Discoveries & Therapeutics. 16(5). 245-250. Available at: https://doi.org/10.5582/ddt.2022.01067 ↑16 Carthy, E., & Ellender, T. (2021). Histamine, neuroinflammation and neurodevelopment: a review. Frontiers in neuroscience. 15. 680214. Available at: https://doi.org/10.3389/fnins.2021.680214 ↑17 Branco, A. C. C. C., Yoshikawa, F. S. Y., Pietrobon, A. J., & Sato, M. N. (2018). Role of histamine in modulating the immune response and inflammation. Mediators of inflammation. 2018(1). 9524075. Available at: https://doi.org/10.1155/2018/9524075 ↑18, ↑20 Grace, S. A., Sutton, A. M., Abraham, N., Armbrecht, E. S., & Vidal, C. I. (2017). Presence of mast cells and mast cell degranulation in scalp biopsies of telogen effluvium. International journal of trichology. 9(1). 25-29. Available at: https://doi.org/10.4103/ijt.ijt_43_16 ↑19 Arck, P. C., Handjiski, B., Hagen, E., Joachim, R., Klapp, B. F., & Paus, R. (2001). Indications for a brain‐hair follicle axis: inhibition of keratinocyte proliferation and up‐regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. The FASEB Journal. 15(13). 2536-2538. Available at: https://doi.org/10.1096/fj.00-0699fje ↑21, ↑23 Zhang, X., Zhao, Y., Ye, Y., Li, S., Qi, S., Yang, Y., Cao, H., Yang, J. and Zhang, X. (2015). Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Archives of dermatological research. 307. 319-331. Available at: https://doi.org/10.1007/s00403-015-1539-1 ↑22 Jutel, M., Watanabe, T., Klunker, S., Akdis, M., Thomet, O.A., Malolepszy, J., Zak-Nejmark, T., Koga, R., Kobayashi, T., Blaser, K. and Akdis, C.A. (2001). Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 413(6854). 420-425. Available at: https://doi.org/10.1038/35096564 ↑24, ↑25 Won, C.H., Kwon, O.S., Kim, Y.K., Kang, Y.J., Kim, B.J., Choi, C.W., Eun, H.C. and Cho, K.H. (2008). Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Archives of dermatological research. 300. 147-152. Available at: https://doi.org/10.1007/s00403-007-0826-x ↑26 Wu, C. C., Kim, J. N., Wang, Z., Chang, Y. L., Zengler, K., & Di Nardo, A. (2019). Mast cell recruitment is modulated by the hairless skin microbiome. Journal of Allergy and Clinical Immunology. 144(1). 330-333. Available at: https://doi.org/10.1016/j.jaci.2019.02.033 ↑27 Charlesworth, E. N., Kagey-Sobotka, A., Norman, P. S., & Lichtenstein, L. M. (1989). Effect of cetirizine on mast cell-mediator release and cellular traffic during the cutaneous late-phase reaction. Journal of allergy and clinical immunology. 83(5). 905-912. Available at: https://doi.org/10.1016/0091-6749(89)90104-8 ↑28 Shin, D. W. (2022). The physiological and pharmacological roles of prostaglandins in hair growth. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological Society and the Korean Society of Pharmacology. 26(6). 405-413. Available at: https://doi.org/10.4196/kjpp.2022.26.6.405 ↑29 Peyravian, N., Deo, S., Daunert, S., & Jimenez, J. J. (2020). The inflammatory aspect of male and female pattern hair loss. Journal of inflammation research. 879-881. Available at: https://doi.org/10.2147/JIR.S275785 ↑30, ↑31, ↑32 Zaky, M. S., Abo Khodeir, H., Ahmed, H. A., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: a case‐controlled study. Journal of Cosmetic Dermatology. 20(4). 1154-1159. Available at: https://doi.org/10.1111/jocd.13940 ↑33, ↑34, ↑35 Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a randomized, single-blind controlled study. Journal of Pharmacy & Pharmaceutical Sciences. 24. 191-199. Available at: https://doi.org/10.18433/jpps31456 ↑36, ↑37 Rossi, A., Campo, D., Fortuna, M.C., Garelli, V., Pranteda, G., De Vita, G., Sorriso-Valvo, L., Di Nunno, D. and Carlesimo, M., 2018. A preliminary study on topical cetirizine in the therapeutic management of androgenetic alopecia. Journal of Dermatological Treatment. 29(2). 149-151. Available at: https://doi.org/10.1080/09546634.2017.1341610 ↑38, ↑39 Bassiouny, E. A., El-Samanoudy, S. I., Abbassi, M. M., Nada, H. R., & Farid, S. F. (2023). Comparison between topical cetirizine with minoxidil versus topical placebo with minoxidil in female androgenetic alopecia: a randomized, double-blind, placebo-controlled study. Archives of Dermatological Research. 315(5). 1293-1304. Available at: https://doi.org/10.1007/s00403-022-02512-2 ↑40 NICE. (No date). Cetirizine hydrochloride. National Institute for Health and Care Excellence. Available at: https://bnf.nice.org.uk/drugs/cetirizine-hydrochloride/ (Accessed: 29 April 2025) ↑41 NHS. (2025). Side effects of cetirizine. National Health Service. Available at: https://www.nhs.uk/medicines/cetirizine/side-effects-of-cetirizine/ (Accessed: 29 April 2025) From thick, scaly patches to itching and irritation, scalp psoriasis is an autoimmune condition that can be both uncomfortable and persistent. But what triggers it, and how can you manage flare-ups effectively? In this article, we’ll explore what scalp psoriasis is, its symptoms and causes, treatment options, how it affects hair health, and simple ways to support your scalp long-term.
Key Takeaways
- What is it? Scalp psoriasis is a chronic autoimmune skin condition that causes the skin on the scalp to produce cells too quickly, resulting in thick, scaly patches. These plaques can be itchy and painful and extend beyond the scalp to the forehead, neck, and ears. It’s a form of plaque psoriasis and is driven by an overactive immune response influenced by genetic and environmental factors.
- What are the symptoms? Common symptoms include raised red, salmon-colored, or purple patches, depending on skin tone, covered with silvery or gray scales. Other signs are flaking (often mistaken for dandruff), intense itching, soreness, burning, and sometimes temporary hair loss due to inflammation or scratching. In more severe cases, plaques can cover the entire scalp and cause sleep disturbances or discomfort.
- What is the prevalence? Scalp psoriasis affects an estimated 50–80% of people with psoriasis. While one study reported a 7.6% prevalence among psoriasis patients in Malaysia, this likely underrepresents the global burden. It can develop at any age, though most cases are diagnosed in adulthood, with women being slightly more affected than men.
- What are the most common treatments? Treatment often starts with topical corticosteroids and vitamin D analogs to reduce inflammation and slow cell turnover. Medicated shampoos with salicylic acid, coal tar, or ketoconazole are widely used. In more persistent or severe cases, systemic medications like methotrexate or biologics may be prescribed. Combination therapies such as Enstilar foam are also effective.
- How else can I improve it? Using gentle hair care products, avoiding scratching or picking, and alternating medication with moisturizing shampoos can help. Natural remedies like aloe vera or apple cider vinegar may provide soothing relief. Managing stress, avoiding known triggers (like smoking, certain medications, or cold weather), and making dietary adjustments may also reduce flare-ups.
- Final thoughts. Scalp psoriasis is a complex and often frustrating condition, but with the right treatment plan and supportive care, it can be effectively managed. Taking a proactive approach by addressing both medical treatment and lifestyle adjustments can significantly reduce symptoms and improve overall scalp health and quality of life.
What is Scalp Psoriasis?
Scalp psoriasis is a chronic autoimmune skin condition affecting the scalp but often spreading below the hairline to the forehead, ears, and neck.[1]Leong, W.C., Tang, J.J. (2022). Scalp psoriasis and Dermatology Life Quality Index: A retrospective study based on 12-year data from the Malaysian Psoriasis Registry. Malaysian Family Physician. … Continue reading It causes an overproduction of skin cells, leading to the formation of thick, scaly patches on the skin.
Scalp psoriasis is a form of plaque psoriasis that specifically targets the scalp region. It results from an overactive immune system, genetics, and environmental factors (which we will cover below). The condition causes skin cells to reproduce too quickly, creating raised, discolored plaques that can be dry, itchy, and irritating.[2]National Institute of Arthritis and Musculoskeletal and Skin Diseases. (no date). Psoriasis. NIH. Available at: https://www.niams.nih.gov/health-topics/psoriasis (Accessed: March 2025)
How Does Scalp Psoriasis Present?
Macroscopic Presentation
Scalp psoriasis typically presents as raised reddish or salmon-colored patches with white scales on light to medium skin tones.[3]NHS. (no date). Psoriasis). National Health Service. Available at: https://www.nhs.uk/conditions/psoriasis/ (Accessed: March) On darker skin, the patches may appear purple with gray scales. These lesions can be a single patch or multiple patches, or they may affect the entire scalp and extend to the forehead, back of the neck, and behind and inside the ears.[4]Blakely, K., Gooderham, M. (2016). Management of scalp psoriasis: current perspectives. Psoriasis. 29(6). 33-40. Available at: https://doi.org/10.2147/PTT.S85330
This condition can range from mild and almost unnoticeable to severe, with thick, crusted sores. Intense itching is common and can significantly impact sleep and daily life.[5]AAD. (no date). Scalp Psoriasis: Symptoms. American Academy of Dermatology Association. Available at: https://www.aad.org/public/diseases/psoriasis/treatment/genitals/scalp-symptoms (Accessed: March) Scalp psoriasis often results in skin flaking, which may be mistaken for dandruff, but it also includes additional symptoms such as red or purple bumpy patches, soreness, and burning.
Scalp psoriasis is typically diagnosed clinically by a dermatologist through a physical examination of the skin, scalp, and nails.[6]National Psoriasis Foundation. (2025). Scalp Psoriasis. National Psoriasis Foundation. Available at: https://www.psoriasis.org/scalp (Accessed: March 2025) Your healthcare provider may also refer you for a biopsy to confirm the diagnosis and rule out other conditions.
Figure 1: Scalp psoriasis in the scalp showing a silvery plaque extending outwards from the hairline.[7]Elghblawi, E., Stanway, A., Coulson, I. (2022). Scalp Psoriasis. DermNet. Available at: https://dermnetnz.org/topics/scalp-psoriasis (Accessed: March 2025)
Microscopic Presentation
Scalp psoriasis shows distinct microscopic features that reveal its underlying inflammatory nature and accelerated skin turnover.
The epidermal changes are most noticeable with a thickened skin layer, known as regular acanthosis, with elongated “finer-like” extensions into the deeper tissue, referred to as rete ridges.[8]Raychaudri, S.K., Maverakis, E., Raychaudhri, S.P. (2014). Diagnosis and classification of psoriasis. Autoimmune Review. 13(4-5). 490-495. Available at: https://doi.org/10.101016/j.autrev.2014.01.008
Other common features are patchy areas with reduced or excess skin cell maturation (hypo- or hypergranulosis) and thinning of the upper skin layers, or supra papillary plates, making blood vessels more visible.[9]Basir, H.R.G., Alirezaei, P., Hamian, Z., Khanlarzadeh, E. (2018). Are quantitative histopathologic criteria capable of differentiating psoriasis from chronic dermatitis? Clinical, Cosmetic, and … Continue reading
Figure 2: Epidermal features of psoriasis.[10]DermNet. (2009). Psoriasis Overview. Available at: https://dermnetnz.org/cme/scaly-rashes/psoriasis-overview (Accessed: March 2025)
Furthermore, people with scalp psoriasis typically experience abnormal skin shedding with an accumulation of immature skin cells (parakeratosis) mixed with neutrophil immune cells, forming tiny abscesses called Munro microabscesses.[11]Butacu, A.I., Toma, C., Negulet, I-E., Manole, I., Banica, A.N., Plesea, A., Badircea, I.A., Iancu, I., Tiplica, G-S. (2024). Updates on Psoriasis in Special Areas. Journal of Clinical Medicine. … Continue reading
In the dermal layer, there are also significant changes. Immune cells accumulate around blood vessels in the middle skin layers, forming a perivascular lymphocytic infiltrate. The blood vessels themselves are dilated and twisted, contributing to the typical redness seen in psoriasis.
Other unique diagnostic clues help differentiate scalp psoriasis from other conditions. For instance, there are “spongy” pustules in the spinous later, known as Kogoj pustules, which are collections of neutrophils.[12]Mirza, H.A., Badri, T., Kwan, E. (2025). Generalized Pustular Psoriasis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: … Continue reading Another unique feature is the atrophy (shrinking) of sebaceous glands, which contrasts with the normal scalp, where these glands are active.[13]Werner, B., Brenner, F.M., Boer, A. (2008). Histopathologic Study of Scalp Psoriasis: Peculiar Features Including Sebaceous Gland Atrophy. The American Journal of Dermatopathology. 30(2). 93-100. … Continue reading
Figure 3: A-D: Atrophic sebaceous glands in scalp psoriasis. E-F: More “normal” looking sebaceous glands with larger lobules.[14]Werner, B., Brenner, F.M., Boer, A. (2008). Histopathologic Study of Scalp Psoriasis: Peculiar Features Including Sebaceous Gland Atrophy. The American Journal of Dermatopathology. 30(2). 93-100. … Continue reading
The epidermal surface also becomes irregular with scalp psoriasis, leading to wart-like bumps (papillomatosis) scaling around hair follicles and pinpoint bleeding (Auspitz’s sign).[15]Dias, M.F.R.G., Loures, A., El Kadi, N., Aranha, N.S., Machado, A., Fontinha, P.R.B., Dutra, H., Trueb, R.M. (2024). Trichoscopic pearl: dynamic trichoscopy sequence of scalp psoriasis before and … Continue reading
Figure 4: Left side: patients with scalp psoriasis showing thick scaling. Right side: Manifestation of Auspitz’s sign, showing as twisted capillary loops, comma vessels, and red dots.[16]Dias, M.F.R.G., Loures, A., El Kadi, N., Aranha, N.S., Machado, A., Fontinha, P.R.B., Dutra, H., Trueb, R.M. (2024). Trichoscopic pearl: dynamic trichoscopy sequence of scalp psoriasis before and … Continue reading
Affected areas of the scalp may also show reduced hair density, and the hair strand itself may have a fragile, damaged cuticle. Moreover, biofilms form on the scalp surface, consisting of clusters of bacteria and yeast linked by sticky strands.[17]Stepanova, A.A., Kornisheva, V.G., Raznatovskiy, K.I., Semolina, O.A. (2020). Scalp Psoriasis and Biofilms: Electron Microscopy. Journal of Microbiology & Experimentation. 8(5). 179-182. … Continue reading These biofilms can contribute to the disease’s persistence and severity.
What is the Difference Between Scalp Eczema and Scalp Psoriasis?
We cover some distinctions between scalp eczema and scalp psoriasis in our Scalp Eczema Ultimate Guide – here’s a summary:
Visual Distinctions
Scalp eczema and psoriasis have subtle visual differences.
Scalp eczema causes patches of dry skin that may be itchy and inflamed. The size and shape of these patches can change over time. The rash tends to blend into the surrounding skin and may be less well-defined.
Scalp psoriasis typically presents as thicker white scales with more well-defined areas of involvement. It often causes red patches with silvery scales that appear thick and raised. Psoriasis plaques usually extend beyond the hairline.
Differences in Causes and Triggers
The underlying causes and triggers for both conditions differ significantly.
Scalp eczema conditions can be caused by a combination of genetic and environmental factors, triggered by skin irritants like soaps, detergents, and disinfectants, exacerbated by allergens like dust, pollen, and certain foods, and heat exposure and increased sweating can lead to flare-ups.
Scalp psoriasis, however, results from an overactive immune system and is classified as an autoimmune disease.[18]Medical News Today (2025). How to tell the difference between scalp eczema and psoriasis. Available at: https://www.medicalnewstoday.com/articles/scalp-eczema-vs-psoriasis (Accessed: March 2025) It can be triggered by factors like stress, infections, skin injuries, and certain medications.
Treatment Approaches
Common treatments used to treat both scalp eczema and scalp psoriasis include:
- Topical corticosteroids to reduce inflammation and itching.
- Medicated shampoos.
- Moisturizers.
- Light therapy (phototherapy).
- Lifestyle changes to avoid triggers and reduce stress.
Several treatments for the two conditions differ, however.
Treatments typically used to treat scalp eczema include:
- Antihistamines to relieve itching.
- Topical calcineurin inhibitors (e.g., tacrolimus, pimecrolimus) for non-steroidal treatment.
- Wet wrap therapy for severe cases.
- Prescription anti-inflammatory creams.
Treatments typically used to treat scalp psoriasis include:[19]American Academy of Dermatology Association. (no date). Scalp Psoriasis: Shampoos, Scale Softeners, and Other Treatments. AAD. Available at: … Continue reading
- Salicylic acid-based products to help remove scales.
- Vitamin D analogs (e.g., calcipotriene) to slow skin cell growth.
- Tazarotene (a topical retinoid) slows cell growth and reduces inflammation.
- Systemic medications for severe cases, including methotrexate, cyclosporine, and biologics (e.g., adalimumab, etanercept, and ustekinumab).
- Excimer laser treatment for targeted therapy of psoriasis plaques.
What Are the Causes of Scalp Psoriasis?
Scalp psoriasis is a complex condition influenced by multiple factors.
Genetic predisposition plays a significant role, as the condition tends to run in families. Over two dozen genes have been identified that may contribute to psoriasis development, with the HLA-C*6:02 gene being the strongest genetic risk factor.[20]Patel, H.A., Revankar, R.R., Pedroza, S.T., Graham, S., Feldman, S.R. (2023). The Genetic Susceptibility to Psoriasis and the Relationship of Linked Genes to Our Treatment Options. International … Continue reading Other genetic variants, such as those in interleukins 17A, 12B, and 10 genes, are also associated with an increased risk of developing psoriasis.
Scalp psoriasis is mainly caused by a dysfunction in the immune system. In this condition, the immune system mistakenly attacks healthy skin cells, resulting in rapid cell turnover. T-cells, which are normally responsible for fighting infections, begin to target healthy skin cells instead.[21]Schadler, E.D., Ortel, B., Mehlis, S.L. (2019). Biologics for the primary care physician: Review and treatment of psoriasis. Disease-a-month:DM. 65(3). 51-90. Available at: … Continue reading This immune dysfunction leads to the overproduction of skin cells, which causes the characteristic plaques and scaling associated with psoriasis.
Several environmental triggers can also initiate or exacerbate scalp psoriasis. These include infections, particularly strep throat and skin infections, as well as skin trauma like cuts, scrapes, bug bits, or severe sunburns.[22]Zhou, S., Yao, Z. (2022). Roles of Infection in Psoriasis. International Journal of Molecular Sciences. 23(13). 6955. Available at: https://doi.org/10.3390/ijms23136955 Stress is another significant trigger, as it can initiate or worsen psoriasis symptoms.[23]Yang, H., Zheng, J. (2020). Influence of stress on the development of psoriasis. Clinical and Experimental Dermatology. 45(3). 284-288. Available at: https://doi.org/10.1111/ced.14105
Additionally, cold, dry weather conditions, smoking, exposure to secondhand smoke, and heavy alcohol consumption can also contribute to flare-ups.[24]Zheng, X., Wang, Q., Luo, Y., Lu, W., Jin, L., Chen, M., Zhu, W., Kuang, Y. (2021). Seasonal Variation of Psoriasis and Its Impact in the Therapeutic Management: A Retrospective Study on Chinese … Continue reading,[25]Miao, M., Yan, J., Sun, Y., Liu, J., Guo, S. (2025). Psoriasis: Unraveling Disease Mechanisms and Advancing Pharmacological and Nontechnological Treatments. Journal of Inflammation Research. 10(18). … Continue reading
In some cases, medications can trigger or worsen scalp psoriasis:
- Lithium.[26]Mayo Clinic. (no date). Psoriasis. Available at: https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes/syc-20355840 (Accessed: March 2025)
- High blood pressure medications like ACE inhibitors.
- Antimalarials.
- Rapid withdrawal of oral or injected corticosteroids.
- Some anti-inflammatory medicines, including ibuprofen.[27]NHS. (2022). Psoriasis. Available at: https://www.nhs.uk/conditions/psoriasis/causes/ (Accessed: March 2025)
It should be noted that while these factors may contribute to the pathogenesis of scalp psoriasis, the condition usually results from a combination of multiple factors. Not everyone with genetic risk factors will develop scalp psoriasis, and the severity and frequency of flare-ups can vary widely among individuals.
What is the Prevalence of Scalp Psoriasis?
According to a study in Malaysia, scalp psoriasis is estimated to affect about 7.6% of patients with psoriasis in places other than the scalp.[28]Leong, W.C., Tang, J.J. (2022). Scalp psoriasis and Dermatology Life Quality Index: A retrospective study based on 12-year data from the Malaysian Psoriasis Registry. Malaysian Family Physician. … Continue reading However, this figure may be underestimated, as other sources suggest that scalp involvement occurs in 50-80% of psoriasis patients.[29]Blakely, K., Gooderham, M. (2016). Management of scalp psoriasis: current perspectives. Psoriasis. 29(6). 33-40. Available at: https://doi.org/10.2147/PTT.S85330
Scalp psoriasis can occur at any age, but it typically follows patterns similar to general psoriasis:
- The mean age of onset is ~29 years of age for women and ~36 years for men.
- More common in adults, but about one-third of cases are diagnosed before age 20.
- Slightly more prevalent in women, with a male-to-female ratio of 1:1.56.
What is the Impact of Scalp Psoriasis on Hair Loss?
While scalp psoriasis doesn’t directly cause permanent hair loss, there is a relationship between the condition and increased hair shedding.
Correlation with Hair Loss Conditions
Scalp psoriasis is associated with increased hair shedding and decreased hair density, particularly in areas affected by psoriatic plaques. These lesions can lead to diffuse hair loss, which may be exacerbated by inflammation and itching.[30]Almeida, M.C., Romiti, R., Doche, I., Valente, N.Y.S., Donati, A. (2013). Psoriatic scarring alopecia. Anais Brasileiros de Dermatologia. 88(6 Suppl 1). 29-31. Available at: … Continue reading It can also result in localized alopecia where the psoriatic plaques are located and generalized telogen effluvium.[31]Song, B., Liu, X., Jin, H. (2024). Ixekizumab Improved Refractory Erythrodermic Psoriasis with Comorbid Diffuse Alopecia: A Case Report with 52-Week Follow-Up. Clinical, Cosmetic and Investigational … Continue reading
The mechanism of hair loss in scalp psoriasis includes inflammatory destruction of the hair follicle, as seen in scarring alopecias. Histological studies have shown inflammatory destruction of the infundibular (upper) region of the hair follicle, leading to the loss of the hair follicle (follicular drop-out) and fibrosis.[32]Wright, A.L., Messenger, A.G. (1990). Scarring alopecia in psoriasis. Acta Dermato-Venereologica. 70(2). 156-159. Available at: PMID:1969203
Factors That May Worsen Hair Loss
- Scratching: Persistent itching and scratching damage hair shafts and follicles, potentially leading to scarring or infections that worsen hair loss.
- Harsh Treatments: Overuse of strong medicated shampoos or aggressive removal of scales can weaken hair and increase shedding.
- Plaque Build-Up: Thick plaques block follicles, preventing new hair growth and increasing breakage.
Preventive Measures
To minimize hair loss:
- Use gentle medicated shampoos and topical treatments.
- Avoid scratching or picking at plaques, and keep nails trimmed and smooth.
- Alternate between medicated and non-medicated shampoos to prevent dryness
- Avoid aggressive hair regrowth treatments that might increase local inflammation, like microneedling, topicals containing retinoic acid, scalp massages, PRP, and mesotherapy.
How Can I Treat Scalp Psoriasis?
Medical Treatments
There are a diverse array of medical treatments for scalp psoriasis. Topical corticosteroids and vitamin D analogs are commonly prescribed.[33]Kim, G.K. (2010). The Rationale Behind Topical Vitamin D Analogs in the Treatment of Psoriasis. Journal of Clinical and Aesthetic Dermatology. 3(8). 46-53. Available at: PMID: 2087752 Corticosteroids help reduce inflammation, while vitamin D analogs like calcipotriene (Dovonex) can be applied at night to promote healthy skin cell growth. Combination products such as Talconex Scalp or Enstilar Foam, which contain both a vitamin D analog and a strong steroid, are also effective.
Medicated shampoos play an important role in managing scalp psoriasis. Shampoos containing salicylic acid help soften and remove scales, while coal tar shampoos can reduce itching and inflammation.[34]National Psoriasis Foundation. (no date). Best Psoriasis Shampoos Your Scalp Will Love. Available at: https://www.psoriasis.org/advance/best-psoriasis-shampoos (Accessed: March 2025) Ketoconazole shampoos may be recommended for their anti-inflammatory properties.
For moderate to severe cases, systemic medications like biologics or small molecules may be prescribed. These include methotrexate, ciclosporin, and oral retinoids, which are used by about 10-20% of people with moderate or severe psoriasis.[35]Sbidian, E., Chaimani, A., Garcia-Doval, I., Doney, L., Dressler, C., Hua, C., Hughes, C., Naldi, L., Afach, S., Le Cleach, L. (2021). Systemic pharmacological treatments for chronic plaque … Continue reading
Natural and Home Remedies
Natural remedies can complement medical treatments by providing relief and soothing the scalp. Aloe vera gel and apple cider vinegar can help to relieve some of the irritation and assist in maintaining moisture levels.[36]Medical News Today. (2019). Can aloe vera treat psoriasis? Available at: https://www.medicalnewstoday.com/articles/320081 (Accessed: March 2025)
Stress management techniques like meditation or yoga may help prevent flare-ups. Some people report symptom improvement after removing dairy or gluten from the diet, although this varies from person to person.[37]National Psoriasis Foundation. (no date). Dietary Modifications. Available at: https://www.psoriasis.org/dietary-modifications (Accessed: March 2025)
Final Thoughts
Scalp psoriasis is a chronic, immune-mediated condition that can significantly impact the quality of life due to the inflammation and discomfort it causes. Rooted in genetic susceptibility and driven by immune dysfunction, scalp psoriasis is often worsened by environmental and lifestyle triggers. While it doesn’t directly cause permanent hair loss, inflammation, itching, and harsh treatments can lead to increased hair thinning. Managing scalp psoriasis effectively often requires a combination of medical therapies and supportive care, including gentle scalp practices and trigger avoidance.
References[+]
References ↑1, ↑28 Leong, W.C., Tang, J.J. (2022). Scalp psoriasis and Dermatology Life Quality Index: A retrospective study based on 12-year data from the Malaysian Psoriasis Registry. Malaysian Family Physician. 17(3). 84-88. Available at: https://doi.org/10.51866/oa.146 ↑2 National Institute of Arthritis and Musculoskeletal and Skin Diseases. (no date). Psoriasis. NIH. Available at: https://www.niams.nih.gov/health-topics/psoriasis (Accessed: March 2025) ↑3 NHS. (no date). Psoriasis). National Health Service. Available at: https://www.nhs.uk/conditions/psoriasis/ (Accessed: March) ↑4, ↑29 Blakely, K., Gooderham, M. (2016). Management of scalp psoriasis: current perspectives. Psoriasis. 29(6). 33-40. Available at: https://doi.org/10.2147/PTT.S85330 ↑5 AAD. (no date). Scalp Psoriasis: Symptoms. American Academy of Dermatology Association. Available at: https://www.aad.org/public/diseases/psoriasis/treatment/genitals/scalp-symptoms (Accessed: March) ↑6 National Psoriasis Foundation. (2025). Scalp Psoriasis. National Psoriasis Foundation. Available at: https://www.psoriasis.org/scalp (Accessed: March 2025) ↑7 Elghblawi, E., Stanway, A., Coulson, I. (2022). Scalp Psoriasis. DermNet. Available at: https://dermnetnz.org/topics/scalp-psoriasis (Accessed: March 2025) ↑8 Raychaudri, S.K., Maverakis, E., Raychaudhri, S.P. (2014). Diagnosis and classification of psoriasis. Autoimmune Review. 13(4-5). 490-495. Available at: https://doi.org/10.101016/j.autrev.2014.01.008 ↑9 Basir, H.R.G., Alirezaei, P., Hamian, Z., Khanlarzadeh, E. (2018). Are quantitative histopathologic criteria capable of differentiating psoriasis from chronic dermatitis? Clinical, Cosmetic, and Investigational Dermatology. 11. 239-244. Available at: https://doi.org/10.2147/CCID.S160697 ↑10 DermNet. (2009). Psoriasis Overview. Available at: https://dermnetnz.org/cme/scaly-rashes/psoriasis-overview (Accessed: March 2025) ↑11 Butacu, A.I., Toma, C., Negulet, I-E., Manole, I., Banica, A.N., Plesea, A., Badircea, I.A., Iancu, I., Tiplica, G-S. (2024). Updates on Psoriasis in Special Areas. Journal of Clinical Medicine. 13(24). 7549. Available at: https://doi.org/10.3390/jcm13247549 ↑12 Mirza, H.A., Badri, T., Kwan, E. (2025). Generalized Pustular Psoriasis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK493189/ (Accessed: March 2025) ↑13, ↑14 Werner, B., Brenner, F.M., Boer, A. (2008). Histopathologic Study of Scalp Psoriasis: Peculiar Features Including Sebaceous Gland Atrophy. The American Journal of Dermatopathology. 30(2). 93-100. Available at: https://doi.org/10.1097/dad.0b013e31816421fd ↑15, ↑16 Dias, M.F.R.G., Loures, A., El Kadi, N., Aranha, N.S., Machado, A., Fontinha, P.R.B., Dutra, H., Trueb, R.M. (2024). Trichoscopic pearl: dynamic trichoscopy sequence of scalp psoriasis before and after the development of Auspitz’s sign. Archives of Dermatological Research. 316(39). 1-3. Available at: https://doi.org/10.1007/s00403-023-02788-y ↑17 Stepanova, A.A., Kornisheva, V.G., Raznatovskiy, K.I., Semolina, O.A. (2020). Scalp Psoriasis and Biofilms: Electron Microscopy. Journal of Microbiology & Experimentation. 8(5). 179-182. Available at: https://doi.org/10.15406/jmen.2020.08.00306 ↑18 Medical News Today (2025). How to tell the difference between scalp eczema and psoriasis. Available at: https://www.medicalnewstoday.com/articles/scalp-eczema-vs-psoriasis (Accessed: March 2025) ↑19 American Academy of Dermatology Association. (no date). Scalp Psoriasis: Shampoos, Scale Softeners, and Other Treatments. AAD. Available at: https://www.aad.org/public/diseases/psoriasis/treatment/genitals/scalp-shampoo (Accessed: March 2025) ↑20 Patel, H.A., Revankar, R.R., Pedroza, S.T., Graham, S., Feldman, S.R. (2023). The Genetic Susceptibility to Psoriasis and the Relationship of Linked Genes to Our Treatment Options. International Journal of Molecular Sciences. 24(15). 12310. Available at: https://doi.org/10.3390/ijms241512310 ↑21 Schadler, E.D., Ortel, B., Mehlis, S.L. (2019). Biologics for the primary care physician: Review and treatment of psoriasis. Disease-a-month:DM. 65(3). 51-90. Available at: https://doi.org/10.1016/j.disamonth.2018.06.001 ↑22 Zhou, S., Yao, Z. (2022). Roles of Infection in Psoriasis. International Journal of Molecular Sciences. 23(13). 6955. Available at: https://doi.org/10.3390/ijms23136955 ↑23 Yang, H., Zheng, J. (2020). Influence of stress on the development of psoriasis. Clinical and Experimental Dermatology. 45(3). 284-288. Available at: https://doi.org/10.1111/ced.14105 ↑24 Zheng, X., Wang, Q., Luo, Y., Lu, W., Jin, L., Chen, M., Zhu, W., Kuang, Y. (2021). Seasonal Variation of Psoriasis and Its Impact in the Therapeutic Management: A Retrospective Study on Chinese Patients. Clinical Cosmetic and Investigational Dermatology. 10(14). 459-465. Available at: https://doi.org/10.2147/CCID.S312556 ↑25 Miao, M., Yan, J., Sun, Y., Liu, J., Guo, S. (2025). Psoriasis: Unraveling Disease Mechanisms and Advancing Pharmacological and Nontechnological Treatments. Journal of Inflammation Research. 10(18). 2045-2072. Available at: https://doi.org/10.2147/JIR.S506103 ↑26 Mayo Clinic. (no date). Psoriasis. Available at: https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes/syc-20355840 (Accessed: March 2025) ↑27 NHS. (2022). Psoriasis. Available at: https://www.nhs.uk/conditions/psoriasis/causes/ (Accessed: March 2025) ↑30 Almeida, M.C., Romiti, R., Doche, I., Valente, N.Y.S., Donati, A. (2013). Psoriatic scarring alopecia. Anais Brasileiros de Dermatologia. 88(6 Suppl 1). 29-31. Available at: https://doi.org/10.1590/abd1806-4841.20132241 ↑31 Song, B., Liu, X., Jin, H. (2024). Ixekizumab Improved Refractory Erythrodermic Psoriasis with Comorbid Diffuse Alopecia: A Case Report with 52-Week Follow-Up. Clinical, Cosmetic and Investigational Dermatology. 8(17). 1811-1814. Available at: https://doi.org/10.2147/CCID.S471582 ↑32 Wright, A.L., Messenger, A.G. (1990). Scarring alopecia in psoriasis. Acta Dermato-Venereologica. 70(2). 156-159. Available at: PMID:1969203 ↑33 Kim, G.K. (2010). The Rationale Behind Topical Vitamin D Analogs in the Treatment of Psoriasis. Journal of Clinical and Aesthetic Dermatology. 3(8). 46-53. Available at: PMID: 2087752 ↑34 National Psoriasis Foundation. (no date). Best Psoriasis Shampoos Your Scalp Will Love. Available at: https://www.psoriasis.org/advance/best-psoriasis-shampoos (Accessed: March 2025) ↑35 Sbidian, E., Chaimani, A., Garcia-Doval, I., Doney, L., Dressler, C., Hua, C., Hughes, C., Naldi, L., Afach, S., Le Cleach, L. (2021). Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Systematic Reviews. 2021(4): CD011535. Available at: https://doi.org/10.1002/14651858.CD011535.pub4 ↑36 Medical News Today. (2019). Can aloe vera treat psoriasis? Available at: https://www.medicalnewstoday.com/articles/320081 (Accessed: March 2025) ↑37 National Psoriasis Foundation. (no date). Dietary Modifications. Available at: https://www.psoriasis.org/dietary-modifications (Accessed: March 2025) Methylene blue, a compound originally developed as a medical dye, has recently been stirring up attention due to its potential therapeutic applications beyond its initial purpose, particularly in the realm of age-related concerns such as cognitive and skin health. However, some are now exploring its potential use in hair regrowth.
Chemical structure of methylene blue.[1]PubChem. (no date). Methylene Blue. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Methylene-Blue (Accessed: March 2025)
This research aligns with a broader trend in the pharmaceutical industry: repurposing old compounds for new indications. This offers several advantages, including reduced development time, lower costs, and decreased risk due to established safety trials. Approximately 30% of repurposed drugs typically gain approval compared to only 10% of new drug applications, making this strategy increasingly attractive for pharma companies.[2]Hernandez, J.J., Pryszlak, M., Smith, L., Yanchus, C., Kurji, N., Shahani, V.M., Molinski, S.V. (2017). Giving Drugs a Second Chance: Overcoming Regulatory and Financial Hurdles in Repurposing … Continue reading
In this article, we will examine the evidence surrounding methylene blue and whether it could translate to better hair growth outcomes.
Key Takeaways
- What Is It? Methylene blue is a medical dye with antioxidant, mitochondrial-supporting, and anti-inflammatory properties. It has been studied for neurodegenerative diseases, skin health, and aging, but its role in hair regrowth is unproven.
- Clinical Data. There are no human clinical trials testing methylene blue for hair regrowth. Most research comes from in vitro and animal studies. One study in hair transplants found no benefit over saline.
- Evidence Quality. Methylene blue scored 6/100 for evidence quality by our metrics.
- Safety. While generally safe at therapeutic doses, methylene blue can cause nausea, headaches, and urine discoloration. More serious risks include serotonin syndrome (when combined with SSRIs/SNRIs) and hemolytic anemia in G6PD-deficient individuals. The long-term effects of cosmetic use remain unknown.
- Best Practices. Given the lack of evidence and potential risks, methylene blue should not be considered a proven hair loss treatment. For those seeking regrowth solutions, clinically tested treatments remain the safest bet.
All-Natural Hair Supplement + Topical
The top natural ingredients for hair growth, all in one serum & supplement.
Take the next step in your hair growth journey with a world-class natural serum & supplement. Ingredients, doses, & concentrations built by science.

Let’s start off by exploring why people are so excited about methylene blue.
Methylene blue is purportedly a potent mitochondrial-targeting antioxidant that effectively scavenges reactive oxygen species (ROS).[3]Xue, H., Thaivalappil, A., Cao, K. (2021). The Potentials of Methylene Blue as an Anti-Aging Drug. Cells. 10(3379). 1-12. Available at: https://doi.org/10.3390/cells10123379 According to the free radical theory of aging, ROS are closely linked to cellular aging. ROS accumulation can cause a cellular state called oxidative stress, which damages DNA, protein, and lipids.[4]Labunskyy, V.M., Galdyshev, N.M. (2013). Role of Reactive Oxygen Species-Mediated Signaling in Aging. Antioxidants & Redox Signaling. 19(12). 1362-1372. Available at: … Continue reading
What Beneficial Properties Might Methylene Blue Have?
Methylene blue has shown promise for improving several aging pathways. Let’s take a look at the pre-clinical evidence and see how it might translate into improvements in hair regrowth.
Anti-Aging
In cultured human skin, fibroblasts derived from healthy donors and patients with progeria (a genetic disorder leading to rapid aging in children) were used to evaluate the antioxidant activity of methylene blue.[5]Xiong, Z-M., O’Donovan, M., Sun, L., Choi, J.Y., Ren, M., Cao, K. (2017). Anti-aging potentials of methylene blue for human skin longevity. Scientific Reports. 7(2475). 1-12. Available at: … Continue reading Compared to other widely used mitochondrial-targeting antioxidants, the researchers found that methylene blue reduced levels of ROS (MitoSOX) and improved skin fibroblast proliferation more effectively (Figure 1).
Figure 1: Effect of 100 nM methylene blue (MB) compared to 100 µMm N-acetyl-cysteine (NAC) (A,B), 100 nM MitoQ (C,D), and 100 nM mTEM (E,F) on mitochondrial-specific superoxide (MitoSOX) levels and cell growth after four weeks of treatment. (*p < 0.05, **p < 0.01).[6]Xiong, Z-M., O’Donovan, M., Sun, L., Choi, J.Y., Ren, M., Cao, K. (2017). Anti-aging potentials of methylene blue for human skin longevity. Scientific Reports. 7(2475). 1-12. Available at: … Continue reading
The researchers also found that methylene blue could reduce signs of aging in old skin cells. Before treatment, the old fibroblasts (harvested from two subjects over 80 years old) showed signs of cellular senescence, including increased ꞵ-galactosidase (a marker of senescence) and higher levels of mitochondrial ROS than younger cells (harvested from two subjects below 30 years old). Both the old and young cells were grown in a medium supplemented with methylene blue for four weeks. Compared to a control, methylene blue significantly reduced levels of mitochondrial ROS, reduced ꞵ-galactosidase marker signal and decreased the gene expression of senescence marker p16 in the old cells. It also improved cell growth in both old and young cells (Figure 2).
Figure 2: Effect of methylene blue on molecular signs of cell aging in young (1-YM, 2-YF) and old (3-OM, 4-OF) fibroblasts. A = ꞵ-galactosidase (blue) staining reduced in cells treated with methylene blue, B = p16 gene expression (a gene that is upregulated in senescence) is significantly reduced in both young and old fibroblasts, C = MitoSOX levels in cells treated with methylene blue were significantly decreased in the two older fibroblasts, D = both young and old fibroblast cells grew better in medium supplemented with methylene blue than without.[7]Xiong, Z-M., O’Donovan, M., Sun, L., Choi, J.Y., Ren, M., Cao, K. (2017). Anti-aging potentials of methylene blue for human skin longevity. Scientific Reports. 7(2475). 1-12. Available at: … Continue reading
In addition to this, the researchers analyzed the effect of methylene blue on the expression of key antioxidant response genes. Methylene blue was found to upregulate the expression of Nrf2, which is an important transcription factor involved in the antioxidant response. In 3D reconstructed human skin, it was also found to promote wound healing, increase tissue viability, skin thickness and hydration, and upregulate elastin and collagen 2A1, which are important for maintaining skin elasticity and thickness.
UV Protection
Another in vitro (in cells) study was conducted in which primary human keratinocytes were irradiated with UV rays and treated with methylene blue to test if it had any protective effects against UV-induced DNA damage and cell death.[8]Xiong, Z-M., Mao, X., Trappio, M., Arya, C., el Kordi, J., Cao, K. (2021). Ultraviolet radiation protection potentials of methylene blue for human skin and coral reef health. Scientific Reports. … Continue reading
The cells were treated with 100 nM of methylene blue for two weeks and then exposed to 0, 5, 10, and 20 seconds of UVB rays at 1 W/cm2 (Watt per cm2). A common marker for DNA damage, ℽH2AX, was measured, with methylene blue treated cells showing significantly reduced expression at each dosage compared to the control (PBS) (Figure 3).
Figure 3: Methylene blue reduces DNA damage induced by UVB irradiation in human keratinocytes. A = The experimental timeline starting from pre-incubation with methylene blue or PBS for 2 weeks, then exposure to UVB light and collection at specific time points, B = Western blot images showing the protein expression of DNA damage markers H2AX and ℽH2AX, C = graph representation of the western blot results, showing that methylene blue reduces DNA damage after UVB irradiation.[9]Xiong, Z-M., Mao, X., Trappio, M., Arya, C., el Kordi, J., Cao, K. (2021). Ultraviolet radiation protection potentials of methylene blue for human skin and coral reef health. Scientific Reports. … Continue reading
The researchers also found that it prevented human keratinocytes from undergoing UVB irradiation-induced cell death and that it was more effective at blocking high-energy UVB/C rays than a common UV blocker used in most sunscreens, oxybenzone.
They also found that methylene blue was a more robust scavenger of ROS (meaning that it can effectively neutralize and remove ROS) than oxybenzone, Vitamin A, and Vitamin C in keratinocytes. A separate experiment was also conducted on young and old fibroblasts derived from males and females treated with either PBS, DMSO (controls), methylene blue, Vitamin A, Vitamin C, or a combination of Vitamin C and methylene blue. The combination treatment showed increased cell growth in both young and old cells (male and female) compared to all other treatments. When it came to ROS levels, the methylene blue alone treatment and the combination treatment both showed the most effective reductions in ROS levels (Figure 4).
Figure 4: Methylene Blue is a more effective ROS scavenger than Vitamin A and Vitamin C in human skin fibroblasts. A and B = young and old fibroblast cell growth during fourteen-day treatment with PBS, DMSO, 100 nM methylene blue, 100 nM Vitamin A, 100 µM Vitamin C, and 100 nM methylene blue + 100 µM Vitamin C. C and D = Comparison of MitoSOX levels in young and old male fibroblasts after treatment with PBS, DMSO, 100 nM methylene blue, 100 nM Vitamin A, 100 µM Vitamin C, and 100 nM methylene blue + 100 µM Vitamin C for four weeks.[10]Xiong, Z-M., Mao, X., Trappio, M., Arya, C., el Kordi, J., Cao, K. (2021). Ultraviolet radiation protection potentials of methylene blue for human skin and coral reef health. Scientific Reports. … Continue reading
Anti-Inflammatory
One study has shown that methylene blue decreases inflammation by reducing serum levels of interleukin-6 (a pro-inflammatory cytokine that plays a significant role in both acute and chronic inflammatory responses) and inhibiting the activation of STAT3 in the skin.[11]Li, Y., Ying, W. (2023). Methylene blue reduces the serum levels of interleukin-6 and inhibits STAT3 activation in the brain and the skin of lipopolysaccharide-administered mice. Frontiers in … Continue reading STAT3 is implicated in the pathogenesis of alopecia areata.[12]Roche, F.C., Hedberg, M.L. Fischer, A.S., Ray, A., Dentchev, T., Rice, X., Taylor, S.C., Seykora, J.T. (2023). Activation of STAT3 in lymphocytes associated with central centrifugal cicatricial … Continue reading
Mice were split into four groups: control (PBS injected intraperitoneally every day for 3 days), and 5 mg/kg, 10 mg/kg, and 20 mg/kg methylene blue groups (injected intraperitoneally every day for 3 days). Another three groups of mice were treated with lipopolysaccharide (1 mg/kg) to induce inflammation, with two groups also being treated with 10 mg/kg of methylene blue and 20 mg/kg of methylene blue.
Mice treated with both 10 and 20 mg/kg methylene blue alongside LPS showed a significant reduction in serum levels of LPS-induced IL-6 (Figure 5) and STAT3 (Figure 6). Other markers of inflammation that were reduced after methylene blue treatment were iNOS (inducible nitric oxide synthase, which can induce or enhance the inflammatory response) and COX2 (an enzyme implicated in inflammation by producing prostaglandins).
Figure 5: Effect of methylene blue on serum levels of LPS-induced IL-6 levels on mice treated with methylene blue alone or combined with LPS. The mice treated with LPS and both 10 and 20 mg/kg showed significant reductions in serum IL-6 compared to LPS alone.[13]Li, Y., Ying, W. (2023). Methylene blue reduces the serum levels of interleukin-6 and inhibits STAT3 activation in the brain and the skin of lipopolysaccharide-administered mice. Frontiers in … Continue reading}
Figure 6: Effect of methylene blue on LPS-induced p-STAT3/STAT3 ratios in the skin tissue of mice treated with methylene blue alone or combined with LPS. Methylene blue significantly reduced LPS-induced increases in pSTAT3/STAT3 ratio.[14]Li, Y., Ying, W. (2023). Methylene blue reduces the serum levels of interleukin-6 and inhibits STAT3 activation in the brain and the skin of lipopolysaccharide-administered mice. Frontiers in … Continue reading
Based on the positive effects seen in these studies, methylene blue could assist in hair growth through several mechanisms:
- Reduction of Oxidative Stress (ROS Regulation)
Hair follicle stem cells and dermal papilla cells are highly susceptible to oxidative stress, which can impair hair follicle cycling and contribute to hair loss. Methylene blue has demonstrated the ability to effectively scavenge reactive oxygen species (ROS), reducing oxidative damage and potentially improving hair follicle resilience and function.
- Enhancement of Mitochondrial Function and ATP Production
Hair follicles require significant energy (ATP) for active growth during the anagen phase. Methylene blue has been shown to enhance mitochondrial function, increase ATP production, and improve cellular energy metabolism, which may support hair follicle growth and maintenance.
- Stimulation of Fibroblast Proliferation and Extracellular Matrix Support
Dermal fibroblasts and dermal papilla cells play crucial roles in hair follicle anchoring and signaling. Methylene blue can enhance fibroblast proliferation and extracellular matrix production, which could promote a supportive environment for robust hair follicle development.
- Anti-Senescence Effects
Cellular senescence in hair follicle stem cells is linked to age-related hair thinning and loss. Methylene blue has been found to reduce markers of cellular senescence, such as p16 and β-galactosidase expression, which may help maintain hair follicle stem cell viability and prolong the anagen phase in aging individuals.
- Upregulation of Antioxidant Response (Nrf2 Activation)
Methylene blue activates Nrf2, a transcription factor involved in cellular defense against oxidative stress. Since Nrf2 is associated with protection against chemotherapy-induced alopecia and inflammatory hair loss conditions (e.g., lichen planopilaris), its activation by methylene blue could offer therapeutic benefits for hair regrowth.
- Improvement in Wound Healing and Scalp Thickness
A healthy scalp environment is critical for hair follicle regeneration. Methylene blue has been shown to promote wound healing, increase skin thickness, and enhance tissue hydration, which may contribute to a more favorable microenvironment for hair growth.
- Reduction of Inflammatory Markers
Chronic inflammation, mediated by IL-6 and STAT3 activation, is implicated in autoimmune and scarring alopecia (e.g., alopecia areata, central centrifugal cicatricial alopecia (CCCA)). Methylene blue can significantly reduce inflammatory markers such as IL-6 and STAT3, potentially mitigating inflammatory hair loss conditions.
What Clinical Evidence Shows Methylene Blue’s Positive Effects?
The only evidence that we could find in humans showing positive effects from methylene blue are those surrounding neurodegenerative diseases and cognitive enhancement.
Neurodegenerative diseases
A double-blind, dose-finding Phase II clinical trial involving 321 patients with mild-to-moderate Alzheimer’s disease tested three doses of methylene blue.[15]Wischik, C.M., Staf, R.T., Wischik, D.J., Bentham, P., Murray, A.D., Storey, J., Kook, K.A., Harrington, C.R. (2015). Tau Aggregation Inhibitor Therapy: An Exploratory Phase 2 Study in Mild or … Continue reading
The study met its predefined primary efficacy endpoint at 24 weeks of reduction in cognitive decline at the 138 mg/day dose. The beneficial effect was sustained for 50 weeks in both mild and moderate subjects at this dose, with a 90% reduction in the rate of cognitive decline overall.
Cognitive enhancement
In a randomized controlled trial involving 26 healthy subjects aged 22-62, low-dose methylene blue (280 mg) improved memory performance and increased brain activity in regions associated with attention and short-term memory.[16]Rodriguez, P., Zhou, W., Barrett, D.W., Altmeyer, W., Gutierrez, J.E., Li, J., Lancaster, J., Gonzalez-Lima, F., Duong, Q.T. (2016). Multimodal Randomized Functional MR Imaging of the Effects of … Continue reading
Do These Results Show That Methylene Blue Can Regrow Hair?
While methylene blue has shown potential in preclinical studies for various therapeutic applications, its efficacy in promoting hair regrowth remains unproven. The enthusiasm surrounding the potential benefits of methylene blue is primarily based on in vitro and animal studies, which often do not translate directly to human outcomes.
As you can see above, preclinical studies have demonstrated that methylene blue possesses antioxidant properties, improves mitochondrial function, and exhibits anti-inflammatory effects. However, we could not find any preclinical or clinical studies looking at its potential effects on hair regrowth, so we can’t say for sure whether it will work or not.
In fact, the only study we could find that included methylene blue and hair follicles is a pilot study testing methylene blue as a storage solution during hair transplants. In this study, normal saline solution outperformed methylene blue in graft survival rates at 8, 12, and 18 months post-surgery.[17]Tangjaturonrusamee, C., Thientaworn P., Arunrattanapong, N., Castillejos, D.K.O., Patjomvanich, D. (2016). Methylene blue: Its Efficacy and Safety as a Storage Solution in Hair Transplantation. … Continue reading
Potential Safety Risks
While methylene blue is considered to be safe at therapeutic doses, it can cause some side effects:[18]Michael. (2025). Is Methylene Blue Safe for Long-Term Use? Covenant Health Products. Available at: https://covenanthealthproducts.com/our-blog/is-methylene-blue-good-for-you (Accessed: March 2025),[19]Drugs.com. (2024). Methylene Blue Side Effects. Drugs.com. Available at: https://www.drugs.com/sfx/methylene-blue-side-effects.html (Accessed: March 2025)
- Common: Skin/urine discoloration, nausea, dizziness, and headaches.
- Severe: Serotonin syndrome (when combined with SSRIs/SNRIs), hemolytic anemia in G6PD-deficient individuals, and rate causes of organ toxicity.
There is also some evidence that prolonged use may strain liver and kidney function due to metabolism demands. Furthermore, there have been no rigorous studies confirming the safety of chronic, low-dose methylene blue use for cosmetic purposes.
Risk vs. Reward: Is It Worth It?
Considering the potential side effects, drug interactions and lack of evidence, we would say that it is currently not worth trying methylene blue to counteract your hair loss. Long-term safety data for methylene blue has not been collected at this point, so it is worth waiting until new research has been conducted.
Final Thoughts
While methylene blue shows promise in anti-aging and cellular health, there’s no clinical evidence supporting its use for hair regrowth. Most findings come from in vitro and animal studies, which don’t always translate to real-world results.
Beyond the lack of evidence, potential risks—including drug interactions and unknown long-term effects—make it a high-risk, low-reward option for hair loss. Until human trials confirm its efficacy and safety, methylene blue remains speculative. For now, clinically tested treatments are the safer bet.
References[+]
References ↑1 PubChem. (no date). Methylene Blue. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Methylene-Blue (Accessed: March 2025) ↑2 Hernandez, J.J., Pryszlak, M., Smith, L., Yanchus, C., Kurji, N., Shahani, V.M., Molinski, S.V. (2017). Giving Drugs a Second Chance: Overcoming Regulatory and Financial Hurdles in Repurposing Approved Drugs as Cancer Therapeutics. Frontiers in Oncology. 7(273). Available at: https://doi.org/10.3389/fonc.2017.00273 ↑3 Xue, H., Thaivalappil, A., Cao, K. (2021). The Potentials of Methylene Blue as an Anti-Aging Drug. Cells. 10(3379). 1-12. Available at: https://doi.org/10.3390/cells10123379 ↑4 Labunskyy, V.M., Galdyshev, N.M. (2013). Role of Reactive Oxygen Species-Mediated Signaling in Aging. Antioxidants & Redox Signaling. 19(12). 1362-1372. Available at: https://doi.org/10.1089/ars.2012.4891 ↑5 Xiong, Z-M., O’Donovan, M., Sun, L., Choi, J.Y., Ren, M., Cao, K. (2017). Anti-aging potentials of methylene blue for human skin longevity. Scientific Reports. 7(2475). 1-12. Available at: https://doi.org/10.1038/s41598-017-02419-3 ↑6, ↑7 Xiong, Z-M., O’Donovan, M., Sun, L., Choi, J.Y., Ren, M., Cao, K. (2017). Anti-aging potentials of methylene blue for human skin longevity. Scientific Reports. 7(2475). 1-12. Available at: https://doi.org/10.1038/s41598-017-02419-3 Figure used in line with the Creative Commons License: https://creativecommons.org/licenses/by/4.0/ ↑8 Xiong, Z-M., Mao, X., Trappio, M., Arya, C., el Kordi, J., Cao, K. (2021). Ultraviolet radiation protection potentials of methylene blue for human skin and coral reef health. Scientific Reports. 11(10871). 1-9. Available at: https://doi.org/10.1038/s41598-021-89970-2 ↑9, ↑10 Xiong, Z-M., Mao, X., Trappio, M., Arya, C., el Kordi, J., Cao, K. (2021). Ultraviolet radiation protection potentials of methylene blue for human skin and coral reef health. Scientific Reports. 11(10871). 1-9. Available at: https://doi.org/10.1038/s41598-021-89970-2 Figure used in line with the Creative Commons License: https://creativecommons.org/licenses/by/4.0/ ↑11 Li, Y., Ying, W. (2023). Methylene blue reduces the serum levels of interleukin-6 and inhibits STAT3 activation in the brain and the skin of lipopolysaccharide-administered mice. Frontiers in Immunology. 14(1181932). Available at: https://doi.org/10.3389/fimmu.2023.1181932 ↑12 Roche, F.C., Hedberg, M.L. Fischer, A.S., Ray, A., Dentchev, T., Rice, X., Taylor, S.C., Seykora, J.T. (2023). Activation of STAT3 in lymphocytes associated with central centrifugal cicatricial alopecia. 89(6). 1245-1246. Available at: https://doi.org/10.1016/j.jaad.2023.01.045 ↑13, ↑14 Li, Y., Ying, W. (2023). Methylene blue reduces the serum levels of interleukin-6 and inhibits STAT3 activation in the brain and the skin of lipopolysaccharide-administered mice. Frontiers in Immunology. 14(1181932). Available at: https://doi.org/10.3389/fimmu.2023.1181932 Figure used in line with the Creative Commons License: https://creativecommons.org/licenses/by/4.0/ ↑15 Wischik, C.M., Staf, R.T., Wischik, D.J., Bentham, P., Murray, A.D., Storey, J., Kook, K.A., Harrington, C.R. (2015). Tau Aggregation Inhibitor Therapy: An Exploratory Phase 2 Study in Mild or Moderate Alzheimer’s Disease. 44(2). 705-720. Available at: https://doi.org/10.3233/JAD-142874 ↑16 Rodriguez, P., Zhou, W., Barrett, D.W., Altmeyer, W., Gutierrez, J.E., Li, J., Lancaster, J., Gonzalez-Lima, F., Duong, Q.T. (2016). Multimodal Randomized Functional MR Imaging of the Effects of Methylene Blue in the Human Brain. Radiology. 281(2). 516-526. Available at: https://doi.org/10.1148/radiol.2016152893 ↑17 Tangjaturonrusamee, C., Thientaworn P., Arunrattanapong, N., Castillejos, D.K.O., Patjomvanich, D. (2016). Methylene blue: Its Efficacy and Safety as a Storage Solution in Hair Transplantation. International Society of Hair Restoration Surgery. 26(5). 194-196. Available at: https://doi.org/10.33589/26.5.0194 ↑18 Michael. (2025). Is Methylene Blue Safe for Long-Term Use? Covenant Health Products. Available at: https://covenanthealthproducts.com/our-blog/is-methylene-blue-good-for-you (Accessed: March 2025) ↑19 Drugs.com. (2024). Methylene Blue Side Effects. Drugs.com. Available at: https://www.drugs.com/sfx/methylene-blue-side-effects.html (Accessed: March 2025) Seborrheic dermatitis is a common, chronic inflammatory skin condition that primarily affects areas with a high concentration of sebaceous (oil-producing) glands, such as the scalp, face, upper back, and chest. It is characterized by red, itchy patches of skin covered with greasy, yellowish scales or flakes.
In the scalp, seborrheic dermatitis is also called dandruff. It can vary widely in symptoms and severity, and a number of treatment options are marketed to treat and prevent dandruff. But what is actually happening to your scalp and hair follicles when seborrheic dermatitis hits, and what are the best treatment combinations? Let’s find out below.
Key Takeaways
- What is it? Seborrheic dermatitis is a chronic inflammatory skin condition that affects areas rich in oil glands, like the scalp, causing red, itchy patches with greasy, yellowish flakes.
- What are the symptoms? Symptoms include visible flakes (dandruff), itchiness, redness, and, in severe cases, crusting and hair shafts around hair follicles.
- What Is The Prevalence of Seborrheic Dermatitis in Patients with AGA? One study found that out of 250 patients with AGA, 56.9% also had seborrheic dermatitis.
- What are the most common treatments? Antifungal topicals are most often used (e.g., ketoconazole and selenium sulfide), topical corticosteroids, and newer options like roflumilast foam to reduce inflammation and yeast overgrowth.
- How else can I improve it? Lifestyle changes, like reducing stress, eating a balanced diet, and avoiding known triggers like harsh hair products or cold weather, can help manage symptoms.
- Final thoughts. Managing seborrheic dermatitis often involves trial and error with different therapies, but with the right combination, most people can achieve long-term symptom control.
What Are the General Features of Seborrheic Dermatitis?
- Appearance: The condition manifests as oily patches with yellow or white scales, which may appear darker or lighter in people of color and redder in those with white skin.
- Common locations: Scalp, face, sides of the nose, eyebrows, ears, eyelids, chest, armpits, and groin area.
- Symptoms: Itching, flaking skin, and sometimes a ring-shaped (annular) rash called petaloid seborrheic dermatitis.
Why Does Seborrheic Dermatitis of the Scalp Happen?
Seborrheic dermatitis of the scalp (also called dandruff) occurs due to a combination of factors, one of which is sebum production. The condition primarily affects areas with a high concentration of sebaceous glands, including the scalp.[1]Ro, B.I., Dawson, T.L. (2005). The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. The Journal of Investigative Dermatology. … Continue reading Excess sebum creates a favorable environment for the growth of Malassezia yeasts, which are naturally present on the skin.
Another factor is the overgrowth of Malassezia yeasts. Malassezia species, particularly M.globosa and M.restricta, are commonly found on the scalps of individuals with seborrheic dermatitis. These yeasts are normally harmless but can trigger an inflammatory response when they overgrow.[2]Wikramanayake, T.C., Borda, L.J., Miteva, M., Paus, R. (2019). Seborrheic dermatitis-looking beyond Malassezia. Experimental Dermatology. 28(9). 991-1001. Available at: … Continue reading
Malessezia lipase breaks down human sebum, releasing free fatty acids (FFAs) and other metabolites. These FFAs can penetrate the stratum corneum (the outer layer of the skin), altering skin barrier permeability and leading to inflammation and abnormal keratinization, which are key features of seborrheic dermatitis.[3]Dawson Jr. (2007). Malassezia globosa and restricta: breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. Journal of … Continue reading
Genetic predisposition is also a contributing factor in the pathogenesis of seborrheic dermatitis.[4]Karakadze, M.A., Hirt, P.A., Wikramanayake, T.C. (2018). The genetic basis of seborrheic dermatitis: a review. Journal of the European Academy of Dermatology and Venereology. 32(4). 529-536. … Continue reading Several gene mutations and protein deficiencies have been associated with the condition or similar phenotypes. The affected genes are involved in the immune response (e.g., ACT1, C5, IKBG/NEMO) and epidermal maturation (differentiation) (e.g., ZNF750, MPZL3).
Other genetic mutations that can affect the complement system, which is part of the immune response, have been associated with an increased risk of seborrheic dermatitis. This dysfunction can lead to an inability to effectively restrict the growth of Malassezia.[5]Adalsteinsson, J.A., Kaushik, S., Muzumdar, S., Guttman-Yassky, E., Ungar, J. (2020). An update on the microbiology, immunology, and genetics of seborrheic dermatitis. Experimental Dermatology. … Continue reading
In addition to these, a number of other elements can influence the development of seborrheic dermatitis and its exacerbation. These include immune system abnormalities, such as reduced numbers of helper T cells.[6]Bergbrant, I.M., Johansson, S., Robbins, D., Scheynius, A., Faergemann, J., Soderstrom, T. (1991). An immunological study in patients with seborrheic dermatitis. Clinical and Experimental … Continue reading Hormonal changes can also aggravate the condition.[7]Kashiri, A., Maghsoudloo, N. (2024). Exploring the Impact of Vitamin D and Zinc Deficiencies on Sebhorreic Dermatitis: A Comparative Study. Health Science Reports. 7(12). E70283. Available at: … Continue reading Furthermore, cold weather can worsen symptoms, and stress can trigger or exacerbate flare-ups.
What Are the Symptoms of Seborrheic Dermatitis in the Scalp?
We can split the symptoms of seborrheic dermatitis into three levels: cosmetic, symptom, and microscopic levels.
Cosmetic Level
At the cosmetic level, the most noticeable sign of seborrheic dermatitis on the scalp is the presence of visible flakes on the scalp, which may fall onto clothing. These flakes can range from mild dandruff to more severe scaling.[8]Schwartz, R.A., Janusz, C.A., Janniger, C.K. (2006). Seborrheic dermatitis: an overview. American Family Physician. 74(1). 125-130. Available at: PMID:16848386 The scales often appear greasy and may have a yellow-brown color. In more pronounced cases, you might see white or yellowish scales covering patches of skin on the scalp. These scales can sometimes form crusts, especially in areas where the scalp meets the hairline or behind the ears. The flakiness can be accompanied by noticeable redness (erythema) and bumps or pustules.[9]Saunte, D.M., Gaitanis, G., Hay, R.J. (2020). Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Frontiers in Cellular and Infection Microbiology. 10. 112. Available at: … Continue reading
Figure 1: Scalp seborrheic dermatitis can present as scaling and redness.[10]DermNet. (no date). Seborrheic Dermatitis. Available at: https://dermnetnz.org/imagedetail/2050-seborrhoeic-dermatitis (Accessed: February 2025)
Symptom Level
On a symptom level, itchiness (pruritis) is often the most bothersome aspect of seborrheic dermatitis.[11]Zhang, F., Li, Y., Ren, W., Li, S. (2023). Establishment of clinical evaluation criteria for scalp seborrheic dermatitis. Journal of Cosmetic Dermatology. 22(11). 3042-3046. Available at: … Continue reading The itching can range from mild to intense and may lead to scratching, which can further irritate the scalp and, in some cases, cause hair loss.[12]National Eczema Associations. (no date). Seborrheic Dermatitis. Available at: https://nationaleczema.org/eczema/types-of-eczema/seborrheic-dermatitis/ (Accessed: February 2025) Some people may experience soreness or tenderness in the affected areas, especially if the condition is severe and there has been excessive scratching. In the most severe cases, the rash may weep or ooze, leading to the formation of crusts.
Microscopic Level
At the microscopic level, several distinctive factors characterize seborrheic dermatitis. One of the most notable is the presence of dandruff casts, which are accumulations of dead skin cells and sebum around hair shafts.[13]Franca, K., Villa, R.T., Silva, I.R., de Carvalho, C.A., Bedin, V. (2011). Hair Casts or Pseudonits. International Journal of Trichology. 3(2). 121-122. Available at: … Continue reading These casts are typically white, firm, and tubular in shape and can range from 2 to 7 mm in length.
Figure 2: Presence of hair casts in a 12-year-old girl with seborrheic dermatitis.[14]Kaliyadan, F., Ashique, K.T. (2019). Hair Casts and Nits – Differentiating Using Dermoscopy. Images in Clinical Practice. 85(4). 434-435. Available at: https://doi.org/10.4103/ijdvl.IJDVL_815_17
Several characteristic vascular patterns can be observed in seborrheic dermatitis under dermoscopy, a noninvasive imaging tool.[15]Kim, G.W., Jung, H.J., Ko, H-C., Kim, M.B., Lee, W-J., Lee, S-J., Kim, D-W., Kim, B-S. (2011). Dermoscopy can be useful in differentiating scalp psoriasis from seborrheic dermatitis. British Journal … Continue reading These include arborizing red lines (ARL), which appear as branching blood vessels, and twisted red loops (TRL), which are coiled blood vessels specific to seborrheic dermatitis. Comma vessels (CV) and short-curved blood vessels are also indicative of this condition. These vascular patterns help differentiate seborrheic dermatitis from other skin conditions.
Figure 3: Aborizing vessels (indicated by yellow arrows) and atypical red vessels (indicated by black arrows) in seborrheic dermatitis.[16]Kim, G.W., Jung, H.J., Ko, H-C., Kim, M.B., Lee, W-J., Lee, S-J., Kim, D-W., Kim, B-S. (2011). Dermoscopy can be useful in differentiating scalp psoriasis from seborrheic dermatitis. British Journal … Continue reading
In addition to these, other microscopic characteristics include spongiosis (buildup of fluid between skin cells in the epidermis) in acute seborrheic dermatitis lesions, psoriasiform hyperplasia (thickening of the epidermis), swelling, and infiltration of antibodies.[17]Park, J-H., Park, Y.J., Kim, S.K., Kwon, J.E., Kang, Y.H., Lee, E-S., Choi, J.H., Kim, Y.C. (2016). Histopathological Differential Diagnosis of Psoriasis and Seborrheic Dermatitis of the Scalp. … Continue reading In some cases, the opening of a hair follicle can become blocked with excess skin cells (follicular plugging), and maturation (differentiation) of keratinocytes can become impaired, leading to cells in the stratum corneum retaining nuclei (shoulder parakeratosis). This contributes to the scaling characteristic of seborrheic dermatitis.
As epidermal cells differentiate through the skin layers, they usually lose their nuclei and become filled with keratin. By the time they reach the stratum corneum, they are typically flat, dead cells without nuclei, forming a protective barrier.[18]Alberts, B., Johnson, A., Lewis J. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Epidermis and Its Renewal by Stem Cells. Available from: … Continue reading
Figure 4: Seborrheic dermatitis can cause (B) shoulder parakeratosis (where an abnormally large number of keratinocytes retain their nuclei in the stratum corneum) and (D) follicular plugging (where the opening of the hair follicle becomes blocked with excess epidermal cells).[19]Park, J-H., Park, Y.J., Kim, S.K., Kwon, J.E., Kang, Y.H., Lee, E-S., Choi, J.H., Kim, Y.C. (2016). Histopathological Differential Diagnosis of Psoriasis and Seborrheic Dermatitis of the Scalp. … Continue reading
How Common is Seborrheic Dermatitis?
A comprehensive meta-analysis published in JAMA Dermatology in 2024 found that the global pooled prevalence (meaning all body locations, including the scalp) of seborrheic dermatitis is 4.38%, which is higher than previous large-scale global estimates.[20]Polaskey, M.T., Chang, C.H., Daftary, K., Fakhraie, S., Miller, C.H., Chovatiya, R. (2024). The Global Prevalence of Seborrheic Dermatitis: A Systematic Review and Meta-Analysis. JAMA Dermatology. … Continue reading In the US, the prevalence is 5.86%, which appears to be middle-of-the-road compared to places like South Africa, which has a prevalence of 8.82%, and India, with a prevalence of 2.62%.
Figure 5: The prevalence of seborrheic dermatitis (%) split by age, community, or country.[21]Polaskey, M.T., Chang, C.H., Daftary, K., Fakhraie, S., Miller, C.H., Chovatiya, R. (2024). The Global Prevalence of Seborrheic Dermatitis: A Systematic Review and Meta-Analysis. JAMA Dermatology. … Continue reading
Is Seborrheic Dermatitis More Common in People with Pattern Hair Loss?
Recent studies have shown a significant association between seborrheic dermatitis and androgenic alopecia (AGA).
- One five-year study from 2006 to 2010 found that seborrheic dermatitis was the most commonly associated disease in both men and women with AGA.[22]Jang, W.S., Son, I.P., Yeo, K.I., Park, K.Y., Li, K., Kim, B.J., Seo, S.J., Kim, M.N., Hong, C.K. (2013). The Annual Changes of Clinical Manifestation of Androgenetic Alopecia Clinic in Korean Males … Continue reading
- A retrospective study published in 2024 found that out of 250 patients with AGA, 56.9% also had seborrheic dermatitis.[23]Faghihkhorasani, A., Sadeghzadeh, A., Goodarzi, A., Rohaninasab, M. (2024). The Relationship between Seborrheic Dermatitis and Androgenetic Alopecia in Patients Referred to a Skin Clinic in Tehran, … Continue reading
- A significant correlation was found between the pattern of AGA in men and the severity of seborrheic dermatitis.
- A significant relationship was also observed between dandruff symptoms and AGA patterns.
- In men, the highest severity of seborrheic dermatitis (grade 3) was related to the Hamilton Norwood type 2 pattern of hair loss (triangular hair loss on both sides of the frontoparietal line).
- In women, the highest severity of seborrheic dermatitis (grade 2) was observed in the Ludwig type 2 hair loss pattern (thinning of hair about 1-3 cm behind the hairline).
What Is the Biology Behind This Correlation?
Although there is no established explanation, there are several factors that could explain the correlation between seborrheic dermatitis and AGA.
- Androgen sensitivity: Both conditions are influenced by androgens, particularly dihydrotestosterone (DHT). The enzyme 5-alpha reductase (5-AR) converts testosterone to DHT, which affects both sebum production and hair follicle miniaturization.[24]Kim, B.J., Kim, J.Y., Eun, H.C., Kwon, O.S., Kim, M.N. (2006). Androgenetic alopecia in adolescents: A report of 43 cases. The Journal of Dermatology. 33(10). 696-699. Available at: … Continue reading
- Sebum production: Increased sebum production is characteristic of both conditions. In AGA, there are increased levels of 5-AR in the follicles of the temporal, frontal, and crown regions.[25]Kure, K., Isago, T., Hirayama, T. (2015). Changes in the sebaceous gland in patients with male pattern hair loss (androgenic alopecia). Journal of Cosmetic Dermatology. 14(3). 178-184. Available at: … Continue reading This excess sebum creates an environment conducive to the overgrowth of Malassezia yeast.
- Microbiome imbalance (dysbiosis): Patients with AGA exhibit scalp dysbiosis, with an increased abundance of Cutibacterium and a decreased abundance of Corynebacterium.[26]Suzuki, K., Inoue, M., Cho, O., Mizutani, R., Shimizu, Y., Nagahama, T., Sugita, T. (2021). Scalp microbiome and sebum composition in Japanese male individuals with and without androgenetic alopecia. … Continue reading This altered microbiome may contribute to inflammation and exacerbate both conditions.
- Malassezia yeast: Malassezia restricta is more abundant on the scalps of patients with AGA and is also implicated in seborrheic dermatitis, which may exacerbate the condition.
While there is no established causality for AGA and seborrheic dermatitis, these underlying factors mean that seborrheic dermatitis and AGA can exacerbate one another.
So, what can you do to treat seborrheic dermatitis on the scalp?
What Are the Typical Treatments for Seborrheic Dermatitis on the Scalp?
Seborrheic dermatitis of the scalp can be managed through various treatment options. It can be attacked in multiple ways, such as through the use of antifungals, anti-inflammatories, treatments that suppress sebum, corticosteroids, and topical keratolytic agents.
Figure 6: Therapeutic Targets in Seborrheic Dermatitis.[27]Mangion, S.E., Mackenzie, L., Roberts, M.S., Holmes, A.M. (2023). Seborrheic dermatitis: topical therapeutics and formulation design. European Journal of Pharmaceutics and Biopharmaceutics. 185. … Continue reading
FDA-Approved Options
Among the FDA-approved treatments, Roflumilast foam 0.3% (Zoryve) is a recent addition, approved on December 2023, for treating seborrheic dermatitis in individuals aged 9 and older.[28]Arcutis Biotherapeutics. (no date). FDA Approves Arcutis’ ZORYVE (roflumilast) Topical Foam, 0.3% for the Treatment of Seborrheic Dermatitis in Individuals Aged 9 Years and Older. Available at: … Continue reading This treatment is a topical non-steroidal phosphodiesterase 4 (PDE4) inhibitor. PDE4 plays a key role in the inflammatory response, so by inhibiting this, it reduces inflammation and associated symptoms like redness, scaling, and itching.[29]Zirwas, M.J., Draelos, Z.D., DuBois, J., Kircik, L.H., Moore, A.Y., Gold, L.S., Alonso-Llamazares, J., Bukhalo, M., Bruce, S., Eads, K., Green, L.J., Guenthner, S., Ferris, L.K., Forman, S.B., … Continue reading
Ketoconazole is FDA-approved for patients 12 years of age and older with healthy immune systems. It acts through multiple mechanisms to treat seborrheic dermatitis in the scalp. It inhibits the production of lanosterol, a precursor for ergosterol biosynthesis, which is essential for fungal membrane integrity.[30]Tynes, B.E., Johnson, C.D., Vaish, M.H., Abbott, B., Vucenovic, J., Varrassi, G., Potharaju, P., Torres, Y.L., Lee, Z., Ahmadzadeh, S., Shekoohi, S., Kaye, A.D. (2024). Ketoconazole Shampoo for … Continue reading
This halts the growth of Malassezia yeasts associated with seborrheic dermatitis. It also strongly binds to the cytochrome p450 mono-oxygenase complex, hindering the fungal biosynthesis of triglycerides and phospholipids and shifting sebum secretion in the stratum corneum, addressing the hypersecretion of sebum characteristic of the condition.[31]Borgers, M., Degreed, H. (2007). The Role of Ketoconazole in Seborrheic Dermatitis. Therapeutics for the Clinician. 80. 359-363. Available at: … Continue reading
Ketoconazole also exhibits anti-inflammatory properties and antiproliferative effects and may favor biotin-producing bacteria, which could improve the skin microbiome.[32]Goularte-Silva, V., Paulino, C.L. (2021). Ketoconazole beyond antifungal activity: Bioinformatics-based hypothesis on lipid metabolism in dandruff and seborrheic dermatitis. Experimental Dermatology. … Continue reading
Figure 7: Effect of Ketoconazole on Malassezia lipid metabolism and biotin-producing bacteria.[33]Goularte-Silva, V., Paulino, C.L. (2021). Ketoconazole beyond antifungal activity: Bioinformatics-based hypothesis on lipid metabolism in dandruff and seborrheic dermatitis. Experimental Dermatology. … Continue reading
Ciclopirox shampoo is approved for people 16 years of age and older with seborrheic dermatitis on the scalp. It works differently from other common antifungals. It acts like a magnet for certain metals, especially iron and aluminum, which are important for fungal survival.[34]LOPROX® (cicloporox) Shampoo 1%. (2006). MEDICIS Pharmaceutical Corp. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021159s009lbl.pdf (Accessed: February 2025) By grabbing these metals, ciclopirox prevents important fungal enzymes from working properly, leading to peroxide build-up and cellular damage.
Other Treatments
Some over-the-counter treatments are used to treat seborrheic dermatitis. These are often antifungal shampoos containing ingredients such as:
- Selenium sulfide: A treatment with antifungal, cytostatic, keratolytic, anti-inflammatory, and sebum regulatory properties. In clinical studies, 2.5% selenium sulfide shampoo improved dandruff scores by 78.3% after 4 weeks.[35]Godse, G., Godse, K. (2024). Safety, Efficacy and Attributes of 2.5% Selenium Sulfide Shampoo in the Treatment of Dandruff: A Single-Center Study. Cureus. 16(3). E57148. Available at: … Continue reading
- Coal tar: This helps to reduce inflammation and redness associated with seborrheic dermatitis and has antifungal and keratolytic properties. It also slows the rate of skin cell production, helping to minimize the buildup of scales and dry patches. However, it has been found to be less effective than ciclopirox and ketoconazole.[36]Davies, D.B., Boorman, G.C., Shuttleworth, D. (1999). Comparative efficacy of shampoos containing coal tar (4.0% w/w; Tarmed), coal tar (4.0% w/w) plus ciclopirox olamine (1.0% w/w Tarmed AF), and … Continue reading
- Salicylic acid: Salicylic acid helps to slough off dead skin cells and remove flakes from the scalp, which can be beneficial for those with seborrheic dermatitis. It also has keratolytic, oil-controlling, anti-inflammatory, and anti-fungal effects.[37]Ge, L., Liu, Z., Xu, S., Li, C., Jin, M., Luo, Y., Kong, Y., Meng, J., Zheng, G., Gao, J., Wang, P., Bai, W., Na, H., Zhou, X., Jin, Z., Pi, L. (2025). A Cohort Clinical Study on the Efficacy of … Continue reading
- Zinc pyrithione: Zinc pyrithione increases cellular copper levels, which damages iron-sulfur clusters of proteins essential for fungal metabolism. This, alongside its antifungal, antibacterial, anti-inflammatory, and sebum-regulatory properties, contributes to its effectiveness in treating seborrheic dermatitis. One study found that a shampoo containing 1% zinc pyrithione was effective in controlling dandruff when used on half of the scalp.[38]Marks, R., Pearse, A.D., Walker, A.P. (1985). The effects of a shampoo containing zinc pyrithione on the control of dandruff. British Journal of Dermatology. 112(4). 415-422. Available at: … Continue reading
- Piroctone olamine: A number of clinical studies have used piroctone olamine alongside other treatments to improve seborrheic dermatitis, with one study showing a clinical improvement of 80%.[39]Ge, L., Liu, Z., Xu, S., Li, C., Jin, M., Luo, Y., Kong, Y., Meng, J., Zheng, G., Gao, J., Wang, P., Bai, W., Na, H., Zhou, X., Jin, Z., Pi, L. (2025). A Cohort Clinical Study on the Efficacy of … Continue reading
Prescription medications such as topical corticosteroids and calcineurin inhibitors may also be used off-label.[40]Paula Ludmann. (2024). Seborrheic Dermatitis: Diagnosis and Treatment. American Academy of Dermatology Association. Available at: … Continue reading UVB light therapy can be used for widespread rash and scales. Other treatments include fluconazole 2%, naftifine hydrochloride 1% gel, and climbazole.[41]Dall’Oglio, F., Nasca, M.R., Gerbino, C., Micali, G. (2022). An Overview of the Diagnosis and Management of Seborrheic Dermatitis. Clinical, Cosmetic and Investigational Dermatology. 6(15). … Continue reading
Lifestyle and Dietary Modifications
In addition to these medical treatments, dietary and lifestyle changes can help manage seborrheic dermatitis.
One case-control study involving 257 participants found associations between white bread, carbonated drinks, and daily fast food, with a higher percentage of seborrheic dermatitis compared to those without the condition.[42]Alshaebi, M., Zahed, L., Osalyan, M., Sulaimani, S., Albahlool, A., Abduljabbar, M.H., Hariri, J. (2023). Association Between Diet and Seborrheic Dermatitis: A Case-Control Study. Cureus. 15(11). … Continue reading The same study reported that increased fruit consumption was associated with a lower risk of seborrheic dermatitis. Furthermore, adherence to a Western diet has been associated with a higher risk of seborrheic dermatitis in female patients.[43]Woolhiser, E., Keime, N., Patel, A., Weber, I., Adelman, M., Dellavalle, R.P. (2024). Nutrition, Obesity, and Seborrheic Dermatitis: Systematic Review. JMIR Dermatology. E50143. Available at: … Continue reading
It is recommended to increase the intake of high-fiber carbohydrates and lean protein foods, as well as foods rich in monounsaturated and omega-3 fatty acids.
Should You Avoid Certain Hair Loss Treatments if You Have Seborrheic Dermatitis?
In our experience, those with seborrheic dermatitis may have problems if they use:
- Topicals containing retinoic acid
- Microneedling
- Scalp massaging
- PRP
- Mesotherapy
These may actually lead to more inflammation, so we would recommend avoiding these until you get your seborrheic dermatitis under control.
Which Treatments Are Best Used for Which Disease Severity?
Management of seborrheic dermatitis of the scalp often involves rotating or combining a number of therapies to target multiple aspects of the disease.
Mild to Moderate Severity
For patients with mild to moderate seborrheic dermatitis, first-line treatments typically include over-the-counter antifungals combined with topical anti-inflammatory agents.
This can include:
- Anti-dandruff shampoos containing 2.5% selenium sulfide or 1-2% zinc pyrithione, used daily or every other day.[44]Paula Ludmann. (2024). Seborrheic Dermatitis: Diagnosis and Treatment. American Academy of Dermatology Association. Available at: … Continue reading
- Ketoconazole shampoo (1-2%) applied for 5-10 minutes before rinsing, used daily initially, then twice weekly for maintenance.
- Topical corticosteroids, like hydrocortisone 1% cream, applied once or twice daily until inflammation clears, then as needed.
- One clinical trial found that the usage of traditional Chinese medicine can also show significant efficacy in treating mild to moderate seborrheic dermatitis without relapse, especially for those with moderate severity.[45]Zhang, F., Li, Y-H., Ren, W., Li, S-R., Chen, Y-C. (2023). Clinical efficacy of a combination treatment of traditional Chinese medicine for scalp seborrheic dermatitis. Journal of Cosmetic … Continue reading
Moderate to Severe
For more severe or persistent cases, treatment may include:
- Oral antifungal medications like itraconazole (200 mg/day for one week, followed by a maintenance dose).[46]Goldenberg, G. (2013). Optimizing Treatment Approaches in Seborrheic Dermatitis. Journal of Clinical and Aesthetic Dermatology. 6(2). 44-49. Available at: PMID: 23441240
- UVB light therapy, with 3 treatment sessions per week for up to 8 weeks. However, some studies have shown relapse within 1 month.[47]Jaalouk, D., Pulumati, A., Algarin, Y.A., Kircik, L., Issa, N.T. (2024). Dermatologic Procedures for the Treatment of Seborrheic Dermatitis. Journal of Drugs in Dermatology. 23(10). 819-824. … Continue reading
- Combination therapy of clobetasol propionate shampoo (0.05%) and ketoconazole (2%) twice weekly has been shown to provide greater efficacy than ketoconazole alone, with a more sustained effect in the treatment of moderate to severe seborrheic dermatitis.[48]Ortonne, J-P., Nikkels, A.F., Reich, K., Oliver, R.M.P., Lee., J.H., Kerrouche, N., Sidou, F., Faergemann, J. (2011). Efficacious and safe management of moderate to severe scalp seborrheic dermatitis … Continue reading
- A recent study conducted on 20 patients with moderate to severe seborrheic dermatitis combined salicylic acid, piroctone olamine, zinc PCA gel, and a cleansing lotion, finding that the combination significantly reduced dandruff, itchiness, redness, and greasiness scores over 4 weeks. Patients with moderate and those with severe seborrheic dermatitis improved to mild with an overall clinical improvement of 80%.[49]Ge, L., Liu, Z., Xu, S., Li, C., Jin, M., Luo, Y., Kong, Y., Meng, J., Zheng, G., Gao, J., Wang, P., Bai, W., Na, H., Zhou, X., Jin, Z., Pi, L. (2025). A Cohort Clinical Study on the Efficacy of … Continue reading
Shampoo Rotations
For people dealing with treatment-resistant seborrheic dermatitis, rotating shampoos may be beneficial. Some of our members have consulted with Dr. Donovan, a leading hair specialist and have experienced success with his approach.
The key to this method is using different active ingredients rather than specific brands. If certain additives in a product don’t suit you, you can opt for alternative with the same active ingredients and concentrations.
- 2% Ketoconazole
- Selenium sulfide
- Coal tar
- Zinc pyrithione
Finding the most effective treatment often requires a trial-and-error approach.[50]University of Utah Health. (no date). Seborrheic Dermatitis. Available at: https://healthcare.utah.edu/dermatology/conditions/seborrheic-dermatitis (Accessed: February 2025) You might be advised by your doctor to start with a basic regimen and assess response after 2-4 weeks. If no improvement has been observed, then your doctor might increase the strength, change the treatment, or add complementary therapies. Once control has been achieved, you can gradually reduce treatment frequency to find the minimal effective maintenance regimen.
When Should You See a Healthcare Professional?
While mild seborrheic dermatitis can be effectively treated with over-the-counter or off-the-shelf treatments, you should see a healthcare professional if any of the following occur:
- Symptoms persist or worsen: If your symptoms don’t improve after using over-the-counter dandruff shampoos for at least two weeks or if they suddenly get worse.[51]Cleveland Clinic. (2020). Seborrheic Dermatitis. Cleveland Clinic. Available at: https://my.clevelandclinic.org/health/diseases/14403-seborrheic-dermatitis (Accessed: February 2025)
- Severity interferes with daily life: When the condition causes anxiety, embarrassment, or disrupts your daily routine.
- Signs of severe disease appear: If you develop yellow-red scaling papules along the hairline, behind the ears, on the eyebrows, or in the others of the face and body.[52]National Eczema Association. (2025). Seborrheic Dermatitis. National Eczema Association. Available at: … Continue reading
- Suspected complications: If you notice signs of skin infection.
- Difficulty in diagnosis: If you are unsure if it is seborrheic dermatitis or another skin condition.
- Presence of other skin conditions: If you suspect you might have seborrheic dermatitis along with other skin conditions, this can complicate diagnosis and treatment.
Final Thoughts
Seborrheic dermatitis of the scalp is a complex condition influenced by factors such as sebum production, Malassezia overgrowth, genetics, and immune responses. While a range of treatments, from over-the-counter antifungal shampoos to prescription medications like roflumilast and ketoconazole, are available, their effectiveness varies depending on the individual and the severity of the condition. Finding the right approach often involves trial and error, with combination therapies frequently offering the best results. Ultimately, consistent management, lifestyle adjustments, and working closely with healthcare providers are key to keeping symptoms under control. If one treatment isn’t working, don’t get discouraged; there are plenty of options to explore.
References[+]
References ↑1 Ro, B.I., Dawson, T.L. (2005). The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. The Journal of Investigative Dermatology. Symposium Proceedings. 10(3). 194-197. Available at: https://doi.org/10.1111/j.1087-0024.2005.10104.x. ↑2 Wikramanayake, T.C., Borda, L.J., Miteva, M., Paus, R. (2019). Seborrheic dermatitis-looking beyond Malassezia. Experimental Dermatology. 28(9). 991-1001. Available at: https://doi.org/10.1111/exd.14006 ↑3 Dawson Jr. (2007). Malassezia globosa and restricta: breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. Journal of Investigative Dermatology. Symposium Proceedings. 12(2). 15-19. Available at: https://doi.org/10.1038/sj.idsymp.5650049. ↑4 Karakadze, M.A., Hirt, P.A., Wikramanayake, T.C. (2018). The genetic basis of seborrheic dermatitis: a review. Journal of the European Academy of Dermatology and Venereology. 32(4). 529-536. Available at: https://doi.org/10.1111/jdv.14704 ↑5 Adalsteinsson, J.A., Kaushik, S., Muzumdar, S., Guttman-Yassky, E., Ungar, J. (2020). An update on the microbiology, immunology, and genetics of seborrheic dermatitis. Experimental Dermatology. 29(5). 481-489. Available at: https://doi.org/10.1111/exd.14091 ↑6 Bergbrant, I.M., Johansson, S., Robbins, D., Scheynius, A., Faergemann, J., Soderstrom, T. (1991). An immunological study in patients with seborrheic dermatitis. Clinical and Experimental Dermatology. 16(5). 331-338. Available at: https://doi.org/10.1111/j.1365-2230.1991.tb00395.x. ↑7 Kashiri, A., Maghsoudloo, N. (2024). Exploring the Impact of Vitamin D and Zinc Deficiencies on Sebhorreic Dermatitis: A Comparative Study. Health Science Reports. 7(12). E70283. Available at: https://doi.org/10.1002/hsr2.70283 ↑8 Schwartz, R.A., Janusz, C.A., Janniger, C.K. (2006). Seborrheic dermatitis: an overview. American Family Physician. 74(1). 125-130. Available at: PMID:16848386 ↑9 Saunte, D.M., Gaitanis, G., Hay, R.J. (2020). Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Frontiers in Cellular and Infection Microbiology. 10. 112. Available at: https://doi.org/10.3389/fcimb.2020.00112 ↑10 DermNet. (no date). Seborrheic Dermatitis. Available at: https://dermnetnz.org/imagedetail/2050-seborrhoeic-dermatitis (Accessed: February 2025) ↑11 Zhang, F., Li, Y., Ren, W., Li, S. (2023). Establishment of clinical evaluation criteria for scalp seborrheic dermatitis. Journal of Cosmetic Dermatology. 22(11). 3042-3046. Available at: https://doi.org/10.1111/jocd.15804 ↑12 National Eczema Associations. (no date). Seborrheic Dermatitis. Available at: https://nationaleczema.org/eczema/types-of-eczema/seborrheic-dermatitis/ (Accessed: February 2025) ↑13 Franca, K., Villa, R.T., Silva, I.R., de Carvalho, C.A., Bedin, V. (2011). Hair Casts or Pseudonits. International Journal of Trichology. 3(2). 121-122. Available at: https://doi.org/10.4103/0974-7753.90834 ↑14 Kaliyadan, F., Ashique, K.T. (2019). Hair Casts and Nits – Differentiating Using Dermoscopy. Images in Clinical Practice. 85(4). 434-435. Available at: https://doi.org/10.4103/ijdvl.IJDVL_815_17 ↑15 Kim, G.W., Jung, H.J., Ko, H-C., Kim, M.B., Lee, W-J., Lee, S-J., Kim, D-W., Kim, B-S. (2011). Dermoscopy can be useful in differentiating scalp psoriasis from seborrheic dermatitis. British Journal of Dermatology. 164(3). 652-656. Available at: https://doi.org/10.1111/j.1365-2133.2010.10180.x ↑16 Kim, G.W., Jung, H.J., Ko, H-C., Kim, M.B., Lee, W-J., Lee, S-J., Kim, D-W., Kim, B-S. (2011). Dermoscopy can be useful in differentiating scalp psoriasis from seborrheic dermatitis. British Journal of Dermatology. 164(3). 652-656. Available at: https://doi.org/10.1111/j.1365-2133.2010.10180.x ↑17 Park, J-H., Park, Y.J., Kim, S.K., Kwon, J.E., Kang, Y.H., Lee, E-S., Choi, J.H., Kim, Y.C. (2016). Histopathological Differential Diagnosis of Psoriasis and Seborrheic Dermatitis of the Scalp. Annals of Dermatology. 28(4). 427-432. Available at: https://dx.doi.org/10.5021/ad.2016.28.4.427 ↑18 Alberts, B., Johnson, A., Lewis J. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Epidermis and Its Renewal by Stem Cells. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26865/ (Accessed: February 2025) ↑19 Park, J-H., Park, Y.J., Kim, S.K., Kwon, J.E., Kang, Y.H., Lee, E-S., Choi, J.H., Kim, Y.C. (2016). Histopathological Differential Diagnosis of Psoriasis and Seborrheic Dermatitis of the Scalp. Annals of Dermatology. 28(4). 427-432. Available at: https://dx.doi.org/10.5021/ad.2016.28.4.427 ↑20 Polaskey, M.T., Chang, C.H., Daftary, K., Fakhraie, S., Miller, C.H., Chovatiya, R. (2024). The Global Prevalence of Seborrheic Dermatitis: A Systematic Review and Meta-Analysis. JAMA Dermatology. 160(8). 846-855. Available at: https://doi.org/10.1001/jamadermatol.2024.1987 ↑21 Polaskey, M.T., Chang, C.H., Daftary, K., Fakhraie, S., Miller, C.H., Chovatiya, R. (2024). The Global Prevalence of Seborrheic Dermatitis: A Systematic Review and Meta-Analysis. JAMA Dermatology. 160(8). 846-855. Available at: https://doi.org/10.1001/jamadermatol.2024.1987 ↑22 Jang, W.S., Son, I.P., Yeo, K.I., Park, K.Y., Li, K., Kim, B.J., Seo, S.J., Kim, M.N., Hong, C.K. (2013). The Annual Changes of Clinical Manifestation of Androgenetic Alopecia Clinic in Korean Males and Females: A Outpatient-Based Study. Annals of Dermatology. 25(2). 181-188. Available at: https://doi.org/10.5021/ad.2013.25.2.181 ↑23 Faghihkhorasani, A., Sadeghzadeh, A., Goodarzi, A., Rohaninasab, M. (2024). The Relationship between Seborrheic Dermatitis and Androgenetic Alopecia in Patients Referred to a Skin Clinic in Tehran, Iran: A Retrospective Study. Journal of Health Reports and Technology. 10(1). E144076. Available at: https://doi.org/10.5812/jhrt-144076 ↑24 Kim, B.J., Kim, J.Y., Eun, H.C., Kwon, O.S., Kim, M.N. (2006). Androgenetic alopecia in adolescents: A report of 43 cases. The Journal of Dermatology. 33(10). 696-699. Available at: https://doi.org/10.1111/j.1346-8138.20106.00161.x ↑25 Kure, K., Isago, T., Hirayama, T. (2015). Changes in the sebaceous gland in patients with male pattern hair loss (androgenic alopecia). Journal of Cosmetic Dermatology. 14(3). 178-184. Available at: https://doi.org/10.1111/jocd.12153. ↑26 Suzuki, K., Inoue, M., Cho, O., Mizutani, R., Shimizu, Y., Nagahama, T., Sugita, T. (2021). Scalp microbiome and sebum composition in Japanese male individuals with and without androgenetic alopecia. Microorganisms. 9(10). 2132. Available at: https://doi.org/10.3390/microorganisms9102132 ↑27 Mangion, S.E., Mackenzie, L., Roberts, M.S., Holmes, A.M. (2023). Seborrheic dermatitis: topical therapeutics and formulation design. European Journal of Pharmaceutics and Biopharmaceutics. 185. 148-164. Available at: https://doi.org/10.1016/j.ejpb.2023.01.023 ↑28 Arcutis Biotherapeutics. (no date). FDA Approves Arcutis’ ZORYVE (roflumilast) Topical Foam, 0.3% for the Treatment of Seborrheic Dermatitis in Individuals Aged 9 Years and Older. Available at: https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-topical-foam-0-3-for-the-treatment-of-seborrheic-dermatitis-in-individuals-aged-9-years-and-older/ (Accessed: February 2025) ↑29 Zirwas, M.J., Draelos, Z.D., DuBois, J., Kircik, L.H., Moore, A.Y., Gold, L.S., Alonso-Llamazares, J., Bukhalo, M., Bruce, S., Eads, K., Green, L.J., Guenthner, S., Ferris, L.K., Forman, S.B., Kempers, S.E., Lain, E., Lynde, C.W., Pariser, D.M., Toth, D.P., Yamauchi, P.S., Higham, R.C., Krupa, D., Burnett, P., Berk, D.R. (2023). Efficacy of Roflumilast Foam, 0.3%, in Patients with Seborrheic Dermatitis. JAMA Dermatology. 159(6). 613-620. Available at: https://doi.org/10.1001/jamadermatol.2023.0846 ↑30 Tynes, B.E., Johnson, C.D., Vaish, M.H., Abbott, B., Vucenovic, J., Varrassi, G., Potharaju, P., Torres, Y.L., Lee, Z., Ahmadzadeh, S., Shekoohi, S., Kaye, A.D. (2024). Ketoconazole Shampoo for Seborrheic Dermatitis of the Scalpe: A Narrative Review. Cureus. 16(8). E67532. Available at: https://doi.org/10.7759/cureus.67532 ↑31 Borgers, M., Degreed, H. (2007). The Role of Ketoconazole in Seborrheic Dermatitis. Therapeutics for the Clinician. 80. 359-363. Available at: https://cdn.mdedge.com/files/s3fs-public/Document/September-2017/080040359.pdf (Accessed: February 2025) ↑32 Goularte-Silva, V., Paulino, C.L. (2021). Ketoconazole beyond antifungal activity: Bioinformatics-based hypothesis on lipid metabolism in dandruff and seborrheic dermatitis. Experimental Dermatology. 31(5). 821-822. Available at: https://doi.org/10.1111/exd.14505 ↑33 Goularte-Silva, V., Paulino, C.L. (2021). Ketoconazole beyond antifungal activity: Bioinformatics-based hypothesis on lipid metabolism in dandruff and seborrheic dermatitis. Experimental Dermatology. 31(5). 821-822. Available at: https://doi.org/10.1111/exd.14505 ↑34 LOPROX® (cicloporox) Shampoo 1%. (2006). MEDICIS Pharmaceutical Corp. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021159s009lbl.pdf (Accessed: February 2025) ↑35 Godse, G., Godse, K. (2024). Safety, Efficacy and Attributes of 2.5% Selenium Sulfide Shampoo in the Treatment of Dandruff: A Single-Center Study. Cureus. 16(3). E57148. Available at: https://doi.org/10.7759/cureus.57148 ↑36 Davies, D.B., Boorman, G.C., Shuttleworth, D. (1999). Comparative efficacy of shampoos containing coal tar (4.0% w/w; Tarmed), coal tar (4.0% w/w) plus ciclopirox olamine (1.0% w/w Tarmed AF), and ketoconazole (2.0% w/w Nizoral) for the treatment of dandruff/seborrheic dermatitis. Journal of Dermatological Treatment. 10(3). 177-183. Available at: https://doi.org/10.3109/09546639909056025 ↑37, ↑39, ↑49 Ge, L., Liu, Z., Xu, S., Li, C., Jin, M., Luo, Y., Kong, Y., Meng, J., Zheng, G., Gao, J., Wang, P., Bai, W., Na, H., Zhou, X., Jin, Z., Pi, L. (2025). A Cohort Clinical Study on the Efficacy of Topical Salicylic Acid/Piroctone Olamine Dandruff Pre-Gel and Cleanser in Improving Symptoms of Moderate to Severe Seborrheic Dermatitis of the Scalp. Journal of Cosmetic Dermatology. 24(1). E16742. Available at: https://doi.org/10.1111/jocd.16742 ↑38 Marks, R., Pearse, A.D., Walker, A.P. (1985). The effects of a shampoo containing zinc pyrithione on the control of dandruff. British Journal of Dermatology. 112(4). 415-422. Available at: https://doi.org/10.1111/j.1365-2133.1985.tb02314.x. ↑40 Paula Ludmann. (2024). Seborrheic Dermatitis: Diagnosis and Treatment. American Academy of Dermatology Association. Available at: https://www.aad.org/public/diseases/a-z/seborrheic-dermatitis-treatment (Accessed: February 2025) ↑41 Dall’Oglio, F., Nasca, M.R., Gerbino, C., Micali, G. (2022). An Overview of the Diagnosis and Management of Seborrheic Dermatitis. Clinical, Cosmetic and Investigational Dermatology. 6(15). 1537-1548. Available from: https://doi.org/10.2147/CCID.S284671 ↑42 Alshaebi, M., Zahed, L., Osalyan, M., Sulaimani, S., Albahlool, A., Abduljabbar, M.H., Hariri, J. (2023). Association Between Diet and Seborrheic Dermatitis: A Case-Control Study. Cureus. 15(11). E48782. Available at: https://doi.org/10.7759/cureus.48782 ↑43 Woolhiser, E., Keime, N., Patel, A., Weber, I., Adelman, M., Dellavalle, R.P. (2024). Nutrition, Obesity, and Seborrheic Dermatitis: Systematic Review. JMIR Dermatology. E50143. Available at: https://doi.org/10.2196/50143 ↑44 Paula Ludmann. (2024). Seborrheic Dermatitis: Diagnosis and Treatment. American Academy of Dermatology Association. Available at: https://www.aad.org/public/diseases/a-z/seborrheic-dermatitis-treatment (Accessed: February 2025) ↑45 Zhang, F., Li, Y-H., Ren, W., Li, S-R., Chen, Y-C. (2023). Clinical efficacy of a combination treatment of traditional Chinese medicine for scalp seborrheic dermatitis. Journal of Cosmetic Dermatology. 22(11). 3072-3077. Available at: https://doi.org/10.1111/jocd.15818 ↑46 Goldenberg, G. (2013). Optimizing Treatment Approaches in Seborrheic Dermatitis. Journal of Clinical and Aesthetic Dermatology. 6(2). 44-49. Available at: PMID: 23441240 ↑47 Jaalouk, D., Pulumati, A., Algarin, Y.A., Kircik, L., Issa, N.T. (2024). Dermatologic Procedures for the Treatment of Seborrheic Dermatitis. Journal of Drugs in Dermatology. 23(10). 819-824. Available at: https://doi.org/10.36849/JDD.2024.8116 ↑48 Ortonne, J-P., Nikkels, A.F., Reich, K., Oliver, R.M.P., Lee., J.H., Kerrouche, N., Sidou, F., Faergemann, J. (2011). Efficacious and safe management of moderate to severe scalp seborrheic dermatitis using clobetasol propionate shampoo 0.05% combined with ketoconazole shampoo 2%: a randomized controlled study. British Journal of Dermatology. 165(1). 171-176. Available at: https://doi.org/10.1111/j.1365-2133.2011.10269.x. ↑50 University of Utah Health. (no date). Seborrheic Dermatitis. Available at: https://healthcare.utah.edu/dermatology/conditions/seborrheic-dermatitis (Accessed: February 2025) ↑51 Cleveland Clinic. (2020). Seborrheic Dermatitis. Cleveland Clinic. Available at: https://my.clevelandclinic.org/health/diseases/14403-seborrheic-dermatitis (Accessed: February 2025) ↑52 National Eczema Association. (2025). Seborrheic Dermatitis. National Eczema Association. Available at: https://nationaleczema.org/eczema/types-of-eczema/seborrheic-dermatitis/#h-management-and-treatment (Accessed: February 2025) While the medical literature clearly advises women to avoid finasteride before conception during pregnancy, and while breastfeeding, the guidance for men is less definitive. The question of whether men can safely continue using finasteride during the conception period remains a topic of debate among healthcare professionals.
However, a recent large dataset statistical analysis has sent ripples through the hair loss space and regulatory world, as it appears to show further evidence that the use of finasteride in men leads to a congenital anomaly called “cryptorchidism” (undescended testicles). But what does the data actually say? And can we link statistical association to causality? In this article, we will take a deep dive into what the paper shows (or doesn’t show) and whether we think it is a cause of major concern for men.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Let’s first take a look at what the study is all about.
The Study
We’ll begin by summarizing the data from the study and then go into detail about what it all means, how the statistics are analyzed, and whether this is a result to be concerned about.
The researchers analyzed data from the FDA Adverse Event Reporting System (FAERS) from 2010 to 2022 to assess potential safety concerns related to paternal drug exposure on fertility, pregnancy outcomes, and offspring health.[1]Zeng, Y., Lin, W., Zhuang, W. (2024). Safety concerns of paternal drug exposure on fertility, pregnancy, and offspring: an analysis based on the FDA adverse event reporting system. Andrology. 1-12. … Continue reading The FAERS is a computerized database that supports the FDA’s post-marketing safety surveillance program for approved drugs and therapeutic biologic products.[2]US Food and Drug Administration. (no date). FDA Adverse Events Reporting System (FAERS) Public Dashboard. US FDA. Available at: … Continue reading It collects and stores information on adverse events, medication errors, and product quality complaints that may be associated with FDA-approved products.
Reporting to FAERS can be done in two ways:
- Mandatory reporting: Manufacturers are required by law to report adverse events to FAERS within 14 days of becoming aware of them.
- Voluntary reporting: Healthcare professionals (such as physicians, pharmacists, and nurses) and consumers (including patients, family members, and lawyers) can voluntarily submit reports.
Importantly, anyone can submit to FAERS directly; it does not need to be done by a doctor. The FDA provides multiple options for voluntary reporting:
- Online submission through the FDA’s Electronic Submission Gateway (ESG) or Safety Reporting Portal (SRP).
- Using FDA Form 3500, which can be submitted electronically or by mail.[3]US Food and Drug Administration (2024). Reporting Serious Problems to FDA. MedWatch. Available at: … Continue reading
What Were The Study Results?
The researchers conducted a disproportionality analysis, specifically the Reporting Odds Ratio (ROR), to identify drugs disproportionately associated with reproductive-related adverse events.
The study analyzed 16,180,533 total reports; 3,210 cases related to paternal drug exposure were identified, with 7,808 associated adverse events (e.g., spontaneous abortions and small babies). The study found that drugs used to treat rheumatoid arthritis, cancer, infections, and psychotropic conditions were the most frequently implicated.
However, one of the strongest links between treatment and adverse health events was between finasteride and cryptorchidism, with an ROR of 891.7 based on 11 reports. This suggests that cryptorchidism was reported for finasteride-exposed fathers at a much higher rate than for most other drugs in the database.
Figure 1: A graph showing the ROR of different drugs and their adverse event pairs. Finasteride and cryptorchidism appear to have a noticeably larger ROR than others.[4]Zeng, Y., Lin, W., Zhuang, W. (2024). Safety concerns of paternal drug exposure on fertility, pregnancy, and offspring: an analysis based on the FDA adverse event reporting system. Andrology. 1-12. … Continue reading
Understanding the Data
Before we go into what the data means, let’s explain some key concepts that are important to the study.
What is a Disproportionality Analysis?
A disproportionality analysis is a tool used in drug safety monitoring. It analyzes large databases of reported side effects from various medications and looks for unusual patterns – like if a particular side effect is reported much more often with one drug than others.[5]Fusaroli, M., Salvo, F., Begaud, B., AlShammari, T.M, Bate, A., Battini, V., Brueckner, A., Candore, G., Carnovale, C., Crisafulli, S., Cutroneo, P.M., Dolladille, C., Drici, M.D., Faillie, J.L., … Continue reading
Imagine a concert where five people in the front row get food poisoning after eating hotdogs from a nearby stall, while only one person in the entire back section reports feeling ill. You might suspect that the front-row hot dogs are bad—but this doesn’t prove the food stand actually caused the sickness. Maybe those five people already had food poisoning before coming. Disproportionality analysis works in a similar way: it flags patterns, i.e., five people sick in the front row, but it doesn’t prove causation.
What is the Reporting Odds Ratio (ROR)?
The ROR is a key statistical measure used in disproportionality analyses. It compares how often a specific side effect is reported for a particular drug versus all other drugs.[6]Rothman, K.J., Lanes, S., Sacks, S.T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiology and Drug Safety. 13(8). 519-523. Available at: … Continue reading If the ROR is high, it means the side effects are being reported more often for that drug than you’d normally expect.
Think about flipping a coin 10 times, and imagine it lands on “heads” 7 times. You might think the coin is biased. But if you had flipped it 1,000 times, the results might have evened out to 50/50. The Reporting Odds Ratio (ROR) works similarly: when there are only a few cases, even small variations make the number seem exaggerated. A few extra reports in a small dataset can make an effect look much larger than it actually is.
Unlike incidence, which measures new cases over time, or prevalence, which measures total cases at a given time, ROR does not provide information about the actual risk of an adverse event occurring. Instead, it indicates whether there’s a disproportionate association between a drug and an adverse event in the reporting database.
The important point to remember is that ROR is primarily a hypothesis-generating tool, helping prioritize which drug-event combinations may need further investigation.
What Do The Study Results Mean?
The ROR calculation was based on reports from the FAERS, which, as we mentioned above, is a system for self-reporting adverse events that anyone can report to. This means that more severe or unusual side effects are more likely to be reported, and as such, FAERS is at risk of reporting bias. This was acknowledged as a limitation in the study.
Furthermore, unlike controlled clinical studies, FAERS does not track how many people take a drug; it only tracks how many people report an issue.
To calculate the true risk of cryptorchidism from finasteride, you need two numbers:
- The number of reported cases (numerator) – FAERS provides this (11 cases).
- The total number of fathers who took finasteride (denominator) – FAERS does not provide this.
Since FAERS does not track drug exposure rates, it is impossible to calculate the incidence or prevalence of an event. This means we can’t determine what percentage of fathers taking finasteride had children with cryptorchidism or whether this percentage is actually higher than background rates in the general population.
Think about it this way: a phone company gets 100 customer complaints in a month. Without knowing how many total customers they have, you can’t tell if that’s a serious problem. If they have 200 customers, that’s bad (50% complaint rate). However, if they have 2 million customers, it’s insignificant (0.005% complaint rate). FAERS has the same issue—it counts events but doesn’t count the total number of people taking the drug.
Furthermore, the disproportionality analysis does not compare finasteride to a control group. Instead, it compares the number of cryptorchidism reports for finasteride (11) to the number of cryptorchidism reports for all other drugs (likely much lower because most drugs do not affect male hormone pathways).
This is problematic for several reasons:
1. This is Not a Matched Control Population
-
- Fathers who took finasteride should be compared to fathers who did not take finasteride while keeping other factors (like age, lifestyle, genetics) constant.
- FAERS instead compares finasteride to all other drugs – but as we just mentioned, most drugs don’t affect male reproductive hormones, making the comparison flawed.
2. Selective Reporting Bias in FAERS
-
- Finasteride users are already aware of post-finasteride syndrome (PFS) and reproductive side effect concerns.
- If a father taking finasteride has a child with any birth defect, they may be more likely to report it than a father taking, say, an antibiotic or a painkiller.
- This could artificially inflate the number of finasteride-related reports, making cryptorchidism appear more common than it is.
3. The Numerator is Small, But the ROR is Huge:
-
- FAERS recorded only 11 cryptorchidism cases for finasteride from 2010 – 2022.
- Cryptorchidism naturally occurs in 2-3% of all full-term male births.[7]Leslie, S.W., Sajjad, H., Villanueva, C.A. (2024). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470270/ … Continue reading
This creates a statistical illusion. If very few cryptorchidism cases are reported for other drugs, the ROR for finasteride skyrockets, even if the actual risk is low.
People fear shark attacks because they’re widely reported in the news, but statistical analyses have shown that the odds of dying from a falling vending machine are higher than being killed by a shark.[8]United States Consumer Product Safety Commission. (1995). CPSC, Soda Vending Machine Industry Labeling Campaign Warns of Deaths and Injuries. US CPSC. Available at: … Continue reading The reason we think sharks are deadlier is that their attacks make headlines while vending machine accidents don’t. Similarly, if only one case of cryptorchidism is reported for another drug, but 11 cases are reported for finasteride, the ROR can look massive—even if the actual risk is tiny.
In 2022, about 2,626,865 men in the United States were estimated to have taken finasteride.[9]ClinCalc. (no date). Finasteride Drug Usage Statistics, United States, 2013-2022. ClinCalc. Available at: https://clincalc.com/drugstats/Drugs/Finasteride (Accessed: January 2025) Even if all 11 reports of cryptorchidism were from distinct individuals and occurred solely in 2022 (instead of over 12 years), this would equate to an incidence rate of 0.00042% (or 4.2 per million users). This is notably below the background risk of cryptorchidism in the general population (2-3%), suggesting that the observed reports in FAERS are likely to be an artifact of a small sample size rather than evidence of a causal relationship.
So, to summarize, this study, while showing a statistical association between men taking finasteride and their children having cryptorchidism, does not show any causal relationship – meaning that it doesn’t actually show that finasteride causes this congenital anomaly.
Why Is This Study Even A Discussion?
Finasteride’s potential impact on reproductive health has been debated for years, largely due to animal studies and regulatory warnings for women. While the FAERS data discussed above suggest a statistical association between finasteride use in men and cryptorchidism in offspring, the underlying question is whether this link is biologically plausible or supported by stronger evidence.
Finasteride is classified as an FDA Pregnancy Category X drug, meaning that it is strictly contraindicated in pregnant women due to teratogenic effects observed in animal studies.[10]Merck & Co. Inc. (no date). Propecia Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf (Accessed: January 2025) These concerns stem from its mechanism of action: blocking the conversion of testosterone into dihydrotestosterone (DHT) by inhibiting the 5ɑ-reductase enzyme. Since DHT is crucial for normal male fetal development, any disruption during pregnancy can result in genital abnormalities.[11]Bormann, C.L., Smith, G.D., Padmanabhan, V., Lee, T.M. (2011). Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reproduction 142(1). Available at: … Continue reading
Studies in rodent and primate models have shown that exposure to high doses of finasteride during pregnancy can lead to:
- Hypospadias – a condition where the urethral opening is misplaced on the underside of the penis instead of at the tip.[12]An, N., Peng, J., He, G., Fan, X., Li, F., Chen, H. (2018). Involvement of activation of mitogen-activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) signaling pathway in … Continue reading
- Cryptorchidism – as mentioned above, is an anomaly in which one or both testicles fail to descend into the scrotum during fetal development, a condition later associated with fertility issues and an increased risk of testicular cancer.
- Reduced anogenital distance (AGD) – a key marker of disrupted androgen signaling during development and is often used as a biomarker for endocrine disruption.[13]Clark, R.L., Anderson, C.A., Prahalada, S., Robertson, R.T., Lochry, E.A., Leonard, Y.M., Stevens, J.L., Hoberman, A.M. (1993). Critical developmental periods for effects on male rat genitalia … Continue reading
One study in rhesus monkeys found that continuous finasteride exposure throughout gestation resulted in severe genital malformations in male offspring.[14]Prahalada, S., Tarantal, A.F., Harris, G.H., Ellsworth, K.P., Clarke, A.P., Skiles, G.L., MacKenzie, K.I., Kruk, L.F., Ablin, D.S., Cukierski, M.A., Peter, C.P., vanZwieten, M.J., Hendrickx, A.G. … Continue reading This was particularly concerning because monkeys have hormonal systems that more closely resemble humans compared to rodents. Due to these findings, the FDA issued strong warnings that pregnant women should never ingest or even handle crushed finasteride tablets, as skin absorption could theoretically lead to fatal exposure.
For more information, see our article diving into the science behind finasteride & conception, and our article about how to use finasteride and minimize its exposure to your partner.
Can These Findings in Animals Be Directly Applied to Humans?
Although these studies raise legitimate concerns, there are important reasons why their findings may not directly apply to human males taking finasteride. For example, the doses used in animal studies are often much higher than those used in human treatment. The studies also involve direct maternal exposure during pregnancy, whereas paternal exposure (via sperm) results in much lower fetal exposure (we’ll discuss this further below). Furthermore, while rodents and monkeys have similar endocrine systems, their sensitivity to 5ɑ-reductase inhibition differs from that of humans.
Therefore, while animal models provide a biological basis for concern, they do not conclusively prove that paternal finasteride exposure affects offspring in humans.
Is It Biologically Plausible That Finasteride Could Cause Issues in Men?
To determine whether paternal finasteride use could biologically contribute to congenital anomalies in humans, we need to look at:
- How much finasteride reaches semen and whether these lead to enough exposure in females to impact fetal development.
- Whether finasteride alters sperm in a way that could lead to birth defects.
How Much Finasteride Reaches Semen?
One way finasteride could hypothetically impact fetal development is through semen exposure during conception or early pregnancy. However, semen levels of finasteride are extremely low.
Clinical data from GlaxoSmithKline (internal studies) measured finasteride concentrations in the semen of men taking a 50 mg dose (which is 10 x higher than the standard dose for hair loss). The result? Only 0.26 ng/mL of finasteride was detected in semen.[15]US Food and Drug Administration. (no date). Propecia (finasteride). US FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020788s017lbl.pdf (Accessed: January 2025) At the standard 1 mg dose used for hair loss, semen concentrations would likely be even lower.
How does this compare to doses known to cause fetal harm?
In the rhesus monkey studies, oral doses of finasteride given directly to pregnant females (at levels over 5,000 x higher than those found in human semen) caused genital abnormalities in male offspring. Therefore, the amount of finasteride that pregnant human women might absorb from semen exposure is likely so low that it falls below the threshold needed to affect fetal development.
Conclusion: There is no compelling evidence to suggest that the trace amounts of finasteride in semen are enough to cause congenital defects in humans.
Can Finasteride Affect Sperm Quality or DNA?
Another possible concern is whether finasteride negatively affects sperm itself, leading to:
- Reduced fertility.
- DNA damage that could increase congenital anomaly risk.
Some studies suggest finasteride can reduce sperm concentration and motility at higher doses (5 mg for prostate treatment), but these effects are reversible after stopping the medication, and studies in men taking the 1 mg dose for hair loss do not show significant sperm abnormalities.
For example, a 2013 study by Samplaski et al. found that men taking an average of 1.04 mg/day of finasteride experienced an 11.6-fold increase in sperm count after stopping the drug, with no participants experiencing a decrease in sperm count post-discontinuation.[16]Samplaski, M.K., Lo, K., Grober, E., Jarvi, K. (2013). Finasteride use in the male infertility population: effects on semen and hormone parameters. Fertility and Sterility. 100(6). 1542-1546. … Continue reading
Similarly, a 1999 study found that 1 mg/day of finasteride did not significantly affect spermatogenesis or semen production in healthy men.[17]Overstreet, J.W., Fuh, V.L., Gould, J., Howards, S.S., Lieber, M.M., Hellstrom, W., Shapiro, S., Carroll, P., Corfman, R.S., Petrous, S., Lewis, R., Toth, P., Shown, T., Roy, J., Jarow, J.P., … Continue reading
A 2007 study observed that taking 5 mg/day of finasteride exhibited mild reductions in semen volume, sperm concentration, and motility, though these parameters returned just below normal after stopping.[18]J.K., Amory., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J., Clark, R.V. (2007). The effect of 5alpha-reductase inhibition with dutasteride and … Continue reading
Figure 2: Percent changes in sperm count, semen volume, sperm concentration, motility, and morphology at weeks 26 and 52 of treatment with finasteride or dutasteride and at the follow-up after stopping.[19]J.K., Amory., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J., Clark, R.V. (2007). The effect of 5alpha-reductase inhibition with dutasteride and … Continue reading
Could Finasteride Cause Epigenetic Changes to Sperm?
Some scientists have speculated that finasteride could cause epigenetic modifications to sperm, meaning it might alter gene expression without changing the DNA sequence. However, no studies have directly confirmed this in humans. Animal studies suggest some potential for altered gene expression, but these findings haven’t been replicated in human sperm research.[20]Kolasa, A., Roginska, D., Rzeszotek, S., Machalinski, B., Wiszniewska, B. (2021). Paternal finasteride treatment can influence the testicular transcriptome profile of male offspring – … Continue reading
In 2011 and 2012, two separate case reports documented men struggling with fertility issues after long-term finasteride use. While their sperm morphology appeared normal, both had elevated sperm DNA fragmentation-a marker of DNA damage. After stopping finasteride, one saw fragmentation drop from 30% to 16.5% within six months, while the other improved and successfully conceived.[21]Salvarci, A., Istanbulluoglu, O. (2012). Secondary infertility due to use of low-dose finasteride. International urology and nephrology. 45(1). 83-85. Available at: … Continue reading,[22]Tu, H.Y.V., Zini, A. (2011). Finasteride-induced secondary infertility associated with sperm DNA damage. Fertility and sterility. 95(6). e13-4. Available at: … Continue reading
These reports suggest finasteride may contribute to sperm DNA damage, but since they lack controls, other lifestyle changes such as diet, exercise, or better sleep could also explain the improvements. You can read more about our thoughts on these case studies here.
The bottom line is that:
- Finasteride can mildly impact sperm quality, but this effect is reversible and not linked to congenital anomalies.
- No strong evidence exists that finasteride causes long-term DNA changes that could harm offspring.
So, is it biologically plausible that finasteride causes birth defects when taken by men? We think the current evidence for paternal risk is weak.
Is There Any Other Evidence That Finasteride Causes Congenital Anomalies?
To date, no large-scale, well-controlled studies have confirmed an increased risk of congenital anomalies from paternal finasteride use.
Danish Nationwide Study (2017)
Study Type: A large-scale epidemiological study using data from national birth registries.[23]Anderson, J.T., Jenson, T.B., Horwitz, H., Clausen, S.S. (2019). Paternal exposure to finasteride – Before and during pregnancy. Pharmacoepidemiology and Drug Safety. 28(16). Conference … Continue reading
Findings: No increased risk of congenital anomalies in children of men who had taken finasteride before conception. The risk of miscarriage was also not elevated.
Conclusion: No strong evidence linking paternal finasteride use to birth defects.
Case Reports and Small Studies
Some isolated case reports have suggested a potential association between paternal finasteride use and reproductive issues in offspring.[24]Ahn, K.H., Shin, J., Hong, S.C., Han, JY., Lee, E.H., Lee, J.S., Oh, M.J., Kim, H.J. (2015). Pregnancy Outcomes with Paternal Exposure to Finasteride, a Synthetic 5-Alpha-Reductase Inhibitor: A Case … Continue reading However, these reports often lack proper controls and fail to rule out other factors such as genetics, environmental exposures, or maternal health conditions.
Final Thoughts
While the study we have discussed in this article highlights a statistical association between paternal finasteride exposure and cryptorchidism, it does not establish causation. The limitations of FAERS data, including reporting bias and the absence of a control population, make it difficult to draw firm conclusions. Additionally, biological plausibility remains weak, given the extremely low levels of finasteride in semen and the lack of strong evidence linking paternal exposure to congenital anomalies. Ultimately, while more research is warranted, current data does not suggest a major cause for concern. Men using finasteride should, however, discuss any reproductive concerns with their healthcare provider before making any decisions.
References[+]
References ↑1 Zeng, Y., Lin, W., Zhuang, W. (2024). Safety concerns of paternal drug exposure on fertility, pregnancy, and offspring: an analysis based on the FDA adverse event reporting system. Andrology. 1-12. Advance online publication. Available at: https://doi.org/10.1111/andr.13790 ↑2 US Food and Drug Administration. (no date). FDA Adverse Events Reporting System (FAERS) Public Dashboard. US FDA. Available at: https://fis.fda.gov/extensions/FPD-FAQ/FPD-FAQ.html#:~:text=Healthcare%20professionals%2C%20consumers%2C%20and%20manufacturers,members%2C%20lawyers%20and%20others). (Accessed: January 2025) ↑3 US Food and Drug Administration (2024). Reporting Serious Problems to FDA. MedWatch. Available at: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program/reporting-serious-problems-fda (Accessed: January 2025) ↑4 Zeng, Y., Lin, W., Zhuang, W. (2024). Safety concerns of paternal drug exposure on fertility, pregnancy, and offspring: an analysis based on the FDA adverse event reporting system. Andrology. 1-12. Advance online publication. Available at: https://doi.org/10.1111/andr.13790 ↑5 Fusaroli, M., Salvo, F., Begaud, B., AlShammari, T.M, Bate, A., Battini, V., Brueckner, A., Candore, G., Carnovale, C., Crisafulli, S., Cutroneo, P.M., Dolladille, C., Drici, M.D., Faillie, J.L., Goldman, A., Hauben, M., Herdeiro, M.T., Mahaux, O., Manlik, K., Montastruc, F., Noguchi, Y., Noren, G.N., Noseda, R., Onakpoya, I.J., Pariente, A., Poluzzi, E., Salem, M., Sartori, D., Trinh, N.T.H., Tuccori, M., van Hunsel, F., van Puijenbroek., E, Raschi, E., Jhouri, C. (2024). The Reporting of Disproportionality Analysis for Drug Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Development and Statement. Drug Safety. 47(6). 575-584. Available at: https://doi.org/10.1007/s40264-024-01421-9 ↑6 Rothman, K.J., Lanes, S., Sacks, S.T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiology and Drug Safety. 13(8). 519-523. Available at: https://doi.org/10.1002/pds.1001 ↑7 Leslie, S.W., Sajjad, H., Villanueva, C.A. (2024). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470270/ (Accessed: January 2025) ↑8 United States Consumer Product Safety Commission. (1995). CPSC, Soda Vending Machine Industry Labeling Campaign Warns of Deaths and Injuries. US CPSC. Available at: https://www.cpsc.gov/Newsroom/News-Releases/1996/CPSC-Soda-Vending-Machine-Industry-Labeling-Campaign-Warns-Of-Deaths-And-Injuries (Accessed: January 2025) ↑9 ClinCalc. (no date). Finasteride Drug Usage Statistics, United States, 2013-2022. ClinCalc. Available at: https://clincalc.com/drugstats/Drugs/Finasteride (Accessed: January 2025) ↑10 Merck & Co. Inc. (no date). Propecia Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf (Accessed: January 2025) ↑11 Bormann, C.L., Smith, G.D., Padmanabhan, V., Lee, T.M. (2011). Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reproduction 142(1). Available at: https://doi.org/10.1530/REP-10-0210 ↑12 An, N., Peng, J., He, G., Fan, X., Li, F., Chen, H. (2018). Involvement of activation of mitogen-activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) signaling pathway in proliferation of urethral plate fibroblasts in finasteride-induced rat hypospadias. Medical Science Monitor. 24. 8984-8992. Available at: https://doi.org/10.12659/MSM.911271 ↑13 Clark, R.L., Anderson, C.A., Prahalada, S., Robertson, R.T., Lochry, E.A., Leonard, Y.M., Stevens, J.L., Hoberman, A.M. (1993). Critical developmental periods for effects on male rat genitalia induced by finasteride, a 5 alpha-reductase inhibitor. Toxicology and Applied Pharmacology. 119(1). 34-40. Available at: https://doi.org/10.1006/taap.1993.1041 ↑14 Prahalada, S., Tarantal, A.F., Harris, G.H., Ellsworth, K.P., Clarke, A.P., Skiles, G.L., MacKenzie, K.I., Kruk, L.F., Ablin, D.S., Cukierski, M.A., Peter, C.P., vanZwieten, M.J., Hendrickx, A.G. (1997). Effects of finasteride, a type 2 5-alpha reductase inhibitor, on fetal development in the rhesus monkey (Macaca mulatta) 55(2). 119-131. Available at: 10.1002/(SICI)1096-9926(199702)55:2<119::AID-TERA1>3.0.CO;2-Z. ↑15 US Food and Drug Administration. (no date). Propecia (finasteride). US FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020788s017lbl.pdf (Accessed: January 2025) ↑16 Samplaski, M.K., Lo, K., Grober, E., Jarvi, K. (2013). Finasteride use in the male infertility population: effects on semen and hormone parameters. Fertility and Sterility. 100(6). 1542-1546. Available at: https://doi.org/10.1016/j.fertnstert.2013.07.2000 ↑17 Overstreet, J.W., Fuh, V.L., Gould, J., Howards, S.S., Lieber, M.M., Hellstrom, W., Shapiro, S., Carroll, P., Corfman, R.S., Petrous, S., Lewis, R., Toth, P., Shown, T., Roy, J., Jarow, J.P., Bonilla, J., Jacobsen, C.A., Wang, D.Z. Kaufman, K.D. (1999). Chronic treatment with finasteride daily does not affect spermatogenesis or semen production in young men. Journal of Urology. 162(4). 11295-300. Available at: PMID: 10492183 ↑18 J.K., Amory., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J., Clark, R.V. (2007). The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. The Journal of Clinical Endocrinology & Metabolism. 92(5). 1659-1665. Available at: https://doi.org/10.1210/jc.2006-2203 ↑19 J.K., Amory., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J., Clark, R.V. (2007). The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. The Journal of Clinical Endocrinology & Metabolism. 92(5). 1659-1665. Available at: https://doi.org/10.1210/jc.2006-2203 ↑20 Kolasa, A., Roginska, D., Rzeszotek, S., Machalinski, B., Wiszniewska, B. (2021). Paternal finasteride treatment can influence the testicular transcriptome profile of male offspring – preliminary study. Current issues in molecular biology. 43(2). 868-886. Available at: https://doi.org/10.3390/cimb43020062 ↑21 Salvarci, A., Istanbulluoglu, O. (2012). Secondary infertility due to use of low-dose finasteride. International urology and nephrology. 45(1). 83-85. Available at: https://doi.org/10.1007/s11255-012-0315-9 ↑22 Tu, H.Y.V., Zini, A. (2011). Finasteride-induced secondary infertility associated with sperm DNA damage. Fertility and sterility. 95(6). e13-4. Available at: https://doi.org/10.1016/j.fertnstert.2010.12.061 ↑23 Anderson, J.T., Jenson, T.B., Horwitz, H., Clausen, S.S. (2019). Paternal exposure to finasteride – Before and during pregnancy. Pharmacoepidemiology and Drug Safety. 28(16). Conference Abstract. Available at: https://doi.org/10.1002/pds.4864 ↑24 Ahn, K.H., Shin, J., Hong, S.C., Han, JY., Lee, E.H., Lee, J.S., Oh, M.J., Kim, H.J. (2015). Pregnancy Outcomes with Paternal Exposure to Finasteride, a Synthetic 5-Alpha-Reductase Inhibitor: A Case Series. Journal of Clinical Toxicology. 5(2). Available at: https://doi.org/https://doi.org/10.1002/pds.4864 Imagine if your genes could predict how much hair you’ll regrow from minoxidil… or whether you could get side effects from finasteride. This would be a breakthrough in hair loss treatments. And yet, according to some genetic testing companies, this breakthrough is already here and available (for a price).
Certain telehealth companies now offer to sequence your DNA to help you create a more tailored, personalized treatment plan for your hair loss.[1]TrichoTest. (no date). Personalizing alopecia treatment. Fagron Genomics. Available at: https://gxsciences.com/trichotest/. (Accessed: October 2024) Depending on your genetics, they might recommend substituting finasteride for dutasteride, oral over topical minoxidil, or adding corticosteroids – all based on the latest scientific data.
Who wouldn’t want to do this? It would enable you to create the highest-value and lowest-risk treatment plan for yourself.
However, the quality of the evidence surrounding this approach isn’t so cut and dry. That’s why we conducted a four-month research project in which we identified 12 genes that some companies say are associated with treatment efficacy. We looked at the available literature to determine what the science says, the quality behind it, and whether it is even relevant to hair loss.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Single Nucleotide Polymorphisms (SNPs)
SNPs are changes at a single position in a DNA sequence. They can occur within genes or in the regions between genes. SNPs are the most common type of genetic variation in humans, occurring once in every 100-300 nucleotides in the human genome.[2]Nelson, M.R., Marnellos, G., Kammerer, S., Hoyal, C.R., Shi, M.M., Cantor, C.R., Braun, A. (2004). Large-scale validation of single nucleotide polymorphisms in gene regions. Genome Research. … Continue reading
In coding regions of genes, SNPs can be:
- Synonymous, i.e., they do not affect the protein sequence
- Nonsynonymous, i.e., they change the protein’s amino acid sequence [3]Sun, Y., Zhang, Y., Zhang, X. (2019). Synonymous SNPs of viral genes facilitate virus to escape host antiviral RNAi immunity. RNA Biology. 16(12). 1697-1710. Available at: … Continue reading
SNPs in non-coding regions of genes can affect gene expression, gene splicing, or other regulatory processes.[4]Degtyareva, A.O., Antontseva, E.V., Merkulova, T.I. (2021). Regulatory SNPs: Altered Transcription Factor Binding Sites Implicated in Complex Traits and Diseases. International Journal of Molecular … Continue reading Like genes, SNPs are inherited from parents to their children and contribute to genetic differences between people.
SNPs in certain genes may increase susceptibility to specific diseases. For example, a large-scale genome-wide association study identified 71 significantly associated loci for male pattern baldness, indicating a genetic aspect to the pathogenesis of androgenic alopecia.[5]Pirastu, N., Joshi, P.K., deVries, P.S., Cornerlis, M.C., McKeigue, P.M., Keum, N., Franceschini, N., Colombo, M., Giovannucci, E.L., Spiliopoulou, A., Franke, L., North, K.E., Kraft, Morrison, A.C., … Continue reading
So, now that we’ve discussed genes and SNPs, let’s examine what we found.
We’ve created a table of every gene we analyzed to give you an overview of the articles. Below, we will summarize each gene and its potential for advising treatment efficacy.
Gene SNP What the Genetic Testing Companies Say What the Evidence Says Treatment Relevance (1-5) ACE rs4341 People with a deletion variant of this polymorphism (CG or GG) may want to try treatments that improve blood flow to the scalp. The deletion allele (G) is associated with increased ACE activity, which leads to vasoconstriction and reduced blood flow. No studies show an association between this polymorphism and the response to hair treatments that may improve blood flow.
1 rs4343 People with a deletion variant of this polymorphism (AG or GG) may want to try treatments that improve blood flow to the scalp. The deletion allele (G) is associated with increased ACE activity, which leads to vasoconstriction and reduced blood flow. No studies show an association between this polymorphism and the response to hair treatments that may improve blood flow.
BTD rs13078881 People with the CC or CG variants may be good candidates for biotin supplementation. The CC and CG genotypes were associated with biotinidase deficiency in a study of 19 children. Adults with this polymorphism exhibited biotinidase deficiency but presented no symptoms.
No studies show an association between this polymorphism and hair loss or the response to biotin supplementation.
1 COL1A1 rs1800012 People with the GT variant may benefit from supplementation that supports collagen formation. People with the GT variant were found to have an increased ratio of α1 to α2 chains, which could lead to instability of the collagen molecules. No studies show an association between this polymorphism and the response to supplements that support collagen formation.
1 CRABP2 rs12724719 People with the AA variant may not benefit from standard retinoic acid supplementation and may require an increased dose or alternative treatment. Newborn babies with the AA variant were found to have increased retinoic acid levels in their umbilical cord blood. It is not known if the same effect is seen in adults. No studies show an association between this polymorphism and the response to retinoic acid supplementation.
1 CYP19A1 rs2470152 People with the TC variant may benefit from treatment with replacement hormones (e.g., estradiol) or anti-androgens. People with the TC variant were found to exhibit increased testosterone levels and a reduced ratio of estradiol to testosterone. No studies show an association between this polymorphism and hair loss or the response to replacement hormones and anti-androgens.
1 rs700519 People with the CC variant may be a good candidate for the typical or higher dosages of 5α-reductase inhibitors. People with the CT or TT variants may be good candidates for lower dosages of 5α-reductase inhibitors.
People with the CT or TT variant were shown to respond better to treatment with dutasteride. However, some people with CT or TT were still classified as being poor responders to dutasteride treatment. GPR44 rs533116 People with the AA variant may be good candidates for treatment with PGD2 inhibitors. People with the AA variant exhibited increased GPR44 expression in their white blood cells, which may increase sensitivity to PGD2. No studies show an association between this polymorphism and the response to treatment with PGD2 inhibitors.
1 rs545659 People with the GG variant may be good candidates for treatment with PGD2 inhibitors. People with the GG variants exhibited greater GPR44 mRNA stability, which may increase PGD2 activity. No studies show an association between this polymorphism and the response to treatment with PGD2 inhibitors.
GRα/GRꞵ (NR3C1) rs6198 People with the GG variant may not respond as well to glucocorticoid treatment. People with the GG variant exhibited a form of the glucocorticoid receptor that does not bind as well to glucocorticoids. No studies show an association between this polymorphism and the response to glucocorticoid treatment for hair loss.
1 IGF1R rs2229765 People carrying at least one A allele may be good candidates for IGF-1 supplementation. People with the AG or AA genotype were found to exhibit lower levels of IGF-1 in their plasma. No studies show an association between this polymorphism and the response to supplementation with IGF-1.
1 PGTFR rs10782665 People carrying at least one T allele have an increased probability of a positive response to treatment with Latanoprost. The presence of a G allele was found to increase the probability of a positive response to Latanoprost treatment for intraocular pressure in mice. No studies show an association between this polymorphism and hair loss response to treatment with Latanoprost.
2 rs1328441 People carrying at least one G allele have an increased probability of a positive response to treatment with Latanoprost. The presence of a G allele was found to increase the probability of a positive response to Latanoprost treatment for intraocular pressure in mice. No studies show an association between this polymorphism and hair loss response to treatment with Latanoprost.
rs6686438 People carrying at least one G allele have an increased probability of a positive response to treatment with Latanoprost. The presence of a G allele was found to increase the probability of a positive response to Latanoprost treatment for intraocular pressure in mice. No studies show an association between this polymorphism and hair loss response to treatment with Latanoprost.
PTGES2 rs13283456 People with the CT variant have reduced PTGES2 enzymatic activity, which reduces PGE2 levels. This makes them better candidates for treatment with minoxidil, which increases PGE2 levels. The CT variant is associated with reduced BMI in males. However, no direct evidence exists that it is associated with PTGES2 activity or PGE2 levels. There are no studies that show an association between this polymorphism and the response to treatment with minoxidil.
1 SRD5A1 &
SRD5A2
rs248793 People carrying at least one C allele have increased levels of DHT, which may make them good candidates for treatment with 5α-reductase inhibitors. The presence of a C allele in adults was found to be associated with an increased DHT/T ratio. No studies show an association between this polymorphism and the response to treatment with 5α-reductase inhibitors.
2 rs523349 People with the GG variant have increased 5α-reductase activity, which may make them good candidates for treatment with 5α-reductase inhibitors. The GG variant is associated with increased 5α-reductase activity, which may increase DHT levels. No studies show an association between this polymorphism and the response to treatment with 5α-reductase inhibitors.
SULT1A1 rs9282861 People carrying at least one A allele have reduced sulfotransferase activity, which may alter their response to minoxidil. Sulfotransferase activity was reduced in people with the GA variant and further in those with the AA variant. Sulfotransferase catalyzes the conversion of minoxidil to its active form, minoxidil sulfate.
Small-scale human studies have shown that people with the GG variant may respond better to minoxidil treatment for hair loss.
3 Angiotensin-converting enzyme (ACE)
ACE is a key enzyme in blood pressure regulation. It converts angiotensin I to angiotensin II, causing vasoconstriction. The ACE gene has an insertion/deletion (I/D) polymorphism that affects enzyme activity, with the deletion (D) allele associated with higher ACE levels and increased vasoconstrictions.[6]Wong, M. K. S. (2016). Angiotensin Converting Enzymes. In Handbook of Hormones. pp. 263-e29D-4. Elsevier. Available at: https://doi.org/10.1016/B978-0-12-801028-0.00254-3
Some research suggests a link between ACE gene polymorphisms and androgenic alopecia (AGA). Certain genetic testing companies propose that individuals with specific ACE polymorphisms may benefit from treatments that improve scalp blood flow. The theory is that higher ACE activity leads to increased vasoconstriction, potentially reducing blood flow to hair follicles. Therefore, treatments like minoxidil or caffeine, which may work partly through vasodilation, could be more effective for individuals with these genetic variants.
Figure 1: Comparison between AGA patients and controls regarding ACE genotypes. The insertion/deletion (ID) and deletion/deletion (DD) genotypes were significantly increased in AGA patients compared to controls.[7]Ibrahim, M.A., Ezzat, I.S., Mostafa, G.Y., Fathy, A.H.N., Eman, F., Samir, E.S.O. (2021). Association between angiotensin-converting enzyme gene insertion-deletion polymorphism and androgenetic … Continue reading
However, while this hypothesis is intriguing, no direct evidence demonstrates that people with particular ACE polymorphisms respond differently to hair loss treatments that improve blood flow.
Read our ACE gene article here.
Biotinidase (BTD)
The BTD gene encodes biotinidase, an enzyme crucial for recycling and utilizing biotin (Vitamin B7) in the body. Biotin is vital in various biological functions, including hair health, through its involvement in protein synthesis and keratin production.[8]Leon‐Del‐Rio, A. (2019). Biotin in metabolism, gene expression, and human disease. Journal of Inherited Metabolic Disease, 42(4), 647-654. Available at: https://doi.org/10.1002/jimd.12073
Mutations in BTD can lead to biotinidase deficiency, which can cause biotin deficiency or dependency. This condition can result in various symptoms, including hair abnormalities and alopecia.
According to some, certain BTD polymorphisms might predict the efficacy of biotin treatment for hair loss. However, the evidence supporting this claim is limited and inconclusive.[9]Bhattarai, D., Banday, A. Z., Sadanand, R., Arora, K., Kaur, G., Sharma, S., & Rawat, A. (2021). Hair microscopy: an easy adjunct to diagnosis of systemic diseases in children. Applied … Continue reading Studies have shown conflicting results regarding the impact of this polymorphism on biotinidase activity in adults and children, and none specifically address hair loss.
Read our BTD gene article here.
Collagen Type 1 Alpha 1 Chain (COL1A1)
COL1A1 encodes one of the chains of type I collagen, the most abundant collagen in the body. Type I collagen is crucial in supporting tissue structure throughout the body.[10]Ricard-Blum, S. (2011). The collagen family. Cold Spring Harbor perspectives in biology, 3(1), a004978. Available at: https://doi.org/10.1101/cshperspect.a004978
COL1A1 gene expression is upregulated in people with AGA.[11]Michel, L., Reygagne, P., Benech, P., Jean‐Louis, F., Scalvino, S., Ly Ka So, S., Hamidou, Z., Bianovici, S., Pouch, J., Ducos, B. and Bonnet, M., 2017. Study of gene expression alteration in male … Continue reading Furthermore, studies have shown the SNPs in the COL1A1 gene can affect collagen stability.[12]Mann, V., Hobson, E.E., Li, B., Stewart, T.L., Grant, S.F., Robins, S.P., Aspden, R.M. and Ralston, S.H. (2001). A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by … Continue reading
Figure 2: Collagen protein and mRNA levels in osteoblasts (cells that form new bones) cultured from patients with different genotypes. Different genotypes of the rs1800012 polymorphism are shown. SS genotype = GG genotype; Ss genotype = GT genotype.[13]Mann, V., Hobson, E.E., Li, B., Stewart, T.L., Grant, S.F., Robins, S.P., Aspden, R.M. and Ralston, S.H. (2001). A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by … Continue reading
While these findings suggest a potential role for COL1A1 in hair growth and loss, the exact mechanisms are not fully understood. More research is needed to clarify how variations in COL1A1 might influence hair loss and treatment responses. Currently, limited evidence supports targeting COL1A1 specifically for hair loss treatments.
Read our COL1A1 gene article here.
CRAPB2
CRABP2 is one of two genes in the cellular retinoic acid-binding protein family and is crucial for regulating retinoic acid by transporting it within cells and aiding its metabolism. Retinoic acid is important for hair health, but its deficiency and excess can lead to hair loss, indicating a dose-dependent relationship.[14]Wei, L. N. (2016). Cellular retinoic acid binding proteins: Genomic and non-genomic functions and their regulation. The Biochemistry of Retinoid Signaling II: The Physiology of Vitamin A-Uptake, … Continue reading
This gene is highly expressed in dermal papilla cells, which are vital for hair follicle growth and development.[15]He, M., Lv, X., Cao, X., Yuan, Z., Quan, K., Getachew, T., Mwacharo, J.M., Haile, A., Li, Y., Wang, S. and Sun, W. (2023). CRABP2 Promotes the Proliferation of Dermal Papilla Cells via the … Continue reading Overexpression of CRABP2 promotes cell growth, suggesting a positive role in hair maintenance. However, increased CRABP2 expression and elevated retinoic acid levels have also been associated with hair loss conditions like alopecia areata and AGA.[16]Duncan, F.J., Silva, K.A., Johnson, C.J., King, B.L., Szatkiewicz, J.P., Kamdar, S.P., Ong, D.E., Napoli, J.L., Wang, J., King Jr, L.E. and Whiting, D.A. (2013). Endogenous retinoids in the … Continue reading
Despite this paradox, treatments using tretinoin have shown promise, stimulating hair regrowth in over half of patients, with further improvements observed after combination with minoxidil.[17]Bazzano, G. S., Terezakis, N., & Galen, W. (1986). Topical tretinoin for hair growth promotion. Journal of the American Academy of Dermatology, 15(4), 880-893. Available at: … Continue reading
Figure 3: Level of hair regrowth after treatment with either a placebo, minoxidil (0.5%), tretinoin (0.025%), or a combination of tretinoin and minoxidil.[18]Bazzano, G. S., Terezakis, N., & Galen, W. (1986). Topical tretinoin for hair growth promotion. Journal of the American Academy of Dermatology, 15(4), 880-893. Available at: … Continue reading
One large study involving over 25,000 AGA patients found an association between this polymorphism and AGA, suggesting that genetic differences in CRABP2 could impact hair health and treatment efficacy.[19]Francès, M. P., Vila-Vecilla, L., Russo, V., Caetano Polonini, H., & de Souza, G. T. (2024). Utilising SNP Association Analysis as a Prospective Approach for Personalising Androgenetic Alopecia … Continue reading Ultimately, further research is needed to show exactly how it might affect treatment efficacy.
Read our CRABP2 gene article here.
CYP19A1
CYP19A1 encodes the enzyme aromatase, which converts androgens like testosterone into estrogens like estradiol. Reduced expression of CYP19A1 and lower enzyme activity have been associated with female pattern hair loss (FPHL) and AGA. Aromatase levels are higher in non-balding scalp regions and significantly higher in women than men, possibly explaining gender differences in hair loss patterns.[20]Nebert, D. W., Wikvall, K., & Miller, W. L. (2013). Human cytochromes P450 in health and disease. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1612), 20120431. … Continue reading
Aromatase helps decrease levels of DHT, and aromatase inhibitors have been linked to hair thinning, indicating a role for the enzyme in hair health. Treatments like minoxidil may increase aromatase activity, which can improve hair growth by increasing estradiol and reducing DHT levels.[21]Gallicchio, L., Calhoun, C., & Helzlsouer, K. J. (2013). Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast cancer research and treatment, 142, 435-443. Available at: … Continue reading
While certain genetic variations have been associated with better response to treatments like dutasteride, the study also showed that some people with this SNP can be poor responders, indicating that it is probably a combination of SNPs that affects treatment response.
Figure 4: Genotypic landscape of 42 patients, the cumulative effect of each allele count, and their positive or negative effect. Boxes represent SNPs that exhibited a positive (blue) or negative (red) effect on the patient’s response to dutasteride. Light-colored boxes represent heterozygous SNPs (a variation where an individual has two different versions of a specific DNA sequence at a particular location in the genome), dark-colored boxes represent homozygous SNPs (a variation where an individual has two identical versions of a specific DNA sequence at a particular location in the genome). Patient responses to dutasteride improve from left to right.[22]Rhie, A., Son, H.Y., Kwak, S.J., Lee, S., Kim, D.Y., Lew, B.L., Sim, W.Y., Seo, J.S., Kwon, O., Kim, J.I. and Jo, S.J. (2019). Genetic variations associated with response to dutasteride in the … Continue reading
So, while there is evidence that CYP19A1 SNPs can affect treatment efficacy, studies have not been done to determine the relationship between these and hair growth.
Read our CYP19A1 gene article here.
GPR44
The GPR44 gene encodes G-protein-coupled receptor 44 (the prostaglandin D2 receptor or DP2). GPR44 is significant because it mediates the effects of prostaglandin D2, a lipid compound involved in inflammation and the immune response.[23]National Library of Medicine. (2024). PTGDR2 Prostaglandin D2 Receptor 2 [Homo sapiens (human)]. NIH. Available at: https://www.ncbi.nlm.nih.gov/gene/11251 (Accessed: 12 July 2024.)
PGD2 levels are elevated in balding scalps compared to haired scalps. Additionally, PGD2 inhibits hair growth in isolated human hair follicles and mouse models. Overexpression of PGD2 has been shown to lead to premature hair follicle regression and alopecia in mice. Furthermore, mice lacking the GPR44 receptor did not exhibit hair loss when exposed to PGD2, indicating that GPR44 is important for PGD2’s hair growth inhibition.[24]Nieves, A., Garza, L.A. (2014). Does Prostaglandin D2 Hold the Cure to Male Pattern Baldness? Experimental Dermatology. 23(4). 224-227. Available at: https://doi.org/10.1111/exd.12348,[25]Garza, L.A., Liu, Y., Alagesan, B., Lawson, J.A., Norberg, S.M., Loy, D.R., Zhao, T., Blatt, H.B., Stanton, D.C., Carrasco, L., Ahluwalia, G., Fischer, S.M., Fitzgerald, G.A., Cotsarelis, G. (2012). … Continue reading
Figure 5: Figure 2. Hair length 10 days after topical PGD2 (1 ug), 15-dPGJ2 (1 ug) or vehicle treatment.[26]Stanton, D.C., Carrasco, L., Ahluwalia, G., Fischer, S.M., Fitzgerald, G.A., Cotsarelis, G. (2012). Prostaglandin D2 Inhibits Hair Growth and is Elevated in Bald Scalp of Men with Androgenetic … Continue reading
However, one clinical trial using setipiprant, a GPR44 antagonist, showed no significant improvement in hair growth compared to a placebo in men with AGA, indicating that PGD2 signaling through GPR44 alone might not be sufficient for treating hair loss.[27]DuBois, J., Bruce, S., Stewart, D., Kempers, S., Harutunian, C., Boodhoo, T., Weitzenfeld, A, Chang-Lin, J.E. (2021). Setipiprant for Androgenetic Alopecia in Males: Results from a Randomized, … Continue reading
SNPs in GPR44 have been associated with increased receptor expression and asthma severity, hinting that similar mechanisms might affect hair loss.[28]Campos Alberto, E., Maclean, E., Davidson, C., Palikhe, N.S., Storie, J., Tse, C., Brenner, D., Mayers, I., Vliagoftis, H., El-Sohemy, A., Cameron, L. (2012). The Single Nucleotide Polymorphism CRTh2 … Continue reading,[29]Huang, J.L., Gao, P.S., Mathias, R.A., Yao, T.C., Chen, L.C., Kuo, M.L., Hsu, S.C., Plunkett, B., Togias, A., Barnes, K.C., Stellato, C., Beaty, T.H., Huang, S.K. Sequence Variants of the Gene … Continue reading
Nonetheless, conflicting study results and poorly controlled variables make it unclear whether targeting GPR44 is effective for treating hair loss.
Read our GPR44 gene article here.
IGF1R
IGF1R encodes the insulin-like growth factor-1 receptor, which mediates the effects of IGF-1, a protein crucial for hair follicle development and the hair growth cycle. IGF-1 promotes cell growth, division, and survival in hair follicles and regulates the transition between the growth (anagen) and red (catagen) phases.[30]Ahn, S. Y., Pi, L. Q., Hwang, S. T., & Lee, W. S. (2012). Effect of IGF-I on hair growth is related to the anti-apoptotic effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Annals of … Continue reading
Studies have linked low levels of IGF-1 to AGA and hair loss. For instance, individuals with Laron syndromes, characterized by deficient IGF-1 production, often experience thinner hair and alopecia in adulthood.[31]Lurie, R., Ben-Amitai, D., & Laron, Z. (2004). Laron syndrome (primary growth hormone insensitivity): a unique model to explore the effect of insulin-like growth factor 1 deficiency on human … Continue reading IGF-1 is also significantly reduced in balding compared to non-balding scalps.[32]Panchaprateep, R., & Asawanonda, P. (2014). Insulin‐like growth factor‐1: roles in androgenetic alopecia. Experimental dermatology, 23(3), 216-218. Available at: … Continue reading Additionally, middle-aged women with lower circulating IGF-1 levels have a higher risk of developing hair loss.[33]Noordam, R., Gunn, D. A., Drielen, K. V., Westgate, G., Slagboom, P. E., Craen, A. D., & Heemst, D. V. (2016). Both low circulating insulin‐like growth factor‐1 and high‐density lipoprotein … Continue reading
Genetic variation in IGF1R influences plasma IGF-1 levels, with some maintaining normal levels and some exhibiting reduced levels. This suggests a potential role of IGF-1 supplementation in those with lower IGF-1 levels.[34]Bonafè, M., Barbieri, M., Marchegiani, F., Olivieri, F., Ragno, E., Giampieri, C., Mugianesi, E., Centurelli, M., Franceschi, C. and Paolisso, G. (2003). Polymorphic variants of insulin-like growth … Continue reading
Figure 6: Plasma IGF-1 levels depending on IGF1R gene variation. The AA and AG genotypes are associated with lower plasma IGF-1 levels. In contrast, the GG genotype is associated with normal IGF-1 levels.[35]Bonafè, M., Barbieri, M., Marchegiani, F., Olivieri, F., Ragno, E., Giampieri, C., Mugianesi, E., Centurelli, M., Franceschi, C. and Paolisso, G. (2003). Polymorphic variants of insulin-like growth … Continue reading
However, no direct link has been established between the rs2229765 SNP and specific hair loss disorders.
Read the full IGF1R gene article here.
NR3C1
NR3C1 encodes the glucocorticoid receptor (GR), which mediates the action of glucocorticoids, impacting metabolism, immune response, and stress response. There are two main isoforms: GRɑ, which binds glucocorticoids, and GRβ, which can inhibit GRɑs activity.[36]Oakley, R.H., Cidlowski, J.A. (2013). The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. Journal of Allergy and Clinical Immunology. 132(5). 1033-1044. … Continue reading
GRs regulate the transitions between different phases of the hair cycle. They can influence the transition from anagen (active growth) to catagen (regression) phases. Furthermore, GRs are expressed in various components of the hair follicle, including dermal papilla cells, outer root sheath keratinocytes, and hair matrix cells.[37]Kwack, M.H., Hamida, O.B., Moon, K.K., Kim, J.C., Sung, Y.K. (2022). Dexamethasone, a Synthetic Glucocorticoid, Induces the Activity of Androgen Receptor in Human Dermal Papilla Cells. Skin … Continue reading
Certain genetic variants in NR3C1 have been linked to glucocorticoid resistance due to altered mRNA stability, affecting GR function. While these variations do impact glucocorticoid activity, there is no evidence to connect these effects to responsiveness to corticosteroids in hair loss, as the study was conducted in children with acute lymphoblastic leukemia.[38]Gasic, V., Zukic, B., Stankovic, B., Janic, D., Dokmanovic, L., Lazic, J., Krstovski, N., Dolzan, V., Jazbec, J., Pavlovic, S., Kotur, N. (2018). Pharmacogenomic Markers of Glucocorticoid Response in … Continue reading
Figure 7: Genotype frequencies associated with glucocorticoid response. The bold numbers indicate a significant difference. NR3C1 6198 shows a significant association with glucocorticoid resistance.[39]Gasic, V., Zukic, B., Stankovic, B., Janic, D., Dokmanovic, L., Lazic, J., Krstovski, N., Dolzan, V., Jazbec, J., Pavlovic, S., Kotur, N. (2018). Pharmacogenomic Markers of Glucocorticoid Response in … Continue reading
Read more about the NR3C1 gene here.
PGTFR
The PGTFR gene encodes the prostaglandin F2 alpha receptor and plays a key role in hair follicle health and pigmentation. This receptor also involves broader physiological processes, including reproduction, inflammation, and cancer. Still, its influence on hair growth has made it a target in hair loss research.[40]Ricciotti, E., FitzGerald, G.A. (2011). Prostaglandins and Inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 31(5). 986-1000. Available at: … Continue reading Studies in animal models have shown that PGF2ɑ and its analogs, such as latanoprost, stimulate the hair follicle and melanocyte growth, hinting at potential therapeutic benefits for hair loss treatments.
Genetic variants within PGTFR have been linked to varied responses to latanoprost in glaucoma treatment. Those with one genotype have been associated with greater response to latanoprost, while others were associated with reduced effectiveness.[41]Sakurai, M., Higashide, T., Takahashi, M., Sugiyama, K. (2007). Association between Genetic Polymorphisms of the Prostaglandin F2ɑ Receptor Gene and Response to Latanoprost. Ophthalmology. 114(6). … Continue reading Similarly, other SNPs have corresponded with lower receptor activity, indicating decreased response to PGF2ɑ analogs.
Figure 8: Latanoprost responsiveness based on single nucleotide polymorphism.[42]↑4 Sakurai, M., Higashide, T., Takahashi, M., Sugiyama, K. (2007). Association between Genetic Polymorphisms of the Prostaglandin F2ɑ Receptor Gene and Response to Latanoprost. Ophthalmology. … Continue reading
In the context of hair loss, we know that some evidence exists to show that latanoprost might benefit hair regrowth.[43]Blume-Peytavi, U., Lonngors, S., Hillmann, K., Bartels, N.G. (2012). A Randomized, Double-Blind, Placebo-Controlled Pilot Study to Assess the Efficacy of a 24-Week Topical Treatment by Latanoprost … Continue reading However, no studies have linked any SNPs to treatment efficacy in the hair follicle.
Read the PGTFR gene article here.
PTGES2
The PTGES2 gene encodes prostaglandin E synthase 2, an enzyme that converts prostaglandin H2 (PGH2) to prostaglandin E2 (PGE2). This enzyme is critical in the prostaglandin synthesis pathway and plays a role in inflammation and hair growth. PGE2 levels are higher in non-balding scalp regions than in balding ones, suggesting that PTGES2 and its product PGE2 might contribute to hair preservation and growth.[44]Garza, L.A., Liu, Y., Alagesan, B., Lawson, J.A., Norberg, S.M., Loy, D.R., Zhao, T., Blatt, H.B., Stanton, D.C., Carrasco, L., Ahluwalia, G., Fischer, S.M., Fitzgerald, G.A., Cotsarelis, G. (2012). … Continue reading
Studies on AGA patients have found that PTGES2 expression increases in balding areas, likely as a compensatory response.[45]Villareal-Villareal, C.D., Sinclair, R.D., Martinez-Jacobo, L., Garza-Rodriguez, V., Rodriguez-Leon, S.A., Lamadrid-Zertuche, A.C., Rodriguez-Gutierrez, R., Ortiz-Lopez, R., Rojas-Martinez, A., … Continue reading Genetic association studies have also linked a PTGES2 SNP to AGA. However, this does not appear to correlate with the severity of hair loss.[46]Frances, M.P., Vila-Vecilla, L., Russo, V., Polonini, H.C., de Souza, G.T. (2024). Utilizing SNP Association Analysis as a Prospective Approach for Personalising Androgenetic Alopecia Treatment. … Continue reading
Figure 9: Prostaglandin mean levels (ng/g tissue) in patients with AGA comparing bald areas with non-balding.[47]Chovarda, E., Sotiriou, E., Lazaridoi, E., Vakirlis, E., Ioannides, D. (2021). The Role of Prostaglandins in Androgenetic Alopecia. International Journal of Dermatology. 60. 730-735. Available at: … Continue reading
Interestingly, some research suggests PTGES2 could influence responses to minoxidil as it has been shown to increase PGE2 production in hair follicle cells.[48]Michelet, J.F., Commo, S., Billoni, N., Mahe, Y.F., Bernard, B.A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating … Continue reading This indicates that individuals with PTGES2 variants possibly associated with lower enzyme activity might benefit more from minoxidil, as the treatment could help elevate PGE2 levels. However, general genetic findings should be interpreted carefully, as gene expression patterns in hair follicles may differ significantly from those in other tissues.
Read the PTGES2 gene article here.
SRD5A1 & SRD5A2
The SRD5A1 and SRD5A2 genes encode type I and type II 5ɑ-reductase enzymes, which are crucial for converting testosterone into dihydrotestosterone (DHT).[49]Scaglione, A., Montemiglio, L.C., Parisi, G., Asteriti, I.A., Bruni, R., Cerutti, G., Testi, C., Savino, C., Mancia, F., Lavia, P. and Vallone, B. (2017). Subcellular localization of the five members … Continue reading Inhibitors targeting these enzymes, particularly finasteride, and dutasteride, are central to AGA treatment. Genetic variations in SRD5A1 and SRD5A2 have shown potential in influencing the effectiveness of these treatments.
In a study of AGA patients treated with dutasteride, certain SNPs in SRD5A1 were positively associated with treatment response, meaning individuals with these variants responded better. However, even patients with these SNPs displayed varied responses, suggesting that the overall genetic profile, rather than individual SNPs alone, likely plays a role in treatment efficacy.[50]Rhie, A., Son, H.Y., Kwak, S.J., Lee, S., Kim, D.Y., Lew, B.L., Sim, W.Y., Seo, J.S., Kwon, O., Kim, J.I. and Jo, S.J. (2019). Genetic variations associated with response to dutasteride in the … Continue reading Interestingly, no SRD5A2 SNPs were found to significantly impact dutasteride response in this study, although variations in SRD5A2 may still affect DHT production and, consequently, treatment outcomes.
Additional research hints that SRD5A gene variants influence enzyme activity. In particular, individuals with higher DHT levels due to SRD5A1 or SRD5A2 variants might require higher doses or longer treatment durations for 5ɑ-reductase inhibitors to show efficacy. Lab studies have further suggested that specific variants of SRD5A2 might respond differently to finasteride versus dutasteride, emphasizing that personalized treatment approaches could optimize hair loss management.[51]Makridakis, N.M., di Salle, E., and Reichardt, J.K. (2000). Biochemical and pharmacogenetic dissection of human steroid 5α-reductase type II. Pharmacogenetics and Genomics, 10(5), 407-413. Available … Continue reading
While further clinical studies are needed to confirm these findings, early data suggest that SRD5A1 and SRD5A2 genotyping could improve the efficacy of 5ɑ-reductase inhibitor treatments.
Read the full SRD5A1 and SRD5A2 gene article here.
SULT1A1
The SULT1A1 gene encodes the enzyme sulfotransferase 1A1, part of the sulfotransferase family. This enzyme plays a critical role in the metabolism and detoxification of various compounds. In hair loss treatment, SULT1A1 is especially important because it activates minoxidil through sulfonation, converting it into minoxidil sulfate, the active form needed to stimulate hair growth.[52]Goren, A., Castano, J.A., McCoy, J., Bermudez, F., Lotti, T. (2014). Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatologic Therapy. 27. 171-173. … Continue reading
Evidence suggests that specific SULT1A1 gene variants correlate with varying levels of enzyme activity and minoxidil responsiveness. Some genetic variations have been shown to lead to higher sulfotransferase activity and greater hair growth response after minoxidil treatment, whereas those with other genotypes exhibited lower enzyme activity and may have a weaker response to the drug.[53]Raghad, N.A., Al-Gazally, M.E., Ewahd, W.A. (2017). Assessment the effect of different genotypes of sulfotransferase 1A1 gene on the response to minoxidil in patients with androgenic alopecia. … Continue reading
Figure 10: (Top) The proportion of participants in the R0 (“non-responder”) and R1 (“responder”) groups with each allele variation. (Bottom) The proportion of participants in the R0 (“non-responder”) and R1 (“responder”) groups with each genotype variation.[54]Raghad, N.A., Al-Gazally, M.E., Ewahd, W.A. (2017). Assessment the effect of different genotypes of sulfotransferase 1A1 gene on the response to minoxidil in patients with androgenic alopecia. … Continue reading
Recent studies have begun using SULT1A1 genotyping as a tool to optimize minoxidil treatment, especially in female-pattern hair loss. However, some people with the “favorable” gene variations still fail to respond, while others with “less favorable” gene variations show substantial hair regrowth. This highlights the need for larger validation studies..[55]Ramos, P.M., Gohad, P., McCoy, J., Wambier, C., Goren, A. (2021). Minoxidil Sulfotransferase Enzyme (SULT1A1) genetic variants predict response to oral minoxidil treatment for female pattern hair … Continue reading
Read more about these studies in the SULT1A1 gene article here.
Should We Be Targeting Genes for Hair Loss Treatment Efficacy?
Based on the evidence in the articles, SULT1A1 currently has the most evidential support. This is especially true as it is one of (if not the only) gene that we have examined that actually examined these SNPs in the hair follicles of people with hair loss.
Aside from this, we don’t see the utility in using our genes to predict hair loss treatment efficacy. That is not to say that we won’t eventually see a use for this, however. As the research evolves, new information might come to light, which we will keep you updated on.
References[+]
References ↑1 TrichoTest. (no date). Personalizing alopecia treatment. Fagron Genomics. Available at: https://gxsciences.com/trichotest/. (Accessed: October 2024) ↑2 Nelson, M.R., Marnellos, G., Kammerer, S., Hoyal, C.R., Shi, M.M., Cantor, C.R., Braun, A. (2004). Large-scale validation of single nucleotide polymorphisms in gene regions. Genome Research. 14(8).1664-1668. Available at: https://doi.org/10.1101/gr.2421604 ↑3 Sun, Y., Zhang, Y., Zhang, X. (2019). Synonymous SNPs of viral genes facilitate virus to escape host antiviral RNAi immunity. RNA Biology. 16(12). 1697-1710. Available at: https://doi.org/10.1080/15476286.2019.1656026 ↑4 Degtyareva, A.O., Antontseva, E.V., Merkulova, T.I. (2021). Regulatory SNPs: Altered Transcription Factor Binding Sites Implicated in Complex Traits and Diseases. International Journal of Molecular Sciences. 22(12). 6454. Available at: https://doi.org/10.3390/ijms22126454 ↑5 Pirastu, N., Joshi, P.K., deVries, P.S., Cornerlis, M.C., McKeigue, P.M., Keum, N., Franceschini, N., Colombo, M., Giovannucci, E.L., Spiliopoulou, A., Franke, L., North, K.E., Kraft, Morrison, A.C., Esko, T., Wilson, J.F. (2017). GWAS for male-pattern baldness identifies 71 susceptibility loci, explaining 38% of the risk. Nature Communications. 8. 1584. Available at: https://doi.org/10.1038/s41467-017-01490-8 ↑6 Wong, M. K. S. (2016). Angiotensin Converting Enzymes. In Handbook of Hormones. pp. 263-e29D-4. Elsevier. Available at: https://doi.org/10.1016/B978-0-12-801028-0.00254-3 ↑7 Ibrahim, M.A., Ezzat, I.S., Mostafa, G.Y., Fathy, A.H.N., Eman, F., Samir, E.S.O. (2021). Association between angiotensin-converting enzyme gene insertion-deletion polymorphism and androgenetic alopecia susceptibility among Egyptian patients: A preliminary case-controlled study. Journal of Cosmetic Dermatology. 21(6). 2629-2634. Available at: https://doi.org/10.1111/jocd.14434 ↑8 Leon‐Del‐Rio, A. (2019). Biotin in metabolism, gene expression, and human disease. Journal of Inherited Metabolic Disease, 42(4), 647-654. Available at: https://doi.org/10.1002/jimd.12073 ↑9 Bhattarai, D., Banday, A. Z., Sadanand, R., Arora, K., Kaur, G., Sharma, S., & Rawat, A. (2021). Hair microscopy: an easy adjunct to diagnosis of systemic diseases in children. Applied Microscopy, 51, 1-12. Available at: https://doi.org/10.1186/s42649-021-00067-6 ↑10 Ricard-Blum, S. (2011). The collagen family. Cold Spring Harbor perspectives in biology, 3(1), a004978. Available at: https://doi.org/10.1101/cshperspect.a004978 ↑11 Michel, L., Reygagne, P., Benech, P., Jean‐Louis, F., Scalvino, S., Ly Ka So, S., Hamidou, Z., Bianovici, S., Pouch, J., Ducos, B. and Bonnet, M., 2017. Study of gene expression alteration in male androgenetic alopecia: evidence of predominant molecular signalling pathways. British Journal of Dermatology, 177(5), 1322-1336. Available at: https://doi.org/10.1111/bjd.15577 ↑12 Mann, V., Hobson, E.E., Li, B., Stewart, T.L., Grant, S.F., Robins, S.P., Aspden, R.M. and Ralston, S.H. (2001). A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. The Journal of Clinical Investigation, 107(7), 899-907. Available at: https://doi.org/10.1172/JCI10347 ↑13 Mann, V., Hobson, E.E., Li, B., Stewart, T.L., Grant, S.F., Robins, S.P., Aspden, R.M. and Ralston, S.H. (2001). A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. The Journal of clinical investigation, 107(7), 899-907. Available at: https://doi.org/10.1172/JCI10347 ↑14 Wei, L. N. (2016). Cellular retinoic acid binding proteins: Genomic and non-genomic functions and their regulation. The Biochemistry of Retinoid Signaling II: The Physiology of Vitamin A-Uptake, Transport, Metabolism and Signaling, 163-178. Available at: https://doi.org/10.1007/978-94-024-0945-1_6 ↑15 He, M., Lv, X., Cao, X., Yuan, Z., Quan, K., Getachew, T., Mwacharo, J.M., Haile, A., Li, Y., Wang, S. and Sun, W. (2023). CRABP2 Promotes the Proliferation of Dermal Papilla Cells via the Wnt/β-Catenin Pathway. Animals, 13(12), 2033. Available at: https://doi.org/10.3390/ani13122033 ↑16 Duncan, F.J., Silva, K.A., Johnson, C.J., King, B.L., Szatkiewicz, J.P., Kamdar, S.P., Ong, D.E., Napoli, J.L., Wang, J., King Jr, L.E. and Whiting, D.A. (2013). Endogenous retinoids in the pathogenesis of alopecia areata. Journal of Investigative Dermatology, 133(2), 334-343. Available at: https://doi.org/10.1038/jid.2012.344 ↑17 Bazzano, G. S., Terezakis, N., & Galen, W. (1986). Topical tretinoin for hair growth promotion. Journal of the American Academy of Dermatology, 15(4), 880-893. Available at: https://doi.org/10.1016/S0190-9622(86)80024-X ↑18 Bazzano, G. S., Terezakis, N., & Galen, W. (1986). Topical tretinoin for hair growth promotion. Journal of the American Academy of Dermatology, 15(4), 880-893. Available at: https://doi.org/10.1016/S0190-9622(86)80024-X ↑19 Francès, M. P., Vila-Vecilla, L., Russo, V., Caetano Polonini, H., & de Souza, G. T. (2024). Utilising SNP Association Analysis as a Prospective Approach for Personalising Androgenetic Alopecia Treatment. Dermatology and Therapy, 14(4), 971-981. Available at: https://doi.org/10.1007/s13555-024-01142-y ↑20 Nebert, D. W., Wikvall, K., & Miller, W. L. (2013). Human cytochromes P450 in health and disease. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1612), 20120431. Available at: https://doi.org/10.1098/rstb.2012.0431 ↑21 Gallicchio, L., Calhoun, C., & Helzlsouer, K. J. (2013). Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast cancer research and treatment, 142, 435-443. Available at: https://doi.org/10.1007/s10549-013-2744-2 ↑22 Rhie, A., Son, H.Y., Kwak, S.J., Lee, S., Kim, D.Y., Lew, B.L., Sim, W.Y., Seo, J.S., Kwon, O., Kim, J.I. and Jo, S.J. (2019). Genetic variations associated with response to dutasteride in the treatment of male subjects with androgenetic alopecia. Plos one, 14(9), e0222533. Available at: https://doi.org/10.1371/journal.pone.0222533 ↑23 National Library of Medicine. (2024). PTGDR2 Prostaglandin D2 Receptor 2 [Homo sapiens (human)]. NIH. Available at: https://www.ncbi.nlm.nih.gov/gene/11251 (Accessed: 12 July 2024.) ↑24 Nieves, A., Garza, L.A. (2014). Does Prostaglandin D2 Hold the Cure to Male Pattern Baldness? Experimental Dermatology. 23(4). 224-227. Available at: https://doi.org/10.1111/exd.12348 ↑25 Garza, L.A., Liu, Y., Alagesan, B., Lawson, J.A., Norberg, S.M., Loy, D.R., Zhao, T., Blatt, H.B., Stanton, D.C., Carrasco, L., Ahluwalia, G., Fischer, S.M., Fitzgerald, G.A., Cotsarelis, G. (2012). Prostaglandin D2 Inhibits Hair Growth and is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Science Translational Medicine. 4(126). 1-21. Available at: https://doi.org/10.1126/scitranslmed.3003122 ↑26 Stanton, D.C., Carrasco, L., Ahluwalia, G., Fischer, S.M., Fitzgerald, G.A., Cotsarelis, G. (2012). Prostaglandin D2 Inhibits Hair Growth and is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Science Translational Medicine. 4(126). 1-21. Available at: https://doi.org/10.1126/scitranslmed.3003122 ↑27 DuBois, J., Bruce, S., Stewart, D., Kempers, S., Harutunian, C., Boodhoo, T., Weitzenfeld, A, Chang-Lin, J.E. (2021). Setipiprant for Androgenetic Alopecia in Males: Results from a Randomized, Double-Blind, Placebo-Controlled Phase 2a Trial. Clinical Cosmetic and Investigative Dermatology. 14. 1507-1517. Available at: https://doi.org/10.2147/CCID.S319676 ↑28 Campos Alberto, E., Maclean, E., Davidson, C., Palikhe, N.S., Storie, J., Tse, C., Brenner, D., Mayers, I., Vliagoftis, H., El-Sohemy, A., Cameron, L. (2012). The Single Nucleotide Polymorphism CRTh2 rs533116 is Associated with Allergic Asthma and Increased Expression of CRTh2. Allergy. 67(11). 1357-1364. Available at: https://doi.org/10.1111/all.12003 ↑29 Huang, J.L., Gao, P.S., Mathias, R.A., Yao, T.C., Chen, L.C., Kuo, M.L., Hsu, S.C., Plunkett, B., Togias, A., Barnes, K.C., Stellato, C., Beaty, T.H., Huang, S.K. Sequence Variants of the Gene Encoding Chemoattractant Receptor Expressed on Th2 Cells (CRTH2) are Associated with Asthma and Differentially Influence mRNA Stability. Human Molecular Genetics. 13(21). 2691-2697. Available at: https://doi.org/10.1093/hmg/ddh279 ↑30 Ahn, S. Y., Pi, L. Q., Hwang, S. T., & Lee, W. S. (2012). Effect of IGF-I on hair growth is related to the anti-apoptotic effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Annals of dermatology, 24(1), 26-31. Available at: https://doi.org/10.5021/ad.2012.24.1.26 ↑31 Lurie, R., Ben-Amitai, D., & Laron, Z. (2004). Laron syndrome (primary growth hormone insensitivity): a unique model to explore the effect of insulin-like growth factor 1 deficiency on human hair. Dermatology, 208(4), 314-318. Available at: https://doi.org/10.1159/000077839 ↑32 Panchaprateep, R., & Asawanonda, P. (2014). Insulin‐like growth factor‐1: roles in androgenetic alopecia. Experimental dermatology, 23(3), 216-218. Available at: https://doi.org/10.1111/exd.12339 ↑33 Noordam, R., Gunn, D. A., Drielen, K. V., Westgate, G., Slagboom, P. E., Craen, A. D., & Heemst, D. V. (2016). Both low circulating insulin‐like growth factor‐1 and high‐density lipoprotein cholesterol are associated with hair loss in middle‐aged women. British Journal of Dermatology, 175(4), 728-734. Available at: https://doi.org/ 10.1111/bjd.14529 ↑34, ↑35 Bonafè, M., Barbieri, M., Marchegiani, F., Olivieri, F., Ragno, E., Giampieri, C., Mugianesi, E., Centurelli, M., Franceschi, C. and Paolisso, G. (2003). Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. The Journal of Clinical Endocrinology & Metabolism, 88(7), 3299-3304. Available at: https://doi.org/10.1210/jc.2002-021810 ↑36 Oakley, R.H., Cidlowski, J.A. (2013). The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. Journal of Allergy and Clinical Immunology. 132(5). 1033-1044. Available at: https://doi.org/10.1016/j.jaci.2013.09.007 ↑37 Kwack, M.H., Hamida, O.B., Moon, K.K., Kim, J.C., Sung, Y.K. (2022). Dexamethasone, a Synthetic Glucocorticoid, Induces the Activity of Androgen Receptor in Human Dermal Papilla Cells. Skin Pharmacology and Physiology. 35(5). 299-304. Available at: https://doi.org/10.1159/000525067 ↑38 Gasic, V., Zukic, B., Stankovic, B., Janic, D., Dokmanovic, L., Lazic, J., Krstovski, N., Dolzan, V., Jazbec, J., Pavlovic, S., Kotur, N. (2018). Pharmacogenomic Markers of Glucocorticoid Response in The Initial Phase of Remission Induction Therapy in Childhood Acute Lymphoblastic Leukemia. Radiology and Oncology. 52(3). 296-306. Available at: https://doi.org/10.2478/raon-2018-0034 ↑39 Gasic, V., Zukic, B., Stankovic, B., Janic, D., Dokmanovic, L., Lazic, J., Krstovski, N., Dolzan, V., Jazbec, J., Pavlovic, S., Kotur, N. (2018). Pharmacogenomic Markers of Glucocorticoid Response in The Initial Phase of Remission Induction Therapy in Childhood Acute Lymphoblastic Leukemia. Radiology and Oncology. 52(3). 296-306. Available at: https://doi.org/10.2478/raon-2018-0034 ↑40 Ricciotti, E., FitzGerald, G.A. (2011). Prostaglandins and Inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 31(5). 986-1000. Available at: https://doi.org/10.1161/ATVBAHA.110.207449. ↑41 Sakurai, M., Higashide, T., Takahashi, M., Sugiyama, K. (2007). Association between Genetic Polymorphisms of the Prostaglandin F2ɑ Receptor Gene and Response to Latanoprost. Ophthalmology. 114(6). 1039-1045. Available at: https://doi.org/10.1016/j.ophtha.2007.03.025. ↑42 ↑4 Sakurai, M., Higashide, T., Takahashi, M., Sugiyama, K. (2007). Association between Genetic Polymorphisms of the Prostaglandin F2ɑ Receptor Gene and Response to Latanoprost. Ophthalmology. 114(6). 1039-1045. Available at: https://doi.org/10.1016/j.ophtha.2007.03.025. ↑43 Blume-Peytavi, U., Lonngors, S., Hillmann, K., Bartels, N.G. (2012). A Randomized, Double-Blind, Placebo-Controlled Pilot Study to Assess the Efficacy of a 24-Week Topical Treatment by Latanoprost 0.1% on Hair Growth and Pigmentation in Healthy Volunteers with Androgenetic Alopecia. Journal of the American Academy of Dermatology. 66(5). 797-800. Available at: https://doi.org/10.1016/j.jaad.2011.05.026. ↑44 Garza, L.A., Liu, Y., Alagesan, B., Lawson, J.A., Norberg, S.M., Loy, D.R., Zhao, T., Blatt, H.B., Stanton, D.C., Carrasco, L., Ahluwalia, G., Fischer, S.M., Fitzgerald, G.A., Cotsarelis, G. (2012). Prostaglandin D2 Inhibits Hair Growth and is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Science Translational Medicine. 4(126). 1-21. Available at: https://doi.org/10.1126/scitranslmed.3003122 ↑45 Villareal-Villareal, C.D., Sinclair, R.D., Martinez-Jacobo, L., Garza-Rodriguez, V., Rodriguez-Leon, S.A., Lamadrid-Zertuche, A.C., Rodriguez-Gutierrez, R., Ortiz-Lopez, R., Rojas-Martinez, A., Ocampo-Candiani, J. (2019). Prostaglandins in androgenetic alopecia in 12 men and four female. Journal of the European Academy of Dermatology and Venereology. 33(5). E214-e215. Available at: https://doi.org/10.1111/jdv.15479 ↑46 Frances, M.P., Vila-Vecilla, L., Russo, V., Polonini, H.C., de Souza, G.T. (2024). Utilizing SNP Association Analysis as a Prospective Approach for Personalising Androgenetic Alopecia Treatment. Dermatology and Therapy (Heidelb). 14(4). 971-981. Available at: https://doi.org/10.1007/s13555-024-01145-y ↑47 Chovarda, E., Sotiriou, E., Lazaridoi, E., Vakirlis, E., Ioannides, D. (2021). The Role of Prostaglandins in Androgenetic Alopecia. International Journal of Dermatology. 60. 730-735. Available at: https://doi.org/10.1111/ijd.15378 ↑48 Michelet, J.F., Commo, S., Billoni, N., Mahe, Y.F., Bernard, B.A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. Journal of Investigative Dermatology. 108(2). 205-209. Available at: https://doi.org/10.1111/1523-1747.ep12334249 ↑49 Scaglione, A., Montemiglio, L.C., Parisi, G., Asteriti, I.A., Bruni, R., Cerutti, G., Testi, C., Savino, C., Mancia, F., Lavia, P. and Vallone, B. (2017). Subcellular localization of the five members of the human steroid 5α-reductase family. Biochimie open, 4, 99-106. Available at: https://doi.org/10.1016/j.biopen.2017.03.003 ↑50 Rhie, A., Son, H.Y., Kwak, S.J., Lee, S., Kim, D.Y., Lew, B.L., Sim, W.Y., Seo, J.S., Kwon, O., Kim, J.I. and Jo, S.J. (2019). Genetic variations associated with response to dutasteride in the treatment of male subjects with androgenetic alopecia. Plos one, 14(9), e0222533. Available at: https://doi.org/10.1371/journal.pone.0222533 ↑51 Makridakis, N.M., di Salle, E., and Reichardt, J.K. (2000). Biochemical and pharmacogenetic dissection of human steroid 5α-reductase type II. Pharmacogenetics and Genomics, 10(5), 407-413. Available at: https://doi.org/10.1097/00008571-200007000-00004 ↑52 Goren, A., Castano, J.A., McCoy, J., Bermudez, F., Lotti, T. (2014). Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatologic Therapy. 27. 171-173. Available at: https://doi.org/10.1111/dth.12111 ↑53 Raghad, N.A., Al-Gazally, M.E., Ewahd, W.A. (2017). Assessment the effect of different genotypes of sulfotransferase 1A1 gene on the response to minoxidil in patients with androgenic alopecia. Journal of Global Pharma Technology. 10(9). 144-149 ↑54 Raghad, N.A., Al-Gazally, M.E., Ewahd, W.A. (2017). Assessment the effect of different genotypes of sulfotransferase 1A1 gene on the response to minoxidil in patients with androgenic alopecia. Journal of Global Pharma Technology. 10(9). 144-149 ↑55 Ramos, P.M., Gohad, P., McCoy, J., Wambier, C., Goren, A. (2021). Minoxidil Sulfotransferase Enzyme (SULT1A1) genetic variants predict response to oral minoxidil treatment for female pattern hair loss. Journal of the European Academy of Dermatology and Venererology. 35(1). E24-e26. Available at: https://doi.org/10.1111/jdv.16765 IngredientsPharmaceuticalsNatural RemediesCompany ReviewsBy Michael Williams, PhDNov 12, 2025Finasteride and Dry Eye Disease: Is There A Connection?
Finasteride is a well-established treatment for hair loss, but some reports suggest it may contribute to dry eye disease. This article examines how finasteride’s hormonal effects may impact tear production, reviews current research findings, and provides practical steps for managing or preventing ey...By Perfect Hair Health TeamNov 11, 20255 Ways to Prevent Hair Loss While Taking Testosterone
Testosterone replacement therapy can transform energy, mood, and libido, but it often comes with an unwelcome side effect: accelerated hair loss. This guide breaks down the science of how TRT ramps up DHT and outlines five evidence-based strategies to protect your hair without sacrificing hormonal b...By Sarah King, PhDNov 8, 2025Minoxidil Side Effects: Everything You Need To Know
Minoxidil is an FDA-approved treatment for androgenic alopecia, but its use can come with side effects – from mild scalp irritation to cardiovascular risks. This comprehensive guide assess common and rare side effects from topical minoxidil and oral minoxidil – along with mitigation strategies....By Perfect Hair Health TeamNov 8, 2025Parts of the Scalp: A Guide to the Anatomy, Mechanics, and New Treatment Possibilities
This article takes a detailed tour of scalp anatomy – the regions of the scalp, the muscles, the arteries, the nerves, and the scalp layers. Then we’ll explore the evidence supporting a possible connection between relaxed muscles, scalp mechanics, and hair growth.
By Perfect Hair Health TeamNov 8, 2025Hims vs Keeps vs Roman: Which Hair Growth Subscription Service Wins?
Hair growth subscription services are sprouting up everywhere online. Among them, three companies have emerged from the pack: Hims, Keeps, and Roman. Each company offers two FDA-approved hair loss treatments, as well as other add-on products (supplements, gummies, etc). In this post, the Perfect Hai...By Perfect Hair Health TeamNov 8, 2025Vitamin B1 for Hair Loss: Good or Bad for Hair Loss?
Vitamin B1 – also known as thiamine (or thiamin) – is part of the B-complex of vitamins. In mouse models, severe vitamin B1 deficiencies lead to rapid weight loss, neurological decline, and hair loss. However, thiamine repletion studies (as well as studies on humans) suggest that this hair loss seem...By Perfect Hair Health TeamNov 8, 2025Nutrafol Review: Half-Truths, Cherrypicked Data, & Misleading Study Results
Nutrafol is a hair loss supplement praised by media, endorsed by doctors, and supported by clinical studies. At first glance, it seems like the perfect option for anyone interesting in fighting hair loss naturally. Unfortunately, during our investigation, we found that (1) Nutrafol’s 2018 clin...By Perfect Hair Health TeamNov 8, 2025Microneedling For Hair Loss: What’s The Best Needle Size?
When it comes to microneedling for hair regrowth, researchers aren’t yet sure which needle size elicits the best results. In clinical studies, needle lengths of 0.6mm to 2.5mm (used once weekly or bi-weekly) have been shown to improve hair counts in men with androgenic alopecia (AGA). Even sti...By Perfect Hair Health TeamNov 8, 2025Oral Minoxidil: What’s The Best Dose (mg) For Men With Pattern Hair Loss?
Oral minoxidil improves hair growth in men with androgenic alopecia (AGA), with dosages of 0.25mg, 2.5mg, and 5.0mg showing positive effects. But the higher the dose, the higher your risk of side effects: namely, body hair growth and lower limb edema (swelling). So, which dose is best for you? While...By Perfect Hair Health TeamNov 8, 2025Rosemary Oil For Hair Loss? Not So Fast… (See Photos)
In 2015, a clinical study showed that rosemary oil improved pattern hair loss and was just as effective as 2% minoxidil (Rogaine®). Since then, interest in rosemary oil has exploded – with many hair loss sufferers switching from Rogaine® to rosemary as a more natural solution. But is this a good mov... - Mission Statement
 Scroll Down
Scroll Down