- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
Articles
Topical finasteride is an effective treatment for androgenic alopecia – particularly for those interested in better localizing the drug’s effects to the scalp. Unfortunately, prescriptions for topical finasteride can cost as much as $50-$100 per month.
This prices out a lot of people who would’ve otherwise committed to the topical had it not been for its costs. It’s also led others to ask, “Can I make topical finasteride at home? Can’t I just crush up my finasteride pills or dilute another topical finasteride?”
The answer to each question is yes, with caveats. While topical finasteride can be made at home – and at a low cost – anyone who intends to try this must formulate the product properly. This might entail:
- Removing the coating of finasteride pills prior to crushing them
- Matching carrier agents when diluting already-existing topicals
- Knowing how much finasteride to add to a formulation to maximize scalp DHT reductions while minimizing the risk of systemic absorption.
- Adjusting finasteride dilutions to control for total daily drug exposure, particularly for those with diffuse vs. localized hair loss.
In this article, we’ll reveal a step-by-step process for how to make topical finasteride – either by crushing finasteride pills or diluting an already-purchased topical. We’ll also provide a topical finasteride calculator that automatically calculates step-by-step instructions for you, all depending on your desired dilutions and starting ingredients. It’s also 100% free. See below.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Oral Finasteride: Efficacy & Side Effects
Oral finasteride is an effective FDA-approved drug used to treat androgenic alopecia (AGA). It reduces a hormone known as type II 5-α dihydrotestosterone (DHT) – which is causally linked to the balding process. Between 0.2 mg to 1.0 mg of finasteride daily can lower DHT levels by 70%, which is enough suppression to therapeutically improve AGA outcomes in 80-90% of male users in two years.[1]https://www.sciencedirect.com/science/article/pii/S0022202X15529357
While most men and women tolerate finasteride without issue, clinical studies consistently show that finasteride adversely impacts a small portion of users. Between 5% to 15% of men trying the drug report mild-to-moderate side effects ranging from diminished libido to brain fog. This is because finasteride does not just reduce DHT in scalp levels, but also across all other tissues in the body such as the brain and testes. For a portion of men and women, DHT reductions of this magnitude across all body tissues can cause undesired effects.
Topical Finasteride: Is It Still Effective? Does It Have A Better Safety Profile?
To reduce the risk of side effects from oral finasteride, many people opt to try finasteride delivered topically. After all, clinical studies demonstrate that compared to 1 mg daily of oral finasteride, daily use of 1 mL x 1% topical finasteride is “non-inferior” to the oral formulation.[2]https://pubmed.ncbi.nlm.nih.gov/19172031/
With topical delivery, users often believe that since they are isolating finasteride to the scalp skin, they can expect less finasteride to reach other parts of the body – thereby preserving DHT levels in other organ sites beyonds the scalp and lowering their risk of side effects.
But is this true?
On the one hand, clinical studies corroborate that some formulations of topical finasteride appear to reduce the risk of side effects versus oral finasteride. On the other hand, the dose per mL of topical finasteride influences this risk – as does the amount of topical finasteride applied daily along with the carrier ingredients used inside the topical formulation.
1. Daily Drug Exposure & Influence On Systemic Absorption
We can use reductions in blood levels of DHT as a proxy to estimate how much topical finasteride leaks from the scalp tissues into the blood stream, thereby traveling to other parts of the body and potentiating DHT reductions other organs.
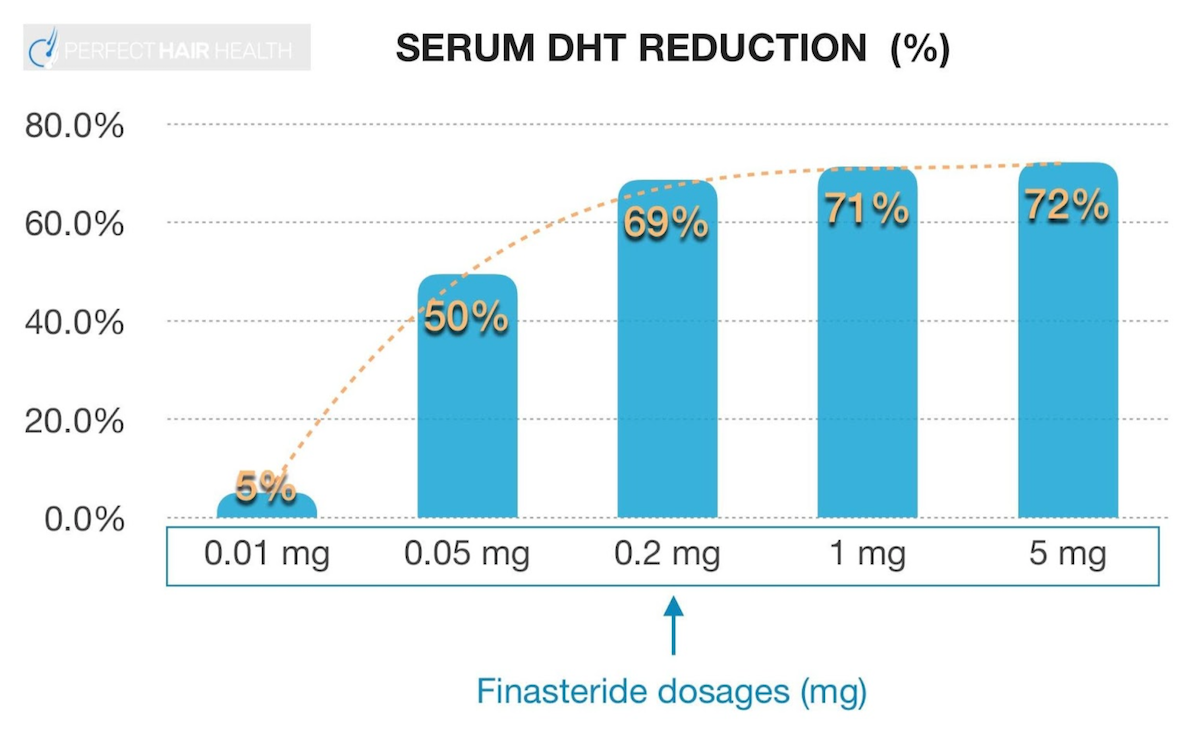
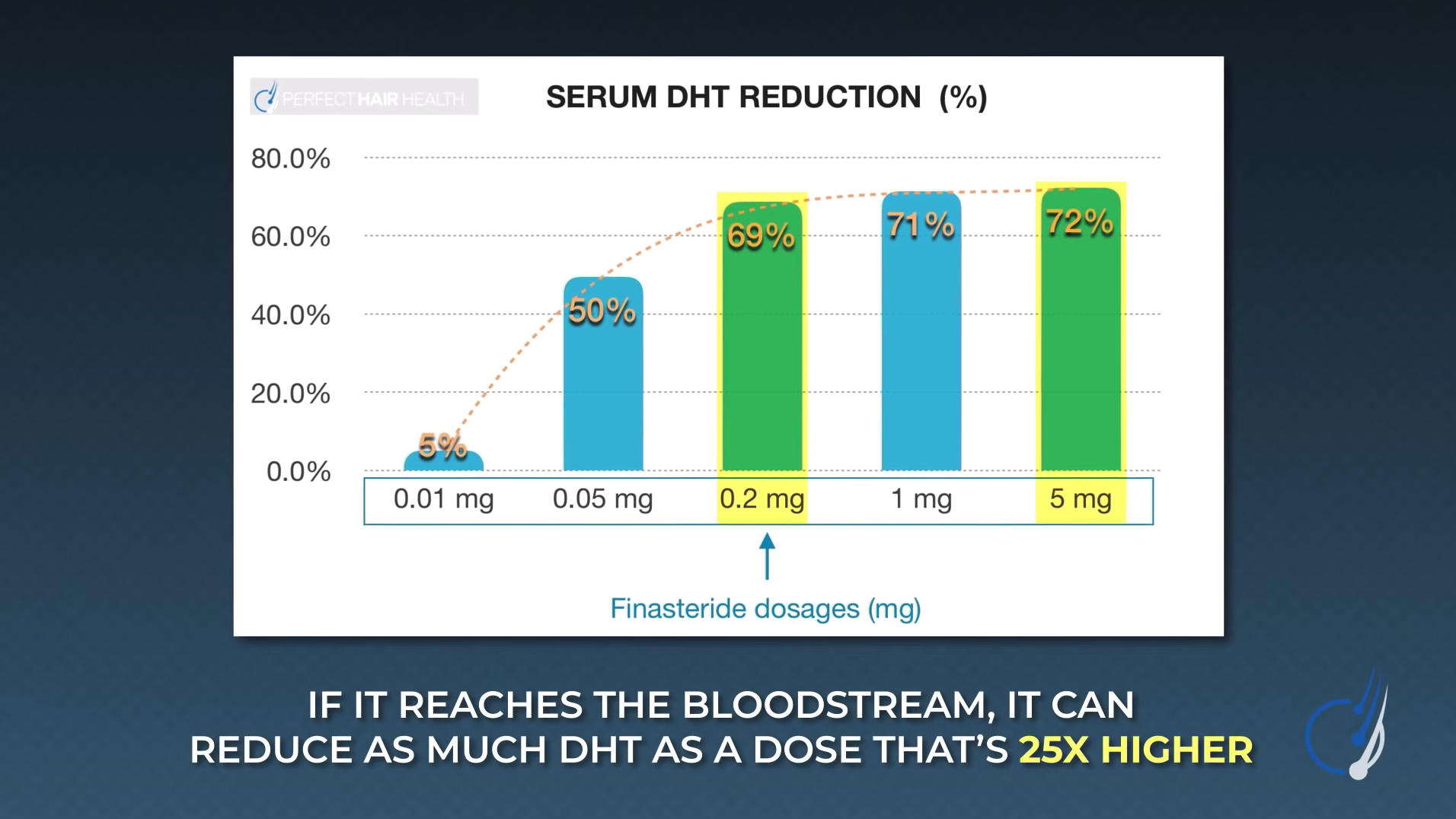
For reference, see the following chart which summarizes clinical studies on topical finasteride in relation to its impact on DHT levels in the blood. Keep in mind that all daily exposure volumes of topical finasteride in this chart were clinically effective at improving hair. On the y-axis, we have the total daily exposure of finasteride (in mg) as a topical. On the x-axis, we show reductions to serum DHT, which acts to estimate the amount of systemic exposure of the drug (even when applied topically).
A graph representing the daily dose exposure of topical finasteride (y-axis) versus the amount of serum DHT reductions in participants (x-axis). Across all studies referenced in the graph, topical finasteride led to hair parameter improvements.
As we can see, according to the clinical literature, the only daily exposure volume of topical finasteride that improves hair loss but does not impact serum DHT levels is 0.005% x 2 mL of topical finasteride daily, which equates to just under 0.1 mg daily of finasteride applied to the scalp.
Therefore, if you intend to make topical finasteride at home with the goal of (1) improving hair loss while (2) minimizing systemic drug absorption, consider finasteride formulations of ~0.1mg daily. At higher daily doses, you can still achieve hair regrowth, but this might come at the expense with more systemic drug absorption and thereby a higher risk of side effects.
2. Carrier Agent Influence On Systemic Drug Exposure
Beyond the daily drug exposure of topical finasteride, the carrier agents used in a topical finasteride formulation also impact the amount of drug absorbed into the skin, and consequently the blood stream.
The outermost layer of the scalp’s epidermis is known as the stratum corneum. This skin layer acts as a barrier for what can enter the skin, and what can escape. It’s also a critical barrier of protection for human survival. Without a stratum corneum, human skin would absorb much more of the outside world – i.e., any nutrients, viruses, microorganisms, and/or pollutants we touch – and we’d be at a much higher risk of exogenous threats.
Unfortunately, the stratum corneum also creates a challenge for topical drug delivery. So, to bypass the stratum corneum, product formulators often add what are called “carrier ingredients” to a topical in order to allow for that topical’s active ingredients to penetrate beyond this layer and deeper into the skin — where that active ingredient can reach target areas and have a therapeutic effect.
Some common carrier ingredients are:
- Alcohol
- Propylene glycol
- Liposomes
But not all carrier ingredients are equally effective at their job. Depending on the active ingredient of a topical, some carrier ingredients do a better job than others at carrying a drug into deeper layers of the skin.
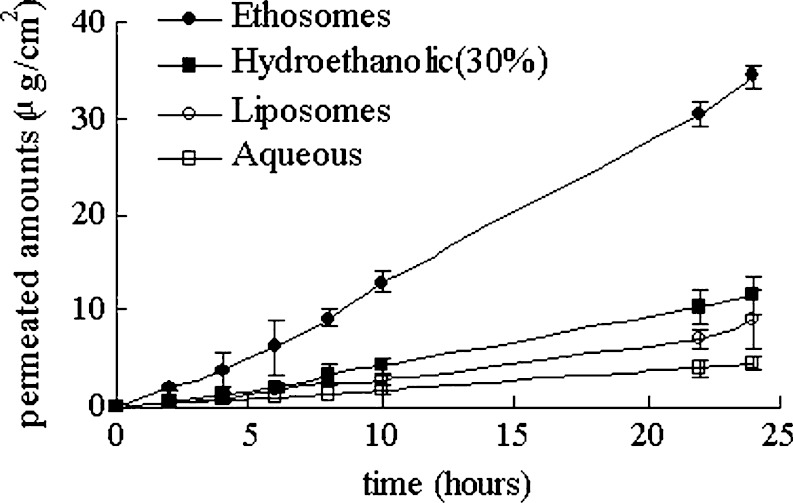
This is particularly true for topical finasteride. Just see this chart from an in vitro study measuring topical finasteride absorption across a 24-hour period, controlling for different carrier agents: ethosomes, hydroethanolic acid, liposomes, and water:[3]https://ncbi.nlm.nih.gov/pmc/articles/PMC2977015/
Penetration profiles of finasteride permeating through human skin from different preparations (mean ± SD, n = 4)
Keep in mind: the more finasteride that permeates into skin tissues, the more finasteride will absorb into the blood stream. Therefore, it is critical to be aware of this relationship when formulating your own topical finasteride at home.
Moreover, if you are diluting topical finasteride from a pre-existing topical, you must ensure that the carrier ingredients used in your new formulation match those used in the already-purchased topical. For instance, if your pre-purchased prescription of topical finasteride uses a liposomal base – which is a gel – but you’re trying to dilute that gel into a liquid formulation (with propylene glycol), the formulations won’t mix well, and you’ll just be wasting product.
How To Make Homemade Topical Finasteride
Here’s a recap of key factors to consider before opting to make topical finasteride at home:
- Control for daily drug exposure. Topical finasteride can still leak into the blood stream, and at higher daily doses, the effects of serum DHT can be as dramatic as if we were using oral finasteride. Formulations greater than 0.1mg daily appear to appreciably lower scalp DHT levels, but also serum DHT levels. With exposure volumes of 1.0 mg daily and higher, serum DHT can become reduced to the same level as oral finasteride.
- Control for daily application volume. Compared to people with localized hair loss (i.e., only temple recession and/or crown thinning), those with diffuse hair loss have a much larger area affected by pattern hair loss, and will thereby need to apply more topical (in mL) versus those with smaller areas of hair loss. Under these circumstances, finasteride dilutions may also need to be titrated in order to maintain a consistent daily exposure volume of the drug. For instance, if we want to keep total topical finasteride exposure consistent at 0.1mg daily, people with localized hair loss may only need 0.01% x 1 mL formulations, whereas those with diffuse hair loss may need to titrate their dilution to 0.005% x 2 mL in order to keep daily drug exposure constant while enabling an extra mL of liquid to cover all areas regions affected by hair loss.
- Select the right carrier ingredients. Different carrier ingredients have different capacities to bring finasteride through the stratum corneum. Over 24-hour penetration periods, one study found that topical finasteride formulated with ethosomes or hydroethanol outperformed formulations of liposomes or water. Having said that, better skin penetration also comes with higher rates of systemic absorption of finasteride into the blood stream.
- If making topical finasteride by diluting a pre-purchased topical, match the carrier ingredients. If you plan on diluting a pre-purchased topical finasteride, it is critical to match carrier ingredients between the old and new formulations.
- If making topical finasteride by crushing up oral pills, peel off any pill coatings prior to crushing. Failure to do this may resort in unwanted debris in your topical formulation and an incomplete dissolution of the finasteride pills in your topical.
If these factors feel overwhelming to control for, we completely understand. For these reasons, we decided to work with developers to build an interactive calculator that generates step-by-step instructions for you to make topical finasteride calculator.
DIY Topical Finasteride: Use Our Free Topical Finasteride Calculator
The following topical finasteride calculator not only controls for daily drug exposure, topical formulations, and carrier ingredients, but it also generates step-by-step instructions for your formulation based on whether you intend to make topical finasteride by crushing finasteride pills or by diluting an already-purchased topical.
Access the free topical finasteride calculator right here.
We hope it helps!
When Homemade Finasteride Is Not Recommended
Technically speaking, making DIY topical finasteride is never advised. After all, finasteride is a prescription drug, and prescription drugs require careful manufacturing and dosing control from compounding pharmacies.
For these reasons, if you’re not so price sensitive, it’s best to leave topical finasteride formulating to the professionals and take yourself out of the equation. Otherwise, you open yourself up to the risks of improper dosing and/or inadvertently exposing others in your household to a drug they never intended to use in the first place.
So, consider getting a prescription of topical finasteride from a dermatologist or telehealth company as your first (and only) option.
Nonetheless, we also recognize that people are going to do whatever they want to do, and that despite us strongly recommending against DIY topical finasteride, people will do it anyway. While this page (and the calculator) are for educational purposes only, and while we obviously recommend seeking medical advice before doing anything, we hope that the resources here help guide those who are making topical finasteride toward doing so as safely and effectively as possible.
Final Thoughts
While we don’t recommend making topical finasteride at home, we also recognize that DIY topical finasteride can save consumers money, enable better control over daily drug exposure, reduce systemic DHT reductions, and lower the risk of adverse events associated with the drug.
Users can make topical finasteride by crushing pills or diluting pre-purchased topical finasteride. Our 100% free calculator can generate step-by-step instructions for you.
Want To Take Your Hair Regrowth To The Next Level?
If you’re looking for personalized support in your battle against hair loss, our membership community is the place for you.
This is where we offer hair loss sufferers one-on-one support – all in an effort to save them years of time, money, and hair. In the first month of membership, community members are often able to simplify their regimen, improve their odds of success, and set themselves up to save tens of thousands of dollars over a lifetime. It starts with an education-first approach that prioritizes the interests of the individual over product sales.
Not a member? Join now to find your path to hair recovery.
We don’t affiliate with any products. Instead, we monetize this site through our personal services. We’ve found that this approach is far more effective for consumers looking to escape information overwhelm online, along with all of the product bombardments on their social media feeds about the latest and greatest “hair loss breakthrough” that, in most cases, is scientifically baseless.
We hope you consider joining. Otherwise, please continue enjoying our free resources.
References[+]
In the medical literature, it’s well established that women should withdraw from finasteride prior to conceiving, or abstain from finasteride throughout pregnancy and/or while breastfeeding. But what about men? Can they continue using finasteride during conception? What about during their partners’ pregnancy — when indirect finasteride exposure (via semen) might expose the female and fetus to the drug?
The answers and evidence aren’t so straightforward. Fortunately, for those concerned of adverse effects from finasteride on either sperm parameters or developing fetuses, there are strategies to mitigate these risks and continue protecting your hair. This article discusses the data, and elucidates a finasteride dosing strategy for men looking to keep their hair while also growing their family.
Interested in Oral Finasteride?
Oral finasteride & minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Is it Safe for Women to Use Finasteride During Conception?
Probably not. Here’s why.
Drugs like finasteride and dutasteride can bypass the placenta, where they can begin to inhibit 5-alpha reductase activity in a developing fetus and interfere with their hormonal profile.[1]https://rep.bioscientifica.com/configurable/content/journals$002frep$002f155$002f3$002fREP-17-0380.xml The hormone that these drugs reduce — dihydrotestosterone — is less relevant in adulthood, but is critical for early development — particularly for males. Subsequently, animal studies have shown that prolonged finasteride use in pregnant females may mutate and/or inhibit the development of male fetus genitalia.[2]https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f96c6acd-4d02-4ece-bd54-2d5a35aab7f5#section-12.2
Since it takes ~30 days for finasteride to clear the body, doctors recommend that women trying to conceive should discontinue the drug at least one month before conception. Some doctors even advise their pregnant female patients to avoid any finasteride exposure whatsoever — including any handling of the medication. This is out of an abundance of caution for the safety of the developing fetus.
What About Men? Can Men Use Finasteride During Conception?
When it comes to men using finasteride during windows of conceptions opinions on whether or not to discontinue the drug are split.
Most providers recommend that men stop using finasteride for at least one month prior to conceiving. Other doctors claim that quitting finasteride is unnecessary, and come at the expense of lost hair. They even go so far as to say there’s “no evidence” that finasteride use for men during windows of conception leads to different health outcomes for their future offspring.
So, which position holds more merit? We’ll detail both sides of the scientific argument below and provide our own take on the data. Then, we’ll reveal a strategy men can employ to temporarily quit finasteride — within a window that shouldn’t compromise any hair gains — and use that window to conceive without (hopefully) losing additional hair.
It all boils down to the length of time it takes for finasteride’s hair growth-promoting effects to wear off versus the length of time the medication takes to clear from the system. Fortunately, there’s a difference here that is in favor of a reproductive window.
Should Men Stop Using Finasteride Before Or During Conception?
In one research article 2001, a group of family physicians examine the perceived versus realized risk of men using oral finasteride during windows of conception.[3]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2018472/pdf/11785276.pdf
First, the authors review evidence surrounding just how much of a daily 1mg finasteride pill ends up detectable in semen:
“In one study, semen levels were measured in 35 men taking 1 mg of finasteride daily for 6 weeks. Highest level measured was 1.52 ng/mL; mean level was 0.26 ng/mL.”
Then, the authors sought to use these numbers to estimate the total exposure of finasteride to a developing fetus — throughout a pregnancy — due to daily unprotected sex.
“Assuming a 100% vaginal absorption through a 5-mL ejaculate per day, women would be exposed to 7.6 ng/d, a negligible amount.”
Finally, the authors contextualized that risk by comparing that exposure level of finasteride to the amount that was required to cause birth defects in monkey studies.
“This level is 750 times lower than the “no effect” level for developmental abnormalities in rhesus monkeys.”
For these reasons, the authors conclude that finasteride use — at least in men — probably doesn’t need to be stopped during pregnancy.
The authors go on to state that, at least for men, the major risk of using finasteride during conception might instead be the drug’s temporary reductions to semen counts, which may impact the ability to conceive, but not the actual health of the baby (more on this later). Thus, while on finasteride, it may be more difficult to conceive within the first few months of using finasteride (when sperm parameters decline) if a male has borderline-low semen levels prior to starting the medication.
Beyond this, the authors seem far less concerned about about finasteride’s effects on the actual conception, such as potential congenital disabilities or risks to male fetal development. In fact, they assert that there’s no data that, during conception, the use of finasteride (for males) interferes with reproductive or fetal outcomes. They recommend that men taking oral finasteride keep taking the drug throughout conception.
Unfortunately, this review was conducted in 2001 — when epigenetics was still an emerging field, and when research groups weren’t necessarily aware of other markers worth measuring that might affect the health of fetal development. Today, in order to completely quell concerns of conception windows and finasteride use in men, what we really need to know is:
- Does finasteride use affect sperm parameters in men?
- Is finasteride use associated with epigenetic and/or DNA damage in sperm, and at doses prescribed for androgenic alopecia?
- Are there actual prospective, long-term studies measuring the outcomes of children whose fathers were using finasteride when they were conceived?
In exploring the answers to these questions, we’ll realize the whole debate over finasteride’s use in men during conception is not as clearcut as it may seem.
Finasteride Use In Men While Conceiving: Affect On Children (Unstudied!)
First, it is true that there are no prospective clinical studies tracking birth outcomes across men using vs. not using finasteride compared to (1) their success with conception, and (2) the health of their offspring. So, does that mean we should assume using finasteride as a male — during conception — is safe?
No. It simply means the question hasn’t been studied. Given that the life and health of a newborn is at stake, our position is that it’s probably better to exercise caution than it is to presume the absence of evidence is a signal suggesting that a behavior that has not been adequately studied must be safe.
Secondly, there’s newer data (from later than 2001) suggesting that finasteride may do more than temporarily lower the ability for men to reproduce via reduced sperm counts, and that these changes may come with a heightened risk of reproductive health.
Finasteride Use In Men: Affect On Sperm Parameters (Studied)
The effects of finasteride use on sperm parameters has been studied in humans at doses of 1-5mg. Here’s what the evidence says.
Overstreet et al (1999)
A 1999 study tested the effects of 1mg finasteride daily on sperm parameters in men. After one year, those using finasteride had an 11% decline in ejaculate volume, compared to 8% in the placebo group. The researchers also noted no significant changes to “sperm concentration, total sperm per ejaculate, sperm motility or morphology.” Resultantly, the team concluded that 1mg daily finasteride use “does not affect spermatogenesis of semen parameters” in men.[4]https://pubmed.ncbi.nlm.nih.gov/10492183/
Given that the study was randomized, double-blinded, placebo-controlled, used the standard dose of finasteride for androgenic alopecia, and ran a full year — many people use this study as justification that finasteride use (in men) probably does not have deleterious effects on reproduction.
Unfortunately, there are two problems with this reasoning.
The first problem is that the study doesn’t actually measure reproductive outcomes, nor does it measure epigenetic changes to sperm. Rather, the study only measure changes to sperms’ histological features: its concentration, sperm per ejaculate, sperm motility, sperm morphology, and semen volume. From a safety perspective, the clinical results are a positive signal. But without long-term studies on fetal outcomes for men using finasteride during conception, they don’t actually answer the question of whether finasteride use in men affect the way their offspring develop.
The second problem is that there are other studies on finasteride that add nuance to finasteride’s safety on sperm parameters — particularly at 5mg doses.
Amory et al (2007)
A 2007 study tested the effects of daily use of 5mg finasteride or 0.5mg dutasteride on semen parameters in healthy men versus placebo. Over six months, the authors found that daily doses of 5mg of finasteride or 0.5mg of dutasteride significantly reduced sperm counts, and by 25-35%. However, at the 12-month mark of continued use, sperm counts for finasteride were still below baseline (i.e., -14.5%), but no longer statistically significantly lower than when the study began.[5]https://academic.oup.com/jcem/article/92/5/1659/2598215
For these reasons, some researchers claim that the effects of finasteride on sperm parameters — if any — are temporary and resolve with continued use at 5mg daily doses, if those changes even exist at all at lower doses (i.e., 1mg).
This should be the end of the story, right? After all, most men are prescribed finasteride at 1mg daily for androgenic alopecia. So, if at 1mg daily there appears to be no effect on sperm parameters, and at 5mg daily the effect seems to diminish with continued use, then men must be in-the-clear to use finasteride while conceiving. Correct?
No.
Again, there are no randomized controlled clinical trials measuring long-term outcomes of children whose fathers were using finasteride before/during their conception. Moreover, sperm counts, motility, and morphogenesis are all histological features of sperm. What isn’t measured in these study is the effect of DNA expression on sperm (i.e., epigenetics). Keep in mind that you can have plenty of sperm that looks healthy, swims effectively, appears normal… but still carries with it genetic expressions that insinuate damage or a heightened potential of birth defects for the fetus.
So, is there any data giving us insights into the potential for finasteride to cause epigenetic changes to sperm (or damage to sperm) that might otherwise affect reproductive capacity and/or fetal outcomes? And no, we’re not talking mouse models that administer high-dose finasteride (which aren’t always applicable to human research, despite what hair loss forums may tell you).[6]https://www.mdpi.com/1467-3045/43/2/62 We’re talking about human evidence.
Yes, there is evidence. And while that evidence isn’t high-quality, it’s still worth discussing.
Finasteride And Infertility In Men: Tu HY, Zini A. (2011), Şalvarci A, Istanbulluoğlu O. (2012)
In 2011 and 2012, two separate case reports were published — each on a male using finasteride long-term who was having trouble conceiving with his respective partner.
Researchers examined sperm morphology — as the studies above did — which appeared normal. But then they examined the sperm through another endpoint: a sperm DNA fragmentation index. This is a measurement to approximate DNA damage (i.e., fragmentation). In both cases, sperm DNA fragmentation was elevated. After discontinuing finasteride, one case report saw sperm DNA fragmentation reduced from 30% to 16.5% within six months.[7]https://pubmed.ncbi.nlm.nih.gov/21292254/ The other case report noted similar improvements to DNA fragmentation, and the successful conception of a baby after discontinuing the drug.[8]https://pubmed.ncbi.nlm.nih.gov/23070721/ The implication: that finasteride use in some men may damage the DNA of sperm, and that discontinuing the drug can improve these outcomes.
Please note: these are case reports, and with such uncontrolled (and unrobust data), there is always the possibility that something aside from the discontinuance of finasteride might be explaining these results. For instance, upon receiving this news, both men featured in the case reports might not have only stopped finasteride, but also addressed other aspects of health to reduce their risk of sperm DNA fragmentation. Examples include supplementing with vitamin D, incorporating more daily activity into their lives, quitting alcohol, getting more consistent sleep, etc.
As such, there’s always the possibility that finasteride was not the majority causative agent in either outcomes.
Our Perspectives: Should Men Continue Using Finasteride While Trying To Have a Baby?
Given the balance of evidence, we feel that men using finasteride should perhaps exercise caution about continuing the drug while also trying to grow their families.
Unfortunately, this position puts many men in an uncomfortable situation. They might feel as though they need to withdraw from a hair-saving drug in order to minimize risks that aren’t necessarily clear, based on the clinical data. Again, those long-term studies on children whose fathers conceived them while using finasteride haven’t yet occurred. They may never occur.
At the same time, this “abundance of caution” comes at a tall expense: lost hair. After all, clinical studies show that after quitting finasteride, any hair that was preserved by the drug is lost and, soon after, hair loss continues at its normal rate.
These risks-benefits are not ours to make for anyone. We’re just here to communicate the data, and the debate. Risk tolerances vary depending on the person, and for many couples, the continued use of finasteride might be the decision made by the male during the windows of conception. This is a decision that shouldn’t be made by us; it should be made by you along with the counseling of your doctor(s).
Nonetheless, if you are looking for an approach to minimize the risks to a developing fetus while simultaneously preserving hair, there is a happy middle ground… and perhaps a way to get the best of both worlds.
Consider Quitting Finasteride Temporarily During Conception
Finasteride is a drug that has a terminal half-life of 5-7 hours, and a biological half-life of around two weeks. In other words, while it takes 5-7 hours for half of the finasteride in your bloodstream to metabolize, it takes 2+ weeks for half of the effects from finasteride use — i.e., the lowering of dihydrotestosterone — to go away. For these reasons, it is estimated that after quitting finasteride, it still takes ~30 days for finasteride (and its effects) to completely leave the body.
This is why most physicians recommend beginning trying to conceive after 30+ days away from finasteride (if female). But after quitting finasteride, how long does it take before hair loss starts to pick back up again?
According to the clinical literature, longer than four weeks. In fact, one study showed that, after one full year of use, men who quit finasteride were still above their baseline hair counts a year after leaving the medication.[9]https://www.sciencedirect.com/science/article/pii/S0022202X15529357 Another study showed that after one year of finasteride use, men who switched to every-other-month of daily medication had the same hair growth outcomes as men who still used the drug every day of the year.[10]https://pubmed.ncbi.nlm.nih.gov/15319158/
Taken together, these studies imply that finasteride’s terminal and biological half-lives might enable a key window whereby men can transition off the drug, conceive without detectable levels of finasteride in their sperm (and/or any adverse effects on sperm from finasteride use), then hop back on the drug — all with little (if any) risk to their hair.
Based on the current data, this window appears to be 1-3 months after quitting finasteride. Within that time, hair loss from withdrawal of the drug should be relatively minimal. And with a two-month reproductive window, this should hopefully be enough time to give couples a good chance to conceive.
What About Topical Finasteride?
For women, the use of topical finasteride within a month before conceiving, during pregnancy, or while breastfeeding is not recommended. This is because a portion of the drug will still go systemic, thereby potentiating adverse events to the developing baby.
For men, there is not yet any data to answer this question — but out of caution, many physicians will just say, “Don’t do it” However, it’s likely that the risks with topical finasteride — especially at lower exposure volumes (0.1 mg daily) — are much smaller than any risks with the oral formulations of the drug (due to less systemic exposure and thereby lower concentrations in the semen).
That said, for those using topical finasteride while starting a family, the Perfect Hair Health team recommends doing everything possible to minimize topical finasteride exposure to a partner while they are pregnant. We’ll have articles on how to do this in the near-future.
References[+]
References ↑1 https://rep.bioscientifica.com/configurable/content/journals$002frep$002f155$002f3$002fREP-17-0380.xml ↑2 https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f96c6acd-4d02-4ece-bd54-2d5a35aab7f5#section-12.2 ↑3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2018472/pdf/11785276.pdf ↑4 https://pubmed.ncbi.nlm.nih.gov/10492183/ ↑5 https://academic.oup.com/jcem/article/92/5/1659/2598215 ↑6 https://www.mdpi.com/1467-3045/43/2/62 ↑7 https://pubmed.ncbi.nlm.nih.gov/21292254/ ↑8 https://pubmed.ncbi.nlm.nih.gov/23070721/ ↑9 https://www.sciencedirect.com/science/article/pii/S0022202X15529357 ↑10 https://pubmed.ncbi.nlm.nih.gov/15319158/ What’s the Prevalence Of Sexual Side Effects From Finasteride?
Does finasteride lower libido? The true incidence of the sexual side effects of finasteride remains up for debate. Finasteride is the world’s best-studied hair loss drug. Over the past 30 years, it has been clinically tested in over 30,000 men – with dozens of randomized, double-blinded, placebo-controlled studies converging on relatively consistent efficacy and safety profiles.
While finasteride is generally well-tolerated, some users have reported sexual side effects after starting the drug. These reports are well documented in the clinical literature. However, their prevalence varies greatly depending on the study referenced.
In well-controlled clinical studies lasting 1-5 years, the incidence of these reports is generally under 7%, with 3-4% of people in the placebo group (sugar pill group) also reporting reductions to libido.[1]https://pubmed.ncbi.nlm.nih.gov/30206635/ In fact, one randomized, double-blinded, placebo-controlled clinical trials in ~2,000 men showed – over one year – that 1 mg daily of finasteride led to reduced libido in 1.8% of users versus 1.3% in the placebo group.[2]https://www.sciencedirect.com/science/article/pii/S0022202X15529357
This seems to indicate the risk of sexual side effects from finasteride is very low. However, other (smaller) clinical studies have put the incidence of sexual side effects as high as 25-30%. It is important to note that these smaller studies are generally of lower quality, so their results should be interpreted with caution.[3]https://pubmed.ncbi.nlm.nih.gov/17655657/ Nonetheless, these studies and the side effects they mention do exist.
Having combed through nearly all the available literature on the subject, we estimate the true incidence of noticeable sexual side effects for finasteride users likely hovers around 3-15%.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Why Does It Seem Like Everyone Online Says Finasteride Lowers Libido?
While side effects from hair loss drugs do occur, their perceived prevalence is often overstated on natural health websites and online forums. There are (at least) two reasons why:
- Financial incentives. Many natural websites fearmonger over hair loss drugs because they want to sell people natural alternatives – supplements and serums – that they claim are free of sexual side effects (which often isn’t true).
- The “Yelp Effect”. The Yelp Effect explains why a restaurant is far more likely to receive reviews from patrons who are dissatisfied (rather than happy) with their experience. Anger motivates us to take action more than contentment does. The same applies to FDA-approved drugs on hair loss forums (especially in online spaces like HairLossTalk and Reddit, where anonymity is preserved).
Can We Reduce the Risk of Finasteride’s Sexual Side Effects?
Yes. There are strategies to potentially reduce the risk of finasteride’s sexual side effects, while also still regrowing hair. These strategies include the following:
- Reduce the dose. As a hair loss drug, finasteride is typically prescribed orally at 1mg daily. However, there is evidence that 0.2mg orally daily is nearly just as effective at improving hair counts.[4]https://pubmed.ncbi.nlm.nih.gov/10495375/ This dose also simultaneously reduces total drug exposure by 80%. For many people, this coincides with a reduction in perceived side effects.
- Try a topical formulation. Studies show that – when formulated properly – topical finasteride may reduce the risk of side effects by 30-90%. One 16-month study on 0.005% topical finasteride demonstrated significant hair improvements, no drug-associated side effects, and no impact on blood hormonal levels.[5]https://www.tandfonline.com/doi/abs/10.3109/09546639709160517 This suggests that, at this dose, there is not enough leakage of finasteride from the scalp into the bloodstream to appreciably alter hormonal profiles – at least in the men and women in this study (though some people inside our membership have reported a different experience).
- Try intradermal delivery methods. Also known as mesotherapy, intradermal delivery methods inject finasteride into the scalp. But rather than use finasteride, consider using another 5-alpha reductase inhibitor: dutasteride. Evidence suggests that scalp injections of 0.01% x 1-2 mL of dutasteride – once every 1-3 months – do not appreciably alter serum hormones, nor do they result in any reported sexual side effects. They do, however, still lead to statistically significant hair improvements. Mesotherapy is more commonly done with dutasteride than finasteride, as dutasteride has a longer half-life (days-to-weeks versus 5-7 hours). This means fewer mesotherapy sessions are required to lower scalp tissue DHT (the hormone lowered by finasteride) for sustained periods.
For topical formulations and intradermal injections, testing personal blood levels of DHT before and during treatment can offer peace of mind. By quantifying the exact changes to serum DHT levels, it’s possible to see just how much topical finasteride (if any) is going systemic. In general, DHT fluctuations smaller than 20% are considered biologically insignificant. For more information on how to test serum DHT, look inside our ultimate guide to finasteride treatment.
So, consider the data first and foremost before giving up entirely on finasteride. It’s by no means guaranteed that finasteride lowers libido. Regardless, there are ways to leverage the power of this effective drug and simultaneously mitigate its risks.
References[+]
What is Finasteride, and Which Finasteride is Best?
Finasteride is a drug approved by the FDA to treat benign prostate hyperplasia and androgenic alopecia. It is prescribed as a 1 mg daily tablet for men with androgenic alopecia. It is also prescribed in higher dosages for women suffering from female pattern hair loss. This ranges from 1.0-5.0 mg daily.
Finasteride is available in different formulations. The best option is determined on a patient-by-patient basis.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What Formulations of Finasteride are Available?
There are two main finasteride formulations: oral and topical. Doctors typically prescribe oral finasteride, as it’s a time-tested formulation with a high success rate. Many telehealth providers have sprouted up in recent years, offering topical and oral versions of the drug. Topical finasteride has become increasingly popular as more studies confirm its efficacy and relative safety versus oral finasteride.
As concluded in one study:
Topical finasteride significantly improves hair count compared to placebo and is well tolerated. Its effect is similar to that of oral finasteride, but with markedly lower systemic exposure and less impact on serum DHT concentrations. [1]https://pubmed.ncbi.nlm.nih.gov/34634163/
Clinical studies have shown that oral and topical formulations improve hair parameters equivalently in target area hair counts.[2]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9297965/ As such, many people looking to minimize their risk of side effects from finasteride often prefer the topical formulation, and they rationalize that decision by arguing that topical finasteride (1) is just as effective as oral finasteride, and (2) remains localized to the scalp, so it must not have any systemic effects elsewhere in the body.
In reality, both of these arguments are wrong.
- While studies do show that topical finasteride is equivalent to oral finasteride in “target area hair counts”, hair count changes outside of these target zones have not yet been measured. Therefore, it is possible that topical finasteride may not protect against hair loss wherever it isn’t applied, whereas the oral formulation tends to provide global protection across the entire scalp. In fact, if topical finasteride does offer hair loss protection in non-applied scalp regions, the most likely reason is that the drug went systemic (i.e., entered into the bloodstream), traveled throughout the body, and redistributed to those non-applied areas. In that regard…
- Topical finasteride can go systemic, depending on the dose. Several studies show that topical finasteride also lowers blood levels of DHT, particularly for daily doses totaling greater than 0.1 mg of finasteride exposure. While the amount of drug in circulation is still far less than that of oral finasteride, people trying topical finasteride should know this, and titrate their topical formulations accordingly. Just see this chart:
A graph representing the daily dose exposure of topical finasteride (y-axis) versus the amount of serum DHT reductions in participants (x-axis). Across all studies referenced in the graph, topical finasteride led to hair parameter improvements.
Finasteride 1mg Oral Tablets
As mentioned, finasteride is typically prescribed as a once-daily 1mg tablet. At 1mg daily, finasteride is sometimes branded as Propecia®. Using more than 1mg per day isn’t likely to improve results.[3]https://pubmed.ncbi.nlm.nih.gov/10495375/
However, it may increase the risk of side effects. Nearly all clinical studies use 1mg, as it’s the gold standard for treating male pattern baldness. 5 mg finasteride is typically used to treat men diagnosed with benign prostatic hyperplasia (under the label Proscar®).
Oral Propecia® (i.e., 1mg daily of finasteride) is prescribed under its brand name and as a generic formulation through many telehealth companies. Generic versions of the drug typically deliver similar results, and often at a fraction of the cost.
Finasteride Topical Formulations
Finasteride topicals include gels, liquid solutions, and liquid sprays. Foams are available as well.
A previous post centered on the best topical finasteride dosage determined that finasteride has a highly-sensitive and dose-dependent response curve.
In other words, 0.01 mg of finasteride barely reduces any DHT, while 0.2 mg reduces almost as much DHT as 5 mg, a much larger dose.[4]https://onlinelibrary.wiley.com/doi/10.1111/jdv.17738 1% topical formulations essentially guarantee systemic absorption.
Those aiming to avoid the side effects may want to consider a formula with lower percentages of the active drug.
Which Finasteride Is Best for Hair Loss?
It depends on two factors: (1) the presence of side effects, and (2) whether a patient has diffuse thinning or localized hair loss.
Side effects
When weighing the pros and cons of finasteride formulas, doctors often have patients start with oral finasteride. This is because oral finasteride has the strongest clinical evidence for treating male pattern hair loss, and it provides some degree of protection across all balding-prone areas.
If side effects occur on oral finasteride at 1 mg daily, doctors may consider lowering the dose to 0.2 mg daily to see if this reduces side effects. If issues persist, other options can be explored – such as topical formulations.
Under these circumstances, users may introduce topical finasteride at a 1-2 mL daily of 0.025% to 0.3% finasteride. If side effects persist, it may be necessary to lower that dose all the way to 0.005% x 2 mL daily, and start tracking serum DHT levels to measure – as a proxy – how much finasteride is actually going systemic (as these levels vary greatly depending on the person and any adjuvant treatments that might be influencing topical absorption – i.e., retinoic acid, microneedling, etc.).
Hair loss patterning
If someone wants to use topical finasteride, they should recognize that topical formulations of the drug are most appropriate for people who have localized hair loss (i.e., hair loss only at the temples and/or crown), rather than people with diffuse thinning (i.e., hair loss throughout the entire scalp).
This is because diffuse thinners have a larger area of the scalp to cover with a topical. That requires a higher amount of mL per application daily of topical finasteride to cover all zones. When holding constance the percentage dilution of topical finasteride, the more mL applied daily, the higher likelihood some of that additional finasteride will leak into the bloodstream and cause systemic effects – thereby defeating the whole effort of the topical in the first place.
For these reasons, diffuser thinners need to take extra care to titrate down their topical finasteride doses, or perhaps consider oral formulations of finasteride to maximize their scalp coverage and thereby improve their odds of long-term success.
For those who don’t experience any sexual side effects, long-term use of oral finasteride may be advisable, given its success rate. And for those who experience adverse systemic effects of oral finasteride, or those wary about potential issues with the oral formulation, topical finasteride may be the better option.
References[+]
Finasteride and dutasteride are drugs that lower the hormone dihydrotestosterone (DHT), which is a hormone that is causally linked to both benign prostatic hyperplasia and male pattern hair loss. While both drugs tend to be effective hair loss treatments for men, they do come with a risk of side effects –most commonly sexual side effects and the growth of male breast tissue (gynecomastia).
These side effects are believed to occur because of finasteride and dutasteride’s inhibitory effects on 5-alpha reductase – an enzyme that helps convert free testosterone into dihydrotestosterone. By inhibiting this enzyme, finasteride and dutasteride are able to therapeutically lower DHT levels to improve the symptoms of an enlarged prostate and/or regrow hair. However, the inhibition of 5-alpha reductase can come with undesired side effects in 5-15% of men using these drugs – mainly due to the hormonal shifts that occur throughout the body when 5-alpha reductase activity is suppressed.
Interested in Oral Finasteride?
Oral finasteride & minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Sexual Side Effects and Hair Loss Drugs
Based on clinical data (so far), there aren’t yet reliable blood tests to determine someone’s risk of sexual side effects from drugs like finasteride or dutasteride. Having said that, clinical studies show that men who have low levels of free testosterone and/or high levels of sex hormone binding globulin tend to be at the highest risk of “low libido”.[1]https://pubmed.ncbi.nlm.nih.gov/25800960/
As such, some clinicians have argued (anecdotally) that patients reporting side effects from finasteride and dutasteride tend to already have hormonal imbalances associated with reduced libido prior to starting the drug. As such, these same clinicians sometimes suggest that by taking measures to (1) improve free testosterone, and/or (2) reduce sex hormone binding globulin – these men tend to see improvements to libido and, as a consequence, sport a higher tolerability for drugs like finasteride and dutasteride.
So, if you’re worried about sexual side effects from finasteride and dutasteride, there is at least some anecdotal and observational evidence suggesting that testing free testosterone and sex hormone binding globulin might help to predict your actual risk tolerance. With that said, it’s important to note that the data here remains limited.
Gynecomastia and Hair Loss Drugs
Gynecomastia is the growth of male breast tissue. It results from prolonged, elevated levels of the hormones prolactin and/or estrogen.
When it comes to the use of finasteride and dutasteride, blood tests can likely be used to determine someone’s risk of gynecomastia from both drugs.
Gynecomastia is estimated to affect between 0.25% to 1% of healthy people using 5-alpha reductase inhibitors, with 5-year retrospective studies in men with benign prostate hyperplasia suggesting an incidence of up to 3% to 5%. [2]https://pubmed.ncbi.nlm.nih.gov/23067029/
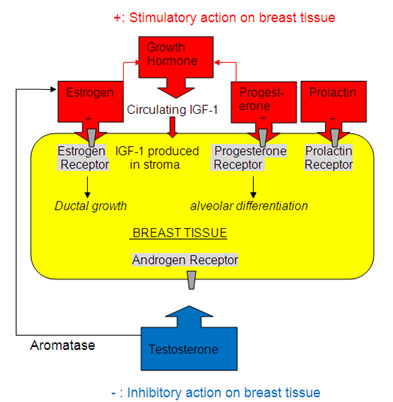
Interestingly, those who start finasteride and/or dutasteride while already having elevated levels of prolactin and estrogen might be at a higher risk of developing gynecomastia. This is because drugs like finasteride and dutasteride can raise blood levels of both testosterone and/or estrogen by 10-20%, depending on the dose.[3]https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf[4]https://journals.sagepub.com/doi/abs/10.1177/2051415820926301 Consequently, as blood levels of estrogen and/or prolactin rise, these hormones can stimulate the growth of ductal tissue and alveolar differentiation – both of which relate to the growth of male breast tissue.[5]https://www.ncbi.nlm.nih.gov/books/NBK279105/
Hormones affecting growth and differentiation of breast tissue. Adapted from “Gynecomastia: Etiology, Diagnosis, and Treatment” (2019)[6]https://www.ncbi.nlm.nih.gov/books/NBK279105/
So, for those starting the drug with borderline-high estrogen, the additional lift in estrogen levels may put someone in the “danger zone” for gynecomastia.
Blood Tests for Finasteride and Dutasteride
For peace of mind, people can always order blood tests for prolactin and estrogen prior to starting finasteride or dutasteride. Additional tests can be performed further down the line.
If levels are within range, the risk of gynecomastia is likely much lower. This can be done with a primary care physician. Those based in the U.S. (and other countries that offer direct-to-consumer lab testing), can order tests through the links below.
- Direct-To-Consumer Lab Test: Prolactin (U.S. only)[7]truehealthlabs.com/product/prolactin
- Direct-To-Consumer Lab Test: Estrogen (U.S. only)[8]truehealthlabs.com/product/estradiol-e2
- Finasteride: Ultimate Guide (Member’s Only)
Not a member? Sign up today to become part of the Perfect Hair Membership Community. You’ll gain instant access to a customized hair regrowth plan, 50+ guides, 30+ product reviews, 60+ case studies, community support forums, expert interviews, and more.
What a Blood Test for Finasteride Can Do
While it’s up for debate if blood tests can actually predict someone’s risk of sexual side effects from finasteride or dutasteride, there is evidence that estrogen and prolactin levels pre-hair loss drugs might give some insights into the risk of developing gynecomastia. The totality of evidence suggests that finasteride and dutasteride may raise estrogen levels by 10-20%. Therefore, if your pre-finasteride levels of estrogen and/or prolactin are within 10-20% of the upper limit, it’s probably best to find ways to lower these levels before committing to the drug.
Diet, lifestyle, and environmental changes are often enough to normalize these hormones in many men.
What about Blood Tests to Predict Hair Regrowth from Finasteride?
The best predictor of hair regrowth from finasteride comes not from a blood test, but from an accurate hair loss diagnosis. After all, two-year clinical studies show that in otherwise healthy men with androgenic alopecia that presents in its standard horseshoe pattern, response rates for finasteride tend to hover around 80-90%.[9]https://www.sciencedirect.com/science/article/pii/S0022202X15529357
Recently, marketers have begun pushing genetic testing to determine someone’s response rate to finasteride and dutasteride. Preliminary data from poorly designed clinical studies suggests that perhaps there are some genes associated with higher-magnitude responses from both drugs, and also a better success rate. For instance, one study suggested that genetic “CAG repeat score” might help determine the response rate to finasteride, and that this data could be collected through blood draws.[10]https://pubmed.ncbi.nlm.nih.gov/31949455/
But again, the evidence here is limited and preliminary. Given the overwhelmingly high odds of a response to finasteride overall, we tend to place more weight on an accurate diagnosis than on genetic testings for either drug.
References[+]
References ↑1 https://pubmed.ncbi.nlm.nih.gov/25800960/ ↑2 https://pubmed.ncbi.nlm.nih.gov/23067029/ ↑3 https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf ↑4 https://journals.sagepub.com/doi/abs/10.1177/2051415820926301 ↑5, ↑6 https://www.ncbi.nlm.nih.gov/books/NBK279105/ ↑7 truehealthlabs.com/product/prolactin ↑8 truehealthlabs.com/product/estradiol-e2 ↑9 https://www.sciencedirect.com/science/article/pii/S0022202X15529357 ↑10 https://pubmed.ncbi.nlm.nih.gov/31949455/ I Am Experiencing Side Effects From Minoxidil. What Should I Do?
If experiencing side effects from minoxidil, it’s best to speak with the prescribing physician as soon as possible to discuss next steps. Discontinuing use, however, is not the only option. There are strategies to reduce minoxidil’s side effects (while still benefiting from the drug) in both topical and oral formulations. This article will focus on reducing adverse events from topical minoxidil, depending on the side effect you’re experiencing:
- Skin irritation, dandruff, and/or dermatitis
- Water retention, skin aging, and/or bags under eyes
- Headaches and/or heart palpitations
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Topical Minoxidil: Reducing the Side Effects
For the most part, side effects from minoxidil are minor. Here are a few adverse events reported in the clinical literature (and online), the percent of people they tend to affect, and strategies on how to go about reducing or resolving them.
Skin Irritation, Dandruff, and/or Dermatitis (2-7% of users)
Skin irritation, dandruff, and/or dermatitis are the most frequently reported side effect from minoxidil. These tend to be reported by 2-7% of topical minoxidil users.
One study demonstrated that 80% of these reports were not actually due to minoxidil, but the carrier ingredient used to help minoxidil penetrate into the dermis: propylene glycol.[1]thaiscience.info/…ticle/JMAT/10986429.pdf
In these cases, simply switching to a minoxidil product without propylene glycol solved most reports of skin irritation. As such, if you’re experiencing these problems, you may want to experiment with switching minoxidil brands or formulations — specifically to a product that does not contain propylene glycol. Examples include:
- Essengen-5 NO PG FAST DRY (from MinoxidilMax)
If skin irritation persists on these new formulations, consider titrating the dose of topical minoxidil. You can achieve this by moving from twice-daily 5% minoxidil to once-daily 5% minoxidil. If that doesn’t work, try moving from once-daily 5% minoxidil to once-daily 2% minoxidil. If that doesn’t work, consider trying oral minoxidil at doses from 0.25mg to 5.0mg (more on this below).
Join the Community
Not a member? Join the Perfect Hair Health Membership Community to build your customized regrowth roadmap and receive personalized minoxidil recommendations.
Water Retention, Skin Aging, and/or Under-Eye Bags (prevalence unknown)
Some topical minoxidil users have reported under-eye bags and/or signs of accelerated skin aging. However, these reports are not reflected in the clinical literature; they’re anecdotal. Even still, while there’s currently no evidence (to which we’re aware) that minoxidil accelerates aging, there is a mechanistic argument to be made that topical minoxidil might increase the perception of skin aging and/or under-eye bags. This is likely due to two factors:
- Skin irritation/dryness from applying the drug, which can make the skin appear to be “more aged”. Keep in mind that studies suggest that 80% of these side effects are caused by propylene glycol and not the minoxidil itself. Therefore, switching brand formulations should resolve this dryness, and thereby the perception of accelerated skin aging.
- The drug leading to more water retention near the areas where it is applied. If this occurs in facial tissues, that water retention comes with the possibility of exaggerating the appearance of under-eye bags, and thereby skin aging.
If these side effects reflect your own experiences with topical minoxidil, consider the following:
- Wait and watch. For many people, the water-retaining effects of minoxidil are transient and go away with continued use of the drug.
- Switch formulations. Finding a minoxidil formulation without propylene glycol should go a long way toward resolving side effects related to dryness or skin irritation.
- Reduce salt intake. Salt is also water retentive, and it’s possible that people consuming lots of salt and using topical minoxidil might be at a higher risk of experiencing more exaggerated water-retentive effects from the drug. By lowering salt intake, this may be enough to resolve excessive water retention and diminish the presence of under-eye bags while still using minoxidil.
In most cases, these changes make enough of an impact to reduce, mitigate, or even eliminate these side effects altogether.
Headaches and/or Heart Palpitations(~1% of users)
In rarer cases, topical minoxidil results in heart palpitations or headaches. In some cases, these side effects are related to the formulation of minoxidil; in others, they’re due to the drug itself. If you don’t intend on quitting the drug outright after having experienced these effects, it’s critical to tease out which category you fall into — and to approach troubleshooting very carefully.
Headaches
If you’re experiencing a headache after topical minoxidil applications, the first question to ask is: is it the minoxidil itself, or an ingredient applied alongside the minoxidil that’s causing this experience?
In many cases, the scent of topical minoxidil is what’s causing someone’s headache after applying the drug topically. This can be due to a scent added to the formulation, or even the off-gassing of the alcohol (if you’re using a topical that contains ethanol or an alcohol as a carrier ingredient).
Under these circumstances, simply switching topical minoxidil brands to something that is (1) unscented, and (2) does not contain alcohol should be enough to resolve symptoms. If this doesn’t work, it’s likely that the headaches are a direct result of the minoxidil itself.
If this is the case, consider titrating the dose of minoxidil from twice-daily 5% minoxidil to once-daily 5% minoxidil. If that doesn’t work, consider trying nanoxidil — a minoxidil analogue that has a lower molecular weight and may confer a slightly better safety profile (at least according to very biased research from the company selling nanoxidil, DS Laboratories). Anecdotally, members of our community who have made this switch have mostly reported resolution of headaches secondary to topical minoxidil after switching to topical nanoxidil. So it’s not a bad idea.
If that doesn’t work, minoxidil may not be the right medication for you — at least when it comes to fighting hair loss. The good news is there are many other options.
Heart Palpitations
If you’ve noticed that, after applying topical minoxidil, your heart feels as though it “skips a beat” or begins beating irregularly, these signs are indication of a drug sensitivity to minoxidil itself. These effects are rare, but they likely impact up to 1% of people who have tried topical minoxidil.
If this is your experience, please speak to a medical professional and strongly consider discontinuing the medication. We do know of people who’ve managed these side effects by titrating the dose of topical minoxidil from twice-daily 5% to once-daily 5%, and even lower. Having said that, it’s critical to remember that our heart is more important than our hair. There are many other treatment options aside from topical minoxidil for your hair loss, and if you’re getting heart palpitations after applying the medication, it’s probably best to start exploring those rather than manage a medication that — despite being FDA-approved — still has lower qualities of evidence supporting its long-term use.
References[+]
References ↑1 thaiscience.info/…ticle/JMAT/10986429.pdf Topical Finasteride
Finasteride is a hair loss drug for treatment of androgenic alopecia. It inhibits the 5-alpha reductase type II enzyme that converts testosterone into dihydrotestosterone, or DHT.
Topical finasteride formulations (think: solution, spray, or gel) are growing in popularity because of their localized approach. Oral finasteride reduces DHT everywhere in the body. Topical finasteride reduces DHT in the area that it is applied while minimizing systemic absorption. That is, if you apply it correctly. The question of how to apply topical finasteride for the greatest benefit and the least systemic absorption has no single, easy answer.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Applying Topical Finasteride to the Scalp
Strategies for applying topical finasteride depend on its formulation (spray, solution, serum, or gel). We’ll review those first, then explain how long topical finasteride needs to stay on the scalp in order to absorb and have an effect (hint: it depends on the dosage and carrier agent).
How to Apply Topical Finasteride Spray
Many hair loss sufferers opt for topical finasteride spray. These sprays are often used to disperse the drug over wide surface areas of scalp skin, and are most appropriate for people with (1) thinning over widespread areas who also (2) are keeping their hair very short so that the scalp skin is easily accessible.
If you have diffuse thinning and longer hair, sprays might not be right for you. This is because too much of the topical finasteride will end up on the hair (where it does nothing) rather than the scalp (where hair grows from).
Users are advised to hold the spray bottle 2-3 inches from the scalp and apply the prescribed amount on the crown, top, and front of their heads. The hands can then be used to rub in any liquid blocked by existing hairs. This will ensure that a considerable amount of the formula has reached the scalp, particularly in problem areas.
Some users prefer to spray the formula into their palms and apply it manually with their fingertips. As with other formulas, parting sections of hair may make it easier to apply.
How to Apply Topical Finasteride Solution
Topical finasteride is also prescribed in the form of a liquid solution. These variations may include minoxidil as a combination therapy. Such formulas typically include a dropper for easy application. To apply the solution, part the hair in sections, apply a few drops along the part, then rub the solution around the scalp skin with the fingertips. Additional drops can then be applied to key problem areas (crown, top, and both sides of the front).
Alternatively, the prescribed amount can be dispensed into the hand and applied manually.
How to Apply Topical Finasteride Gel
Gel formulations are often made of bases of silica and/or liposomes. They’re very popular, as they are easy to apply. For best results, users can start with a few pumps of the gel, applying with their hands to provide even coverage across each problem area. The gel should gently be massaged into the scalp for 30 seconds or more.
No matter the treatment form, it’s only necessary to cover the areas affected by hair loss. Users should always follow their doctor’s recommendations and consult the directions included in their formula.
Any excess formula should immediately be washed off of hands and other areas of the body.
How Long Should Topical Finasteride be left on Scalp?
How long to let topical finasteride sit on your scalp depends on several factors, including:
- The dilution (the % finasteride in your topical formulation)
- The daily mL applied (alongside % dilution, this will determine your daily total exposure volume of finasteride in mg)
- The carrier ingredients in your topical (this will determine how much – and how quickly – topical finasteride will move from outside of the scalp into the dermis, where it can actually elicit an effect on hair follicles)
To understand more of this process, we also need to understand the stages of absorption when applying any drug to our scalp skin:
- Stage #1: the drug sits on the epidermis (i.e., the outermost layer of skin). Over several hours, some of the drug will absorb into the dermis, and some will evaporate.
- Stage #2: the drug absorbs percutaneously. This is when some of the drug moves from the epidermis into the dermis, where it can begin to affect hair follicles.
- Stage #3: the drug absorbs systemically. This is when some of the drug moves from the dermis into the bloodstream, where it can have systemic effects.
An in vitro study measured percutaneous absorption of topical finasteride across a variety of different formulations: ethosomes, ethanol, liposomes, and aqueous (water). [1]ncbi.nlm.nih.gov/pmc/articles/PMC2977015
This chart shows the percent of topical finasteride that percutaneously absorbs (enters the dermis) over a 24-hour period:
Penetration profiles of finasteride permeating through human skin from different preparations (mean ± SD, n = 4)
There are three big takeaways from this chart:
- The longer topical finasteride is left on the scalp, the more drug will be absorbed percutaneously.
- Across carrier ingredients, absorption rates appear to be linear. At 10 hours of contact, 2-13 micrograms of finasteride are absorbed into the skin, depending on the carrier. At 20 hours, those numbers double to 4-26 micrograms.
- When it comes to finasteride skin penetration, carriers rank from best to worst as ethosomes > hydroethanol > lipsomes > water.
Finasteride Absorption Rates
Leaving finasteride on the scalp for 10 hours allows for 5 micrograms per square centimeter of percutaneous absorption across most carrier agents. Is this enough finasteride to therapeutically lower scalp DHT levels? More importantly, is it enough to regrow hair?
There is not a clinical study that attempts to answer this. However, there is some surrogate data to help us approximate the answer.
A certain percentage of topical finasteride will absorb into the dermis. The bloodstream will absorb some of this later. Time-dependent DHT reductions in the bloodstream can be used to “ballpark” how much DHT is likely also being reduced in the scalp. This is because there is more topical finasteride that percutaneously absorbs than systemically absorbs. For lower dosages, the effects we see in the system can be used as signals for what’s happening – at a minimum – in the scalp skin.
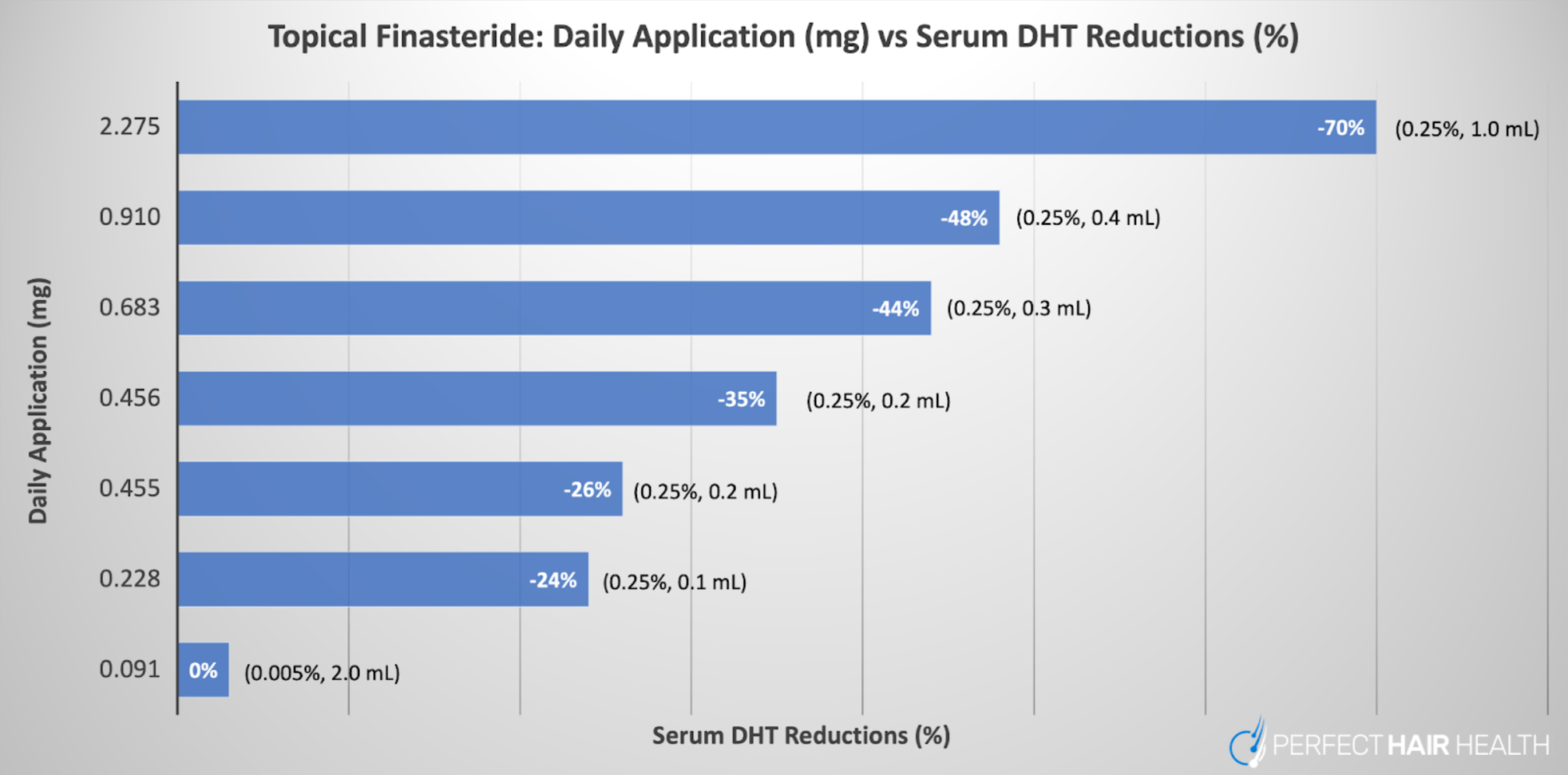
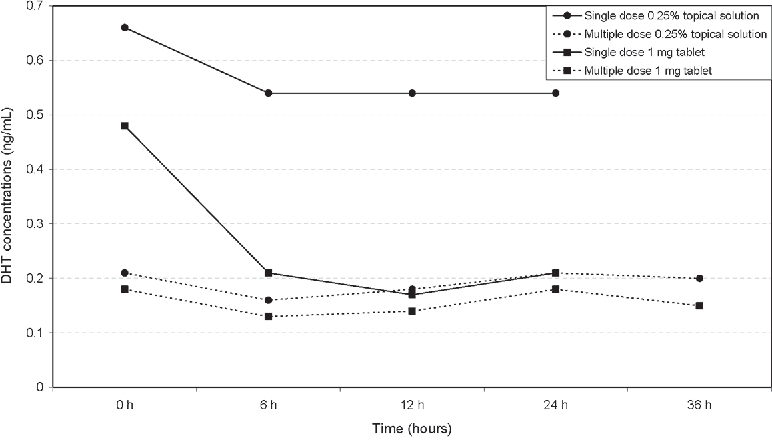
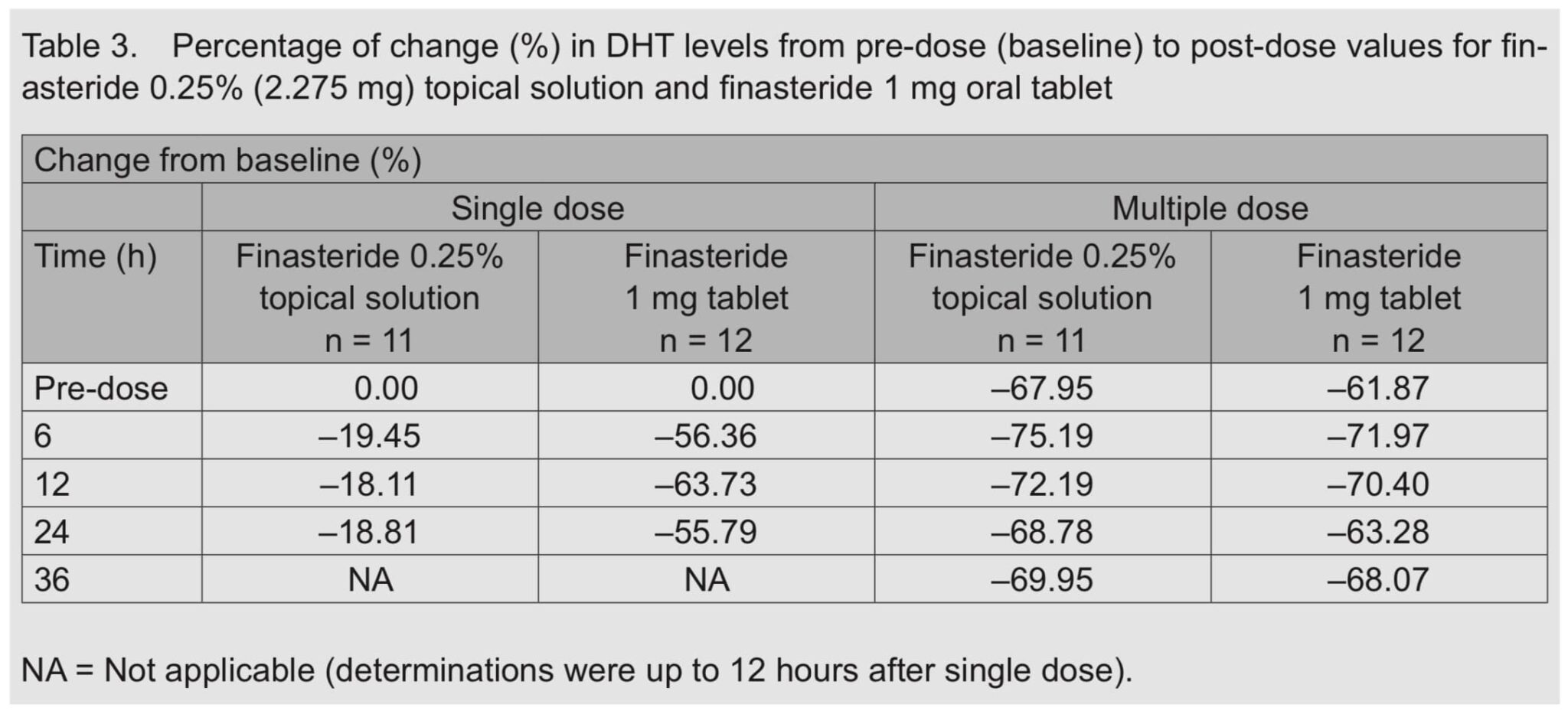
This figure from a 2014 study measures the effects of one versus two applications of 1 mL of 0.25% topical finasteride on serum DHT levels. [2]https://pubmed.ncbi.nlm.nih.gov/25074865/
Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. International journal of clinical pharmacology and therapeutics, 52(10), 842–849.
Optimal Amount of Time
Six hours after applying 1 mL or 2 mL applications of 0.25% topical finasteride (i.e., 2.275-4.550 mg of finasteride), serum DHT reductions flatline. By the 6-hour mark, the 2ml application group experienced roughly the same serum DHT reductions as would be expected from a 1mg oral dose of finasteride.
Finasteride has an upper limit for its effects on serum DHT reduction. After ~70% reduction in serum DHT, adding more finasteride doesn’t reduce more serum DHT. The same is true with scalp DHT reductions.
Topical finasteride applications of 2.275 mg or higher (with a hydroxypropyl chitosan delivery vehicle) achieve systemic reductions in DHT. Furthermore, the larger dose of topical finasteride reduces systemic DHT levels on par with oral finasteride.
Serum DHT reductions are only achieved after scalp DHT reductions occur. Because of this, the conclusion can be drawn that 6-12 hours after applying topical finasteride, there’s likely enough percutaneous absorption to therapeutically lower scalp DHT levels for hair regrowth. However, this time window depends on a number of factors, including:
- Finasteride dilution (%). After all, the percent of finasteride, in part, determines your total daily exposure of finasteride. Remember that finasteride’s absorption is linear, so the higher the dilution, the more finasteride will absorb.
- The daily mL applied. Along with the percentage dilution of finasteride, the mL of topical applied will determine our total daily exposure of finasteride placed on the scalp.
- The carrier ingredients used. As we learned in the previous study, water-based carriers perform the worst at carrying finasteride into the skin, while ethosomes and water-alcohol mixes perform the best.
The next logical question becomes, just how low of a dilution of finasteride can you apply to evoke hair growth outcomes, while still potentially preserving serum DHT levels to the best of your ability? Interestingly, there seems to be a “sweet spot” for this at ~0.1 mg daily of topical finasteride mixed with alcohol and propylene glycol as carriers.
A 1997 study corroborates this. The study suggests that 2 mL daily of 0.005% topical finasteride (i.e., 0.0912 mg of finasteride exposure) improved hair parameters for men with AGA. This was achieved without affecting serum DHT levels – even after 16 months of treatment. [3]https://www.tandfonline.com/doi/abs/10.3109/09546639709160517
For Most Topical Finasteride Formulations, 6-12 Hours is Long Enough
Most big-brand topical finasteride companies are:
- Selling dilutions mixed with alcohol, propylene glycol, or glycerin
- Selling dilutions of 0.1% to 0.3% finasteride
- Advising patients to apply at least 1-2 mL daily
For most users, this will equate to 1 mg to 6 mg of topical finasteride exposure daily – which is more than 100x the minimum viable dose of topical finasteride. At these dosage ranges, the evidence suggests that topical finasteride will only need 6-12 hours on the scalp to therapeutically lower scalp DHT levels and start encouraging hair growth.
Topical finasteride users should use this time window to their advantage! But they should also keep in mind that, at most big-brand dose ranges, they’re exposing themselves to just as much (if not more) finasteride than the oral formulations. So they might be overpaying for topical finasteride in hopes of “localizing” its effects, only to also have just as much – if not more – of that drug going systemic.
To get the true benefits of localization, users will likely need to drop their dose as low as 0.005% x 2 mL daily, and periodically track serum DHT levels to ensure systemic effects are minimal.
The instructions on the packaging aren’t always as precise as the research. Best to trust the science when it comes to how to apply topical finasteride for maximum effectiveness, and minimal side effects.
References[+]
According to 2021 estimates, over 20% of people in the U.S. use antidepressants or antianxiety medications. Within this group of medications, hair loss is sometimes listed as a known side effect.
Mostly, these drugs cause temporary hair loss that goes away after a period of acclimation or after the drug is discontinued. But for the 1 in 5 people who use these drugs to support mental wellbeing, there’s a very real fear that mood stabilization might only be available at the expense of their hair.
The good news is that hair loss from anxiety medications is relatively uncommon. And while it can occur, there are ways to mitigate the risk of hair fall.
This article takes a scientific look at the relationship between the most commonly prescribed psychotropic drugs and hair loss, revealing science-based recommendations for those who are affected by hair loss and worried that their antidepressants are to blame.
- What type of hair loss is associated with psych meds?
- What evidence shows a link between psychopharmaceuticals and hair loss?
- Is there indeed a cause and effect relationship?
- What does this mean for those prescribed to these medications?
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Types of Drug-Induced Hair Loss
Drug-induced alopecia typically falls into two main categories: telogen effluvium and anagen effluvium. These names refer to the stage in the hair cycle where growth is interrupted.[1]https://link.springer.com/article/10.2165/00002018-199410040-00005
Telogen Effluvium
Telogen effluvium is the most common type of drug-induced hair loss. With this condition, hair follicles are prematurely triggered to enter their resting (telogen) phase, which induces early shedding. The condition typically becomes noticeable within 2-4 months of beginning treatment.
Anagen Effluvium
Anagen effluvium occurs during hair’s growth (anagen) phase. Hair cells that should be rapidly dividing are triggered to abruptly stop. This type of drug-related hair loss occurs within days or weeks of treatment and is not limited to the scalp. It is most commonly associated with chemotherapy drugs.
Medication-induced alopecia, in particular telogen effluvium, is a known side effect of some psychopharmaceuticals. Hair loss is typically temporary and may resolve on its own, or with a reduction in dosage. Discontinuation of these drugs nearly always leads to hair regrowth.[2]https://pubmed.ncbi.nlm.nih.gov/10798824/
What About Androgenic Alopecia (AGA)?
Psychopharmaceuticals do not cause androgenic alopecia (male pattern baldness), but may temporarily accelerate the condition. If several hair follicles in AGA-prone regions suddenly enter the telogen phase, hair follicle miniaturization can increase, speeding the progression of AGA.
Psychotropic Medications and Hair Loss: The Research
It’s important to note that just because hair loss is listed as a potential side effect of certain medications does not mean it will happen to everyone. But, it can still happen. What’s most important is the percent of people reporting hair loss in a clinical trial for any of these drugs.
Among the class of drugs referred to as psychopharmaceuticals, hair loss is most frequently associated with long-term use of lithium and valproic acid.
As many as 19% of lithium users and 12% of valproic acid users report the unwanted side effect.[3]https://pubmed.ncbi.nlm.nih.gov/10798824/ For most other drugs, risk is much lower, and possibly as low as 0.01%.
To learn more about the actual connection between antidepressants, anti-anxiety medications, mood stabilizers and hair loss, the Perfect Hair Health team combed through hundreds of case studies.
The Process
First, the team looked at the most commonly prescribed medications and then ran those drug names through a research database to pull up any and all studies that mentioned hair loss. Below is an overview of what was found.
If a drug is not the list, it’s either because no reports of hair loss were not found or commonly prescribed. Others were simply not worth mentioning for various reasons. To jump to a particular drug, use the links below.
Antidepressants
- SSRIs
- Escitalopram (Lexapro)
- Fluoxetine (Prozac)
- Paroxetine (Paxil)
- Sertraline (Zoloft)
- Atypical Antidepressants
- Bupropion (Wellbutrin)
- Atypical Antipsychotics
- Olanzapine (Zyprexa)
- Quetiapine (Seroquel)
Anti-Anxiety Medications
- Benzodiazepines
- Clonazepam (Klonopin)
- Buspirone
- Antimantic Agents
- Lithium
- Valproic Acid
Evaluation Of Evidence Quality
Of the several studies cited below, most consist of case reports that reference just a single individual. What’s more, these case reports often make it to publication precisely because of their uniqueness. In these reports, researchers often make reference to ‘the first known case’ or ‘the only known case.’
Case studies rank relatively low on the hierarchy of evidence, as they are very anecdotal, and not supported by double-blind research, for example. This makes it hard to know the true incidence or prevalence of hair loss from many of these drugs.
Antidepressants and Hair Loss
Antidepressants are of several different classes, namely selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), MAOIs, and atypical medications.
SSRIs and Hair Loss
Selective serotonin reuptake inhibitors (SSRIs) are among the most commonly prescribed antidepressants and are sometimes used to treat anxiety disorders. Of these, hair loss is associated with the following medications:
- escitalopram (Lexapro)
- fluoxetine (Prozac)
- paroxetine (Paxil)
- sertraline (Zoloft)
The team did not find any studies linking two other common SSRIs, protriptyline (Vivactil) and amitriptyline (Elavil) with hair loss. Take a closer look at what was found.
Escitalopram (Lexapro)
2021 Case Study:
A male patient diagnosed with major depressive disorder experienced hair loss with escitalopram. The patient discontinued escitalopram when he could no longer tolerate the hair loss. The hair loss stopped within one month.[4]https://www.psychiatria-danubina.com/UserDocsImages/pdf/dnb_vol33_no2/dnb_vol33_no2_187.pdf
2020 Case Report:
A female patient on 5mg escitalopram reported minor hair loss. After her dose was increased to 10mg, hair loss became ‘significant.’ After quitting the drug due to intolerable hair loss her symptoms ‘dramatically’ resolved within one week.[5]https://www.psychiatrist.com/pcc/depression/escitalopram-induced-hair-loss/
2016 Case Study:
This case study is unusual because the female patient presented with eyelash loss 12 weeks after beginning treatment with escitalopram. Her eyelashes returned to near normal 5 weeks after she stopped taking the medication.
While SSRIs are known to cause alopecia, this is the first reported case of eyelash loss. Researchers noted the timing of the patient’s presentation was consistent with the growth cycle of eyelashes, suggesting the mechanism of loss was interruption of the hair growth cycle.[6]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035805/
Norton DJ, Cates C. Eyelash Loss Secondary to Escitalopram But Not to Sertraline: A Case Report. Prim Care Companion CNS Disord. 2016;18(3):10.4088/PCC.15l01887. Published 2016 May 19.
2011 Case Study:
Woman suffering from major depressive disorder noticed hair loss 3 weeks after beginning escitalopram. She discontinued the drug due to intolerable hair loss. Two weeks later, the hair loss stopped.
Several months later she tried the drug again as her depressive symptoms had returned. After 2 weeks? Her hair loss returned.[7]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3219523/
Fluoxetine (Prozac)
2021 Case Study:
Six weeks after beginning fluoxetine, a male patient reported hair loss in the frontal region of the skull. His complaints ended after he stopped taking the drug. This case appears to be the first evidence of fluoxetine-related hair loss in men.[8]https://pubmed.ncbi.nlm.nih.gov/34355374/
2019 Case Study:
Six weeks after beginning fluoxetine, a female patient reported hair loss in the frontal region of the skull.[9]https://pubmed.ncbi.nlm.nih.gov/31599441/
2018 Case Report:
Female patient notices significant hair loss 2 weeks after beginning fluoxetine. By 18 months, she had lost all her scalp and body hair. Researchers are aware of just one other similar case, and suggest there may be a wider spectrum of fluoxetine-related hair loss than previously known.[10]https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry/article/hair-loss-associated-with-fluoxetine/D2A55E09DDCF393399C14DB9289BAADE
2004 Case Study:
Female patient reports slight hair loss 3 months after beginning fluoxetine 20mg treatment. Reducing the drug to 10mg had no effect on her hair loss. After 1 year she stopped treatment due to intolerable hair loss on the scalp and body. 4 weeks later, her hair returned to normal thickness.[11]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC514846/
1992 Drug Trial:
A group of 15 young adults, ages 16-24, trialed fluoxetine for the treatment of their depression. Researchers noted that the side effect of alopecia appeared more commonly than in adult studies.[12]https://pubmed.ncbi.nlm.nih.gov/19630647/
Paroxetine (Paxil)
2021 Case Report:
Male with social anxiety disorder reports hair loss after beginning treatment with paroxetine. The hair loss stopped after discontinuing the drug, then recurred when treatment with paroxetine began again.[13]https://pubmed.ncbi.nlm.nih.gov/34181746/
2006 Case Study:
Over 60% of patients with alopecia areata (AA) have a psychiatric comorbidity. Researchers hypothesize that treating this depression may have a positive effect on hair growth. In this case study, a female patient had complete hair regrowth 7 weeks after beginning paroxetine treatment. One month after discontinuing the drug, her symptoms of AA had returned.[14]https://sci-hub.se/https://pubmed.ncbi.nlm.nih.gov/16922952/
2001 Double-Blind Randomized, Placebo-Controlled Trial:
A group of 13 patients presenting with AA and a psychiatric comorbidity were studied to see if treatment with SSRIs could lead to regrowth of hair. Researchers observed the complete regrowth of hair in two patients treated with paroxetine, while four showed partial regrowth. Meanwhile, only one patient from the placebo group had similar regrowth.[15]https://pubmed.ncbi.nlm.nih.gov/11737460/
2000 Case Report:
‘Massive’ hair loss was reported in a woman being treated with paroxetine, which improved soon after she stopped taking the drug.[16]https://pubmed.ncbi.nlm.nih.gov/10883182/
1999 Case Report:
A female complained of ‘moderate’ hair loss after beginning paroxetine treatment. The hair loss stopped after discontinuing the drug, and began again when the drug was reintroduced.[17]https://pubmed.ncbi.nlm.nih.gov/10442258/
Sertraline (Zoloft)
2015 Case Study:
A male patient reports hair loss 2 weeks after beginning sertraline treatment. His hair loss improved after he stopped taking the drug.[18]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4589582/
2008 Case Study:
A woman complained of hair loss during sertraline treatment. This case is unique because she had previously been treated with fluoxetine, but reported no hair loss during that treatment.[19]https://pubmed.ncbi.nlm.nih.gov/18664165/
2006 Literature Review:
Researchers reviewed all reports of SSRI-induced hair loss in the national Swedish database and the database for the World Health Organization. Reports of sertraline-induced hair loss were nearly double those for citalopram, although still considered a ‘rare’ side-effect.[20]https://pubmed.ncbi.nlm.nih.gov/16783834/
2005 Case Study:
A 14 year old boy reports hair loss after 5 years of sertraline treatment. The drug was gradually discontinued and the hair loss stopped.[21]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000200/
Atypical Antidepressants
Bupropion (Wellbutrin)
Bupropion is an atypical antidepressant. It is not an SSRI or an SNRI, but an NDRI. It’s used not only to treat depression, but sometimes prescribed to help with smoking cessation.
2018 Research:
In a study that followed over 1 million patients, researchers tracked exclusive users of antidepressants to better understand hair loss risk. They found that compared with bupropion, all other antidepressants had a lower risk of hair loss. Fluoxetine and paroxetine ranked as being lowest risk, with the highest level of confidence.
The researchers concluded that of all the SSRIs and SNRIs, bupropion has the highest risk of hair loss, while paroxetine has the lowest.[22]https://pubmed.ncbi.nlm.nih.gov/28763345/
Atypical Antipsychotics
Olanzapine and quetiapine are classified as atypical antipsychotics. Both are used in the treatment of bipolar disorder, while quetiapine is also sometimes used to treat major depressive disorder.
Olanzapine (Zyprexa)
2002 Case Report:
After increasing her daily dose of olanzapine from 5mg to 15mg daily, a woman reports increasing hair loss. Hair loss discontinued after switching from olanzapine to risperidone. Researchers report that this is the first known case of olanzapine-induced hair loss, and note the manufacturer, Eli Lily Canada, estimates hair loss occurs among just 0.01% of users.[23]https://sci-hub.se/https://pubmed.ncbi.nlm.nih.gov/12500769/
Quetiapine (Seroquel)
2007 Literature Review:
Researchers reviewed all case reports of alopecia following quetiapine treatment reported to the New Zealand Intensive Medicines Monitoring Programme and the World Health Organization. They found 17 cases total, some of which support a causal relationship between quetiapine and hair loss. Researchers note that while hair loss has previously been associated with both olanzapine and risperidone, it has not yet been described with quetiapine.[24]https://pubmed.ncbi.nlm.nih.gov/17293712/
Anti-Anxiety Medications and Hair Loss
Benzodiazepines and Hair Loss
Benzodiazepines are classified as depressants and can help patients who struggle with anxiety. Of this class of drugs, only clonazepam seems to be associated with hair loss. There were no studies connecting alprazolam (Xanax), diazepam (Valium) or lorazepam (Ativan) with hair loss.
Clonazepam (Klonopin)
2009 Case Study:
A woman complains of hair loss one week after beginning treatment with clonazepam. The hair loss stopped when she stopped taking the drug. Researchers note they reviewed the literature and found only one other case of hair loss associated with clonazepam. Also noted, she had been taking escitalopram (which has a more well-documented association with hair loss) prior to her hair shedding and continued with this drug even as her hair grew back.[25]https://pubmed.ncbi.nlm.nih.gov/19471188/
Buspirone
Busiprone is categorized as an anxiolytic, and is used to treat both short-term and chronic anxiety.
2013 Case Report:
A woman being treated with buspirone and sertraline reports significant hair loss. Her treatment team discontinued buspirone, but not sertraline (which is also associated with hair loss). The patient reported her hair loss stopped 3-5 days later, although researchers noted they couldn’t confirm.[26]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3579479/
Antimanic Agents
Antimanic agents are mood stabilizers. They are used in the treatment of bipolar disorders and can calm those in states of mania or extreme anxiety. Of this class of drugs, lithium and valproic acid are most frequently associated with hair loss.
Lithium
2013 Literature Review:
A meta analysis on the side effects of lithium finds no statistically significant increase in risk of hair loss with lithium treatment. Researchers mention that lithium suppresses the thyroid, which could be related to reports of hair loss.[27]https://pubmed.ncbi.nlm.nih.gov/23525591/
2012 Literature Review:
A review of 385 abstracts were screened for reports of lithium toxicity, including lithium-induced alopecia. Researchers found no significant risk of alopecia associated with lithium.[28]https://pubmed.ncbi.nlm.nih.gov/22265699/
1994 Case Study:
A woman reports hair loss after beginning lithium treatment. 2 months after discontinuing treatment, her alopecia resolved. Researchers note this side effect is rare.[29]https://pubmed.ncbi.nlm.nih.gov/7995585/
1988 Case Study:
A patient reports total hair loss after 2 months of lithium treatment. Researchers discuss a possible connection between the drug and alopecia totalis.[30]https://pubmed.ncbi.nlm.nih.gov/3126095/
1984 Literature Review:
Researchers review all cases since 1970, when lithium-related hair loss was first reported. They suggest patients experiencing hair loss after lithium treatment be checked for hypothyroidism, because lithium ions may concentrate in the thyroid, disrupting normal processes in this area.[31]https://pubmed.ncbi.nlm.nih.gov/6519870/
1983 Case Report:
Of 100 patients on lithium therapy, 12 report hair loss. 1 patient was diagnosed with hypothyroidism, others experienced hair regrowth after discontinuing treatment.[32]https://pubmed.ncbi.nlm.nih.gov/6838778/
1983 Case Study:
Researchers present 2 cases of hair loss attributed to lithium therapy. They recommend thyroid tests to exclude hypothyroidism as the cause of hair loss.[33]https://pubmed.ncbi.nlm.nih.gov/6404546/
1982 Case Report:
Researchers study 7 cases of hair loss associated with lithium. They mention other findings that for those with scalp psoriasis, lithium can aggravate this condition. They report findings consistent with telogen effluvium and conclude lithium can cause increased hair shedding. [34]https://pubmed.ncbi.nlm.nih.gov/6809028/
Valproate/Valproic Acid
2018 Case Study:
A review of over 400,000 patients being treated with psychotropic drugs in German-speaking countries found that Valproic acid was related to the highest risk of hair loss. That said, researchers found just 43 cases, a number distinctly lower than expected.[35]https://pubmed.ncbi.nlm.nih.gov/30193142/
2018 Literature Review:
A review of literature finds Valproate-induced hair loss is diffused, nonscarring, and dose-related. The drug may also cause graying and changes to hair texture. Researchers note that topical valproic acid may help hair regeneration and suggest further study into the difference between oral and topical administration as they relate to changes in hair growth.[36]https://pubmed.ncbi.nlm.nih.gov/30386073/
2017 Research:
Hair loss is a well-known side effect of valproic acid. It leads to telogen effluvium and appears to be dose-dependent, meaning hair loss increases with increased dosage. Paradoxically, valproic acid can be applied topically to help regrow hair. Researchers explore this paradox.[37]https://pubmed.ncbi.nlm.nih.gov/29061425/
2017 Research:
Valproic acid is often administered to patients undergoing radiation therapy for brain tumors to help manage seizures. Doctors note that delay or prevention of hair loss in this population seems to be a positive side effect.[38]https://pubmed.ncbi.nlm.nih.gov/27889835/
1996 Literature Review:
A 1996 literature review finds that alopecia is a common side effect of treatment with antimanic agents, and is expected in up to 12% of patients undergoing treatment with valproate, and 10% of patients being treated with lithium.[39]https://pubmed.ncbi.nlm.nih.gov/8899137/
1992 Case Report:
Two reports with seemingly conflicting results. In one, lithium-induced hair loss improved when lithium was replaced by valproate. In the other, hair loss improved only after stopping valproate.[40]https://pubmed.ncbi.nlm.nih.gov/1486112/
Establishing Cause and Effect
The above case studies offer evidence that the relationship between psychopharmaceuticals and hair loss may be more nuanced than expected.
Case reports are justifiably considered a weaker form of evidence. In other words, they rank low on the hierarchy of evidence. While case studies (i.e., n=1 published anecdotes) offer ‘signals’ for scientists to explore in future randomized controlled clinical trials, they also come with a high risk of bias. Case studies don’t establish prevalence rates, and it’s always possible that patients in these reports were using other medications and/or experienced additional life events that might also explain their hair loss. While they indeed contribute to future research, using case studies to interpret cause and effect is difficult.
The bottom line? It’s not always easy to say, ‘this drug causes hair loss.’
Hair loss can have many causes, making a causal relationship between a single drug and hair loss very hard to prove. In some of the above studies on SSRIs, researchers tried to hone in on this relationship by stopping treatment, seeing if the hair grew back, and then rechallenging the patient with the same medication to see if their hair loss then returned.
While this process occurred in a few of the case studies found, more research must still be done, as the sample size in the studies mentioned here consists of just a few people.
Another way to explore the existence of cause-and-effect is to dive more deeply into possible causes outside of the drug. Basically, one must rule out the following:
Are other medications present?
Patients receiving treatment for depression and anxiety may have other comorbidities or may be taking more than one medication.
Some of the case studies above report this, others make it clear the patient was an ‘exclusive user’ of the drug in question, while others make no such reference to either.
It’s possible that even in cases where one medication was discontinued and hair loss subsequently stopped, that it was the interaction between one or more drugs, and not a single drug, that triggered the onset of hair shedding.
Are other skin conditions present?
Several of the studies on lithium, which is widely described as a drug which ‘causes’ hair loss, mentioned patients who had pre-existing skin conditions, particularly scalp psoriasis. While it may be true that they experienced alopecia only after beginning lithium treatment, the root cause of hair shedding could have been their psoriasis.
Several patients in the above case studies had also experienced alopecia in the past. Alopecia is an embarrassing condition which can lead to depression and anxiety. Also, those with depression and anxiety may be predisposed to alopecia. Researchers estimate that up to 30% of patients with skin conditions have psychiatric comorbidity.[41]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7756276/
Is the patient trustworthy?
Of the case reports presented here, researchers often (but not always) confirmed the patient’s own reports of hair loss and hair regrowth. In just one instance was there mention of blood drawn to confirm the patient was indeed taking only the medication prescribed, as directed.
Particularly regarding the anti-anxiety medications, some patients presented with symptoms of schizophrenia. Hair pulling, or trichotillomania, must be ruled out in those cases and others.
Understanding the Mechanisms of Action
Understanding the mechanisms of action can also help establish cause and effect. While it’s still unknown exactly how these medications induce hair loss, the following are a sample of the most plausible theories.
Melatonin and Hair Cycle Interruption
Psychotropic drugs typically cause diffuse, reversible alopecia by influencing hair’s telogen (shedding) phase.[42]https://pubmed.ncbi.nlm.nih.gov/10798824/ The exact mechanism via which this works has yet to be revealed, although it may be connected to the relationship between serotonin and melatonin.
Serotonin is a precursor to melatonin, but the two also work in tandem to regulate a healthy circadian cycle. SSRIs in particular may decrease melatonin, which plays a role in hair cycle control.[43]https://pubmed.ncbi.nlm.nih.gov/16217127/
Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ. On the role of melatonin in skin physiology and pathology. Endocrine. 2005 Jul;27(2):137-48.
Hypothyroidism
Lithium and other antimanic agents may exacerbate hypothyroidism. While lithium cannot seem to shake its reputation as a cause of hair loss, researchers do not necessarily believe that lithium is the direct cause. Rather, it seems that hypothyroidism (caused by lithium) is to blame. Insufficient thyroid hormone can trigger telogen effluvium.
Dose Dependency
With lithium and some other psychopharmaceuticals, dosage plays a role in determining risk for hair loss. Some patients who experience hair loss may find that reducing their dosage allows their hair loss to abate. Lithium in particular, rarely has to be discontinued entirely.[44]https://pubmed.ncbi.nlm.nih.gov/3157663/
Understanding more about the relationship between dose and hair loss may help researchers learn more about the mechanisms behind cause and effect.
Women vs Men
Hair loss related to psychopharmaceuticals is overwhelmingly experienced by women, not men. In one literature review, nearly 89% of the reports came from women.[45]https://pubmed.ncbi.nlm.nih.gov/16783834/ Understanding why women seem to be more affected by this type of hair loss may offer clues into the mechanism of action.
Multidirectional Relationships
Some drugs have a multi-directional relationship to hair loss, for reasons that aren’t yet entirely understood. Valproate and valproic acid, for example, can lead to hair loss when orally administered but may lead to hair growth when applied topically. In two of the case studies mentioned above, alopecia areata improved with paroxetine treatment, a drug that has induced hair loss in others.
These multidirectional relationships may shed light on cause and effect, but also, offer insight into how complicated it is to point to any one drug as a cause of hair shedding.
Hair loss, particularly telogen effluvium, may be related to trauma, stress, anxiety, or depression. Some people may find their hair loss improves as their anxiety and depression get better. On the other hand, researchers acknowledge it’s possible that cases of hair loss are underreported, either due to self-neglect or because the patient has experienced it in the past as a result of emotional stress and does not relate the occurrence to the drug.
What Can Be Done?
In general, the incidence rate for these reports of hair loss associated with antidepressants, anti-anxiety medications and mood stabilizers, appears to be quite low. It’s not a given that hair loss is an outcome with any of these drugs.
That said, hair loss can be devastating for the one person that experiences it. And in some cases, may lead to non-compliance with what otherwise are very effective and life-improving drugs.
Before deciding to forego or discontinue treatment, it’s worth it to consider that in all the cases mentioned above, patients reported their hair shedding stopped after an adjustment to dosage, or once treatment ended.
For those who have recently begun taking a new medication and have noticed hair thinning or hair shedding, it would be advisable to speak with a doctor about switching to another medication. Remember, there were several antidepressants and anti-anxiety medications that did not make the list because there was no evidence linking them to hair loss.
That said, if a medication on this list lands in the medicine cabinet, the risk of hair loss remains very low.
Summary
It’s true there are case studies linking some psychopharmaceuticals to hair loss. But generally, the incidence rates are low, and hair loss tends to stop when dosages are lowered or the use of the drug is discontinued.
That said, for the individual experiencing hair loss, it’s of little comfort to know that risk is low. Maintaining healthy hair can be an important part of maintaining emotional and psychological health. If treating anxiety or depression is having a negative effect on hair, it may be time to talk to a doctor or dermatologist about an alternative treatment plan.
While happiness and health should always supersede hair growth, in this case, people shouldn’t have to sacrifice either.
References[+]
References ↑1 https://link.springer.com/article/10.2165/00002018-199410040-00005 ↑2, ↑3, ↑42 https://pubmed.ncbi.nlm.nih.gov/10798824/ ↑4 https://www.psychiatria-danubina.com/UserDocsImages/pdf/dnb_vol33_no2/dnb_vol33_no2_187.pdf ↑5 https://www.psychiatrist.com/pcc/depression/escitalopram-induced-hair-loss/ ↑6 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035805/ ↑7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3219523/ ↑8 https://pubmed.ncbi.nlm.nih.gov/34355374/ ↑9 https://pubmed.ncbi.nlm.nih.gov/31599441/ ↑10 https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry/article/hair-loss-associated-with-fluoxetine/D2A55E09DDCF393399C14DB9289BAADE ↑11 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC514846/ ↑12 https://pubmed.ncbi.nlm.nih.gov/19630647/ ↑13 https://pubmed.ncbi.nlm.nih.gov/34181746/ ↑14 https://sci-hub.se/https://pubmed.ncbi.nlm.nih.gov/16922952/ ↑15 https://pubmed.ncbi.nlm.nih.gov/11737460/ ↑16 https://pubmed.ncbi.nlm.nih.gov/10883182/ ↑17 https://pubmed.ncbi.nlm.nih.gov/10442258/ ↑18 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4589582/ ↑19 https://pubmed.ncbi.nlm.nih.gov/18664165/ ↑20, ↑45 https://pubmed.ncbi.nlm.nih.gov/16783834/ ↑21 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000200/ ↑22 https://pubmed.ncbi.nlm.nih.gov/28763345/ ↑23 https://sci-hub.se/https://pubmed.ncbi.nlm.nih.gov/12500769/ ↑24 https://pubmed.ncbi.nlm.nih.gov/17293712/ ↑25 https://pubmed.ncbi.nlm.nih.gov/19471188/ ↑26 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3579479/ ↑27 https://pubmed.ncbi.nlm.nih.gov/23525591/ ↑28 https://pubmed.ncbi.nlm.nih.gov/22265699/ ↑29 https://pubmed.ncbi.nlm.nih.gov/7995585/ ↑30 https://pubmed.ncbi.nlm.nih.gov/3126095/ ↑31 https://pubmed.ncbi.nlm.nih.gov/6519870/ ↑32 https://pubmed.ncbi.nlm.nih.gov/6838778/ ↑33 https://pubmed.ncbi.nlm.nih.gov/6404546/ ↑34 https://pubmed.ncbi.nlm.nih.gov/6809028/ ↑35 https://pubmed.ncbi.nlm.nih.gov/30193142/ ↑36 https://pubmed.ncbi.nlm.nih.gov/30386073/ ↑37 https://pubmed.ncbi.nlm.nih.gov/29061425/ ↑38 https://pubmed.ncbi.nlm.nih.gov/27889835/ ↑39 https://pubmed.ncbi.nlm.nih.gov/8899137/ ↑40 https://pubmed.ncbi.nlm.nih.gov/1486112/ ↑41 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7756276/ ↑43 https://pubmed.ncbi.nlm.nih.gov/16217127/ ↑44 https://pubmed.ncbi.nlm.nih.gov/3157663/ Histamine and Hair Loss
Our hair follicles are part of a niche of cells intimately involved in proper immune function. For example, right next to the dermal papilla, the type of cell that grows our hairs, there are a number of immune cells including:
- Macrophage, which helps remodel our extracellular matrix and clean up waste.
- Basophils, which help neutralize foreign particles.
- Neutrophils, which keep pesky microorganisms present on our scalps, in check.
- And in reference to today’s topic, Mast cells which secrete histamine to neutralize foreign invaders.
Chen, Chih-Lung & Huang, Wen-Yen & Wang, Eddy & Tai, Kang-Yu & Lin, Sung-Jan. (2020). Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. Journal of Biomedical Science. 27.
Mast cells can become counterproductive though when too much histamine is secreted. They induce immunoglobulin secretion by our B-cells (those same cells that act as antibodies to viruses), such as the release of IgE – the same immunoglobulin that can create anaphylactic shock.
Histamine is also a pro-inflammatory cytokine (a signaling molecule), that can generate a number of unwanted cell responses that tend to progress hair loss.
In this article, we take a look to see just how much are mast cells and histamine to blame for hair loss, and what can be done about this. We also take a look at the evidence for the use of antihistamines as a hair loss reversal tool.
Interested in Topical Cetirizine?
Support your hair with full-strength topical cetirizine, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What Is The Role Of Histamine In Hair Loss?
How Histamine Works
Histamine is counterintuitively not a hormone, instead, it’s actually a simple amino acid. We get histamine thanks to consuming its precursor amino acid histidine. And it is vitally important for maintaining normal physiology.
Histamine also binds to four receptors. Some of which are located on our skin, some in our gastrointestinal system, and some in our brain. Histamine in the brain is also important for acting as a neurotransmitter.
It’s the excess of histamine signaling that becomes problematic.
A perfect example of excessive histamine signaling being bad is in the case of seasonal allergies, or food allergies. Anyone who has had experience will know how quickly and fiery the body responds to these allergens. This is all due to the release of histamine and how it interacts with those receptors.
With excess histamine signaling, people become easily irritated and prone to inflammation. Body temperature also increases and the vascular system becomes more permeable, causing immune cells to leak into unwanted places.
This opening of our vascular system and leaking of immune cells in unwanted places is actually one way histamine connects to hair loss.
The Histamine-Hair Loss Connection
Because hair follicles and scalp act as a breeding ground for a variety of microorganisms, it is also very prone to exacerbated histamine signaling if the scalp biome balance is thrown off. Further, because histamine responds to changes in hormone levels, it’s very possible that the shift experienced with androgenic alopecia also increases histamine production.
Both androgenic alopecia and alopecia areata also show signs of dysregulated histamine signaling. Histamine in excess can induce pro-inflammatory cascades that cause premature apoptosis of cells, especially as it relates to the dermal papilla.
This rapid apoptosis can extend to cells (such as stem cells) sitting on our epidermis. Stem cells in the subcutaneous tissue and on the hair follicle’s outer root can bulge. Premature apoptosis of these cells makes it increasingly difficult to regrow hair since the stem cells which contribute to hair renewal are no longer present.
The Evidence For Histamine Contributing To Hair Loss
While the discussion on histamine specifically is rather sparse in relation to hair loss, there have been some advances in the past years on the role of histamine overall. For example …
- When humans experience stress, the body tends to release histamine in much larger amounts. This excess of histamine is believed to affect our hair follicles and act as part of the pathophysiology of stress-induced telogen effluvium. In the following study, patients with telogen effluvium had significantly higher numbers of mast cells and the enzyme tryptase.[1]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5514792/.
Tryptase is an enzyme involved in the degradation of proteins, specifically by removing lysine, histidine, and arginine. Higher tryptase is also a hallmark of mast cell activation.
Grace SA, Sutton AM, Abraham N, Armbrecht ES, Vidal CI. Presence of Mast Cells and Mast Cell Degranulation in Scalp Biopsies of Telogen Effluvium. Int J Trichology. 2017;9(1):25-29
- Alopecia areata is one of the more commonly accepted forms of hair loss due to the immune system going awry. In alopecia areata, there is a large change in immune function due to the loss of immune privilege. Immune privilege is a mechanism the body uses to keep immune cells out of certain sensitive tissues.
When the hair follicles experience a collapse of immune privilege, they also experience a dramatic increase in a variety of immunological factors. Specifically, a rise in lymphocytes like CD8+ T-cells, and IgE. Both of which are either triggers/ secrete (T-cells) histamine or can be triggered by histamine release from mast cells.
Specifically, the deep perivascular and perifollicular regions of those with alopecia areata, when compared to controls, had higher CD4+ T cell count, and CD8+ T cell count. They also had more mast cells.
Zhang X, Zhao Y, Ye Y, Li S, Qi S, Yang Y, Cao H, Yang J, Zhang X. Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Arch Dermatol Res. 2015 May;307(4):319-31. doi: 10.1007/s00403-015-1539-1. Epub 2015 Feb 1. PMID: 25638328.
These findings reached multiple orders of magnitude in terms of significance. Interestingly, this was not really something of concern when it came to the upper dermal region. Indicating that unless we look further down the dermis, we are unlikely to see any noticeable changes in immune profiles.
- Androgenic alopecia is often discussed as only ever being due to the increase in androgen signaling. Specifically, the production of DHT from testosterone via the 5AR enzyme and its binding to the androgen receptor. However, what isn’t often discussed is what happens afterward?
It’s not as if the androgen and the androgen receptor are initiating hair loss. Instead, the androgen receptor with DHT translocates into our cell’s nucleus and then tells the cell to change genetic expression. Some of those genes (but not all), happen to be genes for the production of extracellular matrix.
This can lead to the overproduction of elastin, leading to a feeling of hard tissue often referred to as fibrosis of the scalp. In another case-control study, the increase in collagen fibers, elastin, and the lower diameter of hair follicles, was apparently clear in those with androgenic alopecia.[2]https://pubmed.ncbi.nlm.nih.gov/18286292/
Won CH, Kwon OS, Kim YK, Kang YJ, Kim BJ, Choi CW, Eun HC, Cho KH. Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Arch Dermatol Res. 2008 Mar;300(3):147-52. doi: 10.1007/s00403-007-0826-x. Epub 2008 Feb 20. PMID: 18286292.
Figure (a) and the graph to the right of it are indicating the increase of collagen bundles in the occiput and vertex of either controls or those with AGA. While the occiput didn’t differ much, the vertex was clearly different.
Figure (b) is indicating the increase in elastin seen in those with androgenic alopecia as compared to control.
And when it came to mast cells, those with androgenic alopecia also had a higher level of the enzyme tryptase (again indicative of mast cell activation). Interestingly, tryptase is also a well known factor involved in the activation of TGF-ß and collagen remodeling, potentially pointing to a role of mast cells in the fibrosis of those with AGA.
Won CH, Kwon OS, Kim YK, Kang YJ, Kim BJ, Choi CW, Eun HC, Cho KH. Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Arch Dermatol Res. 2008 Mar;300(3):147-52. doi: 10.1007/s00403-007-0826-x. Epub 2008 Feb 20. PMID: 18286292.
It’s important to keep in mind that these findings were for a total of 10 patients and 5 controls. A greater sample size range is required to definitively tell if this is either a correlation or a fluke.
Interestingly, the increase in TGF-ß signaling appears to be contradictory when it comes to alopecia areata. In androgenic alopecia it’s implicated as a fibrosis-inducing agent, while in alopecia areata, the loss of TGF-ß is a precursor step to loss of immune privilege.
This change in activity of TGF-ß is known to be induced by a shift in mast cells from an immune-inhibitory role to a pro-inflammatory role.[3]https://pubmed.ncbi.nlm.nih.gov/24832234/
One research group had actually investigated this separately with a mice model and determined with incredible accuracy that one of the reasons for the differential response of mast cells as either good or bad, is partly due to the scalp microbiome and dermal fibroblasts.[4]https://pubmed.ncbi.nlm.nih.gov/30928651/
The mice study showed that the scalp microbiome activates toll-like receptors (a type of immune recognition receptor) and amplifies progenitor cells from the keratinocyte to become mast cells. Further, a change in how the mast cells behaved was seen. One of immune inhibition into one of pro-inflammation and immune cell recruitment.
It’s possible that for alopecia areata, a change in how the scalp microbiome interacts with immune cells is the prelude to the disease. While with androgenic alopecia, a change in how mast cells behave is a prelude to premature apoptosis of the dermal papilla cells.
Do Antihistamines Work Against Hair Loss?
The next step in assessing the role of mast cells in hair loss is to consider if anyone has looked at the use of antihistamines for hair loss. After all, while mechanistic and observational data connecting histamines to hair loss are important, interventional studies are really the only way to ascertain cause and effect.
Luckily, there are some research papers that addressed this question.
The first study investigated the antihistamine drug cetirizine (if you’ve ever tried claritin or zyrtec, this is the same active ingredient), because of its known inhibitory actions on histamine-1 receptors and PGD2 (a proinflammatory prostaglandin shown to worsen androgenic alopecia).[5]https://onlinelibrary.wiley.com/doi/abs/10.1111/jocd.13940
What the authors did was give 30 participants a 1% solution of topical cetirizine at 1mL doses. The second group which served as a control group was given a placebo solution. After six months these were the results:
Zaky, M. S., Abo Khodeir, H., Ahmed, H., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: A case‐controlled study. Journal of Cosmetic Dermatology, 20(4), 1154–1159.
Essentially, the control group showed no improvement with 4/30 of them seeing worsening responses. While the treatment group saw sparse improvements in photographic assessments and self assessments. Nothing too crazy, but still better than the control group.
In a second study, researchers compared 1% cetirizine vs. 5% minoxidil. In this randomized controlled trial, both groups saw great results in terms of hair density, hair diameter and other hair loss parameters. Minoxidil however, outperformed topical cetirizine.[6]https://journals.library.ualberta.ca/jpps/index.php/JPPS/article/view/31456/21623
This is expected since minoxidil works by promoting the anagen phase of hair follicles. Cetirizine simply works by blocking the uptake of histamine in the histamine-1 receptor. This is fascinating to note though as by simply blocking the cellular actions of histamine, cetirizine has comparability to minoxidil.
Hossein Mostafa, D., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of Cetirizine 1% Versus Minoxidil 5% Topical Solution in the Treatment of Male Alopecia: A Randomized, Single-blind Controlled Study. Journal of Pharmacy & Pharmaceutical Sciences, 24, 191–199.
Their outcomes showed some of the following features:
- Although baseline levels of hair density were higher in the minoxidil group, the cetirizine group had close efficacy. Keeping up with the results of the minoxidil group.
- Minoxidil had better results in terms of vellus hair density, despite starting with a higher baseline. And minoxidil had equal efficacy as cetirizine did in regards to terminal hair density.
- Cetirizine group had a greater reduction in telogen hair follicles 16-weeks in compared to minoxidil. But this effect rebounded slightly during the 24-week mark. Why this is the case is hard to determine.
Nonetheless, a combined therapy may be even more potent at addressing hair loss…
In the final study on cetirizine, patients were once again administering 1mL of a 1% solution of cetirizine topically. After six months, a dramatic improvement in hair regrowth was seen for the participants when compared to the control group.[7]https://pubmed.ncbi.nlm.nih.gov/28604133/
Rossi, A., Campo, D., Fortuna, M. C., Garelli, V., Pranteda, G., De Vita, G., … Carlesimo, M. (2017). A preliminary study on topical cetirizine in the therapeutic management of androgenetic alopecia. Journal of Dermatological Treatment, 29(2), 149–151.
The top row indicates the beginning of the study for the participants. While the bottom row indicates the end of the study. A clinical difference can be seen in all of the patients’ hairlines. In all cases, there were no known side effects too.
What’s Our Take On The Data?
There is a clear rationale for the induction of mast cells in the pathology of possibly all forms of hair loss, including a connection to immune defects like telogen effluvium. Mast cells play a critical role in feedback from the scalp microbiome to our scalp cell niche.
When mast cells go awry, they release histamine, tryptase, and a number of other factors that cause extracellular matrix remodeling, recruitment of immune cells, and a vicious cycle progressively worsening with time.
Addressing the excess histamine production by interfering with the histamine-1 receptor with cetirizine, abrogates most of the negatively associated responses and actually does seem to allow regeneration of hair follicles.
Based On The Evidence
The use of topical cetirizine can be a very appropriate tool to implement with androgenic alopecia and alopecia areata. The rise of mast cells and the enzyme tryptase in patients with telogen effluvium as compared to controls also provides a very compelling argument as to how stress correlates with hair shedding disorders.
It is with the totality of the current evidence, prior knowledge on the mechanisms of mast cells and their roles in our bodies, as well as the efficacy of antihistamines in improving hair loss, that we believe the weight of the evidence largely supports a causal role of mast cells in the development of various forms of hair loss.
What Then Should Be Done?
Since topical cetirizine consistently shows a net benefit in all the current clinical trials, the most logical addition should be topical cetirizine for any hair loss disorder. It may be that in conjunction with minoxidil, large improvements can be acquired.
Further, with topical anti-inflammatories, cetirizine can dramatically reduce the apoptosis rate of precious stem cells surrounding our hair follicles. There are alternatives to cetirizine that operate in similar mechanisms, reducing histamine release and subsequent binding to histamine-1 receptors.
Some of the useful ingredients that can reduce histamine production and the effects of histamine include:
- Vitamin B6.
- Copper.
- Vitamin C.
- Iron.
- Vitamin D.
- Ginger.
- Garlic.
- MSM.
And many more. A majority of the micronutrients and vitamins are also key cofactors for proper function of the enzymes involved in histamine degradation – such as Diamine oxidase and Histamine-N-Methyl Transferase enzymes.
Product Recommendations
Although we can’t generally acquire topical cetirizine, one can be made by dissolving 1 g in 100mL of water. Forming 1% w/v cetirizine, just as outlined in the studies. The only difference would be that the study used a form of alcohol instead of water.
A simple formulation you can make at home includes:
- Take 1 g of Cetirizine, crush it in a mortar, and pestle very finely.
- Dissolve it in 100mL of water.
- Add 1-5mL of ethyl alcohol (70-90%).
- Add 1-5mL of Coco glucoside to help with absorption, or more depending on the consistency you would like.
- Thoroughly mix the ingredients and store it in a dark brown bottle with a 1mL serving pump.
*Note: If you plan on using oral antihistamines, remember that they distribute widely throughout the body, not just the skin. The compounds prevent histamine from binding to the brain which is one of the reasons for the drowsy feeling many experience with Zyrtec or Claritin.
References[+]
References ↑1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5514792/ ↑2 https://pubmed.ncbi.nlm.nih.gov/18286292/ ↑3 https://pubmed.ncbi.nlm.nih.gov/24832234/ ↑4 https://pubmed.ncbi.nlm.nih.gov/30928651/ ↑5 https://onlinelibrary.wiley.com/doi/abs/10.1111/jocd.13940 ↑6 https://journals.library.ualberta.ca/jpps/index.php/JPPS/article/view/31456/21623 ↑7 https://pubmed.ncbi.nlm.nih.gov/28604133/ To some extent, every hairline is unique. But there are identifiable hairline patterns, primarily based on age, gender and genetics. As men age, their hairline will typically recede a bit, known as ‘maturing.’ While this can understandably make men nervous, it’s not always a sign of male pattern balding. In this post, we’ll review the following:
- What defines a mature hairline
- What defines a receding hairline
- The difference between a mature and receding hairline
- How to treat a receding hairline
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is a Mature Hairline?
The hairline is the boundary between hair follicles and the forehead. Everyone’s hairline is unique, although there are some patterns that occur throughout our lives.
According to observational studies, prepubescent men and women both experience concave shaped hairlines. This generally concave hairline is similar across all races and ethnicities. Around the age of 10, a small percentage of children develop a widow’s peak, but this is not an effect of recession at the temples.[1]https://www.researchgate.net/publication/256479799_Phenotype_of_Normal_Hairline_Maturation
Full citation: Rassman, William & Pak, Jae & Kim, Jino. (2013). Phenotype of Normal Hairline Maturation. Facial plastic surgery clinics of North America. 21. 317-24. 10.1016/j.fsc.2013.04.001.
As young teens, when hair is fullest, there’s typically a stark boundary between the hair on the head and the forehead. In men especially, this changes with age. It’s believed that hormonal changes trigger the expression of certain genes, causing men’s hairlines to mature between mid-adolescence and middle-age. This maturation means the hairline shifts a few centimeters further back on the forehead.
This shift may take place uniformly, following the rounded shape of the juvenile hairline, or may be more noticeable at the temples, resulting in a hairline that looks more like a letter M. There appears to be a relationship between the muscles of the forehead, the frontalis muscle, and the height of the hairline. Most adults with high mature hairlines have presented with high foreheads their entire lives. The varying degrees of normal hairline maturation can be seen in the image below.
Full citation: Rassman, William & Pak, Jae & Kim, Jino. (2013). Phenotype of Normal Hairline Maturation. Facial plastic surgery clinics of North America. 21. 317-24. 10.1016/j.fsc.2013.04.001.
As hairlines mature with age, some men hardly notice this change, as it occurs slowly over a period of 10 years or more. In others, this change happens more rapidly, causing concern.
Regardless of when or how quickly it happens, what characterizes a mature hairline is that the recession is limited to just a few centimeters, and then it stops. The hairline does not continue to recede, and remains well-defined, with little to no hair thinning.
A maturing hairline is a natural part of aging, and not indicative of androgenic alopecia (AGA), otherwise known as male pattern baldness.
At What Age Does Mature Hairline Stop?
If and when a juvenile hairline begins to recede, the most commonly asked question is – when does it typically stop?
Just as there’s no telling if and when the hairline will mature, there’s no predicting when a mature hairline will stop. A slightly different, but better question is – how do you differentiate between a maturing hairline, which will eventually stop receding, and receding hairline, which is a sign of male pattern baldness?
To answer this, we begin with an understanding of receding hairlines.
Take the guesswork out of growing hair.
Bypass years of trial and error. Get a personalized Regrowth Roadmap tailored to your hair loss type and treatment preferences. And leverage our support to implement it effectively.
Join Now
What is a Receding Hairline?
A receding hairline is among the earliest signs of androgenic alopecia. AGA often follows a predictable pattern. This pattern begins with a receding hairline, which is especially noticeable at the temples. Simultaneously or sequentially, hair then disappears from the crown of the head. Eventually, complete baldness occurs as the hairline recedes far enough back, and/or baldness from the crown of the head meets the hairline.
The Norwood-Hamilton scale is used by dermatologists to assess progression of AGA. By stage 2, minor recession of the hairline exists, although it’s barely differentiated from a maturing hairline. By stage 3, the hairline has receded much further at the temples, characteristic of androgenic alopecia.
What Causes a Receding Hairline?
Hair shedding is a common part of the normal hair growth cycle. Most people lose somewhere between 50-100 hairs each day, which typically goes unnoticed. When hair loss is localized to the front of the scalp and regrowth does not occur, the hairline recedes.
Age, genetics, gender, hormones, or how you care for and style your hair can all contribute to a receding hairline.
Age: While AGA can occur in children, it’s very rare. Most of the hairline changes that occur with age are not reflective of AGA, until after the age of 17. As we age, the chances of developing a receding hairline increase. Androgenic alopecia is more common in those who are middle-aged or older.
Genetics: Research suggests that there is no single gene involved in AGA. Rather, pattern hair loss is likely a polygenic disorder, meaning there are many gene variances that are involved in the predisposition of its development. Exactly which genes are responsible is still unknown, although a family history of androgenic alopecia is still its single greatest predictor.
Gender: Men are more likely than women to have a receding hairline. Although women do experience AGA, it more often presents as overall hair thinning and baldness that begins at the top of the scalp, and not a receding hairline.
Hormones: While the hormone dihydrotestosterone (DHT), is responsible for growth of hair on the body, this testosterone derivative also contributes to hair follicle miniaturization, and ultimately, hair loss. Although it’s unknown why AGA forms the pattern it does, the answer might have to do with androgen receptor density and 5α-reductase activity at the hairline.
Lifestyle: The onset of a receding hairline may be accelerated from certain forms of stress, medications, scalp environment, and/or hair styling — such as pulling hair too tight. Hair loss may arise from hormonal conditions unrelated to DHT, such as hypothyroidism or hyperthyroidism. Gut dysbiosis, heavy metal toxicity, and vitamin deficiency can all contribute to hair loss.
Unlike maturing hairlines, receding hairlines are not part of the normal aging process, but a sign of androgenic alopecia, another form of hair loss, or both.
Mature vs Receding Hairlines
A mature hairline doesn’t always become a receding hairline. So what indicates the difference between the two, and how can we tell the difference between early AGA and a simple shift in the hairline?
Timing of Hair Loss: Hairlines tend to mature in late adolescence or early adulthood. Generally, receding hairlines start later in life.
Pace of Hair Loss: A receding hairline tends to progress at a faster pace. While a maturing hairline may go unnoticed – a receding hairline is more likely to attract attention.
Shape of Hair Loss: A receding hairline tends to move more towards an M-shape, with hair loss at the temples far more pronounced. A mature hairline, on the other hand, will move back more evenly.
Extent of Hair Loss: As the hairline matures, it may move back 1-2 centimeters. With a receding hairline, this shift can be 1 inch or more.
Thinning of Hair: As a hairline matures, hair maintains its original thickness. Receding hairlines, on the other hand, are accompanied by hair thinning.
Diagnosing a Receding Hairline
A receding hairline is characteristic of androgenic alopecia, but it’s possible to have more than one type of hair loss, especially if the hairline is receding and hair is thinning or balding in other areas.
In the shower, when washing your hair, take all the hairs you shed onto your hands and stick them to the wall using the steam from the shower.
- Are the hairs of varying diameters? This is indicative of hair follicle miniaturization, a defining characteristic of AGA.
- Are the hairs all equal in thickness? This suggests no hair follicle miniaturization, and an AGA diagnosis is less likely. You could have a hair shedding disorder.
A doctor or dermatologist can help identify exactly which type of hair loss is present. This is important, for treatment protocols will vary depending on the cause of hair loss.
How to Prevent Further Receding
There isn’t one single solution for hair loss, which makes diagnosing the cause and type of hair loss important. But in general, a comprehensive solution will address the following:
- Regrowth Regimens: Depending on personal preferences, this could include medications or other science-based hair regrowth regimens.
- General Health: This includes specific dietary and lifestyle interventions based on age, gender, and type of hair thinning to address any conditions linked to the hair loss. While poor general health may accelerate hair loss, for most people, it’s very much a secondary factor in terms of influence over a receding hairline.
Of course, no treatment is always an option. Many choose to change hair styles in an effort to hide a receding hairline, or are comfortable living with it as is. If choosing treatment, factors to consider include how much time and money to invest on hair regrowth, tolerance for side-effects, and personal preference.
A few possible interventions are listed below (and not in order of importance or clinical efficacy).
Massaging: Massaging the scalp for 15 minutes, twice daily has the ability to activate the body’s innate healing responses, and reduce scalp tension. Our own study showed that 75% of people who massaged consistently for 8 months reported a stop or partial reversal in their hair thinning.[2]https://link.springer.com/article/10.1007/s13555-019-0281-6
English, R.S., Barazesh, J.M. Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results. Dermatol Ther (Heidelb) 9, 167–178 (2019).
Shampoos: Ketoconazole shampoo is not actually FDA-approved for pattern hair loss, but is a popular treatment for the condition nonetheless. It’s not yet very well studied, but may work well in combination with other therapies.
Natural Topicals: Jojoba, castor, rosemary, peppermint, and saw palmetto extract are just a few of the natural essential oils that have been marketed for hair regrowth. There are a few small studies showing that natural topicals may improve certain hair loss disorders.
Microneedling: Microneedling the scalp to treat hair loss is typically done biweekly. Microneedling evokes very low levels of inflammation which evoke a healing response from the body. Studies show microneedling can be successful when done alone, or when performed alongside other therapies. [3]https://www.tandfonline.com/doi/abs/10.1080/14764172.2017.1376094?journalCode=ijcl20
Semsarzadeh N, Khetarpal S. Platelet-Rich Plasma and Stem Cells for Hair Growth: A Review of the Literature. Aesthet Surg J. 2020 Mar 23;40(4):NP177-NP188.
Low-Level Laser Therapy: Low-level laser therapy (LLLT) is perhaps the most popular non-drug hair loss treatment and has FDA-clearance as a hair loss treatment for both men and women. The expensive and time consuming treatment does improve hair count for those with AGA.
Medications: Minoxidil and Finasteride are the only 2 FDA-approved medications for hair loss. Each treats AGA either orally or topically. While these medications have been proven effective to treat hair loss, they’re not for everyone. Both lead to side effects in some people, and generally require life-long use.
Botox: Botox is a neuromodulator which relaxes muscles and may also reduce certain inflammatory signaling proteins. Over the last decade, a few studies have been published measuring the hair-promoting effects of Botox on men with AGA.
Platelet-Rich-Plasma Therapy: Platelet-rich plasma therapy (PRP) is offered by thousands of dermatologists as a natural intervention for all types of hair loss. It is effective for androgenic alopecia and alopecia areata, but also expensive and ongoing injections are required to maintain results.
Stem Cell Therapy: Stem cell therapy is an expensive; relatively new therapy that is still under investigation. It’s also not a one-and-done treatment and requires multiple appointments. Early findings seem to suggest that 90% of subjects respond to stem cell therapy. And for AGA subjects, increases in hair count seem to hover around 20-30%.[4]https://pubmed.ncbi.nlm.nih.gov/31111157/
Effective treatment protocols often include some combination of the above. For example, studies suggest microneedling + minoxidil + finasteride, offers a response rate of 80-90% – with hair count increases ranging from 25-40% within 6-24 months.
Summary
As a man’s hairline matures, it will recede slightly from its juvenile position. This can be alarming, but it’s not always an early sign of male pattern baldness.
A receding hairline differs from a maturing hairline in that it may recede at a much faster pace, recede further back, and will recede more at the temples than in the center, resulting in an M-shaped hairline.
A receding hairline is characteristic of androgenic alopecia, but could be related to other types of hair loss too, especially if hair is thinning or balding in other areas.
Diagnosing a receding (vs maturing) hairline allows treatment to begin before baldness further progresses. Treatment options which have been proven effective include massaging, microneedling and medications. Efficacy improves when these methods are combined.
References[+]
References ↑1 https://www.researchgate.net/publication/256479799_Phenotype_of_Normal_Hairline_Maturation ↑2 https://link.springer.com/article/10.1007/s13555-019-0281-6 ↑3 https://www.tandfonline.com/doi/abs/10.1080/14764172.2017.1376094?journalCode=ijcl20 ↑4 https://pubmed.ncbi.nlm.nih.gov/31111157/ For men with pattern hair loss who want to combat pattern hair loss, the conflicting advice online can be overwhelming, particularly when it comes to finding the best DHT blockers.
After performing a quick Google search on the topic of DHT blockers, one might navigate to this Healthline article. The piece mentions dietary solutions like coconut oil, onions, turmeric, pumpkin seeds, and edamame.
Numerous extracts are mentioned as well, including green tea, saw palmetto, reishi mushroom, EGCG, propacil, and zinc.
There are also mentions of pharmaceuticals: finasteride, spironolactone, fluridil, ketoconazole, and dutasteride.
What’s effective? What’s dangerous? Could some DHT blockers actually make hair loss worse?
It’s time to navigate to the facts, not fiction, about DHT blockers. And determine which ones work and which ones don’t. This article outlines 6 DHT blockers, ranked from worst to best.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

But first, what is DHT?
DHT is short for dihydrotestosterone, which is a hormone made from testosterone. It’s also the main hormone implicated in pattern hair loss, one of the world’s most common hair loss disorders.
This article is not going to dive into the nuances of the DHT-pattern hair loss connection. That’s for a later article.
For now, readers should note that studies have determined:
- DHT is elevated in balding scalps [1]https://www.jdsjournal.com/article/S0923-1811(03)00249-4/fulltext
- Men who can’t produce DHT never go bald [2]https://www.amjmed.com/article/0002-9343(77)90313-8/pdf
- Human hair follicle dermal papillae cell clusters undergo cell death and miniaturization when exposed to DHT [3]https://www.karger.com/Article/Abstract/95251
- By reducing DHT levels in the scalp, many men can stop the progression of hair follicle miniaturization, and even regrow hair.[4]https://www.jaad.org/article/S0190-9622(98)70007-6/fulltext
Needless to say, if men want to fight pattern hair loss, they should consider lowering DHT. But herein lies the problem: DHT isn’t just a hormone linked to hair loss; it’s also a hormone that is critical for male development.
Moreover, in adulthood, DHT seems to be protective against high estrogen levels. As such, men who lower their DHT levels will sometimes report side effects, including weak erections, brain fog, and even gynecomastia – the growth of male breast tissue.
That’s why, when picking a DHT blocker, one must weigh power against safety. In other words, for any DHT reducer, hair density tends to increase in tandem with a heightened risk of side effects.
So, for this article, the six DHT reducers mentioned below are ordered from the lowest clinical efficacy and highest safety profile to the highest clinical efficacy and lowest safety profile.
1 – Saw Palmetto
Saw palmetto, a palm plant grown in the Southeastern U.S. is one of the most popular herbal DHT reducers, and not without reason. In one clinical study lasting two years, saw palmetto was shown to stop pattern hair loss in 90% of men taking the supplement.[5]https://journals.sagepub.com/doi/pdf/10.1177/039463201202500435 Other studies have shown a bit of regrowth when combining saw palmetto as a supplement and a topical.[6]https://www.karger.com/Article/FullText/509905
Based on Perfect Hair Health member results, saw palmetto, by itself, isn’t really that effective as a standalone treatment. But it’s better than nothing, and overall, studies show that the supplement is relatively safe – even over five-year time horizons.
For example, a meta-analysis examined 14 randomized, placebo-controlled studies that had reported adverse events, and found that saw palmetto’s risk of side effects was small, comparable to placebo groups. When events did occur, they were mainly upset stomachs or headaches experienced after taking the supplement on an empty stomach.[7]https://link.springer.com/article/10.2165%2F00002018-200932080-00003 Better yet, another study saw no concerning changes to bloodwork… with the majority of side effects reported in the placebo group – or in other words, people who weren’t even taking saw palmetto.[8]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2518869/ Lastly, out of thousands of participants taking saw palmetto, only a small handful have ever reported reductions in libido. This suggests that the risk of sexual side effects is much less than 1%.
Taken together, this puts saw palmetto first on our list of DHT reducers for men. It has relatively low efficacy, but a high safety profile – and it still does something. In other words, it appears very safe and might help to slow down or stop pattern hair loss. But don’t expect any miracles.
Keep the following tips in mind when taking saw palmetto:
- Take 320mg daily. According to the evidence, effective dosages for hair loss seem to range from 200mg – 320mg daily, depending on the extraction method.
- Split dosages. The volatile acids inside saw palmetto have short half-lives. So, it’s probably best to split up that 320mg daily dosage to half in the morning, half at night.
- Combine with ingredients to enhance absorption and efficacy. Specifically, beta-sitosterol, lecithin, inositol, phosphatidylcholine, and perhaps even niacin and biotin.
These recommendations just scratch the surface, especially in terms of which saw palmetto brands to avoid. But that’s for a later article.
2 – Ketoconazole Shampoo
Ketoconazole is an anti-fungal medication. It’s used to treat skin conditions like dandruff, seborrheic dermatitis, and even jock itch. When formulated as a shampoo, there’s some evidence that it can improve hair counts, increase hair diameters, and potentially even lower scalp DHT levels in men – all without impacting hormone levels elsewhere in the body.
In fact, studies show that 2% ketoconazole, when used properly, boasts an 80% response rate with an average hair density increase of around 5%. Again, that’s no miracle, but with just 1-7% of people reporting side effects ranging from scalp itchiness to scalp dryness, ketoconazole can be considered a low cost, low effort, and moderately effective hair loss intervention.
Two quick notes for those who want to try ketoconazole:
- Get the 2% formulation. The 1% variety is available in supermarkets and drug stores, yet this dilution isn’t clinically effective at fighting hair loss. The 2% version is recommended. The product can be purchased without a prescription at NizoralShop.com.
- Use as directed. In most cases, that’s 2-4 times weekly, with a scalp contact time of 5-10 minutes. Failure to do this might result in wasted money and negligible results.
3 – Herbal DHT Reducers
Saw palmetto isn’t the only natural DHT reducer out there. There are studies showing that in cell cultures, other substances and herbal extracts can inhibit 5-alpha reductase, the enzyme that converts free testosterone into DHT, and in doing so, potentially lower DHT in humans.
Herbal DHT reducers include:
- Astaxanthin
- Azelaic acid
- Reishi mushroom extract
- Lycopene
- Green tea extract
- Beta-sitosterol
- Alpha-linolenic acid
- Zinc
- Curcumin
- Pumpkin seed oil
And the list goes on.
If we combine these all together, can we reduce more DHT than saw palmetto alone and see increased hair growth?
The logic is understandable. However, in biology, taking more of something doesn’t always equate to greater improvements – especially when taking things that all target the same pathway for DHT reduction: 5 alpha-reductase.
For instance, in one study, taking 3x the daily dose of saw palmetto for one year did not lead to 3x better improvements to an enlarged prostate; It led to the same improvements as a standard 320 mg dose.[9]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3326341/
Similarly, another study showed that mega-dosing saw palmetto and astaxanthin did not reduce blood levels of DHT any better than a small amount of saw palmetto and astaxanthin.[10]https://jissn.biomedcentral.com/articles/10.1186/s12970-014-0043-x And with this information in mind, when looking at clinical data on supplements like Nutrafol – which combines many natural potential DHT reducers into one pill – the hair regrowth isn’t really any more impressive than what we expect from a typical dose of saw palmetto alone.
Which begs the question: why mention the combination herbal extracts above both saw palmetto and ketoconazole?
There’s not a good answer.
All three of these options are similar in efficacy. But for these herbal combinations, where they differ probably isn’t efficacy; it’s safety.
There are very few long-term studies evaluating the safety profiles of herbal extracts for blocking DHT, particularly at the mega-dosages featured in best-selling DHT blockers on Amazon.
Adding in all these extra natural DHT-reducing herbs might have some effect, but one must be very selective in going about it. And those diminutive hair gains might not be worth the massively higher costs or the safety risks versus just taking saw palmetto alone.
Now on to pharmaceutical territory.
4 – Topical Finasteride
For men, finasteride is considered the gold standard treatment for pattern hair loss. Studies show that it can stop pattern hair loss in 80-90% of men, increase hair counts by 10%, and thicken miniaturizing hair – which all in, often equates to 20-30% improvements in hair density.[11]https://www.sciencedirect.com/science/article/pii/S0022202X15529357 The drug does this by inhibiting an enzyme called type II 5 alpha-reductase, thereby lowering DHT levels by 60-70%.
The problem is that oral finasteride reduces DHT everywhere, and not just on the scalp. In other words, it’s a systemic DHT reducer. And it’s this systemic lowering of DHT that some people use as a surrogate to predict a risk of side effects.
So people often ask: what if we could just localize finasteride’s effects to only the scalp? Well, that’s what topical finasteride attempts to do. It formulates oral finasteride as a topical – the medication can be applied directly to the scalp and, hopefully, reduce its risks of going systemic.
So, does this actually work?
Yes, to a degree.
Studies suggest that topical finasteride, at a 1% formulation, is “non-inferior” (or equivalent) to 1mg oral finasteride tablets in terms of hair regrowth.[12]https://pubmed.ncbi.nlm.nih.gov/19172031/ But concentrations as low as 0.005% have been shown to improve hair growth in men with pattern hair loss.[13]https://www.tandfonline.com/doi/abs/10.3109/09546639709160517 So, what about the systemic absorption part? Well, this is where things get complicated…
GF Mazzarella, GF Loconsole, GA Cammisa, GM Mastrolonardo & Ga Vena (1997) Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course, Journal of Dermatological Treatment, 8:3, 189-192
There are studies showing that topical finasteride at a 0.25% dilution can reduce scalp DHT by 24-75%, depending on the amount applied. And while larger applications reduce more scalp DHT, they also appear to have systemic effects on DHT. [14]https://pubmed.ncbi.nlm.nih.gov/25074865/
This is because finasteride has a dose-dependent logarithmic effect on DHT reduction. In other words, if just a tiny amount of the drug reaches the bloodstream, it can reduce just as much DHT as a dose that is 25 times higher.
That’s important, because depending on the percent of topical finasteride, the body may be exposed to more of the drug than if taking 1mg of finasteride orally. For example, applying 1mL per day of a 0.25% topical finasteride solution translates to the application of roughly 2.5mg of finasteride to the scalp.
That’s 2.5-fold more finasteride exposure than a daily 1mg oral dose. And again, just 0.2mg of that dose needs to enter the bloodstream to produce the same DHT-reducing effects everywhere as the oral medication.
For these reasons, many clinicians estimate that topical finasteride is roughly as effective as oral finasteride, but that it only reduces the risk of side effects by 30-50% compared to oral finasteride. There might be ways to lower this risk even further by changing the delivery vehicle of topical finasteride; however, it’s best to address that in a separate article.
5 – Oral Finasteride
Compared to topical finasteride, oral finasteride confers unique advantages: it’s easier to use, it affects all hair follicles rather than just the follicles where the topical is applied, and it’s supported by better clinical data. Across hundreds of studies and tens of thousands of participants, finasteride has demonstrated consistently impressive hair growth outcomes and a decent safety profile.
Better yet, the drug seems much more effective than its herbal alternatives. In one head-to-head study, it demonstrated significantly better hair growth outcomes over two years with finasteride versus saw palmetto. [15]https://pubmed.ncbi.nlm.nih.gov/23298508/ It’s this data that firmly cements finasteride as one of the more powerful DHT reducers on our list.
6 – Oral Dutasteride
Those looking for an even greater DHT-reducing effect may want to consider oral dutasteride. This medication is an inhibitor of type I and type II 5-alpha reductase and it’s prescribed off-label for those looking to treat pattern hair loss at the highest level.
Depending on the dose, dutasteride can reduce DHT levels by up to 95%. This makes it significantly more powerful than finasteride, with short-term studies showing that 0.5 to 2.5 mg of dutasteride regrows hair 2-5 times faster than finasteride, and even leads to more robust increases in hair counts.
Lee WS, Ro BI, Hong SP et al. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol 2007; 57: 37–46.
Interestingly, this meta-analysis showed that when used as a hair loss treatment, dutasteride’s risk of side effects was actually comparable to finasteride, despite lowering more DHT.[16]https://www.dovepress.com/getfile.php?fileID=48173 However, the risk of certain side effects – like gynecomastia – increases with dutasteride versus finasteride.
Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., & Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis. Clinical interventions in aging, 14, 399–406. https://doi.org/10.2147/CIA.S192435
So, while it’s likely more effective, it also may come with a slightly higher risk of side effects.
Before concluding, there are a few more things worth mentioning.
First, the only scientifically honest way to compare effectiveness and side effect profiles across DHT reducers is to test them within the same study. This is because patient populations, hair counting methodologies, and side effect questionnaires all vary across hair loss studies. That makes crude comparisons across two random studies really hard to do.
Since a single study comparing all of these DHT reducers does not yet exist, we can’t claim that this analysis is perfect.
Second, it’s important to keep in mind that in the absolutes, every DHT mentioned here is relatively safe. Finasteride and dutasteride are taken by millions of men every day – most of whom report no issues.
Hair loss sufferers shouldn’t let these relative comparisons scare them away from trying these pharmaceuticals. If someone cannot tolerate a drug, they can always hop off and try something else.
Third, a few DHT reducers were left out: topical dutasteride, RU58841, procapil, and others. This was intentional: these treatments rely too much on experimental data and unpublished clinical trials to accurately gauge efficacy and safety profiles.
These topics will be covered, at length, in future articles.
References[+]
References ↑1 https://www.jdsjournal.com/article/S0923-1811(03)00249-4/fulltext ↑2 https://www.amjmed.com/article/0002-9343(77)90313-8/pdf ↑3 https://www.karger.com/Article/Abstract/95251 ↑4 https://www.jaad.org/article/S0190-9622(98)70007-6/fulltext ↑5 https://journals.sagepub.com/doi/pdf/10.1177/039463201202500435 ↑6 https://www.karger.com/Article/FullText/509905 ↑7 https://link.springer.com/article/10.2165%2F00002018-200932080-00003 ↑8 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2518869/ ↑9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3326341/ ↑10 https://jissn.biomedcentral.com/articles/10.1186/s12970-014-0043-x ↑11 https://www.sciencedirect.com/science/article/pii/S0022202X15529357 ↑12 https://pubmed.ncbi.nlm.nih.gov/19172031/ ↑13 https://www.tandfonline.com/doi/abs/10.3109/09546639709160517 ↑14 https://pubmed.ncbi.nlm.nih.gov/25074865/ ↑15 https://pubmed.ncbi.nlm.nih.gov/23298508/ ↑16 https://www.dovepress.com/getfile.php?fileID=48173 Viviscal® is one of the most popular hair loss supplements in the world. What sets it apart from its competition? Clinical research. Viviscal has conducted 12+ human studies on its supplement line, all showing that its proprietary blend of marine extracts can improve hair growth in men and women, and with a variety of types of hair loss.
So, does this make Viviscal the go-to supplement for anyone interested in tackling hair loss naturally?
Maybe, maybe not. If there’s anything we’ve learned from other product reviews, it’s that clinical studies on a supplement rarely tell the full story.
More often than not, these studies can be designed to manufacture biased results; those results can create a false sense of hope; that hope can be transmitted to millions through advertising; that advertising won’t usually convey that the results from those clinical studies aren’t applicable to most of the people watching that ad.
That’s why it’s important to look beyond the marketing of a supplement to really see what that data says.
Before purchasing hair loss products, it’s important to examine the company’s products, marketing, and studies to find out whether this supplement is worth the investment.
Here’s the Perfect Hair Health approach to product reviews:
- Buy the product. Send the supplement to a third-party laboratory to test for impurities, heavy metals, and pathogenic microbes.
- Examine the ingredients. Compare the company’s rationale for these ingredients versus what the totality of research says.
- Read all published clinical studies. Evaluate their methodologies and results, identify areas of bias, and determine if these results align with the claims made in marketing.
The findings are surprising. The hope is that people fighting hair loss can make better decisions as to whether Viviscal is right for them.
Key takeaways
- Product: Supplement
- Effort: Low (once- or twice-daily supplement)
- Expectations: Clinical trials suggest improvement in as little as 3 months, however, Viviscal™ recommends at least 6 months of supplementation to see results.
- Response rate*: According to the clinical trials:
- Male pattern hair loss: 85%
- Women with temporary hair loss from stress, poor diet, or menstruation: 75-100%
- Autoimmune hair loss: 30-90%
- Regrowth rate*: According to the clinical trials:
- Male pattern hair loss: 0% (i.e., stabilization)
- Women with temporary hair loss from stress, poor diet, or menstruation: 10% to 32%
- Autoimmune hair loss: 5% to 85%. (Autoimmune hair loss is known to spontaneously fully reverse, so it’s hard to delineate how much of this is due to Viviscal versus the mysteries of biology.)
- Cost: $39.99/month
- Problems: Results contingent upon lifelong use; some biased methodologies in clinical research; hundreds of negative reviews online – with most seemingly complaining the product wasn’t effective after 3 months of use (despite one study showing a minimum 32% average increase in hair count after 3 months); for a product sold to millions of people, it’s nearly impossible to find clearcut before-and-after photos submitted by their customers.
All-Natural Hair Supplement
The top natural ingredients for hair growth, all in one supplement.
Take the next step in your hair growth journey with a world-class natural supplement. Ingredients, doses, & concentrations built by science.

*These response and regrowth rates likely do not reflect reality. Response rates and regrowth rates were calculated using Viviscal’s clinical studies. However, many of these studies came with biased methodologies. Normally, adjustments can be made for these biases in estimates. But the difference between clinical research versus customer reviews was so vast in this case, it’s hard finding a middle ground. The fact that so few before-after photos exist in Viviscal’s customer testimonials – after 25+ years of business – should be a telling sign to consumers.
Ordering experience
- Checkout: 7/10: One-page checkout made the process relatively easy, but coupon popups and the absence of an autofill or PayPal option made form filling a bit more cumbersome than other checkout pages.
- Fulfillment: 9/10: An email receipt was generated instantly upon receipt of the order, and later to announce that the product had shipped.
- Arrival: 8/10: Arrived within 5 days and without issues.
Highlights
Compared to other hair loss supplements, Viviscal has its perks.
- The company has conducted 12 clinical trials testing Viviscal™ in men and women with many types of hair loss. All of them show positive results.
- Viviscal’s supplements don’t contain a lot of extraneous ingredients. This is a positive; many hair loss supplements are chock-full of dozens of vitamins and minerals – many of which are harmful if over-consumed.
- The supplement contains ingredients that may address some underlying causes of hair loss.
- The supplement passed our product purities testing for heavy metals, pollutants, and pathogenic microbes. In other words, what you see on the label is what you get.
Having said that, when looking more closely at Viviscal’s clinical trials, marketing efforts, and product ingredients, a lot of issues emerged. Here are just a few.
- For a company that has conducted 12 clinical studies and been in business for 25+ years, there are almost no before-after photos from customers demonstrating clear, discernible improvements.
- Most of Viviscal’s studies are either unpublished or poorly designed.
- At least 33% of their studies were never published in a scholarly journal.
- Of the studies that were published, there are glaring typos and selection biases that make the results less impressive than perceived at first glance. For example, many of Viviscal’s studies use participants who are losing their hair temporarily as a result of stress, bad diets, or menstruation-related nutrient deficiencies. These participants are most likely to see hair regrowth from a nutritional supplement and would’ve also likely seen just as impressive hair growth with better stress management and nutritional planning. Moreover, in the developed world, this hair loss from these causes is relatively less common – and it’s also avoidable with simple dietary and lifestyle adjustments. Therefore, it’s disingenuous (and perhaps deceptive) to market the results of these participants to the general population of hair loss sufferers.
- Viviscal’s genesis story cannot be verified. Viviscal says that their product was developed after a Scandinavian professor observed that the Inuit peoples’ healthy, long-lasting hair was the result of their diets rich in marine foods. This professor allegedly was able to isolate the compounds responsible for the Inuit’s healthy hair, from which Viviscal was born.
However, none of this is recorded in any of the clinical studies published by Viviscal. Moreover, Viviscal has not responded to our emails requesting the name of the professor. Rather, research actually points to Viviscal being developed from an earlier product targeting to improve aging skin in women.
A full analysis of Viviscal’s company (and supplements) can be found below.
What is Viviscal®?
Viviscal™ is a hair loss supplement endorsed by hundreds of doctors in the U.S. It’s supported by 12 clinical trials and 25 years of research. It goes without saying that Viviscal is the most heavily researched hair loss supplement out there.
Viviscal® Man
What makes Viviscal unique?
There’s one “ingredient” that makes Viviscal unique from all other hair loss supplements: its proprietary blend of shark cartilage and oyster extract powder, known as their AminoMar™ Marine Complex.
According to the company’s FAQ’s:
“The groundbreaking, clinically proven marine complex available exclusively in Viviscal supplements. Derived from key marine protein molecules combined with a blend of Horsetail (Stem) Extract and naturally occurring Silica, it provides essential nutrients needed to promote existing hair growth from within.”
In fact, this proprietary blend (AminoMar™) has been the focus of every clinical study on Viviscal. Its clinical effectiveness is what makes Viviscal so enticing to consumers looking for a natural solution to thinning hair.
Product Offerings
Viviscal™ offers two product lines based on gender.
For women, they offer a Viviscal™ supplement as well as a shampoo, conditioner, and topical. For men, they offer a Viviscal™ supplement (with slightly different ingredients) and a shampoo.
This review focuses exclusively on Viviscal’s supplements. After all, the supplements are the products that have been clinically studied.
Viviscal Hair Growth Supplement – Ingredients
Both Viviscal™ for Women and Viviscal™ for Men contain:
- Vitamin C
- Calcium
- Zinc
- Horsetail extract
- AminoMar™ (Viviscal™’s properietary blend of shark cartilage and oyster extract power).
However, Viviscal™ for Women also contains niacin, iron, biotin, and millet seed whereas Viviscal™ for Men contains flaxseed. Here’s the full list of ingredients.
Viviscal Woman vs. Viviscal Man: product ingredients
Viviscal™ for Women Viviscal™ for Men Vitamin C Vitamin C Calcium Calcium Zinc Zinc AminoMar™ AminoMar™ Niacin Flaxseed Iron Biotin Millet seed There are also slight differences in the number of ingredients in each of the male and female supplements. Here are the labels.
Viviscal™ for Women
Viviscal™ for Men
How might these ingredients improve hair loss?
Viviscal’s clinical studies focus on their AminoMar™ proprietary blend. But their auxiliary ingredients – like flaxseed, biotin, and iron – may also help with hair growth for certain people, and in different ways.
Below is a list of Viviscal’s key ingredients and the company’s rationale for their inclusion. The team found some of Viviscal’s claims to be nondescript. These claims were compared against what the actual research says.
AminoMar™
What Viviscal® says
“The groundbreaking, clinically proven marine complex available exclusively in Viviscal supplements. Derived from key marine protein molecules combined with a blend of Horsetail (Stem) Extract and naturally occurring Silica, it provides essential nutrients needed to promote existing hair growth from within.”
What the research says
What’s Viviscal’s original rationale for how AminoMar™ might work?
After all, their website just says that this marine extract, “promote[s] existing hair growth from within.” That’s a bit vague.
Viviscal’s first study (1993) referenced three earlier studies that tested a similar marine extract.[1]https://pubmed.ncbi.nlm.nih.gov/1286738/ These studies focused on skin health, not hair health. But interestingly, they found that women using this marine extract also reported improvements to their brittle hair, at least in a survey that asked them about brittle hair.
But when we read these three studies, none of them explained or speculated about the ways in which the marine extract worked, either. To quote from the earliest study we could find:
“Although the mode of action of Imedeen® [the marine extract] is unclear, the results of the present study indicate that there are certain agents in the extract which seem to have a repairing effect on degenerated elastic and collagen tissue in the dermis.”[2]https://www.ncbi.nlm.nih.gov/pubmed/1864451
Again, this is very vague, and very unusual – especially for a scientific journal. But it seems that more recent research papers (i.e., those after 2010) have tried to offer better explanations.
For instance, this 2019 review attributes Viviscal’s hair-promoting effects to the fatty acids, polysaccharides, and cartilage proteins inside the marine proprietary blend.[3]https://www.karger.com/Article/FullText/492035
Specifically, the reviewers discuss a polysaccharide (i.e., sugar) called glycosaminoglycan. These sugars are found near the “powerhouse” of a hair follicle: the dermal papilla. This is near the hair follicle base, and it’s where a hair connects to its blood supply.
A hair follicle: note where the blood supply connects to its dermal papilla
When a hair follicle is growing, glycosaminoglycan levels surrounding the dermal papilla increase.[4]https://www.ncbi.nlm.nih.gov/pubmed/1610689 And when a hair sheds, glycosaminoglycan levels in these regions decrease. Thus, one hypothesis is that AminoMar™ increases glycosaminoglycan levels surrounding our hair follicles, and in doing so, helps a hair grow longer and shed less frequently.
There might be some truth to this, too. In clinical studies on AminoMar™, the extract helps elongate our hair growth cycle – shifting more hairs into their growth stages, and fewer hairs into their shedding stages (more on this later).
Flaxseed (Viviscal® for Men)
What Viviscal® says
According to Viviscal, flaxseed extract is included in their male supplement because it contains vitamin E and omega 3 fatty acids, two substances that they say promote hair health.
“Vitamin E helps to nourish your roots and scalp and is found in Flaxseed. Flaxseed is one of the best foods to eat to combat hair loss, or to give your hair an extra boost of nutrients. Omega-3 fatty acid is in this amazing little, yet powerful, nutrient, helping to prevent your hair from drying out and losing its shine.”
What the research says
There is evidence that vitamin E supplementation might improve hair growth. And vitamin E is found inside flaxseed extract. According to NutritionData, one tablespoon of flaxseed oil (i.e., 14 grams) contains 2.4 milligrams of vitamin E, or 12% of our recommended daily intake.[5]https://nutritiondata.self.com/facts/nut-and-seed-products/3163/2
Unfortunately, Viviscal supplements include just 0.05 grams of flaxseed extract. Adjusting for the size difference, this implies that Viviscal has only a fraction of 1% of our recommended vitamin E intake. Given that the studies on vitamin E for hair growth used supplements containing 667% our recommended daily intake, the amount in Viviscal is negligible, and likely has zero effect on our hair.[6]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3819075/
As far as omega 3 fatty acids are concerned, this 2015 study showed that omega 3 supplementation (alongside antioxidants like vitamin E) increased hair counts by ~ 5% in women with general thinning. But again, the amount of omega 3 + vitamin E in that study is dozens of times higher than the amount found in Viviscal, so results really cannot be compared.[7]https://onlinelibrary.wiley.com/doi/abs/10.1111/jocd.12127
The bottom line: in some cases, vitamin E and omega 3 from flaxseed extract might improve hair loss, but probably not in the negligible amounts found in a Viviscal supplement.
Biotin (Viviscal® for Women)
What Viviscal® says
“Also known as Vitamin H, it is a water-soluble Vitamin B complex (Vitamin B7) that helps the body to metabolize carbohydrates, fats and amino acids, which are the building blocks of protein and thus essential in the formation of the hair structure.”
What the research says
Low-grade biotin deficiencies aren’t uncommon in the U.S. In fact, during pregnancy, about 50% of women will develop what’s known as a marginal biotin deficiency. This is when biotin levels drop slightly below what’s considered normal.
Having said that, marginal biotin deficiencies aren’t linked to hair loss. Rather, biotin-related hair loss is only seen in what’s known as a “profound deficiency”. These occur as a result of genetic mutations, chronic alcoholism, chronic antibiotic abuse, and/or a diet that is completely devoid of biotin for years.
So, just how common are partial or profound biotin deficiencies in the developed world?
Incredibly rare. According to worldwide neonatal screening surveys:
“…The incidence of profound biotin deficiency is one in 112271, and the incidence of partial deficiency is one in 129282.”[8]https://www.ncbi.nlm.nih.gov/books/NBK547751/
In other words, severe biotin deficiencies occurs in less than .001% of people. And again, only severe biotin deficiencies are causally linked to hair loss.
This begs the question: how useful is it to supplement with biotin for hair?
According to some research groups, not very useful at all. In fact, one investigation team has recommended to “reject the practice of supplementing with high doses of biotin for treating hair loss” unless there is a lab-confirmed deficiency, and that the deficiency is severe enough to be of concern.[9]https://perfecthairhealth.com/nutrient-deficiency-hair-loss/#biotin
Thus, biotin supplementation (even at the 400% recommended daily intake found in Viviscal®) probably won’t be that helpful, at least for the overwhelming majority of people taking the supplement.
Vitamin C and Iron (iron in Viviscal® for Women only)
What Viviscal® says
“[Vitamin C:] A powerful antioxidant that helps to absorb more Iron into the blood, which in turn promotes hair growth. Vitamin C in Viviscal supplements is sourced from the acerola cherry.”
“[Iron:] An essential mineral that has several important roles in the body, Iron helps to make red blood cells, which carry oxygen around to cells in the body, including hair follicles. Thinning hair can be one of the visible symptoms of anemia (Iron deficiency).”
What the research says
Viviscal’s rationale for including both vitamin C and iron center around an effort to enhance iron levels in their customers.
Vitamin C helps to increase iron absorption. This vitamin is included in both Viviscal® Man and Viviscal® Woman. However, supplemental iron is only found inside the female formulation of Viviscal. And this is because, unlike men, women are at a much higher risk of developing low iron (i.e., anemia) as a result of iron loss from menstruation.
There’s some evidence to support these positions. For instance, studies have linked mild-to-severe iron deficiencies in women to a variety of hair loss disorders – ranging from female pattern hair loss to telogen effluvium.[10]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3678013/ [11]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6385517/ And while there’s still debate over how effective (and how high) iron levels need to raise in order to improve hair loss, it goes without saying that a lot of women have experienced great improvements to hair growth by restoring their iron to adequate levels.
Unfortunately, supplemental iron also harbors some risks. This is because iron is oxidative – meaning that in excess, its presence can create oxidative stress, also known as inflammation.
In fact, evidence indicates that vitamin C and iron salts (like the ferrous fumarate used in Viviscal™ for Women) may have a dangerous and synergistic interaction. Specifically, research suggests that vitamin C can “propel” the oxidative action of iron, meaning that vitamin C may enhance the inflammation that excess iron causes.[12]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC340385/ This suggests that in cases of longterm, excessive iron + vitamin C supplementation, we’re more likely to experience problems like ulcerations, inflammation, exacerbation of chronic disease, and, potentially even cancer.
Customer testimonials
It’s one thing to look at a supplement’s ingredients, then build a case for whether the research shows these ingredients will or won’t help with hair loss. It’s another thing to measure whether all of these ingredients – when taken together – actually improve hair growth in men and women with thinning hair.
To do this, one can analyze two pieces of evidence:
- Customer testimonials. Viviscal has been in business for 25+ years. They claim to average one product sale every single minute of the day. That equates to millions of customers, and (hopefully) thousands of before-after photos.
- Clinical research. Viviscal is the most heavily studied hair loss supplement in the world. If Viviscal’s clinical research proves as promising as claimed in their marketing, then customer testimonials should be validated in the data: increases to hair counts, hair diameters, etc.
This section focuses on that first lever: customer testimonials and before-after photos.
12 clinical studies and 25+ years of business. But where are the before-and-after photos?
When it comes to hair loss, before-and-after photosets are probably the most powerful tool used to validate the effectiveness of any approach. Yes, clinical trials are great (and often required). As will be discussed later, clinical research is only valid when a study is properly designed. Thus, if a product truly works, one can expect to see a combination of both clinical evidence + customer testimonials.
On review websites, customer reviews of Viviscal are mostly negative. However, these reviews were left out of this product review. Why? Because reviews can be written by anyone – including competitors or Viviscal employees. And given the inability for review websites to verify and rank the validity of any review, the focus remained on hard evidence: that of which is presented by Viviscal on their own website.
So, as one of the best-selling hair loss supplements on the planet, how does Viviscal do in the customer success department?
Puzzlingly, Viviscal has almost no customer before-and-after photos (at least on their website).
And of the ones that are featured, it’s as if they’re designed to be intentionally misleading. These can be segmented segment this by the photos featured on Viviscal’s (1) main website, (2) female site, and (3) male site.
The photos featured on Viviscal® appear to have had their backgrounds trimmed and/or recolored. This masks any lighting differences across photos; it makes fair photo comparisons impossible.
The success featured on Viviscal® Woman aren’t actually focused on hair; rather, they’re simply photos of women wearing different hairstyles before and after starting the supplement.
And the few customer photos featured on Viviscal® Man either face the same problems above or were taken from impossible-to-compare angles and lightings.
Why is this concerning?
Well, in their marketing, Viviscal claims that one packet of Viviscal is sold every single minute. This should equate to 10+ million customers over a 25-year period. And if the product were truly as effective as advertised, one would expect Viviscal to present significantly more before after-photos, and of significantly better quality.
For instance, let’s assume that Viviscal only has one million consistent customers (a fraction of what is suggested in their marketing). Now assume that just 0.1% of those customers (or one in 1,000) decide to take before-after photos, then share those photos with Viviscal. That leaves Viviscal with 1,000 before-after photos from which to share on their website.
For some reason, Viviscal seems to only have ~20 photosets total across men and women. And instead of high-quality photo comparisons, we’re left with misleading photosets.
What about before-after photos from Viviscal’s clinical studies?
It’s true that Viviscal’s clinical studies have higher-quality photosets, and that in some cases, these photosets demonstrate clear, discernible hair regrowth. But it’s also important to contextualize these photosets – because not all photos are from people with common hair loss types.
For men with androgenic alopecia (AGA) – the most common form of hair loss in adult men – the highest-quality before-after photoset available from a Viviscal study only showed stabilization after 180 days of use. In fact, this is the one advertised on their website.
For women, the highest-quality before-after photosets come from their website (here) and clinical studies [13]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3509882/
But these women only had temporary forms of hair loss due to stress, poor diet, and nutrient deficiencies due to menstruation. In other words, these before-after photos don’t represent what the average Viviscal customer should expect, because hair loss from these causes are lesser common, and likely do not represent the average customer of Viviscal.
Now for Viviscal’s clinical studies …
Clinical studies
When it comes to clinical studies on a hair loss supplement line, Viviscal® is the industry leader.
According to their marketing materials, Viviscal has conducted 12 clinical trials – more human studies on their product than any other hair loss supplement out there.
And even better, these clinical trials aren’t limited to just one population, or one type of hair loss. Of the 12 clinical trials, seven were conducted on women, two were done men with androgenic alopecia (AGA), and two were completed on men and women with autoimmune hair loss disorders like alopecia areata, alopecia totalis, and alopecia universalis.
In other words, they’ve not only got the most clinical studies, but they’ve also studied their supplement on the majority of types of hair loss out there. And amazingly, all of the studies have appeared to reap great results.
Viviscal’s clinical studies: summarized
Viviscal™’s Clinical Trials, Their Subjects, and the Results Study #1 (2014) Females with Self-Perceived Hair Thinning “• 32% increase in terminal hairs after 3 months • 39% decrease in hair shedding
• After 3 months there was significant self-perceived improvements in overall hair volume, scalp coverage and hair strength.”
Study #2 (2014) Females with Self-Perceived Hair Thinning • 57% increase in terminal hairs after 3 months • 80% increase in terminal hairs
• 12% increase in hair diameter after 6 months
• After 3 and 6 months there was a significant self-perceived improvement in overall hair volume, scalp coverage, hair and nail strength.
Study #3 (2014) Females with Subclinical Hair Thinning “• a 7.4% increase in hair diameter after 6 months • an 18% reduction in hair shedding after 3 months.”
Study #4 (2014) Females with Self-Perceived Hair Thinning • 94% in hair volume • 92% hair thickness
• 91% in nail growth rate and
• 92% in nail strength
After 6 months.
Study #5 (2012) Females with Self-Perceived Hair Thinning • 111% increase in terminal hairs after 3 months versus no change amongst the placebo subjects. • 125% increase in terminal hairs after 6 months versus no change amongst the placebo subjects.
• After 3 and 6 months there were significant self-perceived improvements in overall hair volume, thickness, and scalp coverage.
Study #6 (2011) African American Females with Self-Perceived Hair Thinning “Rapid changes in hair growth and appearance in as little as 2 months.” Study #7 (2010) Females with Self-Perceived Hair Thinning “After a 10 week period, there was an average 46% reduction in hair loss reported. 75% saw an increased thickness in the body of their hair and 75% saw an increase in overall hair volume.” Study #8 (1997) Men with AGA • 75.3% of patients observed a significant decrease in hair loss • 14.6% of patients showed partial regrowth
Study #9 (1996) Males and Females with Alopecia Areata, Alopecia Totalis, or Alopecia Universalis • 92% of areata group showed regrowth of permanent hair. After four months of treatment: • 83.3% of totalis group showed regrowth of permanent hair and
• 31.8% of universalis group showed regrowth of permanent hair after five months.
• Complete cure was observed in 14% of areata, 25% of totalis and 5% of universalis.
Study #10 (1994) Men with AGA “Hair loss stopped for 100% of subjects after two months treatment. 43% showed total regrowth, 23% showed three quarter regrowth, 13% showed half regrowth & 13% showed 30-50% regrowth.” Study #11 (1992) Men with AGA “100% of treated subjects reported that hair loss had stopped after 2 months of treatment. Mean increase in non-vellus hair of 38% was recorded in patients after 6 months treatment. 95% of subjects showed both clinical and histological cure.” Study #12 (1992) Males and Females with Alopecia Areata, Alopecia Totalis, or Alopecia Universalis “85% of subjects with Alopecia Areata were completely cured & 10% showed significant improvement. 25% of subjects with Alopecia Totalis were completely cured & 20% showed significant improvement.” At a glance, this body of clinical research seems to position Viviscal as the go-to supplement for anyone with thinning hair. But as the Perfect Health team learned from its investigation into Nutrafol, clinical trial results can be misleading.
For instance, studies can pick participants who most likely have nutrient deficiency-related hair loss, and are thereby most likely to see hair regrowth from taking any nutritional supplement (not just the brand being investigated. Studies can also use biased hair counting methodologies to make it appear as though a supplement regrows a ton of hair, even when the results don’t translate to visual improvements to hair density.
So, do Viviscal’s studies have any of these biases?
In many cases, yes.
Whether or not these biases invalidate the body of research of Viviscal remains to be seen. It would be unproductive to go through each Viviscal study and dissect every little problem. Doing this would turn this already-long product review into a full-fledged novel.
In focusing more on the larger issues across these studies, hair loss sufferers can decide for themselves whether these concerns are worrying enough to influence purchasing decisions.
Problem #1: at least 33% of Viviscal’s clinical trials were never published
It’s one thing to conduct a clinical trial, analyze the data, write the results into a manuscript, submit that manuscript to a journal, get it reviewed by experts, receive feedback, make revisions, resubmit, and then finally have your study accepted for publication in a scholarly journal.
It’s another thing to conduct a clinical trial, analyze the data, write the results into a manuscript, and then bypass peer review and, instead, just publish the results as part of your marketing materials.
According to Perfect Hair Health research, 33% of Viviscal’s clinical trials fall under that secondary category. Of the 12 studies listed on Viviscal’s website, our investigation showed that four studies (#4, #6, #7, and #12) never made it into a scholarly journal. In fact, 3 of those 4 studies were never even submitted for publication.
Why not publish the results of a clinical trial in a scholarly journal, especially if the results are positive?
This is actually more commonplace than most people realize. In fact, conducting a clinical trial and not publishing the results in a scholarly journal happens all the time – especially for companies studying (and selling) natural health products.
Just take the companies selling low-level laser therapy devices. Companies in that hair loss sector have a history of registering clinical trials containing biased hair counting methods (e.g.., grouping vellus + terminal hair counts). These companies never intend to submit their results to a scholarly journal. Rather, they conduct these studies so that when their great (manufactured) results come in, they can legally claim their devices can “increase hair thickness by over 200%”.
In these cases, the goal isn’t to prove that the clinical research is legitimate; the goal is to avoid getting sued over false claims.
As such, it’s not a priority for these companies to submit their clinical data for peer review. If they did, reviewers would likely find the flaws in the study design, and then reject the study for publication.
Therefore, the most common reason why a company won’t publish clinical results in a journal – at least when those results are favorable – is that they know that the study is biased, and that the paper won’t survive peer review. So, instead, these companies will just write up their results to look like a paper, and then use it as part of their marketing.
(Note: this is different from burying unfavorable clinical trial results – or in other words, never publicizing the findings. This is what Nutrafol is suspected of doing for one of the clinical trials that they completed, but never published.)
For Viviscal, favorable unpublished clinical trial results make up 33% of the studies advertised on their website.
Now, this isn’t the end of the world… because that still leaves 8+ studies that have gone through peer review, and have been published in journals.
So, how do these published trials stack up?
Well, these published studies also have issues. This leads to another problem.
Problem #2: of Viviscal’s published studies, most of them were published in low-ranking journals
Low-ranking journals, and why they should be avoided
If the four studies Viviscal never published were ignored in favor of the eight studies that survived peer review, there would still be a great deal of noise that makes Viviscal’s emphasis on their clinical research problematic.
For starters, these eight clinical studies weren’t published in high-ranking dermatology journals. Rather, they were published in lower-ranking journals in dermatology and medicine. These are journals that rank below the top quartile, according to SciMago Journal Rankings.
Now, one might wonder, “Who cares about a journal’s ranking? Peer review means peer review!” But unfortunately, this isn’t true. This is because not all peer review is created equal.
Quality reviewers – those of reputable institutions and research centers – generally reject review requests from unknown or lesser-respected journals. Why? Because the papers submitted there are often of lower quality. This forces lower-quality journals to send mass emails of review requests –until they either find someone to accept the review or they decide to make a decision on the paper without any external review at all.
In the case of low-ranking journals, too often are their reviewers people with zero expertise regarding the manuscript in which they’re reviewing.
For example, Perfect Hair Health has published two papers about androgenic alopecia. A list of the last three review requests from low-ranking journals? Manuscripts about:
- Dapsone-loaded micro sponge gel for acne management
- Business management practices
- R&D teams: how innovation and performance varies between large American and Chinese firms
All three review requests were declined because the company only publishes research on hair.
So, just think of how far down a list someone has to get before they contact Perfect Hair Health: someone with zero experience, let alone expertise, in any of these fields.
What’s worse: a quick Google search just revealed that two of these three papers passed peer review, made it into a journal, and are now indexed in scholarly databases. That suggests they were reviewed by someone! If not me, then who? Someone with an equally absent level of expertise? Or nobody at all – given some journals’ incentives to collect authors’ payments in exchange for publications?
Now, back to Viviscal.
The company did, in fact, publish their clinical studies in lower-ranking journals. Knowing this, is there evidence that these studies weren’t properly reviewed?
Yes. In fact, one can identify – just by reading these papers – errors so big, they should’ve been flagged by literally anyone reading the paper.
Example 1: grammatical errors… in the first sentence of a paper
See the first of Viviscal’s 2015 study, published in International Journal of Trichology:
“Since skin and hair quality are potent vitality signals, and hair growth deficiency can cause significant psychological morbidity.”
That’s not even a complete sentence.
Example 2: hair count increases of 1,381%
In Viviscal’s first-ever study, investigators show the results of Viviscal supplementation on hair counts of men with androgenic alopecia (AGA) after six months. Their final hair count increase? A jump from 1,238 to 17,101 hairs per measured region… an increase of 1,381%.
Obviously, this is just a typo. The investigators clearly meant to write 1,710 and not 17,1010 (as indicated by the hair count differences listed in the final row). But a serious reviewer should’ve caught this.
This is the difference between publishing in reputable journals, and publishing just so you can tell people that your product has a peer-reviewed clinical study. The former actually matters; the latter is just for marketing.
Problem #3: Many of Viviscal’s studies have selection bias. Many were done on people with a type of hair loss that doesn’t represent the majority of Viviscal consumers.
It doesn’t just matter how people write up a clinical study; it also matters how that study was conducted. And in this regard, many of Viviscal’s published studies have serious problems. This is especially true in the way that they’ve selected participants for their study.
For reference, there are dozens of types of hair loss, and each type has a different set of causes. The most common types of hair loss are (1) androgenic alopecia (AGA) – also known as male and female pattern hair loss, and (2) telogen effluvium.
Current census is that AGA is caused by a combination of hormones and genetics. It’s chronic, progressive, and without treatment, it worsens. Conversely, telogen effluvium is a form of temporary hair loss. It’s got a wide set of causes – stress, medication use, hypothyroidism, etc. – and once the underlying cause of telogen effluvium is resolved, usually the hair will return in 3-6 months.
For women, one common cause of telogen effluvium is a nutrient deficiency. And one of the most common nutrient deficiencies in women of “childrearing age” is iron – because women lose iron each month during menstruation.
Therefore, this is a percentage of women losing their hair from telogen effluvium – a temporary form of hair loss. And a percentage of those women have telogen effluvium due to low iron stores. If these women correct their low iron stores, they’ll often regrow their hair.
So, let’s look at some of the best Viviscal studies: the ones published in journals that are lower-ranking, but still high enough to likely find reputable reviewers.
These studies were conducted in the U.S. That means they had to register the trials on clinicaltrials.gov. And whenever a trial registers on this site, they have to outline the selection criteria for participants. In other words, they have to tell you how the investigators selected people to include (or exclude) as part of their study.
So, when it comes to participant selection, what do all three of Viviscal’s US-based clinical trials have in common?
All of the studies selected women with temporary forms of hair thinning – mainly the result of poor diets, stress, or menstrual cycles – of which would’ve resolved without a nutritional supplement if the person had just taken measures to get more of those nutrients into their diets.
To quote directly from the trial setups:
“Inclusion Criteria: Females with self-perceived thinning hair associated with poor diet, stress, hormone influences or abnormal menstrual cycle.”
All of these clinical trials showed statistically significant hair count increases to Viviscal after 3-6 months of use. Not surprisingly, at least two of these trials were conducted by the Ablon Group – the research institution that used nearly the same selection criteria for Nutrafol’s clinical study – which sufferers from the same problems will be illustrated.
Why is this such a problem?
For two reasons.
Firstly, this selection criteria is incredibly narrow. Women with telogen effluvium from a nutrient deficiency represent only a fraction of people with hair loss. While there’s no data on the incidence of nutrient deficiency-driven telogen effluvium, it probably responsible for less than 10% of hair loss cases in the U.S.
This means that if Viviscal advertises its “clinical results” to all U.S. hair loss sufferers – the results from their clinical trial won’t apply to 90% of them. But consumers don’t know this. They read the words “clinically proven”, and expect that this product applies to them and will reap positive results.
That’s the first problem.
The second problem is that if the women in this study had just reduced their stress levels, improved their diets, and/or made an effort to get more iron, they likely would’ve seen the same degree of hair regrowth… and without the aid of a supplement that costs $39.99 every month.
After all, studies on women with nutrient deficiency-related telogen effluvium have shown equivalent increases in hair counts – without Viviscal’s proprietary ingredients – and all with a basic nutritional supplement.[14]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6136400/
Are there any studies that fairly assess Viviscal?
Yes.
There is one clinical study on Viviscal that appears to meet this criteria. It was done on men with androgenic alopecia (AGA) – the most common cause of hair thinning for adult males.
It was a 1997 study conducted on 200 men with AGA, all of whom were instructed to take Viviscal® for Men twice daily for six months. The study wasn’t published in a high-ranking journal, but it also didn’t have the same selection criteria biases identified in Viviscal’s more recent studies.
Moreover, the study had clearly defined methodologies. The researchers used objective measurements for results – like hair count changes using a trichogram, or hairline changes by calculating the distance between the hairline and the eyebrows before and after treatment. In addition, the researchers also had participants complete a self-assessment survey to standardize their opinions of the supplement.
Over the six-month period, 32 men dropped out of the study. But of the 178 men who completed the trial, here were their results:
- Across all Viviscal users, hair counts increased by 4% (statistically insignificant)
- 75% of men reported a decrease in hair loss (i.e., the rate of hair loss slowed)
- 15% of men showed partial hair density improvements
These results aren’t that impressive. But, in our opinion, they’re the fairest results ever published on Viviscal. Why? Because they set realistic expectations of the supplement.
Males with AGA who are taking Viviscal should set their expectations that the supplement simply slows down the progression of hair loss; the supplement will not fix the problem. And for those who (1) are happy with that expectation, (2) understand the effects only last with continued use, and (3) don’t mind paying $39.99 per month indefinitely – then this supplement might be a good choice.
Viviscal’s clinical research: the bottom line
- Viviscal boasts over 12 clinical studies on their product line – more studies than any other hair loss supplement.
- However, of these 12 studies, four of them were never published in a scholarly journal.
- Of the studies that were published, these clinical trials were published in low-ranking journals that tend to have really easy peer-review procedures,
- Resultantly, these studies were published with problems that would’ve been flagged – and likely would’ve led to a rejection – in reputable journals: for example, typos in the opening sentences and selection biases for participants.
- This is most evident with three of Viviscal’s published studies: the ones that tested the supplement on women with temporary hair thinning as a result of stress, a poor diet, or menstruation-related iron loss. These types of hair loss sufferers are most likely to benefit from a basic nutritional supplement – particularly ones containing iron, zinc, and antioxidants.
- Moreover, hair loss due to a nutrient deficiency is relatively uncommon compared to all hair loss causes. This type of hair loss accounts for just a small fraction of hair loss cases in the U.S. – likely less than 10%. Therefore, it’s unethical to advertise these results as “clinically effective” to all people with hair loss, when the results only apply to a small portion of a people hearing the claim.
- There is one study on Viviscal that seems fairly conducted: a trial on 200 men with androgenic alopecia (AGA). This is the most common form of hair loss in adult men. The trial found that Viviscal did not statistically improve hair counts, but that the supplement did help decrease the rate of hair loss for these men.
As such, our takeaways on Viviscal’s clinical research are the following:
- The product is great for females with hair loss due to a poor diet and/or nutrient loss from menstruation.
- The product is great for males with AGA who want to take a natural approach to improving their hair and only want to slow down the rate of hair loss – and are comfortable spending $39.99 per month to do so.
Otherwise, we think that Viviscal by itself is not an effective solution for most people with hair loss, and that this money could be better invested elsewhere.
Product purity: heavy metals, microbes, and contaminants
While Viviscal’s clinical studies and testimonials aren’t as impressive as they look at first glance, there is good news. The company makes a high-quality supplement… at least in terms of product purity.
The Perfect Hair Health team bought Viviscal® For Men and sent it to a third-party lab testing facility to test for contaminants often found in consumer supplement products: heavy metals, pathogenic microbes, and impurities related to materials processing.
There were no red flags. By all means, this supplement appears to be made well. What you see on the ingredients list is what you get.
Viviscal® Man purity: heavy metals, microbes, and pesticide residues
This is a positive, as some products that the Perfect Hair Health team has reviewed (i.e., Hairguard Anti-Hair Loss Essentials Supplement) have demonstrated an absence of the ingredients they list on their product labels.
BONUS: How did Viviscal start?
This isn’t related to our analysis of the supplement, but it’s a part of our investigation worth sharing.
Viviscal says that the company all began with the discovery (and development) of AminoMar™ Marine Complex – a proprietary blend of shark cartilage and oyster extract power. According to their website:
“AminoMar is a proprietary marine complex available exclusively in Viviscal hair growth* supplements. In the 1980s, a professor from Scandinavia discovered that the Inuits’ great hair and skin was the result of their fish- and protein-rich diet. He isolated the key protein molecules in their diet and it was from these origins that AminoMar was created. Since then, Viviscal has been tried and tested by clinicians around the world.”
The story sounds almost too good to be true. An unnamed Scandinavian researcher… An observation made amongst the Inuits…. The identification of diet as responsible for the Inuit’s great hair… The isolation of key protein molecules from that diet… The packaging of these molecules into a clinically supported supplement.
Is there any validity to this story? The Perfect Hair Health team attempted to identify this mysterious Scandinavian researcher, his early research on the Inuits, and how this research eventually culminated into the product Viviscal.
These efforts just led to more confusion.
Viviscal’s beginnings: fact or fiction?
Research began by combing through Viviscal’s earliest published studies from the 1990s. It was assumed that in these early papers, Viviscal would discuss their novel proprietary blend: how they came across it, how the blend’s suspected effects might improve hair growth, and why the company has decided to study the blend in a clinical setting.
Unfortunately, the team couldn’t find any references mentioning the story that Viviscal explains in their marketing.
The closest thing identified was a 2019 literature review on alternative hair loss treatments, “Complementary and Alternative Treatments for Alopecia: A Comprehensive Review.” According to that review:
“Marine proteins, including extracellular matrix components from sharks and mollusks, have been produced for over 15 years to enhance hair growth. A Scandinavian researcher first described the exceptionally healthy skin and hair of the Inuit peoples to be a result of their fish- and protein-rich diet [36, 37]”.[15]https://www.karger.com/Article/FullText/492035
But when checking the review’s references (i.e., studies “[36, 37]”), it was found that those studies didn’t actually support the claims made in that quote.
For instance, that first reference is to Viviscal’s first-ever study.[16]https://www.ncbi.nlm.nih.gov/pubmed/1286738 That study had already been read and there was no mention of Inuits or a Scandinavian researcher. The second reference (i.e., “37”) couldn’t be found either. Why? Because according to Viviscal, that reference is to a clinical trial that was never even published.
So, the digging continued. The only thing to do next: read all of the studies mentioned in Viviscal’s first study from 1992. In other words, read the papers within a paper.
Still no references to a Scandinavian researcher or the Inuit… or anything about how this marine extract was developed or its purported mechanisms of action.
A how-to guide on passing peer review
The closest thing the team could find were studies on a product called Imedeen® – a marine extract supplement that was shown to improve skin health in females. These studies were done from 1991-1992 – one year before Viviscal’s first study – but these studies also don’t mention what prompted anyone to start studying these marine extracts.
What is Viviscal’s true genesis story?
This remains unknown. Perfect Hair Health reached to Viviscal® in a good faith effort to obtain the name of this Scandinavian professor, so that the team could verify the story they tell in their marketing. No reply has been recieved.
Interestingly, a 2001 clinical study on HairGain® – the earlier brand name of Viviscal® – was found.[17]https://www.researchgate.net/publication/12056799 This study showed that the supplement led to impressive hair regrowth in men with androgenic alopecia. The study was done by a man named Erling Thom – a Scandinavian researcher who was later accused of falsifying data in the studies he conducted on alternative health products.
The team couldn’t find any evidence tying Erlin Thom to Viviscal’s earlier clinical research. But there also wasn’t any evidence that he wasn’t involved. After all, Viviscal’s elusive genesis story seems to have no paper trail – which is odd for a product involved in so much clinical research.
It appears as though Viviscal was created from Imedeen® – an earlier product that contained a similar marine extract, but was positioned for improving aging skin rather than hair loss. Survey portions of Imedeen® studies in the early 1990’s showed that women also reported improvements to their brittle hair. So, our guess is that marketers of Imedeen® saw an opportunity to retarget the product, and either sold off or repositioned the marine extract into a hair supplement and later started testing it on hair loss sufferers.
But as far as this Scandinavian professor, his observations on the Inuits, and the actual identification of why these marine extracts might prove helpful to skin or hair… this part of the story seems to remain lost in time (and clinical research).
Viviscal®: who are the best candidates?
Viviscal® might be right for …
- Females with hair loss due to a poor diet and/or nutrient loss from menstruation.
- Males with AGA who want to take a natural approach to improving their hair and only want to slow down the rate at which they’re losing hair – and are comfortable spending $39.99 per month to do so.
Viviscal® might not be a good fit for …
- Male with AGA with expectations any higher than the above.
- Females with hair loss related to thyroid dysfunction, PCOS, childbirth, stress, vitamin D deficiency… or virtually anything else outside of the nutrient deficiencies that Viviscal® Woman helps to address (i.e., fatty acids, iron, and zinc).
The bottom line
Viviscal is the most clinically studied hair loss supplement on the planet. But that doesn’t mean it’s the best hair loss supplement available. This is obvious at the outset with Viviscal’s relatively low number of customer before-after photos featured on their website – which, for a company with millions of customers over 25+ years, is a little unusual.
Moreover, Viviscal’s clinical research isn’t as impressive as conveyed in its marketing. For starters, at least 33% of their clinical studies were never published in a scholarly journal. Of the ones that were published, all of them were published in low-ranking dermatology journals – with many of those studies containing glaring typos and selection biases that would’ve led to their rejection from reputable journals.
In addition, the Viviscal studies producing the clearest before-after photos were done on women with temporary hair loss due to stress, a poor diet, and/or nutrient deficiencies as a result of menstruation. This specific type of hair loss is the one that is best positioned to respond to a nutritional supplement, and it’s also a type of hair loss that is (1) relatively uncommon among hair loss sufferers in the developed world, and (2) one that can be improved without a $39.99 supplement – and just through lifestyle and dietary changes.
Lastly, Viviscal®’s genesis story of a Scandinavian professor – his observations on Inuits, their hair-healthy diets, and the isolation of marine compounds responsible for the Inuit’s great hair – is either exaggerated or fabricated. After review, it can be assumed that this product was developed from an earlier skincare product (with a similar marine extract) after survey research found that women thought that product also improved their brittle hair.
On the positives – Viviscal® Man did pass Perfect Hair Health’s third-party laboratory testing for heavy metals, pollutants, and pathogenic microorganisms. In other words, what you see on the label is what you get. But unfortunately, this isn’t enough to earn a recommendation – especially given its price point and the discrepancies in Viviscal’s real-world results versus their clinical research.
Questions? Comments? Please reach out in the dedicated forum thread below
References[+]
References ↑1 https://pubmed.ncbi.nlm.nih.gov/1286738/ ↑2 https://www.ncbi.nlm.nih.gov/pubmed/1864451 ↑3, ↑15 https://www.karger.com/Article/FullText/492035 ↑4 https://www.ncbi.nlm.nih.gov/pubmed/1610689 ↑5 https://nutritiondata.self.com/facts/nut-and-seed-products/3163/2 ↑6 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3819075/ ↑7 https://onlinelibrary.wiley.com/doi/abs/10.1111/jocd.12127 ↑8 https://www.ncbi.nlm.nih.gov/books/NBK547751/ ↑9 https://perfecthairhealth.com/nutrient-deficiency-hair-loss/#biotin ↑10 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3678013/ ↑11 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6385517/ ↑12 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC340385/ ↑13 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3509882/ ↑14 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6136400/ ↑16 https://www.ncbi.nlm.nih.gov/pubmed/1286738 ↑17 https://www.researchgate.net/publication/12056799 IngredientsPharmaceuticalsNatural RemediesCompany ReviewsBy Ben Fletcher, PhDSep 8, 2024BTD: Can This Gene Predict Regrowth From Biotin?
The BTD gene, responsible for encoding the enzyme biotinidase, is crucial for biotin metabolism. Biotin, or vitamin B7, plays a significant role in hair health, and biotinidase deficiency—caused by BTD polymorphisms—can lead to hair loss conditions like alopecia. While some studies suggest that biot...By Ben Fletcher, PhDSep 4, 2024COL1A1: Can This Gene Predict Regrowth From Collagen Support?
The COL1A1 gene, responsible for producing a key component of type 1 collagen, has been associated with hair growth and structure. Certain genetic variations in COL1A1 might affect the balance of collagen chains, potentially influencing hair loss and the response to treatments. However, the current ...By Sarah King, PhDSep 4, 20242dDR For Hair Loss: What Do We Know So Far About This Sugar?
2dDR Hair introduces hair care products featuring 2-deoxy-D-ribose (2dDR), a naturally occurring sugar, claiming it can support hair regrowth. While a recent mouse study shows favorable results, there is currently no clinical evidence in humans to support its effectiveness or safety. In fact, after ...By Ben Fletcher, PhDSep 4, 2024CYP19A1: Can This Gene Predict Regrowth From Hormone Therapy?
Can your genes predict the effectiveness of hormone therapy for hair loss? The CYP19A1 gene, which encodes the enzyme aromatase, has been linked to hair growth and hair loss, particularly in androgenetic alopecia (AGA). Some studies suggest that certain CYP19A1 variants might influence the effective...By Ben Fletcher, PhDAug 27, 2024ACE: Can This Gene Predict Regrowth From Treatments Targeting Blood Flow?
This article explores the potential link between angiotensin-converting enzyme (ACE) activity and hair loss, particularly in the context of androgenic alopecia (AGA) and alopecia areata (AA). While ACE plays a crucial role in blood pressure regulation and vasoconstriction, there is limited evidence ...By Sarah King, PhDAug 21, 2024NR3C1: Can This Gene Predict Responsiveness to Glucocorticoids?
The NR3C1 gene codes for the glucocorticoid receptor, a key player in the regulation of metabolism, immune response, and stress adaptation. Variations in the NR3C1 gene, such as the rs6198 SNP, have been linked to differences in glucocorticoid sensitivity with some variants leading to increased expr...By Sarah King, PhDAug 21, 2024PTGES2: Can This Gene Predict Regrowth from Minoxidil?
The PTGES2 gene encodes the enzyme prostaglandin E synthase 2, which is crucial for converting prostaglandin H2 to prostaglandin E2 (PGE2). This enzyme plays a significant role in various physiological processes, including inflammation and hair growth. Research has indicated that an SNP in the PTGES...By Sarah King, PhDAug 21, 2024PGTFR: Can This Gene Predict Regrowth From PGF2a Analogs?
Can your genes predict the effectiveness of PGF2α analogs for hair loss? The PGFTR gene, encoding the prostaglandin F2 alpha (PGF2α) receptor, is a potential target in hair loss treatment. Prostaglandin F2α and its analogs like latanoprost have been shown to stimulate hair follicle growth, making PG...By Ben Fletcher, PhDAug 20, 2024IGF1R: Can This Gene Predict Regrowth From IGF-1?
The IGF1R gene encodes the insulin-like growth factor-1 receptor, which is integral to the IGF signaling pathway, particularly in processes like growth and development. Variations in the IGF1R gene, such as the rs2229765 polymorphism, have been linked to differences in circulating IGF-1 levels, with...By Ben Fletcher, PhDAug 6, 2024SRD5A1 & 2: Can These Genes Predict Regrowth From 5-Alpha-Reductase Inhibitors?
Can your genes predict the effectiveness of 5α-reductase inhibitors for hair loss? The SRD5A1 and SRD5A2 genes, encoding type I and type II 5α-reductase respectively, are central to DHT production, a key factor in the pathogenesis of androgenic alopecia. Genetic testing companies suggest certain SR5... - Mission Statement
 Scroll Down
Scroll Down