- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
7 Best Oils for Hair Growth
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
Articles
In less than a decade since its founding, Hims has grown into an internationally recognized brand. With a range of products available in the USA that can boost testosterone, help with weight loss, and tackle issues in the bedroom, Hims is, unsurprisingly, targeted directly at men. In keeping with this approach, they also have a strong selection of products to tackle hair loss and androgenic alopecia (AGA). As a telehealth provider, Hims offers prescription medications alongside shampoos and supplements.
The brand positions itself as providing highly individualized care: “It’s more than a plan, it’s personal.” Given the diversity of ways in which hair loss can present, and the sometimes unpredictable nature of men’s response to treatment, this approach is often a strong route to a successful regrowth. But how does Hims’ offering stack up? We’ll examine the range of products available through Hims’ telehealth service in the USA, assess the clinical evidence supporting each option, and explore whether the brand can support effective hair loss management.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Key Takeaways
- Hims offers proven medications like finasteride and minoxidil in convenient formats, which can be effective for many users.
- Hims promises personalization and individualized plans. However, this personalization is mostly limited to a smaller assortment of Rx options than the involvement of their assessment surveys might lead users to believe, with fewer than 5 Rx products for each gender (male and female) from which to choose.
- The absence of treatment options, escalation routes, and dosing changes makes sustained management more difficult – especially under circumstances where side effect troubleshooting is warranted. While Hims’ Rxproducts surely help boost hair growth, the limits of customization might still mean many of its customers may lack the options needed to resolve side effects, address scalp inflammation, or even further boost hair growth.
What Does Hims Offer?
Hims offers a range of prescription hair loss drugs, as well as over-the-counter products, including 5% minoxidil and anti-dandruff shampoos. The Rx offering features reliable, clinically proven pharmaceuticals delivered in an impressive range of vehicles: oral supplements are available in pills and “chews”, while topical formulations are available as sprays, serum, and foams.
We’ll take a tour of the product range and see what the Rx catalog offers. Does the science back up the use of the selection to tackle hair loss? And to what extent is it “personal”?
Finasteride (from $22 a month)
Starting off simple, 1 mg a day of finasteride is FDA-approved for the treatment of androgenic AGA in men. There’s a reason finasteride is one of the most popular treatments for hair loss globally. It has been widely studied both for its efficacy in reversing the impact of AGA and for its potential long-term side effects. It’s reliable, widely available, and relatively cheap.
Finasteride lowers levels of a hormone called dihydrotestosterone (DHT), which is known to be involved in AGA. An enzyme called 5-alpha reductase converts testosterone into DHT, which then binds to androgen receptors in susceptible hair follicles in the scalp. This leads to shortening of the growth phase in hair follicles, resulting in miniaturizing the follicles until they produce thin vellus‑like hairs or none at all.[1]Makridakis, N., & Reichardt, J. K. V. (2005). Pharmacogenetic analysis of human steroid 5α-reductase type II: comparison of finasteride and dutasteride. *Journal of Molecular Endocrinology.* … Continue reading
Finasteride blocks 5AR, thereby reducing levels of DHT and inhibiting its effect on hair follicles. Clinical studies suggest that dutasteride can reduce serum levels of DHT (levels circulating in the blood) by around 50%.[2]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Long-term clinical trials have shown that 1 mg finasteride daily stops AGA progression in 80-90% of men and can lead to a 10% increase in hair count on average after two years.[3]Shapiro, J., & Kaufman, K. D. (2003). Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). *Journal of Investigative Dermatology Symposium Proceedings.* … Continue reading
Because finasteride is a hormonally active drug, it can cause side effects throughout the body. This can include decreased libido, sexual dysfunction, breast enlargement or tenderness, and issues with mood in some rare cases. Long-term studies suggest that some level of side effects are experienced by between 2.6% and 13.4% of individuals, although these normally stop after treatment is halted.[4]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading, [5]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading,[6]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading
It’s because of these potentially serious side effects that finasteride is prescription-only and should be taken with medical supervision.
Outside of their sleek branding, there is nothing unique about Hims’ offering here, and the generic finasteride pill is the same you would receive from any vendor.
Finasteride & Minoxidil + Supplement Blend (from $35 a month)
Things start getting more interesting when we look at combination therapies. Clinical data and anecdotal evidence from our members’ point to combinations of therapies as potentially powerful routes to address AGA. The impact of multiple drugs targeting different causes of hair loss can be additive and may lead to improved results.[7]Hu, R., Xu, F., Sheng, Y., Qi, S., Han, Y., Miao, Y., Rui, W., & Yang, Q. (2015). Combined treatment with oral finasteride and topical minoxidil in male androgenetic alopecia: a randomized and … Continue reading
Finasteride with minoxidil is a popular combination. While finasteride works by regulating hormonal causes of AGA, the mechanism of action of minoxidil is less clear-cut. The drug appears to shorten the resting phase in hair follicles, promoting earlier entry into the growth phase.[8]Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 150(2). 186–194. Available at: … Continue reading
Evidence suggests that minoxidil also increases local blood flow to the follicles, activates the Wnt/β-catenin signaling pathway involved in follicular cell proliferation and differentiation, and may have cytoprotective and anti-inflammatory effects.
Minoxidil is most commonly applied in topical formulations, while finasteride is typically taken orally. That rule isn’t universal, however, and there is plenty of clinical evidence supporting oral minoxidil and topical finasteride. You can read more about oral minoxidil and topical finasteride in our in-depth articles.
Hims’ combination approaches offer either oral blends or topical blends. This will simplify the production of the drugs, and also help adherence: it’s much easier to take a single pill every day or apply a single lotion than remember to use a combination of both.
Does Combination Oral Finasteride and Minoxidil Work?
Their finasteride-minoxidil combination includes 1.2 mg finasteride and 3 mg minoxidil, with the option to add a supplemental blend.
There hasn’t been much research into the impact of the combination of oral finasteride and minoxidil on hair loss. One retrospective study, involving 502 men, found that, after one year of 1 mg finasteride and 2.5 mg minoxidil daily, 92.4% of men were stable or improved, while 57.4% showed overt regrowth. This is a retrospective study with no control group, so we can’t make any comparisons to individual therapies.[9]Johnson, H., Huang, D., Clift, A. K., Ângela, B. F., & Guimarães, G. A. (2025). Effectiveness of combined oral minoxidil and finasteride in male androgenetic alopecia: a retrospective service … Continue reading
Figure 3. Response of patients to the combination 1 mg finasteride and 2.5 mg minoxidil daily. The study is retrospective with no control groups, so improvement is compared to baseline (before treatment). Adapted from Figure 2.[10]Johnson, H., Huang, D., Clift, A. K., Ângela, B. F., & Guimarães, G. A. (2025). Effectiveness of combined oral minoxidil and finasteride in male androgenetic alopecia: a retrospective service … Continue reading Image used under Creative Commons license.
The lack of direct evidence doesn’t mean that this combination won’t be effective, and we know that both therapies are effective independently. What we still lack, however, is long-term safety data on low-dose oral minoxidil.
We’ve discussed the potential side effects of finasteride, and oral minoxidil also raises safety concerns. Unlike topical minoxidil, which is FDA-approved for hair loss, oral formulations can act systemically throughout the body. The most common side effect is hypertrichosis, or unwanted hair growth outside the scalp, including on the face. Other commonly reported side effects include lightheadedness, fluid retention, an elevated heart rate, and headache.[11]Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair … Continue reading
This leads us to a major issue with Hims’ offerings for hair loss. The brand is marketed as highly personalizable, and there is a range of options available to suit your practical needs: do you prefer a pill or a chew, would you rather spray on or apply a topical solution? However, this customization doesn’t stretch to the actual treatment itself. If you find that the oral minoxidil and finasteride combination is causing side effects, there is no route to decrease your concentration or find a safer option.
Similarly, if you find the plan tolerable or your regrowth plateaus and you want to escalate your treatment, there is no path to do so with Hims. Dutasteride is a very common escalation route from finasteride: it has been shown to better reduce DHT and can lead to better regrowth, but is not available here.[12]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
A one-size-fits-all treatment plan goes against Hims’ stated aim of providing a personalized approach. In the absence of alternatives or proper guidance, many men will simply persevere with their (potentially harmful) treatment or stop treating their hair loss altogether.
Does the Supplement Blend Help?
Hims’ oral finasteride and minoxidil are available with a supplement including biotin, vitamin B12, vitamin C, resveratrol, and zinc to “support healthy hair”. It’s unlikely that any supplement will have any substantial impact on hair growth compared to the highly active pharmaceutical ingredients. Instead, these ingredients are typically included to improve the condition and appearance of existing hair.
Biotin is commonly found in supplements and shampoos, but there is limited data to support its use for supporting hair growth. In fact, data supporting the use of biotin for hair loss comes almost exclusively from studies done on children with a biotin deficiency. Because biotin is a component of keratin, it is essential for hair growth. For people who can’t absorb dietary biotin, supplementation can make a real difference. Unfortunately, this only accounts for around 1 in 110,000 people.[13]Patel, D. P., Swink, S. M., & Castelo-Soccio, L. (2017). A review of the use of biotin for hair loss. *Skin Appendage Disorders.* 3(3). 166–169. Available at: https://doi.org/10.1159/000462981
Other inclusions, like vitamin B12, vitamin C, and zinc, are also unlikely to provide boosts in growth to anyone without a vitamin deficiency. For Hims customers in the US, the likelihood of deficiencies in these nutrients is highly unlikely. Instead, these ingredients are typically added to increase the value of the average purchase, rather than because of any proven gains.
Rx Hair Loss Spray (from $35 a month)
Hims’ Hair Loss Spray pairs 6% minoxidil with 0.3% finasteride. Topical formulations are often used to avoid some of the more severe, systemic side effects associated with oral pharmaceuticals. Because treatment is localized to the scalp, off-target effects on other organs are minimized.
Topical minoxidil at 6% is higher than the over-the-counter dose of 5%. There is some clinical data suggesting that increased dosing can improve results for some people, though there is diminishing returns at concentrations of 10% and higher, while the risk of side effects such as irritation and unwanted hair growth increases.[14]Singh, S., Patil, A., Kianfar, N., Waśkiel-Burnat, A., Rudnicka, L., Sinclair, R., & Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical … Continue reading, [15]Ghonemy, S., Alarawi, A., & Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a … Continue reading
Topical finasteride is a relatively recent development and has shown promising results in clinical trials. One study suggested that improvements from 1% topical solution were comparable to 1 mg oral finasteride.[16]Hajheydari, Z., Akbari, J., Saeedi, M., & Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. *Indian Journal of … Continue reading
Given the power of topical minoxidil and finasteride to combat hair loss independently, there has been clinical research into the potential of combined therapies. A meta-analysis, which pools the results from multiple studies to find if results are repeatable, found that the combination was superior to minoxidil alone.[17]Li, Y., Huang, Q., Zhou, Z., & Zhang, Y. (2025). Comparing minoxidil-finasteride mixed solution with minoxidil solution alone for male androgenetic alopecia: a systematic review and meta-analysis … Continue reading As such, there’s reason to believe that Hims’ Hair Loss Spray can provide meaningful improvements for many users.
Where Does Hims’ Rx Hair Loss Spray Come Up Short?
Unfortunately, we find the same problems with the Hair Loss Spray offering as we did with the tablet and chew. A one-size-fits-all approach doesn’t provide the nuance that is essential to provide effective care. There is no standard, safe dosing regimen for topical finasteride, and one study has shown that aa 0.25% solution can reduce serum DHT by 35%. While this is lower than oral (55%), it does suggest that there is still a risk of side effects.[18]Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic … Continue reading
Some users may find that the high concentration used here is not sustainable, but will have no route to try alternatives. Furthermore, Hims’ Hair Loss spray includes propylene glycol, which is unfortunately common in hair growth treatments. Propylene glycol causes irritation in around 7% of users and can add to inflammation in the scalp. This acts to worsen, rather than improve, hair growth outcomes.[19]Patel, K., Palmer, A., & Nixon, R. (2023). Allergic contact dermatitis from propylene glycol: a case series from Australia. *Contact Dermatitis.* 89(2). 79–84. Available at: … Continue reading
Sprays also cause issues for users. Particularly for men who wear their hair longer, sprays are often absorbed by the hair and cannot reach the follicles, where drugs are active. This is also likely to increase the proportion of the drug that does not reach the target areas, thereby increasing systemic absorption and the likelihood of further reductions in serum DHT.
What Else is Missing?
As we’ve noted above, dutasteride is a common route to escalate therapy when finasteride isn’t having the expected impact. Dutasteride can provide stronger DHT reduction, with a similar incidence of side effects.[20]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading,[21]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading.
In fact, the FDA recently issued a warning for topical finasteride, noting that “Absorption of finasteride through the skin into the bloodstream is expected, and the reports describe adverse events that are consistent with those reported in association with the approved oral finasteride products”.[22]U.S. Food and Drug Administration. (n.d.). FDA alerts health care providers, compounders, and consumers about potential risks associated with compounded drugs. *FDA.* Available at: … Continue reading This further highlights the need for ongoing physician involvement and expert input.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

There is also no option to include add-ons to help promote the activity of the pharmaceutical components. Tretinoin, for example, can help minoxidil penetrate into the skin and hair follicles, enhancing its activity
Rx Hair Loss Serum (from $35 a month)
Hims’ Hair Loss Serum contains the same ingredients as the Hair Loss Spray, except for the absence of propylene glycol. This would reduce the chances of scalp irritation. It is also applied directly to the scalp, avoiding the issues associated with spray-on topicals. The serum should, therefore, help with adherence and increase the proportion of active ingredients reaching the scalp when compared to the spray.
Where to Find Real Personalization
Unfortunately, the issues found in Hims’ products are systemic in the hair loss industry. Over-standardized dosing, limited escalation paths, and a lack of meaningful medical oversight are widespread and pose a major hurdle for men looking to combat hair loss.
Ulo was launched in response to these issues. Rather than offering fixed, one-size-fits-all protocols, Ulo was designed to give patients and physicians the flexibility needed to manage hair loss as it presents and was built around three core pillars: evidence, personalization, and consumer safety.
At a practical level, this personalization is built-in in several important ways:
- True dosing flexibility
- Ingredient-level customization
- Access to escalation pathways
- Formulations designed for long-term use
- Ongoing physician involvement
Where many brands promise personalization but deliver rigid protocols, Ulo was built to support informed medical decision-making over time.
Final Remarks
Hims offers a broad, accessible entry point into hair loss treatment, with clinically proven medications and tested delivery methods. For many men, these products may slow or stabilize androgenic alopecia, particularly when well tolerated.
However, beneath the convenience and branding, we find consistent limitations: rigid dosing, limited escalation pathways, high-concentration formulations with unclear long-term safety margins, and a lack of meaningful personalization once side effects or plateaus emerge. These issues are not unique to Hims, but they underscore broader problems in telehealth hair loss care.
References[+]
References ↑1 Makridakis, N., & Reichardt, J. K. V. (2005). Pharmacogenetic analysis of human steroid 5α-reductase type II: comparison of finasteride and dutasteride. *Journal of Molecular Endocrinology.* 34(3). 617–623. Available at: https://doi.org/10.1677/jme.1.01725 ↑2, ↑4 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑3 Shapiro, J., & Kaufman, K. D. (2003). Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). *Journal of Investigative Dermatology Symposium Proceedings.* 8(1). 20–23. Available at: https://doi.org/10.1046/j.1523-1747.2003.12167.x ↑5, ↑20 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑6 Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. *Annals of Dermatology.* 34(5). 349. Available at: https://doi.org/10.5021/ad.22.027 ↑7 Hu, R., Xu, F., Sheng, Y., Qi, S., Han, Y., Miao, Y., Rui, W., & Yang, Q. (2015). Combined treatment with oral finasteride and topical minoxidil in male androgenetic alopecia: a randomized and comparative study in Chinese patients. *Dermatologic Therapy.* 28(5). 303–308. Available at: https://doi.org/10.1111/dth.12246 ↑8 Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 150(2). 186–194. Available at: https://doi.org/10.1111/j.1365-2133.2004.05785.x ↑9, ↑10 Johnson, H., Huang, D., Clift, A. K., Ângela, B. F., & Guimarães, G. A. (2025). Effectiveness of combined oral minoxidil and finasteride in male androgenetic alopecia: a retrospective service evaluation. *Cureus.* 17(1). Available at:https://doi.org/10.7759/cureus.77549 ↑11 Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. *Journal of the American Academy of Dermatology.* 84(6). 1644–1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑12 Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 70(3). 489–498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑13 Patel, D. P., Swink, S. M., & Castelo-Soccio, L. (2017). A review of the use of biotin for hair loss. *Skin Appendage Disorders.* 3(3). 166–169. Available at: https://doi.org/10.1159/000462981 ↑14 Singh, S., Patil, A., Kianfar, N., Waśkiel-Burnat, A., Rudnicka, L., Sinclair, R., & Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit?. *Clinical and Experimental Dermatology.* 47(11). 1951–1955. Available at: https://doi.org/10.1111/ced.15338 ↑15 Ghonemy, S., Alarawi, A., & Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. *Journal of Dermatological Treatment.* 32(2). 236–241. Available at: https://doi.org/10.1080/09546634.2019.1654070 ↑16 Hajheydari, Z., Akbari, J., Saeedi, M., & Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. *Indian Journal of Dermatology, Venereology and Leprology.* 75. 47. Available at: https://doi.org/10.4103/0378-6323.45220 ↑17 Li, Y., Huang, Q., Zhou, Z., & Zhang, Y. (2025). Comparing minoxidil-finasteride mixed solution with minoxidil solution alone for male androgenetic alopecia: a systematic review and meta-analysis of randomized controlled trials. *Frontiers in Medicine.* 12. 1632139. Available at: https://doi.org/10.3389/fmed.2025.1632139 ↑18 Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. *Journal of the European Academy of Dermatology and Venereology.* 36(2). 286–294. Available at: https://doi.org/10.1111/jdv.17738 ↑19 Patel, K., Palmer, A., & Nixon, R. (2023). Allergic contact dermatitis from propylene glycol: a case series from Australia. *Contact Dermatitis.* 89(2). 79–84. Available at: https://doi.org/10.1111/cod.14325 ↑21 Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. *Annals of Dermatology.* 34(5). 349. Available at: https://doi.org/10.5021/ad.22.027 ↑22 U.S. Food and Drug Administration. (n.d.). FDA alerts health care providers, compounders, and consumers about potential risks associated with compounded drugs. *FDA.* Available at: https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-providers-compounders-and-consumers-potential-risks-associated-compounded (Accessed: November 2025) A rising interest has grown in topical finasteride as a middle ground between oral dihydrotestosterone (DHT) blockers and non-hormonal treatments like minoxidil. This shift is largely driven by individuals seeking similar efficacy to oral finasteride while minimizing the risk of systemic side effects.
Online transformation photos showcasing impressive regrowth have further fueled the trend, but these often highlight the most exceptional cases rather than typical results. As a result, perceptions of topical finasteride’s effectiveness can be skewed by outliers, making it difficult for prospective users to understand what to expect in real-world use.
Source: u/MrDiabeticGinger via r/tressless.
We’ll take a closer look at this one later.
This article explores how topical finasteride fits within today’s hair loss treatment landscape, clarifying what current evidence supports, how much regrowth is realistically achievable, and how it compares to both oral finasteride and non-DHT-based alternatives like minoxidil.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Topical Finasteride?
Topical finasteride is a 5ɑ-reductase type II inhibitor formulated for direct scalp application. It is designed to inhibit DHT locally in the scalp. By blocking DHT conversion in the scalp, the hope is that it will reduce miniaturization and hair loss in androgenic alopecia (AGA) while minimizing the risk of sexual and hormonal side effects seen with oral administration.
Mechanism of Action
Topical finasteride selectively inhibits the type II 5ɑ-reductase enzyme, decreasing local DHT levels. Topical finasteride has been found to reduce serum DHT by approximately 24-48% depending on the dose, which is notably less than the 60-70% reduction seen with oral finasteride. Lower doses primarily inhibit scalp DHT, potentially minimizing systemic side effects.[1]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading But, as the data shows, complete elimination of systemic impact is not guaranteed.
Formulations and Dosing
Compound strengths for topical finasteride vary, typically ranging from 0.005% to 0.2% (as Ulo does), with other companies offering doses as high as 2.5%; however, the higher the dose, the higher the risk of systemic absorption.
Why Expectation Setting Matters
Expectation setting is important when starting topical or oral finasteride for hair loss because online forums and before-and-after images tend to spotlight the most extreme results, rather than typical user outcomes. While finasteride has demonstrated high effectiveness on average, regrowth is generally gradual, with noticeable changes taking six months or more and reaching a plateau around 12-18 months.[2]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray … Continue reading,[3]Rossi, A., Magri, F., D’Arino, A., Pigliacelli, F., Muscianese, M., Leonici, P., Caro, G., Federico, A., Fortuna, M.C., Carlesimo, M. (2020). Efficacy of Topical Finasteride 0.5% vs 17ɑ-Estradiol … Continue reading
Overestimating likely results can lead to frustration, disappointment, and ultimately premature abandonment of therapy when expectations are unmatched by personal experience. Conversely, underestimating the drug’s potential may lead individuals to dismiss a therapy that could offer substantial stabilization or regrowth if given enough time and proper use.
Balanced, evidence-based expectations help users remain committed through the plateau period and recognize that slow, steady improvement, rather than dramatic changes overnight, is the most common pattern for most finasteride users.
What’s Possible ≠ What’s Probable
While topical finasteride can occasionally deliver dramatic regrowth, most users should expect moderate thickening and stabilization in hair density rather than extreme transformations.
Outcomes are best thought of as falling along a distribution curve:
- A small portion of users are “hyper-responders” who achieve big, visible transformations.
- The majority see moderate, steady improvement, such as visible thickening or a reduction in hair loss.
- Some individuals are non-responders with little to no benefit, reflecting the variability in biology and clinical response.
Several factors influence where an individual might fall on this spectrum:
- Duration of hair loss: Early intervention typically yields better results, while longer-standing balding zones respond less robustly.
- Consistency: Adhering reliably to a treatment schedule increases the chances of improvement, as missed doses may lead to suboptimal outcomes.
- Adjunct therapies: Combining topical finasteride with minoxidil or microneedling can enhance benefits, especially in difficult cases.
- Genetic factors: Variations in androgen receptor (AR) gene sensitivity and baseline DHT levels can also help dictate who will be a strong or weak responder.[4]Wakisaka, N., Taira, Y-I., Ishikawa, M., Nakamizo, Y., Kobayashi, K., Uwabu, M., Fukuda, Y., Taguchi, Y., Hama, T., Kawakami, M. (2005). Effectiveness of finasteride on patients with male pattern … Continue reading
Stronger Evidence = Greater Predictability
Finasteride is one of the most thoroughly studied hair loss medications, with clinical trial data supporting its reliability and predictability. This evidence base translates into a narrower distribution of user responses; most people see consistent moderate results, with fewer extremes compared to newer drugs.
By contrast, topical dutasteride has been studied less, limiting the ability to accurately predict outcomes and resulting in a wider variation of response. Clinical trials consistently show that dutasteride is more potent in DHT suppression and regrowth than finasteride, yet variability in regrowth and side effect profiles remains higher, especially in real-world use due to less standardization and a less robust evidence base.[5]Almudimeegh, A., AlMutairi, H., AlTassan, F., AlQuraishi, Y., Nagshabandi, K.N. (2024). Comparison between dutasteride and finasteride in hair regrowth and reversal of miniaturization in male and … Continue reading
Figure 1: For topical dutasteride, a wide, shallow curve represents a much wider spread of outcomes. For finasteride, a narrow bell curve shows predictable outcomes.
Finasteride’s consistent results and well-documented safety profile make it the first-line treatment for most men with AGA, as clinicians and users can anticipate a predictable, steady benefit with a lower risk of surprises. This predictability helps set realistic user expectations and avoids over- or under-estimation of results.
Real Case Studies: Topical Finasteride Before and After
Case #1: Male (32), 24 Months on Topical Finasteride 0.025% (1 ml nightly) + Minoxidil 5% Added After 12 Months
Source: u/Ecstatic-Nature-1631 via r/tressless.
This 32-year-old male’s current protocol includes a 0.025% topical finasteride solution, dosed at 1 mL nightly. He incorporated 5% topical minoxidil at month 12. Adjuncts included weekly dermastamping, a conditioner-only washing routine, and occasional argan oil usage. He reported stabilization of hair shedding from months 0-3, strong thickening from months 3-12, and maintained density, improved texture, and shine from months 12-24. He reported no sexual or systemic side effects from topical finasteride.
Case #2: Male (23) – 3.5 Months on Topical Finasteride 0.025% (Twice Daily) + Minoxidil 5% (twice daily)
Source: u/MrDiabeticGinger via r/tressless.
This 23-year-old male applied a topical finasteride 0.025% solution compounded with minoxidil 5%, in addition to occasional, inconsistent derma-rolling over 3.5 months. He reported an initial shedding phase at months 1-2 of the protocol, followed by a sudden improvement in hairline density and thickness at month 3. By month 3.5, he noted significant regrowth and richer color and texture along the frontal hairline. The user noted that the topical regimen has been well-tolerated with no systemic side effects.
Case #3: Male (34) – 12 Months on Topical Finasteride 0.3% + Minoxidil 6% (twice daily)
Source: u/iTsundere via r/tressless.
This 34-year-old male had tried numerous natural and supplement-based approaches (including pumpkin seed oil, saw palmetto, and rosemary) without success before starting pharmacologic therapy. He used a topical finasteride 0.3% spray compounded with topical minoxidil 6%, applied twice daily. He additionally used microneedling (1.5 mm depth) every 10 days. He described the first 2 months as a “rough” period, marked by noticeable shedding and even mild chest tenderness.
At months 3-6, he noted steady improvement and early thickening, especially near the temples and mid-scalp. By months 7-8, he observed significant progress, and in months 9-12, coverage stabilized, with occasional light shedding cycles. At this point, he transitioned to an oral regimen for convenience.
Case #4: Male, 8 Months on Topical Finasteride 0.25% (1 mL twice daily) + Minoxidil 8% (1 mL twice daily) + Microneedling 0.25mm (every three days)
Source: u/coldmoney21 via r/tressless.
This male user reported on his 8-month progress using topical finasteride, minoxidil, and microneedling. He applied a topical finasteride 0.25% solution compounded with topical minoxidil 8% twice daily to the target areas, with 0.25 mm microneedling sessions every 3 days. At month 1, he reported a mild, brief shedding period, and his first noticeable improvement with thicker strands by month 3. From months 4-8, the user continued to show thickening and increased density. He reported no side effects beyond scalp redness attributed to dermarolling.
Case #5: Male (37) – 4 Months on Topical Finasteride 0.1% + Minoxidil 5% ( 1mL Daily)
Source: u/Far-Instance268 via r/tressless.
This 37-year-old male opted for a topical combination for safety and convenience. The solution contained topical finasteride 0.5% and topical minoxidil 5%, which he applied 1 mL once daily over 4 months, exclusively to the crown. He noted a 4-week shedding phase from week 3 of treatment, followed by continued shedding and apparent thinning at month 2. By month 2.5, shedding ceased, and months 3-4 marked a period of steady regrowth and improved crown density.
The user reported maintaining consistent adherence despite the discouraging shedding phase and did not report any systemic or local irritation or side effects. While he did not apply the solution outside the crown, he reported slight thickening near the frontal region.
Case #6: Male (23) – 3 Months on Topical Finasteride 0.3% + Minoxidil 6% (4 Sprays Nightly) + Microneedling 1 mm (Weekly)
This 23-year-old male chose a combination therapy of topical finasteride 0.3%/topical minoxidil 6%, which he applied as a spray 4 times nightly for 3 months. Additionally, he used 1 mm dermastamp once weekly, from which he reports mild temporary redness. At month 1, he reported mild shedding, with no change in his appearance. At month 2, he noticed the first signs of regrowth and thickening, especially along the temples. Month 3 was marked by significant visible improvement. The user described his hair as visibly denser and fuller and reported satisfaction after only 3 months of combination therapy.Source: u/[deleted] via r/tressless.
Case #7: Male (46) – 4+ Months on Topical Finasteride 0.1% (0.5 mL front + 0.5 mL crown twice daily, TrichoSol) + Minoxidil 5% + Dermastamp 0.8–1 mm Weekly
Source: u/Hypergrade46 via r/tressless.
Due to long-standing crown thinning, this 46-year-old male commenced a combination of topical finasteride 0.1% and minoxidil 5%, applied twice daily for 4 months. He complemented with microneedling (initially daily, then weekly), occasional rosemary oil, and regular biotin. He noted an initial skin reaction at the start of therapy, which caused him to stop it for around 4 weeks. He did not note any ongoing side effects since restarting, nor any shedding or unrelated side effects. He noted improvement in the mid-scalp despite limiting the application to that area, suggesting a possible systemic effect or local spread.
Case #8: Male (24), 4 Months on Topical Finasteride 0.3% + Minoxidil 6%
Source: u/Aromatic_Tangerine33 via r/tressless.
This 24-year-old male started the topical combination of finasteride 0.3% and minoxidil 6%, applied mainly to his hairline and temples via a serum. He did not observe any side effects, save for transient greasiness and a distinct smell from the serum. Between baseline and month 4 of therapy, he reported visible baby hairs and an early fill across his hairline. He did not notice any shedding across the 4 months.
Case #9: Male, 9 Weeks on Low-Dose Topical Finasteride (0.01–0.02%) + Topical Minoxidil 5% (Daily)
Source: u/Yellow_Icicle via r/tressless.
This male documented his results after 9 weeks on low-dose topical finasteride combined with minoxidil. He created a 0.02% topical finasteride solution using crushed finasteride tablets, which he took from month 2 after initially trialing a 0.01% solution. He also applied a 5% minoxidil solution with a standard alcohol-based carrier. He noted a brief shedding phase at week 4 of the protocol, which he interpreted as a positive response indicator. From weeks 6-9, he noted visible regrowth at the front and thickening of the crown. He did not note any systemic or dermatologic adverse events.
Case #10: Male (43), 3 Months on Topical Finasteride 0.025% (1 ml daily) + Topical Minoxidil 5% (daily) + Microneedling 0.75mm (once every two weeks)
Source: u/siritchie via r/tressless.
This 43-year-old male sought combination finasteride and minoxidil therapy to improve his hair loss. Over three months, he applied a 0.025% topical finasteride solution once daily to the affected areas, along with 5% minoxidil combined with the same solution. Additionally, he applied Dermastamp 0.75 mm every 2 weeks, avoiding topical application on stamping days. Additionally, he supplemented with magnesium, zinc, B-complex, vitamin D3+K2, lecithin, and followed a generally healthy diet with regular exercise.
At months 1-2, he noted early thickening and density improvements, and by month 3, he achieved increased coverage and shaft thickness at the vertex, with no shedding or side effects reported.
Probable Regrowth Timeline
Based on the available data, we’ve created a probable timeline of regrowth.
Months 0-6: No Cosmetic Changes
Most users will see little to no visible improvement in the first six months. During this period, finasteride is actively reducing DHT and stabilizing hair follicles, but hair regrowth is not yet apparent. Some individuals may notice decreased shedding, while others may experience temporary increased shedding as old hairs make way for newer ones.
Months 6-12: First Cosmetic Improvements
Between 6 and 12 months, initial improvements become visible for most responders. Hair density may be noticeably improved, with thicker strands and slower progression of hair loss.[6]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray … Continue reading
Months 12-24: Noticeable Regrowth in Responders
During the second year, responders typically achieve their most significant gains. Hair regrowth becomes more noticeable, and improvements in overall density continue to build. For many, this period yields the best results, though those who started with advanced hair loss may see stabilization rather than dramatic regrowth.[7]Rossi, A., Magri, F., D’Arino, A., Pigliacelli, F., Muscianese, M., Leonici, P., Caro, G., Federico, A., Fortuna, M.C., Carlesimo, M. (2020). Efficacy of Topical Finasteride 0.5% vs 17ɑ-Estradiol … Continue reading
Months 24+: Maintenance and Plateau
After two years, most responders maintain their gains if they consistently use the medication. Regrowth typically plateaus, with ongoing use required to sustain results. Discontinuation leads to a gradual loss of regained hair within 6-12 months, returning to baseline levels. Some individuals may see marginal further improvement with combination or adjunct therapies.
For most users, topical finasteride is a long-term commitment with gradual, steady gains and sustained maintenance. The timeline highlights the importance of patience and expectations aligned with clinical reality.
How To Maintain Realistic Expectations
Most topical finasteride users experience visible, but not dramatic, improvements in hair density and preservation. Rather than achieving total restoration, finasteride is more likely to “turn back the clock” by 1-3 years.
Patience is important as results often take between 6 to 12 months to become noticeable. Initial changes may be subtle, such as reduced shedding or minor thickening, before more visible regrowth stabilizes over the course of a year.
To accurately assess progress, you can track your progress with photographs. However, it’s important to use the same lighting and angles each time you photograph your scalp. This approach reduces bias and helps clearly identify gradual changes that might otherwise go unnoticed day-to-day. Setting and maintaining realistic expectations can improve satisfaction and help sustain adherence to the long-term treatment plan.
Practical Takeaways
- Topical finasteride offers a clinically proven, lower-risk alternative to oral formulations for treating AGA.
- Multiple studies demonstrate that its efficacy is comparable to oral finasteride.
- The key safety advantage of topical finasteride is its reduced systemic DHT suppression, potentially leading to a lower incidence of sexual and hormonal side effects commonly associated with oral therapy.
- Consistency in application and patience over many months remain the most important predictors of treatment success, as improvements are typically gradual and require ongoing commitment to daily use.
Final Thoughts
When evaluating topical finasteride, it’s important to consider both its proven efficacy and its measured expectations. Clinical studies consistently show that topical finasteride can deliver meaningful stabilization and regrowth with a lower likelihood of systemic side effects compared to oral therapy. However, like all hair loss treatments, results develop gradually and vary between individuals.
By grounding expectations in clinical averages rather than exceptional before-and-after photos, users can better appreciate the steady, cumulative improvements that typically occur over months, not weeks. Patience, consistency, and realistic goal setting are key; most users will experience visible thickening, reduced shedding, and sustained density when topical finasteride is used correctly and maintained long term.
Ultimately, topical finasteride represents a balanced, evidence-backed option in the modern hair restoration toolkit: one that offers both reliability and tolerability for those seeking to preserve and strengthen their existing hair with confidence.
References[+]
References ↑1 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑2 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑3, ↑7 Rossi, A., Magri, F., D’Arino, A., Pigliacelli, F., Muscianese, M., Leonici, P., Caro, G., Federico, A., Fortuna, M.C., Carlesimo, M. (2020). Efficacy of Topical Finasteride 0.5% vs 17ɑ-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients. Dermatology Practical & Conceptual. 10(2). E2020039. Available at: PMID: 32363101 ↑4 Wakisaka, N., Taira, Y-I., Ishikawa, M., Nakamizo, Y., Kobayashi, K., Uwabu, M., Fukuda, Y., Taguchi, Y., Hama, T., Kawakami, M. (2005). Effectiveness of finasteride on patients with male pattern baldness who have different androgen receptor gene polymorphism. The Journal of Investigative Dermatology. Symposium Proceedings. 10(3). 293-294. Available at: https://doi.org/10.1111/j.0022-202X.2005.10123.x ↑5 Almudimeegh, A., AlMutairi, H., AlTassan, F., AlQuraishi, Y., Nagshabandi, K.N. (2024). Comparison between dutasteride and finasteride in hair regrowth and reversal of miniaturization in male and female androgenetic alopecia: a systematic review. Dermatology Reports. 16(4). 9909. Available at: https://doi.org/10.4081/dr.2024.9909 ↑6 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 Finasteride Side Effects
Finasteride is the most powerful, well-studied, FDA-approved drug for androgenic alopecia (AGA). It stops AGA progression in 80-90% of men and, on average, leads to a 10% increase in hair count over two years. For men wanting a hands-off approach to hair maintenance, finasteride is often an excellent option.
That said, finasteride isn’t for everyone. While its risk of side effects are often overstated online, the drug appears to reduce libido in a certain percentage of men and may induce gynecomastia. The drug can also temporarily lower sperm counts, which might make conception more difficult during its first six months of use. In some men, the use of finasteride appears to increase anxiety and/or depression.
The true incidence and magnitude of these reports are hard to discern. Depending on the study we cite and the questionnaire design, these effects can range from 1% to 40%. Whether or not one should worry about finasteride side effects may depend on their current hormonal profile and mental health. Keep reading to learn more.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Gynecomastia
Gynecomastia is the growth of male breast tissue. It results from elevated hormones such as prolactin and estrogen. While gynecomastia is estimated to only affect between 0.25-1.00% of people on 5-alpha reductase inhibitors (such as finasteride), those who start the drug with already elevated levels of prolactin and estrogen are likely at a higher risk of its development.
Drugs like finasteride can raise blood levels of both testosterone and estrogen by 10-30%, depending on the dose. So, for those starting the drug with borderline-high estrogen, the additional lift in estrogen levels may put someone at greater risk of gynecomastia.
Sexual Side Effects
Because it lowers DHT levels (a hormone that helps maintain male sex characteristics), some studies have shown that a percentage of finasteride users report sexual side effects ranging from decreased sex drive to reduced semen volume to erectile dysfunction. Studies vary widely, but incidents could be as low as 1-2% of users.
Female users should be aware that Finasteride can potentially mutate and/or inhibit the development of male fetus genitalia.[1]dailymed.nlm.nih.gov/dailymed/lookup.cfm As such, pregnant women are neither prescribed finasteride nor advised to even handle the medication.
Cognitive Side Effects
On hair loss forums, some men taking finasteride have complained of changes to cognition, specifically, a feeling of brain fog since starting therapy. To date, this hasn’t been reported to significant degrees in clinical studies. This may be because even if the effects do exist, it’s likely a subtle, gradual side effect that many may not attribute to the drug.
Animal models have demonstrated that finasteride can indeed change brain chemistry, especially after transitioning off of the drug. But it’s important to note that animals in those studies take dosages of finasteride thousands of times greater than what is prescribed to humans.
In any case, it’s not unreasonable that those with depression, anxiety, and/or bipolar disorder should proceed with caution and speak with their doctor to closely monitor the effects of finasteride use.
How To Reduce Side Effects Of Finasteride
Regardless of the specific side effect that’s being targeted to reduce, the strategies are all similar: find ways to (1) reduce daily drug exposure, (2) localize the drug’s effects to the scalp, and (3) do lab tests to determine personal risk for certain side effects like gynecomastia. These strategies are outlined in detail below:
Limit Daily Drug Exposure
As a hair loss drug, finasteride is typically prescribed orally at 1mg daily. Having said that, there’s evidence that 0.2mg daily is nearly just as effective at improving hair counts while simultaneously reducing total drug exposure by 80%. For many people, this coincides with a reduction in perceived side effects.
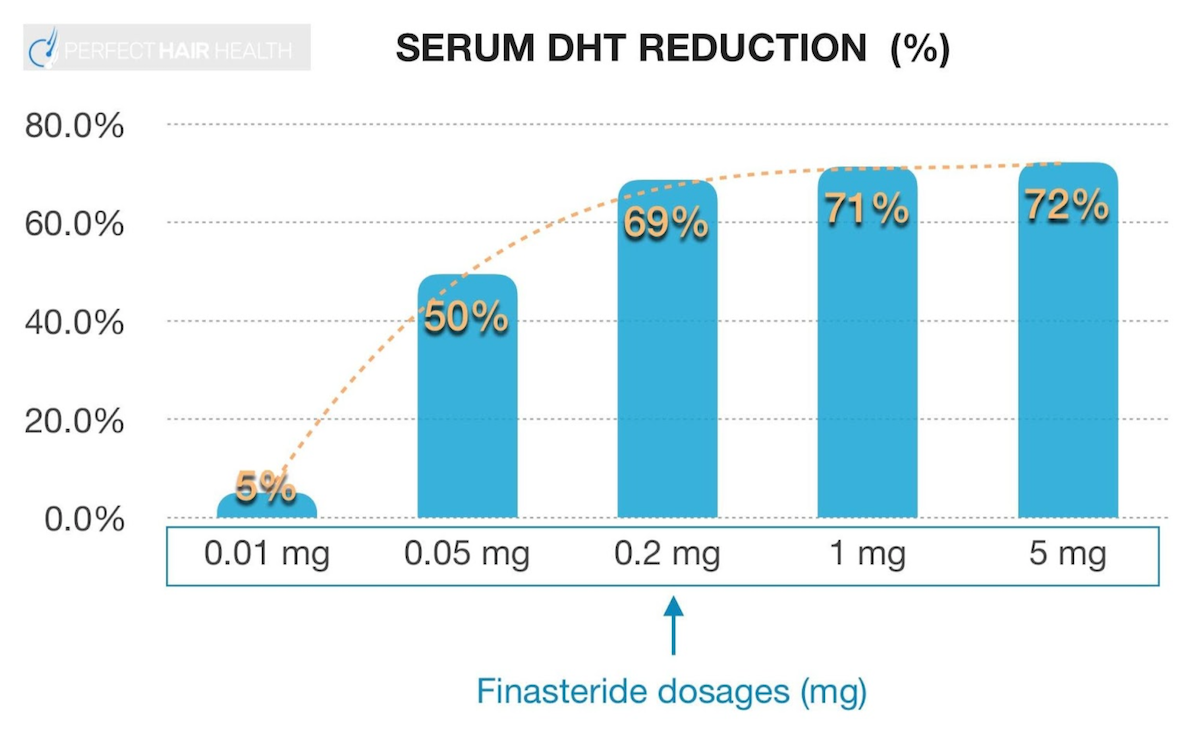
Finasteride’s dose-dependent, logarithmic response curve on serum DHT reduction
If side effects, or anticipatory anxiety, are a concern, try lowering the dose of the drug from 1mg daily to 0.2mg daily. After all, small clinical studies have demonstrated that doses as low as 0.2mg daily still improve hair counts, and may also confer a slightly smaller magnitude and/or severity of side effects (at least anecdotally).[2]https://pubmed.ncbi.nlm.nih.gov/10495375/
Unfortunately, finasteride is a hair loss drug that must be continued indefinitely for its effectiveness to remain. When finasteride treatment is stopped, men typically lose what hair regrowth they gained within 3-12 months. For these reasons, it’s not advised to drop below doses equating to 0.2mg daily – as efficacy may rapidly diminish below this threshold.
If finasteride side effects don’t go away with reduced usage, most users report any lingering side effects go away after discontinuing the drug within 2-3 weeks, and for some, up to a few months. If problems persist beyond that, it’s important to contact your prescribing physician.
Localize Finasteride’s Effects to the Scalp
There are two primary ways to localize finasteride’s effects to the scalp. These methods minimize the amount of the drug that circulates throughout the bloodstream, thus minimizing the side effects of finasteride. Both entail switching from an oral to topical formula.
Try a topical formulation
Studies show that – when formulated properly – topical finasteride may reduce the risk of side effects by 30-90%. One 16-month study on 0.005% topical finasteride demonstrated significant hair improvements, no drug-associated side effects, and no impact on blood hormonal levels.[3]https://dx.doi.org/10.3109%2F09546639709160517 This suggests that any absorption of the drug beyond the scalp was metabolized quickly enough to not impact serum DHT levels.
Other studies have demonstrated that higher doses of topical finasteride also confer benefit, and at smaller reductions of serum DHT (i.e., a proxy for systemic absorption) and perhaps side effects, too.[4]https://onlinelibrary.wiley.com/doi/full/10.1111/jdv.17738 Having said that, if minimizing side effects are a top priority, we still recommend starting with the minimum viable dose of topical finasteride – 0.005% x 2 mL daily – and working your way up from there.
Try intradermal delivery methods (dutasteride)
Also known as mesotherapy, intradermal delivery methods inject finasteride into the scalp. While there aren’t yet clinical studies of mesotherapy finasteride in reputable journals, there are clinical studies of mesotherapy dutasteride – a drug that is more powerful at reducing DHT levels versus finasteride and that also has a longer half-life (which makes it a better candidate for less-frequent injections into the scalp – since the drug will stay active for longer).
A small number of clinical studies suggest that scalp injections of ~0.01% x 1-2 mL of dutasteride, once every 1-3 months, do not appreciably alter serum hormones, nor do they result in any reported cognitive or sexual side effects. They do, however, lead to statistically significant hair improvements.
Determine Your Risk for Finasteride Side Effects
Determining personal risk ahead of time, and getting a baseline measure of hormonal health, can help people decide if finasteride is right for them. It can also help people keep track of how the drug is influencing their serum hormones.
Testing for Sexual Side Effect Risk
There aren’t yet clinical studies demonstrating the predictability of finasteride side effects related to lowered libido or sexual dysfunction. There are, however, clinical studies suggesting men who are experiencing reduced libidos tend to also have low levels of free testosterone and/or high levels of sex hormone binding globulin.
For these reasons, some clinicians recommend getting these hormones tested prior to starting finasteride. While finasteride isn’t known to have a major influence over these hormones, borderline-abnormal tests may make someone more likely to report these problems, irrespective of whether they’re using hair loss drugs.
Testing for Gynecomastia Risk
For peace of mind, blood tests for prolactin and estrogen can be ordered prior to starting finasteride. Clinical studies do show that finasteride and dutasteride can slightly increase levels of estrogen, and that a rise in estrogen and/or prolactin can be pathogenically linked to the development of gynecomastia, also known as male breast development.[5]https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf[6]https://journals.sagepub.com/doi/abs/10.1177/2051415820926301[7]https://www.ncbi.nlm.nih.gov/books/NBK279105/
If you’re worried about this side effect, test your estrogen and prolactin levels. If they’re within 10-20% of the upper limit for normal, then finasteride or dutasteride use may put you “over the edge” and into that risk category of gynecomastia. Under these circumstances, consider dietary, lifestyle, and/or environmental interventions to lower levels of these into a normal range prior to starting finasteride, and rechecking these hormones regularly or if breast tenderness begins to develop.
Also keep in mind that blood tests for hormones are just proxies for what might be occurring elsewhere in the body. Therefore, it’s entirely possible for serum blood tests to mislead us into thinking we have “normal” levels of hormones, when our tissue hormonal profiles might be out-of-range. The inverse is also true: out-of-range blood hormones don’t always signify out-of-range tissue hormones. So, treat any laboratory test as preliminary, and recognize the science supporting these tests – at least for their predictability of finasteride side effects – is still in its infancy.
Anyone interested in doing these lab tests can do so with the help of their primary care physician. Or, those based in the U.S. (or any other country that offers direct-to-consumer lab testing) may be able to order tests through the links below.
For more information, see these resources (no affiliate links):
- Direct-To-Consumer Lab Test: Prolactin (U.S. only) [8]truehealthlabs.com/product/prolactin
- Direct-To-Consumer Lab Test: Estrogen (U.S. only) [9]truehealthlabs.com/product/estradiol-e2
Can We Localize Finasteride Entirely To The Scalp?
Topical finasteride can still go systemic. Having said that, clinical studies also show that daily doses of topical finasteride as low as 0.005% x 2 mL can still produce positive hair parameter changes over 16 months, and without impacting serum DHT levels (a proxy for systemic circulation of the drug).
For those worried about the sexual side effects of finasteride, this formulation of topical finasteride might be most appropriate. However, if you go down this route, you also may want to consider periodically testing serum DHT levels – as members inside our membership community have found that even at these ultra-low dilutions of finasteride, serum DHT tests can still decline by more than 25%.
Changes to serum DHT levels can help people understand just how much topical finasteride (if any) is going systemic. In general, DHT fluctuations smaller than 20% are considered biologically insignificant.
Directions for how to do this can be found inside our comprehensive finasteride guides, available only to members.
So, consider these options (and the data) before giving up entirely on finasteride. There are many ways to leverage its power, mitigate its risks, and perhaps take your hair regrowth to a new level.
References[+]
References ↑1 dailymed.nlm.nih.gov/dailymed/lookup.cfm ↑2 https://pubmed.ncbi.nlm.nih.gov/10495375/ ↑3 https://dx.doi.org/10.3109%2F09546639709160517 ↑4 https://onlinelibrary.wiley.com/doi/full/10.1111/jdv.17738 ↑5 https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf ↑6 https://journals.sagepub.com/doi/abs/10.1177/2051415820926301 ↑7 https://www.ncbi.nlm.nih.gov/books/NBK279105/ ↑8 truehealthlabs.com/product/prolactin ↑9 truehealthlabs.com/product/estradiol-e2 Dihydrotestosterone (DHT) is a male hormone, and one of the factors that can cause androgenic alopecia (AGA). Naturally, there are many products on the market that use “DHT-blocking” ingredients and offer a “science-backed” way to improve hair loss through use of their formulation.
DHT-blocking has proven effective in treating hair loss. After all, oral finasteride is an FDA-approved medication for androgenic alopecia, and it blocks DHT by inhibiting the DHT-forming enzyme, 5-α-reductase.
We are now seeing more haircare products incorporating “DHT-blocking” ingredients into their formulations, including shampoos. These products offer a milder option to FDA-approved medications that can be incorporated into an everyday routine. But do they really work?
With so many options on the market, sorting the science from the marketing can feel daunting. That’s why we’ve researched the evidence behind key shampoo ingredients, from speculative herbal formulas to clinically proven ingredients. In this article, we break down the 10 best DHT-blocking shampoos and assess whether they are worthy hair loss products to incorporate into your routine.
Thickening Shampoo
The top natural ingredients for hair growth.
Take the next step in your hair growth journey with a world-class shampoo. Ingredients, doses, & concentrations built by science.

What is DHT and DHT-Blocking?
DHT is an androgen, a hormone that is formed from testosterone by the enzyme 5-ɑ-reductase. It is involved in the growth of facial, underarm, pubic, and body hair. While this might sound great, DHT is also implicated in the development of pattern hair loss.
The binding of DHT to androgen receptors causes hair follicles to shrink. It shortens their growth phase while also prolonging their non-growing phase. This leads to hair thinning and shorter hair that may fall out or stop growing.
Those with AGA often have elevated levels of 5-ɑ-reductase, DHT androgen receptors, and DHT itself.[1]Ustuner, E. T. (2013). Cause of Androgenic Alopecia: Crux of the Matter. Plast Reconstr Surg Glob Open. 1(7). e64. Available at: https://doi.org/10.1097/GOX.0000000000000005,[2]Sekhavat, H., Bar Yehuda, S., Asotra, S. (2025). Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss. Int J Mol Sci. 26(21). 10712. Available at: … Continue reading
DHT blockers do what their name suggests – they block DHT. To do this, they often operate in one of two ways, or sometimes a combination of both:
- They inhibit 5-ɑ-reductase, reducing the formation of DHT
- They bind to androgen receptors and block DHT binding
These are not the only ways an ingredient may act to block DHT, but they are the most common.
What Are DHT-Blocking Shampoos?
Elevated DHT is often the primary cause of a balding scalp. So, naturally, there are a plethora of products available that act to block DHT, including DHT-blocking shampoos. These shampoos are formulated with ingredients that have been shown in laboratory research or in human clinical trials to reduce DHT levels.
The Problem With DHT-Blocking Shampoos
A DHT-blocking shampoo may appear like a great addition to your daily routine if you are concerned about hair loss. However, shampoos may not be the best option if you have AGA, and there are several factors to consider before purchasing a shampoo that claims to support hair growth.
Regulation
You’ve probably seen shampoos advertised as “DHT-blocking” or “clinically proven to stop hair loss.” But here’s the truth: there are currently no over-the-counter shampoos that have been clinically proven to lower DHT in humans.
That doesn’t mean the ingredients are useless; it just means the science doesn’t support the marketing claims being made.
Marketing vs. Science
Many hair loss shampoos include ingredients like:
- Saw palmetto
- Green tea extract
- Rosemary oil
- Pumpkin seed extract
Marketers label these as “DHT blockers” because some research suggests these compounds might reduce DHT, but this is where the story gets complicated.
Firstly, laboratory studies are not human studies. Much of the evidence behind these claims comes from petri dish experiments (cells in a lab) or animal models (usually mice).
For example, green tea extract might reduce androgen activity in a dish of cultured cells. But real human scalps are a completely different environment. So if something works in a lab, the safest assumption is actually that it won’t work the same way in people until proven otherwise.
This is why evidence quality is so important, and why we include evidence quality rubrics with every ingredient in our ingredient database.
Delivery Mechanism
Even when ingredients have been studied in humans, most studies use oral supplements or leave-on topical products.
That matters because oral products circulate throughout your entire body, and leave-on topicals sit on your scalp for 12+ hours. But shampoo remains on the scalp for only 1 to 2 minutes, typically 3 to 4 times per week.
That is usually not enough time for meaningful absorption, and even if some does get in, the dose is likely small. So it’s a big leap to take results from pills or leave-on products and assume the same ingredient will work in a rinse-off shampoo.
For this very reason, we organise our product metrics by formulation at Perfect Hair Health.
Hypothesis vs. Reality
Ketoconazole shampoo is often called the “best DHT-blocking shampoo”.
Oral ketoconazole can lower male androgens (like DHT), and ketoconazole shampoo has been shown to improve hair outcomes in patients with androgenic alopecia.[3]Perez, H.B.S. (2004). Ketoconazole as an Adjunct to Finasteride in the Treatment of Androgenetic Alopecia in Men. Medical Hypotheses. 62(1). 112-115. Available at: … Continue reading,[4]Khandpur, S., Suman, M., Reddy, B.S. (2002). Comparative Efficacy of Various Treatment Regimens for Androgenetic Alopecia in Men. Journal of Dermatology. 29(8). 489-498. Available at: … Continue reading,[5]Rafi, A.W., Katz, R.M. (2011). Pilot Study of 15 Patients Receiving a New Treatment Regimen for Androgenic Alopecia: The Effects of Atopy on AGA. ISRN Dermatology. 2011. 241953. Available at: … Continue reading But we don’t actually know whether ketoconazole shampoo helps hair by lowering scalp DHT.
It might help hair for completely different reasons, such as reducing scalp inflammation, killing Malassezia yeast, or improving overall scalp health.[6]Prohic, A., Simic, D., Sadikovic, T.J., Krupalija-Fazlic, M. (2014). Distribution of Malassezia Species on Healthy Human Skin in Bosnia and Herzegovina: Correlation With Body Part, Age and Gender. … Continue reading,[7]Piérard-Franchimont, C., De Doncker, P., Cauwenbergh, G., Piérard, G.E. (1998). Ketoconazole Shampoo: Effect of Long-Term Use in Androgenic Alopecia. Dermatology. 196(4). 474-477. Available at: … Continue reading
While ketoconazole shampoo appears helpful, its benefit may have little or no relationship to DHT.
The 10 Best DHT-Blocking Shampoos
When it comes to DHT-blocking shampoos, the claims go far beyond the science. Laboratory studies do not translate to human results; supplements and leave-on products are not the same as rinse-off shampoos; and even the most promising examples are based on hypotheses, not proven evidence.
Considering this, to say that there was a particular brand of DHT-blocking shampoo that is the “best” would be incorrect. Instead, we can consider ingredients that are common to many of these shampoo products. So here, we rank the 10 most common DHT-blocking ingredients in shampoos. We dissect the science, evidence quality, and growth potential behind each of them as hair loss products, so you can decide whether these ingredients in DHT-blocking shampoos really stack up to their marketing claims.
1. Ketoconazole Shampoo
Ketoconazole was a medication formulated to treat fungal infections, but it has also been shown to treat hair loss. Ketoconazole can inhibit 5‑α‑reductase activity and could disrupt DHT binding to androgen receptors.[8]Hugo Perez, B. S. (2004). Ketocazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Med Hypotheses. 62(1). 112-115. Available at: … Continue reading
Unlike many of the ingredients on this list, there is clinical evidence of ketoconazole shampoo as a product for hair loss.
A 1998 trial of 39 participants with androgenic alopecia directed participants to apply 2% kentoconazole for six months. After six months, participants using the ketoconazole shampoo showed an increase in hair diameter, while those using a placebo shampoo showed a decrease. Hair density improvements were similar between those using the ketoconazole shampoo and a 2% minoxidil therapy.[9]Piérard-Franchimont, C., De Doncker, P., Cauwenbergh, G., Piérard, G. E. (1998). Ketoconazole Shampoo: Effect of Long-Term Use in Androgenic Alopecia. Dermatology. 196(4). 474-477. Available at: … Continue reading
In support of this, a review summarising seven studies using topical ketoconazole concluded that ketoconazole appears to increase hair diameter and result in clinical improvement in those with androgenic alopecia.[10]Fields, J. R., Vonu, P. M., Monir, R. L., Schoch, J. J. (2020). Topical ketoconazole for the treatment of androgenetic alopecia: A systematic review. Dermatol Ther. 33(1). e13202. Available at: … Continue reading
Although more trials are needed, and only prescription-grade 2% ketoconazole has shown to improve hair loss, this promising clinical evidence places ketoconazole at the top of the list for DHT-blocking shampoos.
Our evidence score – 54/100 (shampoo)
Interested in learning more about ketoconazole? Read our article.
Example ketoconazole shampoos:
- Nizoral Anti‑Dandruff Shampoo 1% Ketoconazole – $15.89
- Minoxidil Max Hair Covet Hair Restoration Shampoo – $40
- Ketoconazole 2% Medicated Shampoo – $12.50 to $47.00 (Prescription)
2. Ginseng Root Shampoo
Ginseng root is often derived from the Panax species of plant. Two different extracts of Panax ginseng root have shown to inhibit 5-α-reductase activity by 50% at certain concentrations.[11]Murata, K., Takeshita, F., Samukawa, K., Tani, T. and Matsuda, H. (2012). Effects of ginseng rhizome and ginsenoside Ro on testosterone 5α-reductase and hair re-growth in testosterone-treated mice. … Continue reading In rats, oral administration of ginseng root has been found to reduce DHT levels, suggesting ginseng maintains its inhibitory activity against 5-α-reductase in vivo (in living organisms).[12]Park, J. Y., Park, W. Y., Song, G., et al. (2023). Panax ginseng C.A. meyer alleviates benign prostatic hyperplasia while preventing finasteride-induced side effects. Front Pharmacol. 14. Available … Continue reading,[13]Park, H. K., Kim, S. K., Lee, S. W., et al. (2017). A herbal formula, comprising Panax ginseng and bee-pollen, inhibits development of testosterone-induced benign prostatic hyperplasia in male Wistar … Continue reading
Clinical trials have also shown that oral ingestion of Panax ginseng was found to increase hair density and thickness over 24 weeks in those with androgenic alopecia.[14]Kim, J.H., Yi, S.M., Choi, J.E. and Son, S.W. (2009). Study of the efficacy of Korean red ginseng in the treatment of androgenic alopecia. Journal of Ginseng Research. 33(3). 223–228. Available at: … Continue reading Notably this has limited applicability to topical application, and the study does not specify whether total hair or just terminal hairs (i.e. those that actually contribute to hair looking cosmetically dense) were included, so results should be taken with a pinch of salt.
In another trial, Panax ginseng extracts were found to improve hair density and thickness in those with female pattern alopecia when used in combination with 3% minoxidil compared to 3% minoxidil alone.[15]Ryu, H.J., Yoo, M.G. and Son, S.W. (2014). The efficacy of 3% minoxidil vs. combined 3% minoxidil and Korean red ginseng in treating female pattern alopecia. International Journal of Dermatology. … Continue reading. However, this was again with oral intake, not topical application, and the study appeared to lack a control group to fully validate changes.
In the laboratory, ginseng extracts have been shown to promote hair growth in human hair follicles.[16]Park, G. H., Park, K. Y., Cho, H. I., et al. (2015). Red Ginseng Extract Promotes the Hair Growth in Cultured Human Hair Follicles. J Med Food. 18(3). 354-362. Available at: … Continue reading However, for use topically, there is not yet any clinical evidence to indicate whether Panax ginseng is effective at stopping hair loss or supporting hair growth.
Our evidence score – 8/100 (shampoo)
Example ginseng root shampoos:
- Neofollics Scalp Therapy Exfoliating Shampoo – $32.84
- DS Laboratories REVITA Antioxidant Hair Density CBD Shampoo – $38
- Xyon Health Performance DHT-Blocking Shampoo – $45
3. Ecklonia Cava Shampoo
Also called paddle weed, this is a marine algae found off Japan and Korea. A component of this species, dieckol, has been shown to significantly inhibit 5-ɑ-reductase activity in laboratory tests and reduce DHT levels.[17]Kang, J. I., Kim, S. C., Kim, M. K., et al. (2012). Effect of Dieckol, a Component of Ecklonia cava, on the Promotion of Hair Growth. Int J Mol Sci. 13(5). 6407-6423. Available at: … Continue reading
Figure 4: Inhibition activity of Ecklonia cava against 5-ɑ-reductase. Adapted from Figure 5.[18]Kang, J. I., Kim, S. C., Kim, M. K., et al. (2012). Effect of Dieckol, a Component of Ecklonia cava, on the Promotion of Hair Growth. Int J Mol Sci. 13(5). 6407-6423. Available at: … Continue reading Image used in line with the Creative Commons License.
In the same study, daily topical application of 0.4% Ecklonia cava for 33 days stimulated active hair growth in the hair follicles of mice.[19]Kang, J. I., Kim, S. C., Kim, M. K., et al. (2012). Effect of Dieckol, a Component of Ecklonia cava, on the Promotion of Hair Growth. Int J Mol Sci. 13(5). 6407-6423. Available at: … Continue reading Although hair growth was not as substantial as in mice receiving 5% minoxidil, the study shows the potential of topical Ecklonia cava as a natural hair loss ingredient.
However, clinical trials in humans showing the effectiveness of Ecklonia cava as a DHT blocker and hair loss product are unfortunately absent. Until then, Ecklonia cava can only be stated as an effective DHT blocker in vitro (in the test tube).
Our evidence score – 8/100 (shampoo)
Example Ecklonia cava shampoos:
- Zenluca Hair Growth Stimulating Shampoo – €19,95 ($23.38)
- Neofollics Hair Growth Stimulating Shampoo – $32.84
4. Oleanolic Acid Shampoo
Oleanolic acid is a natural compound found in many plants, fruits, and herbs. It is a natural inhibitor of 5-ɑ-reductase. At a 2014 conference, researchers reported that formulations of 3% oleanolic acid were found to inhibit 5-ɑ-reductase by 68% and inactivated 54% of testosterone conversion to DHT.[20]Ashtiani, H. A., Ehsani, A. H., Brikbin, B., Krimlou, Z., Pouya, F. B. (no date). Effectiveness of the formulation that containing oleanolic acid 3% on inhibition of 5-and#945; reductase activity on … Continue reading
While this report might sound positive, the research has not been submitted for peer review and publication. Thus, the robustness of these findings is questionable.
There is, however, in vivo evidence to support the DHT-blocking activity of oleanolic acid. In a study using mice, topical oleanolic acid was found to not only promote hair growth but to also reduce the levels of 5-ɑ-reductase and DHT in blood.[21]Zhang, B., Zhang, W., Luo, J., et al. (2023). Effects of oleanolic acid on hair growth in mouse dorsal skin mediated via regulation of inflammatory cytokines. J Appl Biomed. 21(1). 48-57. Available … Continue reading
Clinical evidence with humans is also available. In a trial examining the effectiveness of a topical hair loss lotion that included oleanolic acid, participants were found to have reduced hair shedding.[22]Garre Contreras, A., Piquero-Casals, J., Trullas, C., Martinez, G. (2018). Efficacy and Safety of a New Topical Hair Loss-Lotion Containing Oleanolic Acid, Apigenin, Biotinyl Tripeptide-1, … Continue reading Of course, this was a multi-ingredient lotion, so it is hard to attribute this to the effect of oleanolic acid alone. However, this does suggest topical application of oleanolic acid could be promising as a hair loss ingredient, and clinical trials with an oleanolic acid shampoo are warranted.
Our evidence score – 7/100 (shampoo)
Example oleanolic acid shampoos:
- Nourkrin Shampoo – $16.53
- Keeps Thickening Shampoo – $24
- DS Laboratories RADIA Purifying Shampoo for Dry, Sensitive or Irritated Scalp – $32
5. Green Tea Extract Shampoo
Green tea is a very common herbal remedy and has a multifaceted role as a DHT blocker.
Laboratory studies show that green tea extract can bind androgen receptors, preventing the binding of DHT.[23]Siddiqui, I. A., Asim, M., Hafeez, B. B., Adhami, V. M., Tarapore, R. S., Mukhtar, H. (2011). Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 25(4). … Continue reading This would reduce the downstream impact of DHT-mediated hair loss.
Green tea extract has also been shown to be a 5-ɑ-reductase inhibitor in test tubes. However, when testing whether green tea extract could also inhibit 5-ɑ-reductase within cells, this was found not to be the case.[24]Hiipakka, R. A., Zhang, H. Z., Dai, W., Dai, Q., Liao, S. (2002). Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem Pharmacol. 63(6). 1165-1176. … Continue reading,[25]Liao, S. S., Hiipakka, R. A. (1995). Selective-Inhibition of Steroid 5 α-Reductase Isozymes by Tea Epicatechin-3-Gallate and Epigallocatechin-3-Gallate. Biochem Biophys Res Commun. 214(3). 833-838. … Continue reading
What cell studies do show, however, is that green tea extract can dampen the downstream effects of DHT. In hair follicle cells, green tea extract can protect against DHT-induced death and lack of growth.[26]Shin, S., Kim, K., Lee, M. J., et al. (2016). Epigallocatechin Gallate-Mediated Alteration of the MicroRNA Expression Profile in 5α-Dihydrotestosterone-Treated Human Dermal Papilla Cells. Ann … Continue reading
The DHT-blocking mechanisms characterised for green tea appear to translate to in vivo DHT-blocking. Dietary intake of green tea, along with soy compounds, was found to reduce DHT levels in mice. However, intake of green tea alone was found to actually increase DHT.[27]Zhou, J. R., Yu, L., Zhong, Y., Blackburn, G. L. (2003). Soy Phytochemicals and Tea Bioactive Components Synergistically Inhibit Androgen-Sensitive Human Prostate Tumors in Mice. J Nutr. 133(2). … Continue reading This suggests whole green tea, instead of green tea extract, may not effectively block DHT.
For now, there is a lack of large, randomized controlled trials to indicate whether green tea extract, whether topical or oral, can effectively block DHT in humans. Thus, in a shampoo, its role is best considered mechanistically possible but not yet proven.
Our evidence score – 6/100 (shampoo)
Example green tea extract shampoos:
- DrFormulas Best DHT Blocker Shampoo – $20.41
- Keeps Thickening Shampoo – $24
- Neofollics Hair Growth Stimulating Shampoo – $32.84
6. Sulforaphane Shampoo
Sulforaphane is a plant-derived compound known as a phytochemical. It is known for its antioxidant, anti-inflammatory, and even potential anti-cancer properties, and may have a novel mechanism for promoting hair growth as well.
In the laboratory, sulforaphane has been shown to increase the concentration of an enzyme that can degrade DHT, known as 3α-hydroxysteroid dehydrogenase.[28]Park, Y., Choi, K., Kim, H., Lee, J., Park, G., Kim, J., (2021). Sulforaphane, L-Menthol, and Dexpanthenol as a Novel Active Cosmetic Ingredient Composition for Relieving Hair Loss Symptoms. … Continue reading
But does this mean it can actually promote hair growth? In the same study, the hair growth potential of sulforaphane as a conditioner in those with androgenic alopecia was examined in an 18-week clinical trial.
The trial found that there was an 87% increase in visual hair growth at the crown and forehead line, and an increase in the number of hair follicles. While this was found to be statistically significant, it should be noted that this only translated to a difference of +2 hairs on average at 18 weeks compared to day one.
To make conclusions about the potential of sulforaphane against hair loss, we need more robust clinical trials. We also need more evidence of its use as a shampoo; the trial above used sulforaphane as a conditioner, where it would have had minimal contact with the scalp compared with a shampoo.
Figure 4: Five images per time point showing the scalp at baseline (0 week) and following use of a sulforaphane conditioner for 6, 12, and 18 weeks. Adapted from Figure 5.[29]Park, Y., Choi, K., Kim, H., Lee, J., Park, G., Kim, J., (2021). Sulforaphane, L-Menthol, and Dexpanthenol as a Novel Active Cosmetic Ingredient Composition for Relieving Hair Loss Symptoms. … Continue reading. Image obtained in line with the Creative Commons License.
If you want to learn more about sulforaphane for hair loss, read our article here.
Our evidence score – 4/100 (shampoo)
Currently, there are no sulforaphane shampoos on the market.
7. Melatonin Shampoo
Melatonin is not a traditional DHT blocker. It does not inhibit 5‑α‑reductase or bind with androgen receptors. Instead, melatonin appears to reduce the DNA changes that occur when androgen receptors are activated.[30]Hao, L., Dong, Y., Zhang, J. J., et al. (2022). Melatonin decreases androgen-sensitive prostate cancer growth by suppressing SENP1 expression. Transl Androl Urol. 11(1). 91-103. Available at: … Continue reading
This has been studied in relation to prostate cancer, but it could also mean that when DHT binds androgen receptors, the receptors are not able to signal DNA changes that may result in hair loss.
Melatonin has also been shown to reduce androgen levels in cells of the ovary.[31]Yu, K., Wang, R. X., Li, M. H., et al. (2019). Melatonin Reduces Androgen Production and Upregulates Heme Oxygenase-1 Expression in Granulosa Cells from PCOS Patients with Hypoestrogenia and … Continue reading Although not hair follicle cells, this suggests melatonin could similarly reduce androgens (like DHT) at the scalp.
Unfortunately, there isn’t any evidence to show that application of melatonin results in reduced blood DHT levels, but topical application of 0.1% melatonin has shown to decrease the rate of active hair loss for patients with early androgenic alopecia. However, not all results were statistically significant.[32]Fischer, T.W., Burmeister, G., Schmidt, H.W. and Elsner, P. (2004). Melatonin increases anagen hair rate in women with androgenetic alopecia or diffuse alopecia. British Journal of Dermatology. … Continue reading
A subsequent study analysing the results of five clinical studies using topical melatonin concluded that, overall, melatonin is an effective ingredient for androgenic alopecia. Leave-on, topical melatonin was found to not only be safe but also increase hair density, reduce hair loss, and increase hair strength.[33]Fischer, T. W., Trüeb, R. M., Hänggi, G., Innocenti, M., Elsner, P. (2012). Topical Melatonin for Treatment of Androgenetic Alopecia. Int J Trichology. 4(4). 236-245. Available at: … Continue reading
Whether a rinse-off melatonin formula could produce similar results is not yet clear, but the topical evidence thus far indicates that melatonin could be a beneficial ingredient for androgenic alopecia.
Our evidence score – 4/100
Example melatonin shampoos:
- As I am Rosemary Shampoo – $9.99
- RevivSerums RevivHair Stimulating Shampoo – $29.00
- Kenogen Hair Growth Shampoo – $59.00
8. Saw Palmetto Shampoo
Saw palmetto is a plant native to the United States. In the laboratory, extracts of saw palmetto have been found to inhibit the activity of 5‑α‑reductase.[34]Blair, H. A. (2022). Hexanic Extract of Serenoa repens (Permixon®): A Review in Symptomatic Benign Prostatic Hyperplasia. Drugs Aging. 39(3). 235-243. Available at: … Continue reading,[35]Buonocore, D., Verri, M., Cattaneo, L., Arnica, S., Ghitti, M., Dossena, M. (2018). Serenoa repens extracts: In vitro study of the 5α-reductase activity in a co-culture model for Benign Prostatic … Continue reading The effectiveness of saw palmetto has also been shown outside the lab. Oral administration of saw palmetto extracts at 320 mg per day has been shown to reduce DHT levels in male participants, confirming that saw palmetto can indeed inhibit 5‑α‑reductase when ingested.[36]Di Silverio, F., Monti, S., Sciarra, A., et al. (1998). Effects of long-term treatment with Serenoa repens (Permixon®) on the concentrations and regional distribution of androgens and epidermal … Continue reading
In clinical trials, the topical application of saw palmetto for hair loss has received mixed results. Application of saw palmetto as a lotion has shown to marginally increase hair number compared to a placebo lotion after 50 weeks in those with androgenic alopecia.[37]Morganti, P., Fabrizi, G., James, B., Bruno, C. (1998). Effect of gelatin-cystine and serenoa repens extract on free radicals level and hair growth. Journal of Applied Cosmetology. 16, 57-64. … Continue reading. It has also been observed that androgenic alopecia patients had improved hair strength and slightly thicker hair after application of a saw palmetto oil for 16 weeks.[38]Sudeep, H.V., Rashmi, S., Jestin, T.V., Richards, A., Gouthamchandra, K., Shyamprasad, K. (2023). Oral and Topical Administration of a Standardized Saw Palmetto Oil Reduces Hair Fall and Improves the … Continue reading
Based on the evidence, saw palmetto seems a promising natural ingredient for promoting hair growth. But it should be considered that both of the outlined trials appeared to count terminal hairs (i.e., long, thick hairs that contribute to hair fullness) as well as vellus hairs (i.e., fine “peach fuzz” hairs that don’t contribute to hair fullness), and use a leave-on formula, so the results are only tentative and do not justify the purchase of a rinse-off saw palmetto shampoo.
However, the foundation is there for future clinical studies to build on these results and provide the proof-of-concept evidence needed to determine whether a saw palmetto shampoo can be effective against hair loss.
Our evidence score – 4/100 (shampoo)
Example saw palmetto shampoos:
- DrFormulas Best DHT Blocker Shampoo – $20.41
- Advanced Trichology DHT-Blocking Shampoo – $27.95
- Shapiro MD DHT Fighting Shampoo – $39.95
9. Pumpkin Seed Oil Shampoo
In the laboratory, pumpkin seed oil has been identified as a moderate inhibitor of 5-α-reductase and can interact with androgen receptors, preventing the conversion of testosterone to DHT and the binding of DHT. At 5 mg per mL, pumpkin seed oil could inhibit 5-α-reductase 70-95%.[39]Heim, S., Seibt, S., Stier, H., Moré, M. I. (2018). Uromedic® Pumpkin Seed Derived Δ7-Sterols, Extract and Oil Inhibit 5α-Reductases and Bind to Androgen Receptor in Vitro. Pharmacol Pharm. 9(6). … Continue reading
There are no studies demonstrating that pumpkin seed oil reduces DHT levels in humans. Administration of pumpkin seed oil extracts to rats has shown to reduce 5-α-reductase levels, showing that the outcomes of laboratory experiments in vitro are matched in vivo.[40]Kang, X. C., Chen, T., Zhou, J. L., et al. (2021). Phytosterols in hull-less pumpkin seed oil, rich in ∆7-phytosterols, ameliorate benign prostatic hyperplasia by lowing 5α-reductase and … Continue reading
In a clinical trial of 60 women with androgenic alopecia, topical application of a pumpkin seed oil foam for three months increased the number of regrowing hairs compared to baseline, but not as much as 5% minoxidil. It should be noted that upright growing hairs aren’t the best endpoint for assessing outcomes, since these come and go with the natural hair cycle. Additionally, this study featured no placebo group, so whether these results were substantial against no treatment at all is unknown.[41]Ibrahim, I. M., Hasan, M. S., Elsabaa, K. I., Elsaie, M. L. (2021). Pumpkin seed oil vs. minoxidil 5% topical foam for the treatment of female pattern hair loss: A randomized comparative trial. J … Continue reading
Nonetheless, the trial suggests topical pumpkin seed oil has the potential to support hair growth, but without further clinical studies, it is simply a marketing ingredient, not a science-backed one.
Our evidence score – 4/100 (shampoo)
Example pumpkin seed oil shampoos:
- BosleyMD Revive Nourishing Shampoo – $21.25
- Roman Revive Shampoo – $24
- Botanical Green Care Saw Palmetto & Cayenne Anti-Hair Loss Shampoo – $36.99
10. Rosemary Oil Shampoo
Rosemary oil is an essential oil and a common ingredient in products marketed towards hair loss.
In the laboratory, rosemary oil extract has been found to inhibit the activity of 5-ɑ-reductase. Rosemary oil extract has also been shown to potentially prevent the interaction of DHT with androgen receptors, both effects resulting in improved hair growth in mice compared to control.[42]Murata, K., Noguchi, K., Kondo, M., et al. (2013). Promotion of hair growth by Rosmarinus officinalis leaf extract. Phytother Res PTR. 27(2). 212-217. Available at: https://doi.org/10.1002/ptr.4712
Figure 3: Effect of rosemary oil extract (RO-ext) and 1% minoxidil on hair regrowth in mice compared against control groups with no treatment. Adapted from Figure 4.[43]Murata, K., Noguchi, K., Kondo, M., et al. (2013). Promotion of hair growth by Rosmarinus officinalis leaf extract. Phytother Res PTR. 27(2). 212-217. Available at: https://doi.org/10.1002/ptr.4712 Image obtained in line with the Creative Commons License.
Research is needed to test whether the same effect occurs within humans, although clinical trials with this essential oil do indicate that rosemary oil works similarly within the body.
Namely, a clinical trial with androgenic alopecia patients has shown that rosemary oil can increase hair count by a similar amount to 2% minoxidil following topical application for six months. However, while the results sound great, increases were not substantial in either group compared to baseline hair counts.[44]Panahi, Y., Taghizadeh, M., Marzony, E. T., & Sahebkar, A. (2015). Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 13(1). … Continue reading This would suggest:
- Rosemary oil only marginally improves hair count
- There may be quality issues with the data and conclusions, as minoxidil should significantly and progressively support hair growth over this time frame.
As it stands, there is some evidence to suggest that rosemary oil might have some action on DHT, but further clinical studies are needed to validate these results and indicate whether rosemary oil could be equally as effective when applied as a shampoo.
Our evidence score – 4/100 (shampoo)
Example rosemary oil shampoos:
- Ulo Thickening Shampoo – $21.75
- Dandrene Exfoliating Anti-Dandruff Shampoo – $32
- Neofollics Scalp Therapy Exfoliating Shampoo – $32.84
Is a DHT-Blocking Shampoo Worth It?
Unlike supplements or leave-on topical products, shampoos have a short contact time with the scalp and are rinsed off quickly. Shampoos are also often used inconsistently. This does not give ample time or enough repeated exposure for ingredients to properly absorb into the scalp and affect hair growth.
Some DHT-blocking ingredients show promise when used topically. However, there is limited evidence, laboratory or clinical, to show that they would have the same effect in a shampoo, except for ketoconazole.
DHT-blocking shampoos contain a range of herbal extracts that may help hair look healthier and feel softer. If you find your DHT-blocking shampoo gives your hair a physical appearance boost, feel free to keep using it. However, the evidence for the use of DHT-blocking shampoos in hair loss regimens does not stack up with claims of hair regrowth when used alone.
If you are interested in DHT-blocking, seek out FDA-approved oral DHT blockers such as finasteride, dutasteride, bicalutamide, flutamide, or spironolactone. If you want a chemical-free formula, find topical lotions, serums, or oils with DHT-blocking ingredients instead of shampoos. Both of these formulations offer longer contact time in and outside the body.
However, DHT-blocking shampoos could be used alongside FDA-approved hair loss treatments or leave-on products with DHT-blocking ingredients if you choose, just do not rely on these shampoos alone to treat hair loss.
Final Remarks
When considering a DHT-blocking shampoo, ingredient choice matters more than marketing. Ingredients like ketoconazole have the best evidence behind them to support their use in an anti-hair loss product.
But it’s not just the ingredients that matter, it’s the formulation as well. DHT blockers are more often incorporated into supplements and FDA-approved medications. This allows the ingredients to work from deep inside the body. Shampoos instead rely on repeated, short-term exposure to deliver the ingredients.
Shampoo exposure like this may influence local DHT levels at the scalp, generally improve the scalp environment, and slow the progression of androgenic alopecia, but it will not act as a re-growth formula, and use of shampoo is unlikely to resolve androgenic alopecia when used alone.
References[+]
References ↑1 Ustuner, E. T. (2013). Cause of Androgenic Alopecia: Crux of the Matter. Plast Reconstr Surg Glob Open. 1(7). e64. Available at: https://doi.org/10.1097/GOX.0000000000000005 ↑2 Sekhavat, H., Bar Yehuda, S., Asotra, S. (2025). Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss. Int J Mol Sci. 26(21). 10712. Available at: https://doi.org/10.3390/ijms262110712 ↑3 Perez, H.B.S. (2004). Ketoconazole as an Adjunct to Finasteride in the Treatment of Androgenetic Alopecia in Men. Medical Hypotheses. 62(1). 112-115. Available at: https://doi.org/10.1016/s0306-9877(03)00264-0 ↑4 Khandpur, S., Suman, M., Reddy, B.S. (2002). Comparative Efficacy of Various Treatment Regimens for Androgenetic Alopecia in Men. Journal of Dermatology. 29(8). 489-498. Available at: https://doi.org/10.1111/j.1346-8138.2002.tb00314.x ↑5 Rafi, A.W., Katz, R.M. (2011). Pilot Study of 15 Patients Receiving a New Treatment Regimen for Androgenic Alopecia: The Effects of Atopy on AGA. ISRN Dermatology. 2011. 241953. Available at: https://doi.org/10.5402/2011/241953 ↑6 Prohic, A., Simic, D., Sadikovic, T.J., Krupalija-Fazlic, M. (2014). Distribution of Malassezia Species on Healthy Human Skin in Bosnia and Herzegovina: Correlation With Body Part, Age and Gender. Iranian Journal of Microbiology. 6(4). 253-262 ↑7 Piérard-Franchimont, C., De Doncker, P., Cauwenbergh, G., Piérard, G.E. (1998). Ketoconazole Shampoo: Effect of Long-Term Use in Androgenic Alopecia. Dermatology. 196(4). 474-477. Available at: https://doi.org/10.1159/000017954 ↑8 Hugo Perez, B. S. (2004). Ketocazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Med Hypotheses. 62(1). 112-115. Available at: https://doi.org/10.1016/S0306-9877(03)00264-0 ↑9 Piérard-Franchimont, C., De Doncker, P., Cauwenbergh, G., Piérard, G. E. (1998). Ketoconazole Shampoo: Effect of Long-Term Use in Androgenic Alopecia. Dermatology. 196(4). 474-477. Available at: https://doi.org/10.1159/000017954 ↑10 Fields, J. R., Vonu, P. M., Monir, R. L., Schoch, J. J. (2020). Topical ketoconazole for the treatment of androgenetic alopecia: A systematic review. Dermatol Ther. 33(1). e13202. Available at: https://doi.org/10.1111/dth.13202 ↑11 Murata, K., Takeshita, F., Samukawa, K., Tani, T. and Matsuda, H. (2012). Effects of ginseng rhizome and ginsenoside Ro on testosterone 5α-reductase and hair re-growth in testosterone-treated mice. Phytotherapy Research. 26(1). 48–53. Available at: https://doi.org/10.1002/ptr.3511 ↑12 Park, J. Y., Park, W. Y., Song, G., et al. (2023). Panax ginseng C.A. meyer alleviates benign prostatic hyperplasia while preventing finasteride-induced side effects. Front Pharmacol. 14. Available at: https://doi.org/10.3389/fphar.2023.1039622 ↑13 Park, H. K., Kim, S. K., Lee, S. W., et al. (2017). A herbal formula, comprising Panax ginseng and bee-pollen, inhibits development of testosterone-induced benign prostatic hyperplasia in male Wistar rats. Saudi J Biol Sci. 24(7). 1555-1561. Available at: https://doi.org/10.1016/j.sjbs.2015.10.020 ↑14 Kim, J.H., Yi, S.M., Choi, J.E. and Son, S.W. (2009). Study of the efficacy of Korean red ginseng in the treatment of androgenic alopecia. Journal of Ginseng Research. 33(3). 223–228. Available at: https://doi.org/10.5142/JGR.2009.33.3.223 ↑15 Ryu, H.J., Yoo, M.G. and Son, S.W. (2014). The efficacy of 3% minoxidil vs. combined 3% minoxidil and Korean red ginseng in treating female pattern alopecia. International Journal of Dermatology. 53(6). e340–e342. Available at: https://doi.org/10.1111/ijd.12359 ↑16 Park, G. H., Park, K. Y., Cho, H. I., et al. (2015). Red Ginseng Extract Promotes the Hair Growth in Cultured Human Hair Follicles. J Med Food. 18(3). 354-362. Available at: https://doi.org/10.1089/jmf.2013.3031 ↑17, ↑18, ↑19 Kang, J. I., Kim, S. C., Kim, M. K., et al. (2012). Effect of Dieckol, a Component of Ecklonia cava, on the Promotion of Hair Growth. Int J Mol Sci. 13(5). 6407-6423. Available at: https://doi.org/10.3390/ijms13056407 ↑20 Ashtiani, H. A., Ehsani, A. H., Brikbin, B., Krimlou, Z., Pouya, F. B. (no date). Effectiveness of the formulation that containing oleanolic acid 3% on inhibition of 5-and#945; reductase activity on skin of patients with acne. J Clin Exp Dermatol Res. Available at: https://doi.org/10.4172/2155-9554.S1.015 ↑21 Zhang, B., Zhang, W., Luo, J., et al. (2023). Effects of oleanolic acid on hair growth in mouse dorsal skin mediated via regulation of inflammatory cytokines. J Appl Biomed. 21(1). 48-57. Available at: https://doi.org/10.32725/jab.2023.003 ↑22 Garre Contreras, A., Piquero-Casals, J., Trullas, C., Martinez, G. (2018). Efficacy and Safety of a New Topical Hair Loss-Lotion Containing Oleanolic Acid, Apigenin, Biotinyl Tripeptide-1, Diaminopyrimidine Oxide, Adenosine, Biotin and Ginkgo biloba in Patients with Androgenetic Alopecia and Telogen effluvium: A Six-month Open-Label Prospective Clinical Study. J Cosmetol Trichology. 04. Available at: https://doi.org/10.4172/2471-9323.1000132 ↑23 Siddiqui, I. A., Asim, M., Hafeez, B. B., Adhami, V. M., Tarapore, R. S., Mukhtar, H. (2011). Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 25(4). 1198-1207. Available at: https://doi.org/10.1096/fj.10-167924 ↑24 Hiipakka, R. A., Zhang, H. Z., Dai, W., Dai, Q., Liao, S. (2002). Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem Pharmacol. 63(6). 1165-1176. Available at: https://doi.org/10.1016/s0006-2952(02)00848-1 ↑25 Liao, S. S., Hiipakka, R. A. (1995). Selective-Inhibition of Steroid 5 α-Reductase Isozymes by Tea Epicatechin-3-Gallate and Epigallocatechin-3-Gallate. Biochem Biophys Res Commun. 214(3). 833-838. Available at: https://doi.org/10.1006/bbrc.1995.2362 ↑26 Shin, S., Kim, K., Lee, M. J., et al. (2016). Epigallocatechin Gallate-Mediated Alteration of the MicroRNA Expression Profile in 5α-Dihydrotestosterone-Treated Human Dermal Papilla Cells. Ann Dermatol. 28(3). 327-334. Available at: https://doi.org/10.5021/ad.2016.28.3.327 ↑27 Zhou, J. R., Yu, L., Zhong, Y., Blackburn, G. L. (2003). Soy Phytochemicals and Tea Bioactive Components Synergistically Inhibit Androgen-Sensitive Human Prostate Tumors in Mice. J Nutr. 133(2). 516-521. Available at: https://doi.org/10.1093/jn/133.2.516 ↑28, ↑29 Park, Y., Choi, K., Kim, H., Lee, J., Park, G., Kim, J., (2021). Sulforaphane, L-Menthol, and Dexpanthenol as a Novel Active Cosmetic Ingredient Composition for Relieving Hair Loss Symptoms. Cosmetics. 8(3). 63. Available at: https://doi.org/10.3390/cosmetics8030063 ↑30 Hao, L., Dong, Y., Zhang, J. J., et al. (2022). Melatonin decreases androgen-sensitive prostate cancer growth by suppressing SENP1 expression. Transl Androl Urol. 11(1). 91-103. Available at: https://doi.org/10.21037/tau-21-1110 ↑31 Yu, K., Wang, R. X., Li, M. H., et al. (2019). Melatonin Reduces Androgen Production and Upregulates Heme Oxygenase-1 Expression in Granulosa Cells from PCOS Patients with Hypoestrogenia and Hyperandrogenia. Oxid Med Cell Longev. 2019. 8218650. Available at: https://doi.org/10.1155/2019/8218650 ↑32 Fischer, T.W., Burmeister, G., Schmidt, H.W. and Elsner, P. (2004). Melatonin increases anagen hair rate in women with androgenetic alopecia or diffuse alopecia. British Journal of Dermatology. 150(2). 341–345. Available at: https://doi.org/10.1111/j.1365-2133.2004.05685.x ↑33 Fischer, T. W., Trüeb, R. M., Hänggi, G., Innocenti, M., Elsner, P. (2012). Topical Melatonin for Treatment of Androgenetic Alopecia. Int J Trichology. 4(4). 236-245. Available at: https://doi.org/10.4103/0974-7753.111199 ↑34 Blair, H. A. (2022). Hexanic Extract of Serenoa repens (Permixon®): A Review in Symptomatic Benign Prostatic Hyperplasia. Drugs Aging. 39(3). 235-243. Available at: https://doi.org/10.1007/s40266-022-00924-3 ↑35 Buonocore, D., Verri, M., Cattaneo, L., Arnica, S., Ghitti, M., Dossena, M. (2018). Serenoa repens extracts: In vitro study of the 5α-reductase activity in a co-culture model for Benign Prostatic Hyperplasia. Arch Ital Urol Androl Organo Uff Soc Ital Ecogr Urol E Nefrol. 90(3). 199-202. Available at: https://doi.org/10.4081/aiua.2018.3.199 ↑36 Di Silverio, F., Monti, S., Sciarra, A., et al. (1998). Effects of long-term treatment with Serenoa repens (Permixon®) on the concentrations and regional distribution of androgens and epidermal growth factor in benign prostatic hyperplasia. The Prostate. 37(2). 77-83. Available at: https://doi.org/10.1002/(sici)1097-0045(19981001)37:2<77::aid-pros3>3.0.co;2-i ↑37 Morganti, P., Fabrizi, G., James, B., Bruno, C. (1998). Effect of gelatin-cystine and serenoa repens extract on free radicals level and hair growth. Journal of Applied Cosmetology. 16, 57-64. Available at: https://www.researchgate.net/publication/294672324_Effect_of_gelatin-cystine_and_serenoa_repens_extract_on_free_radicals_level_and_hair_growth ↑38 Sudeep, H.V., Rashmi, S., Jestin, T.V., Richards, A., Gouthamchandra, K., Shyamprasad, K. (2023). Oral and Topical Administration of a Standardized Saw Palmetto Oil Reduces Hair Fall and Improves the Hair Growth in Androgenetic Alopecia Subjects – A 16-Week Randomized, Placebo-Controlled Study. Clin Cosmet Investig Dermatol. 16, 3251-3266. Available at: https://doi.org/10.2147/CCID.S435795 ↑39 Heim, S., Seibt, S., Stier, H., Moré, M. I. (2018). Uromedic® Pumpkin Seed Derived Δ7-Sterols, Extract and Oil Inhibit 5α-Reductases and Bind to Androgen Receptor in Vitro. Pharmacol Pharm. 9(6). 193-207. Available at: https://doi.org/10.4236/pp.2018.96015 ↑40 Kang, X. C., Chen, T., Zhou, J. L., et al. (2021). Phytosterols in hull-less pumpkin seed oil, rich in ∆7-phytosterols, ameliorate benign prostatic hyperplasia by lowing 5α-reductase and regulating balance between cell proliferation and apoptosis in rats. Food Nutr Res. 65. 10.29219/fnr.v65.7537. Available at: https://doi.org/10.29219/fnr.v65.7537 ↑41 Ibrahim, I. M., Hasan, M. S., Elsabaa, K. I., Elsaie, M. L. (2021). Pumpkin seed oil vs. minoxidil 5% topical foam for the treatment of female pattern hair loss: A randomized comparative trial. J Cosmet Dermatol. 20(9). 2867-2873. Available at: https://doi.org/10.1111/jocd.13976 ↑42 Murata, K., Noguchi, K., Kondo, M., et al. (2013). Promotion of hair growth by Rosmarinus officinalis leaf extract. Phytother Res PTR. 27(2). 212-217. Available at: https://doi.org/10.1002/ptr.4712 ↑43 Murata, K., Noguchi, K., Kondo, M., et al. (2013). Promotion of hair growth by Rosmarinus officinalis leaf extract. Phytother Res PTR. 27(2). 212-217. Available at: https://doi.org/10.1002/ptr.4712 ↑44 Panahi, Y., Taghizadeh, M., Marzony, E. T., & Sahebkar, A. (2015). Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 13(1). 15–21. Available at: https://pubmed.ncbi.nlm.nih.gov/25842469/ Men’s hair loss is a booming multi-billion-dollar industry, yet the vast majority of over-the-counter products fall short when measured against rigorous clinical evidence. This landscape is cluttered with quick-fix promises, leaving consumers sifting through marketing claims with little scientific support. Among all topical hair regrowth options, minoxidil is one of the most extensively studied. It is also one of two FDA-approved treatments for male pattern hair loss (in its topical form).
In this comprehensive guide, we rank our top minoxidil picks of 2026 across Best Overall, Best Value, and other categories to help you navigate the crowded hair loss market with more clarity and confidence.
Quick Look: Best Minoxidil for Men in 2026
Product Strength Format Customization Price Best: Ulo 7% Solution High $41.65 Overall Kirkland Minoxidil 5% Solution None $17.99 Value Rogaine 5% Solution/Foam None $49.97 Sensitive Scalp Keeps 5% Solution/Foam/Spray Low $16.67 Subscription Convenience Happy Head 5-8% Solution/Gel High $59 Strength What is Minoxidil and How Does it Work?
Minoxidil was originally developed to treat high blood pressure and is now widely used in both topical and oral forms as a first-line therapy for androgenic alopecia (AGA).
What is Minoxidil?
Minoxidil was first introduced in the 1970s as a powerful oral vasodilator for resistant hypertension. Clinicians soon noticed a frequent side effect, generalized increased hair growth (hypertrichosis).[1]Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2021). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: … Continue reading This led to the development of topical formulations specifically for AGA. Today, 2-5% topical minoxidil is FDA-approved for both male and female pattern hair loss and is used off-label for other alopecias.
How Does Minoxidil Work?
Minoxidil is a potassium-channel opener that causes vasodilation and improves microcirculation around hair follicles.[2]Hussein, R.S., Dayel, S.B., Abahussein, O., El-Sherbiny, A.A. (2024). Applications and efficacy of minoxidil in dermatology. Skin Health and Disease. 4(6). E472. Available at: … Continue reading It also acts as a prodrug; sulfotransferase converts it to minoxidil sulfate, which prolongs the anagen (growth) phase and shortens the telogen (resting) phase, shifting more hair follicles into active growth.[3]Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a … Continue reading Additional data suggests that induction of key growth factors like vascular endothelial growth factor (VEGF) and activation of Wnt/ꞵ-catenin signaling further support hair regrowth.[4]Gupta, A.K., Talukder, M., Shemer, A., Piraccini, B.M., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: … Continue reading
Although minoxidil doesn’t target dihydrotestosterone, it can improve the effectiveness of other treatments by targeting multiple other targets. When combined with anti-DHT drugs like finasteride, which reduce the hormonal trigger of AGA, minoxidil provides a complementary, non-hormonal pathway. The two treatments have been shown to provide synergistic improvement in hair density in men.[5]Asad, N., Naseer, M., Ghafoor, R. (2024). Efficacy of Topical Finasteride 0.25% with Minoxidil 5% versus Topical Minoxidil 5% Alone in Treatment of Male Pattern Androgenic Alopecia. Journal of Drugs … Continue reading
OTC vs Prescription-Strength
Over-the-counter (OTC) minoxidil solutions (2% and 5%) can be effective for many men, but absorption and vehicle limits mean “stronger” drug percentages above 5% often add irritation rather than results unless the entire formulation is engineered and supervised medically.
Early minoxidil development work showed a clear dose-response between low strengths (1-2%) and mid-strength (5%) solutions, with 5% yielding larger gains in hair counts and shaft diameter in AGA.[6]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
Other companies offer concentrations of 10% or over; however, recent head-to-head studies have shown that 5% topical minoxidil is moderately superior to the higher concentration.[7]Ghonemy, S., Alarawi, A., Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic … Continue reading Additionally, the 10% minoxidil was associated with more marked irritation.
So, with that in mind, let’s take a look at some of our top minoxidil picks of 2026.
Best Minoxidil Picks for Men
#1 Best Overall: Ulo Minoxidil Rx
Pros: Cons: ✓ Clinically-backed combinations ✗Prescription products are only available in the USA ✓ Ongoing medical monitoring and a user-friendly platform ✓ Quality-tested ingredients free of irritants Ulo’s Minoxidil Rx takes the top spot for men who want a data-driven, medically supervised approach rather than a one-size-fits-all bottle. Custom strengths and evidence-based optional add-ons allow precise targeting for hair regrowth.
Their high-strength concentration can be combined with tretinoin 0.01% to enhance absorption and efficacy, as well as cetirizine 1%, melatonin 0.01% for antioxidant protection, and caffeine 0.2% to boost circulation, which further improves the product’s effectiveness.
This treatment begins at $49 per month, increasing to $54 if you choose to have all of the add-ons.
Bottom Line: Ulo’s customizable, science-driven minoxidil prescription offers one of the most advanced, value-conscious options for men who are serious about long-term hair regrowth.
#2 Best Value – Kirkland Signature Minoxidil 5%
Pros: Cons: ✓ Lowest cost we could find for 6 months’ worth. ✗Contains propylene glycol, which can cause irritation or dryness in some users. ✓ Same core 5% minoxidil strength. ✗ No customization possible for strength or add-on ingredients. ✓ Widely available at multiple stores, including Amazon, Costco, etc. ✗ No medical consultation or tailoring of treatment included. ✓ Simple formula. Kirkland Signature Minoxidil 5% is the classic value choice for me who want an over-the-counter treatment without paying for branding or bundled medical services. You get a standard 5% solution similar in strength to more expensive competitors, making it ideal for cost-conscious users who are happy to follow a simple twice-daily routine and do not need personalized compounding.
Bottom line: If you want the most affordable entry point into 5% minoxidil, Kirkland’s no-frills formula is hard to beat for sheer value per effective dose.
#3 Best for Sensitive Scalps: Rogaine 5% Foam
Pros: Cons: ✓ Clinically proven 5% minoxidil strength ✗On the higher end in terms of price. ✓ Foam vehicle is propylene glycol-free, so it is usually better tolerated on sensitive or irritated scalps. ✗ Fixed strength for men with no customization or add-on actives. ✓ Quick drying, less greasy texture ✗ No medical consultation or tailoring of treatment included. ✓ Widely available OTC Rogaine 5% is a strong pick for men who need minoxidil but find traditional liquid formulas too irritating or cosmetically heavy. The foam format avoids common irritants found in many solutions and tends to be more comfortable for daily use, which can improve adherence and long-term outcomes.
Bottom Line: For sensitive scalps that still need full-strength 5% minoxidil, Rogaine Foam provides a gentler, easy-to-use option that balances proven efficacy with better tolerability.
#4 Best Subscription Convenience: Keeps Minoxidil
Pros: Cons: ✓ Automatic subscription refills so you rarely run out or miss doses. ✗More expensive than buying generic minoxidil in bulk. ✓ Offers both 5% foam and 5% solution to suit different scalp and styling preferences. ✗ Limited to preset strengths and formulas with no true custom compounding. ✓ Option to bundle with finasteride and other treatments for a complete regimen. ✓ Online prescribing and follow-up. Keeps Minoxidil is designed for men who value a set-and-forget routine, trading a price increase ($16.67 for 3 months compared to Kirkland’s $17.99 for 6 months) for automatic deliveries and integrated telehealth support. The ability to pair minoxidil with finasteride and keep everything managed through a single platform could make adherence simpler for busy users.
Bottom Line: If you want 5% minoxidil with minimal hassle, Keeps’ subscription model is a strong fit for convenience-focused men who prefer not to manage refills and prescriptions on their own.
#5 Best High-Strength Formula: Happy Head
Pros: Cons: ✓ Offers high-strength minoxidil concentrations beyond standard 5%. ✗Higher strengths may carry a greater risk of irritation or systemic absorption. ✓ Can be combined with topical finasteride and other actives in a single solution. ✗ More expensive than generic 5% minoxidil and some standard telehealth offerings. ✓ Telehealth model with online consultation and prescription management. ✗Corticosteroids used to offset irritation from propylene glycol. ✓ Multiple formula options aimed at men who have plateaued on basic OTC products. Happy Head is built for men who want to move past entry-level minoxidil and explore higher-strength, prescription-only blends under medical oversight. By pairing elevated minoxidil concentrations (8%) with agents like tretinoin and topical finasteride, Happy Head targets users seeking a more comprehensive protocol. With that said, some ingredients included, like the carrier agent propylene glycol, which is used in their base formula, can cause irritation. To offset this, Happy Head uses corticosteroids; however, this may cause skin thinning long-term.
You can read more about corticosteroid usage in hair loss treatments here.
Bottom Line: Happy Head delivers a good jump in strength for minoxidil users who need to increase efficacy.
Timeline: What Results to Expect from Minoxidil
It’s important that realistic expectations are set for regrowth when starting out on your minoxidil usage journey.
Timeframe What to Expect Clinical Evidence Months 0-3 This is the initial shedding phase, which usually resolves in the first 4-8 weeks. [8]Nohria, A., Desai, D., Sikora, M., Mandal, S., Shapiro, J., Lo Sicco, K. (2024). Combating “dread shed”: The impact of overlapping topical and oral minoxidil on temporary hair shedding during … Continue reading Months 3-6 Early visible improvements, like the appearance of new hairs, can be expected. [9]Amit, K., Mansukh, G., Satyaprakash, M., Dhiraj, D., Hanmant, B. (2023). Real-World Effectiveness, Safety, and Tolerability of Cetosomal Minoxidil 5% Alone and a Fixed Drug Combination of Cetosomal … Continue reading Months 6-12 Cosmetic changes peak, with stronger hair, increased density, and most coverage is seen. [10]Katz, H.I., Hien, N.T., Prawer, S.E., Goldman, S.J. (1987). Long-term efficacy of topical minoxidil in male pattern baldness. Journal of the American Academy of Dermatology. 16(3). 711-718. Available … Continue reading Months 12+ Further gains plateau, continued use needed to maintain improvements. [11]Katz, H.I., Hien, N.T., Prawer, S.E., Goldman, S.J. (1987). Long-term efficacy of topical minoxidil in male pattern baldness. Journal of the American Academy of Dermatology. 16(3). 711-718. Available … Continue reading Consistency is Everything
Minoxidil works by prolonging the anagen phase and shortening the telogen phase. Regular dosing maintains these effects at the follicle level; repeatedly missing applications reduces the cumulative time that hair follicles spend in a pro-growth, anagen-biased state.[12]Messenger, A.G., Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 2(1). 186-194. Available at: … Continue reading
Because human scalp follicles cycle slowly (anagen can last years), many follicles need months of uninterrupted exposure before visible density gains can be seen.[13]Hoover E, Alhajj M, Flores JL. Physiology, Hair. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: … Continue reading This is why most guidance recommends at least 6 months of continuous use before judging the response.
Why Stopping Reverses Progress
Minoxidil is not disease-modifying for AGA, meaning that it does not correct androgen-driven miniaturization but counteracts it while present by improving hair cycle dynamics and follicle size. When treatment is stopped, these pharmacologic effects wane, and follicles gradually revert toward their genetically determined, androgen-sensitive pattern.
Clinical and patient information sources consistently note that any gain from minoxidil is maintained only while therapy continues. After discontinuation, increased shedding and loss of density over months are typical, ultimately leading to a reversal of gains to the original starting point.
What Minoxidil Formulations are Available?
You can buy minoxidil in several different vehicles that differ mainly in tolerability, practicality, and how well they fit a given scalp and routine. Choosing the “best” format is less about raw efficacy and more about matching the base to skin sensitivity and your preference of application.
- Foam – Best for Sensitive Skin: Propylene glycol-free foams were developed to reduce contact dermatitis and dryness seen with classic liquid solutions. Reviews note lower rates of irritation and quicker drying, which makes them better for patients with reactive or dry scalps.[14]Purnak, T., Senel, E., Sahin, C. (2011). Liquid formulation of minoxidil versus its foam formulation. Indian Journal of Dermatology. 56(4). 462. Available at: https://doi.org/10.4103/0019-5154.84714
- Liquid – Best for Cost-Effectiveness: Traditional solutions with alcohol and propylene glycol are widely available, inexpensive, and easy to use, but they may cause increased itching, flaking, and stinging. This formulation may suit users with non-sensitive skin who are price-conscious.
- Spray – Easiest for Coverage: Spray tops or aerosolized formats can make it faster to hit broad thinning areas, especially in longer hair. However, the product may end up more on the hair shafts or adjacent skin.
- Gel – Targeted and Controlled: Gel or cream-gel vehicles (often from compounders) sit where you place them, minimize dripping, and are useful for localized areas such as temples or post-transplant zones.
- Liposomal Delivery: Newer liposomal or phospholipid bases aim to enhance follicular penetration while limiting systemic absorption and irritation. This is usually marketed as an “advanced delivery” method.
Side Effects and Safety
Minoxidil is generally well tolerated, but like any active drug, it can cause local irritation, a transient shedding phase, and very rare systemic effects, especially in people with underlying skin or cardiovascular issues.
Common local side effects include:
- Itching
- Flaking
- Redness
- Burning
- Temporary shedding
Systemic and heart-related side effects are very rare with correctly used topical minoxidil, but case reports and product information advise seeking medical help if symptoms like chest pain, palpitations, dizziness, or unexplained swelling occur, as these could reflect systemic absorption or accidental ingestion. Risk is higher with oral minoxidil or overdose, where hypotension, tachycardia, and even heart failure have been documented.[15]Tripathee, S., Benyovszky, A., Devbhandari, R., Quiza, K., Boris, J. (2024). A Very Bad Hair Day: Minoxidil Ingestion Causing Shock and Heart Failure. Cureus. 16(8). E66039. Available at: … Continue reading
To minimize problems, many clinicians suggest starting once daily and titrating up, choosing foam or gentler vehicles to reduce dermatitis, and using custom compounded formulas (for example, lower alcohol, lower strength, or liposomal bases) in those with sensitive or disease-prone scalps.
People with chronic inflammatory scalp conditions such as active psoriasis, eczema, or severe seborrheic dermatitis are typically advised to get the primary condition under control before adding topical minoxidil or other actives, as applying them to an inflamed barrier increases irritation, may worsen the disease, and can unpredictably alter absorption.[16]Junge, A., Jic-Hoesli, S.R., Bossart, S., Simon, D., de Viragh, P., Hunger, R.E., Heidemeye, K., Seyed Jafari, S.M. (2025). Contact Dermatitis Caused by Topical Minoxidil: Allergy or Just Irritation. … Continue reading
Final Verdict – Best Minoxidil for Men in 2026
In 2026, several minoxidil-based options stand out for different priorities, from cost to customization. All of them rely on the same core ingredient, but vehicle, strength, and add-ons make a real-world difference in adherence and results.
Whichever route you choose, there is strong evidence that consistent minoxidil use can stabilize loss and improve density for many men, and modern platforms make it easier than ever to find a formulation that fits your scalp, schedule, and risk tolerance.
References[+]
References ↑1 Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2021). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: https://doi.org/10.1080/09546634.2021.1945527 ↑2 Hussein, R.S., Dayel, S.B., Abahussein, O., El-Sherbiny, A.A. (2024). Applications and efficacy of minoxidil in dermatology. Skin Health and Disease. 4(6). E472. Available at: https://doi.org/10.1002/ski2.472 ↑3 Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. Advances in Dermatology and Allergology. 39(3). 472-478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑4 Gupta, A.K., Talukder, M., Shemer, A., Piraccini, B.M., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: https://doi.org/10.1159/0000531890 ↑5 Asad, N., Naseer, M., Ghafoor, R. (2024). Efficacy of Topical Finasteride 0.25% with Minoxidil 5% versus Topical Minoxidil 5% Alone in Treatment of Male Pattern Androgenic Alopecia. Journal of Drugs in Dermatology. 23(11). 1003-1008. Available at: https://doi.org/10.36849/JDD.7826 ↑6 Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. Journal of the American Academy of Dermatology. 47(3). 377-385. Available at: https://doi.org/10.1067/mjd.2002.124088 ↑7 Ghonemy, S., Alarawi, A., Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. Journal of Dermatological Treatment. 32(2). 236-241. Available at: https://doi.org/10.1080/09546634.2019.1654070 ↑8 Nohria, A., Desai, D., Sikora, M., Mandal, S., Shapiro, J., Lo Sicco, K. (2024). Combating “dread shed”: The impact of overlapping topical and oral minoxidil on temporary hair shedding during oral minoxidil initiation. JAAD International. 15. 220-224. Available at: https://doi.org/10.1016/j.jdin.2024.03.005 ↑9 Amit, K., Mansukh, G., Satyaprakash, M., Dhiraj, D., Hanmant, B. (2023). Real-World Effectiveness, Safety, and Tolerability of Cetosomal Minoxidil 5% Alone and a Fixed Drug Combination of Cetosomal Minoxidil 5% With Finasteride 0.1% in the Management of Androgenetic Alopecia (Inbilt Study). Cureus. 15(7). E41681. Available at: https://doi.org/10.7759/cureus.41681 ↑10, ↑11 Katz, H.I., Hien, N.T., Prawer, S.E., Goldman, S.J. (1987). Long-term efficacy of topical minoxidil in male pattern baldness. Journal of the American Academy of Dermatology. 16(3). 711-718. Available at: https://doi.org/10.1016/s0190-9622(87)70092-9 ↑12 Messenger, A.G., Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 2(1). 186-194. Available at: https://doi.org/https://doi.org/10.1111/j.1365-2133.2004.05785.x ↑13 Hoover E, Alhajj M, Flores JL. Physiology, Hair. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499948/ (Accessed: November 2025) ↑14 Purnak, T., Senel, E., Sahin, C. (2011). Liquid formulation of minoxidil versus its foam formulation. Indian Journal of Dermatology. 56(4). 462. Available at: https://doi.org/10.4103/0019-5154.84714 ↑15 Tripathee, S., Benyovszky, A., Devbhandari, R., Quiza, K., Boris, J. (2024). A Very Bad Hair Day: Minoxidil Ingestion Causing Shock and Heart Failure. Cureus. 16(8). E66039. Available at: https://doi.org/10.7759/cureus.66039 ↑16 Junge, A., Jic-Hoesli, S.R., Bossart, S., Simon, D., de Viragh, P., Hunger, R.E., Heidemeye, K., Seyed Jafari, S.M. (2025). Contact Dermatitis Caused by Topical Minoxidil: Allergy or Just Irritation. Acta Dermato-Venereologica. 105. 42401. Available at: https://doi.org/10.2340/actadv.v105.42401 Here’s a story we see all the time. You’ve been using finasteride, saw some initial improvement or stabilization, but now your hair regrowth is plateauing, you’re still noticing miniaturization, and wondering whether it’s time to switch to dutasteride.
On the surface, the logic is simple. Dutasteride suppresses DHT more than finasteride, and in head-to-head studies, it usually improves hair counts and thickness more. But switching doesn’t just change potential results. It changes how long the drug continues to suppress DHT after discontinuation, how quickly you can evaluate whether it’s helping (or causing side effects), and how easily you can adjust or exit the treatment if needed.
Many people think of this switch as changing one variable. In reality, switching from finasteride to dutasteride can involve changing multiple variables at once: oral versus topical delivery, dose equivalence, absorption variability, drug persistence (half-life), transition strategy, and expectations for timelines. Each of these can alter both outcomes and tolerability.
This article breaks down the real-world trade-offs of switching from finasteride to dutasteride, compares the most common transition scenarios (oral-to-oral, topical-to-topical, and cross-route switches), explains what to expect, and offers our perspective on which decisions are evidence-based and which require a little more skepticism.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Biology of Finasteride and Dutasteride
DHT is the key androgen driving follicular miniaturization in androgenic alopecia (AGA). An enzyme called 5α‑reductase (5AR) in the hair follicle converts testosterone to DHT. Because DHT is known to drive hair loss progression, many treatments for AGA target 5AR to reduce scalp DHT levels.[1]Asfour, L., Cranwell, W., & Sinclair, R. (2023). Male androgenetic alopecia. *Endotext [Internet].* Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/ Dutasteride blocks both Type I and Type II 5AR, leading to more DHT suppression than finasteride, which only targets Type II.
Oral finasteride is most commonly prescribed at 1 mg daily for AGA, while oral dutasteride is typically taken at 0.5 mg daily. Topical formulations were developed later to change the risk profile without affecting the mechanism. Instead of lowering DHT systemically by default, topical finasteride was designed to concentrate drug activity in the scalp while limiting how much reaches the bloodstream. Topical dutasteride represents the newest and least mature category, but evidence is promising. Because dutasteride is more potent than finasteride, even very low topical concentrations (around 0.01-0.05%) can meaningfully suppress scalp DHT.
Figure 1. The structures of finasteride and dutasteride.[2]Wikimedia Commons. (n.d.). Azasteroid Pharmaceuticals [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Azasteroid_Pharmaceuticals.png (Accessed: November 2025) Image in the Public Domain.
Switching Scenarios
There are many treatment options for both dutasteride and finasteride, each with different trade-offs. We’ll compare the available clinical evidence across formulations and break down the most common switching scenarios, highlighting four core considerations: expected efficacy, side-effect profile, commitment and washout dynamics, and strength of evidence.
Key Takeaways:
- Oral finasteride to oral dutasteride: the most evidence-backed upgrade.
- Oral finasteride to topical dutasteride: one study suggests improvement, but we’re skeptical about the data.
- Topical finasteride to topical dutasteride: largely guesswork, with no direct comparative trials.
- Topical finasteride to oral dutasteride: no comparative studies, but surrogate evidence suggests a gain in efficacy.
- Oral dutasteride to topical finasteride: no direct studies, but surrogate data suggests a likely loss in efficacy.
These answers can all change when you account not just for drug switches or formulation switches, but also the dose.
Oral Finasteride to Oral Dutasteride
This is the cleanest and most evidence-supported switch. Four major comparative randomized controlled trials (RCTs) provide direct comparisons between oral finasteride and oral dutasteride at commonly used doses.
Efficacy
Here is a summary of the clinical trials that compare oral finasteride and dutasteride treatment directly:
Study Study Design and Population Treatments Study #1[3]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Randomized, placebo-controlled, double-blinded trial. 415 men with male pattern hair loss aged 21-45 years old; 24 weeks. 5 mg oral finasteride; 0.05, 0.1, 0.5, or 2.5 mg oral dutasteride; placebo. Study #2[4]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Randomized, placebo-controlled, double-blinded trial. 917 men with AGA aged 20-50; 24 weeks. 1 mg oral finasteride; 0.02, 0.1, or 0.5 mg oral dutasteride; placebo Study #3[5]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Open-label, randomized study; no placebo control. 90 men with AGA aged 18-40; 24 weeks. 1 mg oral finasteride; 0.5 mg oral dutasteride. Study #4[6]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading Retrospective chart review. 600 men over 18 with AGA. 1 mg oral finasteride; 0.5 mg oral dutasteride. Across these studies, dutasteride consistently outperformed finasteride on hair parameters:
- Target-area hair counts: In Study #1 the proportion of participants with at least a 10% increase in hair counts was 41% for finasteride (5 mg), 48% for dutasteride (0.5 mg) and 56% for dutasteride (2.5 mg).[7]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading In Study #3, dutasteride (0.5 mg) significantly increased total hair count and decreased thin hair count per cm2 compared to finasteride (1 mg).[8]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Notably, Study #2 showed that dutasteride (0.5 mg) significantly increased terminal hair count compared to finasteride (1 mg).[9]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
- Hair shaft diameter: Study #2 demonstrated that dutasteride (0.5 mg) was statistically superior to finasteride (1 mg) in increasing hair width.[10]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
- Global photographic assessment: Across studies, investigators were more likely to rate dutasteride (0.5 mg) users as “improved” or “markedly improved” compared to those receiving finasteride (1 mg).[11]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading
- DHT suppression: In Study #1, serum DHT levels were significantly lower with dutasteride (0.5 mg and 2.5 mg) than with finasteride (5 mg). Additionally, finasteride (5mg) decreased scalp DHT by 41%, while dutasteride (0.5 mg) decreased it by 51%, and dutasteride (2.5 mg) decreased it by 79%.[12]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading
Important caveat: Most RCTs lasted only 24 weeks. Finasteride continues to produce gains for up to 48 months in longer studies, so some of dutasteride’s apparent advantage may reflect faster onset rather than an infinite ceiling of benefit. However, longer-term observational data suggest that dutasteride maintains superiority on global classification scales.
Side-Effects
The most frequently discussed side effects of finasteride and dutasteride include:
- Sexual side effects – These include decreased libido, erectile dysfunction, and ejaculation disorders. Across randomized trials, these effects occur in a minority of users (generally in the single-digit percentages), appear most often early in treatment, and frequently resolve spontaneously, even while continuing therapy.[13]Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic … Continue reading
- Breast-related effects – Breast tenderness or enlargement has been reported, but rates are low across both drugs and typically comparable between finasteride and dutasteride.
- Other reported effects: Fatigue, mood changes, or nonspecific symptoms are occasionally reported, but these occur inconsistently, lack clear dose–response relationships, and are difficult to separate from background rates and expectation effects.
Across clinical trials, overall side-effect rates were similar for finasteride and dutasteride, averaging approximately 8-9% for both drugs. Notably, a 2019 systematic review and meta-analysis found no significant difference in adverse event rates between the two treatments, including for sexual side effects such as altered libido, erectile dysfunction, and ejaculation disorders.[14]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The Efficacy And Safety Of Dutasteride Compared With Finasteride In Treating Men With Androgenetic Alopecia: A Systematic Review And … Continue reading
The key difference may not be how often side effects occur, but how long drug-induced hormonal changes persist after stopping treatment. Because dutasteride remains biologically active far longer than finasteride, any changes may take longer to unwind after stopping.
Commitment & Washout Dynamics
In this article, when we discuss commitment or washout dynamics, we are referring to how long a drug continues to suppress DHT after it is stopped, not how long hair regrowth or side effects persist. This reflects how quickly the body can clear the medication and return toward baseline hormone activity once therapy is discontinued.
Dutasteride involves a higher level of commitment than finasteride because it remains biologically active for far longer. Oral dutasteride (0.5 mg daily) has a half-life of around 4-5 weeks, meaning drug activity can persist for months after discontinuation, whereas oral finasteride has a half-life of roughly 6-8 hours.
This means that dose changes or discontinuation of dutasteride lead to much slower changes in systemic DHT suppression compared with finasteride. Dutasteride is not a “try it and see how you feel in two weeks” medication. Its pharmacologic effects unwind gradually, requiring a substantially higher level of decision commitment.
Evidence Strength: Strong
Overall, the oral finasteride to oral dutasteride switch is the most evidence-supported pathway in terms of both efficacy and safety. This consistent superiority in hair outcomes is why oral dutasteride is often considered when finasteride results plateau.
If you’re a member and would like to learn more about oral dutasteride, read our ultimate guide here.
Oral Finasteride to Topical Dutasteride
At the end of 2025, the first RCT involving 135 men with AGA comparing oral finasteride (1 mg) to topical dutasteride (0.01-0.05%) over 24 weeks was published.[15]Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled … Continue reading
The study suggested that topical dutasteride (0.05%) outperformed oral finasteride, which would represent a major shift in how we think about treatment escalation.
But, after we took a closer look at the paper, we think the findings should be interpreted with caution due to multiple concerns with their methodology.
Efficacy
In the study, several hair parameters appeared to improve more in the topical dutasteride (0.05%) group than in those receiving oral finasteride (1 mg). The authors reported an average increase of +75 hairs per cm² with topical dutasteride compared to +41 hairs per cm² with oral finasteride. Additionally, 69% of participants using topical dutasteride were rated as “improved” on global photographic assessment, compared with 21% in the oral finasteride group.
However, we think these results are likely inflated. Here are two of the major problems with this study:
#1 Baseline hair counts defy biology
The study reports baseline densities of 300+ hairs per cm² in balding men. For context, healthy, non-balding scalps have around 100-250 hairs per cm², whereas men with Norwood III-V AGA typically have around 25-100 hairs per cm² in the vertex.
This suggests they likely counted vellus hairs, mis-measured the sampling area, or used unreliable manual counting methods. This could significantly inflate improvements.
#2 The measurement circle moves
In the published images, the target circles change location, size, and shape, they appear hand-drawn, and they clearly do not track the same exact scalp area over time.
A 1-2 mm shift can change hair counts by 50%+. The study’s reported gains (10-30%) fall well within the error range of sloppy circle placement, meaning the entire efficacy signal could be measurement noise.
Figure 2. Representative hair growth images. Adapted from Figure 2.[16]Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled … Continue reading Image used under Creative Commons License.
Side-Effects
Despite large hair-growth claims, systemic hormone changes were minimal. Serum DHT change for oral finasteride was between -11% to -27%, and -9% to -11% for topical dutasteride (0.05%). Plasma dutasteride levels were mostly near the quantification limit, but some samples spiked to 2,555 pg/mL, and dutasteride remained detectable at day 168 while participants were still receiving treatment. This suggests that average exposure was low, but absorption may be unpredictable in some users.
Commitment & Washout Dynamics
While participants were actively using topical dutasteride, most had very low drug levels in the blood, but a small number showed noticeable absorption. This means that applying dutasteride to the scalp does not completely prevent it from entering the bloodstream, and individual responses can vary.
Because the study did not measure drug levels after treatment was stopped, it does not tell us how long topical dutasteride continues to have effects once discontinued. So although average exposure appears lower than with oral finasteride, the length of time its effects persist after stopping remains uncertain.
Evidence Strength: Moderate (but controversial)
This is the first RCT that compared oral finasteride directly with topical dutasteride alone, but it had major pitfalls, including implausible baseline hair counts, unreliable target area placement, and results that contradict years of real-world outcomes. These flaws reduce our confidence in this study’s efficacy claims
If you’d like to read more about our interpretation of the 2025 study comparing oral finasteride to topical dutasteride, read our article here.
Topical Finasteride to Topical Dutasteride
Currently, there are no head-to-head studies comparing topical finasteride with topical dutasteride. That means outcomes are highly dependent on formulation choices, and most conclusions in this switch category are informed speculation rather than solid evidence.
Efficacy
Because there are no comparative trials, we cannot say with confidence that topical dutasteride is more effective than topical finasteride. Any perceived improvement would depend on:
- Drug concentration
- Vehicle (alcohol, liposomal, foam, etc.)
- Volume applied per use
- Application frequency
- Individual scalp absorption
In other words, this switch should be viewed as an experiment, not an upgrade supported by clinical data.
Side-Effects
Topical application does not guarantee that finasteride or dutasteride will remain confined to the scalp. Both agents can suppress serum DHT depending on formulation, dose, vehicle, and application frequency. As a result, systemic effects are still possible, particularly when higher concentrations or larger application volumes are used.[17]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
Figure 3. Multiple daily doses of topical finasteride can reduce serum DHT more than a single dose. Adapted from Figure 3.[18]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
Commitment & Washout Dynamics
Topical finasteride generally leads to lower overall drug exposure than oral finasteride, with smaller reductions in serum DHT, suggesting a shorter washout window and greater flexibility with dose adjustments.[19]Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic … Continue reading
Topical dutasteride still uses a drug with a long half-life and strong binding to 5AR. However, in the trial comparing oral finasteride (1 mg) to topical dutasteride (0.05%), systemic exposure and serum DHT suppression were modest, suggesting that at these doses most of its effect is likely occurring in the scalp rather than through prolonged whole-body exposure.[20]Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled … Continue reading
This implies that topical dutasteride (0.05%) is less committing than oral dutasteride (0.5 mg daily), but may still be more committing than low-dose topical finasteride, especially at higher concentrations or with frequent application, because of dutasteride’s pharmacology. That said, this remains an inference rather than a proven conclusion.
Evidence Strength: Weak
Due to the lack of clinical studies comparing topical finasteride with topical dutasteride, the evidence base for making the switch between these two topical therapies is limited. Any decision to make the switch should be treated as a dose-dependent experiment rather than an evidence-backed upgrade.
Topical Finasteride to Oral Dutasteride
There are no direct comparative studies for this switch. Still, using surrogate data from Study #1-4 comparing oral finasteride to oral dutasteride, we can reasonably expect a gain in efficacy for many users. This transition moves from a variable, dose-dependent topical regimen to a potent and consistently studied systemic DHT-suppression strategy.
While this switch is likely to improve hair outcomes, it also represents a meaningful change in drug exposure. It involves moving from a formulation that often limits systemic absorption to one that produces sustained, whole-body DHT suppression with a drug that remains biologically active for weeks. As a result, dose adjustments and discontinuation lead to slower changes in hormone suppression, and any unwanted effects may take longer to resolve after stopping.
Oral Dutasteride to Topical Finasteride
There are also no head-to-head studies for changing from oral dutasteride to topical finasteride. But we can infer that switching from oral dutasteride to topical finasteride will have the opposite effect to what was seen in Study #1-4 comparing oral finasteride to oral dutasteride.
Switching from oral dutasteride to topical finasteride will likely result in a loss of efficacy for many people, as there will be both a reduction in pharmacologic potency and a move from systemic exposure to a less predictable topical delivery system.
From an exposure standpoint, this switch may generally reduce overall systemic drug levels and shorten the washout window. Topical finasteride typically lowers serum DHT less than oral dutasteride and clears the body more quickly, meaning that changes in dosing or discontinuation tend to result in faster shifts in biological activity.
How To Make the Decision to Switch
Step 1: Are you stable and satisfied using finasteride?
Yes: Don’t fix what isn’t broken. If your hair is stable or slowly improving, the safest move is often staying the course and reassessing with standardized photos every 3-6 months.
No: Go to Step 2.
Step 2: Is the priority more efficacy, or minimizing downside risk?
Efficacy priority: The most evidence-backed switch is oral finasteride to oral dutasteride. This switch is best suited for those with aggressive or rapidly progressing AGA, extensive miniaturization, or those who have clearly plateaued after a consistent finasteride trial.
Risk priority: Consider optimizing finasteride (dose/frequency) or adding adjuncts (minoxidil/microneedling). Finasteride remains the more forgiving option due to its shorter half-life and easier dose adjustment.
Step 3: Do you tolerate oral 5AR inhibitors?
Yes: Oral dutasteride is the cleaner evidence path for improved efficacy.
No: Topical options can work, but treat them as dose-dependent experiments rather than guaranteed upgrades.
If you’d like more information on oral versus topical dutasteride, take a look at our article here.
Step 4: Set the evaluation window before you switch.
Don’t judge a switch in 6-8 weeks. Commit to 6-12 months and take standardized photographs to track your progress.
Avoid switching (or be extra cautious) if:
- You’re trying to conceive soon.
- You’ve previously experienced severe side effects when using 5AR inhibitors.
- You prefer treatments that leave your system quickly if something goes wrong.
Our Final Thoughts
Both finasteride and dutasteride offer real benefits, but each comes with distinct trade-offs. If you’re considering switching, it’s critical to focus on what the evidence actually supports. Currently, the only switch backed by high-quality comparative data is oral finasteride to oral dutasteride, making it the most evidence-based upgrade.
That said, this path comes with a higher level of commitment. Because dutasteride persists in the body for weeks, drug-induced hormonal changes unwind more slowly, which may delay how quickly unwanted effects fade after stopping. Other switching scenarios currently don’t have robust clinical evidence, so they should be approached with caution and treated as informed experiments rather than proven upgrades.
If you’d like a deeper breakdown of finasteride versus dutasteride, read our ultimate guide here.
References[+]
References ↑1 Asfour, L., Cranwell, W., & Sinclair, R. (2023). Male androgenetic alopecia. *Endotext [Internet].* Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/ ↑2 Wikimedia Commons. (n.d.). Azasteroid Pharmaceuticals [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Azasteroid_Pharmaceuticals.png (Accessed: November 2025) ↑3 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑4, ↑9, ↑10 Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 70(3). 489–498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑5 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑6, ↑11 Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. *Annals of Dermatology.* 34(5). 349. Available at: https://doi.org/10.5021/ad.22.027 ↑7, ↑12 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑8 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑13 Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. *The Journal of Clinical and Aesthetic Dermatology.* 9(7). 56–62. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5023004/ ↑14 Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The Efficacy And Safety Of Dutasteride Compared With Finasteride In Treating Men With Androgenetic Alopecia: A Systematic Review And Meta-Analysis. Clinical Interventions in Aging. 14. 399-406. Available at: https://doi.org/10.2147/CIA.S192435 ↑15 Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects With Androgenetic Alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑16, ↑20 Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects With Androgenetic Alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑17 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑18 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑19 Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. *Journal of the European Academy of Dermatology and Venereology.* 36(2). 286–294. Available at: https://doi.org/10.1111/jdv.17738 Female hair loss remains a widely misunderstood and often under-treated condition, affecting nearly one-third of all women at some point in their lives and up to two-thirds after menopause. Unlike male pattern hair loss, research and treatment options for women have historically lagged, resulting in limited specialized solutions and significant emotional distress for those afflicted.
Minoxidil stands as the only FDA-approved medication for female pattern hair loss. Its efficacy is supported by robust data, particularly at the 2 and 5% concentrations for women. In this guide, we will showcase the six best minoxidil products for women, including the best overall, best value, and top specialized choices.
Quick Look: Top 6 Minoxidil Brands for Women 2026
Product Strength Format Customization Price Best Ulo Women’s Rx Minoxidil 7% Solution High $41.65 Overall Musely 8% Solution High $99 Strength Rogaine Women’s Foam 5% Foam None $49.97 Sensitivity Hers 2%-5% Solution/Foam None $30 Value Happy Head (Women’s Formula) 6% Solution High $79 Alternative Winona 7% Solution None $150 Woman-centered care Before we get into our list, let’s first take a look at what female pattern hair loss is and how minoxidil works for women.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Female Pattern Hair Loss?
Female pattern hair loss (FPHL) is a chronic, non-scarring alopecia where genetically susceptible follicles on the central scalp progressively minaturize, leading to reduced hair density over time. Clinically, women usually show diffuse thinning over the crown and midline part with relative preservation of the frontal hairline rather than “bald patches”.[1]Bhat, Y.J., Saqib, N-U., Latif, I., Hassan, I. (2020). Female Pattern Hair Loss – An Update. Indian Dermatology Online Journal. 11(4). 493-501. Available at: … Continue reading
The Ludwig scale grades this pattern from I-III: mild central thinning (I), moderate widening of the part and density loss (II), and advanced, see-through vertex thinning (III). Trichoscopy and histology show increased hair shaft diameter variability and replacement of terminal hairs by finer, vellus-like hairs.[2]Kothari, C.R., Shivakumar, P. (2024). Trichoscopic Features in Female Pattern Hair Loss: 1-Year Hospital-Based Cross-Sectional Study. Clinical Dermatology Review. 8(2). 95-101. Available at: … Continue reading
FPHL arises from a mix of genetics, hormones, low-grade inflammation, oxidative stress, and microvascular and aging-related changes around the follicle. Unlike classic male pattern hair loss, many women have normal serum androgens, suggesting that local androgen sensitivity and non-androgen mechanisms both contribute.[3]Ramos, P.M., Miot, H.A. (2015). Female Pattern Hair Loss: a clinical and pathophysiological review. Anais Brasileiros de Dermatologia. 90(4). 529-543. Available at: … Continue reading
How Minoxidil Works
Minoxidil functions as a potassium channel opener that promotes vasodilation, increasing blood flow and improving microcirculation around hair follicles.[4]Hussein, R.S., Dayel, S.B., Abahussein, O., El-Sherbiny, A.A. (2024). Applications and efficacy of minoxidil in dermatology. Skin Health and Disease. 4(6). E472. Available at: … Continue reading
It is also a prodrug that must be converted by the sulfotransferase enzyme into its active form, minoxidil sulfate. This conversion helps extend the anagen (growth) phase while shortening the telogen (resting) phase, thereby shifting a greater number of follicles into active growth cycles.[5]Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a … Continue reading
Additional evidence indicates that minoxidil stimulates the expression of growth-promoting factors, such as vascular endothelial growth factor (VEGF), and activates the Wnt/β-catenin signaling pathway, both of which further support hair regrowth.[6]Gupta, A.K., Talukder, M., Shemer, A., Piraccini, B.M., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: … Continue reading
While minoxidil does not directly address dihydrotestosterone (DHT), it can enhance the effectiveness of other therapies by targeting different biological pathways involved in hair loss. When used alongside anti-DHT treatments such as finasteride, which reduces the underlying hormonal driver of androgenic alopecia, minoxidil offers a complementary, non-hormonal mechanism of action. Clinical research demonstrates that the combined use of topical finasteride and minoxidil produces greater improvements in hair density in men compared to minoxidil alone.[7]Asad, N., Naseer, M., Ghafoor, R. (2024). Efficacy of Topical Finasteride 0.25% with Minoxidil 5% versus Topical Minoxidil 5% Alone in Treatment of Male Pattern Androgenic Alopecia. Journal of Drugs … Continue reading
Why Women Respond Differently
In women, pattern hair loss is generally less strictly DHT-driven than in men, so microcirculation, oxidative stress, and local inflammatory pathways play a proportionately greater role.[8]Fabbrocini, G., Cantelli, M., Masara, A., Annunziata, M.C., Marasca, C., Cacciapuoti, S. (2018). Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. International Journal … Continue reading This helps explain why a vasodilatory, pro-anagen agent like minoxidil is often effective even when anti-androgens alone are insufficient.
Hormonal transitions like perimenopause/menopause, thyroid dysfunction, and polycystic ovary syndrome (PCOS) commonly unmask or accelerate FPHL by altering estrogen-androgen balance and cycling dynamics.[9]Aksenenko, M., Palkina, N., Komina, A., Ruksha, T. (2019). MiR-92a-1-5p and miR-328-3p Are Up-Regulated in Skin of Female Pattern Hair Loss Patients. Annals of Dermatology. 31(2). 256-259. Available … Continue reading
OTC vs Prescription Minoxidil for Women
Over-the-counter (OTC) minoxidil for women typically comes at 2-5% concentrations, while prescription or compounded products may use 5-8% or higher strengths in customized products.
2% minoxidil is the classic, label-approved strength for women, with clear evidence of a benefit versus placebo.[10]van Zuuren, E.J., Fedorowicz, Z., Schoones, J. (2016). Interventions for female pattern hair loss. Cochrane Database of Systematic Reviews. 2016(5). CD007628. Available at: … Continue reading
Compounded “high-strength” minoxidil (5-8%+) is prescription only and relies in part on carrier agent formulation.[11]Sattur, S.S., Sattur, I.S. (2021). Pharmacological Management of Pattern Hair Loss. Indian Journal of Plastic Surgery. 54(4). 422-434. Available at: https://doi.org/10.1055/s-0041-1739254 Simply raising the percentage does not guarantee better results and may raise the risk of irritation, hypertrichosis, or systemic absorption of the drug.[12]Ghonemy, S., Alarawi, A., Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic … Continue reading
So, with that in mind, let’s take a look at our top six minoxidil brands of 2026.
Best Minoxidil Brands for Women
#1 Best Overall: Ulo Women’s Rx Minoxidil (7%)
Pros: Cons: ✓ Prescription-only 7% minoxidil, higher than standard OTC strengths ✗Prescription products are only available in the USA ✓ Optional evidence-based add-ons ✓ Quality-tested ingredients free of irritants ✓ Includes medical consultation and ongoing monitoring Ulo Women’s Rx Minoxidil+ is our go-to product for topical minoxidil treatment. It is designed for those who want more than “pink-labeled” 2-5% solutions and are comfortable with a prescription approach guided by specialists. The 7% concentrated and layered actives (including cetirizine 1%, tretinoin 0.01%, melatonin 0.01%, and caffeine 0.2%) target multiple facets of hair loss with a level of customization that goes beyond typical off-the-shelf products.
Bottom Line: Ulo offers the most precise, clinically guided minoxidil-based treatment for women in 2026, combining higher-strength therapy with thoughtful formulation and ongoing medical oversight.
#2 Best for Strength: Musely The Hair Topical Solution-Classic
Pros: Cons: ✓ High-strength 8% prescription minoxidil. ✗ Use of corticosteroids increases the risk of skin thinning. ✓ Optional evidence-based add-ons like dutasteride and spironolactone for hormonal control. ✗ Propylene glycol and ethyl alcohol base, which can sting or dry out sensitive scalps. ✓ Additional scalp-supporting actives like tretinoin, ketoconazole, caffeine, and melatonin. ✗ More expensive than simpler OTC or lower-strength prescription options. ✓ Includes periodic medical follow-up. Musely’s 8% “Classic” Hair Topical is designed for women with advanced thinning or those who have plateaued on conventional strengths, combining high-dose minoxidil with potent anti-androgens and supportive ingredients in a single solution, including optional add-ons such as dutasteride 0.3%, spironolactone 0.075%, tretinoin 0.01%, ketoconazole 2%, hydrocortisone 1%, plus adjuncts like caffeine and melatonin.
It functions more as an intensive, prescription-only protocol than a starter product, and is best reserved for users willing to tolerate a stronger propylene glycol/ethyl alcohol vehicle, and possible skin thinning if using long-term due to the addition of corticosteroids.
Bottom line: For women with stubborn, progressive hair loss who have outgrown basic 5% formulas, Musely’s high-strength, multi-active topical offers one of the most powerful at-home options, provided they are comfortable with higher cost and higher irritation risk.
#3 Best for Sensitivity: Rogaine Women’s Foam
Pros: Cons: ✓ Propylene-glycol-free foam vehicle, often better tolerated on sensitive or irritated scalps. ✗No medical consultation or follow-up after buying ✓ Widely available OTC ✗ No customization. Rogaine Women’s Foam is a classic choice for those looking for a gentle-on-the-scalp option of minoxidil. The propylene-glycol-free foam base, robust clinical evidence, and once-daily 5% option make it a gentle yet effective starting point for treating female pattern thinning. It should be noted that if you wanted to try the lower dose option (2%), you need to buy the solution, which contains propylene glycol, and so may not be as beneficial for those with sensitive skin.
Bottom Line: Get the foam if you have a sensitive scalp or are a beginner, wanting a low-irritant minoxidil option.
#4 Best Value: Hers Minoxidil Foam and Solution
Pros: Cons: ✓ Budget-friendly option at around $30 for a 2-month supply. ✗No advanced customization available. ✓ Offers both 2% and 5% strengths at the same price. ✓ Available in solution and foam formats. Hers Minoxidil is designed for women who want a clinically supported minoxidil treatment without paying premium prices for branding or heavy telehealth layering. With standard 2 and 5% options in familiar vehicles, it delivers an approachable, budget-conscious package for those comfortable managing a simple daily routine themselves.
Bottom Line: For cost-conscious women who want an easy, no-frills entry into proven minoxidil therapy, Hers Minoxidil offers standard concentrations with good value.
#5 Best Alternative: Happy Head Minoxidil and Spironolactone for Women
Pros: Cons: ✓ Prescription-strength 6% minoxidil, higher than standard OTC options but below ultra-high-dose protocols. ✗ Uses a propylene glycol-containing vehicle, which can increase irritation, dryness, or stinging on sensitive scalps. ✓ Customizable blends that include spironolactone, tretinoin, and hydrocortisone ✗ Inclusion of topical corticosteroids carries a risk of skin thinning and barrier damage if used long-term. ✓ Strong telehealth model with online prescribing and adjustments to treatments over time. ✗ More expensive than generic 5% minoxidil and some standard telehealth offerings. ✓ Good fit for women who need more than basic 2-5% formulas but do not want to jump straight to 8%+ multi-drug cocktails. Happy Head’s 6% formulas are aimed at women who want a personalized, prescription-only topical that goes beyond standard strengths while still staying below the most aggressive high-dose regimens. By combining minoxidil with spironolactone, retinoic acid, and short-term hydrocortisone in a single bottle, it offers a modular approach that can be tuned to individual tolerance and response rather than a one-size-fits-all solution. It should be noted that Happy Head uses corticosteroids in their topicals to offset the potential irritation from propylene glycol usage. However, this can cause skin thinning with long-term use.
Bottom line: For women who need a tailored, mid-high-strength minoxidil blend with added anti-androgens and supportive ingredients, but prefer not to escalate to ultra-high-dose, steroid-heavy cocktails, Happy Head provides a strong option.
#3 Best for Woman-Centered Care: Winona 7% Minoxidil
Pros: Cons: ✓ Women-focused clinic model that addresses broader menopausal health alongside hair loss. ✗ Uses a propylene glycol-containing vehicle, which can increase irritation, dryness, or stinging on sensitive scalps. ✓ Prescription-strength 7% minoxidil specifically targeted to menopausal and perimenopausal thinning. ✗ No customization ✓ Guided supportive care pathway, with structured programs, check-ins, and follow-up. ✗ Premium pricing at around $150 for a 3-month supply. ✓ Treatment plans that can integrate other menopause therapies where appropriate Winona’s 7% minoxidil is designed for women who want more than a stand-alone bottle and value a structured, woman-centered approach that fits into a broader menopause-care framework. The higher-strength prescription formula, combined with clear guidance, follow-up, and attention to hormonal context, makes it particularly suited to postmenopausal and perimenopausal hair loss rather than general early thinning. However, it should be noted that its carrier agent, propylene glycol, may be irritating for sensitive scalps.
Bottom line: For women seeking a guided, menopause-focused program built around prescription-strength minoxidil, Winona offers a structured, woman-centered care pathway, albeit at a higher price and with a more irritant-prone vehicle.
Regrowth Timeline
Setting realistic expectations is essential when beginning topical minoxidil for female pattern hair loss. Unlike quick cosmetic fixes, minoxidil works by gradually altering the hair growth cycle, and visible changes take time to develop.
Timeframe What to Expect Clinical Evidence Months 0-3 An initial increase in shedding may occur as follicles shift out of the resting (telogen) phase and re-enter growth. This temporary “dread shed” phase typically improves within 4–8 weeks and is considered a normal response to treatment initiation. [13]Nohria, A., Desai, D., Sikora, M., Mandal, S., Shapiro, J., Lo Sicco, K. (2024). Combating “dread shed”: The impact of overlapping topical and oral minoxidil on temporary hair shedding during … Continue reading Months 3-6 Early visible signs of improvement may appear, including reduced shedding and the emergence of fine, new hairs (vellus to terminal transformation), particularly along the part line and crown. [14]Amit, K., Mansukh, G., Satyaprakash, M., Dhiraj, D., Hanmant, B. (2023). Real-World Effectiveness, Safety, and Tolerability of Cetosomal Minoxidil 5% Alone and a Fixed Drug Combination of Cetosomal … Continue reading Months 6-12 More noticeable cosmetic improvements are typically seen, including increased overall density, thickening of existing strands, and improved scalp coverage in areas of diffuse thinning. For many women, this is the point at which changes become easily visible in the mirror and in photos. [15]Katz, H.I., Hien, N.T., Prawer, S.E., Goldman, S.J. (1987). Long-term efficacy of topical minoxidil in male pattern baldness. Journal of the American Academy of Dermatology. 16(3). 711-718. Available … Continue reading Months 12+ Hair density gains typically plateau. At this point, continued use is required to maintain results and prevent gradual regression back toward baseline. Stopping treatment may allow thinning to resume over time. [16]Katz, H.I., Hien, N.T., Prawer, S.E., Goldman, S.J. (1987). Long-term efficacy of topical minoxidil in male pattern baldness. Journal of the American Academy of Dermatology. 16(3). 711-718. Available … Continue reading Why Do I Need to Stay Consistent When Using Minoxidil?
Topical minoxidil works by extending the anagen (growth) phase of the hair cycle and shortening the telogen (resting) phase, thereby increasing the amount of time that follicles spend actively producing hair. Maintaining this effect requires regular, consistent application. When doses are frequently missed, follicles spend less cumulative time in a growth-biased state, which reduces the likelihood of noticeable improvement.[17]Messenger, A.G., Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 2(1). 186-194. Available at: … Continue reading
This principle is especially important in women, whose hair loss is often more diffuse and gradual, making subtle changes harder to notice in the early months. Because the human scalp hair cycle progresses slowly, with the anagen phase potentially lasting several years, many follicles need prolonged, uninterrupted exposure to minoxidil before visible gains in density and coverage can be achieved.[18]Hoover E, Alhajj M, Flores JL. Physiology, Hair. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: … Continue reading
For this reason, most clinical guidance advises committing to at least six months of daily, consistent use before evaluating the effectiveness of treatment, with continued use required to sustain and build upon results.
What Minoxidil Formulations Are Available?
You can find topical minoxidil in several different delivery vehicles, each with its own advantages in terms of scalp tolerability, ease of use, and fit for a woman’s lifestyle and hair type. In most cases, the “best” option is not defined by pure strength alone, but by how well the formula matches skin sensitivity, styling habits, and consistency of application.
Foam – Best for Sensitive Skin
Propylene glycol–free foam formulations were developed to reduce the risk of contact dermatitis, itching, and dryness that can occur with traditional liquid solutions. Clinical observations suggest that foam is generally better tolerated and faster-drying, making it a strong choice for women with sensitive scalps or those who wash and style their hair frequently.[19]Purnak, T., Senel, E., Sahin, C. (2011). Liquid formulation of minoxidil versus its foam formulation. Indian Journal of Dermatology. 56(4). 462. Available at: https://doi.org/10.4103/0019-5154.84714
Liquid – Best for Cost and Precision
Classic liquid solutions, usually formulated with alcohol and propylene glycol, remain widely available and affordable. While effective, they may cause itching, dryness, or flaking in some women. These solutions can work well for individuals without scalp sensitivity who prefer a more targeted, dropper-based application, especially along the part line.
Spray – Best for Quick, Even Coverage
Spray or mist-style applicators make it easier to cover larger areas of diffuse thinning, such as along the crown or upper scalp. However, they can be less precise, with some product landing on the hair shafts or surrounding skin rather than directly on the scalp.
Gel – Best for Targeted Areas
Gel or cream-gel formulations tend to stay in place, minimizing runoff and improving control. These are helpful for women treating specific areas of thinning, such as the temples, frontal hairline, or post-partum thinning zones.
Liposomal Delivery
Some prescription and premium products use liposomal or phospholipid-based carriers that are designed to improve follicular penetration while reducing irritation and systemic absorption. These advanced bases may be especially attractive for women who need stronger formulations but have experienced irritation with traditional vehicles.
How Safe is Minoxidil?
Topical minoxidil is generally well tolerated by women, but like any active medication, it can produce local scalp irritation, a brief increase in shedding during the early treatment phase, and, in very rare cases, systemic side effects, particularly in individuals with underlying skin or cardiovascular conditions.
Common local reactions may include:
- Mild itching
- Flaking or dryness
- Redness
- Burning or stinging
- Temporary increase in shedding
These symptoms are typically mild, tend to improve as the scalp adapts, and can often be reduced by switching to a foam-based formula, lowering concentration, or using a gentler vehicle.
Systemic and heart-related reactions are extremely uncommon with proper topical use. However, isolated case reports and safety data recommend seeking medical attention if symptoms such as chest tightness, heart palpitations, lightheadedness, dizziness, or unexplained swelling develop, as these could indicate increased systemic absorption or accidental ingestion. The risk of cardiovascular effects is significantly higher with oral minoxidil or improper dosing, where hypotension, tachycardia, and even heart failure have been reported.[20]Tripathee, S., Benyovszky, A., Devbhandari, R., Quiza, K., Boris, J. (2024). A Very Bad Hair Day: Minoxidil Ingestion Causing Shock and Heart Failure. Cureus. 16(8). E66039. Available at: … Continue reading
To minimize the risk of side effects, many clinicians recommend starting with once-daily application, gradually increasing only if tolerated. Women with reactive skin often benefit from foam formulations, lower-alcohol bases, or liposomal delivery systems, which can reduce irritation and improve comfort during long-term use.
Individuals with active inflammatory scalp conditions, such as psoriasis, eczema, or severe seborrheic dermatitis, are generally advised to first address the underlying condition before beginning minoxidil therapy. Applying treatment to an inflamed or compromised skin barrier can increase irritation, worsen symptoms, and lead to unpredictable absorption patterns.[21]Junge, A., Jic-Hoesli, S.R., Bossart, S., Simon, D., de Viragh, P., Hunger, R.E., Heidemeye, K., Seyed Jafari, S.M. (2025). Contact Dermatitis Caused by Topical Minoxidil: Allergy or Just Irritation. … Continue reading
Although topical minoxidil has lower systemic absorption than oral formulations, it is generally not recommended during pregnancy or while breastfeeding unless specifically advised by a healthcare provider. Women who are pregnant, trying to conceive, or nursing should consult a qualified medical professional to fully review potential risks and alternative treatment options.
Final Verdict
A range of minoxidil-based options is available to support different needs among women experiencing hair thinning, from strength-focused prescriptions to gentle, sensitivity-friendly alternatives and highly customized compounded formulas. While each product relies on the same core ingredient, differences in concentration, delivery vehicle, add-on actives, and level of medical support can significantly influence both tolerability and long-term adherence.
Whichever option you choose, consistent use of minoxidil remains one of the most evidence-backed ways to slow progression and improve hair density in women with pattern hair loss. With today’s expanded access to prescription platforms, foam-based formulations, and individualized treatment models, it is now easier than ever to find an approach that aligns with your scalp sensitivity, stage of hair loss, and lifestyle needs.
References[+]
References ↑1 Bhat, Y.J., Saqib, N-U., Latif, I., Hassan, I. (2020). Female Pattern Hair Loss – An Update. Indian Dermatology Online Journal. 11(4). 493-501. Available at: https://doi.org/10.4103/idoj.IDOJ_334_19 ↑2 Kothari, C.R., Shivakumar, P. (2024). Trichoscopic Features in Female Pattern Hair Loss: 1-Year Hospital-Based Cross-Sectional Study. Clinical Dermatology Review. 8(2). 95-101. Available at: https://doi.org/10.4103/cdr.cdr_123_21 ↑3 Ramos, P.M., Miot, H.A. (2015). Female Pattern Hair Loss: a clinical and pathophysiological review. Anais Brasileiros de Dermatologia. 90(4). 529-543. Available at: https://doi.org/10.1590/abd1806-4841.20153370 ↑4 Hussein, R.S., Dayel, S.B., Abahussein, O., El-Sherbiny, A.A. (2024). Applications and efficacy of minoxidil in dermatology. Skin Health and Disease. 4(6). E472. Available at: https://doi.org/10.1002/ski2.472 ↑5 Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. Advances in Dermatology and Allergology. 39(3). 472-478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑6 Gupta, A.K., Talukder, M., Shemer, A., Piraccini, B.M., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: https://doi.org/10.1159/0000531890 ↑7 Asad, N., Naseer, M., Ghafoor, R. (2024). Efficacy of Topical Finasteride 0.25% with Minoxidil 5% versus Topical Minoxidil 5% Alone in Treatment of Male Pattern Androgenic Alopecia. Journal of Drugs in Dermatology. 23(11). 1003-1008. Available at: https://doi.org/10.36849/JDD.7826 ↑8 Fabbrocini, G., Cantelli, M., Masara, A., Annunziata, M.C., Marasca, C., Cacciapuoti, S. (2018). Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. International Journal of Women’s Dermatology. 4(4). 203-211. Available at: https://doi.org/10.1016/j.ijwd.2018.05.001 ↑9 Aksenenko, M., Palkina, N., Komina, A., Ruksha, T. (2019). MiR-92a-1-5p and miR-328-3p Are Up-Regulated in Skin of Female Pattern Hair Loss Patients. Annals of Dermatology. 31(2). 256-259. Available at: https://doi.org/10.5021/ad.2019.31.2.256 ↑10 van Zuuren, E.J., Fedorowicz, Z., Schoones, J. (2016). Interventions for female pattern hair loss. Cochrane Database of Systematic Reviews. 2016(5). CD007628. Available at: https://doi.org/10.1002/14651858.CD007628.pub4 ↑11 Sattur, S.S., Sattur, I.S. (2021). Pharmacological Management of Pattern Hair Loss. Indian Journal of Plastic Surgery. 54(4). 422-434. Available at: https://doi.org/10.1055/s-0041-1739254 ↑12 Ghonemy, S., Alarawi, A., Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. Journal of Dermatological Treatment. 32(2). 236-241. Available at: https://doi.org/10.1080/09546634.2019.1654070 ↑13 Nohria, A., Desai, D., Sikora, M., Mandal, S., Shapiro, J., Lo Sicco, K. (2024). Combating “dread shed”: The impact of overlapping topical and oral minoxidil on temporary hair shedding during oral minoxidil initiation. JAAD International. 15. 220-224. Available at: https://doi.org/10.1016/j.jdin.2024.03.005 ↑14 Amit, K., Mansukh, G., Satyaprakash, M., Dhiraj, D., Hanmant, B. (2023). Real-World Effectiveness, Safety, and Tolerability of Cetosomal Minoxidil 5% Alone and a Fixed Drug Combination of Cetosomal Minoxidil 5% With Finasteride 0.1% in the Management of Androgenetic Alopecia (Inbilt Study). Cureus. 15(7). E41681. Available at: https://doi.org/10.7759/cureus.41681 ↑15, ↑16 Katz, H.I., Hien, N.T., Prawer, S.E., Goldman, S.J. (1987). Long-term efficacy of topical minoxidil in male pattern baldness. Journal of the American Academy of Dermatology. 16(3). 711-718. Available at: https://doi.org/10.1016/s0190-9622(87)70092-9 ↑17 Messenger, A.G., Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 2(1). 186-194. Available at: https://doi.org/https://doi.org/10.1111/j.1365-2133.2004.05785.x ↑18 Hoover E, Alhajj M, Flores JL. Physiology, Hair. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499948/ (Accessed: November 2025) ↑19 Purnak, T., Senel, E., Sahin, C. (2011). Liquid formulation of minoxidil versus its foam formulation. Indian Journal of Dermatology. 56(4). 462. Available at: https://doi.org/10.4103/0019-5154.84714 ↑20 Tripathee, S., Benyovszky, A., Devbhandari, R., Quiza, K., Boris, J. (2024). A Very Bad Hair Day: Minoxidil Ingestion Causing Shock and Heart Failure. Cureus. 16(8). E66039. Available at: https://doi.org/10.7759/cureus.66039 ↑21 Junge, A., Jic-Hoesli, S.R., Bossart, S., Simon, D., de Viragh, P., Hunger, R.E., Heidemeye, K., Seyed Jafari, S.M. (2025). Contact Dermatitis Caused by Topical Minoxidil: Allergy or Just Irritation. Acta Dermato-Venereologica. 105. 42401. Available at: https://doi.org/10.2340/actadv.v105.42401 Oral minoxidil has become a widely used off-label option for androgenic alopecia (AGA) in men and women, despite initially being approved only as an antihypertensive medication decades ago.
Oral minoxidil is increasingly offered to address specific unmet needs in AGA treatment:
- Topical non-responders: Some people don’t see satisfactory results with topical minoxidil, possibly due to inadequate absorption, variations in scalp skin properties, or genetic factors affecting drug metabolism.
- Scalp sensitivity/irritation: Topical minoxidil can cause local side effects such as itching, flaking, or dermatitis due to the vehicle (especially propylene glycol) or the active ingredient itself. Switching to oral minoxidil bypasses these local reactions.
- Simpler routines/adherence: Oral dosing eliminates the need for daily topical application, which can be messy, time-consuming, or interfere with hair styling. For some, this leads to better long-term adherence and quality of life.
Regrowth with oral minoxidil can be variable, however. This variability mainly stems from biological differences between patients. Because minoxidil is a prodrug that requires conversion by the enzyme sulfotransferase (SULT1A1) into its active form, individual variation in enzyme activity strongly influences response.[1]Ramos, P.M., Goren, A., Sinclair, R., Miot, H.A. (2020). Oral minoxidil bio-activation by hair follicle outer root sheath cell sulfotransferase enzymes predicts clinical efficacy in female pattern … Continue reading
Interested in Oral Minoxidil?
Low-dose oral minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Unfortunately, what most people see online are extremely successful oral minoxidil before and after photos (which you’ll see below), which can lead people to believe that they will experience more regrowth than they actually might.
Source: u/pomidorich via r/FemaleHairLoss.
In this article, we will explore what the likely (not just possible) results are and what the clinical trials show.
But first, let’s have a quick refresher on what oral minoxidil is.
What is Oral Minoxidil?
Oral minoxidil is a tablet medication originally developed as a vasodilator for severe hypertension, but now increasingly used off-label at low doses to treat AGA and other hair loss conditions. It enhances scalp flow and prolongs the growth phase (anagen) of hair follicles, primarily by increasing prostaglandin E2 and vascular endothelial growth factor (VEGF).
Oral minoxidil acts by opening ATP-sensitive potassium channels in vascular smooth muscle, which increases perifollicular blood flow. It also prolongs the anagen phase of the hair cycle. Unlike topical minoxidil, which requires local sulfotransferase enzyme activity in the scalp for conversion to its active form, oral minoxidil is metabolized systemically via hepatic sulfotransferase, potentially bypassing poor scalp enzyme activity in some individuals.[2]Kaiser, M., Abdin, R., Gaumond, S.I., Issa, N.T., Jiminez, J.J. (2023). Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs. Clinical, Cosmetic and Investigational Dermatology. 16. … Continue reading
For hair loss, oral minoxidil is prescribed at significantly reduced dosages compared to antihypertensive therapy – typically:
- 0.25 mg – 1.25 mg for women
- 2.5 mg – 5 mg for men
- Adverse effects are much less frequent at these low doses but may include hypertrichosis (excess unwanted body hair), fluid retention, and, rarely, cardiovascular effects.[3]Bloch, L.D., Carlos, R.M.D. (2024). Side Effects’ Frequency Assessment of Low Dose Oral Minoxidil in Male Androgenetic Alopecia Patients. Skin Appendage Disorders. 11(1). 14-18. Available at: … Continue reading
While oral minoxidil is not FDA-approved for hair loss, a number of studies show its efficacy.
The Evidence
You can see a complete table of oral minoxidil studies here, but we have picked a few to show.
Study One – AGA (Female)
Vahabi-Amlashi et al (2021) conducted a triple-blind, randomized clinical trial in 72 women with female pattern hair loss, comparing oral minoxidil 0.25 mg daily to 2% topical minoxidil twice daily over 36 weeks.[4]Vahabi-Amlashi, S., Layegh, P., Kiafar, B., Hoseininezhad, M., Abbaspour, M., Khaniki, S.H., Forouzanfar, M., Sabeti, V. (2021). A randomized clinical trial on the therapeutic effects of 0.25 mg oral … Continue reading
- Measured outcomes included hair shedding, Sinclair scores by three independent evaluators, trichoscopy assessments of hair density and diameter, and safety labs (liver, kidney, electrolytes).
- Both groups showed significant improvement in hair diameter and density:
- Group 1 (oral): 13% improvement in hair density.
- Group 2 (topical): 6% improvement in hair density.
- Sinclair and shedding scores decreased significantly in both groups by the end of the study.
- There was no significant difference between groups in any measured endpoint.
- Oral minoxidil showed slightly better improvement in hair density; topical minoxidil showed slightly better improvement in hair diameter.
- Adverse events:
- Group 1 (oral): hirsutism (7.7%), gastrointestinal intolerance (7.7%), weight gain (3.8%), hypotension (3.8%), leading to withdrawal.
- Group 2 (topical): hirsutism (8%).
Study Two – AGA (Male)
Panchaprateep and Lueangarun (2020) conducted an open-label, prospective single-arm study in 30 men with AGA, treated with 5 mg oral minoxidil once daily for 24 weeks.[5]Panchaprateep, R., Lueangarun, S. (2020). Efficacy and Safety of Oral Minoxidil 5 mg Once Daily in the Treatment of Male Patients with Androgenetic Alopecia: An Open-Label and Global Photographic … Continue reading
- Statistically significant increase in total hair count at 24 weeks (+35.1±18.9 hairs/cm2; p=0.003).
- Hair diameter improved by 15.2% from baseline (p<0.001).
- Global photographic assessment: 43% of patients achieved an “excellent improvement” score by study end.
- High rate of hypertrichosis (93.3% at 24 weeks); pedal edema was reported in 3 patients, resolving after 2-3 months in all cases.
- No serious cardiovascular side effects were observed.
Study Three – AGA and TE (Male and Female)
Feaster et al. (2023) performed a multicenter retrospective study in 210 patients (50 men, 160 women) with AGA and/or telogen effluvium (TE), comparing low-dose oral minoxidil (0.625 – 2.5 mg daily) to a control (no minoxidil or other non-minoxidil treatments) over 52 weeks.[6]Feaster, B., Onamusi, T., Cooley, J.E., McMichael, A.J. (2023). Oral minoxidil use in androgenetic alopecia and telogen effluvium. Archives of Dermatological Research. 315(2). 201-205. Available at: … Continue reading
- Group 1 (oral minoxidil): 52.4% of patients showed clinical improvement in hair growth; 41.9% had hair loss stabilization; 5.7% experienced worsening.
- These results were significantly better than the control group (p=0.001).
- The mean duration of oral minoxidil use was 25.1 months (range 12-54); the most common dose was 1.25 mg daily.
- Adverse effects were uncommon (9.5% of patients), generally mild, and included:
- Hypertrichosis (4.8%)
- Edema (1.9%)
- Hair shedding (1%)
- Lower extremity cramping (1%)
- Palpitations (1%)
- Scalp dysesthesia (1%)
- There were no serious side effects or study withdrawals due to adverse events in the analyzed population.
Overall, these studies demonstrate that oral minoxidil is an effective and generally well-tolerated treatment option for both AGA and TE in men and women. Across the studies, clinical improvements were observed, with similar efficacy to topical minoxidil.
However, the results that people show online are usually more dramatic than what is seen in clinical studies.
What’s Possible ≠ What’s Probable
When reviewing outcomes, such as oral minoxidil before and after photos, it’s essential to distinguish what can happen from what is likely to happen. Take a look at the example we have created below. The scattered data points represent the full spectrum of possible individual results, while the central trendline shows the average, reflecting the most common or expected response.
Figure 1: A graph we created showing possible and probable results. The arrows point to possible results, and the trendline shows probable results.
These anecdotes (arrows on the graphs) often highlight “hyper-responders”. These are individuals who experience notably dramatic hair regrowth. They may have higher SULT1A1 activity, leading to higher levels of minoxidil activation.
Figure 2: Anecdotal results often look significantly more dramatic than clinical results.
These accounts, although genuine, illustrate results that surpass what most people can achieve. Problems can then arise when people fixate on these exceptional outcomes and assume that their experience will be similar. If their own hair growth is slower or less impressive, they might discontinue treatment early, or wrongly conclude that it isn’t working.
Figure 3: This leads to high expectations when the reality is that results will often be a lot less dramatic.
Impressive results are more frequently shared than modest improvements as a result of survivorship bias. This bias happens when only the most impressive successes receive attention, while the many cases with typical or less remarkable outcomes are overlooked or not reported.
Survivorship bias can distort our understanding of how hair loss treatments perform. Online narratives often draw attention to the most extreme cases, either astonishing successes or clear failures, while the more typical moderate outcomes that most people experience are rarely seen or discussed.
Now that we have covered the clinical evidence, strategies, and reasonable expectations for oral minoxidil, let’s now look into 10 firsthand accounts of oral minoxidil before and after photos shared by real users on Reddit.
Oral Minoxidil Success Stories
Keep in mind that the experiences below are purely anecdotal in nature and are not subject to any independent verification. They strictly reflect the individual journeys of each poster and should not be construed as reliable clinical evidence or medical advice.
Case 1: Male (29), Oral minoxidil monotherapy (11 months, 5 mg nightly)
Source: u/jeffersonsteelflex94 via r/tressless.
This Redditor began oral minoxidil monotherapy at a dose of 2.5 mg before increasing to 5 mg once nightly. He had switched from topical minoxidil after a year of subpar results. He experienced shedding in the early months of therapy, with visible regrowth by month 3, and a major improvement in hair density by 11 months. Overall, the patient was satisfied with thicker scalp coverage, a fuller beard and brows, and did not report any significant side effects.
Case 2: Male (26), finasteride and oral minoxidil (2 years total; 1 mg finasteride and 2.5 mg oral minoxidil daily)
Source: u/GriffsChoice via r/tressless.
This 26-year-old used a combination of 1 mg finasteride and 2.5 mg oral minoxidil once daily, having switched from topical to oral minoxidil one year into treatment. He reported notable improvement one year into therapy, with visible regrowth by the 4-month mark. He did not report any shedding or other side effects after switching from topical to oral formulations. After two years of treatment, the patient reported marked hair thickening and cosmetic improvement.
Case 3: Female, oral minoxidil monotherapy (2 years; 2.5 mg daily, titrated up from 1.25 mg)
This user reported one her progress with oral minoxidil monotherapy over 2 years. She started at a daily dose of 1.25 mg, before increasing to 2.5 mg. She had tried vitamins, spironolactone, oils, and Nioxin/Nizoral without benefit for one year prior. Shedding started shortly after beginning the protocol and normalized by the fourth month. She reported visible improvement within one year, and by the end of the second year, she had notable improvement in ponytail thickness and part density. She also reported that her hair looked and felt close to pre-loss baseline. The user did not report any significant side effects.Source: u/[deleted] via r/FemaleHairLoss.
Case 4: Female (24), Oral and topical minoxidil (9 months topical 5% foam; 6 months oral 0.625 – 1.25 mg nightly)
Source: u/pomidorich via r/FemaleHairLoss.
This 24-year-old began minoxidil monotherapy with 5% foam nightly, before adding oral minoxidil 0.625 mg three months later, and titrating to 1.25 mg nightly the following month. She continued both nightly for six months. She did not note any noticeable shedding on the foam, nor after incorporating 0.625 mg orally. She reported steady baby hairs and a reduction in hair loss at ~6-9 months alongside thickening compared to the baseline, and mentioned that her hair felt and looked much closer to its pre-loss condition overall. She also reported faster growth of facial/body hair (hypertrichosis), water retention, and bloating after salt meals, and occasional stronger heartbeat at night, after incorporating oral minoxidil, though this last symptom did resolve.
Case 5: Male, oral minoxidil (6 weeks shown; 5 mg daily) and weekly microneedling
Source: u/No_Pop2289 via r/tressless.
This male Redditor used oral minoxidil 5 mg once daily in addition to microneedling once weekly with a pen device. The poster also noted no side effects from finasteride, implying that they might have used it alongside the other treatments. He reported an initial shedding phase at around week 3 and rapid visible coverage by week 6 into treatment. In commenting on the progress to date, he noted fuller coverage compared to baseline and said his beard was slightly thicker without any apparent increase in body hair. He did not report any side effects.
Case 6: Female, oral minoxidil + spironolactone (1 year; 1.25 mg oral minoxidil daily, 50 mg spironolactone daily)
Source: u/monicatheshark5 via r/FemaleHairLoss.
The poster took oral minoxidil 1.25 mg once daily, in addition to spironolactone 50 mg once daily (started at 100 mg but reduced the dose due to drowsiness). She noted visible improvement after about 5 months of consistent use, with shedding slowing markedly around weeks 7-8. At the one-year mark, she reported noticeably fuller hair at the part and temples, with ongoing gradual thickening, and “good hair days”. She reported increased body and facial hair from oral minoxidil, which she managed by waxing, and did not report any noticeable weight gain or low-blood-pressure symptoms.
Case 7: Female, oral minoxidil monotherapy (6 months; 1.25 mg daily, titrated from 0.625 mg)
Source: u/Aggressive_Space_121 via r/FemaleHairLoss.
This female Redditor took oral minoxidil 1.25 mg once daily for six months, having started from the initial dose of 0.625 mg. She supplemented with vitamin B12 injections every two weeks for pernicious anemia, daily shampooing, higher protein intake, and therapy/stress management. She reported steady improvement in hair through month 6, despite a brief “dread shed” in the initial month. Unfortunately, the images don’t show the same hair pulled back, so we can’t see exactly how much improvement was gained. She did report increased facial hair, which she has been managing by shaving/dermaplaning.
Case 8: Female, oral minoxidil monotherapy (1 year; 1.25 mg daily)
Source: u/neptunesummer via r/FemaleHairLoss.
This user took oral minoxidil 1.25 mg once daily for one year. She also used Nizoral shampoo on the same timeline. Her hair loss was first diagnosed as telogen effluvium that did not resolve, and she now suspects AGA. She reported modest shedding in the first 2 months, then gradual thickening over the year. The photos showed some improvement, but the patient stated that she is not fully back to baseline. Regarding side effects, she noted increased body hair growth and mild dizziness in the first month that resolved.
Case 9: Male, self-made oral minoxidil (3 months; ingested diluted topical solution)
Source: u/Frosty_Wedding8706 via r/tressless.
This male Redditor switched from applying topical minoxidil to drinking a diluted solution mixed with water. He reported taking “a few drops” per dose, having discontinued application of the topical preparation to the scalp. At the three-month mark, the Redditor reported clearly greater overall density and said results were still improving. He did not report any side effects, except for some extra body hair.
We should mention that we do not recommend ingesting topical minoxidil – if oral therapy is pursued, it is advised to use a prescribed oral dose under medical supervision to control dosing and avoid health risks.
Case 10: Female, oral minoxidil monotherapy (12 months; dose not reported)
Source: u/blahblahyayah via r/FemaleHairLoss.
This user reported taking oral minoxidil at an unspecified dose for about one year. The photos show modest improvements around the parting. The poster did not report any side effects. Notwithstanding the sparse information provided by this Redditor, multiple commenters observed hair thickening and density gains in the before-and-after photos.
Strategies to Optimize Oral Minoxidil Response
To maximize the benefits of oral minoxidil, consistent use and patience are essential as optimal regrowth often takes 6-8 months to fully manifest. Combining oral minoxidil with other treatments like finasteride or dutasteride, platelet-rich plasma (PRP), or low-level laser therapy (LLLT) can improve results for those who do not respond sufficiently to minoxidil alone. Recent studies support the efficacy of combination regimens, particularly oral minoxidil plus finasteride, with one retrospective study finding significant and clinically meaningful improvements.[7]Johnson, H., Huang, D., Clift, A.K., Bersch-Ferreira, A., Guimaraes, G.A. (2025). Effectiveness of Combined Oral Minoxidil and Finasteride in Male Androgenetic Alopecia: A Retrospective Service … Continue reading
Figure 4: Percentage of patients who are stable or improved, or improved only, across increasing Norwood severities after combination finasteride and oral minoxidil treatment.[8]Johnson, H., Huang, D., Clift, A.K., Bersch-Ferreira, A., Guimaraes, G.A. (2025). Effectiveness of Combined Oral Minoxidil and Finasteride in Male Androgenetic Alopecia: A Retrospective Service … Continue reading Image used in accordance with the PMC Copyright notice.
People using oral minoxidil, especially at 2.5 mg or more and in combination with other treatments, should keep an eye on potential side effects. This can help catch rare but potentially serious side effects like edema or changes in blood pressure. Regular follow-ups can ensure that any adverse effects are detected early and that the regimen can be safely adjusted to maintain a balance between safety and efficacy. This patient-tailored management increases the likelihood of achieving the best possible outcomes while minimizing health risks.
Probable Regrowth Timeline
Based on the available evidence, the expected regrowth timeline for oral minoxidil is around 8 months.
Months 0-3: Possible increased shedding; no visible gains
- Temporary shedding can occur as follicles transition into a new growth cycle.
- This process often resolves within the first 4-8 weeks and is seen as an early sign that the treatment is starting to take effect.
Months 3-6: First positive density changes for many
- Early visible improvements, such as reduced shedding and appearance of fine new hairs, can be expected.
- Thicker, darker regrowth starts to become apparent for most users during this period.
Months 6-8: Maximal visible benefits
- Cosmetic changes peak, with stronger hair, increased density, and the greatest coverage observed.
- This is the period of maximum regrowth for most individuals.
Months 8-12+
- Further gains plateau.
- Continued daily use is needed to maintain improvements.
- Some might consider combination treatment at this point to increase growth outcomes.
Safety and Side Effects
While oral minoxidil can avoid local irritation occurring with the topical formulation, some safety concerns do remain.
Oral minoxidil use does carry a risk of cardiovascular issues like pericardial effusion, especially at the high doses used for blood pressure control. At hair loss doses (0.25 – 5 mg), such events are rare and tend to be individual rather than predictable.
The majority of side effects, such as excess body hair, mild ankle swelling, lightheadedness, or palpitations, are mild and usually resolve with dose adjustments or stopping the medication.
Large safety studies have shown that serious side effects are exceedingly rare in otherwise healthy individuals taking low-dose oral minoxidil, with only a small percentage discontinuing treatment due to adverse effects.
A gradual dose escalation is recommended to minimize side effects with oral minoxidil. Recent studies have shown that a 2.5 mg daily dose is as effective as 5 mg for treating male pattern hair loss, but with a better safety profile, and this supports 2.5 mg as a preferred starting dose for men.[9]Fonseca, L.P.C., Miot, H.A., Chaves, C.R.P., Ramos, P.M. (2025). Oral Minoxidil 2.5 mg vs 5 mg for Male Androgenetic Alopecia: A Double-Blind Randomized Clinical Trial. Journal of the American … Continue reading
If you want to use a higher dose, sublingual minoxidil may be beneficial for reducing the risk of cardiovascular side effects.[10]Gupta, A.K., Bamimore, M.A., Williams, G., Talukder, M. (2025). Comparative Efficacy of Minoxidil and 5-Alpha Reductase Inhibitors Monotherapy for Male Pattern Hair Loss: Network Meta-Analysis Study … Continue reading
Practical Takeaways
- Oral minoxidil is an effective and generally well-tolerated alternative for people who cannot use topical minoxidil or do not respond to it.
- Clinical trial results typically show stabilization or modest regrowth. Hyper-responder cases seen online are possible but not common.
- Careful dose selection (0.25 – 2.5 mg daily) minimizes side effects while maintaining efficacy.
- Consistent, long-term use is key; optimal results generally take 6-8 months to appear and require ongoing treatment to maintain gains.
- Combining oral minoxidil with other therapies such as finasteride, dutasteride, PRP, or LLLT may improve outcomes for partial responders.
Final Thoughts
Oral minoxidil use is growing, but should be recognized as an off-label option (albeit backed by a solid body of emerging evidence.. Most users can expect stabilization or noticeable yet modest regrowth rather than dramatic transformation. The best outcomes come with realistic expectations, consistent use over several months, and regular medical supervision to ensure safety. By focusing on average, evidence-based results rather than rare hyper-responder anecdotes, patients can make balanced decisions and maintain long-term adherence to their treatment plan.
References[+]
References ↑1 Ramos, P.M., Goren, A., Sinclair, R., Miot, H.A. (2020). Oral minoxidil bio-activation by hair follicle outer root sheath cell sulfotransferase enzymes predicts clinical efficacy in female pattern hair loss. Journal of the European Academy of Dermatology and Venereology. 34(1). E40-e41. Available at: https://doi.org/10.1111/jdv.15891 ↑2 Kaiser, M., Abdin, R., Gaumond, S.I., Issa, N.T., Jiminez, J.J. (2023). Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs. Clinical, Cosmetic and Investigational Dermatology. 16. 1387-1406. Available at: https://doi.org/10.2147/CCID.S385861 ↑3 Bloch, L.D., Carlos, R.M.D. (2024). Side Effects’ Frequency Assessment of Low Dose Oral Minoxidil in Male Androgenetic Alopecia Patients. Skin Appendage Disorders. 11(1). 14-18. Available at: https://doi.org/10.1159/00539969 ↑4 Vahabi-Amlashi, S., Layegh, P., Kiafar, B., Hoseininezhad, M., Abbaspour, M., Khaniki, S.H., Forouzanfar, M., Sabeti, V. (2021). A randomized clinical trial on the therapeutic effects of 0.25 mg oral minoxidil tablets on treatment of female pattern hair loss. Dermatological Therapy. 34(6). E15131. Available at: https://doi.org/10.1111/dth.15131 ↑5 Panchaprateep, R., Lueangarun, S. (2020). Efficacy and Safety of Oral Minoxidil 5 mg Once Daily in the Treatment of Male Patients with Androgenetic Alopecia: An Open-Label and Global Photographic Assessment. Dermatology and Therapy. 10. 1345-1357. Available at: https://doi.org/10.1007/s13555-020-00448-x ↑6 Feaster, B., Onamusi, T., Cooley, J.E., McMichael, A.J. (2023). Oral minoxidil use in androgenetic alopecia and telogen effluvium. Archives of Dermatological Research. 315(2). 201-205. Available at: https://doi.org/10.1007/s00403-022-02331-5 ↑7 Johnson, H., Huang, D., Clift, A.K., Bersch-Ferreira, A., Guimaraes, G.A. (2025). Effectiveness of Combined Oral Minoxidil and Finasteride in Male Androgenetic Alopecia: A Retrospective Service Evaluation. Cureus. 17(1). E77549. Available at: https://doi.org/10.7759/cureus.77549 ↑8 Johnson, H., Huang, D., Clift, A.K., Bersch-Ferreira, A., Guimaraes, G.A. (2025). Effectiveness of Combined Oral Minoxidil and Finasteride in Male Androgenetic Alopecia: A Retrospective Service Evaluation. Cureus. 17(1). E77549. Available at: https://doi.org/10.7759/cureus.77549 ↑9 Fonseca, L.P.C., Miot, H.A., Chaves, C.R.P., Ramos, P.M. (2025). Oral Minoxidil 2.5 mg vs 5 mg for Male Androgenetic Alopecia: A Double-Blind Randomized Clinical Trial. Journal of the American Academy Dermatology. 15. Available at: https://doi.org/10.1016/j.jaad.2025.09.031 ↑10 Gupta, A.K., Bamimore, M.A., Williams, G., Talukder, M. (2025). Comparative Efficacy of Minoxidil and 5-Alpha Reductase Inhibitors Monotherapy for Male Pattern Hair Loss: Network Meta-Analysis Study of Current Empirical Evidence. Journal of Cosmetic Dermatology. 62(2). 257-259. Available at: https://doi.org/10.1111/ijd.163737 Finding the right hair loss treatment can feel overwhelming, and expert guidance is essential to identify the best formula for your needs. The PhD-led team at Perfect Hair Health leverages emerging research and close collaborations with top innovators to provide readers with the most accurate and actionable reviews of hair restoration offerings. Topical finasteride is one of the most sought-after treatments on the market, boasting clinical support, targeted results, and easy application.
This comprehensive guide to the top-rated topical finasteride products is based on extensive research, expert recommendations, and the latest consumer feedback. Whether you’re just beginning your hair health journey or searching for better hair loss solutions, this article provides valuable information on safe and effective treatment options. Our recent partnership with Ulo enhances this guide with privileged insights on clinical findings and state-of-the-art protocols.
Read on for specialized guidance, insightful reviews, and proven recommendations of topical finasteride preparations from today’s leading brands.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Quick Look: 5 Best Topical Finasteride Products
Here’s a side-by-side comparison of our top recommended topical finasteride treatments. Read on for more details on each of these excellent products.
Product Price Finasteride % Customization Application Ulo $64+/month 0.005% – 0.2% High Solution Strut Health $59 0.1-0.25% Medium Solution/Gel Happy Head $59-99/month 0.3% Medium Liposomal Gel Roman $50/month 0.3% Low Spray Hims $35+/month 0.3% Low Spray Topical Finasteride: What It Is & Why It’s Better
First, we will briefly explore the science behind topical finasteride, including its mechanism of action in treating hair loss and the advantages of topical formulations.
What Is Topical Finasteride?
Topical finasteride is a clinically proven treatment for androgenic alopecia (AGA), a type of hair loss that affects more than 50% of men by the age of 50.[1]Jang, W.S., Son, I.P., Yeo, I.K., Park, K.Y., Li, K., Kim, B.J., Seo, S.J., Kim, M.N., Hong, C.K. (2013). The Annual Changes of Clinical Manifestation of Androgenetic Alopecia Clinic in Korean Males … Continue reading AGA is caused by dihydrotestosterone (DHT), a testosterone-derived hormone that damages hair follicles, causing them to shrink and produce thinner hair. Finasteride reduces DHT levels by inhibiting the enzyme 5-alpha-reductase, protecting the hair follicles to stop hair loss and encourage new growth.[2]Duran, R, C-D., Martinez-Ledesma, E., Garcia-Garcia, M., Gauzin, D.B., Sarro-Ramirez, A., Gonzalez-Carillo, C., Rodriguez-Sardin, D., Fuentes, A., Cardenas-Lopez. (2024). The Biology and Genomics of … Continue reading
While orally administered finasteride has been in use since 1997, topical formulations are a relatively new development. When applied to the scalp, finasteride provides targeted treatment with a reduced risk of systemic side effects associated with oral preparations. Topical finasteride is shown to reduce DHT levels on the scalp for greater hair count and density with comparable effectiveness to oral finasteride.[3]Lee, S.W, Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in … Continue reading
Numerous studies confirm that topical finasteride slows and reverses hair loss by boosting hair thickness, density, and growth cycles. These results are enhanced when combined with other topical treatments like minoxidil. Due to minimal systemic absorption and attendant side effects, topical formulas are considered a safer choice for prolonged use. Overall, topical finasteride is prized among clinicians and patients as a safe, effective, and convenient solution to pattern hair loss.[4]Lee, S.W, Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in … Continue reading,[5]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray … Continue reading
Why Choose Topical Over Oral?
Because topical finasteride is applied directly to the scalp, it offers localized therapy and avoids the side effects associated with oral finasteride’s systemic exposure. Here is a brief overview of this and other primary advantages of topical formulas:
- Better Safety Profile: Oral finasteride is absorbed into the bloodstream and can have global side effects like sexual dysfunction, hormone disruption, and mood swings. In contrast, topical formulas target the scalp directly and minimally affect plasma levels. Topicals are consistently shown to have fewer systemic side effects. If present, side effects tend to be mild and temporary, such as scalp irritation.
- Individualized Dosing: While the oral finasteride dose is a standardized 1 mg, topical formulas enable tailored dosages and greater precision through potency titration. This is especially important given the dose-dependent outcomes of finasteride treatment. The finasteride concentration in topicals can range from a maintenance dose of 0.005% to a more aggressive protocol of 2.5%.
- Greater Customization: Topical formulas often combine other proven treatments, such as tretinoin, minoxidil, and caffeine. Studies demonstrate that finasteride-minoxidil combinations deliver superior results compared to single-agent treatments. Plus, the range of available delivery methods (e.g., solutions, sprays, gels) can improve convenience, satisfaction, and adherence.
- Long-Term Use: With a better safety profile and lower systemic impact, topical finasteride is more suitable for prolonged use, which is often necessary to maintain the benefits of hair loss treatment.
In short, research data indicate that topical finasteride yields comparable results with a more favorable safety profile than oral finasteride, making it a better option for long-term treatment. Further benefits of topical formulations include greater dose precision, synergistic adjuvants like minoxidil, and a range of delivery methods to support compliance.[6]Lee, S.W, Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in … Continue reading,[7]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray … Continue reading
Evaluation Criteria: What Makes the Best?
When selecting the best topical finasteride products, we adhered to the following evaluation criteria, which are thoroughly supported by clinical findings and customer feedback.[8]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray … Continue reading,[9]Suchonwanit, P., Iamsumang, W., Leerunyakul, K. (2020). Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: a review of the current literature. Journal … Continue reading
✓ Effectiveness
We assessed scientific data, DHT decrease rates, and real-world testimonies of hair count increases. The top products deliver measurable results in 3-6 months with continuous improvements in hair density throughout the first year and beyond.
✓ Safety
Because finasteride is a prescription-only treatment, we verified that providers adhere to all safety regulations, including proper prescribing and monitoring protocols. Leading suppliers also provide full ingredient transparency to help assess potential side effects.
✓ Customization
Premium brands offer treatment customization options tailored to patients’ needs and preferences, maximizing outcomes beyond those achieved with traditional, standardized approaches. These include concentration adjustments, synergistic add-on ingredients such as minoxidil, and various delivery methods.
✓ Convenience
Successful treatment outcomes depend on long-term compliance and a positive user experience. Factors such as ease of application, clear instructions, manageable dose frequency, and responsive support help ensure uninterrupted treatment.
✓ Value
The best providers balance superior quality with transparent and affordable pricing models that cover doctor consultations, prescriptions, and follow-up care. These are especially important concerns in the telehealth landscape, where many subpar platforms employ opaque billing practices to stack on hidden fees and unexpected charges.
How to Choose the Right Product for Your Needs
When choosing the product that best meets your hair loss treatment needs, consider the extent of your hair loss and treatment aims, such as basic upkeep or intensive restoration. Also account for your preferred delivery method (i.e., gel, solution, or spray), desired add-ons, medical support requirements, and budget.
Reviewing the Best Topical Finasteride Products
With the above key factors in mind, let’s turn now to our top recommended options that each offer unique advantages sure to satisfy.
#1: Ulo Finasteride + Minoxidil Plus Rx
Pros: Cons: ✓ Clinically-proven combination therapy ✗ Prescription products are only available in the USA ✓ True personalization with custom concentrations and medical consultations ✓ PhD-scientist formulated ✓ Ongoing medical monitoring and a user-friendly platform ✓ Quality-tested ingredients free of irritants Ulo’s combination formula of high-strength finasteride with prescription-grade minoxidil takes the top spot, reflecting the platform’s commitment to providing safe, scientifically proven hair loss treatments tailored to meet individual needs. Every Ulo product is developed by a team of PhD scientists who deliver innovative interventions in hair restoration.
Their advanced finasteride and minoxidil solution blocks DHT and stimulates new growth in a targeted dual treatment that is proven superior to monotherapies in clinical trials. Ulo’s finasteride formulas are uniquely customizable and available in different concentrations (0.005% for maintenance and 0.2% for stronger protocols), with a series of synergistic add-ons like caffeine to boost circulation, tretinoin for better absorption, and melatonin for antioxidant protection.
The deluxe treatment begins at $64 per month, which includes an onboarding medical consultation to assess each client’s needs, a physician-crafted treatment plan, and continued dermatologist oversight. All Ulo products are strictly sourced from pharmacies that adhere to safety and quality regulations. The clinic’s licensed dermatologists provide customized treatment regimens with free home prescription deliveries and ongoing support.
Bottom Line: Ulo’s individualized hybrid treatment is the ultimate in advanced hair restoration technology. With precise formulation and continuous medical supervision, this tailored solution delivers exceptional results for a low monthly subscription fee starting at $64. Ulo’s guiding dedication to safety and efficacy makes this full-service platform the best option for those who are serious about combating hair loss.
#2 Strut Finasteride Hair Loss Formula
Pros: Cons: ✓ Custom-compounded topical finasteride formulas (with optional add-ons) ✗ Not available internationally, and currently cannot ship to Arkansas ✓ Online consult and prescriptions from U.S. board-certified physicians ✗ Auto-refills by default; some reviews report difficulty canceling and customer service gaps ✓ Multiple formulation options (gel or solution; adjustable finasteride strength) ✓ Convenient telehealth model with free, discreet shipping in most U.S. states. Strut Health is a U.S.-based telehealth provider offering customized topical finasteride for AGA. After an online questionnaire and photo-based evaluation, a board-certified clinician designs a compounded formula that may contain finasteride alone or in combination with other actives like minoxidil, tretinoin, fluocinolone, or biotin. The company’s finasteride hair-loss formulas typically use 0.1-0.25% topical finasteride, with optional minoxidil concentrations up to 7.5% and tretinoin in a gel or solution base, giving flexibility to tailor potency and tolerability.
Our in-house lab testing showed that Strut Health’s finasteride product contained the correct concentrations of ingredients (finasteride, minoxidil, and tretinoin), indicating that they can be trusted to provide a quality product.
However, there are trade-offs. Strut does not accept insurance, and pricing for topical finasteride (often around the mid-range of the telehealth market) may add up for long-term use. The company currently ships to nearly all U.S. states but cannot ship to Arkansas, and there is no international service. Public reviews highlight convenience for many users but also recurring concerns about auto-refills and difficulty canceling, as well as frustration with customer support responsiveness and refund policies.
Bottom Line: Strut Health’s topical finasteride offers a flexible, telehealth-delivered alternative for patients who want DHT-targeted treatment without taking a daily pill, with customizable formulas that can include or exclude minoxidil and other actives. Its convenience, physician oversight, and compounding flexibility are strong advantages, but subscription-based pricing and mixed customer service reviews may give more budget-conscious patients pause.
#3 Happy Head Topical Super Solution: Finasteride, Minoxidil & Liposomal
Pros: Cons: ✓ Medical oversight by board-certified dermatologists ✗ Price rises from $59 to $99 after the first six months ✓ Higher concentrations of 0.3% finasteride + 8% minoxidil ✗ Higher concentrations may increase side effect risk ✓ Made with a delayed-release liposomal base ✗ Gel formula cannot be customized ✓ User-friendly platform and follow-up care ✗ May irritate sensitive skin ✓ Affiliate programs available ✗ Possible shipping and customer service concerns Our next selection is from Happy Head, known for unique formulations and dermatologist oversight. The Topical Super Solution features a more concentrated combination of 0.3% finasteride with 8% minoxidil for targeted hair restoration.
Available in a mess-free pump dispenser, this gel is made with the brand’s proprietary liposomal delivery base that boosts scalp penetration with timed medication release and once-daily application. This helps minimize bloodstream absorption, reducing potential side effects, and is a notable safety feature for higher finasteride doses. That said, some ingredients in the carrier base should not be used on sensitive skin. Other products from the brand are more customizable and can be adapted to sensitive skin.
The starting price of Happy Head’s treatment is competitive at $59 for a monthly subscription that covers dermatologist oversight and prescription deliveries. However, the price increases to $99 after the first six months. Some reviews cite a lack of transparency regarding potential side effects, as well as intermittent shipping delays and inconsistent customer service.
Bottom Line: Happy Head’s potent dual therapy is a useful option for patients with intermediate to severe hair loss and normal skin. The delayed-release gel base increases medication absorption for more targeted treatment while decreasing systemic side effects and requires application only once a day. Advantages include dermatologist support and attractive pricing, but patients should be aware of reviews citing shipping and customer service issues.
#4: Roman (Ro) Hair Solution Rx
Pros: Cons: ✓ Transparent and competitive price of $50 per month ✗ Contains potential irritants not suitable for sensitive skin ✓ High finasteride concentration (0.3%) for aggressive treatment ✗ High fixed concentration with a greater risk of side effects ✓ Includes minoxidil and tretinoin for synergistic effects ✗ Limited customization options ✓ Convenient once-daily application ✗ Standard delivery system lacks advanced features ✓ Established telehealth provider with medical supervision Roman offers a simple topical finasteride formula designed for convenient, once-daily use and focused intervention. The spray contains three active ingredients: high-dose 0.3% finasteride with 6% minoxidil and tretinoin to enhance absorption. This product delivers a potent dose of finasteride on par with premium rivals through a no-frills spray system.
While tretinoin may improve minoxidil absorption, Roman’s spray has some drawbacks for users with sensitive skin or systemic side effect concerns. The 0.3% finasteride concentration cannot be tailored to individual response, and the standard delivery system does not have delayed-release technology to offset the risk of systemic absorption as seen in Happy Head’s liposomal gel. Inactive ingredients like ethyl alcohol and propylene glycol are also known irritants.
Roman is an accessible option for many users. The transparent and fixed monthly fee of $50 covers doctor consultations, prescription deliveries, and unlimited follow-ups. However, users with sensitive skin or more complex treatment needs may find Roman’s standardized and less gentle formulation to be unsuitable for long-term compliance.
Bottom Line: Roman offers a potent and affordable dual finasteride therapy with telehealth support features. Although it is not customizable like leading alternatives, this spray is a convenient choice for individuals with moderate to severe hair loss who can tolerate high dosages and potential irritants.
#5: Hims Topical Finasteride & Minoxidil Spray
Pros: Cons: ✓ Lowest starting rate of $35+/month ✗ Standardized approach that lacks customization ✓ Well-known brand with recognized platform ✗ Minimal ongoing medical support ✓ Simple ordering process and rapid shipping ✗ Contains irritants not suitable for sensitive skin and fixed high dose ✓ No lengthy consultations required ✗ Basic telehealth features without specialist care ✓ High-dose dual treatment ✗ Concerns about the price transparency of subscription tiers Hims is a well-known brand that offers topical finasteride through its streamlined telemedicine platform. Their fixed-dose spray of 0.3% finasteride and 6% minoxidil is a convenient and affordable option without the customization features of premium competitors, appealing to those who value simplicity over tailored care.
Like similar high-dose sprays, this standardized formula from Hims is not suitable for patients with sensitive skin, complex treatment needs, or those requiring a lower maintenance dose.
This provider’s appeal is its wide accessibility. With low prices starting at just $35 per month, quick doctor consultations, and shipping in all 50 states, Hims is a gateway brand for many new hair loss patients. However, this entry-level approach lacks the treatment personalization and close medical supervision of established specialty providers.
Bottom Line: Hims’ topical finasteride spray is an affordable and easily accessible option backed by a recognized brand name. Although Hims fails to offer the precision treatment and close medical oversight of premium alternatives, it is a mainstay for patients who prioritize low costs and convenience.
Final Verdict: Our Topical Finasteride Picks
After a comprehensive evaluation, Ulo emerges as our top choice for topical finasteride in 2026. Their genuine customization, science-backed formulations, and ongoing dermatologist supervision set the gold standard for personalized hair restoration.
While competitors offer standardized protocols or unproven technologies, Ulo delivers verified results through precision medicine tailored to individual needs. For patients seeking the highest quality care, Ulo’s scientific rigor and safety-first approach make it the clear winner.
What to Expect: Timeline and Results
Realistic treatment expectations facilitate long-term compliance and successful outcomes. Medical research has established the following evidence-based benchmarks of expected improvements with topical finasteride use as prescribed to treat androgenetic alopecia.
Clinical Timeline for Topical Finasteride Results
Timeframe What to Expect Clinical Evidence 3 Months Terminal hair count starts to improve; increases in total hair count may not yet be observable [10]B. Piraccini et al. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European … Continue reading,[11]Cheng Zhou et al. (2025). Efficacy and safety of topical finasteride spray solution in the treatment of Chinese men with androgenetic alopecia: A phase III, multicenter, randomized, double-blind, … Continue reading 6 Months Noteworthy increases in hair counts (total and terminal); visible increase in vertex hair growth [12]B. Piraccini et al. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European … Continue reading,[13]Cheng Zhou et al. (2025). Efficacy and safety of topical finasteride spray solution in the treatment of Chinese men with androgenetic alopecia: A phase III, multicenter, randomized, double-blind, … Continue reading,[14]A. Rossi et al. (2020). Efficacy of Topical Finasteride 0.5% vs 17α-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 … Continue reading 12-18 Months Maximum improvement is typically observed; continuous improvements in hair coverage and density [15]A. Rossi et al. (2020). Efficacy of Topical Finasteride 0.5% vs 17α-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 … Continue reading - Early Treatment Phase (Months 1-3): During early treatment, visible changes are minimal, which may be disappointing for new patients. The therapy begins to suppress scalp DHT levels, creating shifts that are not yet cosmetically evident. Studies show that improvements in terminal hair count begin around 3 months, though total hair count is not yet typically impacted.
In the first 6-8 weeks, some experience a temporary increase in shedding as finasteride prompts new growth cycles to start. This is expected and is an encouraging indication that the treatment is taking effect.
- Visible Improvement Phase (Months 3-6): Visible results begin to appear during this period. Studies show notable increases in hair counts and density by 6 months. Patients tend to note particular improvements in the crown or vertex region.
- Optimization Phase (Months 6-18): The most significant benefits occur during this phase. Research indicates that maximum improvements arise around 12 months of continued treatment, as hair density and new growth quality increase.
- Long-Term Maintenance: According to clinical data, benefits stabilize around 18-24 months, and treatment transitions from active regrowth to maintenance. Because treatment cessation can lead to the gradual return of hair loss within 6-12 months, continued daily compliance is essential to maintain long-term therapeutic outcomes.
Topical Delivery Formats
There are several different topical finasteride delivery formats that each offer unique advantages to suit individual needs and preferences, as follows:
- Solutions are liquid formulas typically applied using a dropper for targeted administration on desired scalp areas. These lightweight preparations are valued for their excellent absorption and residue-free finish. Because solutions may contain irritating excipients such as propylene glycol, sensitive patients should opt for gentle blends like Ulo’s prescription topicals.
- Sprays are a lighter, more fluid type of solution applied using an aerosol or pump. This form enables misting over larger or difficult-to-reach scalp areas. However, sprays are not as precise as droppers, as the liquid may run off and fail to effectively reach the hair follicles.
- Gels offer a more controlled application with a thicker consistency than solutions. They often include advanced delivery bases such as liposome carriers with delayed-release technology, designed to enhance penetration and reduce systemic absorption. However, customization options are typically limited due to compounding practices. Additionally, gel formulas can leave a sticky residue that some users find undesirable.
- Foams may be beneficial for patients with sensitive skin, as they dry quickly and generally lack harsh additives. However, their capacity for targeted application is limited compared to other formulations.
- Creams and lotions are appropriate for sensitive or dry scalps, featuring thick, emollient-laden bases with moisturizing benefits. Lotions are generally faster-absorbing, more spreadable, and lighter than creams.
The best formulation choice depends on hair and skin type, lifestyle, and personal preferences. Patients are encouraged to consult a doctor to determine the ideal format for their individual needs.
Safety and Side Effects
Topical finasteride is noted for its favorable safety profile, especially in contrast to oral formulations; however, understanding potential risks and proper monitoring practices is recommended.
- Common Mild Side Effects: The most common side effects are transient and mild scalp irritation or dryness. Though these reactions often resolve on their own, they also respond well to dose adjustment or calming adjunctive treatments. Patients with sensitive skin should avoid formulas containing irritants like propylene glycol or parabens.[16]Lee, S.W, Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in … Continue reading,[17]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray … Continue reading
- When to Contact Your Doctor: More serious side effects or reactions are rare but possible and require prompt medical intervention. These include severe scalp irritation, allergic reactions (blistering, swelling, and redness), and systemic effects like mood changes, sexual dysfunction, or gynecomastia.[18]Suchonwanit, P., Iamsumang, W., Leerunyakul, K. (2020). Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: a review of the current literature. Journal … Continue reading,[19]Cheng Zhou et al. (2025). Efficacy and safety of topical finasteride spray solution in the treatment of Chinese men with androgenetic alopecia: A phase III, multicenter, randomized, double-blind, … Continue reading
- Topical vs. Oral Finasteride Safety: Clinical data show that topical finasteride has a significantly lower risk of sexual side effects, typically less than 2% compared to 8-15% with oral formulations. This is attributed to the targeted delivery of topical preparations that limit systemic exposure while providing localized scalp treatment.[20]Mysore, V. (2012). Finasteride and sexual side effects. Indian Dermatology Online Journal, 3(1), 62. https://doi.org/10.4103/2229-5178.93496.
- Who Should Avoid Topical Finasteride: Topical finasteride is not suitable for women who are pregnant or nursing due to the risk of birth defects or infant harm. Additionally, individuals with known sensitivity, scalp infections, or certain autoimmune diseases may be ineligible for use. Consult with a doctor before beginning treatment.
Closing Thoughts
Topical finasteride is an important development in hair loss therapeutics, providing comparable results with a superior safety profile to oral medications. In today’s market, Ulo’s individualized, clinically rigorous approach sets a benchmark for comprehensive care, outperforming competitors primarily focused on budget or convenience factors.
Successful treatment with topical finasteride requires patience and persistence. Advances in hair restoration technology suggest that tailored medicine, rather than standardized approaches, represents the future. Ready to begin your own journey toward hair restoration?
Visit Ulo today to see how customized topical finasteride can enhance your results.
Frequently Asked Questions
Do I need a prescription for topical finasteride?
Yes, topical finasteride is a prescription-only medication in the USA. This ensures proper diagnosis, medical supervision, dosage, and quality control. Reputable telehealth providers like Ulo make the prescription process quick and easy via video consultations with licensed doctors specialized in hair restoration therapies. These elite platforms also offer ongoing medical supervision to monitor side effects and adjust treatment for optimization.
Avoid online platforms that market finasteride products without a prescription or those that fail to perform a proper medical consultation before prescribing treatment. Reputable platforms employ board-certified doctors licensed to practice in your state of residence with credentials clearly displayed or offered upon request. Sourcing topical finasteride products from unauthorized vendors without a prescription risks hazardous side effects and ineffectiveness.
Can topical finasteride be used with other hair loss treatments?
Yes, topical finasteride can be beneficially combined with treatments like minoxidil, a popular additive in many available products. These formulas often pair finasteride with additional synergistic ingredients such as antioxidants, vasodilators, antifungals, and absorption enhancers.
Topical finasteride can also be safely used in conjunction with most non-prescription hair products, hair transplants, and low-level laser treatments. However, it should not be combined with oral DHT blockers (i.e., oral finasteride or dutasteride) due to the risk of systemic side effects. Medical approval is necessary before starting new treatments.
When will I see results with topical finasteride?
Most patients report stabilization of hair loss after 3-4 months, with notable regrowth in 6-12 months. Shedding within the first 6-8 weeks of starting treatment is considered normal and indicative of the treatment’s efficacy. Because DHT suppression takes time to significantly impact hair growth cycles, consistency and patience are essential for optimal treatment outcomes.
Treatment outcomes can vary between individuals. Consult a doctor to discuss influencing factors and form realistic treatment expectations. This grounded approach facilitates better long-term adherence and more desirable results.
Is topical finasteride suitable for women?
Although the FDA has not yet authorized topical finasteride to treat AGA in women, it is occasionally prescribed for off-label use in postmenopausal patients with female pattern hair loss (FPHL). Because topical finasteride risks fewer hormonal adverse effects than oral finasteride, it is considered a safer option in these cases.
However, women of reproductive age are often recommended to completely avoid finasteride due to the risk of severe birth defects from exposure while pregnant. Non-pregnant women who may plan for pregnancy in the future should abstain from finasteride use unless under strict medical supervision with suitable contraception established. Newer low-dose formulations designed for women show promise, although more study is needed to verify long-term viability.
Ultimately, women considering finasteride therapy should speak with an experienced clinician to go over their medical history, weigh the benefits and risks, and review alternative treatment options, such as FDA-approved topical minoxidil.
What is the cost of topical finasteride without insurance?
The average monthly cost of topical finasteride without insurance can vary from $35 to $90, depending on the source, formulation, and services rendered. Direct-pay telehealth providers tend to offer lower rates than conventional in-person dermatology clinics with combined pharmacy fees. Bespoke formulas with advanced excipients can command higher prices. Patients should account for continued costs to budget for long-term treatment, which is often required for beneficial outcomes.
What will happen if I stop using topical finasteride?
If regular topical finasteride treatment is discontinued, hair loss is expected to return gradually within 2-3 months of cessation. Most patients report a rebound to their baseline hair loss level within 6-12 months. Because this regression is gradual, patients have some flexibility regarding therapy breaks or medication transitions if needed.
Does topical finasteride have any drug interactions?
Compared to oral finasteride, topical formulations have few contraindications due to lower systemic absorption rates. For your safety, inform your prescribing doctor of all current medications, especially blood thinners and other hormonal drugs. While topical finasteride is often safely combined with common medications, there may be exclusions.
How do I store topical finasteride?
Topical finasteride should be kept at room temperature out of direct light, moisture, and heat. With proper storage, most products maintain stability for 12-24 months. While refrigeration isn’t required, it may boost shelf life in temperate climates. Be sure to check expiration dates and safely dispose of expired products.
Can I travel while on topical finasteride?
Yes, prescribed topical finasteride may be stored in carry-on luggage during domestic travel. When traveling internationally, it is recommended to bring the original prescription and label. Be mindful of TSA restrictions on liquid volumes over 3.4 oz.
How is topical finasteride applied?
Topical finasteride is applied directly to a clean and dry scalp with the product applicator. While specific instructions vary with delivery method, in general, the hair should be parted to expose the scalp and allow targeted application to the desired areas. Patients should consult product instructions for precise indications and dosing protocols. Avoid wetting hair for several hours after application, and thoroughly wash hands after use.
References[+]
References ↑1 Jang, W.S., Son, I.P., Yeo, I.K., Park, K.Y., Li, K., Kim, B.J., Seo, S.J., Kim, M.N., Hong, C.K. (2013). The Annual Changes of Clinical Manifestation of Androgenetic Alopecia Clinic in Korean Males and Females: A Outpatient-Based Study. Annals of Dermatology. 25(2). 181-188. Available at: https://doi.org/10.5021/ad.2013.25.2.181 ↑2 Duran, R, C-D., Martinez-Ledesma, E., Garcia-Garcia, M., Gauzin, D.B., Sarro-Ramirez, A., Gonzalez-Carillo, C., Rodriguez-Sardin, D., Fuentes, A., Cardenas-Lopez. (2024). The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia. International Journal of Molecular Sciences. 25(5). 2542. Available at: https://doi.org/10.3390/ijms25052542 ↑3, ↑4, ↑6, ↑16 Lee, S.W, Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in Dermatology. 17(4). 457-463. Available at: PMID: 29601622 ↑5, ↑7, ↑17 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑8 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑9, ↑18 Suchonwanit, P., Iamsumang, W., Leerunyakul, K. (2020). Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: a review of the current literature. Journal of Dermatological Treatment, 33(2), 643–648. Available at: https://doi.org/10.1080/09546634.2020.1782324 ↑10, ↑12 B. Piraccini et al. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology, 36: 286 – 294. https://doi.org/10.1111/jdv.17738 ↑11, ↑13 Cheng Zhou et al. (2025). Efficacy and safety of topical finasteride spray solution in the treatment of Chinese men with androgenetic alopecia: A phase III, multicenter, randomized, double-blind, placebo-controlled study. Chinese medical journal. Available at: https://doi.org/10.1097/CM9.0000000000003495 ↑14, ↑15 A. Rossi et al. (2020). Efficacy of Topical Finasteride 0.5% vs 17α-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients. Dermatology Practical & Conceptual, 10 (2020). https://doi.org/10.5826/dpc.1002a39 ↑19 Cheng Zhou et al. (2025). Efficacy and safety of topical finasteride spray solution in the treatment of Chinese men with androgenetic alopecia: A phase III, multicenter, randomized, double-blind, placebo-controlled study. Chinese medical journal. Available at: https://doi.org/10.1097/CM9.0000000000003495 ↑20 Mysore, V. (2012). Finasteride and sexual side effects. Indian Dermatology Online Journal, 3(1), 62. https://doi.org/10.4103/2229-5178.93496. Oral finasteride remains the most clinically validated medication for androgenic alopecia (AGA). Over 20 years of trials and follow-up data show consistent, predictable outcomes in slowing miniaturization and restoring density, particularly in the vertex and mid-scalp.
Yet, despite this wealth of data, online forums and “miracle” before-and-after photos often distort expectations. These transformations represent the upper end of the response curve, while most users experience more moderate, steady results that unfold over months to years.
This article explores what realistic regrowth looks like with oral finasteride, how to interpret finasteride before and after photos critically, and what to expect when used correctly and consistently.
What is Oral Finasteride?
Oral finasteride is a type II 5ɑ-reductase inhibitor that reduces the conversion of testosterone to dihydrotestosterone (DHT), the key androgen responsible for hair follicle miniaturization in AGA.
Originally approved by the FDA at a dose of 1 mg for male pattern hair loss, oral finasteride has since become widely known as the gold-standard treatment for hair loss. Its primary mechanism is systemic DHT suppression, lowering circulating levels by roughly 60-70% and scalp DHT by up to 65%.[1]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading
Mechanism of Action
As mentioned above, finasteride is a competitive inhibitor of the 5ɑ-reductase enzyme, specifically targeting the type II (and to a lesser extent, type III) isoenzymes.[2]Zito, P.M., Bistas, K.G., Patel, P., Syed, K. (2024). Finasteride. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513329/
Treatment with finasteride leads to a significant decrease in both scalp and serum DHT concentrations. This effect is dose-dependent and has been observed as early as 28 days after starting treatment.[3]Dallob, A.L., Sadick, N.S., Unger, W., Lipert, S., Geissler, L.A., Gregoire, S.L., Nguyen, H.H., Moore, E.C., Tanaka, W.K. (1994). The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp … Continue reading Lower DHT levels in the scalp prevent androgen-dependent miniaturization of hair follicles, promoting the maintenance and improvement of hair follicle size and number in the anagen (growth) phase.
Unlike topical formulations, oral finasteride exerts a systemic effect. More comprehensive, but also more likely to cause side effects in sensitive individuals.
Adverse Effects
The most commonly reported adverse effects of oral finasteride are sexual in nature, including decreased libido, erectile dysfunction, and reduced ejaculatory volume. These occur in a range of users depending on the study and determination of what a side effect is (<1% to 25%).[4]Traish, A.M., Hassani, J., Guay, A.T., Zitzmann, M., Hansen, M.L. (2011). Adverse side effects of 5ɑ-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and … Continue reading
Other potential side effects include psychological and neuropsychiatric effects and post-finasteride syndrome (PFS). PFS refers to persistent sexual, neurological, and physical symptoms that continue after stopping finasteride. However, the existence and prevalence of PFS are debated, with some studies suggesting a possible genetic predisposition.[5]Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the safety of finasteride correlated with its route of administration: topical versus oral? A pharmacovigilance study with data from … Continue reading
You can read in more depth about the potential side effects in our oral finasteride ultimate guide.
Why Expectation Setting Matters
Finasteride is powerful and predictable, but even the best data can get lost when you see incredible results online. Online before-and-after posts tend to showcase outliers, individuals who happen to respond at the extreme high end of the efficacy curve.
Meanwhile, clinical averages show that:
- Most men stop or reverse their hair loss progression.
- Around 65-80% experience visible thickening after 12-24 months.
- Only a small subset achieves “complete restoration”.
Unrealistic expectations can lead to disappointment, early discontinuation, or cycling through treatments unnecessarily. Grounding expectations in clinical probabilities rather than forum anecdotes helps users stay consistent long enough to see results.
What’s Possible ≠ What’s Probable
While oral finasteride can produce impressive regrowth, most users experience gradual thickening and stabilization rather than dramatic restoration.
Treatment outcomes typically follow a distribution pattern:
- A minority are “hyper responders”, achieving rapid and substantial visible improvement.
- The majority notice moderate, progressive gains, such as fuller density or slowed shedding.
- A smaller subset experience arrest of hair loss but remain unsatisfied with new hair growth.[6]Dhurat, R., Mathapati, S. (2015). Response to Microneedling Treatment in Men with Androgenetic Alopecia Who Failed to Respond to Conventional Therapy. Indian Journal of Dermatology. 60(3). 260-263. … Continue reading
Factors influencing response:
- Age and duration of hair loss: Finasteride efficacy is higher in younger patients and those with less advanced disease at treatment initiation.[7]Camacho, F.M., Garcia-Hernandez, M.J., Fernandez-Crehuet, J.L. (2008). Value of hormonal levels in patients with male androgenetic alopecia treated with finasteride: better response in patients under … Continue reading Starting treatment at age 40 or higher is an independent risk factor for insufficient efficacy.[8]Yoshitake, T., Takeda, A., Ohki, K., Inoue, Y., Yamawaki, T., Otsuka, S., Akimoto, M., Nemoto, M., Shimakura, Y., Sato, A. (2015). Five-year efficacy of finasteride in 801 Japanese men with … Continue reading
- Consistency: Missing doses or cycling off can interrupt the stabilization of hair loss.
- Adjunct therapies: Combining finasteride with other therapies like topical minoxidil can improve results.[9]Hu, R., Xu, F., Sheng, Y., Qi, S., Han, Y., Miao, Y., Rui, W., Yang, Q. (2015). Combined treatment with oral finasteride and topical minoxidil in male androgenetic alopecia: a randomized and … Continue reading
- Genetics: Variations in the androgen receptor (AR) gene sensitivity affect responsiveness.[10]Keene, S., Goren A. (2011). Therapeutic hotline. Genetic variations in the androgen receptor gene and finasteride response in women with androgenetic alopecia mediated by epigenetics. Dermatologic … Continue reading
Strong Evidence = Predictable Results
Unlike many newer (and even potentially stronger acting treatments), oral finasteride benefits from decades of standardized data, making outcomes highly predictable. A good example of this is comparing finasteride to dutasteride. Finasteride is extremely well-studied; therefore, we can usually predict the type of results we will see. Dutasteride, on the other hand, has studies showing it can outperform even the likes of finasteride in terms of hair regrowth. But the number of clinical studies is lacking, meaning that it is more difficult to predict a consistent outcome.
Figure 1: For dutasteride, a wide, shallow curve represents a much wider spread of outcomes. For finasteride, a narrow bell curve shows predictable outcomes.
This predictability helps clinicians set clear, realistic expectations and provides users with confidence that consistency will lead to steady results.
Real Case Studies: Oral Finasteride Before and After
The examples below help see what’s possible and do not represent what every user should expect. They are purely anecdotal and not independently verified by us or any qualified third party.
Case Summary #1: Male (23), 3 Months on Oral Finasteride 1 mg (then every other day) + Topical Minoxidil Foam (twice daily)
Source: u/Prize-Leg2544 via r/tressless.
This 23-year-old male took oral finasteride 1 mg daily for 2 months before titrating down to 1 mg every other day due to side effects. He also applied topical minoxidil foam twice daily, initially to the whole scalp but later exclusively to the hairline. He noted an initial shedding phase and early side effects on finasteride in the first month of treatment. In the second month, he described visible thickening while still on 1 mg daily. By month 3, he reduced finasteride to every other day and continued with minoxidil to maintain the progress. He reported marked improvement in hairline and density over the 3 months.
The user also described early transient effects, including testicular ache (first week), watery semen, heightened libido, and less intense orgasm, which resolved after dialing the finasteride down to every other day.
Case Summary #2: Male (25), 1-Year Course of Finasteride (1 mg) + Topical Minoxidil (1–2x Daily) + Monthly Dermarolling
Source: u/zacboring via r/malehairadvice.
This male Redditor started treatment at age 24 after severe crown thinning since he was about 18. His one-year course consisted of finasteride 1 mg daily (generic), topical liquid minoxidil 1–2/day, and dermarolling roughly monthly (post-shower, before nightly minoxidil). He supplemented with vitamin D and occasional biotin, and he regularly used argan oil after showers to limit flaking.
The patient noted a strong early response, but felt his results peaked roughly halfway into treatment. He stopped minoxidil for 1 month and missed some doses in the months leading up to the post. His photos indicated a significant improvement in density compared to baseline, particularly at the hairline and the top. He noted more coverage at the crown but felt it was still noticeably weaker than desired. The patient reported no sexual side effects or skin issues.Case Summary #3: Male (24), 6 Months on Oral Finasteride 1 mg Daily + Oral Minoxidil 2.5 mg Daily
Source: u/LongjumpingDurian429 via r/tressless.
This 24-year-old male’s intervention consisted of oral finasteride 1 mg once daily and oral minoxidil 2.5 mg once daily. The user noted the emergence of small baby hairs at the hairline and temples at month 2 of treatment. By months 4-5, he reported noticeable thickening, with longer eyelashes and denser eyebrows. By month 6, the user showed clear improvement, as evidenced by his wet-hair photos and day-to-day density. He reported visible density gains at the hairline with better coverage under bright light, with his crown improved from baseline, though not fully closed. He reported no side effects, apart from brief mild erectile difficulty early on that resolved.
Case Summary #4: Male (25), 3 Months on Oral Finasteride 1 mg Daily + Oral Minoxidil 2.5 mg Daily (added at month 2) + Ketoconazole Shampoo 3x/week + Microneedling 1.5 mm Weekly
Source: u/Buchaill-Bo via r/tressless.
This 24-year-old male’s intervention was oral finasteride 1 mg daily for 3 months, oral minoxidil 2.5 mg daily (added 2 weeks before the 3-month update), ketoconazole shampoo (Nizoral) about three times weekly, and dermaroller 1.5 mm once weekly over the whole scalp.
The user described gradual thickening over the 3-month finasteride course, with minoxidil introduced at month 2.5. He reported a noticeable improvement in density at the hairline and mid-scalp compared with baseline. He described a possible mild libido reduction on finasteride and thinning of the eyebrows thinned on finasteride, which appeared fuller after starting minoxidil. He reported no other significant effects.
Case Summary #5: Male (38), 7 Months on Oral Finasteride + Topical Finasteride + Topical Minoxidil + Weekly Microneedling
Source: u/Moonrocks321 via r/tressless.
This 38-year-old male started the current treatment with oral finasteride 1 mg daily and topical minoxidil (strength/frequency not specified). He added topical finasteride (concentration not specified) and weekly microneedling at 0.25 mm in month 5. The patient reported a slight but noticeable improvement vs. baseline at month 5 and, at month 7, likewise described his progress as modest. In the 7 months, he achieved stabilization with modest density improvement, most visible anteriorly, with the crown remaining limited. He reported no side effects from finasteride and minoxidil.
Case Summary #6: Male (28), 10 Years on Oral Finasteride + 4–5 Months on Oral Minoxidil
Source: u/DatBronzeGuy via r/tressless.
This 28-year-old male reported on his 10-year treatment progress on oral finasteride 1 mg daily, before adding oral minoxidil 2.5 mg twice daily 4-5 months before the progress report. He confirmed no dermarolling or topical agent application. The patient reported that, between the ages of 18 and 27, he was on finasteride monotherapy, achieving significant regrowth and strong maintenance. Towards the end of that period, he reported subtle renewed thinning, prompting him to add oral minoxidil. Within 4 months thereafter, his hair density and thickness nearly doubled.
He reported good tolerance to finasteride with no noticeable side effects. He added that oral minoxidil caused mild generalized hypertrichosis (slightly darker chest/back hair, fuller beard, higher cheek/temple hairs), but was otherwise tolerated.
Case Summary #7: Male (28), 2–3 Months on Oral Finasteride 1 mg Monotherapy
Source: u/danish0001 via r/tressless.
This 28-year-old male’s intervention included oral finasteride 1 mg daily, weekly microneedling with dermastamp 1.0–1.5 mm, and supplementation with vitamin D3 + K2, zinc, folate, magnesium glycinate, and ketoconazole 2% shampoo. Additionally, he made lifestyle adjustments, including going to the gym 2x/week and starting a higher-protein diet.
He noted a mild shedding phase in the first month of treatment. By month 2, he described the first visible tiny regrowth after a buzz cut, by which point the shedding had reduced. Overall, the patient described early regrowth at the hairline and thinning zones. He reported no side effects on finasteride 1 mg daily, stating that libido and energy have remained unchanged or improved.
Case Summary #8: Male (unspecified age), 3 Years on Oral Finasteride 1 mg Daily + Topical Minoxidil
Source: u/AthleteAfraid7844 via r/tressless.
This male Redditor’s intervention over 3 years was oral finasteride 1 mg daily and topical minoxidil (strength not specified).
In the first year, he noted steady cosmetic improvement, followed by continued thickening and maintenance of gains over years 2-3. Overall, the user reported substantial density gain, with perceived improvement in the hairline and temples over time. He mentioned no significant side effects and confirmed his sex drive had remained unchanged throughout treatment. He noted that the minoxidil sometimes caused dandruff and that on finasteride, he required greater mental focus to push weight-lifting sets to failure.
Case Summary #9: Male (early 30s), 3.5 Months on Oral Finasteride 1.25 mg Daily
Source: u/Ill-Foundation2637 via r/tressless.
This male, in his early 30s, opted for a treatment consisting of oral finasteride monotherapy at 1.25 mg daily (5 mg tablets quartered). He described a mild daily shedding phase within the first month of treatment. By month 2.5, he reported his first visible thickening. At the 3.5-month progress report, the user achieved a fuller crown with reduced scalp show-through. He reported no side effects.
Case Summary #10: Male (25), 5.5 Months on Oral Finasteride 1.25 mg Daily + Oral Minoxidil 5 mg DailySource: u/Dantheghost012 via r/tressless.
This 25-year-old South Asian male’s intervention was oral finasteride 1.25 mg daily (5 mg tablets quartered) and oral minoxidil, starting at 2.5 mg daily, titrated to 5 mg after 3 months. He described a substantial shedding phase, particularly in the first 3 weeks of month 3. The following month, he reported progressive thickening and density gains with improved coverage. As of month 5.5, he is still improving and continuing both agents unchanged. Overall, he reported a marked increase in density, with a thicker texture, and the crown and hairline both looked fuller than baseline. He reported no side effects on either dose level for finasteride or oral minoxidil.
Probable Regrowth Timeline
Based on the available research (which you can take a look at with our research tables here), we have created a probable hair regrowth timeline.
Figure 2: Typical regrowth for oral finasteride 1 mg.
Months 0-3: Adjustment Phase
In the first three months, most users experience minimal visible change. Finasteride actively lowers scalp and serum DHT levels, initiating follicle recovery. Some users notice a temporary increase in shedding as older hairs are shed to make way for new growth, while others observe subtle stabilization of hair loss.
Months 3-6: Reduction in Shedding
By this stage, a clear reduction in hair shedding often becomes noticeable. Although visible thickening remains limited for many, the treatment is beginning to normalize hair cycling. Early responders may report small areas of improved density, particularly in the crown or vertex.
Months 6-12: Early Cosmetic Improvements
Between 6 and 12 months, most responders start to see visible regrowth. Hair density gradually improves, strands appear thicker, and areas of thinning begin to fill in. The crown region typically shows improvement sooner than the frontal hairline, which tends to respond more slowly. Continued adherence is essential during this phase to sustain progress.
Months 12-24: Peak Response Period
During the second year, responders generally achieve their maximum results. Hair density, coverage, and stabilization reach their highest levels, with further visible gains possible in some individuals. Those starting with more advanced thinning usually experience consolidation rather than full growth.
Months 24+: Maintenance and Long-Term Stability
After two years, most users reach a plateau in visible improvement. Continued daily use of finasteride is needed to preserve these gains, as stopping the medication often results in gradual hair loss returning to baseline within 6-12 months. Some people combine treatments at this point to achieve further outcomes.
For most users, oral finasteride provides progressive, steady improvements over time, with long-term commitment being the key to maintaining results.
Setting Realistic Expectations
It’s important to set realistic expectations when starting any treatment. Finasteride primarily stabilizes hair loss during the early months, with noticeable growth typically developing later in the treatment course. True cosmetic improvement usually unfolds gradually over 12 to 24 months rather than weeks, reflecting the natural pace of the hair growth cycle.
Clinical trials suggest that most men experience visible thickening and halting of progression rather than full restoration, effectively “turning back the clock” by two to five years in terms of density and coverage.[11]Kaufman, K.D., Olson, E.A., Whiting, D., Savin, R., DeVillez, R., Bergfeld, W., Price, V.H., Van Neste, D., Roberts, J.L., Hordinsky, M., Shapiro, J., Binkowitz, B., Gormley, G.J. (1998). Finasteride … Continue reading,[12]Price, V.H., Roberts, J.L., Hordinsky, M., Olsen, E.A., Savin, R., Bergfeld, W., Fiedler, V., Lucky, A., Whiting, D.A., Pappas, F., Culbertson, J., Kotey, P., Meehan, A., Waldstreicher, J. (2000). … Continue reading,[13]Rossi, A., Mari, E., Scarno, M., Garelli, V., Maxia, C., Scali, E., Iorio, A., Carlesimo, M. (2012). Comparative effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: a … Continue reading
Consistent photographic tracking using identical lighting and angles can help visualize these incremental gains. Long-term adherence is crucial, as discontinuation typically leads to renewed miniaturization and hair loss within several months of stopping treatment.
Practical Takeaways
- Oral finasteride remains the most effective DHT blocker for male AGA
- Decades of clinical data make results highly predictable and durable
- The safety profile is well-established, with sexual side effects uncommon and often reversible
- Combining treatments can improve outcomes, especially for advanced loss.
Final Thoughts
Oral finasteride remains the most extensively studied and clinically validated treatment for androgenic alopecia, offering predictable outcomes for most users through gradual thickening, stabilization of shedding, and preservation of existing hair rather than dramatic restoration. While newer options like oral minoxidil are gaining popularity, they remain off-label despite growing supportive evidence and should be used under medical supervision.
The key to achieving and maintaining meaningful results lies in consistency, patience, and realistic expectations, recognizing that hair regrowth is a slow, cumulative process measured over months and years. By grounding expectations in clinical evidence rather than anecdotal extremes, patients can make informed, balanced decisions and stay committed to a long-term plan that maximizes both safety and effectiveness.
References[+]
References ↑1 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. International Journal of Clinical Pharmacology and Therapeutics. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑2 Zito, P.M., Bistas, K.G., Patel, P., Syed, K. (2024). Finasteride. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513329/ ↑3 Dallob, A.L., Sadick, N.S., Unger, W., Lipert, S., Geissler, L.A., Gregoire, S.L., Nguyen, H.H., Moore, E.C., Tanaka, W.K. (1994). The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. Journal of Clinical Endocrinology and Metabolism. 79(3). 703-706. Available at: https://doi.org/10.1210/jcem.79.3.8077349 ↑4 Traish, A.M., Hassani, J., Guay, A.T., Zitzmann, M., Hansen, M.L. (2011). Adverse side effects of 5ɑ-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. The Journal of Sexual Medicine. 872-884. Available at: https://doi.org/10.1111/j.1743-6109.2010.02157.x ↑5 Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the safety of finasteride correlated with its route of administration: topical versus oral? A pharmacovigilance study with data from the United States Food and Drug Administration adverse event reporting system. International Journal of Dermatology. Available at: https://doi.org/10.1111/ijd.17957 ↑6 Dhurat, R., Mathapati, S. (2015). Response to Microneedling Treatment in Men with Androgenetic Alopecia Who Failed to Respond to Conventional Therapy. Indian Journal of Dermatology. 60(3). 260-263. Available at: https://doi.org/10.4103/0019-5154.156361 ↑7 Camacho, F.M., Garcia-Hernandez, M.J., Fernandez-Crehuet, J.L. (2008). Value of hormonal levels in patients with male androgenetic alopecia treated with finasteride: better response in patients under 26 years old. British Journal of Dermatology. 158(5). 1121-1124. Available at: https://doi.org/10.1111/j.1365-2133.2008.08509.x. ↑8 Yoshitake, T., Takeda, A., Ohki, K., Inoue, Y., Yamawaki, T., Otsuka, S., Akimoto, M., Nemoto, M., Shimakura, Y., Sato, A. (2015). Five-year efficacy of finasteride in 801 Japanese men with androgenetic alopecia. The Journal of Dermatology. 42(7). 735-738. Available at: https://doi.org/10.1111/1346-8138.12890 ↑9 Hu, R., Xu, F., Sheng, Y., Qi, S., Han, Y., Miao, Y., Rui, W., Yang, Q. (2015). Combined treatment with oral finasteride and topical minoxidil in male androgenetic alopecia: a randomized and comparative study in Chinese patients. Dermatologic Therapy. 28(5). 303-308. Available at: https://doi.org/10.1111/dth.12246 ↑10 Keene, S., Goren A. (2011). Therapeutic hotline. Genetic variations in the androgen receptor gene and finasteride response in women with androgenetic alopecia mediated by epigenetics. Dermatologic Therapy. 24(2). 296-300. Available at: https://doi.org/10.1111/j.1529-8019.2011.01407.x. ↑11 Kaufman, K.D., Olson, E.A., Whiting, D., Savin, R., DeVillez, R., Bergfeld, W., Price, V.H., Van Neste, D., Roberts, J.L., Hordinsky, M., Shapiro, J., Binkowitz, B., Gormley, G.J. (1998). Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. Journal of the American Academy of Dermatology. 39(4 Pt 1), 578-589. Available at: https://doi.org/10.1016/s0190-9622(98)70007-6 ↑12 Price, V.H., Roberts, J.L., Hordinsky, M., Olsen, E.A., Savin, R., Bergfeld, W., Fiedler, V., Lucky, A., Whiting, D.A., Pappas, F., Culbertson, J., Kotey, P., Meehan, A., Waldstreicher, J. (2000). Lack of efficacy of finasteride in postmenopausal women with androgentic alopecia. Journal of the American Academy of Dermatology. 43(5 Pt 1). 768-776. Available at: https://doi.org/10.1067/mjd.2000.107953 ↑13 Rossi, A., Mari, E., Scarno, M., Garelli, V., Maxia, C., Scali, E., Iorio, A., Carlesimo, M. (2012). Comparative effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: a two-year study. International Journal of Immunopathology and Pharmacology. 25(4). 1167-1173. Available at: https://doi.org/10.1177/039463201202500435 Millions worldwide experience hair loss, with androgenic alopecia (AGA) as the leading cause. Also known as male- or female-pattern hair loss, AGA becomes more prevalent with age. It affects a majority of men over the age of 50 and a substantial number of postmenopausal women. Hair thinning and balding due to AGA is not just a cosmetic concern but can significantly detract from self-esteem and overall quality of life.
Among popular hair restoration treatments, topical dutasteride is a breakthrough solution to the serious side effects and plateaued results many experience. This potent DHT blocker offers more targeted therapeutic benefits with minimal systemic exposure and reduced risk. However, not all topical dutasteride products meet quality standards, so specialized guidance is key to selecting the right formula.
The PhD-directed board of experts at Perfect Hair Health draws from the latest clinical data and verified customer feedback to deliver this comprehensive guide to the best topical dutasteride products available in 2026. Through our research collaboration with leading innovators in the field, including our partnership with Ulo, we provide unique insights into the most advanced hair restoration protocols.
Read on for our detailed analysis and evidence-based product recommendations.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Quick View: 8 Best Topical Dutasteride Products
Here’s a brief comparison of our most highly recommended topical dutasteride products. Continue reading for in-depth reviews.
Product Price Dutasteride % Delivery Method Key Feature Ulo $84+/month 0.02%-0.2% Solution PhD-developed extensive customization XYON Health $129+/month 2% Gel Patented controlled-release technology Strut Health $69/month Up to 0.1% Gel Alcohol-free and customizable Blends ~$45/month (90-day supply) 0.3% Gel Proprietary hydrating formula Happy Head $59-89+/month Up to 0.3% Solution Potency and customization Maximus $54.99 0.1% Gel Customizability, including the addition of antihistamines Miiskin $30-59 + Rx cost Variable, custom Solution No subscription required Musely $33/month 0.3% Solution Lowest cost option Science and Benefits of Topical Dutasteride
To begin, we will briefly explore the scientific background of topical dutasteride, including how it works to treat AGA and the distinct benefits of topical preparations.
Mechanism of Action
Topical dutasteride is valued as a breakthrough treatment for AGA due to its powerful and targeted effects on the molecular levels of hair loss. The root cause of AGA is the testosterone-derived hormone dihydrotestosterone (DHT), which adheres to hair follicles and leads to follicular degeneration and hair loss over time. Dutasteride is classified as a DHT blocker, reducing DHT levels on the scalp to combat hair loss.[1]R. Cuevas-Díaz Durán., Martinez-Ledesma, E., Garcia-Garcia, M., Gauzin, D.B., Sarro-Ramirez, A., Gonzalez-Carrillo, C., Rodriguez-Sardin, D., Fuentes, A., Cardenas-Lopez, A. (2024) The Biology and … Continue reading,[2]Dhurat, R., Sharma, A., Rudnicka, L., Kroumpouzos, G., Kassir, M., Galadari, H., Wollina, U., Lotti, T., Golubović, M., Binic, I., Grabbe, S., & Goldust, M. (2020). 5‐Alpha reductase … Continue reading
Among DHT-blocking treatments, dutasteride is considered the most potent due to its dual enzyme activity. Testosterone is converted into DHT through two forms of 5α-reductase enzymes: Type I is located in the skin and sebaceous glands, and Type II is in the hair follicles. Finasteride inhibits only Type II for about 70% DHT reduction, while dutasteride inhibits both types to elicit greater DHT suppression (up to 93% in the skin and 99% in the follicles).[3]Arif, T., Dorjay, K., Adil, M., & Sami, M. (2017). Dutasteride in Androgenetic Alopecia: An Update.. Current clinical pharmacology, 12 1, 31-35. Available at: … Continue reading
Dutasteride’s powerful DHT inhibition leads to greater therapeutic outcomes in hair restoration. Clinical findings indicate dutasteride’s superior effects on hair count and mass in comparison to finasteride in terms of both quantitative and temporal metrics. While finasteride’s benefits tend to plateau after about 12 months of treatment, dutasteride demonstrates continued improvements up to 12 months and beyond.[4]Harcha, W., Martínez, J., Tsai, T., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy … Continue reading
Topical vs. Oral Advantages
Studies show that topical dutasteride is a safer option than oral with comparable and often better treatment outcomes. When applied locally to the scalp, dutasteride delivers targeted treatment with limited systemic exposure and a reduced risk of serious side effects. If present, adverse reactions to topical dutasteride tend to be transient and mild, in contrast to oral dutasteride’s potential systemic side effects like sexual dysfunction and mood disorders.[5]Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 mg) in … Continue reading,[6]Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., & Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology, 240, 833 – 843. Available at: … Continue reading
This and other clinically proposed advantages of topical dutasteride are briefly summarized as follows:
- Safe and Targeted Treatment: Oral dutasteride has systemic exposure through the bloodstream and therefore runs the risk of more serious side effects. Meanwhile, topical treatment targets only the scalp, lowering the risk of severe adverse reactions. Minor irritation at the site of application may occur and tends to resolve quickly.
- Flexible Dosing: Topical formulations offer greater dose flexibility through modified concentrations, in contrast to the usually fixed doses of oral preparations. Common topical offerings range from a maintenance dose of 0.02% dutasteride to advanced therapies of 2%, allowing dose-dependent customization.
- Increased Personalization: Topicals can easily integrate synergistic treatments like vasodilators (minoxidil), absorption enhancers (tretinoin), and anti-inflammatories (melatonin) for combination therapies that outperform dutasteride alone. These add-ons are prescribed based on patients’ individual needs and treatment goals.
Plus, patients have a choice of different delivery methods (e.g., sprays, gels, solutions), which can offer greater convenience and adherence.
- Long-term Outlook: Topical use is considered safer for long-term treatment due to its reduced systemic effects. Because hair restoration treatments’ benefits build with time, their suitability for prolonged use is paramount.
Combined, these factors locate topical dutasteride as a more effective and flexible hair restoration intervention with a greater safety profile than its oral counterpart.[7]Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 mg) in … Continue reading,[8]Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., & Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology, 240, 833 – 843. Available at: … Continue reading,[9]Radhakrishnan, P., Mariyappan, G., Karthi, J., & Jaisumi, K. (2024). Formulating, Optimizing and Evaluation of Dutasteride Loaded Insitu-Emulgel for Androgenetic Alopecia. International Journal … Continue reading
Choosing the Best: What We Looked For
To help navigate the topical dutasteride market, we developed these quality benchmarks grounded in clinical expertise, scientific evidence, and patient feedback.
Clinical Efficacy
Quality dutasteride products demonstrate notable DHT reduction and improved hair growth within 3-6 months, with these effects continuing over 12 months or longer. The products recommended in this article were assessed based on documented DHT reductions, verified patient feedback, and peer-reviewed studies.
Safety Profile and Medical Oversight
Given the prescription status of topical dutasteride, we verified each telehealth provider’s legitimacy in terms of physician supervision, adherence to legal requirements, and adherence to sound safety protocols. These include proper diagnostic procedures before prescribing, as well as close follow-up care to monitor for side effects and adjust the treatment as needed for optimal outcomes. Trustworthy providers exclusively partner with licensed physicians specialized in hair restoration.
When it comes to quality assurance, suppliers must partner only with authorized domestic pharmacies to source their compounded medications. Complete ingredient transparency and labeling of allergens establishes acceptable safety profiles.
Personalization Options
Varied patient needs call for customizable treatments that exceed standard one-size-fits-all protocols. Outstanding brands provide a personalized approach through extensive customization options, including variable concentrations, adjuvant blends (e.g., minoxidil), and various delivery formats (e.g., solutions and controlled-release gels). These tailored therapies are physician-crafted to safely elicit the best results.
User Experience
Prolonged, uninterrupted treatment is necessary to achieve positive results, highlighting the importance of user comfort and satisfaction. Practical considerations, such as application convenience, precise instructions, manageable dose routines, and excellent customer support, all influence treatment adherence.
Cost Transparency
Because treatment sustainability also depends on affordability, we also examined billing models and price transparency. Premium suppliers have honest and reasonable pricing that covers primary treatment costs (prescriptions, consultations, deliveries, and monitoring), free of hidden fees that are common among telehealth providers.
Selecting Your Ideal Treatment
Begin by assessing your hair loss level and treatment objectives, determining whether moderate maintenance or intensive regrowth protocols are needed. Further considerations include application method preferences (gels, solutions, sprays, etc.), helpful adjuvants, and skin sensitivities. Budgetary limitations and your required level of physician involvement also come into play.
With these factors in mind, let’s dive into the following product reviews that highlight distinct approaches.
Our Top 8 Topical Dutasteride Products
#1: Ulo Dutasteride + Minoxidil Plus Rx
Pros: Cons: ✓ PhD-scientist developed with thorough customization ✗ USA-only service for prescription treatments ✓ Customizable concentrations with adjuvant ingredients ✓ Board-certified physician oversight, monitoring, and tailored treatment protocols ✓ Verified quality and safety ✓ Full price transparency and free shipping The top position goes to Ulo, a leader in clinical rigor and precision hair loss medicine. Known for their highly individualized treatments, cutting-edge protocols, and PhD oversight, Ulo’s offerings are among the most comprehensive and patient-centered available. Their dual topical dutasteride solution with minoxidil delivers potent DHT suppression and new growth stimulation, proven to be more effective than either therapy alone.
Ulo’s topical systems feature customization across the spectrum, from dutasteride concentration (0.02% for lighter therapy to 0.2% for more aggressive intervention) to adjuvants like absorption enhancers, antioxidants, and vasodilators. Premium services include licensed dermatologist consultations, tailored treatment plans, and continued medical oversight, with a starting monthly subscription price of $84 and no added fees.
All Ulo safety and medication sourcing practices adhere to the highest regulatory standards, including partnerships with certified domestic pharmacies and strict follow-up protocols. Full ingredient transparency and irritant-free formulations establish excellent safety profiles. Patients enjoy free prescription deliveries and responsive support through Ulo’s sleek telehealth platform.
Our Verdict: Ulo’s advanced dual formula harnesses the power of customized topical dutasteride to deliver the gold standard of hair loss therapy. With PhD-developed formulas, genuine customization, and complete medical oversight, this platform offers proven benefits through a transparent monthly subscription that starts at $84. Their dedication to upholding the highest standards of safe and effective care positions Ulo as the best choice for your hair restoration needs.
#2 XYON Topical Dutasteride
Pros: Cons: ✓ Proprietary SiloxysSystem™ Gel for timed release ✗ Premium pricing at $129/month ✓ Highest concentration (2% dutasteride) for aggressive protocols ✗ Limited customization options ✓ Physician oversight ✗ Not recommended for sensitive skin ✓ Service area includes the US and Canada XYON stands out for its innovative, technology-driven approach, exemplified by its proprietary SiloxysSystem Gel delivery base, which contains delayed-release topical dutasteride at a high concentration of 2%. This controlled-release method allows for a higher dutasteride concentration and more targeted treatment, protecting against systemic exposure and potentially serious side effects.
While this treatment is a strong option for advanced hair loss cases due to its potency, XYON emphasizes its advanced and proven delivery method over treatment customization features. Monotherapy involves a fixed dose of 2% dutasteride, which may be too strong for some patients. Excipients include ingredients like silicone that are not recommended for sensitive skin.
XYON’s premium price point reflects not only its forward-looking strategy but also its high medical standards, as the platform offers qualified physician support throughout the process.
Our Verdict: XYON’s topical dutasteride formulation delivers maximum treatment with minimal systemic risk through its unique delayed-release carrier gel. It is recommended for advanced hair loss patients who can tolerate higher doses and potential irritants, particularly those who don’t mind paying a premium for its advanced technology development.
#3: Strut Health Topical Dutasteride Gel
Pros: Cons: ✓ Good value at $69/month with full customization ✗ Lower maximum dutasteride concentration (0.1%) ✓ Alcohol-free, preservative-free options available ✗ Moderate doses may be insufficient for advanced hair loss cases ✓ Extensive customization of adjuvant ingredients ✗ Occasional gaps in customer service are cited ✓ Licensed medical oversight with hair loss specialization ✗ Service area excludes Arkansas Strut Health is a solid mid-tier option that balances accessible pricing with medically guided customization, emphasizing cost-effectiveness over potency. The preservative- and alcohol-free dutasteride gel contains a moderate 0.1% dutasteride, with tailored add-on options including minoxidil, biotin, and tretinoin at varying concentrations. Capped at such a middling dose, this gentle gel formula is best for mild to moderate hair loss patients with sensitive or dry skin.
Fluocinolone, a corticosteroid, is also available as an adjuvant. While leading providers like Ulo avoid such ingredients due to potential safety concerns, Strut provides appropriate safety disclaimers and maintains an overall emphasis on ingredient transparency, backed by medical oversight.
Strut’s subscription fee of $69 per month for topical dutasteride treatment is a competitive offering, covering consultations, follow-ups, and prescription shipping. Patients cite flexible cancellation policies and rapid home deliveries as notable benefits. We detected some mixed reviews regarding delayed customer service responses and logistical issues.
Our Verdict: Strut Health offers a compelling value proposition with its topical dutasteride gel, priced at only $69 per month, and features that rival those of more expensive providers. The gentle formula with a limited 0.1% dutasteride concentration suits patients with moderate treatment needs who are seeking a budget-friendly option with flexible add-ons and legitimate medical oversight.
#4: Blends Growth PRO Topical
Pros: Cons: ✓ Doctor-founded with hair restoration specialization ✗ 90-day supply requirement may not suit all patients ✓ Competitive pricing at ~$45/month (90-day supply) ✗ Fixed formula despite “personalized” marketing claims ✓ Preservative-free, alcohol-free formula with 5 active ingredients ✗ Includes latanoprost with limited long-term safety data for scalp use ✓ 0.3% dutasteride and 7.5% minoxidil offer powerful DHT inhibition ✗ Lacks true customization compared to leading competitors ✓ Proprietary hydrating gel base with advanced formulation Blends is a physician-founded company with a heavy focus on cutting-edge manufacturing methods and sophisticated formulations. Their small but specialized catalog includes this topical dutasteride treatment made with a proprietary gel base for timed medication release and scalp hydration. Free of alcohol and preservatives, the concentrated 0.3% dutasteride and 7.5% minoxidil formula offers powerful therapeutic effects.
Although Blends asserts the “personalized” and “customized” nature of its products, its Growth Pro Topical gel is actually a comprehensive fixed formula with three adjuvant ingredients in addition to dutasteride and minoxidil: tretinoin, latanoprost, and ketoconazole. Competitively priced at about $45/month (sold in 90-day batches), this offering stands out for its full ingredient transparency and high concentrations suited to aggressive treatment protocols.
However, the use of latanoprost poses concerns due to the lack of long-term studies confirming its safety and efficacy as a topical hair loss treatment. Despite marketing claims of customized protocols, Blends does not allow formulation adjustments to this fixed gel product. Although the monthly cost is lower than that of most leading brands of comparable quality, the minimum purchase requirement of a 90-day supply (~$135) may not suit all patients.
Our Verdict: This physician-crafted gel formula from Blends is competitively priced, featuring high concentrations of synergistic ingredients for advanced hair loss treatment. Its positioning as a mid-level selection rather than a top choice is due to its false personalization claims and unproven add-on ingredient.
#5: Happy Head Topical Rx Dutasteride & Minoxidil
Pros: Cons: ✓ Dermatologist oversight with ongoing support ✗ Limited dutasteride concentration options ✓ 0.3% dutasteride and 8% minoxidil formula with customizable add-ons, including ketoconazole ✗ Pricing increases after the initial months and can reach ~$109 ✓ Established brand with a 6-month money-back guarantee ✗ Some formulations contain potential irritants ✓ User-friendly platform and free consultation ✗ Shipping and customer service inconsistencies Happy Head represents a dependable option for targeted hair restoration, recognized for its dermatologist-backed and customizable treatments. This hybrid dutasteride-minoxidil solution offers a strong dose of 0.3% dutasteride with up to 8% minoxidil for effective DHT reduction. Elective adjuvants include retinoic acid and hydrocortisone. Anti-dandruff agent ketoconazole can be added for an additional fee.
While some clinicians warn against the use of topical corticosteroids like hydrocortisone, Happy Head seems to follow established medical safety protocols with appropriate disclaimers. Inactive ingredients like alcohol and propylene glycol can irritate and should be approached with caution. Highly concentrated DHT blockers with harsh excipients require close medical oversight, and patients with sensitive skin may want to seek other options.
Although transparent about costs, Happy Head’s variable pricing can grow quickly with treatment duration and formulation. Their topical dutasteride solution starts at $59 per month and shifts to $89 after the first six months. The anti-dandruff adjuvant can be added for $20, bringing the total to $109 per month, which exceeds the cost of some comparable providers. Mixed patient reviews cite issues with deliveries and logistical support.
Our Verdict: This combination treatment from Happy Head is a solid choice for patients in need of more aggressive treatment and concentrated formulas. Continued dermatologist oversight, personalization options, and potent formulations can help patients whose results may have stalled on other treatments. However, highly active ingredients and variable pricing may deter those seeking gentler therapies and fixed costs.
#6 Maximus Dutasteride+ Gel
Pros: Cons: ✓ High level of personalization with physician-driven telehealth oversight ✗ Limited long-term safety data for some ingredient combinations ✓ Competitive monthly pricing ✗ Availability restricted to the U.S. ✓ Physician-guided treatment plans and ongoing clinical support ✗ No subscription flexibility (all via 90-day supply) ✓ Proprietary gel base designed for scalp absorption and minimal mess ✓ Evidence-informed protocols supported by board-certified physicians Maximus Tribe’s Max-Absorb Gel is a highly customizable, prescription-only hair loss solution leveraging physician-driven telehealth and evidence-based formulation. At $54.99 for a 90-day supply, it offers a lot of value for those looking for efficacy and clinical support. The core formulation includes dutasteride (0.1%) with optional minoxidil, tretinoin, and fexofenadine, delivered in a proprietary gel base that is designed to minimize mess and maximize scalp coverage.
Maximus prioritizes convenience and targeted care: onboarding and ongoing support are managed by board-certified doctors, and the medication is shipped directly to the patient for easy home application. While ingredient customization and clinical guidance are strengths, choices remain limited in comparison to other telehealth options (like Ulo).
Our Verdict: Maximus offers a well-priced topical dutasteride gel that balances multi-ingredient efficacy with convenient home use and physician oversight.
#7: Miiskin Dutasteride + Minoxidil
Pros: Cons: ✓ Direct board-certified dermatologist consultations ✗ Total cost varies with third-party pharmacy fees ✓ Custom-compounded formulations with up to 3 active ingredients ✗ Basic compounding without advanced delivery systems ✓ No automatic subscriptions—pay per order ✗ Less specialized in hair loss compared to dedicated platforms ✓ Legitimate medical oversight with prescription flexibility ✓ Serves both the USA and Mexico Miiskin differs from the subscription-based providers on this list, as it facilitates direct medical consultations between patients and qualified dermatologists. The pay-as-you-go model allows legitimate medical access without subscription requirements. Miiskin charges $59 for initial consultations and $30 for returning visits. Medication fees vary and are charged separately at the pharmacy where the medication is filled.
While the dermatologist-direct platform increases access to qualified care and prescription hair loss treatments, its simple compounded formulas lack the customization and specialization of leading brands. Ingredient disclosure is limited beyond general mentions of three-active-ingredient formulations and the listing of a combination of dutasteride and minoxidil therapy.
The model does not permit total cost calculation until third-party pharmacy checkout, separate from Miiskin’s billing process, which only covers prescribing dermatologist consultation fees.
Our Verdict: Although Miiskin offers medical legitimacy, the dermatology consultation platform lacks the treatment specialization and transparency of top dutasteride therapy sources. It may be a valuable tool for patients with hair loss to connect with qualified providers and access basic compounded formulas.
#8 Musely The Hair Topical Solution – Classic
Pros: Cons: ✓ Lowest cost option at $33/month ✗ Minimal medical oversight compared to competitors ✓ 0.3% dutasteride concentration in a customizable formula ✗ Limited customization options ✓ Aesthetic medicine platform with dermatologist involvement ✗ Medical rigor lacking ✓ Multi-ingredient formulations ✗ Less specialized hair loss expertise ✗ Some ingredients are not recommended for sensitive skin
Musely’s topical dutasteride solution occupies the final slot on our list as the lowest-priced option at just $33 per month (plus a one-time $20 consultation fee at onboarding). The lower price point reflects less extensive offerings that are well-suited for patients who prefer a more self-directed approach. This tailored prescription formulation combines up to 0.3% dutasteride and 8% minoxidil with optional add-ons of ketoconazole to fight dandruff and hydrocortisone to reduce inflammation.It is less customizable than other options, characteristic of Musely’s simplified strategy. Excipients like propylene glycol and alcohol may deter ingredient-conscious consumers due to irritant potential. While such potent formulas and highly active ingredients offer more powerful treatment, they also carry a greater risk of adverse reactions and call for qualified medical supervision.
However, unlike premium providers, Musely is not known for close medical oversight. In fact, the aesthetic medicine platform has a markedly superficial onboarding and assessment process that falls short of the thorough medical evaluations offered by top providers. Although clinicians are involved in the platform, follow-up care and specialized hair restoration knowledge are lacking, according to customer reviews.
Our Verdict: Musely is included as a purely budget-friendly option for cost-conscious patients who understand the importance of self-monitoring and supplement with appropriate medical consultation.
Bottom Line on Buying Topical Dutasteride Online
According to our thorough analysis, Ulo is the best choice for topical dutasteride therapy in 2026. This platform stands out for its rigorous scientific foundation, highly tailored treatments, and commitment to close medical oversight, outperforming the fixed protocols and misleading marketing claims of its peers.
Rather than standardized approaches, Ulo’s PhD-designed therapies with built-in customization features meet the individual needs of each patient, representing the pinnacle of evidence-based precision care in the rapidly evolving field of hair restoration medicine.
Expectations and Timeline
Clinical findings support the following timeline of expected treatment outcomes in topical dutasteride therapy. Familiarity with these will ground your therapy in a realistic outlook, encouraging patience and prolonged adherence.[10]Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 mg) in … Continue reading,[11]Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., & Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology, 240, 833 – 843. Available at: … Continue reading,[12]Radhakrishnan, P., Mariyappan, G., Karthi, J., & Jaisumi, K. (2024). Formulating, Optimizing and Evaluation of Dutasteride Loaded Insitu-Emulgel for Androgenetic Alopecia. International Journal … Continue reading
- Early Stage (Months 1-3): Within days of beginning treatment, scalp DHT levels start to decline, although cosmetic improvements take longer to show as existing hair cycles complete. Some patients may experience temporary hair shedding in the first 2-8 weeks, which is considered a positive sign that the treatment is working by triggering new growth cycles.
- Emergence Stage (Months 3-6): After about 3 months of continued therapy, subtle visible improvements typically begin to emerge. Existing strands become thicker, and previously inert hair follicles are reactivated to produce new shafts for measurable increases in hair count metrics.
- Maximization Stage (Months 6-18): This phase sees the most considerable improvements, as hair count and diameter continue to increase for a thicker, healthier appearance. The majority of patients reach optimal results within a year.
- Maintenance Stage (Long-Term): After reaching peak outcomes, continuous daily application is necessary to sustain benefits. Studies show that indefinite treatment can maintain positive results, although occasional adjustments may be needed over time to account for typical signs of aging.
Risk Profile and Safety
Topical dutasteride is considered a safe hair loss intervention, particularly when weighed against oral formulas that can carry substantial risk due to systemic exposure. Nonetheless, informed risk assessment and familiarity with safety protocols are recommended before beginning any new treatment. Here is a brief overview of medical data on the safety and side effects of topical dutasteride.[13]Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., & Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology, 240, 833 – 843. Available at: … Continue reading
Common Side Effects
Adverse patient reactions to topical dutasteride are rare and typically limited to mild, localized scalp irritation that resolves in 2-4 weeks, occurring in only about 5-10% of new users. Depending on individual sensitivities and irritating formulation additives like alcohol, scalp dryness, flaking, and contact dermatitis are possible, though the latter is less common. Mild systemic side effects are extremely rare, documented in under 2% of patients.
Serious Considerations
Also rare are severe allergic reactions and pronounced systemic side effects, which can include sexual dysfunction, mood disorders, and gynecomastia. These all warrant prompt medical intervention. Topical dutasteride is not recommended in patients with active scalp infections and hormonal disorders.
Topical dutasteride is strongly contraindicated in pregnant or nursing women and in those planning future pregnancies due to the risk of birth defects. Patients with known hypersensitivity to DHT blockers should also avoid use.
Safety and optimal treatment outcomes depend on regular medical follow-up evaluations, typically at three-month intervals in the first year. This enables early identification of problems and treatment response evaluation.
Conclusion: Best Topical Dustaride Products
Cutting-edge topical dutasteride formulations bridge the gap between pharmacological precision and patient satisfaction in hair restoration therapy. Available products represent a spectrum of targeted, proven treatments that are considered safer and more customizable than previous-generation modalities.
Our thorough review of the evolving market identified clear leaders, with Ulo setting itself apart as an exemplary provider of topical dutasteride. Its scientifically optimized protocols are tailored to meet the individual needs of each patient, with qualified medical supervision to ensure a safe and transformative treatment experience.
As hair restoration technology advances, leading topical dutasteride formulations, such as Ulo’s, are at the forefront, empowering users with convenient and clinically proven solutions that not only enhance appearance but also improve quality of life.
Explore Ulo’s advanced topical dutasteride treatments today.
Frequently Asked Questions
Do I need a prescription for topical dutasteride? Yes, topical dutasteride is a prescription-only treatment in the United States. This ensures proper candidate screening and legitimate medical supervision throughout treatment.
When can I expect results? Initial DHT suppression on the scalp occurs within the first few days, but visible changes in hair growth typically emerge within 3-4 months. Benefits generally reach their peak after 12-18 months of consistent application.
How do I apply topical dutasteride? Use the product applicator to deposit the treatment directly onto clean, dry scalp areas where you are experiencing hair loss. Allow it to absorb fully before using other hair products. Following application, avoid wetting the hair for several hours and wash your hands thoroughly.
Can I combine topical dutasteride with other treatments? Yes, the benefits of topical dutasteride can be enhanced through combination therapies, such as minoxidil. Consult your prescribing doctor to learn about safe and effective hybrid treatments.
What will happen if I stop treatment? When treatment is halted, scalp DHT levels gradually return to starting levels, which typically leads to resumed hair loss after several months.
How much does topical dutasteride cost? Pricing varies from about $30 to $135+ for a monthly supply, depending on concentration, formulation, brand, and telehealth services included. Leading brands provide comprehensive medical oversight and customization, offering transparent and flat-rate monthly subscription fees.
References[+]
References ↑1 R. Cuevas-Díaz Durán., Martinez-Ledesma, E., Garcia-Garcia, M., Gauzin, D.B., Sarro-Ramirez, A., Gonzalez-Carrillo, C., Rodriguez-Sardin, D., Fuentes, A., Cardenas-Lopez, A. (2024) The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia. International Journal of Molecular Sciences, 25. Available at: https://doi.org/10.3390/ijms25052542. ↑2 Dhurat, R., Sharma, A., Rudnicka, L., Kroumpouzos, G., Kassir, M., Galadari, H., Wollina, U., Lotti, T., Golubović, M., Binic, I., Grabbe, S., & Goldust, M. (2020). 5‐Alpha reductase inhibitors in androgenetic alopecia: Shifting paradigms, current concepts, comparative efficacy, and safety. Dermatologic Therapy, 33. Available at: https://doi.org/10.1111/dth.13379. ↑3 Arif, T., Dorjay, K., Adil, M., & Sami, M. (2017). Dutasteride in Androgenetic Alopecia: An Update.. Current clinical pharmacology, 12 1, 31-35. Available at: https://doi.org/10.2174/1574884712666170310111125. ↑4 Harcha, W., Martínez, J., Tsai, T., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia.. Journal of the American Academy of Dermatology, 70 3, 489-498.e3. Available at: https://doi.org/10.1016/j.jaad.2013.10.049. ↑5, ↑7, ↑10 Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 mg) in Treatment of Androgenetic Alopecia. QJM: An International Journal of Medicine. Available at: https://doi.org/10.1093/qjmed/hcae175.207. ↑6, ↑8, ↑11, ↑13 Ding, Y., Wang, C., Bi, L., Du, Y., Lu, C., Zhao, M., & Fan, W. (2024). Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology, 240, 833 – 843. Available at: https://doi.org/10.1159/000541395. ↑9, ↑12 Radhakrishnan, P., Mariyappan, G., Karthi, J., & Jaisumi, K. (2024). Formulating, Optimizing and Evaluation of Dutasteride Loaded Insitu-Emulgel for Androgenetic Alopecia. International Journal of Pharmaceutical Research and Applications. Available at: https://doi.org/10.35629/4494-0906631659. Finasteride is a prescription medication originally created to treat benign prostate hyperplasia (BPH), and now commonly used to treat androgenic alopecia (AGA). It achieves this through targeting 5α‑reductase (5AR), an enzyme that converts testosterone to dihydrotestosterone (DHT), lowering levels of DHT in the scalp. DHT is the key androgen driving follicular miniaturization and is known to drive hair loss. Therefore, the finasteride-induced reduction of DHT results in increased hair growth.[1]Asfour L, Cranwell W, Sinclair R, (2023), Male Androgenetic Alopecia. In: Feingold KR, Adler RA, Ahmed SF, et al., editors, Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; Available at: … Continue reading
Despite the high amount of evidence supporting finasteride use, users may be disincentivized due to its potential side effects, including a loss of libido and erectile dysfunction. In some cases, the symptoms can persist after ceasing finasteride use, a condition known as post-finasteride syndrome.[2]Diviccaro, S., Melcangi, R.C., Giatti, S. (2019). Post-finasteride syndrome: an emerging clinical problem. Neurobiology of Stress. 12. 100209. Available at: https://doi.org/10.1016/j.ynstr.2019.100209
The potential for the finasteride mechanism to lower testosterone levels is a common fear; however, the sexual side effects observed in a minority of finasteride users do not occur due to a finasteride-induced reduction in testosterone. Paradoxically, finasteride causes a rise in testosterone, yet occasional sexual side effects are still seen.
In this article, we will discuss whether finasteride lowers testosterone and what the mechanisms are behind sexual side effects in finasteride users.
Interested in Oral Finasteride?
Oral finasteride & minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

How Does Finasteride Work?
AGA is a progressive remodelling of the hair follicle, which is induced by androgen sensitivity. Activation of the androgen receptor shortens the growth phase of the hair growth cycle, resulting in progressive miniaturization of susceptible hair follicles. The rest phase of the hair growth cycle remains the same or lengthens, altering the ratio of growth to rest from 12:1 to 5:1. As a result of follicle miniaturization, the hair growth is insufficient to penetrate the outer layer of the skin.[3]Ho, C.H., Sood, T., Zito, P.M., (2024), Androgenetic Alopecia. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430924/ (Accessed: 07 January 2026)
DHT is the key androgen driving follicular miniaturization. As mentioned above, the 5AR enzyme metabolizes testosterone to produce DHT. Both 5AR and DHT have been shown to be elevated in balding areas of the scalp compared to the hair-containing scalp.[4]Dallob, A.L., Sadick, N.S., Unger, W., Lipert, S., Geissler, L.A., Gregoire, S.L., Nguyen, H.H., Moore, E.C. and Tanaka, W.K. (1994). The Effect of Finasteride, a 5 Alpha-Reductase Inhibitor, on … Continue reading
Effect of Finasteride on DHT Suppression
Finasteride suppression of DHT can be fit to a non-linear (logarithmic) dose-response curve, i.e., even very low levels of finasteride (≥0.2 mg/day) can produce near-maximal suppression of DHT in both the serum and the scalp.[5]Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W.P., Cotterill, P.C., Thiboutot, D.M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S.J., Griffin, E.I., Weiss, D., … Continue reading The majority of its inhibitory action is achieved at low doses, with higher dosing only providing marginal additional effects.
Clinical trials have demonstrated that finasteride treatment decreases levels of DHT in the serum by 71-72% and in the scalp by 64-69%.[6]Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W.P., Cotterill, P.C., Thiboutot, D.M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S.J., Griffin, E.I., Weiss, D., … Continue reading
Clinical Effectiveness
The clinical effectiveness of finasteride in improving hair growth has been demonstrated in multiple clinical trials. It has been shown to improve hair growth and slow further hair loss across 1-year, 2-year, and 5-year clinical trials, with 65% of finasteride-treated men demonstrating increased hair count at 5 years compared to 0% of the placebo-treated (sugar pill group) men.[7]Finasteride Male Pattern Hair Loss Study Group. (2002). Long-Term (5-Year) Multinational Experience With Finasteride 1 mg in the Treatment of Men With Androgenetic Alopecia. Eur J Dermatol. 12:38-49. … Continue reading,[8]Shapiro, J., Kaufman, K.D. (2003). Use of Finasteride in the Treatment of Men With Androgenetic Alopecia (Male Pattern Hair Loss). J Investig Dermatol Symp Proc. 8(1):20-23. Available at: … Continue reading
Does Finasteride Lower Testosterone?
Myths around finasteride treatment resulting in testosterone deficiency abound; however, there is no evidence supporting this. In fact, research has actually shown that finasteride treatment increases levels of testosterone.
A four-year clinical trial investigating the safety and efficacy of finasteride in patients with BPH demonstrated a ~15% rise in testosterone in the serum compared to the placebo group.[9]Roehrborn, C., Lee, M., Meehan, A., Waldstreicher, J. (2003). Effects Of Finasteride On Serum Testosterone And Body Mass Index In Men With Benign Prostatic Hyperplasia. Urology. 62(5). 894–899. … Continue reading Additional trials have also reported a moderate rise in testosterone (5-33%; not dose-dependent).[10]Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W.P., Cotterill, P.C., Thiboutot, D.M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S.J., Griffin, E.I., Weiss, D., … Continue reading,[11]Uygur, M.C., Arık, A.İ., Altuğ, U., Erol, D., (1998). Effects Of The 5α-Reductase Inhibitor Finasteride On Serum Levels Of Gonadal, Adrenal, And Hypophyseal Hormones And Its Clinical … Continue reading,[12]Amory, J.K., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J. and Clark, R.V. (2007). The Effect Of 5α-Reductase Inhibition With Dutasteride And … Continue reading
Although the rise in testosterone was significantly different between finasteride- and placebo-treated individuals, levels of testosterone consistently remained within the normal physiological range. The increase in testosterone was identified in women as well as men, and was found to be more pronounced in men with low baseline testosterone.[13]Albasher, G., Bin-Jumah, M., Alfarraj, S., Al-Otibi, F., Al-Sultan, N.K., Alarifi, S., Alkahtani, S. and Al-Qahtani, W.S. (2020). Prolonged Use Of Finasteride-Induced Gonadal Sex Steroids … Continue reading,[14]Roehrborn, C., Lee, M., Meehan, A., Waldstreicher, J. (2003). Effects Of Finasteride On Serum Testosterone And Body Mass Index In Men With Benign Prostatic Hyperplasia. Urology. 62(5). 894–899. … Continue reading However, not all patients tested experienced this rise in testosterone.
The evidence therefore supports a short-term rise in testosterone on finasteride treatment, while remaining within the physiological range. Many of these studies were carried out in relation to BPH, where the finasteride doses are higher (generally 5 mg vs 1 mg), so the impact of finasteride for AGA treatment is likely to be even lower.
Why Does Testosterone Increase When DHT Falls?
The inhibition of the 5AR enzyme results in the blocking of the DHT production pathway, resulting in the testosterone that would otherwise have been converted to DHT remaining as testosterone. No large increases in testosterone are seen, as not only do negative feedback adjustments occur, but free testosterone is also thought to be redistributed within the androgen pathway, where it is converted into estradiol.
Can the Rise in Testosterone Have Sexual Side Effects?
The moderate rise in testosterone identified in individuals taking finasteride does not result in a rise above the physiological range, making sexual side effects directly as a result of the rise in testosterone unlikely. In general, a moderate rise in testosterone levels improves sexual function. This appears to contradict the well-characterized (though uncommon) sexual side effects of finasteride treatment. Therefore, another mechanism must be at work to induce these sexual side effects.
Finasteride Sexual Side Effects
What are the Sexual Side Effects of Finasteride?
Sexual side effects have been reported in a small minority of users. These include:
- Erectile dysfunction
- Ejaculatory dysfunction
- Loss of libido
While these symptoms often improve or resolve on their own, persistent sexual dysfunction has been reported in a very small number of finasteride users, in a condition known as post-finasteride syndrome.[15]Hirshburg, J.M., Kelsey, P.A., Therrien, C.A., Gavino, A.C. and Reichenberg, J.S. (2016). Adverse Effects And Safety Of 5-Alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. … Continue reading However, reports of this condition are controversial, and conflicting results are seen in different studies. If you are interested in learning more, see our article exploring whether post-finasteride syndrome is real.
What is the Prevalence of Sexual Side Effects?
In controlled clinical trials, sexual side effects of taking finasteride are uncommon, but exact estimates vary depending on the dose, study design, and population receiving treatment.
- In a 4-year, randomized, placebo-controlled clinical trial assessing the prevalence of sexual adverse experiences (AEs) on the treatment of BPH with 5 mg of finasteride, 15% of finasteride-treated and 7% of placebo-treated men had sexual AEs within the first year, indicating an increase in AEs in the finasteride group. However, no differences between placebo- and finasteride-treated AEs were seen in years 2-4.[16]Wessells, H., Roy, J., Bannow, J., Grayhack, J., Matsumoto, A.M., Tenover, L., Herlihy, R., Fitch, W., Labasky, R., Auerbach, S., Parra, R., Rajfer, J., Culbertson, J., Lee, M., Bach, M.A. and … Continue reading
- A meta-analysis (a study where data from multiple independent studies are analyzed) of 15 clinical trials where AGA was treated with 1 mg of finasteride found that only 5.31% of users had adverse sexual effects compared to 3.05% of those taking a placebo.[17]Lee, S., Lee, Y.B., Choe, S.J. and Lee, W. (2019). Adverse Sexual Effects Of Treatment With Finasteride Or Dutasteride For Male Androgenetic Alopecia: A Systematic Review And Meta-Analysis. Acta … Continue reading
- A different meta-analysis of 17 clinical trials found that men taking finasteride had a 1.29 times higher risk of underactive sexual desire or a 1.47 times higher risk of erectile dysfunction.[18]Corona, G., Tirabassi, G., Santi, D., Maseroli, E., Gacci, M., Dicuio, M., Sforza, A., Mannucci, E. and Maggi, M. (2017). Sexual Dysfunction In Subjects Treated With Inhibitors Of 5α-Reductase For … Continue reading
Meta-analyses and studies with a high number of participants are the gold standard for assessing the risk of sexual side effects. Smaller studies have put the incidence of sexual side effects higher; however, these results should be interpreted with caution due to the limitations of the clinical trials.
Further caution should be taken due to the “nocebo” phenomenon, where a patient may experience negative symptoms or worse outcomes due to their being informed that the product may cause them harm.
A small study investigating this phenomenon in patients treated for BPH with 5 mg finasteride found that the group who received counseling on the sexual side effects (i.e., were informed of these side effects) demonstrated a significantly higher proportion of sexual side effects than the group who did not receive counseling (43.6% vs. 15.3%).[19]Mondaini, N., Gontero, P., Giubilei, G., Lombardi, G., Cai, T., Gavazzi, A. and Bartoletti, R. (2007). Finasteride 5 mg And Sexual Side Effects: How Many Of These Are Related To A Nocebo Phenomenon? … Continue reading
Care should also be taken when interpreting information on natural health websites and online forums, as the perceived prevalence of sexual side effects may seem incredibly high on these. This may occur due to multiple reasons, including:
- Fearmongering due to the desire to sell natural remedies claiming to be sexual side-effect-free (which often isn’t true).
- Users are more likely to post in anger about issues with the product, whereas those content with the product are less likely to post (the “Yelp Effect”).
Why do Sexual Side Effects Occur Despite Higher Testosterone?
As discussed above, increased testosterone (within the physiological range) enhances sexual function. However, despite the slightly raised testosterone seen in men taking finasteride, sexual side effects still occur. Below are three main pathways through which these side effects have been suggested to occur. Please consider that none of these mechanisms have been conclusively proven, and are only suggestions as to how sexual side effects may be occurring.
DHT Depletion
DHT is a potent androgen, binding the androgen receptor with greater strength than testosterone. DHT and testosterone can bind to androgen receptors in the corpora cavernosa (erectile tissue in the penis), causing an upregulation in nitric oxide production and resulting in increased blood flow into the penis and an erection.
It has been suggested that susceptible individuals experiencing decreased levels of DHT on finasteride treatment experience a reduction in nitric oxide production, limiting blood flow into the penis, and, consequently, in erectile dysfunction.[20]Clapauch, R. (2011). Finasteride and Erectile Dysfunction: Fact or Fiction? Brasília Medical (Medical Experience). 48(4), pp.422–427. Available at: … Continue reading,[21]Erdemir, F., Harbin, A. and Hellstrom, W.J.G. (2008). 5-Alpha Reductase Inhibitors And Erectile Dysfunction: The Connection. The Journal Of Sexual Medicine. 5(12), pp.2917–2924. Available at: … Continue reading
DHT is also an important contributor to secretory function and semen volume, as controlled by two glands – the prostate and seminal vesicles. These glands are androgen-dependent. DHT is the primary androgen involved, but testosterone can compensate for reduced DHT.[22]Anitha, B., Inamadar, A.C., Ragunatha, S. (2009). Finasteride-Its Impact on Sexual Function and Prostate Cancer. J Cutan Aesthet Surg. 2(1):12–16. Available at: … Continue reading,[23]Cai, L.Q., Fratianni, C.M., Gautier, T., Imperato-McGinley, J. (1994). Dihydrotestosterone Regulation of Semen in Male Pseudohermaphrodites with 5 Alpha-Reductase-2 Deficiency. Journal of Clinical … Continue reading
Finasteride treatment does not cause low semen or ejaculatory volume in all users, but this does appear in rare cases. This ejaculatory dysfunction could occur as a result of testosterone not sufficiently compensating for DHT’s role in secretory function.
Neurosteroids in the Central Nervous System
In addition to its ability to convert testosterone to DHT, 5AR is also important in the conversion of other molecules into neurosteroids. Neurosteroids are steroids synthesized within the central nervous system (CNS; formed of the brain and spinal cord) that play a key role in controlling activity within the CNS, including anxiety, stress, and depression.
Treatment with 5AR inhibitors, such as finasteride, can cause a decrease in active neurosteroids within the CNS and altered transmission of certain signals within the brain. This disrupted neurosteroid signaling has been suggested as a potential cause of the decreased libido side effect that is occasionally seen in users of finasteride.[24]Irwig, M.S. (2014), Persistent Sexual and Nonsexual Adverse Effects of Finasteride in Younger Men. Sex Med Rev. 2(1):24–35. Available at: https://doi.org/10.1002/smrj.19,[25]Traish, A.M., Hassani, J., Guay, A.T., Zitzmann, M., Hansen, M.L. (2011). Adverse Side Effects of 5 Alpha-Reductase Inhibitors Therapy: Persistent Diminished Libido and Erectile Dysfunction and … Continue reading
Androgen Redistribution
As discussed above, the inhibition of 5AR results in less testosterone being converted into DHT and a mild rise in testosterone. It has been suggested that the increase in free testosterone may be compensated for through androgen redistribution via conversion to estradiol (an estrogen).
Estradiol levels show a minor rise in finasteride users of approximately 15% (while remaining within physiological reference ranges).[26]U.S. Food and Drug Administration, (2022), Propecia (Finasteride) Tablets Label. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020788s030lbl.pdf (Accessed: 07 January 2026) For those with a genetic predisposition to high estrogen or whose diets, lifestyles, and environments have elevated their estrogen levels, a finasteride-induced further rise in estrogen may be sufficient for the development of gynecomastia (the development of male breast tissue).
How Can We Reduce the Risk of Sexual Side Effects?
The first step if side effects occur is to speak to your primary care physician to ensure that side effects are recorded and appropriate management steps are taken. If side effects are severe or rapidly worsening, you should immediately see your physician. The strategies discussed below are only applicable if mild to moderate side effects are experienced, and should only be carried out under clinical supervision. More information on finasteride-associated side effects can be found in our articles on topical finasteride and reducing side effects.
- Lowering your daily dose of finasteride
Finasteride produces the majority of its DHT-lowering effects at relatively low doses. The standard finasteride daily dose is 1 mg. Due to its non-linear dose-response curve, lower doses can still have significant effects on lowering serum and scalp DHT and enhancing hair growth.
Finasteride side effects are often dose-sensitive, meaning that lowering the daily dose could be a first step to reducing side effects, without a significant loss in benefits.
Figure 1. The non-linear dose response curve of finasteride shows that increasing the concentration of the treatment does not translate to an increased reduction in serum DHT.
- Lowering the frequency of dosing
Mild flexibility in finasteride dosing also allows for carefully controlled alterations in dosing frequency. Finasteride has a relatively short half-life (time taken for the amount of drug in the body to halve), but does accumulate with repeated doses.[27]Steiner, J.F. (1996). Clinical Pharmacokinetics and Pharmacodynamics of Finasteride. Clinical Pharmacokinetics. 30(1):16–27. Available at: https://doi.org/10.2165/00003088-199630010-00002
As a result, the frequency of finasteride dosing can be reduced to 1 mg three times per week or 0.5 mg every other day (once steady-state suppression has been achieved) while maintaining sufficient DHT inhibition for significant hair growth effects.
This has the potential to reduce finasteride-associated side effects, particularly sexual side effects associated with hormonal changes.
- Switching to a topical finasteride
Topical, as opposed to oral, formulations deliver the drug directly to the scalp without extensive systemic circulation and therefore reduce overall drug exposure.
Randomized clinical trials have been carried out to assess the effectiveness of oral vs topical finasteride. These have demonstrated comparable clinical benefits, including hair count, hair thickness, and size of bald area.[28]Hajheydari, Z., Akbari, J., Saeedi, M., Shokoohi, L. (2009). Comparing the Therapeutic Effects of Finasteride Gel and Tablet in Treatment of the Androgenetic Alopecia. Indian Journal of Dermatology, … Continue reading,[29]Bhura, M. (2013). Comparative Analysis of Topical and Oral Finasteride in Androgenetic Alopecia: Impact on Hair Growth, Systemic Absorption, and Adverse Effects. Indian Journal of Basic and Applied … Continue reading
Topical application lowers the amount of systemic drug, resulting in reduced side effects. Studies have shown that sexual side effects, in particular, are improved when using finasteride topically instead of orally.[30]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falqués, M., Otero, R., Tamarit, M.L., Galván, J., Tebbs, V., Massana, E., Topical Finasteride Study Group. (2022). Efficacy and Safety … Continue reading
The beneficial effects of topical finasteride require consistent, measured application and strong adherence to the prescribed application regimen. As such, users who are not experiencing side effects may find it easier to use oral finasteride instead.
- Using PDE5 inhibitors to manage sexual side-effects
PDE5 inhibitors can be used to treat erectile dysfunction by relaxing the blood vessels so as to allow increased blood flow into the penis. The effectiveness of these drugs is well studied, and they have also been shown to improve erectile function specifically in men taking finasteride.[31]Casabé, A., Roehrborn, C.G., Da Pozzo, L.F., Zepeda, S., Henderson, R.J., Sorsaburu, S., Henneges, C., Wong, D.G. & Viktrup, L. (2014). Efficacy and Safety of the Coadministration of Tadalafil … Continue reading
While PDE5 inhibitors improve erectile performance and sexual confidence, they do not address other sexual side effects of finasteride, such as libido or ejaculatory volume. In addition, as prescription medications, they should only be used under clinical supervision to reduce the risk of drug interactions.
Final Thoughts
Finasteride does not lower testosterone. In fact, clinical evidence consistently demonstrates that the reduced conversion of testosterone into DHT causes a modest increase in serum testosterone, which remains within the normal physiological ranges. Concerns about finasteride causing testosterone deficiency are therefore not supported by the scientific literature.
Sexual side effects, while real, occur only in a small minority of users and are unlikely to be caused by changes in testosterone. Instead, they are more likely linked to DHT depletion in androgen-sensitive tissues, altered neurosteroid signaling, androgen distribution, or the “nocebo” effect.
For most users, finasteride is effective and well-tolerated. For those who do experience side effects, strategies like dose adjustment or switching to topical use under the guidance of your physician may improve tolerability while maintaining benefits.
References[+]
References ↑1 Asfour L, Cranwell W, Sinclair R, (2023), Male Androgenetic Alopecia. In: Feingold KR, Adler RA, Ahmed SF, et al., editors, Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/ (Accessed: 05 January 2026) ↑2 Diviccaro, S., Melcangi, R.C., Giatti, S. (2019). Post-finasteride syndrome: an emerging clinical problem. Neurobiology of Stress. 12. 100209. Available at: https://doi.org/10.1016/j.ynstr.2019.100209 ↑3 Ho, C.H., Sood, T., Zito, P.M., (2024), Androgenetic Alopecia. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430924/ (Accessed: 07 January 2026) ↑4 Dallob, A.L., Sadick, N.S., Unger, W., Lipert, S., Geissler, L.A., Gregoire, S.L., Nguyen, H.H., Moore, E.C. and Tanaka, W.K. (1994). The Effect of Finasteride, a 5 Alpha-Reductase Inhibitor, on Scalp Skin Testosterone and Dihydrotestosterone Concentrations in Patients with Male Pattern Baldness. J Clin Endocrinol Metab. 79(3), pp.703-706. Available at: https://doi.org/10.1210/jcem.79.3.8077349 ↑5, ↑6, ↑10 Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W.P., Cotterill, P.C., Thiboutot, D.M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S.J., Griffin, E.I., Weiss, D., Carrington, P., Gencheff, C., Cole, G.W., Pariser, D.M., Epstein, E.S., Tanaka, W., Dallob, A.D., Vandormael, K., Geissler, L. and Waldstreicher, J. (1999). The Effects of Finasteride on Scalp Skin and Serum Androgen Levels in Men with Androgenetic Alopecia. J Am Acad Dermatol. 41(4), pp.550-554. Available at: https://pubmed.ncbi.nlm.nih.gov/10495374/ ↑7 Finasteride Male Pattern Hair Loss Study Group. (2002). Long-Term (5-Year) Multinational Experience With Finasteride 1 mg in the Treatment of Men With Androgenetic Alopecia. Eur J Dermatol. 12:38-49. Available at: https://pubmed.ncbi.nlm.nih.gov/11809594/ ↑8 Shapiro, J., Kaufman, K.D. (2003). Use of Finasteride in the Treatment of Men With Androgenetic Alopecia (Male Pattern Hair Loss). J Investig Dermatol Symp Proc. 8(1):20-23. Available at: https://www.sciencedirect.com/science/article/pii/S0022202X15529357 ↑9, ↑14 Roehrborn, C., Lee, M., Meehan, A., Waldstreicher, J. (2003). Effects Of Finasteride On Serum Testosterone And Body Mass Index In Men With Benign Prostatic Hyperplasia. Urology. 62(5). 894–899. Available at: https://pubmed.ncbi.nlm.nih.gov/14624915/ ↑11 Uygur, M.C., Arık, A.İ., Altuğ, U., Erol, D., (1998). Effects Of The 5α-Reductase Inhibitor Finasteride On Serum Levels Of Gonadal, Adrenal, And Hypophyseal Hormones And Its Clinical Significance: A Prospective Clinical Study. Steroids. 63(4). 208–213. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0039128X98000051 ↑12 Amory, J.K., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J. and Clark, R.V. (2007). The Effect Of 5α-Reductase Inhibition With Dutasteride And Finasteride On Semen Parameters And Serum Hormones In Healthy Men. The Journal Of Clinical Endocrinology & Metabolism. 92(5), pp.1659–1665. Available at: https://doi.org/10.1210/jc.2006-2203 ↑13 Albasher, G., Bin-Jumah, M., Alfarraj, S., Al-Otibi, F., Al-Sultan, N.K., Alarifi, S., Alkahtani, S. and Al-Qahtani, W.S. (2020). Prolonged Use Of Finasteride-Induced Gonadal Sex Steroids Alterations, DNA Damage And Menstrual Bleeding In Women. Bioscience Reports. 40(2), BSR20191434. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC7007407/ ↑15 Hirshburg, J.M., Kelsey, P.A., Therrien, C.A., Gavino, A.C. and Reichenberg, J.S. (2016). Adverse Effects And Safety Of 5-Alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. Journal Of Clinical Aesthetic Dermatology. 9(7), pp.56–62. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5023004/ ↑16 Wessells, H., Roy, J., Bannow, J., Grayhack, J., Matsumoto, A.M., Tenover, L., Herlihy, R., Fitch, W., Labasky, R., Auerbach, S., Parra, R., Rajfer, J., Culbertson, J., Lee, M., Bach, M.A. and Waldstreicher, J. (2003). Incidence And Severity Of Sexual Adverse Experiences In Finasteride And Placebo-Treated Men With Benign Prostatic Hyperplasia. Urology. 61(3), pp.579–584. Available at: https://pubmed.ncbi.nlm.nih.gov/12639651/ ↑17 Lee, S., Lee, Y.B., Choe, S.J. and Lee, W. (2019). Adverse Sexual Effects Of Treatment With Finasteride Or Dutasteride For Male Androgenetic Alopecia: A Systematic Review And Meta-Analysis. Acta Dermato-Venereologica. 99(1), pp.12–17. Available at: https://pubmed.ncbi.nlm.nih.gov/30206635/ ↑18 Corona, G., Tirabassi, G., Santi, D., Maseroli, E., Gacci, M., Dicuio, M., Sforza, A., Mannucci, E. and Maggi, M. (2017). Sexual Dysfunction In Subjects Treated With Inhibitors Of 5α-Reductase For Benign Prostatic Hyperplasia: A Comprehensive Review And Meta-Analysis. Andrology. 5(4), pp.671–678. Available at: https://doi.org/10.1111/andr.12353 ↑19 Mondaini, N., Gontero, P., Giubilei, G., Lombardi, G., Cai, T., Gavazzi, A. and Bartoletti, R. (2007). Finasteride 5 mg And Sexual Side Effects: How Many Of These Are Related To A Nocebo Phenomenon? Journal Of Sexual Medicine. 4(6), pp.1708–1712. Available at: https://pubmed.ncbi.nlm.nih.gov/17655657/ ↑20 Clapauch, R. (2011). Finasteride and Erectile Dysfunction: Fact or Fiction? Brasília Medical (Medical Experience). 48(4), pp.422–427. Available at: https://rbm.org.br/details/247/en-US/finasteride-and-erectile-dysfunction–fact-or-fiction ↑21 Erdemir, F., Harbin, A. and Hellstrom, W.J.G. (2008). 5-Alpha Reductase Inhibitors And Erectile Dysfunction: The Connection. The Journal Of Sexual Medicine. 5(12), pp.2917–2924. Available at: https://doi.org/10.1111/j.1743-6109.2008.01001.x ↑22 Anitha, B., Inamadar, A.C., Ragunatha, S. (2009). Finasteride-Its Impact on Sexual Function and Prostate Cancer. J Cutan Aesthet Surg. 2(1):12–16. Available at: https://doi.org/10.4103/0974-2077.53093 ↑23 Cai, L.Q., Fratianni, C.M., Gautier, T., Imperato-McGinley, J. (1994). Dihydrotestosterone Regulation of Semen in Male Pseudohermaphrodites with 5 Alpha-Reductase-2 Deficiency. Journal of Clinical Endocrinology & Metabolism. 79(2):409–414. Available at: https://doi.org/10.1210/jcem.79.2.8045956 ↑24 Irwig, M.S. (2014), Persistent Sexual and Nonsexual Adverse Effects of Finasteride in Younger Men. Sex Med Rev. 2(1):24–35. Available at: https://doi.org/10.1002/smrj.19 ↑25 Traish, A.M., Hassani, J., Guay, A.T., Zitzmann, M., Hansen, M.L. (2011). Adverse Side Effects of 5 Alpha-Reductase Inhibitors Therapy: Persistent Diminished Libido and Erectile Dysfunction and Depression in a Subset of Patients. Journal of Sexual Medicine. 8(3). Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4064044/ ↑26 U.S. Food and Drug Administration, (2022), Propecia (Finasteride) Tablets Label. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020788s030lbl.pdf (Accessed: 07 January 2026) ↑27 Steiner, J.F. (1996). Clinical Pharmacokinetics and Pharmacodynamics of Finasteride. Clinical Pharmacokinetics. 30(1):16–27. Available at: https://doi.org/10.2165/00003088-199630010-00002 ↑28 Hajheydari, Z., Akbari, J., Saeedi, M., Shokoohi, L. (2009). Comparing the Therapeutic Effects of Finasteride Gel and Tablet in Treatment of the Androgenetic Alopecia. Indian Journal of Dermatology, Venereology and Leprology. 75(1):47–51. Available at: https://pubmed.ncbi.nlm.nih.gov/19172031/ ↑29 Bhura, M. (2013). Comparative Analysis of Topical and Oral Finasteride in Androgenetic Alopecia: Impact on Hair Growth, Systemic Absorption, and Adverse Effects. Indian Journal of Basic and Applied Medical Research. 3(1):436–444. Available at: https://www.ijbamr.com/assets/images/issues/pdf/71nag2_6w0c1i_34kUVI_YI8x3j_177107.pdf (Accessed: 07 January 2026) ↑30 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falqués, M., Otero, R., Tamarit, M.L., Galván, J., Tebbs, V., Massana, E., Topical Finasteride Study Group. (2022). Efficacy and Safety of Topical Finasteride Spray Solution for Male Androgenetic Alopecia: A Phase III, Randomized, Controlled Clinical Trial. Journal of the European Academy of Dermatology and Venereology. 36(2):286–294. Available at: https://doi.org/10.1111/jdv.17738 ↑31 Casabé, A., Roehrborn, C.G., Da Pozzo, L.F., Zepeda, S., Henderson, R.J., Sorsaburu, S., Henneges, C., Wong, D.G. & Viktrup, L. (2014). Efficacy and Safety of the Coadministration of Tadalafil Once Daily with Finasteride for 6 Months in Men with Lower Urinary Tract Symptoms and Prostatic Enlargement Secondary to Benign Prostatic Hyperplasia. Journal of Urology. 191(3), pp. 727–733. Available at: https://www.auajournals.org/doi/10.1016/j.juro.2013.09.059 IngredientsPharmaceuticalsNatural RemediesCompany ReviewsBy Sophie Grice, PhDMar 3, 20267 Best Oils for Hair Growth
Natural oils are widely marketed as hair-growth remedies. In this article, we cut through the hype and rank the most popular oils based on evidence quality, regrowth potential, safety profile, and real-world relevance. We’ll also explain common industry pitfalls, offer practical dosing and formulati...By Michael Williams, PhDFeb 13, 2026Hims Hair Growth Reviews: The Pros, Cons, and Real Results
Hims has quickly become one of the most recognizable telehealth brands for men’s health, and its hair loss lineup is no exception. But do the sleek branding and promises of “personalized” care translate into real, sustainable regrowth? In this in-depth review, we break down Hims’ most popular hair g...By Perfect Hair Health TeamFeb 12, 2026Topical Finasteride Before and After: Real Case Studies
Recent studies show that topical finasteride can significantly improve hair density and slow shedding while reducing the risk of systemic side effects seen with oral use. Learn how to interpret before-and-after results realistically, understand what typical regrowth looks like, and optimize your tre...By Perfect Hair Health TeamFeb 8, 2026How to Reduce the Risk of Finasteride Side Effects
Finasteride is an FDA-approved drug for androgenic alopecia. For a portion of men using finasteride, side effects can occur – most often in the form of sexual side effects, enlarged male breasts, lowered sperm counts, and erectile dysfunction. Fortunately, there are several strategies that can be em...By Cassie Hopton, PhDFeb 3, 202610 Best DHT-Blocking Shampoos
DHT-blocking shampoos are widely available, but do they actually work? While blocking DHT is a proven strategy for treating androgenic alopecia, most evidence comes from oral medications and leave-on topicals, not rinse-off shampoos. In this article, we separate science from marketing by breaking do...By Sarah King, PhDJan 31, 2026Best Minoxidil for Men: Top Picks for 2026
With countless hair loss products on the market, choosing the right minoxidil can be overwhelming. This evidence-based guide ranks the best minoxidil treatments for men in 2026, including prescription and over-the-counter options, based on effectiveness, safety, customization, and value. Discover wh...By Sophie Grice, PhDJan 27, 2026Switching From Finasteride to Dutasteride
Switching from finasteride to dutasteride is often framed as a simple upgrade or downgrade. In reality, each switch changes not only DHT suppression but also exposure patterns, timelines for results, washout dynamics, and evidence quality. This article breaks down realistic switching scenarios, oral...By Perfect Hair Health TeamJan 21, 2026Best Minoxidil for Women: Top 6 Brands of 2026
With so many hair-thinning treatments marketed to women, it can be difficult to separate what truly works from what’s just hype. This evidence-based guide ranks the best minoxidil options for women in 2026, including prescription and over-the-counter formulas, based on effectiveness, tolerability, c...By Perfect Hair Health TeamJan 17, 2026Oral Minoxidil: Before and After Photos [2026]
Oral minoxidil is emerging as a popular off-label treatment for hair loss, offering an option for those who can’t use or don’t respond to topical formulas. While some achieve dramatic regrowth, most see more modest improvements. Discover why results vary, how to interpret before-and-after photos rea...By Perfect Hair Health TeamJan 16, 2026Best Topical Finasteride: 5 Top Products of 2026
Topical finasteride is exploding in popularity, but not all formulas are created equal. This guide breaks down the 5 best topical finasteride treatments on the market, comparing strength, safety, customization, and real-world value. From ultra-tailored prescriptions like Ulo to big telehealth brands... - Mission Statement
 Scroll Down
Scroll Down


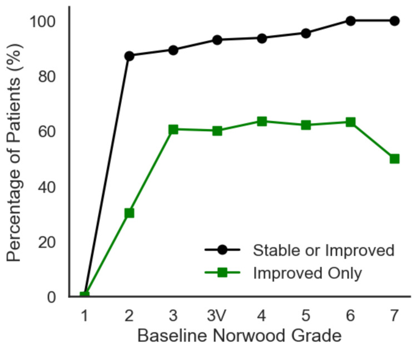



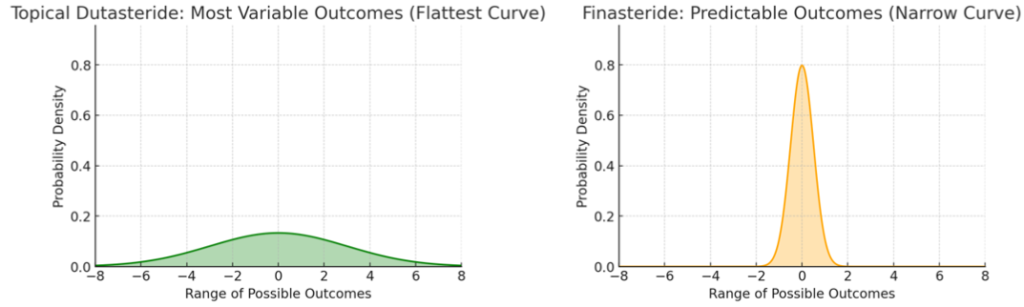

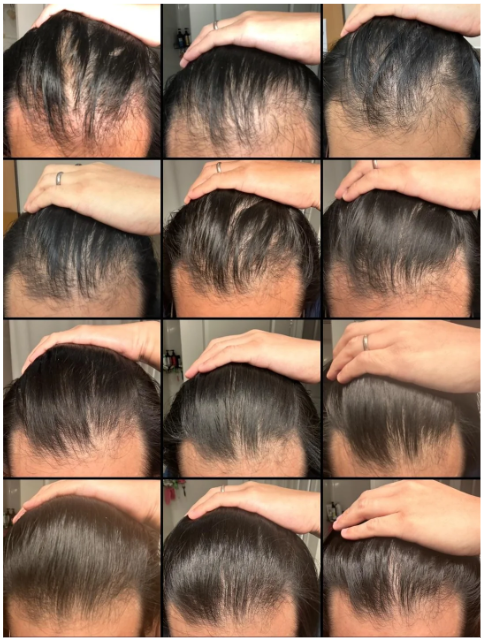
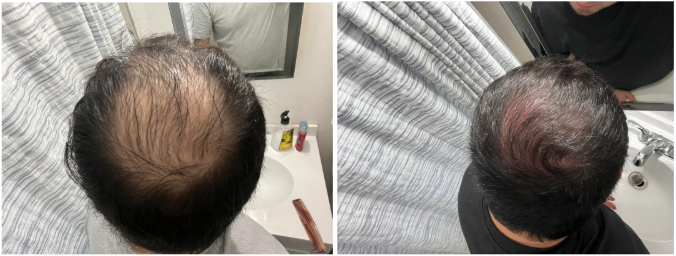
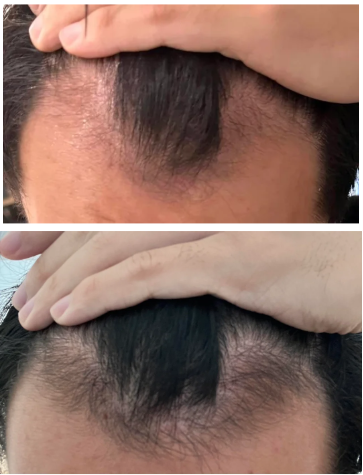
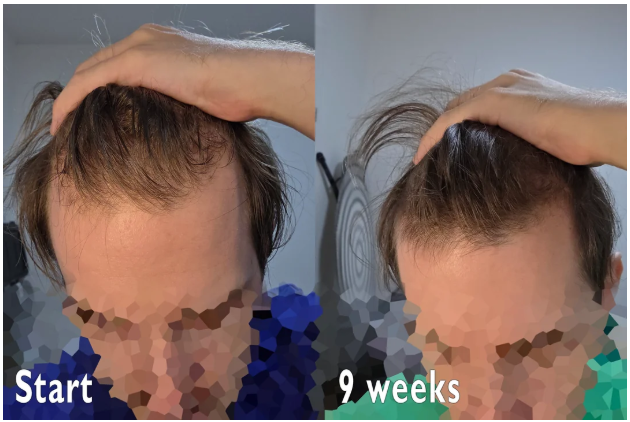









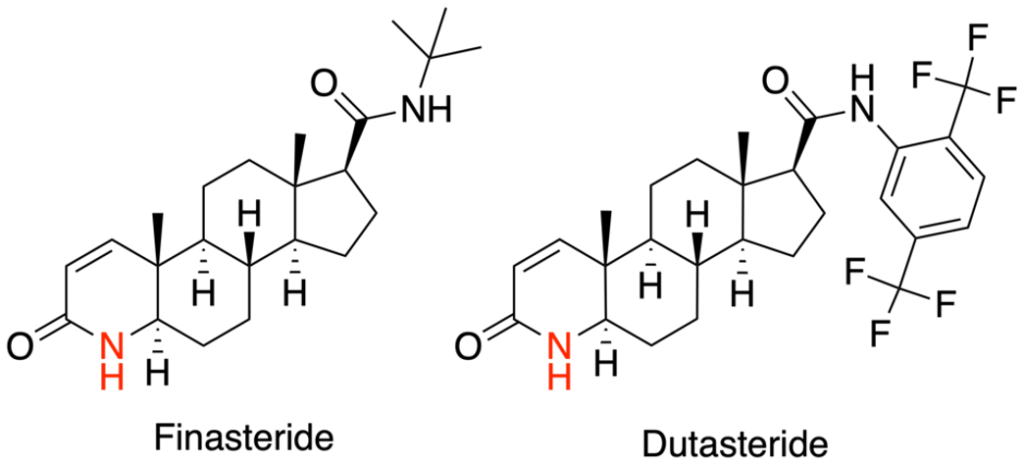
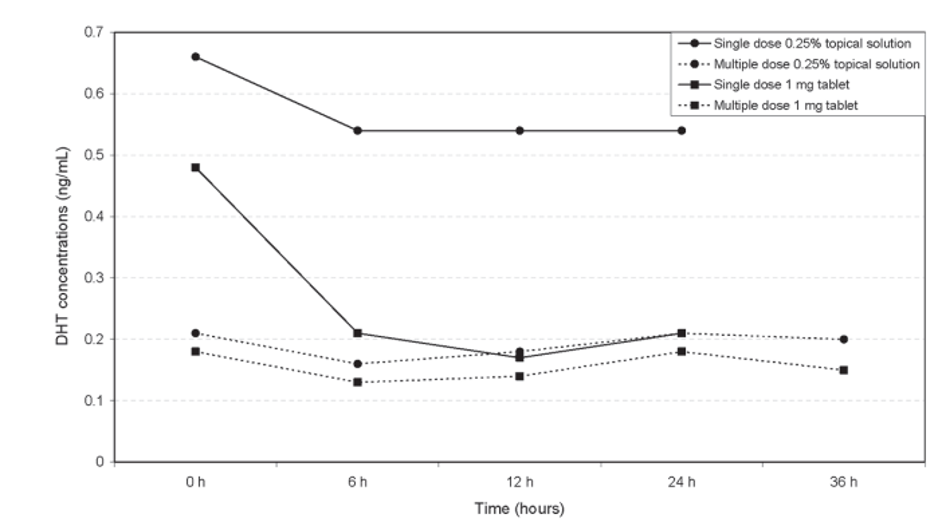





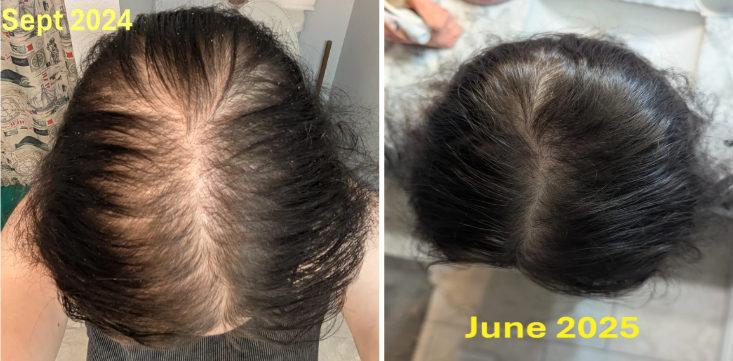
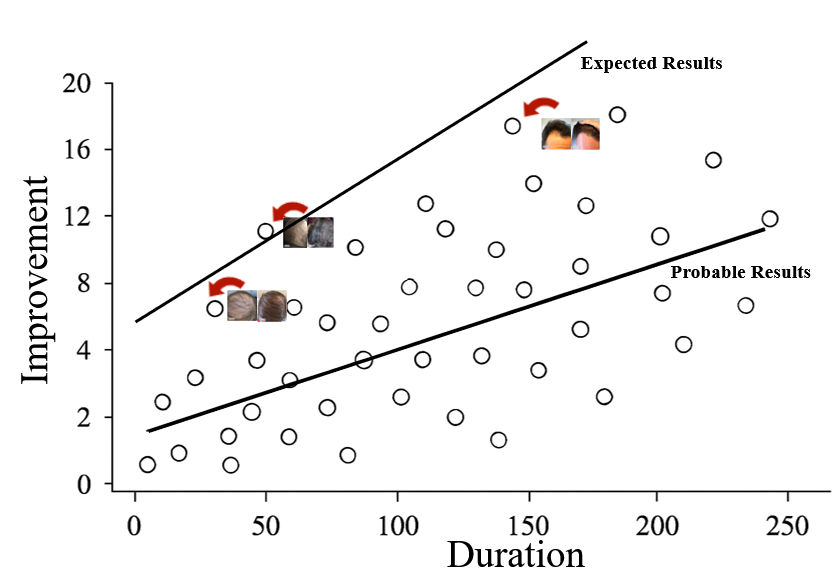
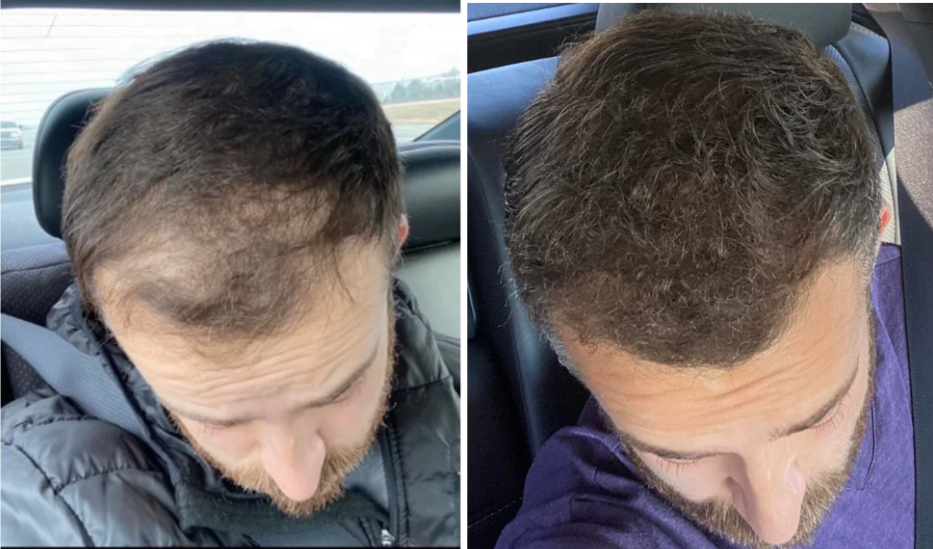

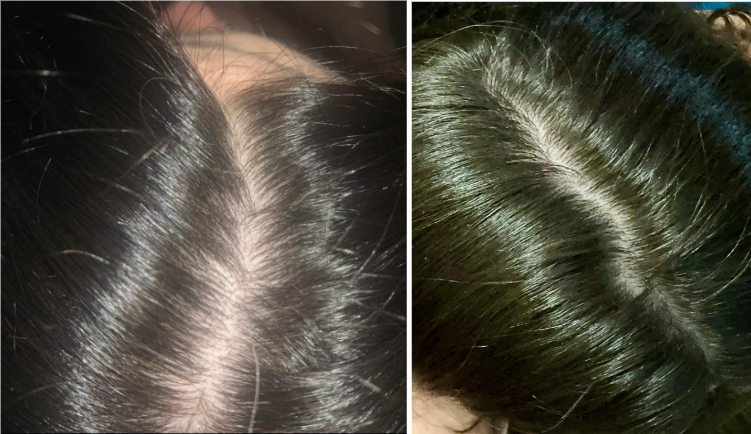
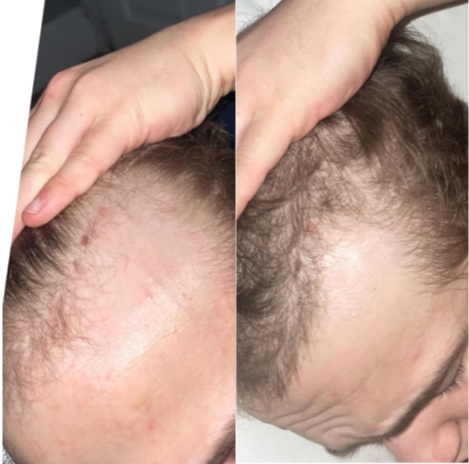

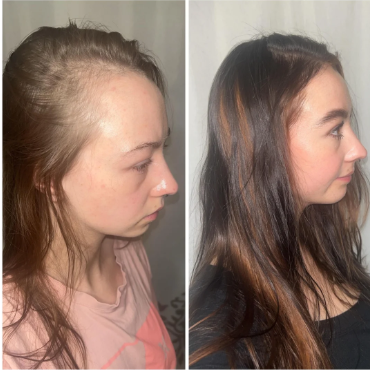

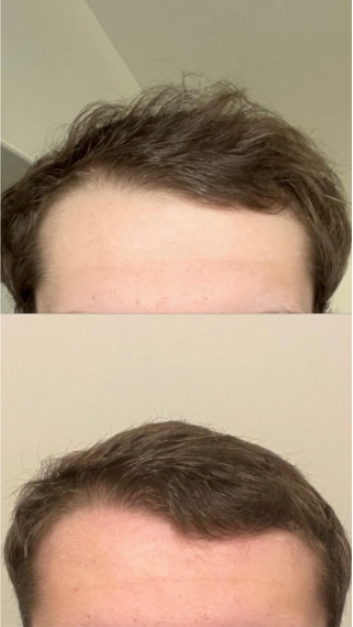
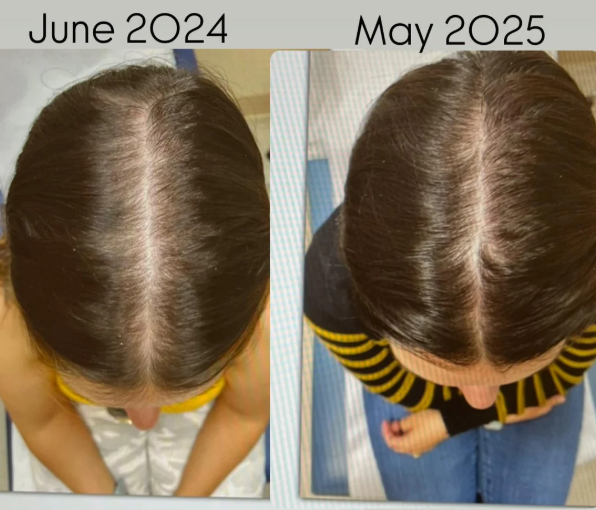
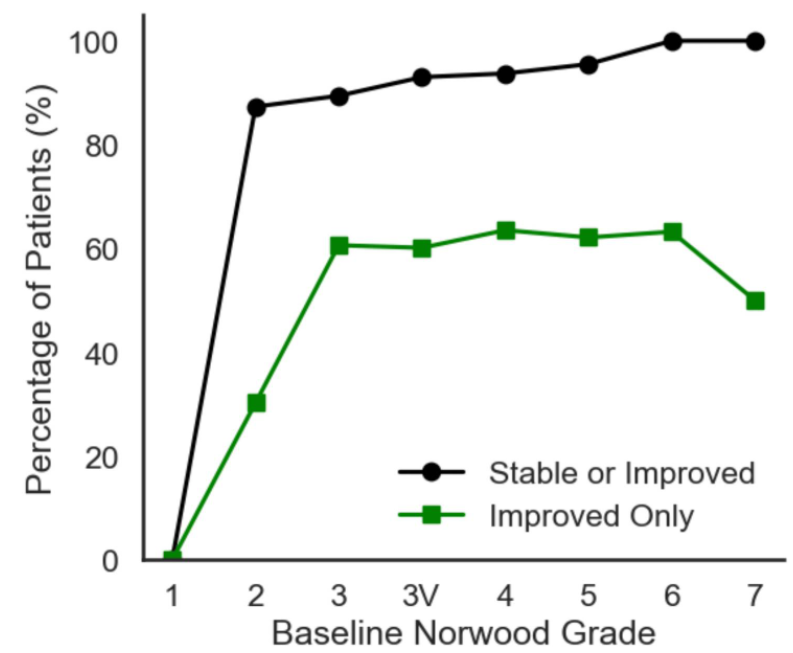






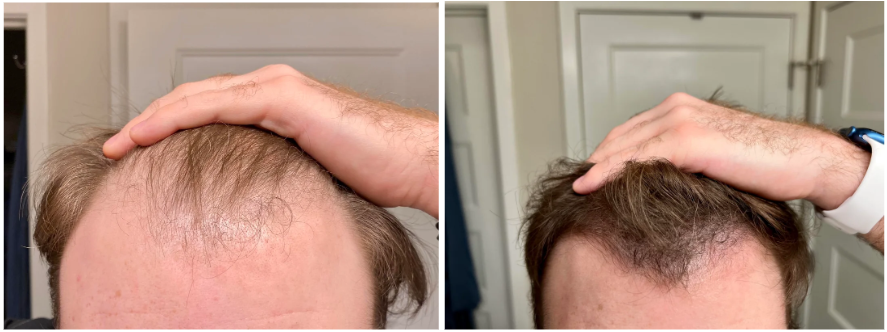
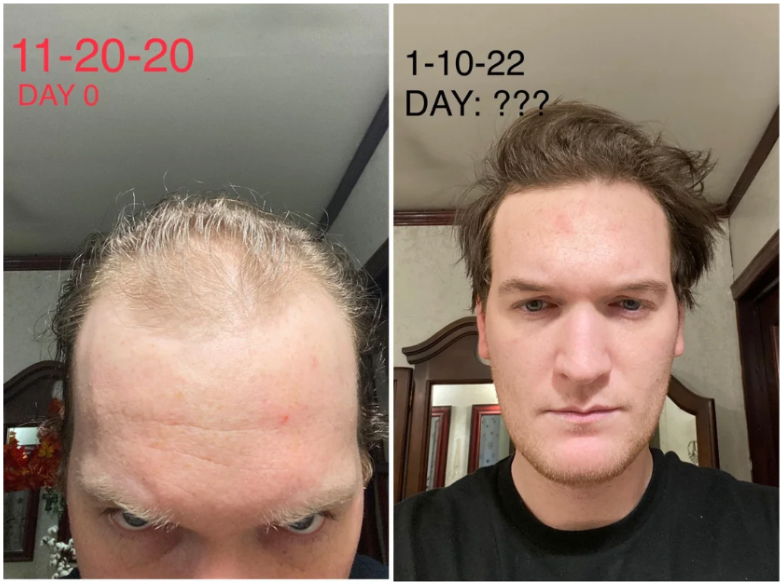

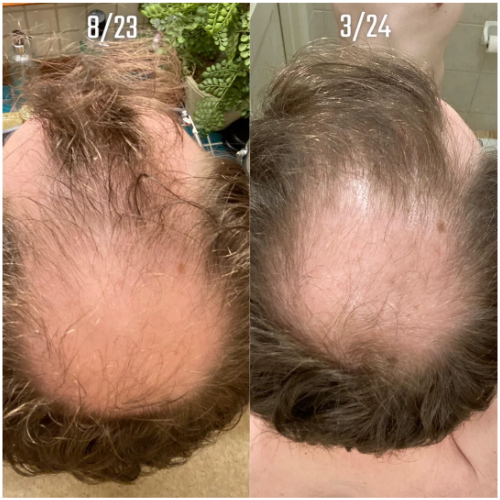
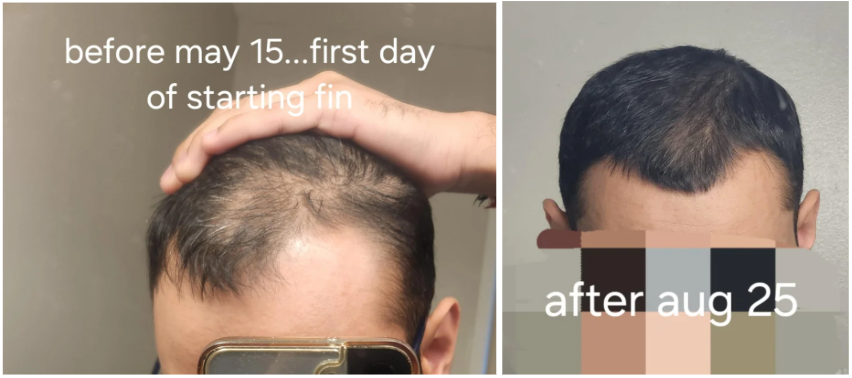

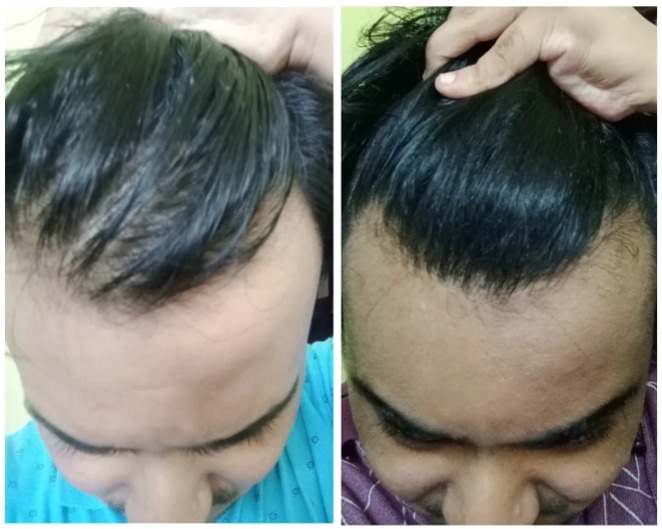
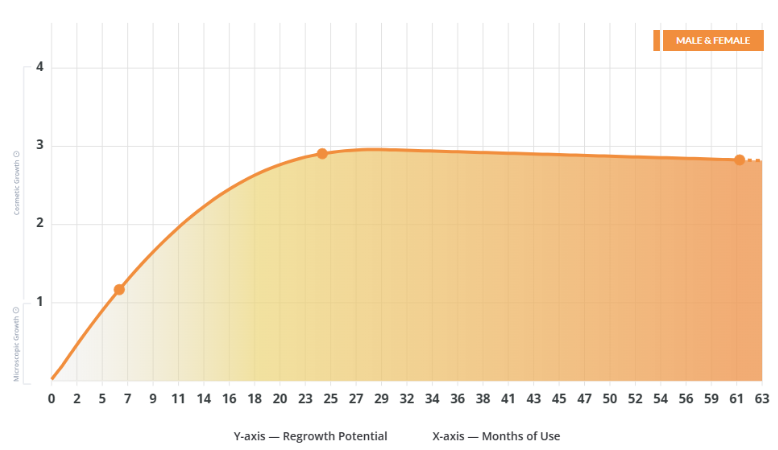








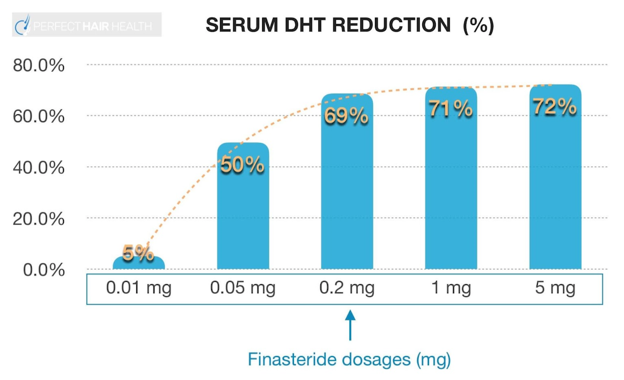
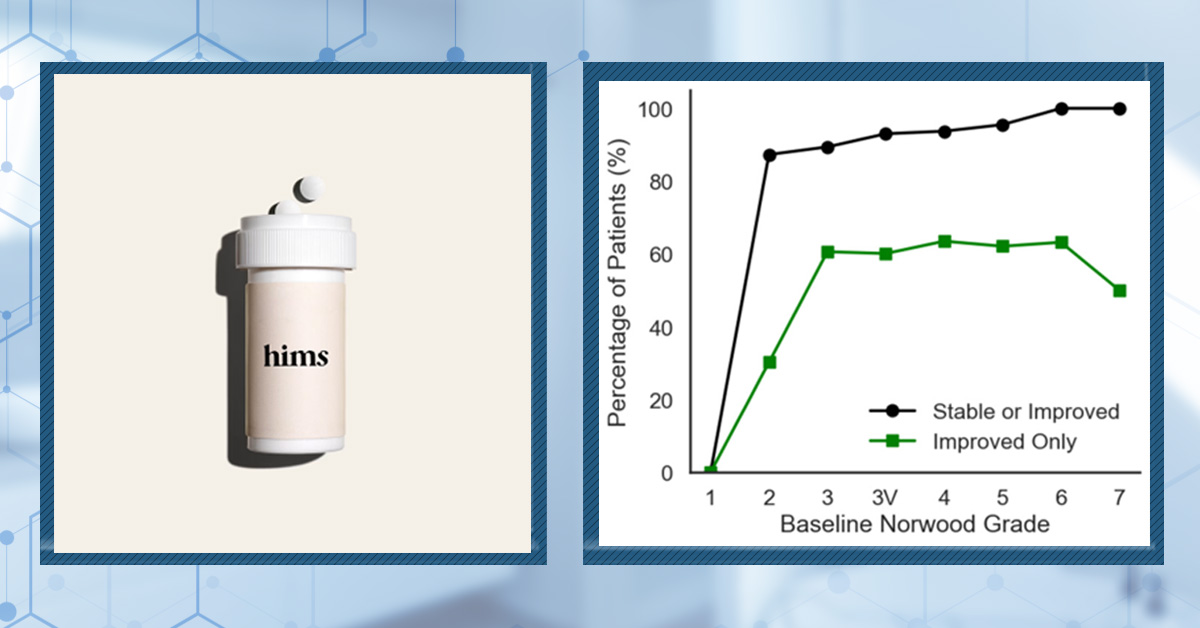
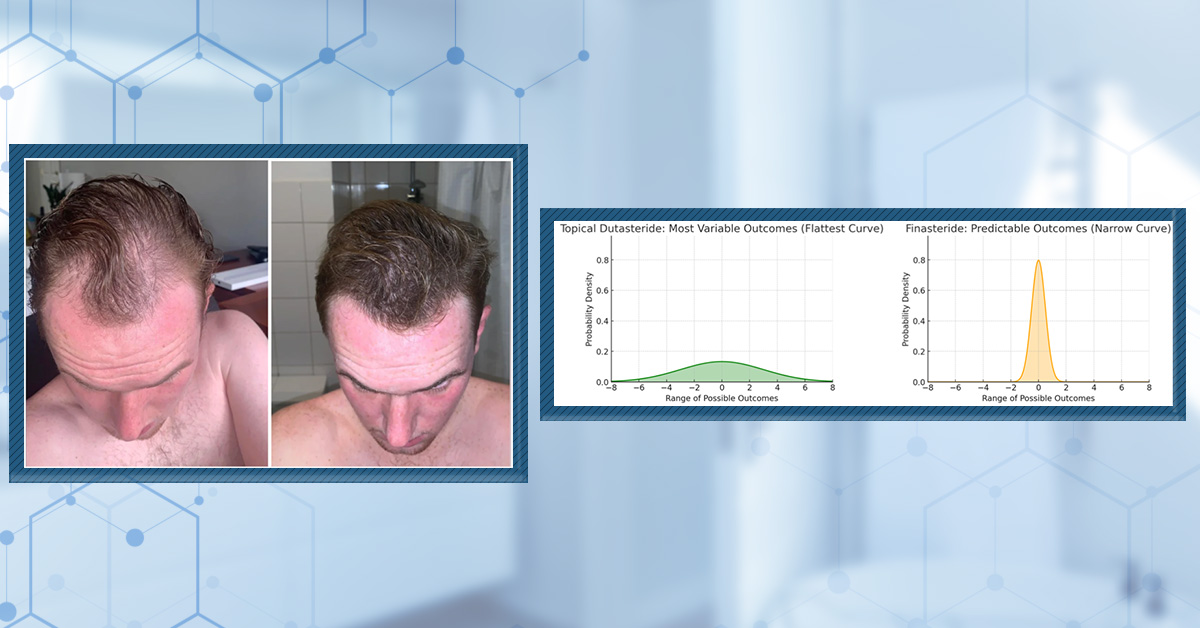



![Oral Minoxidil: Before and After Photos [2026]](https://perfecthairhealth.com/wp-content/uploads/2025/10/93-OM.jpg)
