- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Topical Minoxidil for Hair Growth: Efficacy, Safety, and How to Enhance Results
-
Oral Minoxidil for Hair Growth: Is it Effective and Safe? Our Analysis
-
Introducing Ulo: The Future of Hair Loss Telemedicine
-
OS-01 Hair Review: Does It Live Up to the Hype?
-
Stretching The Truth: 3 Misrepresented Claims From Hair Loss Studies
-
Minoxidil Shedding – What to Expect & When it Stops
-
Does Minoxidil Cause Skin Aging?
-
Thermus Thermophilus Extract Does Not Increase Hair Density By 96.88%, Despite Dermatology Times’ Claims.
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesThe Microbiome and Hair Loss: A Scientific Review [2021]
First Published Feb 23 2021Last Updated Oct 29 2024IngredientsMiscellaneousNatural Remedies Researched & Written By:Perfect Hair Health Team
Researched & Written By:Perfect Hair Health Team Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor![The Microbiome and Hair Loss: A Scientific Review [2021]](https://perfecthairhealth.com/wp-content/uploads/2021/02/Microbiome-min-1-1.jpeg)
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Ten years ago, many researchers dismissed the possibility that gut bacteria could influence hair loss and hair growth. Today, they’re changing their tune. Research teams have now demonstrated that changes to our gut microbiome are linked to both stress-related hair loss and alopecia areata. Fascinatingly, there’s also mechanistic research linking gut bacteria to the production (and regulation) of DHT, and potentially even pattern hair loss. This article uncovers the evidence, mechanisms, and more.
Full Article
Can the bacteria inside of our guts affect hair loss? It’s possible. Studies now link changes to our gut bacteria (i.e., the microbiome) to hair loss disorders such as alopecia areata, telogen effluvium, and maybe even androgenic alopecia (pattern hair loss).
In this article, we’ll dive into the science surrounding the microbiome, its connections to hair health, ways in which gut bacteria might affect hair shedding, and how our gut microbiome might become a new frontier for hair loss research (and hair growth treatments).
If you have any questions, feel free to reach out in the comments.
Note: this is a massive article. If you’re looking for information related to the microbiome and a particular type of hair loss, please use the table of contents below.
Table of contents
What is the gut microbiome?
The gut microbiome is a term to describe the trillions of microorganisms living in our intestines.
Artist’s representation of gut bacteria
Inside our intestines, a pool of bacteria, viruses, protozoa, helminths, and archaea feed off our blood, tissues, and food particles. In exchange, these microorganisms help regulate our immune systems, neutralize infections, and metabolize nutrients (among other things).
In the last decade, researchers have discovered that the gut microbiome affects almost every facet of human health: metabolism, hormone production, immunity, brain function…
This begs the question: can our gut microflora also influence hair loss?
It may seem outlandish, as the gut is a far distance from our scalps. But hair health and gut health appear intertwined. In order to understand why, we’ll need to dive into the microbiome and its potential relationship with the four most common types of hair loss:
- Alopecia areata
- Telogen effluvium
- Scarring alopecias
- Pattern hair loss (androgenic alopecia, or AGA)
Alopecia areata and the microbiome
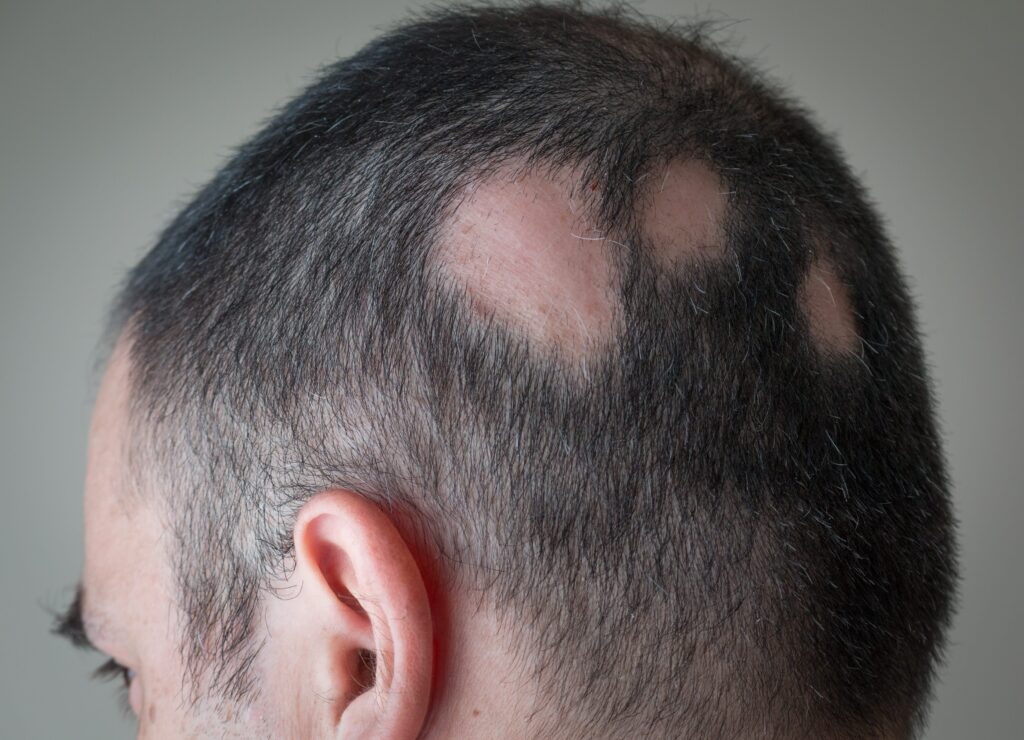
Alopecia areata is an autoimmune type of hair loss affecting up to 2% of people worldwide. It’s caused by a mysterious driver of inflammation that disrupts hair follicle stem cells and triggers shedding. It presents as patchy-related hair loss, often accompanied by exclamation-mark hairs.
Alopecia areata in a male patient
Interestingly, alopecia areata occurs more often in people with irritable bowel syndrome (IBS) [1]. And fascinatingly, people with alopecia areata who receive treatment for IBS have later reported spontaneous hair regrowth [2].
This has led researchers to wonder: is the gut microbiome causally linked to alopecia areata? And if yes, by which mechanisms might our gut flora influence autoimmune forms of hair loss?
How can gut health influence alopecia areata?
There are a few hypotheses, but the best ones involve the immune system: T cells, TH1/TH17 responses, and immune privilege.
Our gut flora is in constant competition: commensal (i.e., benign and/or healthy) and pathogenic (i.e., dangerous) microorganisms compete for fuel (the food we eat).
As such, these bacterial balances are highly sensitive: our food choices, stress, sleep quality, and medication use can significantly alter the balance of good:bad bacteria. If enough negative influencers persist, we can end up seeing a shift away from homeostasis and toward an “imbalanced” gut biome: one that is mostly pathogenic. This is sometimes termed as gut dysbiosis.
Interestingly, in states of gut dysbiosis, we also often see a shift in our immune health. Our T-regulatory responses begin to shut down, while our Th1 and Th17 responses start to increase (to fight off the pathogens) [3].
Th1 and Th17 cells are immune cells with the capacity to elicit inflammation and autoimmunity, and these markers stay elevated for too long of a period, autoimmune conditions can arise whereby the body begins to confuse its own tissue with pathogens (and starts to attack them).
We find this elevated immune profile not only in the gastrointestinal system, but also in the scalps of patients with alopecia areata [4,5].
But how can immune problems in the gut reach all the way to our scalps?
Because the lining of our gastrointestinal system is only one cell layer thick [6]. This one-cell thick wall is essentially what blocks microbes in our guts from traversing into our circulatory and lymphatic systems.
In cases of gut dysbiosis or IBS, increases in TH1 and TH17 begin to create inflammation, which begins to degrade this thin gut barrier. Eventually these one-cell thick linings become so damaged that pathogenic bacteria start escaping the gut and into the circulatory and lymphatic systems… whereby they begin to create the same inflammation elsewhere in the body.
Thus, this Th1 and Th17 reaction follows the bacteria, and immune dysregulation begins to jump from the gut to other tissues. One place in particular? Tissues that are supposed to remain privileged from the immune system: our scalp hair follicles [6].
What is immune privilege?
Certain body tissues have the ability to “hide” from our immune system. Why? In these tissues, even the slightest immune response (i.e., inflammation) might inhibit its ability to regenerate.
Hair follicles are one of these immune-privileged organs. They need protection from the immune system in order to continue the hair cycle: the normal degeneration and regeneration of our hair.
Hair follicles evade our immune systems by “lacking” lock-and-key mechanisms needed to alert the immune system of danger. These systems – like major histocompatability complex (MHC-I and MHC-II complex) – are best understood through organ transplants. After an organ transplant, MHC complexes alert the immune system of a new organ – or foreign invader – and the immune system begins to attack what it perceives to be a dangerous tissue. This is why organ transplants require immune-suppression drugs in order to avoid rejection [7].
When pathogenic bacteria escape the gut and enter into tissues surrounding the hair follicles, our immune system elicits the same reaction. Only this time, this inflammation comes with an additional consequence: immune privilege collapse for the hair follicles [8].
The end-result: the hair follicles are confused as foreign bodies by the immune system, inflammation ensues, and the hair follicles are destroyed. This is likely how alopecia areata develops from IBS and/or gut dysbiosis.
(Note: in cases of telogen effluvium and androgenic alopecia, the horseshoe shape taken on the crown of the head and the shedding of hair is possibly linked to histamine-related immune actions. Histamine gets broken down not only in the kidneys and liver, but also the gut [9,10]. We’ll explore a possible connection between gut health, excess histamine, and hair loss in future articles.)
Does alopecia areata only arise from an unhealthy gut?
No. Microflora don’t just exist in the gut; they also exist on our skin. Therefore, disruptions to our skin biome may also trigger an immune overreaction that may affect our hair follicles – regardless of whether gut dysbiosis is present [11].
That being said, microbiome alterations – whether in the skin or gut – aren’t always present in all alopecia areata patients. There are other underlying factors that can contribute to immune dysregulation and, thereby, the development alopecia areata.
However, for those whose microbiomes are affected, studies show that shifting the bacterial balance in favor of immune balance may lead to massive regrowth. We’ll get into that exact research (and more) below.
Inside the gut microbiome of alopecia areata patients
We have some immunological connections between the gut microbiome, the skin microbiome, and alopecia areata. But aside from theory, is there any evidence of disturbances in the gut microbiome of people with alopecia areata?
Yes. In one recent study, researchers took stool samples of patients affected by alopecia universalis and compared their microbiome to healthy controls. The gut biomes were different – albeit not dramatically different.
But interestingly, the researchers mentioned that bacterial species found in those with alopecia universalis (a more aggressive form of alopecia areata) also happened to be species involved in a number of other disease states.
Two notable mentions:
- Erysipelotrichaceae, which are linked to increased levels of TNF-alpha and gastrointestinal inflammation. TNF-alpha is elevated in some hair loss disorders, including androgenic alopecia.
- Lachnospiraceae, which is implicated in autoimmune conditions like ankylosing spondylitis and sclerosing cholangitis. In times of great stress, these microbes also increase. Interestingly, flare-ups of alopecia areata also often worsen in times of stress. [12].
Moreover, a handful of these alopecia areata subjects also had hypothyroidism – a thyroid condition that is linked to a type of hair loss called telogen effluvium. It shouldn’t be surprising to learn that hypothyroid patients also experience marked disturbances in the gut microbiome.
Certain beneficial microbes such as Bifidobacterium bifidum and Lactobacillus plantarum are shown to be able to bind to thyroid-peroxidase antibodies and thyroglobulin antibodies [13]. These are specific immune antibodies that target and destroy thyroid tissue if left unchecked. They are also a major component of Hashimoto’s thyroiditis, an autoimmune thyroid disease, that tends to present with comorbidity of alopecia in some cases [14].
Therefore, it’s not unreasonable to believe that changes to the gut microbiome not only contribute to alopecia directly, but also indirectly via endocrine disruptions to the thyroid gland itself [15].
Are the rates of alopecia areata increasing?
It’s tough to say.
Alopecia areata is seen as a relatively rare autoimmune disease. Previous associations with alopecia areata have been labeled at a lifetime risk between 1.7-2% [16]. However, this prevalence rate hasn’t been updated since 1989! Reports of alopecia areata are rising – particularly among dermatologists who are seeing evidence of the disease while performing scalp biopsies. So, there is some preliminary evidence to suggest the risk of alopecia areata might be higher than we think.
Interestingly, in 2015, researchers estimated around 1.3% of the entire United States population had received a diagnosis of IBS. While this may seem low, in reality, this statistic translates to over 3 million Americans facing a diagnosable form of gut dysbiosis [17].
It’s likely that the percent of people with IBS is much higher – as most people with poor-performing digestive systems chalk up the symptoms to “middle age”. Yet there is no reason biological why digestive health should dramatically decline after our twenties.
It’s our belief that both IBS and alopecia areata is on the rise, and that the increasing prevalence is a direct consequence of modern day living: high stress, poor sleep, bad food choices, and little-to-no physical activity.
Which brings us to our next question…
How do we restore gut function and (hopefully) reverse alopecia areata?
To reiterate, hair follicles lose immune privilege when pro-inflammatory T-cell responses increase and anti-inflammatory T-cell responses decrease. Thus, if we want to restore hair follicle immune privilege and prevent an autoimmune attack on our hair follicles, we need to find a way to normalize T-cell responses in these tissues.
This is where T-regulatory cells (T-regs) come into play. T-regs are a type of T-cell that help regulate and prevent unwanted immune responses.
Therefore, one potential avenue to restore immune privilege: upregulating T-regs activity. With enough T-regs activity, hair follicle immune privilege may restore, and normal hair growth may ensue.
So, how do we do this?
- Improve gut microbial diversity. Our gut bacteria are the main mediators of T-regs. Specifically, certain species of Clostridia and Bacteroides have been shown to support T-reg activity [18].
- Evaluate your fiber intake. Fiber is a crucial nutrient for our gut microbes. and subsequently create short-chain fatty acids. These bacteria ferment these fibers and, subsequently, produce short-chain fatty acids contribute to immune regulation locally and systemically. Interestingly, these fatty acids can also be produced from certain proteins that have not been degraded [18]. Could this be a connection with individuals consuming a carnivore diet experiencing relief from their digestive system, and even in some cases, hair loss [19]?
- The nuclear option: fecal microbiota transplant. More on this later.
It goes without saying that our diets, lifestyles, and environments all greatly impact T-regs activity (and thereby our predisposition to alopecia areata). For instance, the food we eat feed the microbes within our gastrointestinal tract. Note: if you’re looking for recommendations here, please see our article discussing treatments for alopecia areata.
Moreover, the kinds of food we eat may also affect the immune privilege of hair follicles. For instance, studies have shown that the proteins in gluten (alpha-gliadin) contain shapes and forms of proteins (i.e. epitopes) that look nearly identical to the same proteins that triggers autoimmune reactions in hair follicles (trichochyalin) [16].
For those with tight cell junctions in the intestinal tract, gluten consumption might not be problematic. But for those with leaky gut and/or gut dysbiosis, these cell walls are compromised. Therefore, more gluten may escape the gut and into the circulatory system – which would further dysregulate T-cell activity and worsen autoimmune responses.
Regardless, it’s imperative that we maintain proper gut flora to reduce our risk of autoimmune conditions – including hair loss disorders like alopecia areata.
Summary so far: Alopecia areata is an autoimmune condition characterized by a dysregulation of the immune system which leads to subsequent collapse of immune privilege. The delicate balance between beneficial and pathogenic bacteria within our gut can directly contribute to immune balance and, thus, may influence immune balance in alopecia areata – both by direct (systemic inflammation via dysbiosis + leaky gut) and indirect (bacterial regulation of T cells) mechanisms. Thus, it’s imperative that we support a healthy microbiome – one that favors commensal flora – in order to promote immune balance in alopecia areata.
Telogen effluvium and the microbiome
Telogen effluvium is a type of temporary hair loss caused by a disruption of the hair cycle. It often presents as diffuse thinning – i.e., even thinning throughout the entire scalp.
Unlike alopecia areata, telogen effluvium isn’t the result of the destruction of the hair follicle by way of collapsed immune privilege. Rather, telogen effluvium is often just the result of a delay in time between when a hair sheds and when a new hair grows long enough for you to notice its been replaced.
Telogen effluvium (TE) has a broad range of causes, but most cases relate to stress, nutrient imbalances, and hypothyroidism.
Is there a connection between telogen effluvium and our gut microbiome?
Full disclaimer: there is very little research on TE and the microbiome. However, we can extrapolate from related research and find connection points that are worth considering.
TE is the prevailing type of hair loss in patients with IBS
In a study performed on the frequency of hair loss types in association with IBS, telogen effluvium appeared as the dominant form of hair loss – with 66% of participants suffering from TE [20]. Even more interesting was the association between flare-ups of IBS coinciding with periods of increased hair thinning in TE subjects – suggesting a direct relationship between gut health and hair shedding.
Case reports: TE and IBS / Crohn’s Disease
IBS flare-ups coinciding with TE-related hair shedding have also been reported in a case study of a 10-year-old child suffering from Crohn’s disease. Interestingly, the child showed no nutrient deficiencies, no gluten antibodies, and no abnormal immune, inflammatory, and antibody markers. The only culprit left as a driver of telogen effluvium? Crohn’s disease itself.
This hypothesis was supported after medication use – which put IBS into remission and also stopped the hair loss entirely. When the medication ceased, Crohn’s disease returned (as did the hair loss) [21].
In our opinion, that’s no coincidence.
Additionally, in 1989, researchers had described a similar situation in a 21-year-old woman, who experienced telogen effluvium for six years prior to the onset of Crohn’s in the ileum-colon (the area where the dominant amount of gut microbes are found).
She had experienced bouts of diarrhea during those six years that she had perceived to be normal, and examinations ruled out any other culprit aside from the Crohn’s itself [22]. When medication was prescribed to repair the damage done by Crohn’s, the hair loss had also been completely stopped.
How does this relate to the gut flora? While stool examinations did not show bacteria within the stool, it’s important to note that stool samples aren’t always reflective of the gut microbiome [23]. Further, there are only a number of species that are coated by our intestinal mucous that appear in stool samples [24].
So while they can be helpful, stool samples should not be the only method in determining if microbial species were to blame for IBD-related conditions, and subsequently the hair loss experienced.
This is also precisely what we find when investigating the mucous layer of gastrointestinal tissue affected by Crohn’s: there appears a marked change in the kinds of bacteria within the colon and also a change in the diversity of these bacteria [25].
While no specific bacteria has been found to be the definite cause of Crohn’s (which could connect to specific microbes associated with hair loss), we do know that the overall bacterial density and bacterial diversity is altered towards a handful that retain total dominion over the gut. Additionally, stool samples reflect something entirely different and do not support the changes seen in the mucosal realm of the GI-tract [25].
To really hammer this nail in, when investigating the kind of immunological actions seen in Crohn’s, we see similar immunological players to those seen in alopecia areata, telogen effluvium, and other IBD conditions.
- Higher Th1 and Th17 (associated bad guys)
- Lower T-reg action (the good guy)
- Increased TGF-Beta (the snitch that calls the bad guys)
- Oxidative stress (the mess created by the bad guys) [26,27,28].
Summary so far: telogen effluvium (TE) is a form of hair loss characterized by increased shedding throughout the scalp. It’s usually attributed to underlying conditions like stress, hypothyroidism, and nutrient deficiencies. And while there is very little research on the connection between the microbiome and TE, several case studies and observational studies have shown a connection between TE and two IBD conditions: IBS and Crohn’s – two conditions that may be mediated by microbial changes in the gut.
If low bacterial density and low bacterial diversity are linked to telogen effluvium, are probiotics a possible treatment?
It’s hard to say. But it’s possible.
A 2013 research team wanted to test whether supplementing with commensal bacteria – like L. reuteri – could improve skin and hair quality. So, they conducted a series of tests on mice. They designed a study with three groups of mice fed different foods:
- One group received “regular” feed.
- One group received yogurt – which is known to contain a litany of beneficial microbes.
- Another group received L.reuteri as a probiotic supplement.
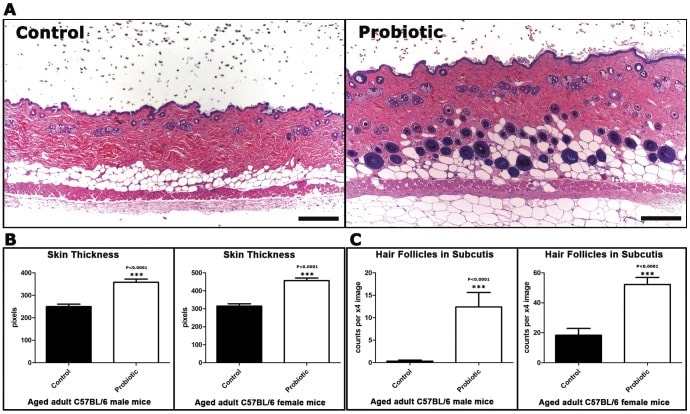
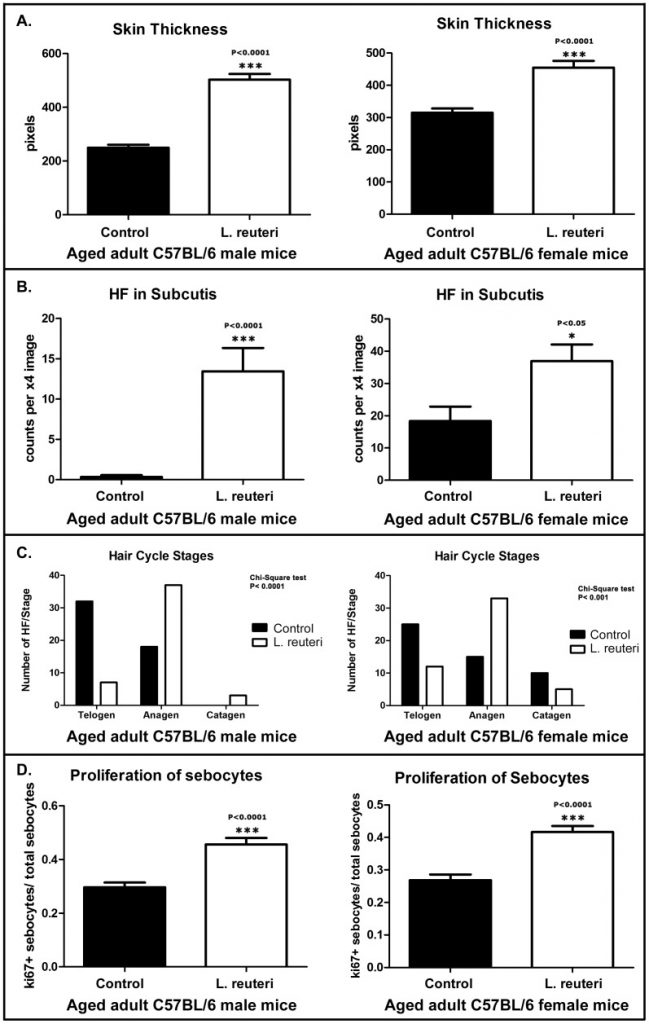
The results were impressive: the mice fed yogurt or L.reuteri had significant increases in hair growth and the luster of their fur. Skin thickness also improved.
Skin thickness & hair follicle in mice fed conventional foods vs. L. reuteri
Most importantly, both groups showed a dramatic preference in hair growth within the anagen phase, and very few within both telogen and catagen phases [29].
This indicates a direct effect from the probiotics in not only preserving hair health, but also at increasing the percentage of hair phases we aim to improve in various forms of alopecia; the anagen phase.
Skin thickness & hair follicle in mice fed conventional foods vs. L. reuteri (continued)
Interestingly, L.reuteri was once a dominant bacterial species in human beings. However, recently, the prevalence of it being found within our guts is around only 10-20% of the total population [30].
Could this be part of a larger picture in the relation of the gut microbiome to hair loss disorders? Maybe, maybe not – we just don’t know.
The problem with probiotics? They probably don’t significantly alter our microbiome
Beyond L. reuteri, there isn’t much research linking probiotic supplementation to hair growth improvements (aside from a kimchi study which we’ll get into later). And while the absence of evidence isn’t evidence against something, it’s also important to recognize that we all know lots of people taking probiotics. Very few of them have reported hair growth as a side effect.
Part of the reason why? Well, probiotics have a hard time colonizing the gut. Moreover, everyone’s guts are different; as such, it’s really the whole concert of bacterial species that act to influence immunity and other health systems… not necessarily one single bacterial species.
To our knowledge, there aren’t any probiotics on-the-market that have actually shown to completely revamp someone’s gut flora. As such, it’s unlikely that most probiotics do much to change anything at all.
Having said that, there are ways to completely revamp our gut microbiomes… but they don’t involve probiotics; rather they involve transplants.
Specifically, stool transplants. And this is where the connection between the gut biome and hair loss becomes incredibly interesting.
Summary so far: low bacterial diversity and low bacterial density are linked to telogen effluvium. However, probiotics are likely limited in their ability to improve gut health here – at least in the long-term. This is because most probiotics lack the capability to inoculate the gut beyond point-of-use – meaning the effects of probiotics generally last only as long as you take them. However, there are more extreme versions of microbiome therapies that could address this problem. They’re called stool transplants, and the next section covers them in detail (as well as their connection to hair loss).
Fecal microbiota transplants: a new frontier for hair health?
Fecal microbiota transplants (FMT) are exactly what they sound like: taking the feces (and its bacteria) of a healthy person and transplanting that feces into someone else with pathological microbiome alterations. If you’re interested in learning more, we’d recommend checking out our article on stool transplants and hair regrowth.
Typically, a doctor receives the donor’s fecal sample, purifies and isolates the bacteria, and then transplants the bacteria into the recipient. There are also circumstances where healthy feces is packaged into supplements and then ingested – or where the bacteria is delivered through an enema.
This type of therapy was originally developed as an experimental “last resort” for people fighting life-threatening, antibiotic-resistant C. difficile infections. Early studies found C. difficile remission rates of 90% – significantly better than most antibiotics. Since those studies, people receiving FMTs have reported puzzling (but fascinating) things: changes in body mass index (BMI) to match the BMI of their “donor”… personality changes similar to their “donor”… and even the reversal of autoimmune-related hair loss disorders (as discussed earlier).
Interestingly, there are now anecdotes of stool transplants improving pattern hair loss – and through completely unidentified mechanisms.
In one instance, one user of a hair loss forum performed a fecal transplant in an attempt to treat his irritable bowel syndrome. He’d also been dealing with male pattern hair loss (unsuccessfully) – having tried finasteride from some time period but without success.
After the transplant, his hair (which had been thinning for the past five years) almost entirely stopped shedding. One year later, he reported significant hair thickening… and some regrowth that occurred in the absence of any other treatment.
Stool transplants & inadvertent hair thickening / hair regrowth (androgenic alopecia)
While there are many others who have attributed improved hair health to their FMTs, these anecdotes are oftentimes confounded by the fact they were also using treatments such as finasteride and minoxidil. As such, it is impossible to fully attribute the hair growth (or pause in shedding) to the fecal transplant.
However, in published case reports, correlations can be better established – with some demonstrating a strong connection between FMTs… and massive hair regrowth.
A few examples (not just related to androgenic alopecia):
- An 86-year old man who had a history of colon cancer, depression and diarrhea. Performing a FMT not only improved a plethora of health markers, but also resulted in the total regrowth of an alopecic patch on the back of his head. Not only that, but his gray hair began to darken again [31].
- A 34-year old woman who had a history of ulcerative colitis and hair loss (for which she used minoxidil to no avail) performed a FMT [32]. After seven sessions, she had a remarkable decrease in hair thinning and improvement in hair growth which was present even after a six-month follow-up.
- A 38-year old man with alopecia universalis (i.e. total hair loss all over the scalp, face, and body) saw dramatic improvements to his hair loss after performing FMT [33].
- A 20-year old man with Clostridium difficile infection resistant to treatment underwent FMT. Prior to the transplant he had alopecia universalis. Post transplant, he grew a full head of hair again, while also growing hair in areas surrounding parts of his body that hadn’t seen hair in months to years [33].
These case studies shine a light on just how powerful the gut microbiome is and how large a role it plays in the overall state of our hair health throughout the entire body, not just the scalp. More importantly, a majority of those studies also demonstrate that FMT’s benefits extend to many other symptoms and aren’t just limited to hair regrowth.
Androgenic Alopecia & The Gut Microbiome
Before we dive into a possible connection between pattern hair loss (AGA) and the gut microbiome – it’s worth noting that research here is incredibly limited. Because of these limitations, most of our commentary here is purely speculative.
Having said that, there are several possible lines of research implicating the gut microbiome as a causative agent and a therapeutic agent for pattern hair loss.
The most plausible connection? The impact our gut microbiome has on our endocrine system: the tissues that regulate the production, secretion, and metabolism of various hormones, including our sex hormones.
DHT, gut bacteria, and pattern hair loss: what’s the connection?
Research demonstrates that there is cross-talk between the gut microbiome and our endocrine system, and that our gut bacteria may play a significant role in hormone production and metabolism. In fact, some researchers argue the gut itself should be considered an endocrine organ.
This is for good reason: the gut flora demonstrates the ability to modulate the entirety of our HPA-axis: the headquarters that controls our stress response and sex hormone production, including androgens [34].
AGA is a hair loss disorder that is mediated by male hormones: specifically, levels of dihydrotestosterone (DHT) in the scalp. Thus, if the microbiome state can alter DHT activity in skin tissues, it’s also possible that it could influence AGA.
It’s important to note: no direct evidence has proven the gut microbiome contributes to AGA, there is one (arguably stunning) study, performed in 2019, showing how important the gut microbiome may influence DHT activity [35].
For the first time, these investigators demonstrated that the gut microbiome of mice could metabolize androgens. They also found that the amount of free DHT (unbound) in the feces and colons of mice was astoundingly high: about 20x that seen in the blood.
This led researchers to investigate DHT levels in the colon of healthy men. The subsequent analysis produced even more dramatic results than the former experiment: unbound DHT levels were close to 70x that of unbound DHT levels measured in the blood.
The rationale? Enzymes produced by bacteria in our gut produce an enzyme called beta-glucuronidase, an enzyme that unconjugates hormones – producing free (unbound) hormones. In other words, they take hormones that have been conjugated by the liver (to enhance excretion) and turn them back into free (unconjugated) hormones. Further supporting this is the fact that DHT in the colons of experimental “germ-free” (i.e. don’t possess bacteria in their guts) was completely conjugated – emphasizing the fact that bacteria are the culprits behind the free DHT.
The most important difference between unconjugated and conjugated hormones like DHT: unconjugated DHT can be used by our cells (and, thus influence AGA processes in hair follicle cells); conjugated DHT cannot be used in the same way.
This begs the question: if the bacteria in our gut can unconjugate massive amounts of DHT in our gastrointestinal tract, can this lead to massive increases in DHT activity elsewhere (like in our scalp), and, thus, contribute to AGA? In other words, can DHT be recirculated back into our body and reach our scalp?
Let’s look at the research.
If gut bacteria can produce and regulate DHT, can they recirculate it back into our blood?
While no research has currently looked at the ability of androgens like DHT to be reabsorbed through the gut, this mechanism has been observed with estrogen. Studies show that variations in gut flora can contribute to an increase in estrogen activity within the body via recycling between the gut and liver [36]. Some researchers even believe that the contribution of this recirculation to estrogen activity is so significant, that they’ve gone as far as to label it the estrogen-gut microbiome axis [37].
Thus, recirculation of hormones is a very plausible contributor to overall hormonal activity in the body. Considering studies have shown this occurs with estrogen, it wouldn’t be far fetched to assume that something similar could occur with DHT and other androgens.
So, what’s the verdict? Can dramatic amounts of unconjugated DHT (present in levels as high as 70-fold of serum measurements) recirculate into the body and thereby potentially contribute to androgen-driven pattern hair loss?
It’s possible. However, this may not be the case for all men with AGA. Why? If gut-generated DHT were a contributor to AGA, we would probably also see high levels of blood DHT levels in men with pattern hair loss. While this is true for some men, not all men with AGA have increased serum DHT levels [38, 39]. So, unless gut-regulated DHT is somehow bypassing the circulatory system to directly influence levels of scalp skin DHT, we don’t really have evidence that this is the case.
So, does this mean that the microbiome is completely unrelated to AGA? Not necessarily. It just means that we don’t have clearcut evidence connecting these things together (yet).
Do changes to our microbiome influence AGA?
There’s one small study that might help answer this question.
In Korea, clinicians performed a pilot study testing whether probiotics + kimchi – a good source of beneficial bacteria (ones that also modulate the beneficial bacteria in our microbiome) – might improve hair counts in men with AGA [40].
After fours months, researchers found that probiotics + kimchi supplementation improved hair counts by 5%, as well as the overall look and feel of hair. At the same time, there was no control group – so it’s tough to discern whether these increases also confounded with other things that influence hair counts – like seasonality or the placebo effect.
In any case, let’s assume that these uncontrolled factors did not influence results, and that the hair count increases were strictly the result of probiotic + kimchi supplementation and, subsequently, bacterial changes in the gut. If true, is it possible that these improvements might’ve led to lower levels of serum DHT?
Maybe.
Studies show that a strain of bacteria produced during fermentation, Lactobacillus acidophilus, can decrease the number of beta-glucuronidase enzymes in the gut of humans. That’s the exact same enzyme responsible for unconjugating DHT [41].
As such, it’s possible that probiotics in the kimchi product used in the study directly reduced beta-glucuronidase activity in the gut, thereby reducing free DHT available for recirculation. If free DHT in the gut truly does contribute to elevated serum DHT, it’s conceivable that the study’s hair density improvements might be attributed to reduced DHT levels by way of reduced free DHT recirculation.
That being said, this is a massive stretch of the imagination. It’s easy to connect a bunch mechanisms together and ask, “What if?” It bears repeating: this hasn’t been proven yet. And unfortunately, those researchers did not measure how kimchi + probiotics affected serum or scalp DHT levels.
Summary so far: studies have shown that androgenic alopecia (AGA) is causally linked to the hormone DHT. Interestingly, bacteria inside our microbiome have the ability to (1) produce DHT, glucuronidate DHT (i.e., convert DHT from lipophilic to hydrophilic so that it can enter into the instestinal tract), and (3) convert DHT into its active or inactive forms. This is further reinforced by studies showing that levels of free DHT are 70x higher in stool samples than in the blood of healthy men – implicating the intestines as a massive reservoir for DHT, with gut bacteria as the gatekeepers. Whether this influences pattern hair loss – we don’t yet know! But technically speaking, it is possible for this unconjugated DHT to recirculate into the serum and have significant influence over a variety of androgen-linked disease states: heart disease, prostate enlargement, and maybe even androgenic alopecia.
In any case, discussing the effects the microbiome on DHT only in the context of free DHT produced in the gastrointestinal tract fails to acknowledge a lot of the big picture. In reality, there are a myriad of other ways the microbiome and, thus, alterations to the microbiome may affect DHT – even if recirculation of DHT isn’t a significant contributor.
Let’s explore these points.
How else might the microbiome be related to AGA?
There are other means by which the microbiome might be related to AGA, and it primarily has to do with metabolic health:
- Women with polycystic ovarian syndrome (PCOS) usually also deal with female pattern hair loss (FPHL, female AGA) [42]. Both FPHL and PCOS are believed to be driven by excess androgens, which can be driven by poor blood sugar control — in which gut microbiome is believed to play a significant role [43, 44]. Thus, pathological alterations to the microbiome may contribute to FPHL. Studies in rats support this, demonstrating that certain diets may increase androgen production by way of microbiome changes. The opposite is also likely true: improvements to the microbiome may reduce androgen production and, in turn, improve FPHL. Interestingly, the same study that examined the effects of kimchi on male AGA also looked at its effects on female AGA [40]. They found that female AGA patients were actually more likely to respond to kimchi than men with AGA, lending support to the idea that a healthier microbiome may improve AGA, potentially by reducing androgen production.
- Similar to PCOS and FPHL, studies show that early-onset AGA in men is associated with metabolic syndrome, a set of co-morbidities including hypertension, insulin resistance, and abnormal cholesterol and triglycerides in the blood [45]. Microbiome alterations are believed to contribute to metabolic syndrome, primarily through increases in low-grade inflammation [46]. Similarly, men with metabolic syndrome show improved lab markers after FMT, directly correlating with improvements to microbiome diversity and short-chain fatty acid production (indicative of an increased abundance of good bacteria). Collectively, the evidence suggests that adverse changes to the microbiome do appear to play a role in metabolic syndrome and that, conversely, improvements to microbiome health translate to improvements in metabolic health. The relevance to male AGA: increased systemic inflammation, which contributes to metabolic syndrome, might also accelerate AGA. As such, its possible that hair health may improve alongside metabolic health, subsequent to microbiome improvements.
In general, it’s important to note that research on the microbiome is still in its infancy. As such, we still have a lot of research to amass before we fully understand the microbiome, let alone how it affects our hair health.
Resultantly, this research should be treated as speculation. Moreover, you shouldn’t rely upon microbiome alterations alone to treat your hair loss.
So, don’t: forego treatments to solely work on microbiome health (until we better understand how to effectively leverage changes to the microbiome for hair growth).
Do: work on microbiome health while simultaneously targeting your hair loss with available, evidence-based treatments.
Is there anything else we should know about the microbiome and AGA?
Yes, but it doesn’t necessarily have to do with how the microbiome (or alterations to its structure) influences AGA. Rather, it has to do with how one of the most common treatments for AGA may affect the microbiome and, resultantly, contribute to lasting side effects.
You might have already guessed what treatment we’re talking about: finasteride, a conventional DHT reducer prescribed to AGA patients.
Finasteride is an incredibly effective treatment: it reduces scalp DHT levels by ~60%, stops pattern hair loss in 80-90% of men, and leads to a 10% increase in hair counts and some hair thickening over a two-year period. For these reasons, many men use this drug and simply forget about balding.
At the same time, one thing that holds back many people from trying finasteride is its risk of side effects. This topic is hotly debated, and we don’t have room to get into it in this article. However, between 1% to 40% of men taking finasteride report side effects after trying finasteride (depending on the quality of the study we reference). And for a fraction of those who experience side effects, they don’t seem to go away after stopping finasteride.
This has led to the coining of a debated condition referred to as post-finasteride syndrome (PFS). Some researchers and clinicians acknowledge it as real, while others dismiss it as a psychosomatic phenomenon. Note: there’s not yet enough research to claim that this condition doesn’t exist. So, anyone who has an absolutist stance on PFS as a real or fake condition probably hasn’t looked hard enough into the literature. It’s complicated.
In any case, there have been some interesting studies related to post-finasteride syndrome patients and the microbiome.
Post-finasteride syndrome and the microbiome
In a pilot study performed on 23 men diagnosed with post-finasteride syndrome, researchers found that their microbiomes were significantly altered. Specifically, they found reductions to both bacterial colonization and bacterial diversity [47].
Studies in animals mirror these effects [48]. Microbiome changes subsequent to finasteride treatment in rats mimic the microbiome changes that occur after castration. Interestingly, these microbiome changes also occur alongside significant mood and brain changes – similar to what is reported in patients with post-finasteride syndrome.
It’s possible that the mood and brain changes simply occur alongside microbiome changes and are only related to the effects of finasteride or castration, possibly through altering brain physiology and physical structure long-term. At the same time, it’s also very well possible that the effects of DHT-suppression on the microbiome contribute to post-finasteride syndrome, given compelling evidence that the microbiome plays a significant role in neurotransmitter activity.
As such, improving microbiome health may not only contribute to improved hair growth in AGA, it might also improve post-finasteride syndrome in men who have used finasteride to treat their AGA.
Summary so far: The microbiome might be a therapeutic tool to improve androgenic-related disease states that often present alongside pattern hair loss – like polycystic ovarian syndrome (PCOS) and metabolic syndrome. Moreover, the microbiome might be a future therapeutic target for individuals suffering from post-finasteride syndrome (PFS). As new research unfolds, we’ll continue to update this article.
Summary
The gut microbiome is intimately tied to human health. Moreover, the gut microbiome might also be a causative and therapeutic agent for the world’s most common hair loss disorders: alopecia areata, telogen effluvium, and androgenic alopecia.
From the ability to modulate our immune system, to its ability to improve intestinal health, to even the ability to alter the production and metabolism of our sex hormones, the gut microbiome could (and likely will) be the next frontier in hair loss research. However, research is still in its infancy – and big advancements in this space are likely decades away.
In the meantime, you can support the health of your microbiome by eliminating processed foods and excess sugars, and beginning to eat a more whole foods-based diet. A few lucky ones might also see hair improvements to alopecia areata and telogen effluvium. But for pattern hair loss (androgenic alopecia), improvements here are likely reserved for more aggressive microbiome interventions (i.e., stool transplants, extreme dietary changes) – and that’s if there is a connection at all.
Have any questions about the microbiome and hair loss? You can reach us in the comments any time.
References
- Patel KV, Farrant P, Sanderson JD, Irving PM. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013 Jul;19(8):1753-63.
- Peterson DM, King BA. Gut instinct: Using tofacitinib to treat alopecia areata in the context of comorbid inflammatory bowel disease. JAAD Case Rep. 2020 Nov 6;7:44-46. doi: 10.1016/j.jdcr.2020.10.027. PMID: 33319003; PMCID: PMC7727289.
- Rahmanpour M, Keramat F, Jourghasemi S, Rashidi G, Abdolmaleki M, Solgi G, Hajilooi M. Direct correlation between Th1 and Th17 responses in immunity to Brucella infection. Microbes Infect. 2019 Dec;21(10):441-448. doi: 10.1016/j.micinf.2019.05.002. Epub 2019 Jun 8. PMID: 31185302.
- Song, T., & Guttman-Yassky, E. (2020). Alopecia Areata: A Complex Cytokine Driven Disease. Journal of Investigative Dermatology Symposium Proceedings, 20(1), S55–S57. doi:10.1016/j.jisp.2020.04.007
- Gálvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014 Mar 25;2014:928461. doi: 10.1155/2014/928461. PMID: 25101191; PMCID: PMC4005031
- Bertolini M, McElwee K, Gilhar A, Bulfone-Paus S, Paus R. Hair follicle immune privilege and its collapse in alopecia areata. Exp Dermatol. 2020 Aug;29(8):703-725. doi: 10.1111/exd.14155. PMID: 32682334.
- Loewendorf, A., & Csete, M. (2013). Concise review: immunologic lessons from solid organ transplantation for stem cell-based therapies. Stem cells translational medicine, 2(2), 136–142. https://doi.org/10.5966/sctm.2012-0125
- Barahmani N, de Andrade M, Slusser JP, Zhang Q, Duvic M. Major histocompatibility complex class I chain-related gene A polymorphisms and extended haplotypes are associated with familial alopecia areata. J Invest Dermatol. 2006 Jan;126(1):74-8. doi: 10.1038/sj.jid.5700009. PMID: 16417220.
- Grace SA, Sutton AM, Abraham N, Armbrecht ES, Vidal CI. Presence of Mast Cells and Mast Cell Degranulation in Scalp Biopsies of Telogen Effluvium. Int J Trichology. 2017 Jan-Mar;9(1):25-29. doi: 10.4103/ijt.ijt_43_16. PMID: 28761261; PMCID: PMC5514792.
- Michel, L., Reygagne, P., Benech, P., Jean-Louis, F., Scalvino, S., Ly Ka So, S., … Hocquaux, M. (2017). Study of gene expression alteration in male androgenetic alopecia: evidence of predominant molecular signalling pathways. British Journal of Dermatology, 177(5), 1322–1336.
- Migacz-Gruszka, K., Branicki, W., Obtulowicz, A., Pirowska, M., Gruszka, K., & Wojas-Pelc, A. (2019). What's New in the Pathophysiology of Alopecia Areata? The Possible Contribution of Skin and Gut Microbiome in the Pathogenesis of Alopecia - Big Opportunities, Big Challenges, and Novel Perspectives. International journal of trichology, 11(5), 185–188.
- Moreno‐Arrones, O. M., Serrano‐Villar, S., Perez‐Brocal, V., Saceda‐Corralo, D., Morales‐Raya, C., Rodrigues‐Barata, R., … Vano‐Galvan, S. (2019). Analysis of the gut microbiota in alopecia areata: identification of bacterial biomarkers. Journal of the European Academy of Dermatology and Venereology
- Zhao, F., Feng, J., Li, J., Zhao, L., Liu, Y., Chen, H., … Wei, Y. (2018). Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid, 28(2), 175–186.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018 Sep 1;41(9). doi: 10.1093/sleep/zsy111. PMID: 29955877.
- Billoni N, Buan B, Gautier B, Gaillard O, Mahé YF, Bernard BA. Thyroid hormone receptor beta1 is expressed in the human hair follicle. Br J Dermatol. 2000 Apr;142(4):645-52. doi: 10.1046/j.1365-2133.2000.03408.x. PMID: 10792213.
- Borde, A., & Åstrand, A. (2018). Alopecia areata and the gut—the link opens up for novel therapeutic interventions. Expert Opinion on Therapeutic Targets, 22(6), 503–511.
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years — United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:1166–1169. DOI: http://dx.doi.org/10.15585/mmwr.mm6542a3external icon.
- Omenetti, S., & Pizarro, T. T. (2015). The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Frontiers in immunology, 6, 639. https://doi.org/10.3389/fimmu.2015.00639
- McClellan, S. W., Du Bois, F. E. (1930). Prolonged meat diets with a study on kidney function and ketosis. Journal of biological chemistry.
- Shah, R., Abraham, B., Hou, J., & Sellin, J. (2015). Frequency and associated factors of hair loss among patients with inflammatory bowel disease. World journal of gastroenterology, 21(1), 229–232. https://doi.org/10.3748/wjg.v21.i1.229
- Rogalidou, M., Tzoufi, M., Katsanos, K., Gaitanis, G., Zioga, A., Tsianos, E., & Siamopoulou-Mavridou, A. (2014). Telogen effluvium as the first symptom of Crohn's disease in a child. Annals of gastroenterology, 27(4), 418–420.
- Schattner, A., & Shanon, Y. (1989). Crohn's ileocolitis presenting as chronic diffuse hair loss. Journal of the Royal Society of Medicine, 82(5), 303–304.
- Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D'hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J. Population-level analysis of gut microbiome variation. Science. 2016 Apr 29;352(6285):560-4. doi: 10.1126/science.aad3503. Epub 2016 Apr 28. PMID: 27126039.
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014 Aug 28;158(5):1000-1010. doi: 10.1016/j.cell.2014.08.006. PMID: 25171403; PMCID: PMC4174347.
- Khanna, S., & Raffals, L. E. (2017). The Microbiome in Crohn’s Disease. Gastroenterology Clinics of North America, 46(3), 481–492.
- Savci U, Senel E, Oztekin A, Sungur M, Erel O, Neselioglu S. Ischemia-modified albumin as a possible marker of oxidative stress in patients with telogen effluvium. An Bras Dermatol. 2020 Jul-Aug;95(4):447-451. doi: 10.1016/j.abd.2020.01.005. Epub 2020 May 6. PMID: 32482549; PMCID: PMC7335859.
- Katagiri K, Arakawa S, Hatano Y. In vivo levels of IL-4, IL-10, TGF-beta1 and IFN-gamma mRNA of the peripheral blood mononuclear cells in patients with alopecia areata in comparison to those in patients with atopic dermatitis. Arch Dermatol Res. 2007 Jan;298(8):397-401. doi: 10.1007/s00403-006-0700-2. Epub 2006 Sep 22. PMID: 17021766.
- Brand, S. (2009). Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut, 58(8), 1152–1167.
- Levkovich, T., Poutahidis, T., Smillie, C., Varian, B. J., Ibrahim, Y. M., Lakritz, J. R., Alm, E. J., & Erdman, S. E. (2013). Probiotic bacteria induce a 'glow of health'. PloS one, 8(1), e53867. https://doi.org/10.1371/journal.pone.0053867
- Sinkiewicz, Gabriela. (2010). Lactobacillus reuteri in health and disease. Malmo university. Health and societal dissertaion.
- Xie, Wen-Rui. et al. (2019). Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: A case report and review of the literature. Department of Gastroenterology.
- Mahajan, R., Midha, V., Singh, A., Mehta, V., Gupta, Y., Kaur, K., Sudhakar, R., Singh Pannu, A., Singh, D., & Sood, A. (2020). Incidental benefits after fecal microbiota transplant for ulcerative colitis. Intestinal research, 18(3), 337–340. https://doi.org/10.5217/ir.2019.00108
- Rebello, D., Wang, E., Yen, E., Lio, P. A., & Kelly, C. R. (2017). Hair Growth in Two Alopecia Patients after Fecal Microbiota Transplant. ACG case reports journal, 4, e107. https://doi.org/10.14309/crj.2017.107
- Clarke, G., Stilling, R. M., Kennedy, P. J., Stanton, C., Cryan, J. F., & Dinan, T. G. (2014). Minireview: Gut microbiota: the neglected endocrine organ. Molecular endocrinology (Baltimore, Md.), 28(8), 1221–1238. https://doi.org/10.1210/me.2014-1108
- Colldén, H., Landin, A., Wallenius, V., Elebring, E., Fändriks, L., Nilsson, M. E., Ryberg, H., Poutanen, M., Sjögren, K., Vandenput, L., & Ohlsson, C. (2019). The gut microbiota is a major regulator of androgen metabolism in intestinal contents. American journal of physiology. Endocrinology and metabolism, 317(6), E1182–E1192. https://doi.org/10.1152/ajpendo.00338.2019
- Adlercreutz H, Pulkkinen MO, Hämäläinen EK, Korpela JT. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J Steroid Biochem. 1984 Jan;20(1):217-29. doi: 10.1016/0022-4731(84)90208-5. PMID: 6231418.
- Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017 Sep;103:45-53. doi: 10.1016/j.maturitas.2017.06.025. Epub 2017 Jun 23. PMID: 28778332.
- Zhang Y, Xu J, Jing J, Wu X, Lv Z. Serum Levels of Androgen-Associated Hormones Are Correlated with Curative Effect in Androgenic Alopecia in Young Men. Med Sci Monit. 2018;24:7770-7777. Published 2018 Oct 30. doi:10.12659/MSM.913116
- Stárka L, Cermáková I, Dusková M, Hill M, Dolezal M, Polácek V. Hormonal profile of men with premature balding. Exp Clin Endocrinol Diabetes. 2004 Jan;112(1):24-8. doi: 10.1055/s-2004-815723. PMID: 14758568.
- Park, D. W., Lee, H. S., Shim, M. S., Yum, K. J., & Seo, J. T. (2020). Do Kimchi and Cheonggukjang Probiotics as a Functional Food Improve Androgenetic Alopecia? A Clinical Pilot Study. The world journal of men's health, 38(1), 95–102. https://doi.org/10.5534/wjmh.180119
- Goldin BR, Swenson L, Dwyer J, Sexton M, Gorbach SL. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J Natl Cancer Inst. 1980 Feb;64(2):255-61.
- Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2(2):189-199.
- Legro, R. S., Bentley-Lewis, R., Driscoll, D., Wang, S. C., & Dunaif, A. (2002). Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. The Journal of clinical endocrinology and metabolism, 87(5), 2128–2133. https://doi.org/10.1210/jcem.87.5.8513
- Dumitrescu R, Mehedintu C, Briceag I, Purcarea VL, Hudita D. The polycystic ovary syndrome: an update on metabolic and hormonal mechanisms. J Med Life. 2015;8(2):142-145.
- Gopinath H, Upadya GM. Metabolic syndrome in androgenic alopecia. Indian J Dermatol Venereol Leprol. 2016 Jul-Aug;82(4):404-8. doi: 10.4103/0378-6323.174421. PMID: 27279298.
- Wang PX, Deng XR, Zhang CH, Yuan HJ. Gut microbiota and metabolic syndrome. Chin Med J (Engl). 2020;133(7):808-816. doi:10.1097/CM9.0000000000000696
- Borgo F, Macandog AD, Diviccaro S, Falvo E, Giatti S, Cavaletti G, Melcangi RC. Alterations of gut microbiota composition in post-finasteride patients: a pilot study. J Endocrinol Invest. 2020 Sep 19. doi: 10.1007/s40618-020-01424-0. Epub ahead of print. PMID: 32951160.
- Diviccaro, S., Giatti, S., Borgo, F., Barcella, M., Borghi, E., Trejo, J. L., … Melcangi, R. C. (2018). TREATMENT OF MALE RATS WITH FINASTERIDE, AN INHIBITOR OF 5ALPHA-REDUCTASE ENZYME, INDUCES LONG-LASTING EFFECTS ON DEPRESSIVE-LIKE BEHAVIOR, HIPPOCAMPAL NEUROGENESIS, NEUROINFLAMMATION AND GUT MICROBIOTA COMPOSITION. Psychoneuroendocrinology.
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Perfect Hair Health Team
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down