- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
Articles
Introduction
Finasteride is one of the most commonly-prescribed medications for treatment of male pattern hair loss—also known as androgenic alopecia (AGA). But it’s also used as an off-label treatment for female pattern hair loss. Evidence suggests this medication can help regrow hair in both sexes.
But what’s the best dose of finasteride for women with AGA? Unfortunately, it’s complicated. This article sets out to evaluate the data, uncover the answers, and provide recommendations based on the current landscape of clinical research.
Interested in Oral Finasteride?
Oral finasteride & minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What Is Finasteride?
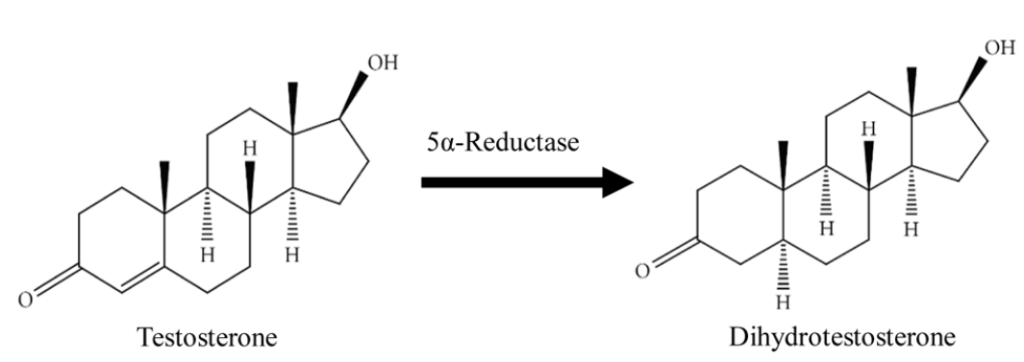
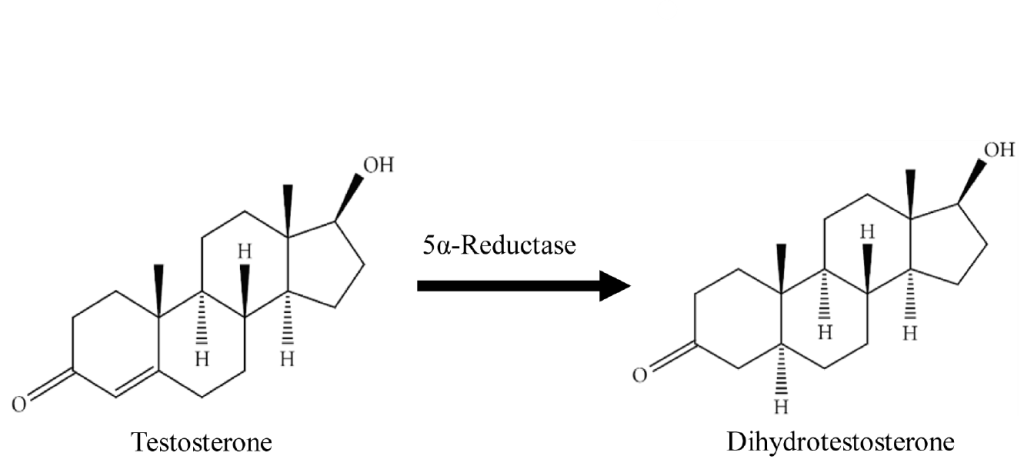
Finasteride is a drug developed to inhibit type II 5-alpha reductase. This is an enzyme in the body that converts free testosterone in dihydrotesterone (DHT).
Essentially, finasteride lowers DHT levels by reducing the amount of type II 5-alpha reductase circulating throughout our bodies. And by taking finasteride at 0.2 to 5.0 mg daily dosages, we can often reduce total DHT levels by 70%. [1]https://www.ncbi.nlm.nih.gov/books/NBK513329/
Why Use a Drug To Reduce DHT?
DHT is not just a metabolite of testosterone; it’s also the primary male hormone causally associated with androgenic alopecia.
We know this because studies have shown that men who cannot produce DHT are nearly fully-protected from going bald throughout a lifetime. Furthermore, clinical studies on DHT-lowering drugs – such as finasteride – show that if DHT levels are suppressed enough, 80-90% of men can arrest the progression of their pattern hair loss and even regrow 10-20% of their lost hair. [2]https://pubmed.ncbi.nlm.nih.gov/29407002/ [3]https://www.ncbi.nlm.nih.gov/books/NBK430924/
Similarly, studies on females with androgenic alopecia have shown that finasteride can also improve their hair loss outcomes. The evidence is less robust than for men, but finasteride is something many women should consider trying in order to improve their pattern hair loss.
Finasteride for Women: What’s the Perfect Dose?
In men, the FDA has approved the use of 1mg of finasteride for pattern hair loss. However, male and female hair loss cases are not always the same. Reducing DHT levels is often of therapeutic interest to fighting AGA – and for both sexes – but some clinical evidence suggests that females might need a different dose of finasteride versus males.
How Much DHT Does Finasteride Reduce?
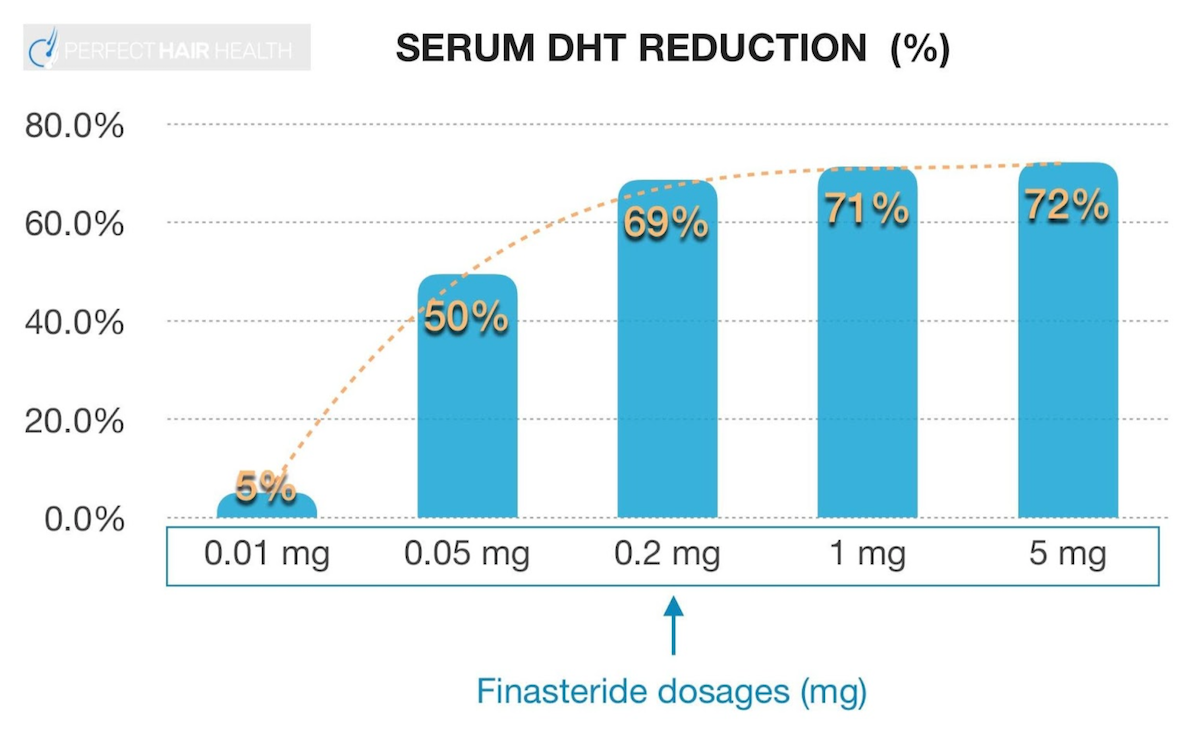
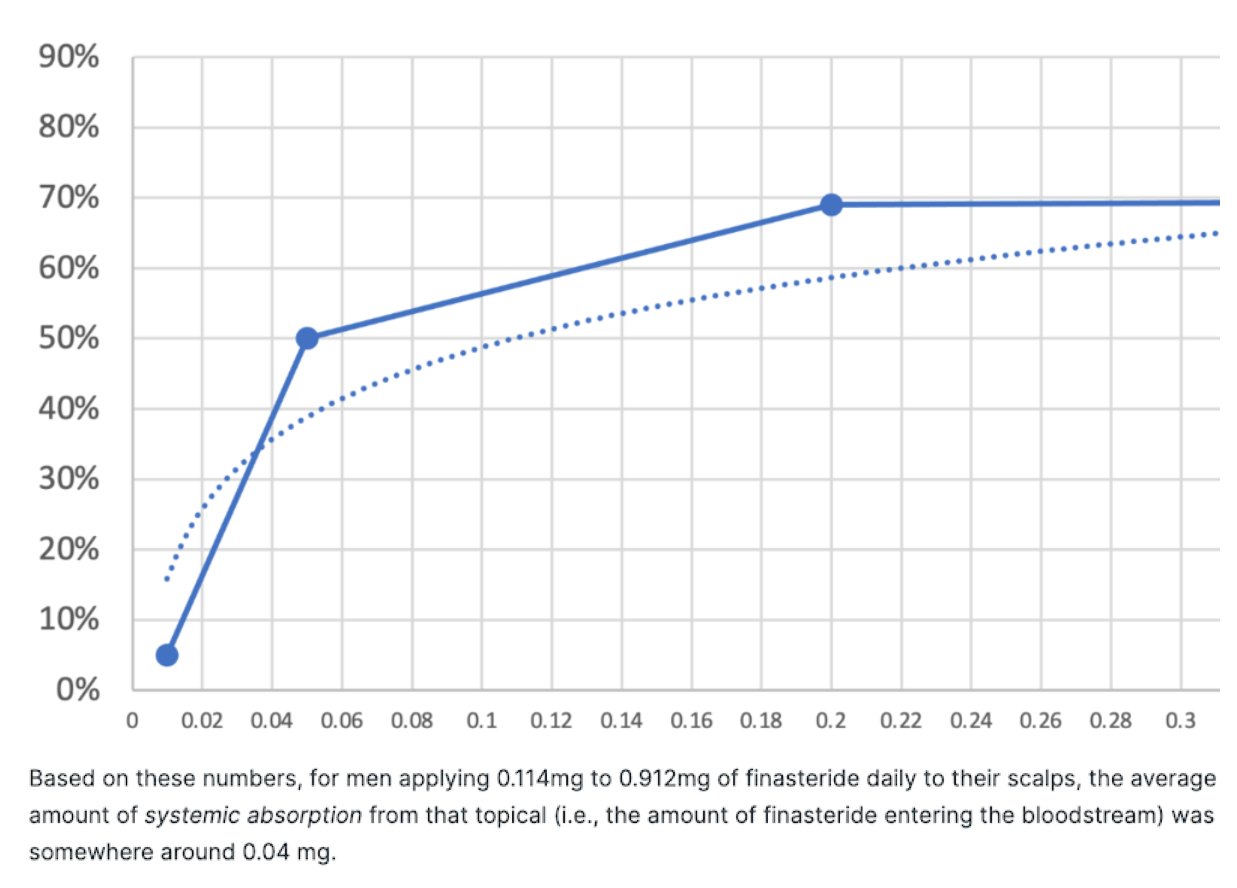
Finasteride has what is known as a dose-dependent, logarithmic response curve for DHT reduction. In other words: a little bit of finasteride reduces nearly as much DHT as a lot of finasteride. For an example, see this chart:
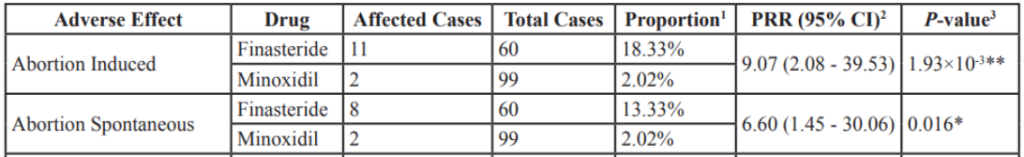
Clinical studies have demonstrated that 0.2 mg and 5.0 mg reduce nearly the same amount of finasteride: 69% versus 72%, respectively.
Because of this, a lot of people actually prefer to use lower dosages of finasteride than what is generally prescribed. This practice is also supported by clinical data. For instance, in men, 0.2mg of finasteride AGA at a statistically similar level as 1.0 mg of finasteride over the course of a year. [4]https://europepmc.org/article/med/15319158
But is the same true with females? Unfortunately, the data is less clear.
Finasteride for Female Pattern Hair Loss: the Clinical Evidence
The FDA approves the use of 1 mg of finasteride for male pattern hair loss.[5]https://www.fda.gov/drugs/information-drug-class/5-alpha-reductase-inhibitor-information But the underlying causes of male and female pattern hair loss cases (androgens such as DHT) are not always the same. Furthermore, while reducing DHT levels is of therapeutic relevance in treating AGA in men and women, some clinical evidence suggests that females might need a different dose of finasteride versus males.
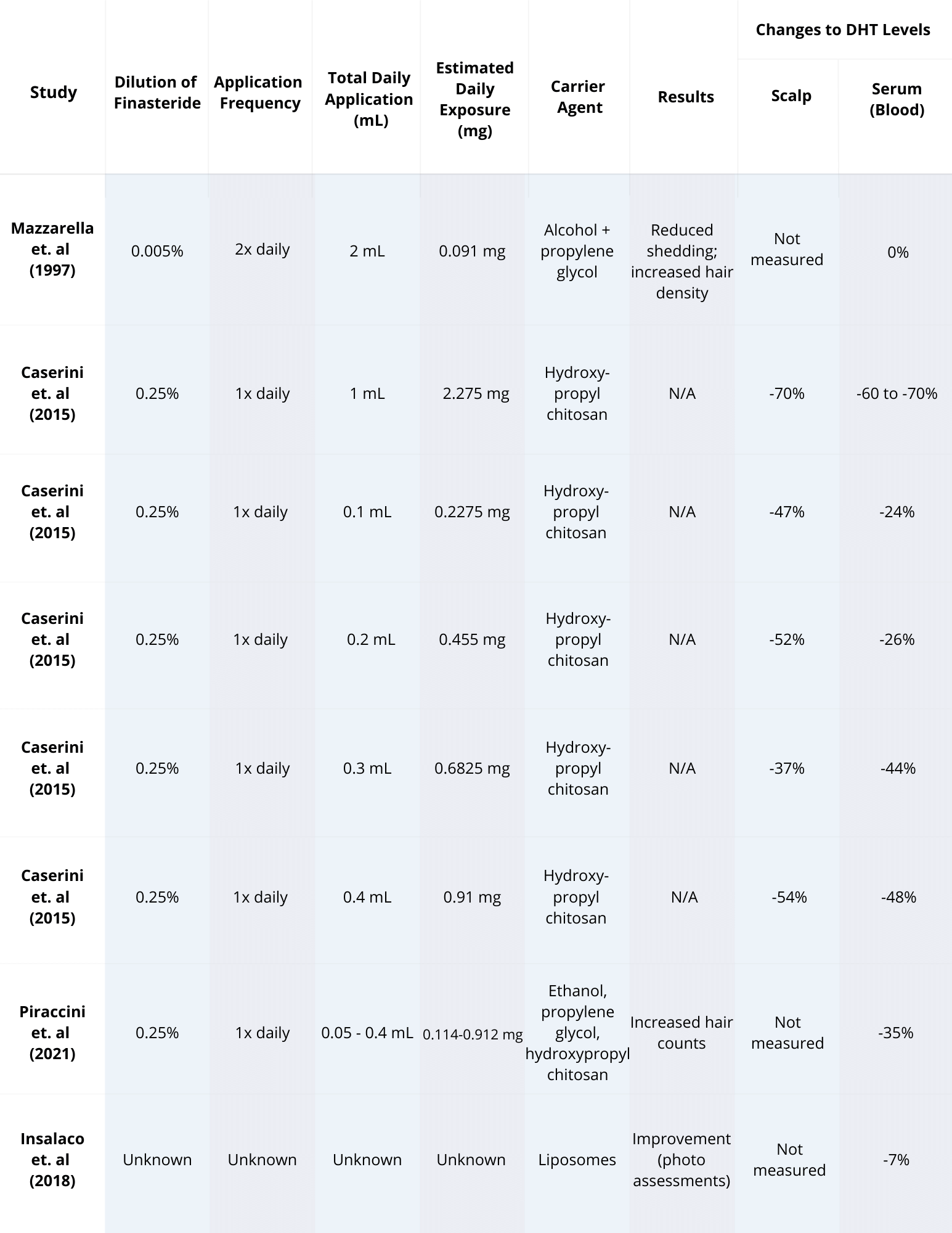
What does the available research tell us? Here are a few of the key studies on finasteride for women, and their main findings (summarized in the table below).
Finasteride for Women: Key Studies
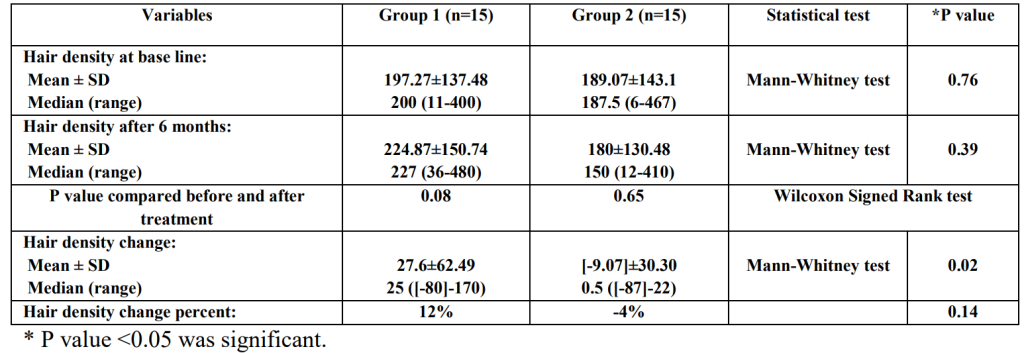
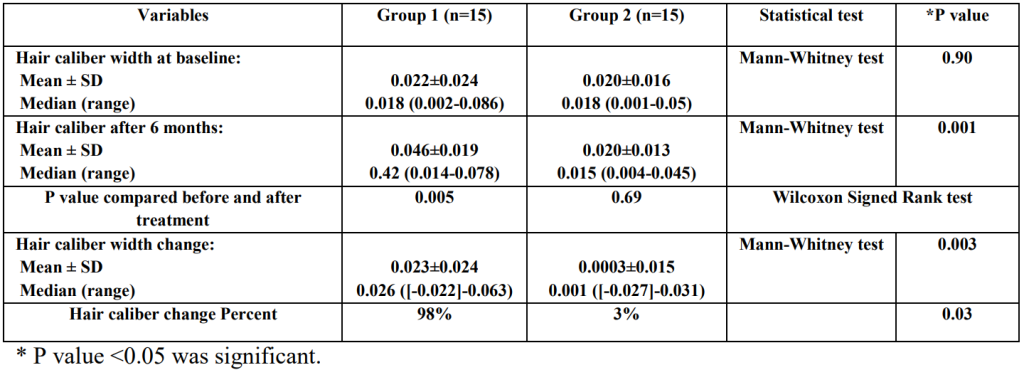
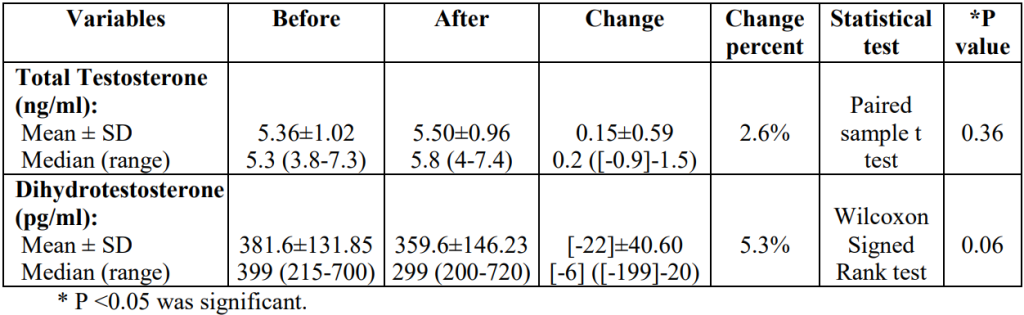
Type of study Number of patients Patient condition Finasteride dosage Length of treatment Assessment parameters Outcomes Reference Double-blind, randomized control trial 67 treated, 70 placebo AGA, post-menopausal, normal serum testosterone 1 mg, daily 12 months Hair counts, photographic assessment, self-assessment, scalp biopsies Serum DHT reduction but no effect on hair loss outcomes compared to placebo [6]https://pubmed.ncbi.nlm.nih.gov/10674382/[7]https://pubmed.ncbi.nlm.nih.gov/11050579/ Randomized, unmasked trial 12 finasteride, 12 flutamide, 12 cyproterone acetate with estradiol, 12 no treatment 48 women, hyperandrogenic, age- and weight matched controls 5 mg, daily 12 months Ludwig classification15 of female hair loss, self-assessment, and investigator assessment No effect with finasteride [8]https://pubmed.ncbi.nlm.nih.gov/11050579/ Single-blind, placebo-controlled trial 24 female patients included AGA, age 23-38 years (mean 33) 1 ml topical application (0.005%), twice daily to affected area 16 months Semi-quantitative investigator assessment, hair shedding quantification, self-assessment Hair count and hair density improvements versus placebo (data not sex-stratified) [9]https://www.tandfonline.com/doi/abs/10.3109/09546639709160517 Small trial 5 treated Post-menopausal, normal androgen levels 2.5 or 5 mg, daily 18 months, review every 6 months Self-assessment, investigator assessment, photographic assessment Overall improvement [10]https://pubmed.ncbi.nlm.nih.gov/15459533/ Small trial 10 treated Post-menopausal 1 mg, daily 52-82 weeks Self-assessment and photographic assessment Overall improvement (9/10 patients) [11]Ahn J, Cho SB, Kim MN, Ro BI. Finasteride treatment of female patterned hair loss in postmenopausal women. Korean J Dermatol. 2006;44:1094-1097. Small trial 6 treated AGA, age 30-76 years (mean 46.5), normal androgen levels 5 mg, daily Weeks (not specified) Retrospective questionnaire (self-assessment) Overall improvement (5/6 patients) [12]https://pubmed.ncbi.nlm.nih.gov/17454167/ Small trial 37 treated Female pattern hair loss, pre-menopausal, age 19-50 years (mean, 33.7) 2.5 mg, daily (+ oral contraceptive drospirenone and ethinyl estradiol) 12 months Self-assessment, photographic assessment, and hair-density scoring Overall improvement by self-assessment (29/37), photographic improvement ((23/37), significant hair density improvement (12/37) [13]https://pubmed.ncbi.nlm.nih.gov/16549704/ Small trial 41 treated AGA, persistent adrenarche syndrome 2.5 mg, daily (+ ethinyl estradiol) 2 years Not specified Overall improvement [14]https://pubmed.ncbi.nlm.nih.gov/19341939/ Small trial 4 treated 36, 40, 60, and 66 years old, elevated testosterone and hyperandrogenism 1.25 mg, daily 6 months to 2.5 years Photographic assessment and self-assessment Stabilization of hair loss within 6-12 months, hair growth improvements in 6 months – 2.5 years [15]https://pubmed.ncbi.nlm.nih.gov/12399766/ Case study 1 treated 47-year-old, ‘male’ pattern hair loss, hysterectomy and ovariectomy, long-term hormone replacement 2.5 mg, daily (+continued testosterone supplementation) 10 months Photographic assessment Hair loss stabilization at 6 months, hair growth improvement at 10 months [16]Hong JB, Chiu HC, Chan JY, Chen RJ, Lin SJ. A woman with iatrogenic androgenetic alopecia responding to finasteride. Br J Dermatol. 2007;156(4):754-755. doi:10.1111/j.1365-2133.2006.07719.x Case study 1 treated 67-year-old, 18 month history of hair thinning, normal androgen levels 5 mg, weekly 12 months Self-assessment and photographic assessment Improvement, hair regrowth [17]https://pubmed.ncbi.nlm.nih.gov/12366441/ Case study 1 treated 51-year-old, 8 month history of hair thinning, normal androgen levels 1 mg, daily 12-13 months Hair density measurements Hair density increased versus baseline [18]https://pubmed.ncbi.nlm.nih.gov/15844649/ Finasteride for Women: Key Studies Takeaway
There is conflicting data regarding the efficacy of finasteride for female pattern hair loss. Some studies report improvements while others do not.
There are some key variables to consider when weighing the available evidence:
- The dose of finasteride used
- The length and frequency of treatment
- Oral administration versus a topical treatment
- Whether finasteride was used in conjunction with other drugs or therapies
- The type of hair loss in the patient groups (e.g., female pattern hair loss versus age-related thinning – and was this accurately determined?)
- Patient age, history, and status (e.g., pre-, or post-menopausal, or abnormal androgen levels)
- How the treatment was assessed (e.g., quantitative hair counting versus patient self-assessment)
- Whether the study contained appropriate controls (i.e., placebo-receiving patients, ideally matched for age, weight and medical history)
- The number of patients in the study (which affects the statistical power; was the study a case report of one patient, or a larger study with a control group?).
Interpreting research data can be difficult and confusing. such as different studies using different dosages of finasteride and for varying lengths of time, measuring different hair loss outcomes, and using different numbers and ages of patients.
The specific type of hair loss is also a crucial variable.[19]https://pubmed.ncbi.nlm.nih.gov/30604525/ Often, studies reporting positive outcomes are based on patient self-reporting, which can be suspect and not meaningfully objective or quantitative in measuring true prevention or reversal of hair loss.
What is the best dose? Explaining the conflicting results.
Given the dose-response relationship between finasteride and DHT levels, shouldn’t 1 mg be as effective as 5 mg? Why aren’t women getting consistent regrowth across doses within these ranges?
Other discrepancies are the time for which finasteride was given. In men, 1 mg of finasteride can be effective in 6-12 months, but it is possible that women require more long-term treatment regimens.[20]https://pubmed.ncbi.nlm.nih.gov/30604525/ Generally, success with finasteride in women has been reported in both the short- and long-term.[21]https://pubmed.ncbi.nlm.nih.gov/12399766/
Alternatively, the difference in observed efficacies between studies may be due to patient background and the type of hair loss. Hair loss in patients suffering from PCOS or adrenarche (i.e., high levels of adrenal gland activity) likely has a clear causal link to abnormal androgen signaling (and therefore suitable for finasteride), where hair loss in post-menopausal women may be more akin to age-related hair ‘thinning’, and not linked to testosterone or DHT, which may explain why some studies find no effect with finasteride.[22]https://pubmed.ncbi.nlm.nih.gov/10674382/[23]https://pubmed.ncbi.nlm.nih.gov/11050579/[24]https://pubmed.ncbi.nlm.nih.gov/12399766/
That said, finasteride is also reportedly effective in patients that are androgen-normal.20 Therefore, there needs to be more careful classification of the type of hair loss and the likely underlying mechanisms, as well as clear standardization of treatment outcome measurements.
Conclusions
Does finasteride work for women suffering from hair loss? If so, what is the ideal dosage?
Finasteride is most beneficial for women when their hair loss occurs alongside elevated androgen levels—much like hair loss in men.
It is likely that DHT is a major factor in a proportion of female hair loss cases. Finasteride is likely to be a beneficial part of a successful hair loss regimen in these cases. However, it is not clear that male and female pattern hair loss universally share the same underlying cause.
Finasteride may be less suitable in contexts of age-related hair thinning. Hair follicles are sensitive to all manner of different hormones and chemical signals, not just androgens such as testosterone and DHT.[25]https://pubmed.ncbi.nlm.nih.gov/32731328/
The best dose is one that maximizes the desired benefits (prevention and reversion of hair loss) while minimizing any undesirable side effects.
Finasteride can be effective at reducing DHT even in small doses. Because of this, experimentation with low levels of finasteride may lead to results with far less exposure to chemicals. Topical treatments may also be considered to further reduce systemic side effects.
Therefore, the best dose will be patient-subjective. Prior consideration of your history, goals regarding hair loss, and experimentation with dosages is needed before you will find the ideal routine for your hair health.
References[+]
References ↑1 https://www.ncbi.nlm.nih.gov/books/NBK513329/ ↑2 https://pubmed.ncbi.nlm.nih.gov/29407002/ ↑3 https://www.ncbi.nlm.nih.gov/books/NBK430924/ ↑4 https://europepmc.org/article/med/15319158 ↑5 https://www.fda.gov/drugs/information-drug-class/5-alpha-reductase-inhibitor-information ↑6, ↑22 https://pubmed.ncbi.nlm.nih.gov/10674382/ ↑7, ↑8, ↑23 https://pubmed.ncbi.nlm.nih.gov/11050579/ ↑9 https://www.tandfonline.com/doi/abs/10.3109/09546639709160517 ↑10 https://pubmed.ncbi.nlm.nih.gov/15459533/ ↑11 Ahn J, Cho SB, Kim MN, Ro BI. Finasteride treatment of female patterned hair loss in postmenopausal women. Korean J Dermatol. 2006;44:1094-1097. ↑12 https://pubmed.ncbi.nlm.nih.gov/17454167/ ↑13 https://pubmed.ncbi.nlm.nih.gov/16549704/ ↑14 https://pubmed.ncbi.nlm.nih.gov/19341939/ ↑15, ↑21, ↑24 https://pubmed.ncbi.nlm.nih.gov/12399766/ ↑16 Hong JB, Chiu HC, Chan JY, Chen RJ, Lin SJ. A woman with iatrogenic androgenetic alopecia responding to finasteride. Br J Dermatol. 2007;156(4):754-755. doi:10.1111/j.1365-2133.2006.07719.x ↑17 https://pubmed.ncbi.nlm.nih.gov/12366441/ ↑18 https://pubmed.ncbi.nlm.nih.gov/15844649/ ↑19, ↑20 https://pubmed.ncbi.nlm.nih.gov/30604525/ ↑25 https://pubmed.ncbi.nlm.nih.gov/32731328/ Topical finasteride has emerged to meet demand for hair loss treatments that promise strong results with fewer systemic side effects than oral finasteride by concentrating action at the scalp while reducing overall drug exposure. Early studies suggest it can improve hair growth and lower scalp dihydrotestosterone (DHT) with less systemic absorption, but side effects are not eliminated, and long-term head-to-head data versus oral finasteride remain limited.
This article will cut through the “same results, fewer side effects” marketing narrative and set realistic expectations by focusing on what current clinical evidence and real-world user patterns actually support.
How Does Finasteride Work?
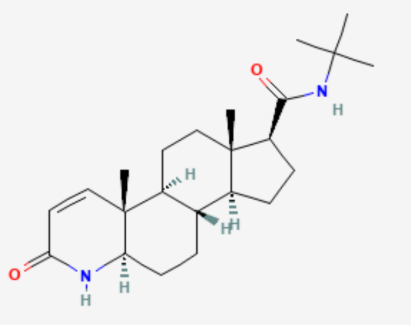
Finasteride is a 5-alpha reductase inhibitor, meaning that it blocks this enzyme from converting testosterone into DHT, the more potent androgen that drives miniaturization of sensitive scalp follicles in androgenic alopecia (AGA). The enzyme catalyzes this conversion in the scalp, skin, liver, and prostate. By inhibiting it, finasteride lowers both local (scalp/prostate) and circulating DHT.[1]Zito PM, Bistas KG, Patel P, et al. Finasteride. [Updated 2024 Feb 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: … Continue reading
Finasteride structure
Why Oral Finasteride is Systemic
A standard 1 mg oral dose reduces DHT by roughly 60-70%, with some studies reporting around 70% suppression at steady state, and intraprostatic DHT falls by about 90%.[2]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading,[3]Smith, A.B., Carson, C.C. (2009). Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Therapeutics and Clinical Risk Management. 5. 535-545. Available at: … Continue reading Because 5 alpha-reductase is expressed in multiple organs, this systemic inhibition affects DHT signaling throughout the body, not only in the scalp.
Why Side Effects Occur
Sexual and mood-related side effects are not purely “in the mind”; they reflect altered androgen physiology in a subset of men whose tissues are more sensitive to a DHT drop, even though testosterone usually stays within the normal range or rises modestly. Reduced DHT can influence libido, erectile function, ejaculate volume, and, in some individuals, mood or energy, consistent with the hormone’s broader role beyond hair follicles.[4]Traish, A.M. (2017). Negative Impact of Testosterone Deficiency and 5ɑ-Reductase Inhibitors Therapy on Metabolic and Sexual Function in Men. Advances in experimental medicine and biology. 1043. … Continue reading
Topical Targeting vs. Spillover
Topical finasteride aims to concentrate drug action in the scalp by delivering finasteride directly to hair follicles, potentially lowering local DHT while limiting how much drug reaches the bloodstream. However, percutaneous absorption still occurs, and pharmacokinetic studies do show measurable reductions in serum DHT with topical formulations, meaning systemic exposure is reduced compared to oral dosing, but not eliminated.[5]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading
What the Research Says About Topical Finasteride Side Effects
The evidence on topical finasteride’s side effect profile shows a nuanced picture – measurable systemic exposure, lower but not zero adverse event rates, and substantial variability between formulations and individuals.
Systemic Absorption
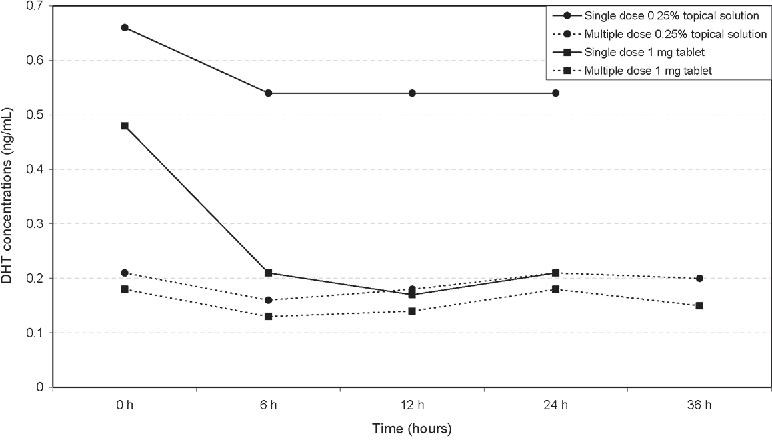
The 2021 Piraccini trial, one of the largest randomized double-blind topical finasteride studies to date, tested a 0.25% finasteride spray applied once daily over 24 weeks in 458 men.[6]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E., Topical Finasteride Study Group. (2022). Efficacy and safety … Continue reading Plasma finasteride concentrations were over 100-fold lower than with oral finasteride. Serum DHT fell by 34.5% with topical use compared to 55.6% with a 1 mg oral tablet.
Caserini found that lower volumes (100-200 μL of 0.25% solution) reduced serum DHT by 24-26%, while higher volumes (300-400 μL) dropped it by 44-48%.[7]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading Even at the lowest tested doses, scalp DHT declined by roughly 47-52%, demonstrating effective local action with more limited systemic spillover.
Other studies report a range of serum DHT suppression with topical formulations, but all confirm that absorption is lower than oral administration, not absent. One hydroxyl-propyl chitosan formulation was designed specifically to retain finasteride in the reticular dermis near hair bulbs; repeated-dose rat experiments showed no detectable plasma finasteride and no accumulation in skin, although human data remain sparse.[8]Monti. D., Tampucci, S., Burgalassi, S., Chetoni, P., Lenzi, C., Pirone, A., Mailland, F. (2014). Topical formulations containing finasteride. Part I: in vitro permeation/penetration study and in … Continue reading
So to summarize, topical finasteride’s systemic exposure is markedly reduced compared to oral dosing, yet reductions in circulating DHT do still occur across most formulations (in human studies), meaning that some degree of systemic exposure is virtually unavoidable.
Reported Side Effect Rates in Studies
Clinical trials have monitored sexual dysfunction, dermatological reactions, and less common systemic complaints, though sample sizes and durations limit the ability to detect rare or delayed adverse events.
Sexual Side Effects
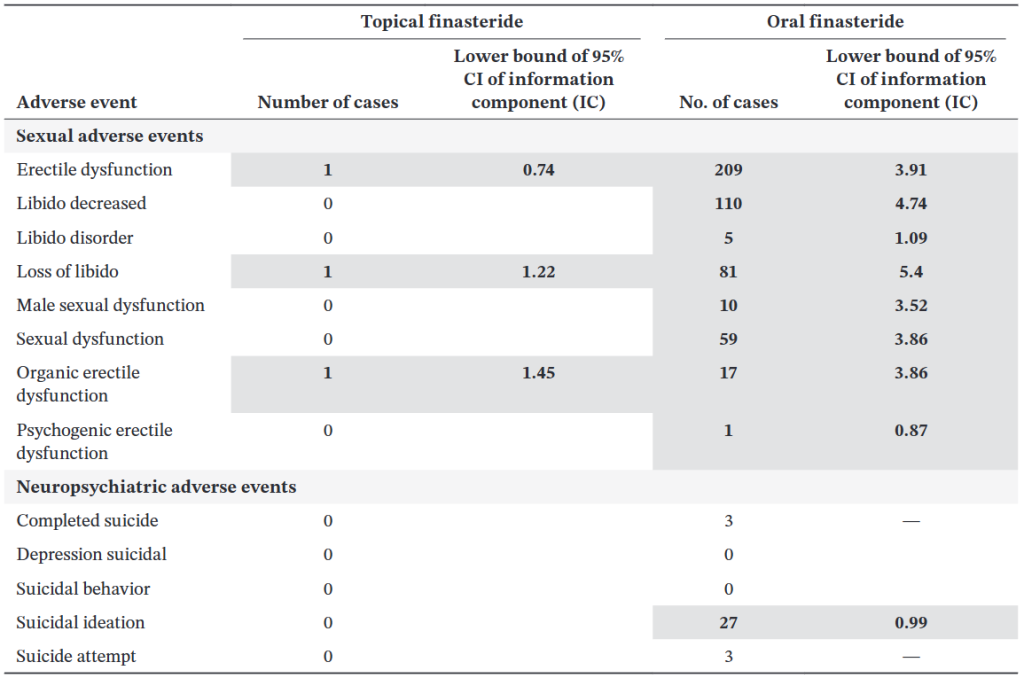
In the 2021 Piraccini study, 2.8% of topical finasteride users reported sexual adverse events (decreased libido, erectile dysfunction, and ejaculatory dysfunction) versus 4.8% on oral finasteride and 3.3% on placebo.
The similarity between the topical and placebo groups led some investigators to suggest that many reports may not be causally linked to the drug. However, a 2025 FDA pharmacovigilance analysis of adverse event reports (2019-2024) identified 32 cases involving topical finasteride formulations, most of which were compounded products, with complaints of erectile dysfunction, anxiety, brain fog, depression, fatigue, insomnia, decreased libido, and testicular pain.[9]US Food and Drug Administration. (2025). FDA alerts health care providers, compounders and consumers of potential risks associated with compounded topical finasteride products. FDA. Available at: … Continue reading What’s more, many of these symptoms reportedly persisted after discontinuation.
A separate analysis found that topical finasteride generated fewer signals for post-finasteride syndrome (PFS) – like events than oral finasteride, but erectile dysfunction remained the most consistently reported adverse event for both routes.[10]Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated With Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study With Data From … Continue reading
Figure 1: Association between finasteride use (topical vs. oral) and occurrence of adverse events (sexual and neuropsychiatric) between 2006and 2011 (inclusive) and across men.[11]Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated With Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study With Data From … Continue reading
Scalp Irritation and Contact Dermatitis
Local reactions are the most common topical-specific side effects. A 2018 systematic review noted reports of erythema, contact dermatitis, and scalp irritation in several studies, though serious cutaneous adverse events were absent.[12]Lee, S.W., Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in … Continue reading
Rates of contact dermatitis ranged from 12-24% in some combination formulations (finasteride + minoxidil), though these were generally mild and did not lead to treatment discontinuation. The irritation effect likely derives from vehicle components (alcohol, propylene glycol) as well as the active drug.
Headaches, Fatigue, and Other Systemic Complaints
Less frequently, trials have documented headaches, feeling about to faint, testicular pain, increased liver enzymes, and even bed-wetting in isolated cases. The 2025 FDA alert highlighted fatigue and insomnia among the systemic complaints associated with topical formulations, reinforcing that absorption can produce effects beyond the scalp.
Trial Limitations: Duration and Sample Size
Most published trials run 12-24 weeks with cohorts of 30-135 participants per arm, which may under-detect rare events (incidence <1%) or adverse effects that emerge only after prolonged exposure. Only a handful of studies have extended beyond six months, and none approach the multi-year observation periods needed to assess phenomena such as PFS.[13]Pereira, A.F.J.R., Coehlo, T, O. de A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001 Recruitment through specialist dermatology centres may also select for patients more tolerant of medication, introducing survivorship bias.
How do the Side Effects Compare with Oral Finasteride?
Head-to-head data have shown that topical finasteride has a numerically lower, but not zero, incidence of systemic adverse events. In Piraccini’s trial, sexual side effects occurred in 2.8% of topical users versus 4.8% of oral users, representing an approximate halving of risk.
There are two important points often overlooked in clinical summaries, however:
- Population averages do not predict individual susceptibility: Even if 97% of topical finasteride users report no sexual side effects in a 24-week trial, that statistic offers little reassurance to someone who is biochemically sensitive to modest DHT reductions. Genetic polymorphisms in 5ɑ-reductase isoforms, androgen receptor sensitivity, and neurosteroid metabolism all vary widely, meaning that a 25% serum DHT drop can be innocuous for one man and symptomatic for another.[14]Pereira, A.F.J.R., Coehlo, T, O. de A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001
- Short trials may miss long-latency or persistent events: Most published studies conclude at 6 months, yet reports to the FDA and patient registries describe symptoms that onset gradually or persist long after stopping treatment. The cases flagged in the FDA alert involved adverse events that continued to persist after product discontinuation, suggesting that systemic absorption, however modest, can trigger durable changes in a subset of users. However, the exact cause of symptom persistence is not yet known.
Why Do Some People Still Get Side Effects From Topical Finasteride?
Despite the reduced systemic exposure, some people still experience sexual, mood, and systemic adverse effects. Let’s take a look at the mechanisms why.
Skin Permeability Varies Significantly Between Individuals
Human skin is not a uniform barrier. Stratum corneum thickness, lipid composition, hydration status, pH, and blood flow differ among individuals and even within scalp regions of a single person.
In vitro permeation studies using human cadaver scalp skin show that finasteride flux ranges of 1.1-20.1 μg/cm2/h, depending on vehicle choice, a roughly 18-fold variation. Live scalp skin likely exhibits even greater heterogeneity due to sebum content, follicle density, and individual differences in dermal blood flow, all of which influence how much drug reaches the bloodstream versus remaining confined to follicular tissue.[15]Farah, A.H., Brown, M.B., McAuley, W.J. (2020). Enhanced Follicular Delivery of Finasteride to Human Scalp Skin Using Heat and Chemical Penetration Enhancers. Pharmaceutical Research. 37(6). 112. … Continue reading
For topical finasteride to minimize systemic exposure, it needs to stay within the area of the hair follicle. Variability in penetration means some individuals absorb more systemically than others, despite using the same concentration and volume.
Vehicle Formulation Dramatically Alters Absorption
The formulation of a topical finasteride product isn’t just a minor detail; it’s one of the biggest determinants of how much of the medication actually enters the bloodstream. Not all vehicles behave the same way, and the differences can be dramatic. For example, in vitro research comparing common topical solvents on human scalp skin shows that propylene glycol (PG) and isopropyl alcohol (IPA) formulations allow far more drug to pass through the skin: roughly 62–81 µg/cm² over 24 hours. In contrast, dimethyl isosorbide (DMI), often marketed as a “low-penetration” carrier, delivered just 17.8 µg/cm² in the same timeframe.[16]Farah, A.H., Brown, M.B., McAuley, W.J. (2020). Enhanced Follicular Delivery of Finasteride to Human Scalp Skin Using Heat and Chemical Penetration Enhancers. Pharmaceutical Research. 37(6). 112. … Continue reading That’s a 4.5-fold reduction in systemic permeation, purely from changing the solvent.
More sophisticated delivery systems behave differently still. Liposomal and niosomal gels, for example, are designed to deposit the medication deeper into hair follicles while limiting diffusion into the bloodstream. And in controlled experiments, these vesicular carriers did exactly that: liquid-state liposomes delivered 2.1–2.3% of the applied dose into the skin, compared to only 0.76% from a standard hydroalcoholic solution.[17]Tabbakhian, M., Tavakoli, N., Jaafari, M.R., Daneshamouz, S. (2006). Enhancement of follicular delivery of finasteride by liposomes and niosomes: 1. In vitro permeation and in vivo deposition studies … Continue reading
In other words, depending on the vehicle, the same dose of finasteride can behave like a local scalp medication or something much closer to an oral drug.
At the opposite end of the spectrum are ethosomes, ethanol-rich vesicles that dramatically increase drug penetration. In one study, finasteride ethosomes produced 7.4-times higher transdermal flux than the same drug in an aqueous solution, reaching all the way into the dermis.[18]Rao, Y., Zheng, F., Zhang, X., Gao, J., Liang, W. (2008). In vitro Percutaneous Permeation and Skin Accumulation of Finasteride Using Vesicular Ethosomal Carriers. 9(3). 860-865. Available at: … Continue reading
This is the kind of penetration behavior that significantly raises the likelihood of systemic absorption.
The bottom line: two topical finasteride products with the same concentration can have completely different side-effect profiles, simply because the vehicles modulate how much drug gets through the skin. Hydroalcoholic sprays tend to penetrate more aggressively; liposomal or niosomal gels are usually more conservative; and individual biology, skin barrier integrity, inflammation, and sebaceous output add yet another layer of variability. This is why real-world responses to topical finasteride are so mixed, and why formulation needs to be taken as seriously as the dose itself.
Concentration and Application Volume Compounds Absorption
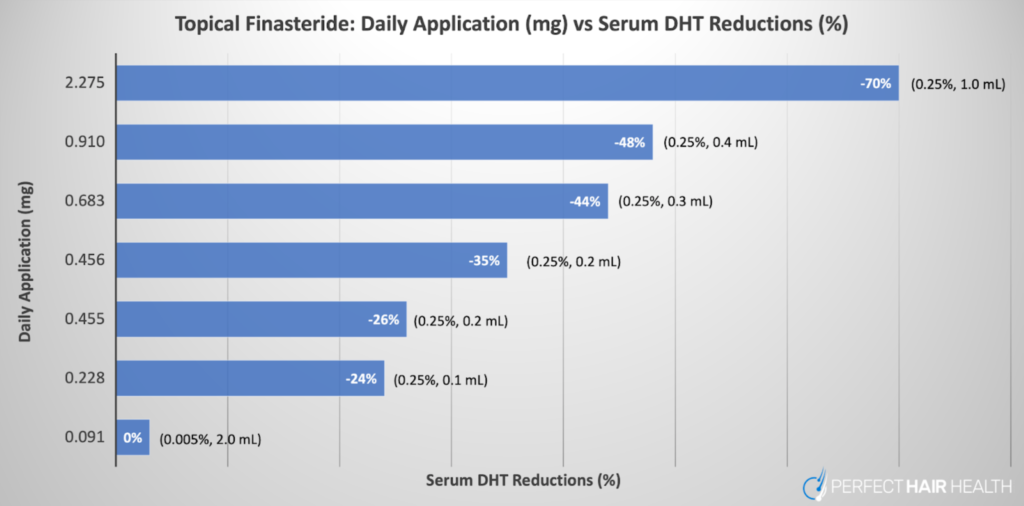
Higher concentrations and larger volumes increase both local scalp DHT reduction and systemic spillover. As mentioned above, Caserini’s dose-response study found that applying 100-200 μL of 0.25% finasteride reduced serum DHT by 24-26%, whereas 300-400 μL volumes reduced it by 44-48%, approaching the efficacy of oral dosing at the higher end.[19]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading
Users who apply larger volumes, cover a wider scalp area, or use higher-concentration formulations absorb more drug systemically, shifting the risk-benefit profile toward greater potential for side effects.
Finasteride’s Half-Life Enables Accumulation
Finasteride has a terminal half-life of 5-6 hours in young men and 8 hours in older men.[20]Mysore, V. (2012). Finasteride and sexual side effects. Indian Dermatology Online Journal. 3(1). 62-65. Available at: https://doi.org/10.4103/2229-5178.93496 Although short, daily topical application leads to steady-state accumulation in plasma and skin tissues.
With topical dosing, measurable finasteride concentrations appear in plasma within hours and remain detectable for a full day; repeated daily application amplifies cumulative levels, particularly if absorption is higher than intended due to vehicle choice or individual skin permeability.[21]Tai, Z., Cui, Z., Shi, X., Li, H., Chai, R., Huang, Y., Fang, Y., Jia, D., Zhu, Q., Chen, Z. (2025). The Pharmacokinetics of Topical Finasteride 0.25% Spray in Chinese Adult Male Volunteers with … Continue reading
DHT Sensitivity Varies Widely Between Individuals
Even when two people experience the same level of DHT reduction, their bodies may not respond the same way. This is where individual biology plays a much bigger role than most clinical studies acknowledge. Variations in genes tied to 5α-reductase activity, androgen receptor sensitivity (including CAG repeat length), and enzymes involved in neurosteroid production, such as 3α-HSD, can all influence how strongly someone reacts to changes in DHT.[22]Cecchin, E., De Mattia, E., Toffoli, G., Mazzon, G., Cauci, S., Trombetta, C. (2014). A Pharmacogenetic Survey of Androgen Receptor (CAG)N and (GGN)N Polymorphisms in Patients Experiencing … Continue reading,[23]Rahimi-Ardabili, B., Pourandarjani, R., Habibollahi, P., Mualeki, A. (2006). Finasteride-induced depression: a prospective study. BMC Clinical Pharmacology. 6(7). Available at: … Continue reading
For one man, a 30% drop in circulating DHT might feel completely benign. For another, that exact same reduction might coincide with noticeable changes in libido, erectile quality, or overall sexual function.
Layered on top of genetics are factors like baseline hormone levels, underlying hypogonadism, and even a person’s psychophysiological sensitivity to small hormonal shifts. Together, these variables help explain why side effects remain so individualized and difficult to predict, and why two people using an identical topical finasteride formulation can walk away with very different experiences. This isn’t meant to alarm anyone, only to highlight that DHT suppression is not a one-size-fits-all phenomenon, and that understanding your own sensitivity is often just as important as choosing the right dose or vehicle.
How Can I Minimize the Risk of Topical Finasteride Side Effects?
Target the Lowest Effective Exposure
- Understand exposure = concentration × volume. The systemic finasteride dose delivered through the scalp depends on both how strong the solution is and how much is applied. A low-concentration solution applied in large volume can expose the body to as much finasteride as a small volume of a stronger solution. Both variables must be considered together when aiming to reduce side effects.
- Start with the minimum viable dose. Evidence suggests that daily exposure to around 0.1 mg (for example, 0.005% solution applied at 2 mL per day) can still achieve measurable scalp DHT reduction with negligible systemic suppression.
- 0.25% remains the most studied concentration. Clinical trials show that 0.25% topical finasteride effectively treats androgenetic alopecia with hair density gains similar to oral finasteride yet significantly lower serum DHT reduction. For most users, starting at or below this level helps balance efficacy and safety.
- Higher isn’t always better. Increasing concentration or volume each raises systemic absorption, but hair-growth benefits tend to plateau. Pushing beyond the optimal concentration can shift the risk-to-benefit ratio unfavorably.
- Consider titrating upward. If highly sensitive to hormonal side effects, begin at lower exposure (e.g., 0.025–0.1% at 1 mL daily), then increase concentration or frequency only if well-tolerated and results plateau. Serum DHT measurements can help assess systemic exposure over time.
Adjust Volume and Frequency to Limit Systemic Load
- Keep volume conservative. Systemic absorption scales with total dose applied. Around 1 mL per application is a common clinical standard; using more typically provides diminishing returns for hair outcomes but notably raises systemic uptake.
- Use the lowest frequency that maintains results. Because twice-daily 0.25% use can suppress serum DHT by up to 70%—similar to oral dosing—while once-daily reduces it by only 20–35%, finding a balance between efficacy and exposure is key. For many users, once-daily application of a lower-strength solution achieves a good safety–efficacy trade-off.
Choose Vehicles Carefully
- Hydroalcoholic vs. liposomal: Standard hydroalcoholic (alcohol-based) solutions enhance drug penetration, which can lead to greater systemic absorption. In contrast, advanced vehicles like liposomal or other nanoparticle-based formulations are designed to target drug delivery to the hair follicle and reduce transdermal flux into the bloodstream.
Implement Smart Application Strategies
- Apply to a dry scalp: Applying topicals to wet skin can enhance absorption. Ensure the scalp is completely dry to create a more robust barrier.
- Target affected areas only: Use the applicator to apply the solution precisely to the areas of thinning hair, not the entire scalp, to limit the total surface area of absorption.
- Avoid compromised barriers: Do not apply the solution immediately after microneedling, on sunburnt skin, or on a scalp with cuts, inflammation, or dermatitis, as a compromised skin barrier significantly increases uptake.
- Wash hands thoroughly: Always wash hands with soap and water after application to prevent accidental transfer to other body parts or individuals.
Important: If you experience any side effects, we recommend first stopping and speaking to your primary care physician.
Who Should Avoid or Be Cautious with Topical Finasteride?
While topical finasteride carries a lower risk profile than its oral counterpart, some people may still need to exercise caution or avoid the drug entirely.
Groups That Should Exercise Caution
- Those with a prior history of persistent sexual dysfunction from oral finasteride.
- People with underlying endocrine disorders or baseline sexual dysfunction.
- Those sensitive to propylene glycol or alcohol-based carrier agents.
It should be mentioned that a history of side effects from oral finasteride does not mean that topical finasteride won’t work well for you; however, it does warrant a personalized, cautious approach. This approach might include starting with lower doses, extended monitoring, and communication with the prescribing physician about any potential symptoms.
Groups That Should Avoid Topical Finasteride
- Women who are pregnant or attempting to conceive.
- People with severe scalp dermatitis or barrier dysfunction.
- Individuals with known allergy/hypersensitivity to finasteride or formulation components.
If I Can’t Tolerate Topical Finasteride, What Should I Do?
For users unable to tolerate topical finasteride due to side effects or scalp irritation, several evidence-based alternatives exist.
Pharmacological Alternatives
Low Dose Oral Minoxidil
Oral minoxidil is a hair growth stimulant that bypasses the hormonal pathway entirely. Studies show high efficacy (e.g., 43% of men achieving excellent results with 5 mg), but it carries systemic risks such as hypertrichosis (excess body hair), fluid retention, and cardiovascular effects like tachycardia.[24]Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., … Continue reading
Alternative Topical Anti-Androgens
Fluridil is a topical anti-androgen designed to degrade in water (i.e., blood), minimizing systemic exposure. Clinical data are limited but suggest efficacy without affecting serum testosterone or sexual function.[25]Sovak, M., Seligson, A.L., Kucerova, R., Bienova, M., Hajduch, M., Bucek, M. (2002). Fluridil, a rationally designed topical agent for androgenetic alopecia: first clinical experience. Dermatologic … Continue reading
Clascoterone (17ɑ-proprionate) is an androgen receptor antagonist that competes with DHT at the follicle rather than reducing DHT production. It offers a different mechanism but is still under investigation for hair loss.[26]Devjani, S., Ezemma, O., Kelley, K.J., Stratton, S., Senna, M. (2023). Androgenetic Alopecia: Therapy Update. Drugs. 83(8). 701-715. Available at: PMID: 37166619
Pyrilutamide is a non-steroidal anti-androgen that binds to the androgen receptor with high affinity. Like fluridil, it is designed to metabolize into an inactive form upon entering systemic circulation, theoretically offering a safety advantage over finasteride.[27]Biospace. (2023). Kintor Pharma’s KX-826 and GT20029 for Treatment of Androgenetic Alopecia (AGA) and Acne Presented at AAD 2023. Biospace. Available at: … Continue reading
Non-Pharmaceutical Options
Microneedling creates micro-injuries in the scalp and stimulates growth factors (PDGF, VEGF) and activates Wnt/ꞵ-catening signaling, promoting hair regeneration even without concurrent drug use.[28]Dhurat, R., Sukesh, M.S., Ayhad, G., Dandale, A., Pal, A., Pund, P. (2013). A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study. International … Continue reading
Low-level laser therapy (LLLT) devices use red light (650-655 nm) to stimulate mitochondrial activity in hair follicles, prolonging anagen and improving density with an excellent safety profile.[29]Egger, A., Resnik, S.R., Aickara, D., Maranda, E., Kaiser, M., Wikramanayake, T.C. (2020). Examining the Safety and Efficacy of Low-Level Laser Therapy for Male and Female Pattern Hair Loss: A … Continue reading
Some botanical supplements have some 5ɑ-reductase inhibitory properties. Saw palmetto and pumpkin seed oil have been shown to have mild 5ɑ-reductase inhibition.[30]Cho, Y.H., Lee, S.Y., Jeong, D.W., Choi, E.J., Kim, Y.J., Lee, J.G., Yi, H.Y., Cha, H.S. (2014). Effect of Pumpkin Seed Oil on Hair Growth in Men with Androgenetic Alopecia: A Randomized, … Continue reading,[31]Pilar, P., 2010. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Advances in Therapy. 27(8). 555-563. Available at: … Continue reading While less potent than finasteride (reducing DHT by ~30-40% vs. >60%), they may provide a viable “middle ground” for those seeking modest stabilization.
Final Thoughts
Topical finasteride offers a compelling middle path for those seeking the hair-growth benefits of 5-α-reductase inhibition with a lower likelihood of systemic side effects. But “lower” does not mean “none,” and responses vary widely depending on formulation, dose, skin permeability, and individual DHT sensitivity. The current research, while promising, remains short-term, and real-world patterns remind us that systemic exposure is still possible.
For many people, topical finasteride can be a safe, effective component of a broader hair-loss program. The key is approaching it strategically: start low, personalize dosing, choose the right vehicle, and monitor closely for changes. For others, especially those prone to hormonal side effects, alternatives ranging from anti-androgen topicals to non-pharmaceutical options may offer a better balance of efficacy and tolerability.
As with any AGA therapy, the best results come from matching the treatment to the individual, not the other way around.
References[+]
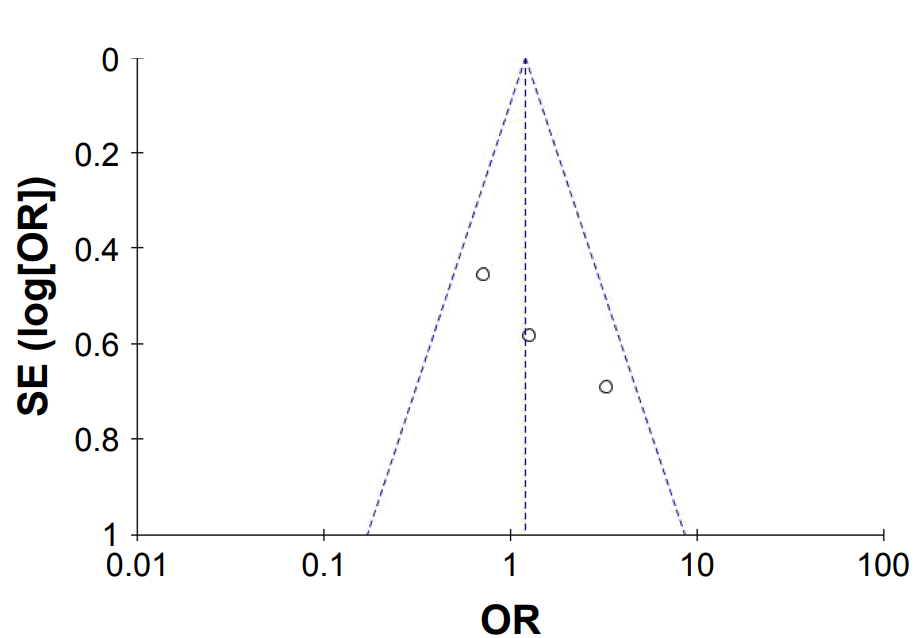
References ↑1 Zito PM, Bistas KG, Patel P, et al. Finasteride. [Updated 2024 Feb 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513329/ (Accessed: December 2025) ↑2 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. International Journal of Clinical Pharmacology and Therapeutics. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑3 Smith, A.B., Carson, C.C. (2009). Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Therapeutics and Clinical Risk Management. 5. 535-545. Available at: https://doi.org/10.2147/tcrm.s6195 ↑4 Traish, A.M. (2017). Negative Impact of Testosterone Deficiency and 5ɑ-Reductase Inhibitors Therapy on Metabolic and Sexual Function in Men. Advances in experimental medicine and biology. 1043. 473-526. Available at: https://doi.org/10.1007/978-3-319-70178-3_22 ↑5 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. International Journal of Clinical Pharmacology and Therapeutics. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑6 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E., Topical Finasteride Study Group. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑7, ↑19 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. International Journal of Clinical Pharmacology and Therapeutics. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑8 Monti. D., Tampucci, S., Burgalassi, S., Chetoni, P., Lenzi, C., Pirone, A., Mailland, F. (2014). Topical formulations containing finasteride. Part I: in vitro permeation/penetration study and in vivo pharmacokinetics in hairless rat. Journal of Pharmaceutical Sciences. 103(8). 2307-2314. Available at: https://doi.org/10.1002/jps.24028 ↑9 US Food and Drug Administration. (2025). FDA alerts health care providers, compounders and consumers of potential risks associated with compounded topical finasteride products. FDA. Available at: https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-providers-compounders-and-consumers-potential-risks-associated-compounded (Accessed: December 2025) ↑10 Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated With Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study With Data From the United States Food and Drug Administration Adverse Event Reporting System. International Journal of Dermatology. Available at: https://doi.org/10.1111/ijd.17957 ↑11 Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated With Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study With Data From the United States Food and Drug Administration Adverse Event Reporting System. International Journal of Dermatology. Available at: https://doi.org/10.1111/ijd.17957 ↑12 Lee, S.W., Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in Dermatology. 17(4). 457-463. Available at: PMID: 29601622 ↑13, ↑14 Pereira, A.F.J.R., Coehlo, T, O. de A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001 ↑15, ↑16 Farah, A.H., Brown, M.B., McAuley, W.J. (2020). Enhanced Follicular Delivery of Finasteride to Human Scalp Skin Using Heat and Chemical Penetration Enhancers. Pharmaceutical Research. 37(6). 112. Available at: https://doi.org/10.1007/s11095-020-02822-y ↑17 Tabbakhian, M., Tavakoli, N., Jaafari, M.R., Daneshamouz, S. (2006). Enhancement of follicular delivery of finasteride by liposomes and niosomes: 1. In vitro permeation and in vivo deposition studies using hamster flank and ear models. International Journal of Pharmaceutics. 1-2(323). 1-10. Available at: https://doi.org/10.1016/j.ijpharm.2006.05.041 ↑18 Rao, Y., Zheng, F., Zhang, X., Gao, J., Liang, W. (2008). In vitro Percutaneous Permeation and Skin Accumulation of Finasteride Using Vesicular Ethosomal Carriers. 9(3). 860-865. Available at: https://doi.org/10.1208/s12249-008-9124-y ↑20 Mysore, V. (2012). Finasteride and sexual side effects. Indian Dermatology Online Journal. 3(1). 62-65. Available at: https://doi.org/10.4103/2229-5178.93496 ↑21 Tai, Z., Cui, Z., Shi, X., Li, H., Chai, R., Huang, Y., Fang, Y., Jia, D., Zhu, Q., Chen, Z. (2025). The Pharmacokinetics of Topical Finasteride 0.25% Spray in Chinese Adult Male Volunteers with Androgenic Alopecia: A Phase I Study. Advances in Therapy. 42(3). 1494-1505. Available at: https://doi.org/10.1007/s12325-025-03106-w. ↑22 Cecchin, E., De Mattia, E., Toffoli, G., Mazzon, G., Cauci, S., Trombetta, C. (2014). A Pharmacogenetic Survey of Androgen Receptor (CAG)N and (GGN)N Polymorphisms in Patients Experiencing Long-Term Side Effects after Finasteride Discontinuation. The International Journal of Biological Markers. 29(4). 310-316. Available at: https://doi.org/10.5301/jbm.500095 ↑23 Rahimi-Ardabili, B., Pourandarjani, R., Habibollahi, P., Mualeki, A. (2006). Finasteride-induced depression: a prospective study. BMC Clinical Pharmacology. 6(7). Available at: https://doi.org/10.1186/1472-6904-6-7 ↑24 Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., Salas-Callo, C.I., Vincenzi, C., Yin, L., Lo-Sicco, K., Waskiel-Burnat, A., Starace, M., Zamorano, J.L., Jaen-Olasolo, P., Piraccini, B.M., Rudnicka, L., Shapiro, J., Tosti, A., Sinclair, R., Bhoyrul, B. (2021). Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. Journal of the American Academy of Dermatology. 84(6). 1644-1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑25 Sovak, M., Seligson, A.L., Kucerova, R., Bienova, M., Hajduch, M., Bucek, M. (2002). Fluridil, a rationally designed topical agent for androgenetic alopecia: first clinical experience. Dermatologic Surgery. 28(8). 678-685. Available at: https://doi.org/10.1046/j.1524-4725.2002.02017.x ↑26 Devjani, S., Ezemma, O., Kelley, K.J., Stratton, S., Senna, M. (2023). Androgenetic Alopecia: Therapy Update. Drugs. 83(8). 701-715. Available at: PMID: 37166619 ↑27 Biospace. (2023). Kintor Pharma’s KX-826 and GT20029 for Treatment of Androgenetic Alopecia (AGA) and Acne Presented at AAD 2023. Biospace. Available at: https://www.biospace.com/kintor-pharma-s-kx-826-and-gt20029-for-treatment-of-androgenetic-alopecia-aga-and-acne-presented-at-aad-2023 (Accessed: December 2025) ↑28 Dhurat, R., Sukesh, M.S., Ayhad, G., Dandale, A., Pal, A., Pund, P. (2013). A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study. International Journal of Trichology. 5(1). 6-11. Available at: https://doi.org/10.4103/0974-7753.114700 ↑29 Egger, A., Resnik, S.R., Aickara, D., Maranda, E., Kaiser, M., Wikramanayake, T.C. (2020). Examining the Safety and Efficacy of Low-Level Laser Therapy for Male and Female Pattern Hair Loss: A Review of the Literature. Skin Appendage Disorders. 6(5). 259-267. Available at: https://doi.org/10.1159/000509001 ↑30 Cho, Y.H., Lee, S.Y., Jeong, D.W., Choi, E.J., Kim, Y.J., Lee, J.G., Yi, H.Y., Cha, H.S. (2014). Effect of Pumpkin Seed Oil on Hair Growth in Men with Androgenetic Alopecia: A Randomized, Double-Blind, Placebo-Controlled Trial. Evidence-Based Complementary and Alternative Medicine. 23. 549721. Available at: https://doi.org/10.1155/2014/549721 ↑31 Pilar, P., 2010. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Advances in Therapy. 27(8). 555-563. Available at: https://doi.org/10.1007/s12325-010-0041-6 After years of anticipation, we finally have the first-ever study testing topical dutasteride as a standalone treatment for androgenic alopecia (AGA). No microneedling, no formulations mixed with other ingredients, just topical dutasteride itself.
And the results?
Frankly, they are shocking.
According to the study, low-dose topical dutasteride outperformed oral finasteride for hair regrowth.
On the face of it, this is so surprising that it borders on unbelievable, especially given the 5+ years of real-world data we’ve collected from our members.
So, in this article, we’ll break down:
- What we previously knew about topical dutasteride.
- How real-world user experiences compare to clinical findings.
- What this new study claims.
- And why two major methodological problems force us to seriously question the results.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What We Knew About Low-Dose Topical Dutasteride Before This Study
Until now, only two small clinical studies have evaluated low-dose topical dutasteride. Both used it alongside microneedling and applied it just once every 1-4 weeks.
-
Nada et al. (2018): Microneedling + Topical Dutasteride vs. Microneedling Alone
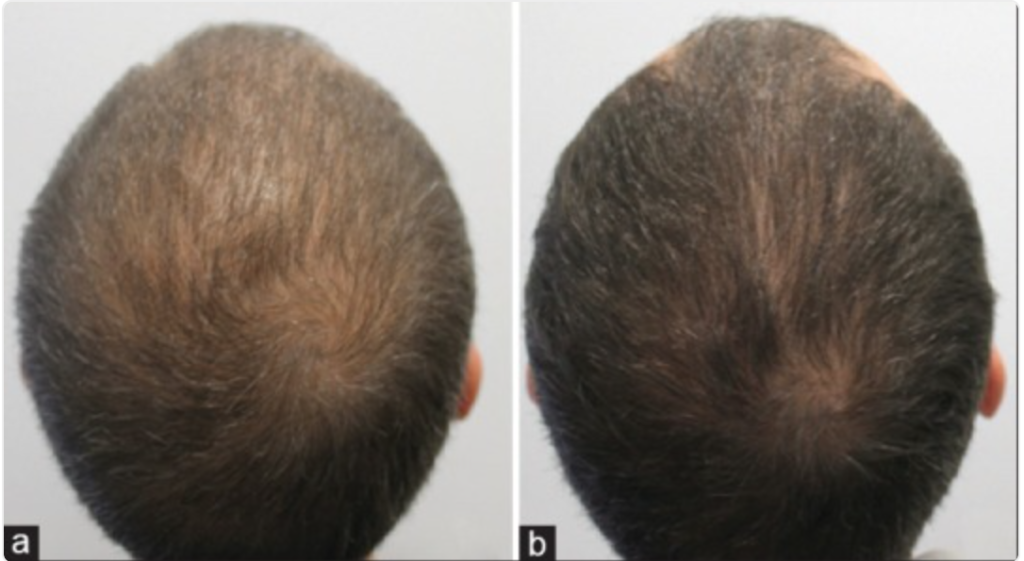
Nada et al. reported that adding low-dose topical dutasteride to a structured microneedling regimen improved hair density and shaft thickness more than microneedling alone, while only slightly reducing serum DHT, likely without meaningful systemic hormonal impact.[1]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmaged, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). Available at: … Continue reading
30 men with AGA were randomized to either microneedling plus topical dutasteride 0.02% or microneedling alone for six months. Both groups received 13 microneedling sessions with a 1.5 mm Dermapen (12-needle cartridge) on a staggered schedule: weekly during the first 8 weeks, then gradually reduced to once every 2-4 weeks through month six. In the combination arm, up to 2 mL of 0.02% dutasteride
-
Sanchez-Meza et al. (2022): Microneedling + Topical Dutasteride vs. Microneedling Alone
Sanchez-Meza et al. found that adding very low-dose topical dutasteride to microneedling produced greater clinical and trichoscopic improvement than microneedling with placebo, without reported sexual side effects and with much lower total dutasteride exposure than earlier work.[2]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-Y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
34 men with AGA were randomized to microneedling plus 1 mL of 0.01% topical dutasteride (~0.1 mg) or microneedling plus 1 mL of saline as a placebo. Both groups received three treatment sessions spaced 4 weeks apart using a Dr. Pen Ultima A6 device set to a 2.5 mm needle depth, and outcomes were assessed over a 16-20 week period.
While both these studies showed mild hair regrowth, the effect is not from topical dutasteride alone, nor from daily use.
Furthermore, low-dose topical dutasteride showed almost no systemic dihydrotestosterone (DHT) suppression.
Across these studies, bloodwork suggested:
- Little to no measurable DHT suppression
- Meaning minimal systemic absorption
- And therefore lower risk of systemic side effects
This makes sense. Dutasteride is a large, lipophilic molecule. At low concentrations, it tends to stay localized to the scalp unless dosing or penetration enhancers are significant.
What Did Real-World Users Experience?
Because early studies were promising, some of our members tried 0.01-0.02% topical dutasteride in the years that followed.
A dozen users also tracked blood DHT levels before and after treatment.
The results? Strong localization with minimal regrowth.
- No meaningful changes in serum DHT (just normal daily variation of 10-15%).
- Even among users applying up to 2 mL daily.
- Meaning: absorption remained low – as expected.
- But…users also reported very little growth.
Instead, most people saw hair maintenance, not cosmetic improvement.
This aligns with the published studies: low-dose topical dutasteride appears to stabilize hair loss, with minimal systemic impact, but not drive substantial growth.
You can get more information about this and comparisons to topical finasteride here:
- Topical Dutasteride Vs. Topical Dutasteride: The Dose Decides Everything (2025)
- Topical Finasteride: Where Do We Stand Today? (2025 Update)
- Topical Finasteride: Same Results, No Side Effects? (2022)
What Happened in the August 2025 Study?
This new randomized, double-blind, placebo-controlled study appeared to be the gold standard of topical dutasteride research.[3]Panuganti, V.K., Mandala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., Rao, S., Dundigalla. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety … Continue reading
It was the first study to test topical dutasteride:
- By itself (no microneedling).
- With daily use.
- Across multiple doses (0.01%, 0.02%, and 0.05%).
- Against both a placebo and oral finasteride.
On paper, this looked like the definitive study that we have long needed.
Study Design
135 men aged 20-60 with Norwood III vertex, IV, or V AGA were randomized across five treatment arms in a 2:2:2:2:1 ratio:
- 0.01% topical dutasteride (n=30)
- 0.02% topical dutasteride (n=30)
- 0.05% topical dutasteride (n=30)
- Oral finasteride 1 mg + placebo topical (n=30)
- Placebo topical + placebo oral (n=15).
All participants were Asian men, average age of ~38 years. Baseline hair loss severity was balanced across groups.
Each 1 mL dose contained:
- 0.01% = 0.1 mg dutasteride
- 0.02% = 0.2 mg dutasteride
- 0.05% = 0.5 mg dutasteride
Importantly, the solution was nearly 30% dehydrated alcohol, a known penetration enhancer. This choice may have implications for systemic absorption and hair-count interpretation, though the authors claim systemic exposure remained minimal.
The researchers measured hair counts (total area hair counts) and hair widths (total area hair widths). Using a Dino-Lite microscope, researchers identified a 1.9 cm2 circular region at the vertex, clipped hairs to 0.5 – 1 mm, marked the center of the circle, and captured macrophotographs at baseline, week 12, and week 24.
The authors do not mention tattooing, ink permanence, or the use of a positioning device, which becomes critically important later.
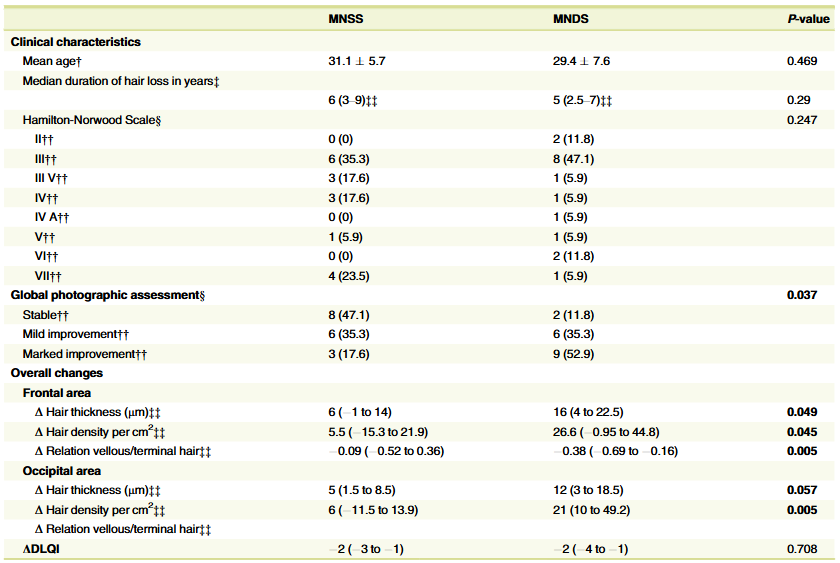
The Study’s Reported Results
Here’s where the shock factor begins.
At 24 weeks, the study reported:
- 0.01% dutasteride: +32.32 hairs/cm2
- 0.02% dutasteride: +27.48 hairs/cm2
- 0.05% dutasteride: +75.52 hairs/cm2
- Oral finasteride: +41.21 hairs/cm2
- Placebo: 0.07 hairs/cm2
The headline claim: “0.05% topical dutasteride significantly outperformed oral finasteride (p=0.0083)”
This is a remarkable result, and one that contradicts every known real-world case we’ve observed at similar dosing.
Hair-Width Improvements
All active groups increased hair thickness, with results very close to oral finasteride:
- 0.01% +6.68 μm.
- 0.02% +9.15 μm.
- 0.05% +11.59 μm.
- Finasteride: +10.68 μm.
- Placebo: +4.00 μm.
Only the 0.05% topical and finasteride groups significantly beat placebo. The 0.05% topical did not significantly outperform finasteride in hair-width metrics.
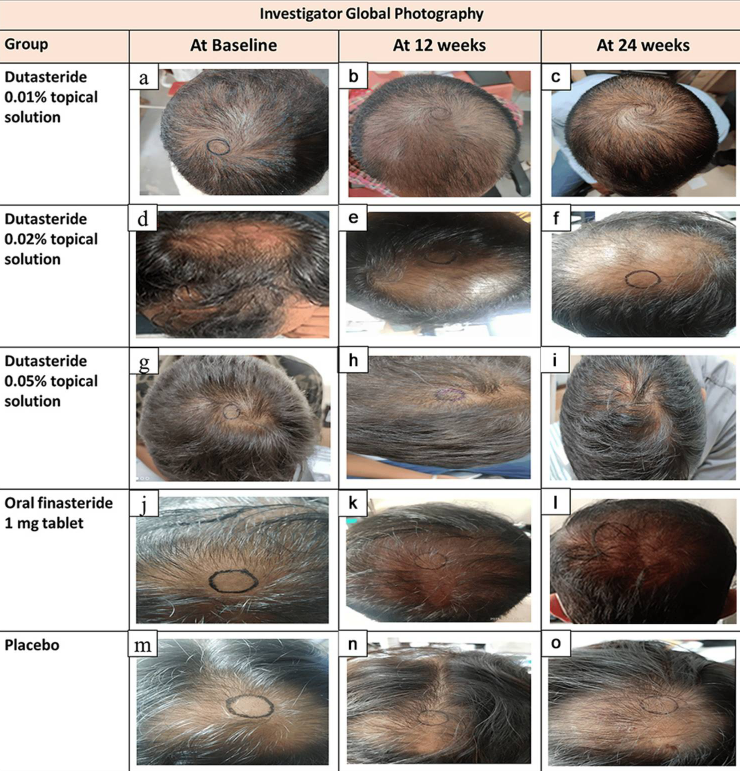
Global Photography Assessment (Investigator-Rated)
At 24 weeks, the percentage of participants rated as having “moderate improvement” or better (GPA ≥ +2) was:
- 0.05% topical: 68.97%.
- Finasteride: 21.43%.
- Placebo: 13.33%.
These numbers suggest a level of regrowth from 0.5 mg/week of topical dutasteride that we’ve simply never witnessed, not in our community, not in the medical literature, and not among clinicians who routinely prescribe topical dutasteride.
Patient-Reported Outcomes (MHGQ)
By week 24, 96.55% of 0.05% topical users were satisfied with the hair on top of their heads. This is far above the finasteride group (71.43%) and far above the placebo (33.33%).
Again, the magnitude of the difference warrants scrutiny.
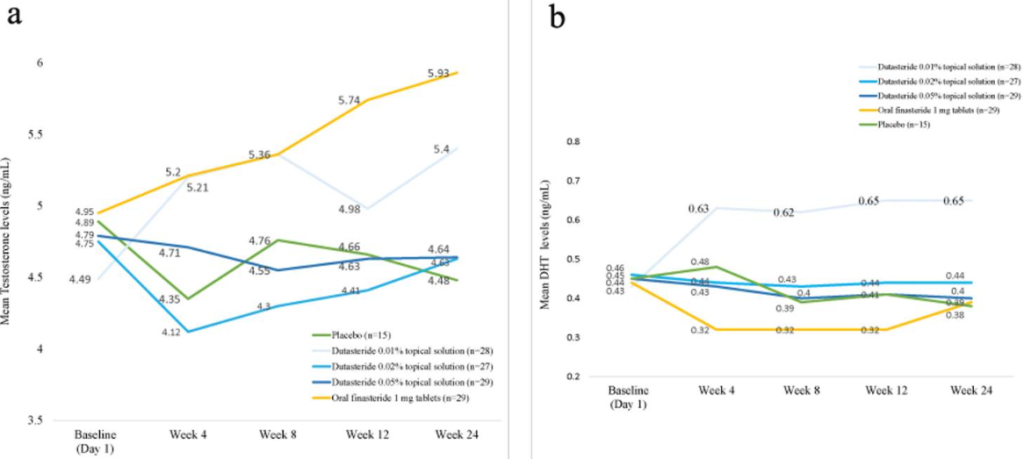
Serum Hormone Changes
This is where things do align with real-world experiences of low-dose topical users.
DHT Reductions
- Finasteride: 11% to -27%.
- 0.05% topical dutasteride: around -9% to -11%.
Testosterone Increases
- Finasteride: +20%.
- Topical dutasteride: modest, non-significant changes.
The authors emphasize that topical dutasteride caused minimal systemic effects.
This is consistent with our members’ lab data – but inconsistent with the hair-growth magnitude reported.
Pharmacokinetics (How Much Enters the Bloodstream)
According to the PK data:
- Plasma dutasteride levels were near or below quantification limits.
- A few values spiked as high as 2555 pg/mL, suggesting occasional high absorption events.
- Dutasteride remained detectable at day 168, implying accumulation over time.
The authors interpret this as “low systemic exposure”. But variability this large raises questions.
Safety Findings
Across the entire study, no serious adverse events were reported, no withdrawals due to safety, minimal skin irritation, and all groups showed mild effects like “glazing” at similar rates.
This matches expectations for low-dose topicals.
So, Why Don’t the Results Match Real-World Experience?
For more than five years, we’ve tracked user outcomes from low-dose topical dutasteride at comparable or even higher weekly doses than used in this study.
Not once have we seen:
- Regrowth exceeding oral finasteride.
- Cosmetic transformation from 0.05% topical alone.
- Hair-count improvements anywhere near +75 hairs/cm2.
Even in dermatology clinics across the world, this simply isn’t observed. So, why would this study find such dramatically different results?
It comes down to two major methodological problems – both related to hair counts.
Problem #1: Baseline Hair Counts That Defy Biology
The average baseline hair density reported in the study was 305-330 hairs/cm2.
For context:
- A healthy adult without hair loss typically has 100-250 hairs/cm2.
- Men with Norwood III-V AGA typically have 25-100 hairs/cm2 in the vertex.
- Yet this study reports 300+ hairs/cm2 in balding men.
This would mean that severely balding scalps had triple the hair density of a normal, non-balding scalp, and that hair counts exceeded what is physiologically plausible.
Why might this happen?
Possible Explanations
Vellus hairs were counted.
- The study never defines a diameter cutoff.
- Best practice is to exclude hairs ≤40 µm.
- Counting vellus hairs inflates numbers dramatically.
The measurement area was bigger than stated.
- The paper claims 1 cm2.
- Even a small mismeasurement could triple hair counts.
Manual counting errors.
- The study doesn’t specify software use.
- Manual hair counting is outdated and error-prone.
None of these possibilities inspires confidence in the baseline data.
Problem #2: The Measurement Circle Moves Between Photos
This is the more serious problem, and the one most likely to invalidate the findings entirely.
When examining the study’s published before-and-after images, the measurement circles:
- Change location.
- Change size.
- Change shape.
- Appear hand-drawn.
- Clearly do not track the same exact scalp area over time.
Figure 1: Representative hair growth images.[4]Panuganti, V.K., Mandala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., Rao, S., Dundigalla. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety … Continue reading
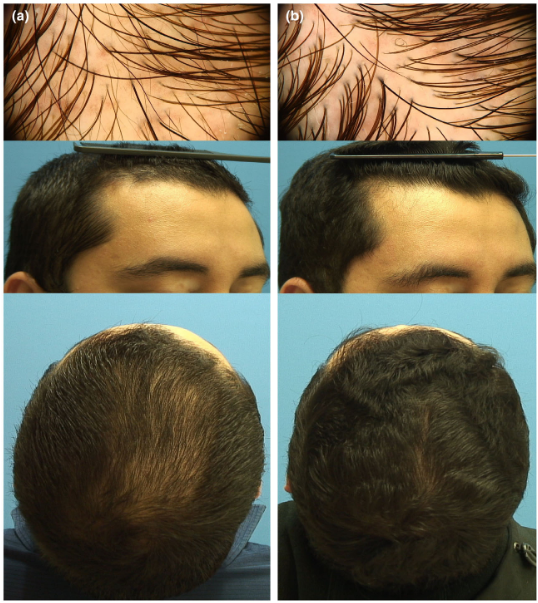
Take a close look at these images; the center point marks do not appear to be consistent (at least from these photos). This means that you really can’t compare the improvements over time, as the follow-up hair counts would have been conducted in slightly different areas of the scalp!
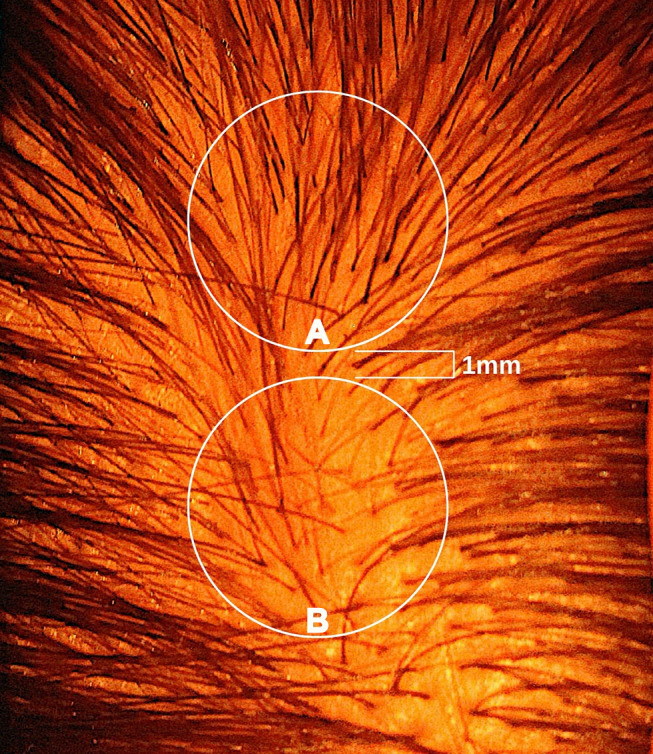
This is an enormous methodological flaw.
A shift of just 1-2 millimetres in circle placement can change hair counts by 50% or more, something we demonstrated in our 2021 publication.[5]Jnr, R.E., Ruiz, S. (2021). Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle … Continue reading
Fig 2: Circle A has 50% more hair than Circle B.[6]Jnr, R.E., Ruiz, S. (2021). Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle … Continue reading
Yet the improvements reported in this study were around 10-30%.
Meaning: These “improvements” could be fully explained by inconsistent circle placement, not actual regrowth.
This alone is enough to call the validity of the hair-count data into question.
Final Thoughts
This is one situation where we at PHH trust the real-world experiences of our members over a randomized, controlled clinical trial, because while the study appears rigorous on the surface, its hair-counting methods reveal inconsistencies significant enough to undermine its conclusions.
All available evidence still supports the following: low-dose topical dutasteride (0.01-0.05%) localizes well to the scalp, minimally suppresses serum DHT, effectively slows or stops hair loss, and rarely produces meaningful regrowth, whereas high-strength topical dutasteride (≥0.1%) is more likely to leak systemically, carry a greater risk of side effects, and generate visible regrowth approaching the results of oral finasteride.
References[+]
References ↑1 Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmaged, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). Available at: https://smj.journals.ekb.eg/article_42083_24b61cbba4be9982db23c318414034c0.pdf (Accessed: December 2025) ↑2 Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-Y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. Journal of the European Academy of Dermatology and Venereology. 36(10). E806-e808. Available at: https://doi.org/10.1111/jdv.18285 ↑3 Panuganti, V.K., Mandala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., Rao, S., Dundigalla. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects with Androgenetic Alopecia. Cureus. 17(8). E89309. Available at: https://doi:10.7759/cureus.89309 ↑4 Panuganti, V.K., Mandala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., Rao, S., Dundigalla. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects with Androgenetic Alopecia. Cureus. 17(8). E89309. Available at: https://doi:10.7759/cureus.89309 ↑5 Jnr, R.E., Ruiz, S. (2021). Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle Miniaturization Partly to Blame? Clinical, Cosmetic and Investigative Dermatology. 14. 357-365. Available at: https://doi.org/10.2147/CCID.S306157 ↑6 Jnr, R.E., Ruiz, S. (2021). Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle Miniaturization Partly to Blame? Clinical, Cosmetic and Investigative Dermatology. 14. 357-365. Available at: https://doi.org/10.2147/CCID.S306157 Finasteride is among the most effective drugs for androgenic alopecia. And while side effects are often overstated, it can lead to reduced libido or lowered sperm counts in some men. For this reason, many choose topical versus oral finasteride, hoping to limit the drug’s DHT-reducing effects to the scalp. But at certain doses, even topical finasteride can become systemic. So, to minimize side effects, which finasteride dosage, formula and application is best?
In this article, we’ll review
- About oral versus topical finasteride
- Why topical finasteride can still go systemic
- Why choosing the lowest percentage solution isn’t the only answer
- How to maximize finasteride gains, while minimizing side effects
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

About Finasteride
Finasteride, also known under the brand name Propecia or Proscar, is a prescription medication approved by the FDA for the treatment of androgenic alopecia (AGA). The anti-androgen works by reducing the production of Dihydrotestosterone (DHT), a hormone linked to pattern hair loss. In use since 1992, Finasteride is among the most powerful and well-studied drugs for hair loss.
Oral Finasteride
Oral finasteride stops AGA progression in 80-90% of men and, on average, leads to a 10% increase in hair count over two years.[1]https://www.sciencedirect.com/science/article/pii/S0022202X15529357 For men wanting a “hands-off” approach to hair maintenance, oral finasteride can be an excellent option. With a once-daily pill, it’s expected that hair loss will stop at approximately 6 months, and thereafter, improve.
However, oral finasteride isn’t for everyone. While the risk of side effects are often overstated online, the drug appears to reduce libido in a certain percentage of men. Oral finasteride can also temporarily lower sperm counts, which might make conception more difficult during its first six months of use. In some men, the use of finasteride appears to increase anxiety and/or depression. While the true incidence and magnitude of these reports are hard to discern, it’s understandable that many are weary of taking this once daily pill.
Fortunately, recent improvements in finasteride’s delivery may mean we no longer need to throw the proverbial baby out with the bathwater.
Topical Finasteride
The biggest reason people seek out topical finasteride (instead of oral finasteride) is because they want to minimize systemic exposure to the drug, and in doing so, localize finasteride’s effects to the scalp.
While research is still in the early stages, evidence suggests that topical finasteride can produce similar levels of hair regrowth compared to oral finasteride, with a significantly reduced incidence of side effects.
Topical finasteride works identically to its oral counterpart: by inhibiting the type II 5-alpha reductase enzyme to reduce DHT. It is designed to target scalp DHT instead of systemic DHT levels.
However, limiting finasteride’s reach to scalp, not serum, DHT is harder than perhaps anticipated. To understand why, let’s take a closer look at scalp versus serum DHT.
Serum vs Scalp DHT
There’s a lot of evidence that the hormone DHT is directly implicated in androgenic alopecia. In fact, research directly links DHT to all three of AGA’s defining characteristics:
- Increased telogen:anagen ratio. DHT’s influence on DKK-1 expression increases hair shedding
- Increased anagen cycling. DHT’s effects on signaling pathways limit the growth phase of hairs
- Hair follicle miniaturization. DHT’s effects on dermal papilla sizing thins hair with each cycle
There are a wide variety of DHT reducers available, including prescription drugs, over-the-counter products, intradermal injections, and even herbal supplements. Finasteride reduces DHT in two ways.
- Oral finasteride reduces DHT everywhere in the body. Beyond just the scalp, it reduces DHT in the blood, brain, and prostate.
- Topical finasteride attempts to reduce DHT only where it is applied – in the scalp – with minimal effects on DHT levels elsewhere.
However, it’s far easier than most realize for topical finasteride to go systemic. And when it does, it potentially reduces DHT everywhere – leading to the very side effects it’s meant to prevent.
Topical Finasteride Can Still Go Systemic
Why? When it comes to lowering DHT (the goal of the drug), finasteride has a highly-sensitive, dose-dependent response curve. This means that while 0.01 mg of finasteride barely reduces any DHT at all, 0.1 mg reduces almost as much DHT as 5 mg, a much larger dose.
This implies that when applying topical finasteride, only a tiny fraction of it needs to go systemic in order to produce the same DHT-lowering effects as oral finasteride. If this happens, the purpose of using the topical formulation is completely defeated.
Research suggests a 1% topical finasteride formulation, applied twice daily, is ‘non-inferior,’ meaning equivalent, to 1 mg oral finasteride tablets.[2]https://www.ncbi.nlm.nih.gov/pubmed/19172031 And while that’s a positive in terms of hair growth, using this amount topically twice daily pretty much guarantees systemic absorption. In other words, if 1% topical is the equivalent of 1 mg oral, we can expect it to reduce serum DHT levels by 71%.
So what if we choose a formula with even lower percentages of the active drug? A study on 0.25% topical finasteride showed just a 35% reduction in serum DHT levels versus 55% for the oral medication, and yet similar outcomes for hair regrowth in both oral and topical groups.[3]https://onlinelibrary.wiley.com/doi/10.1111/jdv.17738
So what if we go even lower? A study on 0.005% alcohol-based topical finasteride instructed participants to use the formulation twice daily, with 1 mL applied per session.[4]https://dx.doi.org/10.3109%2F09546639709160517 This led to no appreciable changes in serum DHT levels… meaning that for this ultra-low topical formulation, there was little-to-no systemic absorption (and presumably, few-to-no side effects). The good news? This group still experienced great hair growth outcomes.
So Just Reach for the Lowest Percentage, Yes?
It’s tempting from the above to infer we’ve reached the end of our story. As long as we reach for the lowest percentage of topical finasteride, we can be free from worries about the drug’s systemic effects. But, reality is a bit more complicated than that.
Systemic absorption isn’t just about dilution percentages. Topical finasteride’s systemic absorption actually depends on (at least) for variables:
- The drug’s formulation. Common carrier agents include alcohol and propylene glycol.
- The amount applied per use. The amount of topical finasteride applied to the scalp greatly varies.
- The application frequency. Applying the drug twice per day can more than double its effects on serum DHT.
- The days of application. Topical finasteride can take many days to “leak” into the blood before its final effects on serum DHT are realized.
For an example, see this figure from a 2014 study measuring how one versus two applications of 1 mL of 0.25% topical finasteride affected serum DHT levels.[5]https://www.ncbi.nlm.nih.gov/pubmed/25074865
Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. International journal of clinical pharmacology and therapeutics, 52(10), 842–849.
Over a 24-hour period, what were the findings?
- One application (i.e., 1 mL of 0.25% topical finasteride) lowered blood DHT levels by ~20%
- Two applications (i.e., 2 mL total) lowered blood DHT levels by ~70%
As such, we need to factor in these variables if we plan on sticking with topical finasteride for the long-term. Otherwise, we risk lowering our DHT levels to the same degree as 1 mg of oral finasteride, which would defeat the purpose of using the topical in the first place.
Topical Finasteride and Serum DHT: What Do The Clinical Studies Say?
Let’s take a closer look at some of the studies mentioned above. In addition to the percentage of active drug in the formula, there are a few more variables to understand. How much of the topical was applied, and which carrier agents were used?
The table below shows how even a .25% formula, for example, can reduce DHT in the serum by 24-70%
Based on this table, our total mg of daily finasteride exposure is probably the biggest factor in determining systemic leakage. And our total daily exposure (in mg) is a function of topical finasteride dilution (%) and the amount (mL) applied daily.
Knowing this, we can turn this table into a chart and sort it by mg of daily exposure. In doing so, we see a clear trend:
The good news? At both extremes of this chart – 0.091 mg and 2.275 mg daily – topical finasteride was shown to produce clinical results in improving hair parameters. Based on this, if we’re going for topical finasteride, we probably want to be prescribed topical finasteride solutions that net us daily exposure volumes of 0.228 mg and lower. After all, at 0.091 mg of topical finasteride daily, no systemic effects on DHT were observed.[6]https://www.tandfonline.com/doi/abs/10.3109/09546639709160517
In other words, low dilutions (i.e., 1-2 mL of 0.005% to 0.02% of topical finasteride) confer significant benefits but at reduced risks of side effects due to lower systemic absorption – provided that guidelines for daily amounts (in mL) are also followed.
So What’s the Perfect Amount and Formula?
Our analyses from member-submitted lab tests and the clinical data suggest that 10-15% of topical finasteride will enter the bloodstream, at least when it’s formulated with alcohol and/or propylene glycol as carrier ingredients (as most compounding pharmacies do). So, if we apply 1-2 mL of 0.005% to 0.02% topical finasteride, this might equate to just 0.01-0.03 mg of systemic drug exposure.
For the overwhelming majority of people, that’s not enough systemic leakage to significantly lower DHT levels in the body. But it is enough to produce great hair loss outcomes.Our partial analysis estimating systemic leakage of topical finasteride from the study: Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial (2021)
Join our Membership Program to get the full analysis.
Long-story short: stick to 1-2 mL of 0.005% to 0.02% topical finasteride solutions. This equates to roughly 0.1-0.2mg of daily finasteride exposure to the scalp. Any more than that, and there’s risk of significant systemic leakage, which defeats the purpose of using topical finasteride altogether.
How to Maximize Gains and Minimize Side Effects
Maximizing gains while minimizing the side effects of finasteride can be done, but it’s not a perfect science. We’ve outlined several tips below that we think might help. Due to individual variance, the most important step is to always start with testing.
1. Get Lab Tests for DHT
The goal with topical finasteride is to reduce the risk of side effects. To do this, we must minimize the amount of finasteride that leaks into the bloodstream. The best way to do this isn’t to rely on estimated metrics from clinical studies, but to collect personal data.
Get a serum DHT test before using the topical to establish a baseline. Then, DHT levels should be retested at one month to gauge just how much is going systemic. Testing is easier than most expect, and in our experience, the expenses are worth the peace of mind.
If future lab tests deviate from baseline, it’s an indication of just how much topical finasteride is going systemic. According to the clinical data, 30 days of application is more than enough time for finasteride to saturate at its maximum levels in the scalp and serum.
As such, measuring DHT levels after one month of finasteride use offers a great reference point to see if topical finasteride is impacting serum DHT levels. If needed, changes can be made to the application or use frequency depending on any changes to your blood levels of DHT.
2. Understand That DHT Levels Fluctuate Diurnally
Keep in mind, some fluctuation in DHT levels is normal. DHT levels fluctuate throughout the day and across seasons. As such, 15-20% differences across tests are normal and expected. Anything beyond 20% suggests that topical finasteride might be having slight systemic effects.
Because of this fluctuation, however, it’s important to get blood draws done at the same time of day – preferably in the morning and while in a fasted state. Also, try not to make drastic changes to diet, lifestyle, or environment prior to testing. Heavy drinking, deviations from a typical daily diet, the introduction of creatine powders, and/or sleep deprivation can all influence DHT levels and muddy test results. In the 3-5 days prior to the second test, try to keep things as they were when you first went in for testing.
3. Avoid supplementing with Quercetin and/or Creatine
Maintaining systemic DHT levels, while maximizing the effects of finasteride, isn’t just about the drug. There are other activities that could potentially affect DHT levels. Supplementing with quercetin and/or creatine is one common mistake that could impact results.
Studies on mice suggest that quercetin can inhibit the DHT-reducing effects of finasteride.[7]https://joe.bioscientifica.com/view/journals/joe/181/3/493.xmlWhile the translatability to humans has not yet been studied, the dosages used in these mouse models were comparable to what humans typically consume from quercetin supplements. As such, it may be best for those using finasteride to avoid this supplement.
When it comes to creatine, one study found that in training athletes, creatine supplementation increased serum DHT levels by over 70%.[8]https://pubmed.ncbi.nlm.nih.gov/19741313/ While the study was small, we have to reconcile these findings with the reality that for the overwhelming majority of training athletes, creatine is unnecessary. Bodybuilders can still look great without using it, and so can the every-day gym goer. Competitive bodybuilders are, however, a different story.
4. Set Realistic Expectations and Track Progress
Finasteride is the best-studied intervention for androgenic alopecia. In order to appreciate its full effects, consider the following:
- Know the timeline for results. Clinical studies show that finasteride improves pattern hair loss outcomes in 80-90% of men. Having said that, cosmetic degrees of hair regrowth don’t often occur until after 10-12 months of treatment. Moreover, the drug’s full effects typically take two years to manifest, with the biggest degrees of cosmetic regrowth occurring between year one and year two. As such, don’t expect any miracles in the first six months, it may take the full two years to see an effect.
- Track progress. It’s hard to know if something is improving without objective measurements. While not all of us have access to trichoscopic hair-counting equipment, most of us do have a smartphone, which can easily be used to take photos to track progress (particularly when the photosets are standardized). This can be done relatively effortlessly.
Following the tips above allows for the best possible shot at measurable hair growth results, while minimizing the potential effects of systemic DHT reduction.
Summary
Oral finasteride works great for male pattern hair loss, but by significantly reducing serum DHT, it can cause unwanted side effects.
Topical finasteride was developed as a solution, with a goal to reduce DHT in the scalp only. But when applied in high percentages or large amounts, it too, can go systemic and reduce serum DHT levels.
To minimize the side effects of finasteride without missing out on the benefits, stick to 1-2 mL of 0.005% to 0.02% topical finasteride solutions. This equates to roughly 0.1-0.2mg of daily finasteride exposure to the scalp.
The best way to ensure a reduced risk for side effects is to track serum DHT levels. Establish a baseline by taking one test before using finasteride, then test again one month later.
References[+]
References ↑1 https://www.sciencedirect.com/science/article/pii/S0022202X15529357 ↑2 https://www.ncbi.nlm.nih.gov/pubmed/19172031 ↑3 https://onlinelibrary.wiley.com/doi/10.1111/jdv.17738 ↑4 https://dx.doi.org/10.3109%2F09546639709160517 ↑5 https://www.ncbi.nlm.nih.gov/pubmed/25074865 ↑6 https://www.tandfonline.com/doi/abs/10.3109/09546639709160517 ↑7 https://joe.bioscientifica.com/view/journals/joe/181/3/493.xml ↑8 https://pubmed.ncbi.nlm.nih.gov/19741313/ Dihydrotestosterone (DHT) is a potent androgen hormone that can significantly impact hair loss. Its activity, however, isn’t just restricted to the scalp. What’s more, hair loss is a multifactorial condition, with a wide range of causes. As such, the solution to hair loss is much more complicated than simply eliminating DHT.
Many natural or pharmaceutical therapies seek to reduce, or rather, rebalance DHT levels. While lowering DHT can slow or even reverse hair loss, the hormone itself plays vital roles in sexual function, mood regulation, and overall well-being. Therefore, the goal should not be total suppression, but rather modulation that considers the balance between hair health and systemic androgen levels.
In this article, we will rank both natural and pharmaceutical DHT blockers from weakest to strongest, based on research, effectiveness, and risk of side effects.
All-Natural Hair Topical
The top natural ingredients for hair growth, all in one serum.
Take the next step in your hair growth journey with a world-class natural serum. Ingredients, doses, & concentrations built by science.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Understanding DHT: Why Blocking It Helps (and Hurts)
DHT is derived from testosterone through the enzyme 5-alpha-reductase. Once formed, DHT binds to androgen receptors and plays a major role in androgenic alopecia (AGA). In hair follicles genetically sensitive to DHT, such as those on the crown and frontal scalp, the hormone acts as a signal for miniaturization. Over time, the follicle shrinks, producing thinner, shorter hairs until it eventually becomes dormant.[1]York, K., Meah, N., Bhoyrul, B., & Sinclair, R. (2020). A review of the treatment of male pattern hair loss. Expert Opinion on Pharmacotherapy. 21(5). 603-612. Available at: … Continue reading
Figure 1. 5α-reductase converts testosterone into dihydrotestosterone. Finasteride is a 5α-reductase inhibitor, meaning it prevents or reduces this activity. Image adapted from Figure 1.[2]Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti-5-alpha-reductase activity: A prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: … Continue reading Image used under Creative Commons License.
By reducing DHT levels or blocking its receptor binding, therapies can interrupt this miniaturization cascade. The goal is to prevent DHT from triggering the cellular changes that cause follicles to shrink and enter a premature resting (telogen) phase. AGA is influenced not just by DHT levels but also by individual receptor sensitivity, local inflammation, and oxidative stress.
However, because of DHT’s wide-reaching activity, side effects of its reduction can include sexual dysfunction, decreased motivation, and mood changes, especially in individuals who are more hormonally sensitive. As such, careful consideration of different therapies and their potential impacts is essential if you choose to use a DHT-blocking treatment.[3]Traish, A. M., Melcangi, R. C., Bortolato, M., Garcia-Segura, L. M., & Zitzmann, M. (2015). Adverse effects of 5α-reductase inhibitors: what do we know, don’t know, and need to know?. Reviews … Continue reading
Ranking the DHT Blockers: From Mild to Potent
We’ll rank DHT blockers based on their potency, as well as on the quality of evidence supporting their use for hair loss. You can learn more about our treatment metrics, including evidence quality, in our article.
Tier 1 – Mild / Natural DHT Blockers
Natural treatments, typically based on oils and herbs, are generally more gentle than pharmaceutical options. They tend to be less potent, but have far fewer side effects and are not typically subject to the same stringent regulation as pharmaceuticals.
EGCG (Evidence Quality – 6%)
Epigallocatechin gallate (EGCG), a catechin compound found in green tea, is another naturally derived compound that has attracted interest for its potential to influence hair follicle biology. As a potent antioxidant and anti-inflammatory molecule, EGCG helps protect follicular cells from oxidative stress and inflammation, both of which are exacerbated by androgen activity.
Figure 2: Molecular structure of EGCG. Adapted from:[4]Wikipedia. (no date). Epigallocatechin gallate. Available at: https://en.wikipedia.org/wiki/Epigallocatechin_gallate (Accessed: November 2025) Image used under Creative Commons License
EGCG appears to enhance cell-survival pathways such as Akt and Erk, supporting follicular cell proliferation while suppressing apoptotic signals that can trigger hair follicle regression.[5]Kwon, O. S., Han, J. H., Yoo, H. G., Chung, J. H., Cho, K. H., Eun, H. C., & Kim, K. H. (2007). Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). … Continue reading
Laboratory studies also indicate that EGCG can partially inhibit 5AR, reducing the local generation of DHT.[6]Hiipakka, R. A., Zhang, H. Z., Dai, W., Dai, Q., & Liao, S. (2002). Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochemical Pharmacology. 63(6). … Continue reading However, while this study showed that EGCG can inhibit 5AR in cell-free experiments, it did not have the same impact in cells. This suggests that it may not be able to enter cells and would have reduced impacts in humans.
This seems to be the case, and clinical evidence for EGCG in the treatment of hair loss remains limited. A small pilot study of topical EGCG found no visible changes in hair, although this research was conducted in people with no hair loss disorders.[7]Kwon, O. S., Han, J. H., Yoo, H. G., Chung, J. H., Cho, K. H., Eun, H. C., & Kim, K. H. (2007). Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). … Continue reading Despite the lack of large-scale human trials, molecular findings do suggest EGCG contributes meaningfully to the cellular environment that supports hair growth and protection.
Catechins from green tea infusions are generally well tolerated. However, supplements should be used cautiously at high doses, particularly above 800 mg per day, as they have been linked to liver enzyme elevations in sensitive individuals.[8]EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Younes, M., Aggett, P., Aguilar, F., Crebelli, R., Dusemund, B., Filipič, M., Frutos, M. J., Galtier, P., Gott, D., … Continue reading
You can read more about the evidence for using EGCG for hair loss in our article here.
Pumpkin Seed Oil (Evidence Quality – 49%)
Pumpkin seed oil (PSO) is one of the most extensively studied non-pharmaceutical treatments for hair loss. In general, it is mild and well-tolerated. Its effects appear to arise from its profile of phytosterols and essential fatty acids, such as linoleic and oleic acid. These compounds have been shown to exhibit mild 5AR inhibitory activity in laboratory studies, potentially reducing local DHT formation in scalp tissues.[9]Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti 5-alpha-reductase activity: a prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: … Continue reading,[10]Liang, T., & Liao, S. (1992). Inhibition of steroid 5α-reductase by specific aliphatic unsaturated fatty acids. Biochemical Journal. 285(2). 557-562. Available at: … Continue reading
Beyond hormonal modulation, pumpkin seed oil provides antioxidant and anti-inflammatory support, which can help protect hair follicles from oxidative damage and microinflammation – two key contributors to progressive miniaturization.[11]Fahim, A. T., Abd-El Fattah, A. A., Agha, A. M., & Gad, M. Z. (1995). Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacological … Continue reading
A clinical trial carried out in Korea made waves in 2014 when it reported a 40% increase in hair count after 24 weeks. However, the formulation, Octa-Sabal Plus, included multiple additional herbal compounds, alongside pumpkin seed powder, not oil. This means we can’t attribute the growth reported to pumpkin seed oil.[12]Cho, Y. H., Lee, S. Y., Jeong, D. W., Choi, E. J., Kim, Y. J., Lee, J. G., & Cha, H. S. (2014). Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: a randomized, … Continue reading
You can read more about the controversial PSO study here.
Cold-pressed pumpkin seed oil is typically taken orally at doses ranging from 400 to 1000 mg daily, with results accumulating gradually over several months. The compound is generally well tolerated, with gastrointestinal discomfort being rare and mild.[13]Hong, H., Kim, C. S., & Maeng, S. (2009). Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutrition Research and Practice. 3(4). … Continue reading Although PSO alone is unlikely to reverse established AGA, it may serve as a gentle adjunct for individuals seeking natural or preventive strategies to support long-term scalp and hair health.
Saw Palmetto (Evidence Quality – 54%)
Saw palmetto (Serenoa repens) is one of the most widely studied natural 5AR inhibitors and is sometimes called “nature’s finasteride.” It appears to reduce DHT by inhibiting Type II 5AR, reducing the binding of DHT to androgen receptor sites and increasing the conversion of DHT to a weaker metabolite called androstanediol.[14]Suzuki, M., Ito, Y., Fujino, T., Abe, M., Umegaki, K., Onoue, S., Noguchi, H., & Yamada, S. (2009). Pharmacological effects of saw palmetto extract in the lower urinary tract. *Acta … Continue reading
Importantly, saw palmetto appears to reduce DHT selectively, with research showing decreases in DHT in the prostate but not in the blood. As such, it may cause fewer side effects than systemic DHT reducers.[15]Fagelman, E., & Lowe, F. C. (2001). Saw palmetto berry as a treatment for BPH. Reviews in Urology. 3(3). 134-138. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1476047/
Clinical studies have shown modest impacts of saw palmetto on hair loss. In one study, participants using oral extracts over several months showed reductions in hair shedding, increases in hair density, and subjective improvements in scalp coverage and hair quality.{ Importantly, however, this was an open-label study, where participants and researchers both knew what product they were receiving, so is not as strong a form of evidence as a randomzied controlled trial (RCT).[16]Rossi, A., Mari, E., Scarno, M., Garelli, V., Maxia, C., Scali, E., Iorio, A., & Carlesimo, M. (2012). Comparative effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: a … Continue reading
In a recent RCT, a standardized, proprietary saw palmetto extract was given as either a 400mg oral capsule or 5 ml of topical formulation and compared to relevant controls. The study found a significant reduction in hair shedding in both oral and topical groups compared to their controls, alongside an increase in hair density of 5% and 7.5% respectively. However, this study also had significant drawbacks. For example, measurements of hair density were not well defined and may therefore include vellus hairs, which do not contribute to visible hair. Despite this, the study does provide some support for the use of saw palmetto in decreasing hair shedding and increasing hair thickness.[17]Sudeep, H. V., Rashmi, S., Jestin, T. V., Richards, A., Gouthamchandra, K., & Shyamprasad, K. (2023). Oral and topical administration of a standardized saw palmetto oil reduces hair fall and … Continue reading
Evidence from benign prostatic hyperplasia suggests that an oral dose of 320 mg per day is optimal, with increases in dose above that resulting in no further improvements. For topical use, concentrations around 0.5 – 1% applied once or twice daily are common. Both routes are well tolerated, with infrequent reports of mild gastrointestinal discomfort or skin irritation.
You can read about the differences between saw palmetto and finasteride in our article here.
Tier 2 – Over-the-Counter Pharmaceutical Options
Over-the-Counter Pharmaceutical Options bridge the gap between gentle natural therapies and prescription-strength DHT blockers, potentially offering clinically supported benefits with relatively low systemic risk.
Ketoconazole Shampoo (Evidence Quality – 54%)
Originally developed as an antifungal agent, ketoconazole helps normalize the scalp environment by reducing inflammation, fungal overgrowth, and excess sebum, all of which can worsen AGA. Beyond its antifungal activity, laboratory and clinical research suggest that ketoconazole exerts mild local antiandrogenic effects, likely through partial inhibition of 5AR and modulation of DHT activity within the scalp.
Clinical evidence for men is stronger than for women. One 21-month study using 2% ketoconazole shampoo showed progressive improvement in hair shaft diameter and percentage of hairs in the growth (anagen) phase, reversing the slow decline seen in untreated AGA.[18]Piérard-Franchimont, C., De Doncker, P., Cauwenbergh, G., & Piérard, G. E. (1998). Ketoconazole shampoo: effect of long-term use in androgenic alopecia. Dermatology. 196(4). 474-477. Available … Continue reading
Another study, which compared Ketoconazole with other shampoos, found that 1% ketoconazole reduced hair shedding by 17%, improved anagen hair by 4.9%, and lowered scalp sebum levels.[19]Piérard-Franchimont, C., Goffin, V., Henry, F., Uhoda, I., Braham, C., & Piérard, G. E. (2002). Nudging hair shedding by antidandruff shampoos: A comparison of 1% ketoconazole, 1% piroctone … Continue reading Studies also show that ketoconazole performs well as an adjunct therapy, with synergistic effects when combined with finasteride.[20]Perez, B. S. H. (2004). Ketoconazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Medical Hypotheses. 62(1). 112-115. Available at: … Continue reading
Evidence in women is limited, with only one published study investigating ketoconazole solution, rather than shampoo. They compared 2% ketoconazole to 2% minoxidil and reported meaningful regrowth in both groups, with ketoconazole producing improvements by the six-month mark and causing far fewer side effects.[21]El-Garf, A., Mohie, M., & Salah, E. (2019). Trichogenic effect of topical ketoconazole versus minoxidil 2% in female pattern hair loss: a clinical and trichoscopic evaluation. Biomedical … Continue reading
Ketoconazole is generally well tolerated. Mild irritation, dryness, or temporary scalp tightness may occur, especially with 2% formulations or frequent use. Most regimens recommend applying it 2–3 times per week, leaving the shampoo on the scalp for 3–5 minutes before rinsing.
You can read our breakdown of the research into ketoconazole shampoos here.
You can also read about why 2% ketoconazole might be a better choice than 1% here.
Tier 3 – Prescription Pharmaceutical DHT Blockers
Prescription Pharmaceutical DHT Blockers represent the most potent and clinically validated options, offering stronger and more predictable suppression of DHT than natural therapies but with a greater need for careful monitoring of side effects.
Topical Finasteride (Evidence Quality – 69%)
Topical finasteride represents a new, more targeted approach to DHT inhibition, delivering the drug directly to the scalp while aiming to minimize systemic exposure. Like its oral counterpart, it blocks the Type II 5-alpha reductase enzyme. However, by focusing this activity locally within the hair follicle, topical formulations can achieve substantial reductions in scalp DHT while maintaining serum levels that are a fraction of those seen with oral dosing. This distinction is clinically important for individuals concerned about systemic side effects such as decreased libido or hormonal shifts.
Clinical research has consistently shown that topical finasteride can achieve similar improvements in hair density and regrowth as oral therapy. One 24-week study showed a 34.5% decrease in DHT, compared to 55.6% for oral finasteride. However, plasma concentrations were 100 times lower, indicating that systemic exposure is greatly reduced.[22]Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., Galván, J., Tebbs, V., Massana, È., on behalf of the Topical Finasteride Study Group. … Continue reading
Topical finasteride is particularly appealing to men who respond well to oral therapy but wish to lower the risk of systemic adverse effects. When applied once daily at concentrations between 0.1% and 0.25%, it can stabilize shedding within four to six months, with visible improvements generally continuing over the course of a year.[23]Mazzarella, G. F., Loconsole, G. F., Cammisa, G. A., Mastrolonardo, G. M., & Vena, G. A. (1997). Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a … Continue reading For optimal results, it can be combined with topical minoxidil to stimulate regrowth through complementary pathways.[24]Wang, H., Chen, B., & Hong, X. (2020). The efficacy and safety of finasteride combined with topical minoxidil for androgenetic alopecia: A systematic review and meta-analysis. *Journal of … Continue reading
Our members can find the ultimate guide to finasteride here.
Dutasteride (Evidence Quality – 77%)
Dutasteride is the most potent 5AR inhibitor currently available, targeting both Type I and Type II isoenzymes. This dual inhibition results in serum DHT reductions of up to 90 – 95%, creating a profoundly anti-androgenic environment within scalp follicles.[25]Roehrborn, C. G., Marks, L. S., Fenter, T., Freedman, S., Tuttle, J., Gittleman, M., Morrill, B., & Wolford, E. T. (2004). Efficacy and safety of dutasteride in the four-year treatment of men … Continue reading Clinically, this translates into faster and often more pronounced regrowth compared to finasteride, particularly in individuals with advanced hair thinning.
Because dutasteride suppresses DHT more comprehensively, it may pose a slightly higher risk of hormonal side effects such as gynecomastia or reduced libido. However, head-to-head studies have suggested superior efficacy for dutasteride in both short-term and long-term endpoints.
A meta-analysis, which combines results from multiple studies, indicated that dutasteride showed better efficacy in treating AGA.[26]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., & Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue reading These results suggest that dutasteride is ~3 times more potent than finasteride in blocking Type II 5α-reductase and up to 100 times more potent against Type I. This dual inhibition reduces scalp DHT levels by over 50%, compared to approximately 41% with finasteride, leading to greater prevention of follicular miniaturization and enhanced regrowth response with similar levels of adverse effects.
Dutasteride has a relatively low evidence quality score compared to finasteride, though this is largely due to there being fewer studies. The studies that do investigate dutasteride have shown higher potency and effectiveness.
Topical dutasteride formulations are now under active investigation. Concentrations ranging from 0.01% to 0.05% have produced significant gains in target-area hair counts, with studies suggesting that improvements associated with oral formulations can be achieved without drops in serum DHT.[27]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., KSSVV, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo and active controlled Phase II study to … Continue reading
Figure 3. Topical Dutasteride supports hair regrowth. Adapted from Figure 2.[28]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., KSSVV, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo and active controlled Phase II study to … Continue reading Image used under Creative Commons License
A 2018 randomized clinical study further demonstrated that topical dutasteride (0.02%) combined with microneedling significantly improved hair shaft thickness and patient satisfaction compared with microneedling alone, without notable systemic absorption or hormonal side effects. These findings suggest that localized delivery of dutasteride may enhance follicular response while minimizing systemic exposure and related adverse effects.[29]Nada, E.A., El Sharkawy, R.E.E.D., Abd El-Maged, W. M., & Abo Elmagd, M. A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. … Continue reading
Similarly, research published in 2024 looked into the impact of microneedling. However, in this study, topical dutasteride was combined with minoxidil. They found similar improvements in hair density and hair width in participants who had topical dutasteride and minoxidil treatment to those in patients who had oral dutasteride and topical minoxidil.[30]Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through … Continue reading These results suggest that, in combination with other therapies (minoxidil or microneedling), topical dutasteride may be able to produce similar results to oral formulations but with a significantly lower chance of side effects.
A newly published Phase II trial has also drawn significant attention because it is the first study to test topical dutasteride alone, without microneedling. The results appear impressive: all topical doses improved hair parameters versus placebo, showed minimal impact on serum DHT (suggesting good scalp localization), and 1 ml daily of 0.05% topical dutasteride reportedly outperformed oral finasteride in key regrowth metrics.[31]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading
However, the study reports unusually high baseline hair counts, as well as inconsistencies in the placement and size of the 1 cm² target areas used for serial hair counts. These issues can dramatically skew results, creating the illusion of improvement even when none exists. Real-world data from users of low-dose topical dutasteride (0.01–0.02% applied daily or near-daily) also paint a more modest picture: good localization and serum DHT stability, but primarily maintenance rather than regrowth exceeding oral finasteride
Oral Finasteride (Evidence Quality – 99%)
As you can see from our evidence quality score, oral finasteride remains the most clinically validated pharmacological treatment for AGA. It acts as a selective inhibitor of the Type II 5AR enzyme, reducing systemic and scalp DHT levels by approximately 60–70%.[32]Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W. P., Cotterill, P. C., Thiboutot, D. M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S. J., Griffin, E. I., Weiss, D., … Continue reading This substantial suppression slows or halts follicular miniaturization, prolongs the growth (anagen) phase, and enables partial regrowth in many users. The standard 1 mg daily dose for AGA has been extensively studied and represents the optimal balance between efficacy and safety for most men.
Clinical trials have consistently confirmed the effectiveness of finasteride in both the short and long term. A 4-year, randomized study found that 1 mg of finasteride led to increased hair weight (46% vs placebo) and hair count (20% vs. placebo).[33]Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. … Continue reading
You can find an in-depth overview of the clinical studies on finasteride here.
While highly effective, oral finasteride is not without potential drawbacks. A small percentage of users report side effects related to sexual function, including reduced libido, erectile difficulty, and diminished ejaculate volume. Most of these effects are mild and resolve upon discontinuation, although persistent symptoms have been reported in rare cases. Some users also report changes in mood or motivation, likely reflecting individual hormonal sensitivity. For those wishing to minimize systemic exposure, transitioning to topical finasteride or titrating to a lower dose (e.g., 0.25-0.5 mg daily) may be a suitable option.[34]Gupta, A. K., Venkataraman, M., Talukder, M., & Bamimore, M. A. (2022). Finasteride for hair loss: a review. Journal of Dermatological Treatment. 33(4). 1938-1946. Available at: … Continue reading
There have also been reports of a condition that’s been named ‘post-finasteride syndrome’. While not widely accepted by the medical community, similar symptoms have been reported in a number of individuals who are taking finasteride. These symptoms can include sexual dysfunction, such as loss of libido and erectile dysfunction, neuropsychiatric symptoms like depression and anxiety, and physical changes like muscle atrophy and fatigue. Importantly, there have been cases where these symptoms have persisted after users have stopped taking the drug.
While the authors who published the original article on post-finasteride syndrome do highlight the lack of consistent evidence for persistent side effects found in clinical trials, they suggest that finasteride may act to worsen symptoms associated with sexual dysfunction and psychiatric conditions for some users who already have these issues.[35]Pereira, A. F. J. R., & Coelho, T. O. A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001
Overall, oral finasteride remains the gold standard for DHT suppression, providing robust and predictable results when used consistently. It’s also important to consider the potential side effects when choosing to take finasteride, and consult with your doctor if symptoms arise.
Honourable Mention – Pyrilutamide
Pyrilutamide (KX-826)
Pyrilutamide takes a different approach to androgen modulation. Rather than reducing DHT production, it acts as a topical androgen receptor antagonist, blocking DHT and testosterone from binding to their receptors in the follicle. By interfering with receptor activation, pyrilutamide aims to suppress the downstream gene expression that leads to follicular miniaturization.[36]Koralewicz, M. M., & Szatkowska, O. A. (2024, July). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 61-70. Available at: … Continue reading
Clinical data show encouraging signals. Trials conducted in China and the United States suggest that topical formulations applied once or twice daily can produce measurable increases in hair counts over several months. Reported side effects are primarily mild local dermatitis, and systemic exposure appears very low. However, many of these results remain unpublished in peer-reviewed journals and have failed to demonstrate statistical superiority over placebo for some measurements.[37]Kintor Pharmaceuticals. (2022). Developing Novel Drugs and Commercialization Platform. Available at: … Continue reading,[38]Kintor Pharmaceutical Limited. (2023, March 28). Kintor Pharma Announces Completion of Subject Enrollment in Phase III Clinical Trial for KX-826 for Treatment of Male Androgenetic Alopecia in China. … Continue reading Because these reports have not been published in peer-reviewed journals, they can only currently be considered pre-releases, rather than academic papers. The absence of published, peer-reviewed, blinded, controlled data means that we can’t draw firm conclusions on pyrilutamide, even in the wake of the many press releases that Kintor has published.
For now, pyrilutamide should be viewed as an experimental therapy. Its mechanism is compelling and localized, offering a theoretical advantage over systemic antiandrogens. Still, robust, peer-reviewed clinical trials confirming efficacy and long-term safety are needed before it can be considered a mainstream treatment. Those interested in exploring it should do so only under medical supervision or within the context of formal clinical research.
Final Thoughts
DHT blockers span a broad spectrum, ranging from gentle, naturally derived modulators like pumpkin seed oil and EGCG to powerful prescription therapies such as finasteride and dutasteride. Natural options may improve scalp health and have a modest influence on hormonal activity, but their effects are typically gradual and limited. In contrast, pharmacological inhibitors act directly on the enzymatic pathways responsible for DHT synthesis, offering stronger and more predictable outcomes. Topical finasteride has emerged as a compelling middle ground, delivering scalp-level efficacy with minimal systemic impact, while dutasteride represents the upper limit of potency for those requiring more aggressive intervention.
Ultimately, the “best” DHT blocker depends on individual tolerance, goals, and the stage of hair loss. A well-balanced regimen typically combines a DHT inhibitor with a growth stimulant, such as minoxidil, alongside scalp care and lifestyle modifications. With consistent use and proper medical guidance, these approaches can collectively slow, halt, or even reverse the progression of AGA, helping individuals maintain fuller, healthier hair over time.
References[+]
References ↑1 York, K., Meah, N., Bhoyrul, B., & Sinclair, R. (2020). A review of the treatment of male pattern hair loss. Expert Opinion on Pharmacotherapy. 21(5). 603-612. Available at: https://doi.org/10.1080/14656566.2020.1721463 ↑2 Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti-5-alpha-reductase activity: A prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: https://doi.org/10.12688/f1000research.51066.3 ↑3 Traish, A. M., Melcangi, R. C., Bortolato, M., Garcia-Segura, L. M., & Zitzmann, M. (2015). Adverse effects of 5α-reductase inhibitors: what do we know, don’t know, and need to know?. Reviews in Endocrine and Metabolic Disorders. 16(3). 177-198. Available at: https://doi.org/10.1007/s11154-015-9319-y ↑4 Wikipedia. (no date). Epigallocatechin gallate. Available at: https://en.wikipedia.org/wiki/Epigallocatechin_gallate (Accessed: November 2025) ↑5, ↑7 Kwon, O. S., Han, J. H., Yoo, H. G., Chung, J. H., Cho, K. H., Eun, H. C., & Kim, K. H. (2007). Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine. 14(7-8). 551-555. Available at: https://doi.org/10.1016/j.phymed.2006.11.003 ↑6 Hiipakka, R. A., Zhang, H. Z., Dai, W., Dai, Q., & Liao, S. (2002). Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochemical Pharmacology. 63(6). 1165-1176. Available at: https://doi.org/10.1016/S0006-2952(02)00848-1 ↑8 EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Younes, M., Aggett, P., Aguilar, F., Crebelli, R., Dusemund, B., Filipič, M., Frutos, M. J., Galtier, P., Gott, D., Gundert-Remy, U., Kuhnle, G. G., Lambré, C., Leblanc, J.-C., Lillegaard, I. T., Moldeus, P., Mortensen, A., Oskarsson, A., Stankovic, I., Waalkens-Berendsen, I., Woutersen, R. A., Andrade, R. J., Fortes, C., Mosesso, P., Restani, P., Arcella, D., Pizzo, F., Smeraldi, C., & Wright, M. (2018). Scientific opinion on the safety of green tea catechins. *EFSA Journal.* 16(4). e05239. Available at: https://doi.org/10.2903/j.efsa.2018.5239 ↑9 Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti 5-alpha-reductase activity: a prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: https://doi.org/10.12688/f1000research.51066.3 ↑10 Liang, T., & Liao, S. (1992). Inhibition of steroid 5α-reductase by specific aliphatic unsaturated fatty acids. Biochemical Journal. 285(2). 557-562. Available at: https://doi.org/10.1042/bj2850557 ↑11 Fahim, A. T., Abd-El Fattah, A. A., Agha, A. M., & Gad, M. Z. (1995). Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacological Research. 31(1). 73-79. Available at: https://doi.org/10.1016/1043-6618(95)80051-4 ↑12 Cho, Y. H., Lee, S. Y., Jeong, D. W., Choi, E. J., Kim, Y. J., Lee, J. G., & Cha, H. S. (2014). Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: a randomized, double-blind, placebo-controlled trial. *Evidence-Based Complementary and Alternative Medicine.* 2014(1). 549721. Available at: https://doi.org/10.1155/2014/549721 ↑13 Hong, H., Kim, C. S., & Maeng, S. (2009). Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutrition Research and Practice. 3(4). 323-327. Available at: https://doi.org/10.4162/nrp.2009.3.4.323 ↑14 Suzuki, M., Ito, Y., Fujino, T., Abe, M., Umegaki, K., Onoue, S., Noguchi, H., & Yamada, S. (2009). Pharmacological effects of saw palmetto extract in the lower urinary tract. *Acta Pharmacologica Sinica.* 30(3). 271-281. Available at: https://doi.org/10.1038/aps.2009.1 ↑15 Fagelman, E., & Lowe, F. C. (2001). Saw palmetto berry as a treatment for BPH. Reviews in Urology. 3(3). 134-138. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1476047/ ↑16 Rossi, A., Mari, E., Scarno, M., Garelli, V., Maxia, C., Scali, E., Iorio, A., & Carlesimo, M. (2012). Comparative effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: a two-year study. International Journal of Immunopathology and Pharmacology. 25(4). 1167-1173. Available at: https://doi.org/10.1177/039463201202500435 ↑17 Sudeep, H. V., Rashmi, S., Jestin, T. V., Richards, A., Gouthamchandra, K., & Shyamprasad, K. (2023). Oral and topical administration of a standardized saw palmetto oil reduces hair fall and improves hair growth in androgenetic alopecia subjects – a 16-week randomized, placebo-controlled study. Clinical, Cosmetic and Investigational Dermatology. 16. 3251-3266. Available at: https://doi.org/10.2147/CCID.S380214 ↑18 Piérard-Franchimont, C., De Doncker, P., Cauwenbergh, G., & Piérard, G. E. (1998). Ketoconazole shampoo: effect of long-term use in androgenic alopecia. Dermatology. 196(4). 474-477. Available at: https://doi.org/10.1159/000017954 ↑19 Piérard-Franchimont, C., Goffin, V., Henry, F., Uhoda, I., Braham, C., & Piérard, G. E. (2002). Nudging hair shedding by antidandruff shampoos: A comparison of 1% ketoconazole, 1% piroctone olamine and 1% zinc pyrithione formulations. International Journal of Cosmetic Science. 24(5). 249-256. Available at: https://doi.org/10.1046/j.1467-2494.2002.00145.x ↑20 Perez, B. S. H. (2004). Ketoconazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Medical Hypotheses. 62(1). 112-115. Available at: https://doi.org/10.1016/S0306-9877(03)00264-0 ↑21 El-Garf, A., Mohie, M., & Salah, E. (2019). Trichogenic effect of topical ketoconazole versus minoxidil 2% in female pattern hair loss: a clinical and trichoscopic evaluation. Biomedical Dermatology. 3(1). 1-8. Available at: https://doi.org/10.1186/s41702-019-0046-y ↑22 Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., Galván, J., Tebbs, V., Massana, È., on behalf of the Topical Finasteride Study Group. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑23 Mazzarella, G. F., Loconsole, G. F., Cammisa, G. A., Mastrolonardo, G. M., & Vena, G. A. (1997). Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. Journal of Dermatological Treatment. 8(3). 189-192. Available at: https://doi.org/10.3109/09546639709160517 ↑24 Wang, H., Chen, B., & Hong, X. (2020). The efficacy and safety of finasteride combined with topical minoxidil for androgenetic alopecia: A systematic review and meta-analysis. *Journal of Dermatological Treatment.* Available at: https://doi.org/10.1007/s00403-020-02100-6 ↑25 Roehrborn, C. G., Marks, L. S., Fenter, T., Freedman, S., Tuttle, J., Gittleman, M., Morrill, B., & Wolford, E. T. (2004). Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology. 63(4). 709-715. Available at: https://doi.org/10.1016/j.urology.2004.01.001 ↑26 Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., & Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis. Clinical Interventions in Aging. 14. 399-406. Available at: https://doi.org/10.2147/CIA.S192435 ↑27 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., KSSVV, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo and active controlled Phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑28 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., KSSVV, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo and active controlled Phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑29 Nada, E.A., El Sharkawy, R.E.E.D., Abd El-Maged, W. M., & Abo Elmagd, M. A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-400. Available at: https://doi.org/10.21608/smj.2018.42083 ↑30 Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 mg) in Treatment of Androgenetic Alopecia. QJM: An International Journal of Medicine. 117(Supplement_2). hcae175-207. Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑31 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. *Cureus.* 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑32 Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W. P., Cotterill, P. C., Thiboutot, D. M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S. J., Griffin, E. I., Weiss, D., Carrington, P., Gencheff, C., Cole, G. W., Pariser, D. M., Epstein, E. S., Tanaka, W. K., Dallob, A. L., Vandormael, K., Geissler, L. A., & Waldstreicher, J. (1999). The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 41(4). 550-554. Available at: https://doi.org/10.1016/S0190-9622(99)80051-6 ↑33 Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. Journal of the American Academy of Dermatology. 55(1). 71-74. Available at: https://doi.org/10.1016/j.jaad.2005.07.001 ↑34 Gupta, A. K., Venkataraman, M., Talukder, M., & Bamimore, M. A. (2022). Finasteride for hair loss: a review. Journal of Dermatological Treatment. 33(4). 1938-1946. Available at: https://doi.org/10.1080/09546634.2021.1959506 ↑35 Pereira, A. F. J. R., & Coelho, T. O. A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001 ↑36 Koralewicz, M. M., & Szatkowska, O. A. (2024, July). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 61-70. Available at: https://doi.org/10.5603/fd.101208 ↑37 Kintor Pharmaceuticals. (2022). Developing Novel Drugs and Commercialization Platform. Available at: https://docs.publicnow.com/viewDoc?hash_primary=BFD6444A4669BD4BE0683A8BCC8B5E80F3D7E430 (Accessed: October 2025)
↑38 Kintor Pharmaceutical Limited. (2023, March 28). Kintor Pharma Announces Completion of Subject Enrollment in Phase III Clinical Trial for KX-826 for Treatment of Male Androgenetic Alopecia in China. Available at: https://www.prnewswire.com/news-releases/kintor-pharma-announced-the-primary-endpoint-of-phase-ii-clinical-study-for-kx-826s-treatment-of-female-androgenetic-alopecia-in-china-was-met-301691321.html (Accessed: November 2025)
Oral finasteride is one of the most widely prescribed treatments for androgenic alopecia (AGA). The drug is a type II 5a-reductase inhibitor, which means it helps reduce dihydrotestosterone (DHT) – the major hormone implicated in pattern hair loss.
At the same time, finasteride isn’t the most powerful DHT-reducing drug available. In fact, there’s a drug that can reduce up to 50% more DHT than finasteride. It’s called dutasteride – and for those who are interested in unlocking another level of hair regrowth, it might be the right choice for them.
So, how much hair regrowth can we expect from dutasteride? Does dutasteride outperform finasteride? How does this drug stack up in terms of side effects? And is there anything else we should know before making the “leap” to a more powerful DHT reducer?
This ultimate guide uncovers the answers.
Interestingly, despite dutasteride’s stronger DHT-reducing effects, this drug doesn’t seem to come with an increased risk of cognitive or sexual side effects – at least when compared to finasteride. In fact, some research indicates that dutasteride might be a better option altogether.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Highlights
- Effort: Low (drug, taken orally, once daily)
- Expectations: Regrowth observed as early as 3 months.
- Regrowth rate: 5-11% in 6 months, depending on the dosage. Some studies suggest that dutasteride leads to twice the hair count increase as finasteride over a six-month timeframe, though it’s unclear whether this is two
- Response rate: 80-95%, depending on the dosage.
- Cost: $14-$160 per month.
- Problems: Results contingent upon lifelong use; if taken at higher dosages and for long periods, DHT may remain suppressed 6+ months after stopping treatment; risk of side effects similar to finasteride
Key Takeaways
Dutasteride is a drug used most commonly in the treatment of benign prostate hyperplasia. But it’s also used off-label as a treatment for male pattern hair loss.
Dutasteride is a dual 5α-reductase inhibitor, meaning that the drug inhibits both the type I and type II isoforms of the 5α-reductase enzyme. This makes it unique from finasteride, a drug that only inhibits type II 5-alpha reductase. And since dutasteride inhibits more 5-alpha reductase, it can reduce DHT levels much more effectively than finasteride. Specifically, dutasteride appears to reduce systemic DHT levels by 92-96% and scalp DHT levels by 51-79%; whereas finasteride reduces systemic DHT by 60-70% and scalp DHT by 50-60%.
When it comes to hair regrowth, short-term studies suggest that dutasteride is anywhere from 2-5 times more effective than finasteride (depending on the dose). However, the studies comparing dutasteride and finasteride are generally over six-month periods. This may not make for a fair comparison, as finasteride tends to take two years to achieve its full effect.
Despite being more effective, dutasteride doesn’t seem to come with an increased risk of side effects relative to finasteride. In fact, in some instances, dutasteride’s risk of side effects is actually lower than that of finasteride… despite a more substantial decrease in systemic DHT. While it’s debated why this is the case, one explanation might involve dutasteride’s molecular weight, which is much heavier than finasteride, so much so that the drug cannot likely cross the blood-brain barrier.
If you opt for dutasteride, research suggests that for treating pattern hair loss, a dosage of 0.5 mg daily is best. At this dose, dutasteride seems to outperform finasteride and not result in long-term DHT suppression after stopping the medication. At higher dosages (i.e., 2.5 mg daily), DHT can remain suppressed for 6+ even after stopping treatment.
What is dutasteride?
Dutasteride is a drug used to treat a variety of androgen-related problems in men. It’s branded as Avodart®, and it’s most commonly prescribed for the treatment of benign prostate hyperplasia (BPH). But since it’s such a powerful DHT-reducing drug, many doctors will prescribe it “off-label” to treat male pattern hair loss, or androgenic alopecia (AGA).
How does dutasteride reduce DHT?
There are dozens of ways to reduce DHT levels – from lowering overall testosterone to inhibiting enzymes to blocking androgen receptors to increasing DHT metabolism (to name just a few). Some of these methods are safe; some of them are dangerous; some of them aren’t studied well enough in humans to consider trying.
The good news? Dutasteride reduces DHT through one of the better studied (and safer) ways… and similarly to finasteride. It inhibits the enzyme that converts testosterone into DHT: the 5-alpha reductase enzyme.
What makes dutasteride unique? It reduces type I and type II 5-alpha reductase
Dutasteride differs from finasteride in one key way: it reduces more DHT than finasteride. This is because dutasteride inhibits both type I and type II 5-alpha reductase, whereas finasteride inhibits only the type II 5-alpha reductase enzyme.
Both isoforms of 5-alpha reductase – type I and type II – help convert free testosterone into DHT. However, both function at different pH levels, and both are found in different tissue locations throughout the body:[1]Gerst, C., Dalko, M., Pichaud, P., Galey, J.B., Buan, B., Bernard, B.A. (2002). Type-1 steroid 5 alpha-reductase is functionally active in the hair follicle as evidenced by new selective inhibitors … Continue reading
- Type I 5-alpha reductase is found in the skin, liver, and in three specific places in the hair follicle: the outer root sheath, the dermal papilla, and the matrix cells (a.k.a the cells that produce the hair shaft).
- Type II 5-alpha reductase is predominantly located in the prostate, genital skin, seminal vesicles, epididymis, and liver. But it’s also found in hair follicles.
When it comes to skin tissues near and within hair follicles, the type I isoform dominates… at least in terms of the quantity of enzymes present. But interestingly, it’s the type II isoform that tends to get all of the attention when it comes to male pattern hair loss (AGA).
This is because type II 5-alpha reductase activity in the scalp seems to be a prerequisite for male AGA. This was confirmed back in the 1970s, when studies showed that men born without the genes that make type II 5-alpha reductase also never lose their hair to AGA later in life.[2]Imperato-McGinley, J., Miller, M., Wilson, J.D., Peterson, R.E., Shackleton, C., Gajdusek, D.C. (1991). A cluster of male pseudohermaphrodites with 5-alpha-reductase deficiency in Papua New Guinea. … Continue reading
In fact, these studies were what built the basis for finasteride – a drug that is now the most popular (and most studied) FDA-approved treatment for AGA. After all, finasteride is a type II 5a-reductase inhibitor, and it’s this specific enzyme that seems directly implicated in DHT-related hair follicle miniaturization.
Dutasteride does the exact same thing as finasteride – except it also inhibits both the type I and type II isoforms of 5-alpha reductase. And, since the body’s total DHT levels are closely linked to the sum of type I and type II isoform activity, dutasteride can technically reduce more DHT than finasteride – around 50% more (depending on the tissue being measured).
If dutasteride also inhibits the type I isoform and reduces more DHT, is it also better than finasteride at regrowing hair?
Maybe.
Several studies have demonstrated that dutasteride reduces DHT levels more than finasteride– at least in tests done on serum (i.e., blood) levels of DHT.[3]Bramson, H.M., Hermann, D., Batchelor, K.W., Lee, F.W., James, M.K., Frye, S.V. (1997). Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. The Journal of pharmacology and … Continue reading In fact, a study found that while finasteride reduces serum DHT levels by ~70%, dutasteride reduces them by ~90% (depending on the dose):[4]Clark, R.V., Hermann, D.J., Cunningham, G.R., Wilson, T.H., Morrill, B.B., Hobbs, S. (2004). Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual … Continue reading
Figure 1: Change in DHT from baseline. Dutasteride vs. finasteride.[5]Clark, R.V., Hermann, D.J., Cunningham, G.R., Wilson, T.H., Morrill, B.B., Hobbs, S. (2004). Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual … Continue reading
But do these results also hold for scalp DHT – the region where DHT reduction matters most?
At least so far, the data suggests yes. Scalp biopsy studies on men taking dutasteride versus finasteride show that at certain dosages, dutasteride reduces much more DHT.[6]Olsen, E.A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M.L., Wilson, T., Rittmaster, R.S., Dutasteride Alopecia Research Team. (2006). The importance of dual 5alpha-reductase … Continue reading
Again, this makes logical sense. After all, type I and type II 5-alpha reductase are found inside and surrounding our hair follicles. And since dutasteride reduces both of these enzymes, this drug should technically reduce more scalp DHT than finasteride.
Thus, it’s totally rational to presume that since:
- DHT is causally linked to pattern hair loss, and…
- Finasteride helps treat pattern hair loss by reducing DHT, and…
- Dutasteride technically reduces more overall DHT + scalp DHT than finasteride…
Then all else equal, dutasteride should be a more effective hair loss drug.
But is this what the clinical research suggests? In order to answer this question, we’ll need to evaluate dutasteride’s hair-regrowing abilities against its risk profile: its reports of side effects, and how this does (or doesn’t) differ from finasteride.
Dutasteride versus finasteride: clinical trials (hair regrowth)
While there have been a handful of studies that have researched dutasteride, there are only four studies that provide data relevant to this question. We’ve compiled these studies as well as their methodologies, results, and conclusions into the chart below.
Studies Comparing the Effectiveness of Dutasteride and Finasteride
High quality study?
Daily dosages used Length of study Average hair count changes with dutasteride (%) Average hair count changes with finasteride (%) Any other notable results? Superior treatment?
Study #1.[7]Olsen, E.A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M.L., Wilson, T., Rittmaster, R.S., Dutasteride Alopecia Research Team. (2006). The importance of dual 5alpha-reductase … Continue reading Yes, randomized, placebo-controlled
Dutasteride: 0.1 mg, 0.5 mg,
2.5 mg
Finasteride:
5 mg
24 weeks 0.1 mg: 8.6% 0.5 mg: 10.1%
2.5 mg: 11.3%
8.3% The amount of patients who achieved at least 10% increase in hair count increases with dutasteride dose. In other words, as dose increased, so did the amount of patients who achieved at least 10% increase in density Dutasteride 0.5 mg and 2.5 mg
Study #2.[8]Shanshanwal, S.J.S., Dhurat, R.S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled … Continue reading Yes, randomized controlled trial
Dutasteride: 0.5 mg Finasteride: 1 mg
24 weeks 11% 2% 24% increase in the number of thick, terminal hairs with dutasteride vs. 4% with finasteride Dutasteride
Study #3.[9]Harcha, W.G., Martinez, B.J., Tsai, T-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy … Continue reading Yes, randomized, placebo-controlled trial
Dutasteride: 0.02 mg 0.1 mg
0.5 mg
Finasteride: 1 mg
24 weeks 0.02 mg: 2.3% 0.1 mg: 8%
0.5 mg: 11.7%
7.5% 0.5 mg dosage of dutasteride was superior to 1mg finasteride in increasing hair thickness. Dutasteride at 0.5 mg
Study #4.[10]Choi, G-S., Sim, W-Y., Kang, H., Huh, C.H., Lee, Y.W., Shantakumar, S., Ho, Y-F., Oh, E-J., Duh, M.S., Cheng, W.Y., Bobbili, P., Thompson-Leduc, P., Ong, G. (2022). Long-Term Effectiveness and Safety … Continue reading Yes, multicenter long-term retrospective study
Dutasteride: 0.5 mg/day Finasteride: 1 mg/day
≥3 years (mean 3.4 years) Hair count data not reported (too sparse; BASP classification used instead) N/A Dutasteride is significantly superior across BASP basic, basic M, and V types. Dutasteride
Here’s what these results tell us about dutasteride:
- Dutasteride, at a dosage of 0.5 mg, has a clear advantage over both 1 mg and 5 mg of finasteride over the course of 24 weeks.
- Much like finasteride, a higher dose doesn’t necessarily equal better results. In fact, among two different studies, a 2.5 mg dose showed no better efficacy over a 0.5 mg dose. However, a higher dose appears to increase the number of users who will achieve at least a 10% increase in density.
- Dutasteride doesn’t just increase the number of hairs present; it also seems to increase the thickness of existing hairs, and more effectively than finasteride. This suggests that dutasteride may be a better drug for reversing hair follicle miniaturization.
The most interesting finding, though? Despite the fact that 2.5 mg of dutasteride reduces scalp DHT by almost 30% more than 0.5 mg, this doesn’t necessarily translate to more hair regrowth. In fact, the difference in efficacy between the two doses doesn’t even reach statistical significance.
This suggests that even when we reduce DHT to a greater degree and where it’s most important (the scalp), we don’t necessarily see evidence that this increases efficacy. In other words, maybe reductions to scalp DHT don’t always correlate to increased hair regrowth. Maybe there is a degree of pattern hair loss that stems directly from serum DHT, not just scalp DHT. Dr. George Cotsarelis (of PGD2 fame) has mentioned this before in interviews. Hopefully, with more research, we’ll have a clearer answer.
Nonetheless, these trials still tell us that dutasteride, at various doses, is more effective than finasteride – and anywhere between 2- and 5-fold more effective (depending on the doses).
The problem with these trials? Many are relatively short (24 weeks)
The one caveat to all but one of these comparative studies is that they were conducted over a 24-week period. This makes it difficult to discern just how effective dutasteride versus finasteride is… mainly because finasteride is notoriously slow at regrowing hair.
For instance, studies show that men taking finasteride generally just start to see improvements around the 3-6 month mark.[11]Price, V.H., Menefee, E., Sanchez, M., Ruane, P., Kaufman, K.D. (2002). Changes in hair weight and hair count in men with androgenetic alopecia after treatment with finasteride, 1 mg, daily. Journal … Continue reading These studies also show that finasteride takes 2+ years to take full effect on our hair, with scalp hair counts increasing an average of 10% over this two-year period before plateauing.
That’s 96 weeks for finasteride to demonstrate full efficacy, not 24 weeks – the length of time for all studies comparing dutasteride to finasteride. If, for unforeseen reasons, dutasteride efficacy plateaus after six months, then maybe finasteride catches up in the long run. If dutasteride continues its trajectory at the same pace as finasteride, then maybe it’s actually 2- to 5-times more effective than finasteride… and we just don’t know it yet.
In any case, we can surmise that:
- Dutasteride works faster than finasteride. Meaning, both will achieve similar density gains, but dutasteride can achieve this in about a quarter of the time.
- It’s very possible (and probable) that dutasteride increases hair counts by even more over a longer period of time. In other words, if we studied dutasteride for the same amount of time as finasteride, there’s a chance it might be more effective. This is further demonstrated by members here who’ve switched from finasteride (after 5+ years of use) to dutasteride, and within months, saw even bigger hair gains (see CradleCap’s case study).
Regardless, there’s still one piece to the puzzle we haven’t examined yet: the incidence of side effects.
After all, if dutasteride is 200%-500% more effective than finasteride, but it also comes with a 200-500% increase in side effects… that may defeat the point of using it. After all, for those dealing with hair loss, we’re always trying to find treatments that strike the right balance between effectiveness versus the risk of side effects.
This begs the question: given that dutasteride reduces more DHT and seems much more effective than finasteride, does it also come with an increased risk of side effects?
The answers might surprise you.
Is there an increased risk of side effects with dutasteride?
We already know that dutasteride reduces serum DHT by larger amounts than finasteride. So, does this come with a higher risk of sexual side effects?
Several studies have attempted to answer this question. Here’s what they have to say:
Incidence of Side Effects
Average incidence of total side effects among dutasteride group vs. finasteride group Decreased libido (%) Ejaculation disorders (%) Erectile dysfunction (%) Breast enlargement (%) Breast tenderness (%) Total serum DHT reduction Study #1
4.6% vs. 2.6% Dutasteride 0.1 mg: 3%
0.5 mg: 1%
2.5 mg: 13%
Finasteride
5 mg: 4%
Dutasteride 0.1 mg: 4%
0.5 mg: 1%
2.5 mg: 1%
Finasteride
5 mg: 3%
Dutasteride 0.1 mg: 0%
0.5 mg: 0%
2.5 mg: 0%
Finasteride
5 mg: 1%
N/A N/A Dutasteride 0.1 mg: 69.8%
0.5 mg: 92%
2.5 mg: 96.4%
Finasteride
5 mg: 73%
Study #2 10% vs 10%
N/A
N/A N/A N/A N/A N/A
Study #3 11.5% vs. 13.4%
Dutasteride
0.02 mg: 8.1%
0.1 mg: 6.9%
0.5 mg: 4.9%
Finasteride
1 mg: 6.7%
Dutasteride 0.02 mg: 2.2%
0.1 mg: 4.8%
0.5 mg: 3.3%
Finasteride
1 mg: 3.9%
Dutasteride 0.02 mg: 4.3%
0.1 mg: 3.7%
0.5 mg: 5.4%
Finasteride
1 mg: 5.6%
Dutasteride 0.02 mg: 0%
0.1 mg: 0.5%
0.5 mg: 0.5%
Finasteride
1 mg: 0.6%
Dutasteride 0.02 mg: 0.5%
0.1 mg: 0.5%
0.5 mg: 0%
Finasteride
1 mg: 0%
N/A
Study #4 7.6% vs. 10.5% Dutasteride: 1.2% Finasteride: 0.7%
N/A Dutasteride: 0.4% Finasteride 0%
N/A N/A N/A
When we examine this data, here’s what we find:
- In one study, 0.5 mg and 2.5 mg of dutasteride reduced serum DHT by 19% and 23% more than finasteride, respectively. Despite this, finasteride still had a higher incidence of side effects than the various doses of dutasteride in some categories (except for the incidence of decreased libido, which appeared to affect about 13% of men in the 2.5mg group). In all other categories, the incidence of side effects was virtually the same.
- Side effects associated with dutasteride don’t appear to follow a dose-response relationship (at least in these three studies). In fact, in studies #1 and #2, a higher incidence of side effects is observed in the lowest In other words, it appears that higher doses don’t necessarily mean a higher risk of side effects.
- Overall, if we average out the total incidence of side effects over the three studies, finasteride and dutasteride have the exact same incidence of side effects: 8.7% for dutasteride and 8.6% for finasteride.
- The conclusion? Dutasteride is more effective at increasing hair counts over a 6-month period, all without a higher risk of side effects.
Moreover, here are some other findings from these studies that are not reported within that table:
- The most common side effect was observed in study #1’s 2.5 mg dutasteride group – about 13% reported decreased libido. What the table doesn’t tell you is that more than half of the men reporting decreased libido also reported that it spontaneously resolved over the course of the study. In other words, it resolved even while taking dutasteride. Moreover, an additional 22% reported libido recovery after cessation of use.
- For men reporting side effects with dutasteride, almost all of these men reported resolution while still taking dutasteride.
- In contrast, men taking finasteride did not see a resolution of side effects during treatment. Most did, however, report resolution after the study was over.
Again, these side effects are only derived from clinical trials conducted over the course of 6 months. So, these studies alone don’t really tell us what might happen over longer periods of use. And considering that, much like finasteride, dutasteride likely has to be taken for a lifetime to sustain its effectiveness – this really matters.
So, is there any insight as to what the incidence of side effects might be after 6 months?
Well, when we examine the research, we find that the incidence of side effects actually decrease over time with dutasteride use. For example, one four-year trial employing a daily 0.5 mg dose demonstrated that the incidence of side effects decreased from 6% in year one to a marginal 0.4% by year four.
We could argue that this shift is simply a consequence of our olfactory wiring + change blindness – whereby we have a tendency to only notice changes (i.e., new smells) when they first arrive, and whereby if changes occur too slowly, we generally won’t notice them at all.
At the same time, we could also argue that with long-term use of dutasteride, our bodies simply rewire and find ways for testosterone (and other hormones) to replace the roles previously fulfilled by DHT. This is generally how the body works – there are dozens of “fallback” mechanisms in case a vitamin, mineral, or hormone is missing. In other words, we’re more durable than we think.
Why does dutasteride come with an equivalent (or lower) risk of side effects?
It’s puzzling that dutasteride seems to come with an equivalent (or lower) risk of side effects… and that over time, these side effects seem to decrease more than with finasteride. This is in spite of dutasteride reducing much more DHT than finasteride. What could explain these paradoxical findings?
Well, one could argue that maybe DHT reduction isn’t necessarily as great of a predictor of side effects as we think. But, there is one additional explanation for these findings, and one that’s much more interesting to explore…
It’s the actual structure of the dutasteride molecule itself… and the fact that the size of this molecule might greatly reduce the drug’s accessibility to certain organs within our body. Specifically, the brain.
Why might the molecular characteristics of dutasteride influence the risk of side effects?
The blood is the ultimate transporter for nutrition. It’s also the transporter for oral drugs.
During circulation, the blood will deposit various compounds into cells, where they then elicit their specific effects. However, in the brain, it isn’t quite as simple. This is because of a membrane called the blood-brain barrier (BBB).
This membrane is comprised of cells that maintain tight cellular junctions. In other words, these cells are packed in close together with minimal space between each cell. As such, only very small compounds or compounds that can bind to specific entry receptors on the BBB can pass through the membrane into the brain.
In terms of molecular size, a given compound has to be smaller than 400 daltons (a measurement of mass) to traverse past this membrane. If any larger? The compounds cannot theoretically enter the brain through blood transportation. Therefore, these molecules may not be able to elicit their specific effects on the brain.
So, what does this have to do with dutasteride and finasteride? And why might this explain the difference in the incidence of side effects?
Interestingly, while both finasteride and dutasteride act similarly with respect to DHT reduction, they are actually quite different on a molecular level – particularly regarding their molecular weight.
While finasteride is only 372 daltons, dutasteride is 528 daltons.
The biggest difference here? Finasteride is small enough to pass through the tight junctions of the blood-brain barrier (BBB). Dutasteride isn’t.
As such, finasteride may elicit effects on the brain itself, which may precipitate major effects on sexual and mental side effects. In contrast, dutasteride may have less of a direct effect on the brain… or a reduced capacity to effect androgen metabolism in the brain relative to finasteride.
Again, this is all hypothetical, and the science is still very much debated. For instance, this study on mice found that dutasteride does influence androgen metabolism in the brain (although that study also found that dutasteride had neuroprotective effects on mice, helping to stave off Parkinsons).[12]Litim, N., Morissette, M., Caruso, D., Melcangi, R.C., Di Paolo, T. (2017). Effect of the 5α-reductase enzyme inhibitor dutasteride in the brain of intact and Parkinsonian mice. The Journal of … Continue reading
At the same time, with dutasteride use, some reduction in brain androgen activity is technically to be expected… even if dutasteride doesn’t technically enter the brain. Why? Because 5-alpha reductase can be transported via the blood (where dutasteride does elicit an effect). And if blood entering the brain has less 5-alpha reductase activity, then this would also change androgen activity in the tissues where that blood eventually gets transported (i.e., the brain).
In any case, our suggestion here is that dutasteride itself has a harder time entering into brain tissues, and that this is why it may confer to a lower risk of side effects (despite more DHT reduction) than finasteride.
So, despite the fact that dutasteride has a more powerful effect on both systemic DHT than finasteride, the lower or on-par incidence of side effects may simply be due to dutasteride’s molecular weight… and how this dampens the drug’s ability to enter brain tissue.
Thus, it appears we can derive faster, more substantial hair regrowth in AGA… all with the same or even lower risk of side effects. Moreover, we can even leverage dosages to both maximize regrowth and minimize the risk of side effects.
Dutasteride: best candidates + best practices (i.e., dosages)
It goes without saying that dutasteride is a drug that is appropriate only for one specific kind of hair loss: androgenic alopecia (pattern hair loss). It also goes without saying that this drug is studied almost exclusively on men. Therefore, men with pattern hair loss are the best candidates.
So, for men with pattern hair loss, what’s the best dose?
Well, revisiting data from the three studies comparing dutasteride to finasteride, we see that larger doses of dutasteride are generally no more effective than a dosage of 0.5 mg. And based on the safety data, this appears to be one of the safest doses, too.
Specifically, the studies show that 0.5 mg can increase hair counts by anywhere between 10 and 11.5% in 6 months, with just a 1.3% overall risk of sexual side effects. In contrast, finasteride’s risk of side effects ranges from 2-13% with regrowth rates only around 7-8%.
So, a daily dose of 0.5 mg of dutasteride orally seems to both maximize hair regrowth and minimize the risk of side effects. Moreover, it does so in a safer and more effective manner than both 1 mg and 5 mg doses of finasteride.
Finally, a 0.5 mg daily dose is also a dose that allows for our DHT levels to bounce back within six months after stopping the drug. Because of dutasteride’s longer half-life, it tends to exert a longer effect in the body. At higher dosages, it seems as though DHT levels remain suppressed for the majority of users, at least for six months of quitting. A 0.5 mg daily dose prevents this from happening, allowing DHT to return to 80% of baseline levels after six months of cessation, and likely 100% baseline levels a few months thereafter.
Want an example of a long-term dutasteride user? See Ashton Kutcher.
If you’d like an example of a long-term dutasteride user, look no further than Ashton Kutcher.[13]Team Coco. (2018). Ashton Kutcher’s Hair Is Starting To Go | CONAN on TBS. Available at: https://www.youtube.com/watch?v=Wv9gu1zJdRo (Accessed: November 2025)
During his 2018 interview with Konan, he revealed that he had used the drug for years, only hopping off to father his children (born in 2014 and 2016). It seems to have worked wonderfully for him. Just look at his hairline in 1998 versus today. While there’s no information about whether he has returned to using dutasteride, it also doesn’t seem like his hairline has changed much over the past several years anyway.
Summary
Finasteride is one of the most powerful and frequently used therapies for AGA. However, emerging research indicates that dutasteride may have a clear advantage over finasteride, at least in terms of (1) scalp DHT reduction, (2) hair regrowth, and (3) side effects.
Specifically, a 0.5 mg daily dose appears to increase hair density by anywhere between 10 and 11.5% over the course of 6 months, which happens to be the hair count increase expected from 2+ years of finasteride use. Moreover, dutasteride seems to achieve this without greatly increasing someone’s risk of cognitive or sexual side effects. In fact, the incidence of side effects with dutasteride seems to decrease over time, with one study demonstrating just a 0.4% incidence of side effects with four years of use.
While higher doses (up to 2.5 mg) do increase the response rate to the treatment, they don’t appear to increase the average amount of hair someone regrows. Moreover, they also increase the likelihood of long-term DHT suppression post-treatment. As such, a 0.5 mg daily dose seems like the best approach when utilizing this drug for hair regrowth.
Considering these overall advantages, cost-effectiveness, and accessibility, dutasteride shows considerable promise for men with AGA.
If you’re interested in taking dutasteride or swapping out your oral finasteride, talk with your doctor about the research. If you’re a good candidate for the drug, ask them to prescribe you a 0.5 mg daily dose, and please keep us posted with your results!
References[+]
References ↑1 Gerst, C., Dalko, M., Pichaud, P., Galey, J.B., Buan, B., Bernard, B.A. (2002). Type-1 steroid 5 alpha-reductase is functionally active in the hair follicle as evidenced by new selective inhibitors of either type-1 or type-2 human steroid 5 alpha-reductase. Experimental Dermatology. 11(1). 52-58. Available at: https://doi.org/10.1034/j.1600-0625.2002.110106.x. ↑2 Imperato-McGinley, J., Miller, M., Wilson, J.D., Peterson, R.E., Shackleton, C., Gajdusek, D.C. (1991). A cluster of male pseudohermaphrodites with 5-alpha-reductase deficiency in Papua New Guinea. Clinical Endocrinology. 34(4). 293-298. Available at: https://doi.org/10.1111/j.1365-2265.1991.tb03769.x. ↑3 Bramson, H.M., Hermann, D., Batchelor, K.W., Lee, F.W., James, M.K., Frye, S.V. (1997). Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. The Journal of pharmacology and experimental therapeutics. 282(3). 1496-1502. Available at: PMID 9316864 ↑4, ↑5 Clark, R.V., Hermann, D.J., Cunningham, G.R., Wilson, T.H., Morrill, B.B., Hobbs, S. (2004). Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. The Journal of Clinical Endocrinology & Metabolism. 89(5). 2179-2184. Available at: https://doi.org/10.1210/jc.2003-030330 ↑6, ↑7 Olsen, E.A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M.L., Wilson, T., Rittmaster, R.S., Dutasteride Alopecia Research Team. (2006). The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. Journal of the American Academy of Dermatology. 55(6). 1014-1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑8 Shanshanwal, S.J.S., Dhurat, R.S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study. Indian Journal of Dermatology, Venereology and Leprology. 83(1). 47-54. Available at: https://doi.org/10.4103/0378-6323.188652 ↑9 Harcha, W.G., Martinez, B.J., Tsai, T-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. Journal of the American Academy of Dermatology. 70(3). 489-498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑10 Choi, G-S., Sim, W-Y., Kang, H., Huh, C.H., Lee, Y.W., Shantakumar, S., Ho, Y-F., Oh, E-J., Duh, M.S., Cheng, W.Y., Bobbili, P., Thompson-Leduc, P., Ong, G. (2022). Long-Term Effectiveness and Safety of Dutasteride versus Finasteride in Patients with Male Androgenic Alopecia in South Korea: A Multicentre Chart Review Study. 34(5). 349-359. Available at: https://doi.org/10.5021/ad.22.027 ↑11 Price, V.H., Menefee, E., Sanchez, M., Ruane, P., Kaufman, K.D. (2002). Changes in hair weight and hair count in men with androgenetic alopecia after treatment with finasteride, 1 mg, daily. Journal of the American Academy of Dermatology. 46(4). 517-523. Available at: https://doi.org/10.1067/mjd.2002.120537 ↑12 Litim, N., Morissette, M., Caruso, D., Melcangi, R.C., Di Paolo, T. (2017). Effect of the 5α-reductase enzyme inhibitor dutasteride in the brain of intact and Parkinsonian mice. The Journal of Steroid Biochemistry and Molecular Biology. 174. 242-256. Available at: https://doi.org/10.1016/j.jsbmb.2017.09.021 ↑13 Team Coco. (2018). Ashton Kutcher’s Hair Is Starting To Go | CONAN on TBS. Available at: https://www.youtube.com/watch?v=Wv9gu1zJdRo (Accessed: November 2025) Dutasteride is one of the strongest off-label treatments for androgenic alopecia. In this guide, we’ll explore the evidence supporting two delivery methods for dutasteride: topical dutasteride and mesotherapy dutasteride. We’ll also provide clear step-by-step instructions for anyone looking to try these therapies at home or find clinicians willing to do the procedures.
Overview: Topical / Mesotherapy Dutasteride
- Effort: Low (once weekly-to-monthly applications, depending on topical or mesotherapy delivery).
- Expectations: Hair improvements within 4-6 months.
- Response rate (i.e., the percent of people who experience a slowing, stopping, or partial reversal of AGA):
- Topical dutasteride: 70-90%
- Topical dutasteride + microneedling: 60-80%
- Mesotherapy dutasteride: 60-80%
- Regrowth rates (i.e., the percent change to hair counts, hair diameters, and hair density (i.e., ∆hair counts x ∆hair diameters)):
- Topical dutasteride: +23-25% increase in hair counts (mean +75 hairs/cm² vs baseline in the 0.05% group over 24 weeks).
- Topical dutasteride + microneedling: 10-15% increase in hair counts, 15-20% increase in hair density (sometimes more)
- Mesotherapy dutasteride: 10-15% increase in hair counts, 15-20% increase in hair density (sometimes more)
- Advantages: Easy to administer; works for the majority of people; minimizes DHT reductions elsewhere in the body; likely confers with a lower side effect profile.
- Disadvantages: Clinical studies are of lower quality; no standardization across mesotherapy needle depths or dutasteride exposure volumes per session; high variability between patients regarding systemic absorptions puts the onus on each patient to test serum DHT levels before/during treatment; expensive.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Key Takeaways
- The Good News:
- Topical / mesotherapy dutasteride works. While the evidence is limited and of lower quality, multiple studies from different research groups all suggest that topical dutasteride and mesotherapy dutasteride improve hair loss outcomes for men and women with androgenic alopecia.
- Topical / mesotherapy dutasteride may minimize unwanted declines in serum DHT. Multiple studies (while not perfectly designed) suggest, when dosing is done properly, that topical dutasteride and/or mesotherapy dutasteride can improve hair parameters while only modestly reducing serum DHT levels (a marker for systemic absorption). This has been confirmed by members here who’ve done serum DHT tests before/after treatment and seen only 10-15% declines in DHT. Moreover, absorption studies testing 1 mL of 0.025% topical dutasteride (i.e., 0.25 mg) applied to the scalp show, after 12 hours, that ~7% of the drug enters the bloodstream while 25% of the drug remains within the dermis of the scalp. This means that within proper exposure volumes, we can maximize DHT reduction in the scalp while minimizing the effects of DHT reduction elsewhere in the body.
- No sexual side effects (yet) reported in the clinical data. While the currently available studies have small sample sizes, all investigations on topical / mesotherapy dutasteride have yet to have a participant report sexual side effects. This experience is echoed by members here who’ve adhered to the usage ad frequency parameters of both topical and mesotherapy dutasteride.
- Critical Unknowns:
- How does topical dutasteride and mesotherapy dutasteride compare to each other (and other therapies)? Due to lacking head-to-head clinical studies, we don’t yet have an answer. However, directional hair count data across studies suggest that topical / mesotherapy dutasteride is probably less powerful than its oral counterpart. (This comparison is unscientific, but it’s all we can currently glean.)
- What’s the best dosage, frequency of application, and carrier agents? While low-exposure volumes of topical / mesotherapy dutasteride show minimal effects on serum DHT, we don’t yet know the perfect dosing schedule to maximize hair regrowth while minimizing serum DHT reductions. It’s likely that leakage from the scalp and systemic absorption vary depending on the individual and the treatment methodologies: age, genetics, mode of delivery, dosage per application, application frequency, and application volume are just a few of the dozens of factors impacting the answers to these questions.
- How does topical dutasteride perform as a standalone therapy? Early data suggest that low-dose topical dutasteride (including 0.05%) can improve hair parameters and may, in some trials, appear comparable or even superior to oral finasteride, but methodological limitations and conflicting real-world experiences mean these findings should be interpreted cautiously until independently replicated.
- Best Practices & Recommendations
- See below.
Key Recommendations
Topical dutasteride and mesotherapy dutasteride are probably most appropriate for people who:
- Have androgenic alopecia
- Don’t want to use oral finasteride or oral dutasteride, but want to leverage a pharmaceutical 5-alpha reductase inhibitor
- Want to better localize the effects of dutasteride to scalp
- Are comfortable experimenting with delivery methods that are lesser explored and have lower levels of clinical support
- Are comfortable investing $50-$400 per month into treatment, depending on the mode of delivery (topical or mesotherapy)
- Don’t have metabolic syndrome (as one study suggested that mesotherapy dutasteride was ineffective for these patients)
Step-By-Step Guide
Topical dutasteride
If trying topical dutasteride:
- Limit topical exposure of dutasteride to 0.1-0.5mg weekly.
- This can be achieved in many ways. Consider 1 mL formulas more appropriate for localized hair loss, and 2 mL formulas more appropriate for diffuse hair loss (for more spread of liquid). Examples:
- 1 mL of 0.01% to 0.05% dutasteride, once weekly. Exposure = 0.1-0.5 mg
- 1 mL of 0.025% dutasteride, twice weekly. Exposure = 0.5 mg
- 2 mL of 0.005% to 0.025% dutasteride, once weekly. Exposure = 0.1-0.5 mg
- 2 mL of 0.005% to 0.0125% dutasteride, twice weekly. Exposure = 0.2-0.5 mg
- This can be achieved in many ways. Consider 1 mL formulas more appropriate for localized hair loss, and 2 mL formulas more appropriate for diffuse hair loss (for more spread of liquid). Examples:
- Leave in scalp for at least 4 hours, and preferably 8-12 hours
- This will allow 25% of the dutasteride applied to penetrate into the percutaneous tissues, so it can have an effect.
- This will minimize the amount of dutasteride that enters the bloodstream, thus reducing systemic absorption.
- The easiest way to do this is to apply topical dutasteride before bed and then wash it out in the morning.
- Consider combining with microneedling
- If combining with microneedling, do not apply topical dutasteride the day of microneedling
- Consider testing serum DHT levels before/during treatment
- This will help track if topical dutasteride is remaining localized to the scalp.
- In tracking DHT levels, you can increase or decrease dutasteride dosing depending on your serum DHT readouts.
- Product recommendations
- MinoxidilMax: 0.1% topical dutasteride, 60 mL.
- You can use this solvent to dilute the formula down to 0.005% to 0.02%. If you’d like help with this, ask us. We’re currently building out a “how to” video for diluting topicals that will make this process simple. But if you want to get a jumpstart on this, please don’t hesitate to ask for our help.
- MinoxidilMax: 0.1% topical dutasteride, 60 mL.
Mesotherapy dutasteride
If trying mesotherapy dutasteride:
- Start with mesotherapy injections once monthly.
- After 6+ months, move to one session every 1-3 months.
- If once monthly injections are infeasible, opt for injections once every three months.
- Limit dutasteride exposure to 0.1-0.2 mg per session.
- This is equivalent to 1-2mL of 0.01% dutasteride
- Consider testing serum DHT levels before/during treatment
- This will help track if topical dutasteride is remaining localized to the scalp.
- In tracking DHT levels, you can increase or decrease dutasteride dosing depending on your serum DHT readouts.
How do I track down a clinician who can provide mesotherapy dutasteride?
It’s not easy, but it can be done.
Step #1: Build a list of practitioners offering mesotherapy in your locale
Run a google search for dermatologists and/or medical spas in your area. When checking out their websites, make sure they offer mesotherapy. It’s okay if the mesotherapy they offer is not related to hair loss. All that matters is that they offer mesotherapy, because that means they will have a mesotherapy gun in their office.
Track down their names and contact information via contact forms and emails listed on their sites. Try to build a list of 5-10 clinicians within travel distance.
Step #2: Send each practitioner this personalized email
Here’s an email script that has given us some success. Please personalize as necessary:
Dear [CLINICIAN],
My name is [YOUR NAME]. I’m reaching out because I’m interested in trying mesotherapy dutasteride for the treatment of androgenic alopecia. The procedures are typically done once monthly for 6+ months. I wanted to see if your offices would be willing to provide the therapy to me.
I’ve been encouraged by the results of this mini-review, suggesting that repeated mesotherapy dutasteride may improve hair loss outcomes for men with androgenic alopecia, and without significantly altering serum DHT levels.[1]Herz-Ruelas, M.E., Alvarez-Villalobos, N.A., Millan-Alanis, J.M., de Leon-Gutierrez, H., Ocampo-Garza, S.S., Grimalt, R. (2020). Efficacy of Intralesional and Oral Dutasteride in the Treatment of … Continue reading I’m planning on tracking serum DHT before/during treatment, and I’d be happy to share the blood work with you (especially as interest in this therapy is growing, it might be nice to have the data).
I’m happy to source the mesotherapy dutasteride serum. All I need is a clinician trained in mesotherapy to administer the procedure. Are you interested? If so, I can come to offices as soon as next week.
All my best,
[YOUR NAME]
[PHONE NUMBER]Step #3: Secure a practitioner
Typically, for every 5-10 clinicians who receive the above email, one will respond to say that they’re interested. Meet with these clinicians, print out and share with them the studies, and show them the methodologies (below) to standardize the procedure, thus making it as easy as possible for the doctor to administer.[2]Herz-Ruelas, M.E., Alvarez-Villalobos, N.A., Millan-Alanis, J.M., de Leon-Gutierrez, H., Ocampo-Garza, S.S., Grimalt, R. (2020). Efficacy of Intralesional and Oral Dutasteride in the Treatment of … Continue reading
Methods For Mesotherapy Dutasteride (With A Clinician):
- Injection depth: intradermal (i.e., between the epidermal and dermal skin layers, roughly 0.5-0.75 mm deep)
- Mesotherapy formulation: 0.01% dutasteride in a 10 mL vial (provider here)
- Injection volume per session: 1-2 mL (i.e., 0.1-0.2 mg of dutasteride)
- Injection frequency: once monthly for six months, then once every 1-3 months thereafter
Can I try mesotherapy at home?
To be clear, we DO NOT recommend taking a DIY approach to mesotherapy. If you cannot find a provider, we recommend trying topical dutasteride + microneedling. Topical dutasteride is far more accessible, and it’s also easily administered at home. It’s also likely that the combination produces similar results to mesotherapy dutasteride.
Under the circumstances that you decide not to heed our warnings, here are DIY instructions for mesotherapy dutasteride.
Methods for Mesotherapy Dutasteride (At-Home):
- Buy this mesotherapy device. Use the 9-needle cartridges and adjust the needle depth to 0.5mm when operating.
- Buy mesotherapy dutasteride from this provider (we lab tested their products; they meet quality and purity standards).
- Follow the instructions from the manufacturer to deliver mesotherapy to your scalp once per month.
- Consider tracking serum DHT levels before/during treatment.
Below is a scientific deep-dive on topical / mesotherapy dutasteride.
What Is Topical Dutasteride and How Does It Work?
Topical dutasteride is topical version of the oral drug, dutasteride. Whereas oral dutasteride tends to have hormonal effects all throughout the body, topical dutasteride is designed to selectively target just the scalp skin – allowing people to reap all the hair-growing benefits of dutasteride, and without the risk of systemic side effects.
At least, that’s the hope.
Specifically, dutasteride lowers the hormone dihydrotestosterone (DHT), which is causally linked to androgenic alopecia. It does so by inhibiting the enzyme that converts testosterone into DHT: 5-alpha reductase.
Dutasteride is one of the most powerful 5-alpha reductase inhibitors available. Whereas hair loss drugs like finasteride inhibit 5-alpha reductase and lower DHT levels by ~70%, dutasteride can lower DHT levels by 95%.
This is because there are three known isoforms of 5-alpha reductase: type I, II, and III. Compared to finasteride, dutasteride is more effective at inhibiting them. In fact, dutasteride is able to target the type I isoform 100x better than finasteride, and the type II isoform 3x better than finasteride.[3]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue reading
How Does Oral Dutasteride Compare To Oral Finasteride for Androgenic Alopecia?
Dutasteride’s larger effects on DHT reduction also confer better hair regrowth outcomes.
A 2019 meta-analysis by Zhou et al. found that oral dutasteride regrew more hair than oral finasteride – even at dosage ranges lower than 0.5 mg daily.[4]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue reading And while the studies in this review were relatively short (i.e., 6 months) and had sample sizes of less than 500 participants, it’s hard to ignore the preliminary evidence suggesting that oral dutasteride packs a big punch for hair regrowth – even at dosages as low as 0.1mg daily.
Figure 1: The studies cited maintain a low risk of bias, but because of the low sample size, it’s difficult to say with confidence that dutasteride is superior to finasteride.[5]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue reading
What About Safety?
Despite reducing more DHT and regrowing more hair, both finasteride and dutasteride appear to carry the same risk of side effects.
Figure 2: Forest plots showing changes in (A) altered libido, (B) erectile dysfunction, and (C) ejaculation disorders.
Abbreviation: M–H, Mantel–Haenszel.[6]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue readingWhy is this? Wouldn’t we expect dutasteride to carry a higher risk of side effects, since it reduces DHT levels more?
Generally, yes. But that’s not currently reflected in the data. Regarding why, there are many hypotheses:
- Biases in sample sizes and study durations. Perhaps dutasteride does carry a larger risk of side effects, as it has been shown to do so in some patient populations (for instance, dutasteride carries a higher risk versus finasteride for gynecomastia in patients with enlarged prostates). But perhaps the sample sizes in these hair loss studies were too small in these studies to pick up the differences. Or, perhaps the studies did not run long enough to capture all risks.[7]Hagberg, K.W., Divan, H.A., Fang, S.C., Nickel, J.C., Jick, S.S. (2017). Risk of gynecomastia and breast cancer associated with the use of 5-alpha reductase inhibitors for benign prostatic … Continue reading
- Differences in isoform side effect attribution. Perhaps the type II 5-AR enzyme (i.e., the one predominantly targeted by finasteride) is the one isoform predominantly responsible for most of the side effects associated with finasteride and dutasteride. (We’ve heard this argument before, but we don’t necessarily believe it.[8]Rahimi-Ardabili, B., Pourandarjani, R., Habibollahi, P., Mualeki, A. (2006). Finasteride induced depression: a prospective study. BMC Clinical Pharmacology. 6(7). Available at: … Continue reading
- Tissue-dependent concentration differences of isoforms. For example, in the prostate and testes, the type II isoform is expressed far more than type I. The opposite is true in brain tissue, where type I 5-AR is predominantly found. Perhaps the side effects of 5-AR inhibitors depend on how much 5-AR is inhibited in specific tissues, and at the dosages administered for dutasteride and finasteride, there just isn’t enough difference in DHT reduction to make one drug more likely to produce sexual side effects than the other.[9]Wang, K., Fan, D-D., Jin, S., Xing, N-Z., Niu, Y-N. (2014). Differential expression of 5-alpha reductase isozymes in the prostate and its clinical implications. Asian Journal of Andrology. 16(2). … Continue reading
Even still, there remains no consensus among clinicians for why this is the case.
Pharmacokinetics
How Does Dutasteride Behave In Our Bodies?
Pharmacokinetics is a term used to describe the behavior of drugs once they enter the body: how quickly they are transported to tissues, how quickly they are metabolized (i.e., their half-life), and more.
Dutasteride and finasteride, despite being structurally similar, differ dramatically in their half-lives. For instance, finasteride has a half-life of 5-8 hours. That means that after ingesting finasteride, it takes 5-8 hours before 50% of that finasteride leaves our bloodstream.
Dutasteride, on the other hand, has a half-life that can last 5+ weeks. In fact, dutasteride’s half-life depends on a lot of factors: age, genetics, and, most importantly, the amount of dutasteride given to an individual at one time.
This was investigated by Gislekog et al. (1998) by giving a dose of dutasteride ranging from 0.01mg to 40mg to 32 healthy males. The findings showed a number of distinctions:
- The half-life of the drug increases with increasing dose.
- Serum concentrations of dutasteride can last for quite a while before it is undetectable.
This is presented in the following table:
Dose Serum Concentration Duration (up to 1ng mL-1) 0.1mg Approximately one day. 1.0mg Averaging around seven days, but can be up to 14 days. 2.5mg Averaging approximately 14-21 days, but can be as long as 28 days. 5.0mg Approximately 21-28 days. 10.0mg Greater than 28 days. 20.0mg Greater than 28 days. 40.0mg Greater than 28 days. And here are the graphs from the study:Concentration of dutasteride in serum is dependent on how large a dose is used. This means that beyond half-life, the general concentration of the drug can last for quite a while when using greater than 2.5mg doses.[10]Gisleskog, P.O., Hermann, D., Hammarlund-Udenaes, M., Karlsson, M.O. (1999). The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. … Continue reading
Can Topical Dutasteride Lower Scalp DHT While Minimally Affecting Hormones Elsewhere In The Body?
This is not only possible, but plausible… provided that we play with the dosages of dutasteride and the frequencies of application.
Since dutasteride rapidly distributes across tissues and has a dose-dependent half-life, the key is to find an exposure volume of topical dutasteride that…
- Is large enough to effectively saturate the epidermal and dermal layers of the scalp (so that we can locally lower DHT)
- Is small enough such that the amount of dutasteride that leaks daily into the bloodstream is rapidly metabolized (so that DHT levels elsewhere are minimally impacted)
Encouragingly, we have some data to suggest there are pharmacokinetic “sweet spots” to achieving this.
A recent analysis measured how much of a 1 mL solution of 0.025% dutasteride (i.e., 0.25mg) applied to the scalp (1) stayed on the skin, (2) penetrated into the dermis, and (3) travelled systemically into the bloodstream.
Across 12 hours of study, here’s what the investigators discovered:
Figure 4: Effect of topical dutasteride on skin absorption and circulation entry.
If the above chart feels overwhelming, don’t worry. For now, just know that 12 hours after applying topical dutasteride:
- 60.8% of the dutasteride remained on the surface of the scalp and was unabsorbed (i.e., 0.152 mg)
- 26.2% of the dutasteride penetrated into the scalp (i.e, 0.06 mg).
- 6.4% of the dutasteride entered into the bloodstream (i.e., 0.016 mg)
This is actually good news. Why? Because 12 hours after applying 1 mL of 0.025% topical dutasteride, 0.06 mg of that dutasteride permeated into balding tissues, while only 0.016 mg entered into the bloodstream.
In balding skin tissue, 0.06 mg of dutasteride is probably:
- Enough drug to appreciably lower scalp DHT levels, and…
- Enough drug to increase the elimination half-life to > 1 day, so that the scalp DHT suppression can last for multiple days.
Simultaneously, with the amount of dutasteride reaching the bloodstream, 0.016 mg of dutasteride is probably:
- Not enough drug to appreciably lower serum DHT levels, and…
- Not enough drug to increase the elimination half-life to > 1 day. As such, any serum DHT suppression will likely be short-lived.
In other words, one application of 1 mL of 0.025% topical dutasteride might lead to impressive reductions to scalp DHT, while having minimal impact on serum DHT (and thereby DHT levels elsewhere in the body).
So, Should We All Apply 1 mL of 0.025% Topical Dutasteride, Once Or Twice Daily?
We wish it were that simple.
The problem is that the above analysis only measures the effects from one application of topical dutasteride. If we apply this dosage daily, dutasteride levels in the scalp skin will accumulate, as will the amount of dutasteride entering the bloodstream.
Additionally, after that 12-hour mark, a portion of the ~25% of dutasteride already absorbed into the skin will continue to leak into the bloodstream. The above study does not capture these effects. So, while the analysis is insightful, it unfortunately does not run long enough to reveal the full extent of systemic absorption from a single 1 mL dose of 0.025% topical dutasteride. That means total bloodstream exposure from 1 mL of 0.025% topical dutasteride is probably a bit higher than the 0.016 mg suggested by the study.
Finally, as blood levels of dutasteride increase, so too will the drug’s half-life. Remember: the more dutasteride present, the longer the half-life. So, after a few weeks of daily application, it’s not unreasonable to expect serum drug exposure to accumulate and thereby serum DHT levels to substantially decrease. This is exactly what most people trying topical dutasteride want to avoid!
Fortunately, we don’t have to rely exclusively on mathematical modeling to figure this out. There are clinical studies testing topical dutasteride for the treatment of androgenic alopecia – some of which measured not only hair growth, but also changes to serum DHT levels before and after treatment.
Topical Dutasteride: Clinical Studies (Hair Counts, Serum DHT, & More)
Nada et al. (2018): Microneedling + Topical Dutasteride vs. Microneedling Alone
In a 2018 clinical study, researchers sought to determine how topical dutasteride + microneedling fared versus microneedling alone.[11]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. … Continue reading
So, they recruited 30 males with androgenic alopecia, randomized them into two groups, and tracked hair parameter changes of a six-month period.
Using a Dermapen with a 12-gauge needle cartridge and a 1.5mm needle length, both groups received 13 microneedling sessions over six months, and using the following schedule:
- Once per week (weeks 0-7)
- Once every two weeks (weeks 9 and 11)
- Once every four weeks (weeks 15, 19, and 23)
However, during each microneedling session, the topical dutasteride group also received up to 2 mL of 0.02% topical dutasteride (i.e., 0.4mg) applied to the scalp.
After six months, the topical dutasteride + microneedling group saw greater improvements in hair density versus the microneedling-only group. This equated to a 12% increase in hair density.
Figure 5: Changes of hair density in the study groups before and after treatment.[12]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. … Continue reading
Additionally, the topical dutasteride + microneedling group also saw hair thickness improve by nearly 100%.
Figure 6: Changes in hair caliber width in the study groups before and after treatment.[13]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. … Continue reading
Finally, hormonal testing revealed that the topical dutasteride + microneedling group did experience a 5.3% reduction in serum levels of DHT. However, this level of DHT reduction is considered biologically insignificant. Keep in mind that serum DHT levels can fluctuate as much as 20-40% daily, depending on if the measurements were taken during the morning or night. So a ~5% change in DHT isn’t very concerning.
Figure 7: Hormone levels before and after treatment.[14]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. … Continue reading
Our Analysis
While these results are encouraging, they’re also problematic. Specifically, the results are obfuscated by a methodological concern: staggered microneedling + topical dutasteride sessions, and no measurements for serum DHT during shorter durations between dutasteride applications.
Throughout the study, time gaps between topical dutasteride applications grew. For the first 8 weeks, topical dutasteride was applied once weekly. But for the last 12 weeks, it was applied once monthly. The investigators only reported changes to serum DHT before/after the study concluded. Specifically, they recorded serum DHT before the study began, and one week after their final dutasteride application. So, are these results really representative of serum DHT levels throughout the study… or were serum DHT levels much lower in the first few months when application frequency was 4x higher?
Unfortunately, the investigators do not give us enough information to know the answers to these questions. We’re reaching out and hope to have answers for you soon.
In the interim, we’ve had several members emulate the methodologies of this study, test serum DHT levels before/during treatment, and adhere to a once-weekly topical dutasteride application rate (at up to 2 mL of 0.025% dutasteride, that’s up to 0.4mg of weekly dutasteride exposure).
The good news is that all of these members reported only 10-15% decreases in serum DHT. While those decreases are likely partly due to serum dutasteride, they’re still within the realm of biologically insignificant. And encouragingly, our members’ results not only align with the ballpark results of this study, but also the previous dosing analysis – at least in terms of expectations for systemic leakage.
In our eyes, these are all positive signs. They’re preliminary signals (validated by some members) that at weekly dosages of up to 2 mL x 0.02% topical dutasteride (i.e., 0.4 mg per week), we can mostly preserve serum DHT levels – even when adding in microneedling.
Encouragingly, a 2022 study also validated this approach – at least in terms of efficacy.
Sanchez et al. (2022): Microneedling + Topical Dutasteride vs. Microneedling Alone
In 2022, investigators conducted another study on topical dutasteride + microneedling, albeit without serum DHT and with slightly different methodologies.[15]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
These investigators randomized 34 men into two groups. One received microneedling + 1 mL of 0.01% topical dutasteride (i.e., 0.1 mg). The other received microneedling + 1 mL of saline solution (i.e., water).
Both groups were treated once every four weeks for a total of three sessions. In both groups, the investigators used a Dr. Pen A6 Ultimate microneedling device and set the needle depth to 2.5mm.
Hair parameters were assessed 4-8 weeks later, thus bringing the study duration to 20 weeks (despite each group only having received three microneedling + topical treatments).
Encouragingly, over 50% of people in the microneedling + topical dutasteride group observed “marked improvement” in their hair loss. Hair thickness, hair density, and the ratio of vellus:terminal hairs also improved versus the microneedling-only group.
Figure 8: Results from a 2022 study on microneedling + dutasteride (left column) versus microneedling alone (right column).[16]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
While serum DHT levels were not measured, there were no reports of sexual side effects from participants.
Our Analysis
This is another positive (albeit preliminary) signal that topical dutasteride can produce results and reduce our risk of adverse events (and drug exposure) – especially when paired with microneedling. Just see these before-and-after photos from one study participant:
Figure 9: (a). Vertex area trichoscopy and clinical photograph of a patient with androgenetic alopecia (Hamilton-Norwood Scale III) at baseline visit. (b). Frontal area trichoscopy and clinical photograph showing marked improvement (HamiltonNorwood Scale II) at week 16 after 3 sessions with microneedling plus topical 0.01% solution of dutasteride in monotherapy.[17]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
Another encouraging component of this study is that the total dutasteride dosage was lower than the 2018 study, yet still produced results. Whereas the 2018 study used dosages as high as 0.4mg weekly (at least for the first 8 weeks), this 2022 study used dosages equating to 0.1mg once every four weeks. If we are to take the minimal serum DHT changes from the 2018 study at face-value, then it’s even more unlikely that the dutasteride volumes used in this 2022 study led to appreciable changes to serum DHT.
Another study published this year (2025) also gave some insight into low topical dosages of dutasteride in people with AGA.
Panuganti et al, 2025: Topical Dutasteride at 0.01%, 0.02%, and 0.05% w/v
The investigators randomized 135 men with AGA into five groups: 0.01%, 0.02%, or 0.05% topical dutasteride (1 mL once daily), 1 mg oral finasteride daily, or a topical placebo. Treatments were continued for 24 weeks, with the topical solutions applied to a marked vertex target area.[18]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate … Continue reading
Baseline characteristics were balanced across groups. Efficacy was assessed via macrophotography and target area hair counts (TAHC), global photographic assessment, and patient satisfaction questionnaires.
At 24 weeks:
- The 0.05% topical dutasteride group showed the largest mean increase in TAHC, reported as +75.5 hairs/cm² vs baseline (p=0.0001), compared with +41.2 hairs/cm² in the oral finasteride group (p=0.0001) and essentially no change in the placebo group (+0.07 hairs/cm², p=0.9957).
- The 0.01% and 0.02% topical groups improved versus placebo, but did not outperform oral finasteride.
- Hair shaft width improved in a dose-dependent manner; 0.05% topical dutasteride increased hair width by 11.6 µm vs placebo at week 24 (p=0.0185).
- Patient satisfaction and investigator global assessments were highest in the 0.05% group.
- The study also evaluated serum testosterone and DHT. Reported changes with topical dutasteride were modest and dose-dependent. In the 0.05% group, serum DHT decreased by 8.9% at week 12 and 11% at week 24, while testosterone changes were described as minor. In contrast, oral finasteride was associated with a 27% DHT reduction at week 12 and 11% at week 24, alongside a gradual increase in serum testosterone (up to ~20% by week 24).
- Across all topical dutasteride arms, the authors state there were no statistically significant changes in serum DHT or testosterone, low systemic exposure, and no sexual side effects or clinically relevant lab abnormalities. The 0.05% group delivered the greatest reported efficacy while appearing to avoid the hormonal shifts seen with oral finasteride.
Figure 10: Representative images of hair growth in male-pattern androgenetic alopecia after treatment with 0.01% dutasteride topical solution at 12 week (b) and 24 week (c) vs baseline (a); 0.02% dutasteride topical solution at 12 week (e) and 24 week (f) vs baseline (d); 0.05% dutasteride topical solution at 12 week (h) and 24 week (i) vs baseline (g); oral finasteride 1 mg tablets at week 12 (k) and week 24 (l) vs baseline (j); placebo at week 12 (n) and week 24 (o) vs baseline (m).[19]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate … Continue reading
Our Analysis
On the surface, the Panuganti trial is extremely attractive: it suggests that low-dose topical dutasteride (0.05%) can outperform 1 mg oral finasteride in hair regrowth while producing only minimal average changes in serum DHT. However, several issues with the design and reporting warrant caution, and in our view, the topline conclusion should not be taken at face value.
Sponsorship and authorship
The trial was sponsored by Shilpa Medicare Limited, and all authors were company employees. Industry sponsorship does not invalidate results, but it does increase the importance of methodological transparency and independent replication—especially when findings are strikingly positive and out of step with real-world experience.
Hair counts that don’t match typical scalp biology
The reported baseline hair counts (≈300–330 hairs/cm²) in men with Norwood III–V vertex thinning are difficult to reconcile with established data. In non-balding adults, typical terminal hair densities are ~100–250 hairs/cm², and they fall substantially with moderate-to-severe AGA. Yet the study presents baseline photos of visibly thinned vertex regions alongside hair counts that exceed even full-density scalps.
The most likely explanations are that:
- Vellus hairs were included in the counts without a clear diameter cut-off (e.g., ≤40 µm), and/or
- The counted area was effectively larger than 1 cm², or hair counts were performed manually rather than via standardized software.
Any of these will inflate absolute hair counts and complicate the interpretation of changes over time.
Questionable consistency of the hair count target area
Accurate hair counting depends on revisiting the exact same 1 cm² area at each time point, typically anchored to a tattoo or permanent scalp mark. In the figures provided, the circular “counting zones” appear to shift in position, shape, and size between baseline, week 12, and week 24 for the same participant.
Even a 1–2 mm shift in the center of a 1 cm² circle can change measured hair counts by 50% or more in patchy thinning regions. Since the study reports changes on the order of ~10–15%, small misalignments in the counting area could fully account for the apparent “regrowth,” particularly in groups with smaller gains.
Limited insight into systemic exposure
Serum DHT and testosterone were reported only as group mean percentage changes, with no measures of variability (e.g., standard deviations) or individual pharmacokinetic correlations. This means:
- A reported 11% mean DHT decrease could still conceal substantial outliers with much larger reductions.
- We can’t assess whether men with the best regrowth had proportionally greater systemic exposure.
For a drug like dutasteride, where individual absorption and metabolism vary widely, this lack of granularity limits the conclusions we can draw about “safety” at the individual level.
Conflict with real-world data and dose–response expectations
Over several years, our community has tracked people using low-dose topical dutasteride (e.g., 0.01–0.02%, 1–2 mL daily, sometimes higher weekly exposure than in the 0.05% arm of this study). Broadly:
- Serum DHT typically changes by ~10–15%, which aligns with the idea of good scalp localization.
- Clinical outcomes are generally hair loss stabilization, with modest regrowth in some cases, not the large average gains suggested to outperform oral finasteride.
- More pronounced regrowth tends to appear only at much higher weekly exposure (e.g., around 8–10+ mg/week or with added microneedling), and at that point, systemic side effects become more common.
Taken together, this creates a tension: the study’s claim that 1 mL of 0.05% topical dutasteride daily can outperform 1 mg oral finasteride on average, without meaningful systemic effects, does not match what we see in practice or what we would expect based on dutasteride pharmacology.
Figure 11: Changes from baseline to week 24 in (a) testosterone levels and (b) DHT levels.[20]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate … Continue reading
Overall, the Panuganti et al. trial supports that low-dose topical dutasteride (0.01–0.05%) has real biologic activity, can improve hair parameters versus placebo, and may keep average serum DHT changes relatively modest. However, issues with baseline hair counts, apparent inconsistencies in how target areas were measured, limited systemic exposure data, and a headline result that conflicts with prior studies and real-world experience mean we don’t think this study justifies expecting low-dose topical dutasteride to reliably outperform oral finasteride. For now, it’s more reasonable to view low-dose topical dutasteride (≤0.05% with limited weekly exposure) as a localized, hair-loss–stabilizing option with modest regrowth and relatively mild systemic impact, while higher-dose topical regimens are more likely to produce cosmetically meaningful gains but also to behave more like oral dutasteride in terms of systemic DHT suppression and potential side effects.
Yet another study came out in 2025, combining skin patting and iontophoresis (a medical procedure using a mild electrical current) with dutasteride gel in male and menopausal female AGA.
Cedirian et al., 2025: 6% Dutasteride Gel, Skin Patting and Iontophoresis
In this single-center pilot study, researchers enrolled 20 adults (10 men, 10 postmenopausal women) with AGA who had failed at least 12 months of standard therapy (topical minoxidil and/or oral finasteride).[21]Cedirian, S., Pampaloni, F., Quadrelli, F., Rapparini, L., Bruni, F., Martelli, G., Piraccini, B.M., Starace, M. (2025). Efficacy of Skin Patting and Iontophoresis with Dutasteride Gel in Male and … Continue reading Participants underwent monthly sessions of a compounded 6% dutasteride gel delivered using a device that combines skin patting (SPi), microneedling, iontophoresis, electrostimulation, and red LED light over a standardized frontal-vertex treatment field for four months.
Hair density and shaft diameter were assessed by trichoscopy at baseline and again 8 weeks after the final session (i.e., roughly 6 months from baseline), alongside a hair pull test and patient-reported satisfaction scores. By the end of follow-up, frontal and vertex hair density increased significantly (p<0.001), hair shaft diameter improved in both regions (vertex p<0.001; frontal p=0.046), and pull test counts nearly halved (p<0.001), pointing to both regrowth and reduced shedding.
Clinically, investigators rated mean global improvement at +1.9 on a 7-point scale, corresponding to moderate regrowth and less shedding. Subjective outcomes were similarly positive: about 70% of participants perceived the treatment as moderately to very effective, mean cosmetic satisfaction was 3.4/4, and 85% described the procedure as pleasant. No adverse events or tolerability concerns were reported during treatment or follow-up.
Our Analysis
On paper, this is an encouraging signal: the participants experienced measurable gains in density, diameter, and shedding. The high satisfaction scores and absence of side effects also support the idea that this approach is, at minimum, well-tolerated.
However, the design makes it difficult to isolate what’s actually driving the benefit. Because the protocol combines several modalities, we can’t separate the relative contribution of dutasteride from the other treatments. Outcome measures were also partly subjective and non-validated, and all assessments were unblinded, which opens the door to expectation and observer bias.
A second major limitation is the complete absence of systemic safety data. Unlike the other studies, a high dosage of topical dutasteride is used (6%), but we don’t know the effect of this on systemic/serum DHT levels.
While lab work is really the only way to get a grip on things, this study suggests that topical dosages as low as 1 mL x 0.01%, and as infrequently applied as once every four weeks, can still produce results for more than 50% of men with androgenic alopecia.
This begs the question: just how low can we go for dosing? While we don’t yet have the answer, studies on a different delivery vehicle for dutasteride – mesotherapy – suggest we can go even lower.
Mesotherapy Dutasteride: Clinical Studies (Hair Counts, Serum DHT, & More)
Mesotherapy dutasteride is also known as intradermal dutasteride. This is different from topical dutasteride.
Whereas topical dutasteride is applied directly to the scalp, mesotherapy dutasteride is when dutasteride is injected directly into the scalp skin – between the epidermis and dermis (i.e., 0.5-0.7mm deep into the scalp).
Whereas only 25% of topical dutasteride is absorbed into the scalp dermis over 12 hours, 100% of mesotherapy dutasteride is absorbed into the scalp dermis, and immediately upon injection. After all, the drug is injected directly into the scalp, leaving no material to evaporate or get wiped away by sweat or during a shower.
That means, across equal dosages, that mesotherapy dutasteride will better saturate the scalp skin, but also lead to more systemic absorption.
The question then becomes: how do the pharmacokinetics of mesotherapy dutasteride change the equation for how much (and how frequently) we should be exposing our scalps to this medication in order to maximize hair gains while minimizing systemic DHT reductions?
While we don’t yet have perfect answers, there are a few studies that suggest the dose is even lower than topical dutasteride.
Corralo et. al (2017): Mesotherapy Dutasteride
A 2017 study by Corralo et al. investigated the effects of mesotherapy dutasteride on people with androgenic alopecia.[22]Saceda-Corralo, D., Rodrigues-Barata, A.R., Vano-Galvan, S., Jaen-Olasolo, P. (2017). Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia. International Journal of Trichology. … Continue reading The researchers selected six participants to receive 1 mL of 0.01% dutasteride (i.e., 0.1 mg of dutasteride) once every three months, totaling three injection sessions at months 0, 3, and 6.
The investigation group also took serum DHT measurements before starting treatment, and one month after the final mesotherapy injection (according to personal email correspondences with the authors).
Three months after the last mesotherapy injection, the investigators measured hair parameter improvements. Encouragingly, 1 mL of 0.01% mesotherapy dutasteride once every three months led to statistically significant hair improvements for all participants. Moreover, no changes to serum hormonal profiles were observed.
Here are the before-and-after photos of a featured patient:
Figure 13: Androgenetic alopecia in a 33-year-old man before (a) and after (b) treatment with dutasteride injections through 6 months with a one-session treatment every 3 months.[23]Saceda-Corralo, D., Rodrigues-Barata, A.R., Vano-Galvan, S., Jaen-Olasolo, P. (2017). Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia. International Journal of Trichology. … Continue reading
Our Analysis
The sample size of this study is incredibly small. With that said, the results are impressive, and they add to a growing number of signals suggesting that mesotherapy dutasteride (and topical dutasteride) improve hair growth for men and women with androgenic alopecia.
Moreover, this study suggests that a dosing schedule as infrequent as 1 mL of 0.01% dutasteride (i.e., 0.1 mg) once every three months can appreciably improve hair parameters, and without altering systemic levels of hormones.
However, just as with the last study measuring serum DHT levels, this investigation group waited a full month after a mesotherapy dutasteride session to read serum levels of DHT. While the results showed no effect on serum hormones, the reality is that serum DHT levels could have dropped during the days and weeks following an injection, only to rebound to baseline by the time these investigators decided to test.
On the other hand, it’s also possible that mesotherapy dutasteride changes the pharmacokinetics of dutasteride itself – perhaps by increasing tissue saturation time and decreasing systemic leakage rates. While this may sound absurd, other studies have demonstrated mesotherapy to exhibit this behavioral shift with other drugs and compounds.[24]Mammucari, M., Maggiori, E., Russo, D., Giorgio, C., Ronconi, G., Ferrara, P.E., Cazona, F., Antonaci, L., Violo, B., Velluci, R., Mediati, D.R., Migliore, A., Massafra, U., Bifarini, B., Gori, F., … Continue reading Therefore, it’s not unreasonable to assume these effects may also carry over to mesotherapy dutasteride, and that for unknown reasons, the drug has longer staying power in the scalp when injected (and less systemic absorption).
Based on the results of these serum DHT tests, preliminary evidence points toward mesotherapy dutasteride perhaps lasting longer in the scalp and conferring slower systemic absorption. But be forewarned: there is likely an upper limit for how much mesotherapy dutasteride we can inject before serum DHT levels start to decline.
Member Experience: Too Much Mesotherapy Dutasteride?
We once had a member test 2 mL of 0.05% dutasteride (i.e., 1.0 mg of dutasteride, or 10x the dosage used in the 2017 study) during his first mesotherapy session. He did a great job tracking serum DHT changes before treatment and 4 days, 4 weeks, and 8 weeks after the injections.
His results? Serum DHT decreases shortly after treatment, which continued to decline up through month one. At that point, the member started experiencing cognitive-related side effects. By the second month, serum DHT levels began to rebound and all side effects went away.
Serum DHT Reported side effects Pre-treatment 51 ng/dL (reference: 30-90 ng/dl) No Post-treatment (4 days) 35 ng/dL No Post-treatment (4 weeks) 25 ng/dL Yes Post-treatment (8 weeks) 31 ng/dL No With that said, there are a few things worth noting about this member’s experience:
- First, these effects occurred at 10x the dosage of the 2017 study by Saceda. So, it’s unlikely that if you follow the study parameters, the same thing will happen to you.
- Secondly, the delayed decline in serum DHT puzzled us, because it didn’t necessarily fit with the pharmacokinetics expected from mesotherapy or the better-studied pharmacokinetic expectations of oral dutasteride. Keep in mind that 1mg of oral dutasteride rapidly distributes across tissues and has a half-life of 7-14 days. With mesotherapy, much of the drug remains in the scalp skin and a lesser amount gets absorbed into the serum. Therefore, even if mesotherapy had delayed the systemic absorption of dutasteride, we shouldn’t expect the drop at 4 weeks to be more dramatic than the drop at 4 days – much less significantly lower at 4 weeks versus 4 days.
- Thirdly, after having a conversation with this member, we learned they also started using an over-the-counter allergy medication at some point after the mesotherapy sessions. Studies suggest that this allergy medication can lower testosterone, which can inadvertently lower DHT. As such, we’re not certain enough to attribute 100% of these serum DHT reductions exclusively to mesotherapy dutasteride – even if we believe the therapy to be responsible for the majority of the drops observed.
- Lastly, regardless of the drop in DHT and side effects, this member did report better hair growth after just one session.
So, by following lower dosage guidelines, we’re confident you can avoid a similar experience. In fact, a 2013 study on mesotherapy dutasteride echoes the experience from our member and shows why mesotherapy dosages, volumes, and frequencies matter a lot.
Sobhy et al. (2013): Low-Dose Vs. High-Dose Mesotherapy Dutasteride
In 2013, a research team in Egypt enrolled 90 men with androgenic alopecia to test mesotherapy dutasteride at varying dosages, with the higher dosage also containing other potential hair growth-promoting ingredients. They randomized the men into three groups, and then set them up with the following treatment:
- Group A: 1.5-2.0 mL of 0.005% mesotherapy dutasteride (i.e., 0.075-0.1 mg of dutasteride per session).
- Group B: 1.5-2.0 mL of 0.05% mesotherapy dutasteride (i.e., 0.75-1.0 mg of dutasteride per session; 10x more than Group A). The 10 mL solution also contained 500 mg of dexapanthenol, 20 mg of biotin 20 mg, and 200 mg of pyridoxine.
- Group C: 1.5-2.0 mL of saline solution (i.e., water; this is a placebo group).
Then, the investigators performed 9 mesotherapy sessions over a span of 19 weeks. The sessions were as follows:
- Once per week (weeks 0-3)
- Once every two weeks (weeks 5 and 7)
- Once every four weeks (weeks 11, 15, 19
Given this dosing schedule, here’s how much dutasteride each group was exposed to over 19 weeks:
- Group A: 0.675-0.9 mg
- Group B: 6.75-9.0 mg (i.e., 10x more than Group A)
- Group C: 0 mg (they’re the placebo group)
Results
After 19 weeks, here were the changes to hair parameters and serum DHT levels:
- Hair Parameters:
- Unsurprisingly, both Group A and Group B saw hair parameter improvements. However, Group B saw more robust improvements relative to Group A. This is probably because they received 10x the dosage of dutasteride.
- Serum DHT:
- Group A saw a significant increase in serum DHT levels before and after the study ended. The authors attributed this change to the preservation of diurnal DHT fluctuations. This suggests that at dosages of as low as 0.9 mg over 19 weeks, serum DHT levels are basically preserved.
- Group B saw a significant decrease in serum DHT levels. While this would be expected given what we’ve just learned about the dosing and the pharmacokinetics of both mesotherapy and dutasteride, the investigation group was also puzzled by the fact that the placebo group also saw a decline in serum DHT levels. Again, the researchers chalked it up to normal diurnal fluctuations to DHT. However, based on members’ experiences and the additional studies published since this 2013 investigation, our best bet is that Group B saw serum DHT levels decline mainly because of higher exposure rates to dutasteride.
The bottom line: stick to mesotherapy dutasteride dosages of 0.1-0.2 mg per month. That’s probably close to the “sweet spot” for maximizing hair gains while minimizing unwanted systemic hormonal effects.
Ultimately, a number of factors determine how much of a substance will be absorbed systemically and how much of that substance will remain saturated in a tissue to exert its maximal effect. These factors include:
- Drug pKa. This is a measurement of the acidity of a drug.
- Drug polarity. this affects the hydrophilic-hydrophobic ratio of a compound in reference to its location within the dermis.
- Location of the mesodermal application. Keep in mind the scalp dermis is distinctly different from the facial dermal layer.
- The diffusion coefficient of a compound.[25]Souto, E.B., Fangueiro, J.F., Fernandes, A.R., Cano, A., Sanchez-Lopez, E., Garcia, M.L., Severino, P., Paganelli, M.O., Chaud, M.V., Silva, A.M. (2022). Physicochemical and biopharmaceutical aspects … Continue reading This is a measurement for how quickly and widely a compound can diffuse in the surrounding tissue.
The above factors are influenced by drug preservatives, carrier agents, temperatures, injection needle depths, aqueous secretions from sweat glands, and even apocrine secretions from sebaceous glands (just to name a few). We can tell you right now: none of the studies on mesotherapy dutasteride made an effort to standardize these factors, or even mention them in their methodologies. In fact, we couldn’t even find standards for just how deep the mesotherapy needles were injected for any study. These are all big limitations for data quality.
(Side note: we’re planning on investigating these factors in early 2023, so that we can develop a better protocol for mesotherapy dutasteride – one that is optimized based on human data across multiple injection frequencies, exposure volumes, carrier agents, and needle depths. Expect more details about this toward the end of 2022.)
Are There Any Other Studies On Mesotherapy Dutasteride?
Yes. However, they mostly repeat the results we’ve discussed above. Moreover, to our knowledge, none of the other studies measured serum DHT levels, so they don’t help us get closer to answering our core question: “What’s the best protocol for mesotherapy dutasteride to maximize hair gains while minimizing systemic hormonal effects?”
With that said, there is one final study that provides some nuanced insights – particularly related to mesotherapy dutasteride and its variability in hair regrowth, depending on whether someone has metabolic syndrome.
Mofta et al. (2021): Mesotherapy Dutasteride In Females With Metabolic Syndrome
In this 2021 study, researchers sought to determine the effects of mesotherapy dutasteride on females with pattern hair loss (androgenic alopecia), and how hair growth outcomes changed depending on whether participants had metabolic syndrome.[26]Moftah, N., Mubarak, R., Abdelghani, R. (2021). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection … Continue reading
The researchers identified 51 females with pattern hair loss. Then, they determined which women also had metabolic syndrome. For reference, the investigators defined metabolic syndrome as meeting at least three of the following criteria:
- Raised waist circumference (population- and country-specific definitions), i.e.,> 92.3 cm in Egyptian women
- Fasting blood glucose ≥ 100 mg/dL or on diabetes treatment
- Blood pressure ≥ 130/85 mm Hg, or on antihypertensive treatment
- Triglycerides ≥150 mg/dL or on treatment
- High-density lipoprotein-cholesterol < 50 mg/dL, or on treatment
This left 26 and 25 females with and without metabolic syndrome, respectively.
Next, the investigators administered mesotherapy dutasteride to all participants. This included 1 mL of 0.02% mesotherapy dutasteride (i.e., 0.2 mg of dutasteride) per session, with eight sessions spanning a period of ~6 months. A few months later, hair parameters were assessed to gauge results.
Results
Surprisingly, females with metabolic syndrome experienced an initial improvement in hair growth at month one, followed by an acceleration of hair loss by month three. Contrastingly, females without metabolic syndrome saw their hair growth increasingly improve throughout each month of treatment.
These results were not only reflected in phototrichograms (i.e., hair counts, hair diameters, and anagen:telogen ratios) but also in global photographs.
Just see these before-and-after photos of two females without metabolic syndrome:
Figure 14: Before and after of woman one without metabolic syndrome.[27]Moftah, N., Mubarak, R., Abdelghani, R. (2019). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection … Continue reading
Figure 15: Before and after of woman two without metabolic syndrome.[28]Moftah, N., Mubarak, R., Abdelghani, R. (2019). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection … Continue reading
Now see this before-and-after photoset of a female with metabolic syndrome:
Figure 16: Before-and-after images of a woman with metabolic syndrome.[29]Moftah, N., Mubarak, R., Abdelghani, R. (2019). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection … Continue reading
On a positive note, no females in either group reported any side effects – sexual or otherwise. But the discrepancy in regrowth between those affected and unaffected by metabolic syndrome was, to put it simply, puzzling.
Why Did Female With Metabolic Syndrome See A Worsening Of Hair Loss With Mesotherapy Dutasteride?
The researchers did not have a definitive answer. However, some studies suggest an association between metabolic syndrome and androgenic alopecia, with mechanistic data suggesting that insulin insensitivity can enhance androgen production in certain tissues – like the ovaries in women and, perhaps, other organs like the skin.
Oral dutasteride is known to have a mildly negative effect on insulin sensitivity. For most healthy people, this isn’t problematic. But the data are less clear for people who are already afflicted with metabolic syndrome and then start taking dutasteride.
Here’s the rationale the authors used to explain their results:
“These findings could be explained by the study conducted by Upreti et al. [59] who confirmed that dual inhibition of 5α reductase I and II by dutasteride lead to modulation of insulin sensitivity in the peripheral tissues. Accordingly, dutasteride induced reduction of insulin sensitivity that is already impaired in both MetS [55, 60] and FPHL [61-63] could be a possible mechanism of deterioration in participants with MetS. This possibility was recently supported by Sadgrove [64] who suggested that insulin resistance could enhance microinflammation in androgenetic alopecia both directly and indirectly by increased microbial colonization. Moreover, an in vitro study carried out by Philpott et al. [39] revealed that removal of insulin from the culture medium of human hair follicles could lead to follicular transformation into catagen state.“
The bottom line: if you’re already insulin resistant, perhaps it is worth getting those levels within normal ranges before trying dutasteride altogether– including via mesotherapy delivery vehicles.
Where Can We Buy Mesotherapy Dutasteride?
Here are a few resources.
- SuperHumanStore (worldwide). We recently lab-tested this product. It passed all tests for purity and sterility. Unfortunately, the store no longer carries that product. So, we’re currently lab-testing their new product. We’ll update this guide when we have the results.
- FUE Clinic (U.S.). This company sells 0.01% mesotherapy dutasteride alongside biotin, pyridoxine, and a blend of “cellular matrix growth factors”. Don’t be fooled by those fancy words; we can assure you that cellular matrix growth factors are currently more so buzzwords than they are clinically-proven hair growth stimulants.
Where Can We Buy Topical Dutasteride?
Here are a few resources.
- MinoxidilMax (worldwide): 0.1% topical dutasteride, 60 mL. On the one hand, MinoxidilMax definitely sells prescription-grade hair loss drugs without requiring a prescription, which we’re 99% sure is illegal. On the other hand, when we lab-tested their products, they contained what they claimed – which is a good sign. We like MinoxidilMax because (1) they ship worldwide, and (2) they sell solvents to pair with their products – so you can dilute their topicals down to lower dosages: 0.005%, 0.01%, 0.02%, etc.
- Happy Head (U.S.). Their products contain topical finasteride, minoxidil and retinoic acid. But they allow customization by including topical dutasteride with or in place of finasteride.
- Regrowth Labs, D57α (U.S.). This is a topical solution containing dutasteride in a 0.5% concentration. Their suggested dose is 1mL which would give a 0.5mg dose topically. However, the company suggests applying this volume twice a day, daily. The accompanying ingredients include azelaic acid, vitamins, phytonutrients and polar carriers to help with absorption.
- CFS Pharmacy (U.S.). This also sells topical dutasteride as a prescription medication. The topical solution comes in a gel. While CFS Pharmacy promotes themselves as capable of making any dosage of topical dutasteride, we believe they require a prescription. So, before you exercise this option, be sure to find a doctor who will write you a prescription for the dutasteride concentration you’d like.
- Bauman Medical, 82D (U.S.). This is a topical dutasteride + minoxidil solution. The amount of dutasteride contained in the product is a 0.75% concentration. There is no mention of what the additional ingredients or carrier solutions are.
References[+]
References ↑1, ↑2 Herz-Ruelas, M.E., Alvarez-Villalobos, N.A., Millan-Alanis, J.M., de Leon-Gutierrez, H., Ocampo-Garza, S.S., Grimalt, R. (2020). Efficacy of Intralesional and Oral Dutasteride in the Treatment of Androgenetic Alopecia: A Systematic Review. Skin Appendage Disorders. 6(6). 338-345. Available at: https://doi.org/10.1159/000510697 ↑3, ↑4, ↑5, ↑6 Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis. Clinical Interventions in Aging. 14. 399-406. Available at: PMID 30863034 ↑7 Hagberg, K.W., Divan, H.A., Fang, S.C., Nickel, J.C., Jick, S.S. (2017). Risk of gynecomastia and breast cancer associated with the use of 5-alpha reductase inhibitors for benign prostatic hyperplasia. Clinical Epidemiology. 9. 83-91. Available at: https://doi.org/10.2147/CLEP.S124674 ↑8 Rahimi-Ardabili, B., Pourandarjani, R., Habibollahi, P., Mualeki, A. (2006). Finasteride induced depression: a prospective study. BMC Clinical Pharmacology. 6(7). Available at: https://doi.org/10.1186/1472-6904-6-7 ↑9 Wang, K., Fan, D-D., Jin, S., Xing, N-Z., Niu, Y-N. (2014). Differential expression of 5-alpha reductase isozymes in the prostate and its clinical implications. Asian Journal of Andrology. 16(2). 274-279. Available at: https://doi.org/10.4103/1008-682X.123664 ↑10 Gisleskog, P.O., Hermann, D., Hammarlund-Udenaes, M., Karlsson, M.O. (1999). The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. British Journal of Clinical Pharmacology. 47. 53-58. Available at: https://doi.org/10.1046/j.1365-2125.1999.00843.x ↑11 Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. Available at: https://smj.journals.ekb.eg/article_42083_24b61cbba4be9982db23c318414034c0.pdf (Accessed: Novemeber 2025) ↑12, ↑13, ↑14 Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. Available at: https://smj.journals.ekb.eg/article_42083_24b61cbba4be9982db23c318414034c0.pdf (Accessed: November 2025) ↑15, ↑16, ↑17 Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. JEADV. 36. c806-c808. Available at: https://doi.org/10.1111/jdv.18285 ↑18, ↑19, ↑20 Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects With Androgenetic Alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑21 Cedirian, S., Pampaloni, F., Quadrelli, F., Rapparini, L., Bruni, F., Martelli, G., Piraccini, B.M., Starace, M. (2025). Efficacy of Skin Patting and Iontophoresis with Dutasteride Gel in Male and Menopausal Female Androgenetic Alopecia: A Pilot Study. Dermatologic Therapy. 15(11). 3419-3424. Available at: https://doi.org/10.1007/s13555-025-01532-w. ↑22, ↑23 Saceda-Corralo, D., Rodrigues-Barata, A.R., Vano-Galvan, S., Jaen-Olasolo, P. (2017). Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia. International Journal of Trichology. 9(3). 143-145. Available at: https://doi.org/10.4103/ijt.ijt_73_16 ↑24 Mammucari, M., Maggiori, E., Russo, D., Giorgio, C., Ronconi, G., Ferrara, P.E., Cazona, F., Antonaci, L., Violo, B., Velluci, R., Mediati, D.R., Migliore, A., Massafra, U., Bifarini, B., Gori, F., di Carlo, M., Brauneis, S., Paolucci, T., Rocchi, P., Cugutti, A., Di Marzo, R., Bomprezzi, A., Santini, S., Giardini, M., Catizzone, A.R., Troili, F., Dorato, D., Gallo, A., Guglielmo, C., Natoli, S. (2020). Mesotherapy: From Historical Notes to Scientific Evidence and Future Prospects. ScientificWorldJournal. 3542848. Available at: https://doi.org/10.1155/2020/3542848 ↑25 Souto, E.B., Fangueiro, J.F., Fernandes, A.R., Cano, A., Sanchez-Lopez, E., Garcia, M.L., Severino, P., Paganelli, M.O., Chaud, M.V., Silva, A.M. (2022). Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon. 8(2). e08938. Available at: https://doi.org/10.1016/j.heliyon.2022.e08938 ↑26 Moftah, N., Mubarak, R., Abdelghani, R. (2021). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection in patients with female pattern hair loss: a prospective cohort study. 32(7). 827-836. Available at: https://doi.org/10.1080/09546634.2019.1708849 ↑27, ↑28, ↑29 Moftah, N., Mubarak, R., Abdelghani, R. (2019). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection in patients with female pattern hair loss: a prospective cohort study. Journal of Dermatological Treatment. 32(7). Available at: https://doi.org/10.1080/09546634.2019.1708849 Oral finasteride has established itself as a highly effective treatment for androgenic alopecia (AGA) and is widely prescribed around the globe. Its success can be largely attributed to its proven ability to slow hair loss and stimulate regrowth by inhibiting the conversion of testosterone to DHT, the hormone chiefly responsible for follicular miniaturization.
However, despite its well-documented efficacy, oral finasteride has become equally notorious for its potentially systemic side effects. Some men experience adverse effects such as decreased libido, erectile dysfunction, mood changes, and even persistent symptoms after discontinuation, a phenomenon called post-finasteride syndrome.[1]Diviccaro, S., Melcangi, R.C., Giatti, S. (2019). Post-finasteride syndrome: an emerging clinical problem. Neurobiology of Stress. 12. 100209. Available at: https://doi.org/10.1016/j.ynstr.2019.100209 This reputation has prompted many to seek alternatives that are both effective and safer for long-term use.
Recently, topical finasteride formulations have garnered attention as a promising alternative. By delivering the medication directly to the scalp, topical finasteride aims to minimize systemic absorption and thereby reduce the likelihood of side effects while still providing the benefits of DHT inhibition where it matters most. This alternative approach has piqued the interest of both patients wary of systemic issues and clinicians seeking tailored treatments.
In this article, we will examine whether topical finasteride can serve as a viable alternative to its oral counterpart. The main focus will be on answering three questions:
- Can topical finasteride effectively regrow hair in those affected by AGA?
- Does it match the efficacy results seen with oral finasteride?
- Can it reduce hair loss while offering a more favorable side effect profile?
Ulo offers finasteride options that range from low to high dose finasteride – allowing you to be flexible in your treatment choices.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Oral vs. Topical Finasteride
Some research directly compares oral finasteride (1 mg daily) with various concentrations of topical finasteride, aiming to match hair regrowth efficacy while minimizing systemic side effects. Let’s take a look at a couple of examples:
Study 1 – Piraccini et al., 2022 (Phase III RCT, 24 weeks)
- Compared topical finasteride (0.25% spray) to oral finasteride (1 mg/day) and placebo in men with AGA.[2]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E., Topical Finasteride Study Group. Efficacy and safety of … Continue reading
- The increase in hair count on a defined scalp area was similar for topical and oral finasteride, both outperforming the placebo, suggesting comparable clinical benefit.
- Systemic finasteride (oral) reduced mean serum DHT by 55.6%, whereas topical finasteride reduced it by 34.5%. Plasma finasteride exposure from topical application was over 100 times lower than from oral administration.
- Both had similar low rates of mild side effects, but sexual side effects, but sexual side effects were more associated with oral treatment.
- Caveats: The “target area” measured may not represent the full scalp effects; although systemic absorption, albeit at a much lower level with topical application, still occurred and varied by formulation.
Study 2 – Bhura, 2013 (RCT, 8 months)
- Parallel-group comparison in male AGA, tracking hair density and systemic DHT.[3]Bhura, M. (2013). Comparative Analysis of Topical and Oral Finasteride in Androgenetic Alopecia: Impact on Hair Growth, Systemic Absorption, and Adverse Effects. Indian Journal of Basic and Applied … Continue reading
- Oral finasteride resulted in slightly superior increases in hair number and thickness, although the difference was not statistically significant.
- Oral finasteride significantly reduced systemic DHT and caused more sexual side effects; topical finasteride led to minimal serum DHT changes and almost no systemic side effects, with most reactions being local (e.g., irritation).
- Those worried about long-term systemic effects preferred topical use, despite slightly less pronounced gains.
Study 3 – Hajheydari, 2009 (RCT, 6 months)
- Compared a 1% topical gel + placebo tablet with oral finasteride 1 mg/day + placebo gel.[4]Hajheydari, Z., Akbari, J., Saeedi, M., Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian Journal of Dermatology, … Continue reading
- Both the gel and tablet produced statistically significant increases in total hair count and terminal hair count.
- There were no significant differences in hair thickness, hair count, or size of the bald area between the two groups.
Mechanism of Action
Finasteride Pharmacodynamics
Finasteride is a competitive inhibitor of 5α-reductase, primarily targeting the type II isoenzyme at therapeutic doses, which predominates in the hair follicles and prostate. As mentioned above, this enzyme catalyzes the conversion of testosterone to DHT.
At higher tissue concentrations, finasteride can also inhibit type I 5α-reductase, which is primarily found in the skin and sebaceous glands; however, its clinical significance for hair loss at standard doses is limited. By selectively inhibiting the type II enzyme, finasteride effectively lowers local and systemic DHT levels.[5]Finn, D.A., Beadles-Bohling, A., Beckley, E.H., Ford, M.M., Gililland, K.R., Gorin-Meyer, R.E., Wiren, K.M. (2006). 12(1). 53-76. A New Look at the 5α-reductase Inhibitor Finasteride. CNS Drug … Continue reading
What is Finasteride’s Effect on DHT Suppression?
Finasteride’s effect on DHT suppression is characterized by a logarithmic dose-response curve, meaning that even very low systemic levels can produce a marked reduction in both serum and scalp DHT. Most of the drug’s inhibitory action is achieved at low doses, with additional dosing yielding only marginal further effect.
As a result, even minimal “leakage” of topically applied finasteride into the bloodstream can decrease DHT levels elsewhere in the body.
Pharmacokinetic studies show that inhibition of type-II 5ɑ-reductase reaches saturation at typical clinical dosage, while type-1 enzyme inhibition requires much higher concentrations. This nonlinear pharmacodynamic profile explains both the strong efficacy and the wide safety margin of topical finasteride: a small amount achieves most of the desired effect, so careful formulation is crucial to maximize scalp delivery while minimizing unwanted systemic exposure.[6]Suzuki, R., Satoh, H., Ohtani, H., Hori, S., Sawada, Y. (2010). Saturable Binding of Finasteride to Steroid 5ɑ-reductase as Determinant of Nonlinear Pharmacokinetics. Drug Metabolism and … Continue reading
What the Science Says
Topical finasteride has been studied across a 200-fold concentration range, from 0.005% solutions to 1% gels. Collectively, these trials demonstrate meaningful reductions in shedding and measurable regrowth; however, systemic exposure increases with dose and vehicle potency.
Study Concentration & Vehicle Hair Growth-Outcomes Systemic/Serum Findings Mazzarella 1997, single-blind, 52 men/women, 16 months. 0.005% hydro-alcoholic solution 73% reported “high effectiveness”; wash-test hair counts improved; slowed shedding by month 6 No significant change in plasma DHT or testosterone; absorption was negligible. Tanglertsampan 2012 RCT, 33 men, 24 weeks. 0.1% lotion + 3% minoxidil The combination arm gained more hairs/cm2 and thicker shafts than minoxidil alone. Local irritation mild; systemic parameters not monitored. Datta 2021 double-blind trial, 35 participants completed, 6 months. 0.1% lotion +5% minoxidil vs oral 1 mg. The topical combination was non-inferior to oral minoxidil for reducing the Hamilton-Norwood stage. Sexual adverse events only occurred in the oral group; topical was well-tolerated. Caserini 2016 OK study, 50 men, 1 week; Piraccini 2022 phase III, 323 completers, 24 weeks. 0.25% solution. -70% scalp DHT after once-daily 1 mL; +20.2 hairs in 1 cm2 target area at 24 weeks – numerically equal to oral 1 mg. 100-200 μL doses reduced serum DHT by only 24-26%; 400 μL treatment reduced levels by 44-48%; Cmax was more than 100 times lower than oral. Rossi 2020 retrospective, 69 women, 12-18 months. 0.5% lotion (postmenopausal women) The Finasteride + minoxidil group scored higher on a 7-point global scale compared to the 17ɑ-estradiol + minoxidil group. No androgenic side effects reported. Hajheydari 2009 DB-RCT, 45 men, 6 months. 1% gel Increases in total and terminal hair counts matched those of oral 1 mg; the bald area remained unchanged. Serum DHT not assayed; clinical side-effects minimal. Low-Dose Takeaways
Even a micro-dose of 0.005% twice daily (~0.1 mg/day) curbed shedding and improved density without measurable systemic suppression, making it an attractive option for highly risk-averse users.[7]Mazzarella, F., Loconsole, F., Cammisa, A., Mastrolonardo, M., Vena, G.A. (1997). Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. … Continue reading
Mid-Range (0.1-0.25%)
Adding 0.1% finasteride to minoxidil amplifies regrowth compared to minoxidil alone, while standalone 0.25% sprays deliver oral-level scalp DHT blockade, maintaining serum exposure roughly one-tenth that of tablets. Dose and volume are critical.[8]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading
High-Dose (0.5-1%)
Topical finasteride options at 0.5% have been shown to extend benefits to female pattern hair loss with good tolerability.[9]Rossi, A., Magri, F., D’Arino, A., Pigliacelli, F., Muscianese, M., Leoncini, P., Caro, G., Federico, A., Fortuna, M.C., Carlesimo, M. (2020). Efficacy of Topical Finasteride 0.5% vs 17ɑ-Estradiol … Continue reading
Why Formulation and Dose Matter
- The vehicle drives absorption: according to the studies, hydro-alcoholic sprays penetrate faster than gels or liposomes, explaining divergent serum DHT curves at equal nominal strengths.
- Daily drug load, not percentage alone, predicts systemic spill over; 0.025% at 400 μL approaches the systemic impact of oral dosing, whereas 100 μL does not.
- Combination therapy often allows for a lower finasteride concentration to achieve equal cosmetic benefits, thereby minimizing exposure.
Minimizing Systemic Absorption
While topical finasteride offers an option for minimizing systemic exposure, several key variables influence the amount that enters the bloodstream, and this can occur surprisingly quickly.
Key Factors Affecting Systemic Absorption
- Carrier Agent: The vehicle used to deliver finasteride dramatically alters absorption. Alcohol-based (ethosomal) carriers enhance drug penetration through the skin and into deeper layers like the dermis much more than conventional liposomes, which tend to remain more superficial. One study found that ethosomes resulted in approximately six times greater dermal accumulation than liposomes, highlighting the direct impact of carrier choice on systemic exposure.[10]Rao, Y., Zheng, F., Zhang, X., Gao, J., Liang, W. (2008). In Vitro Percutaneous Permeation and Skin Accumulation of Finasteride Using Vesicular Ethosomal Carriers. AAPS PharmSciTech. 9(3). 860-865. … Continue reading
- Time on Scalp Before Washing: The longer finasteride remains on the scalp before washing, the greater the chance for absorption through the skin. Washing off too soon reduces drug uptake, but leaving it for extended periods increases both local efficacy and systemic exposure. Guidelines often recommend allowing at least 4-6 hours on the scalp, though this can vary by formulation.
- Frequency of Use: If you can keep topical finasteride on your scalp for at least 10-12 hours, once-daily applications of low-dose topical finasteride might be beneficial. For those who can only tolerate finasteride for 4-6 hours, twice-daily applications may be better. You can read more about how often topical finasteride should be applied here.
- Individual Skin Permeability: Personal factors, including skin thickness, barrier integrity, genetics, and even scalp conditions, can strongly influence how much finasteride penetrates the scalp. Individuals with more permeable skin may absorb a greater amount of the drug systemically, increasing the risk of side effects.[11]Brito, S., Baek, M., Bin, B-H. (2024). Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics. 16(11). 1403. Available at: … Continue reading
How To Maximize Gains and Minimize Risk
To achieve the best results and reduce the likelihood of side effects, there are several strategies you can employ:
- Establish a Baseline with Serum DHT Testing
Before starting topical finasteride, get a baseline measurement of your serum DHT levels through a blood test. This serves as a reference to assess the extent to which systemic DHT is affected by your treatment.
- Retest After 30 Days
After one month of consistent topical use, repeat the serum DHT test under the same conditions (preferably in the morning, fasted, and at a similar time of day). This helps you gauge systemic absorption and adjust your regimen if your serum DHT levels drop excessively. Hormone levels fluctuate based on daily rhythms, food intake, and stress, so always test under similar circumstances: morning, fasted, and ideally before applying the day’s finasteride.
- Avoid Confounding Supplements
Avoid supplements that may directly affect DHT, such as those that increase DHT (e.g., creatine) or quercetin (which may lower it). This ensures your test results and progress are a direct reflection of the topical finasteride.[12]van der Merwe, J., Brooke, N.E., Myburgh, K.H. (2009). Three weeks of creatine monohydrate supplementation affects dihydrotestosterone to testosterone ratio in college-aged rugby players. Clinical … Continue reading,[13]Ma, Z., Nguyen, T.H., Huynh, T.H., Do, P.T., Huynh, H. (2004). Reduction of rat prostate weight by combined quercetin-finasteride treatment is associated with cell cycle deregulation. Journal of … Continue reading
- Be Patient – It Takes Time
Visible improvements often require 12-24 months. Don’t make hasty adjustments if you don’t see immediate changes; hair cycles are slow.
- Track Your Progress Objectively
Use standardized, high-quality photo documentation, same angle, lighting, and distance each time, to objectively monitor changes in hair density and coverage.
Combination Therapies
Robust evidence supports the use of combination therapies for AGA, with multi-modal approaches consistently outperforming monotherapy in terms of efficacy and speed of regrowth.
Finasteride + Minoxidil: Studies show that pairing topical finasteride with 5% minoxidil yields superior hair density and patient satisfaction compared to either agent alone, particularly after 24 weeks of treatment.[14]Apoorva, V.B., Vibhu, M., Singh, R.H., Smita, T. (2023). Comparative Efficacy of Topical Finasteride (0.25%) in Combination with Minoxidil (5%) Against 5% Minoxidil or 0.25% Finasteride Alone in Male … Continue reading,[15]Rossi, A., Caro, G. (2023). Efficacy of the association of topical minoxidil and topical finasteride compared to their use in monotherapy in men with androgenetic alopecia: A prospective, randomized, … Continue reading,[16]Abeck, F., Hansen, I., Kott, J., Schroder, F., Garrahy, E., Veneroso, J., Runger, A., Torster, L., Schneider, S.W., von Buren, J. (2024). Patient-reported outcomes of topical finasteride/minoxidil … Continue reading This combination is effective for both new users and those who have experienced shedding after discontinuing oral finasteride.
Figure 1: Effect of combination minoxidil and finasteride or monotherapy treatment on global photographic assessment score at T3 (3 months) and T6 (six months).[17]Rossi, A., Caro, G. (2023). Efficacy of the association of topical minoxidil and topical finasteride compared to their use in monotherapy in men with androgenetic alopecia: A prospective, randomized, … Continue reading Image obtained in line with the Creative Commons License.
Finasteride + Microneedling: Adding microneedling to topical therapy can significantly enhance outcomes. Clinical trials demonstrated that combining microneedling with minoxidil and/or finasteride increased hair density and shaft diameter more than minoxidil alone, with effects noticeable within just 12 weeks.[18]Chang, Y., Zhang, W., Zhou, J., Lv, L., He, Q., Chen, Y., Wang, P., Zhai, Q. (2025). Clinical efficacy of microneedle combined with 5% Minoxidil solution and finasteride in the treatment of … Continue reading,[19]Adistri, K., Sirait, S.P., Rihatmadja, R., Legiawati, L., Indriatmi, W., Saldi, S.R.F. (2024). Effectiveness and safety of the combination therapy of micro-needling and minoxidil in androgenetic … Continue reading
Triple Topical Therapy: Preliminary studies on formulations that combine finasteride, dutasteride, and minoxidil show promising results, with visible regrowth as early as three months in some cases.[20]Rafi, A.W., Katz, R.M. (2011). Pilot Study of 15 Patients Receiving a New Treatment Regimen for Androgenic Alopecia: The Effects of Atopy on AGA. ISRN Dermatology. 241953. Available at: … Continue reading
So, combination therapy appears to be more effective than monotherapy. Therefore, leveraging two or more topical therapies or adding microneedling can support hair regrowth outcomes.
Who Should (or Shouldn’t) Use Topical Finasteride?
Good Candidates:
- Men diagnosed with AGA.
- Those who tolerate oral finasteride but seek to minimize systemic risks.
Not Ideal For:
- People with hair loss from causes other than AGA (e.g., alopecia areata, telogen effluvium).
- Those who have previously shown a poor response to finasteride.
- Anyone trying to conceive, or with infants, toddlers, or pregnant individuals in close contact.
- Users who are unable or unwilling to follow consistent application routines or medical monitoring.
Final Verdict
Topical finasteride is a legitimate option for hair regrowth. When formulated and used properly, it can rival oral finasteride’s effectiveness with a lower risk of systemic side effects. Success hinges on the right delivery method, correct dilution, and consistent application. While not flawless, it excels as part of a comprehensive regimen, especially when paired with therapies like minoxidil or microneedling.
For those hesitant about oral medication, topical finasteride offers a practical, lower-risk compromise, provided users carefully follow evidence-based protocols to optimize both safety and results. Used strategically, it is a potent addition to hair loss treatment plans.
References[+]
References ↑1 Diviccaro, S., Melcangi, R.C., Giatti, S. (2019). Post-finasteride syndrome: an emerging clinical problem. Neurobiology of Stress. 12. 100209. Available at: https://doi.org/10.1016/j.ynstr.2019.100209 ↑2 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E., Topical Finasteride Study Group. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III randomized, controlled clinical trial. JEADV. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑3 Bhura, M. (2013). Comparative Analysis of Topical and Oral Finasteride in Androgenetic Alopecia: Impact on Hair Growth, Systemic Absorption, and Adverse Effects. Indian Journal of Basic and Applied Medical Research. 3(1). 436-444 ↑4 Hajheydari, Z., Akbari, J., Saeedi, M., Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian Journal of Dermatology, Venereology, and Leprology. 75(1). 47-51. Available at: https://doi.org/10.4103/0378-6323.45220 ↑5 Finn, D.A., Beadles-Bohling, A., Beckley, E.H., Ford, M.M., Gililland, K.R., Gorin-Meyer, R.E., Wiren, K.M. (2006). 12(1). 53-76. A New Look at the 5α-reductase Inhibitor Finasteride. CNS Drug Reviews. Available at: https://doi.org/10.1111/j.1529-3458.2006.00053.x ↑6 Suzuki, R., Satoh, H., Ohtani, H., Hori, S., Sawada, Y. (2010). Saturable Binding of Finasteride to Steroid 5ɑ-reductase as Determinant of Nonlinear Pharmacokinetics. Drug Metabolism and Pharmacokinetics. 25(2). 208-213. Available at: https://doi.org/10.2133/dmpk.25.208 ↑7 Mazzarella, F., Loconsole, F., Cammisa, A., Mastrolonardo, M., Vena, G.A. (1997). Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. Journal of Dermatological Treatment. 8. 189-192. ↑8 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. International Journal of Clinical Pharmacology and Therapeutics. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑9 Rossi, A., Magri, F., D’Arino, A., Pigliacelli, F., Muscianese, M., Leoncini, P., Caro, G., Federico, A., Fortuna, M.C., Carlesimo, M. (2020). Efficacy of Topical Finasteride 0.5% vs 17ɑ-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients. Dermatology Practical & Conceptual. 10(2). E2020039. Available at: https://doi.org/10.5826/dpc.1002a39 ↑10 Rao, Y., Zheng, F., Zhang, X., Gao, J., Liang, W. (2008). In Vitro Percutaneous Permeation and Skin Accumulation of Finasteride Using Vesicular Ethosomal Carriers. AAPS PharmSciTech. 9(3). 860-865. Available at: https://doi.org/10.1208/s12249-008-9124-y ↑11 Brito, S., Baek, M., Bin, B-H. (2024). Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics. 16(11). 1403. Available at: https://doi.org/10.3390/pharmaceutics16111403 ↑12 van der Merwe, J., Brooke, N.E., Myburgh, K.H. (2009). Three weeks of creatine monohydrate supplementation affects dihydrotestosterone to testosterone ratio in college-aged rugby players. Clinical Journal of Sports Medicine. 19(5). 399-404. Available at: https://doi.org/10.1097/JSM.0b013e3181b8b52f. ↑13 Ma, Z., Nguyen, T.H., Huynh, T.H., Do, P.T., Huynh, H. (2004). Reduction of rat prostate weight by combined quercetin-finasteride treatment is associated with cell cycle deregulation. Journal of Endocrinology. 181(3). 493-507. Available at: https://doi.org/10.1677/joe.0.1810493 ↑14 Apoorva, V.B., Vibhu, M., Singh, R.H., Smita, T. (2023). Comparative Efficacy of Topical Finasteride (0.25%) in Combination with Minoxidil (5%) Against 5% Minoxidil or 0.25% Finasteride Alone in Male Androgenetic Alopecia: A Pilot, Randomized, Open-Label Study. International Journal of Trichology. 15(2). 56-62. Available at: https://doi.org/10.4103/ijt_ijt_72_22 ↑15 Rossi, A., Caro, G. (2023). Efficacy of the association of topical minoxidil and topical finasteride compared to their use in monotherapy in men with androgenetic alopecia: A prospective, randomized, controlled, assessor-blinded, 3-arm, pilot trial. Journal of Cosmetic Dermatology. 23(2). 502-509. Available at: https://doi.org/10.1111/jocd.15953 ↑16 Abeck, F., Hansen, I., Kott, J., Schroder, F., Garrahy, E., Veneroso, J., Runger, A., Torster, L., Schneider, S.W., von Buren, J. (2024). Patient-reported outcomes of topical finasteride/minoxidil treatment for male androgenetic alopecia: A retrospective study using telemedical data. Journal of Cosmetic Dermatology. 23(9). 2956-2963. Available at: https://doi.org/10.1111/jocd.16360 ↑17 Rossi, A., Caro, G. (2023). Efficacy of the association of topical minoxidil and topical finasteride compared to their use in monotherapy in men with androgenetic alopecia: A prospective, randomized, controlled, assessor-blinded, 3-arm, pilot trial. Journal of Cosmetic Dermatology. 23(2). 502-509. Available at: https://doi.org/10.1111/jocd.15953 ↑18 Chang, Y., Zhang, W., Zhou, J., Lv, L., He, Q., Chen, Y., Wang, P., Zhai, Q. (2025). Clinical efficacy of microneedle combined with 5% Minoxidil solution and finasteride in the treatment of androgenetic alopecia in males. Archives of Dermatological Research. 317(428). Available at: https://doi.org/10.1007/s00403-025-03891-y ↑19 Adistri, K., Sirait, S.P., Rihatmadja, R., Legiawati, L., Indriatmi, W., Saldi, S.R.F. (2024). Effectiveness and safety of the combination therapy of micro-needling and minoxidil in androgenetic alopecia of Indonesian men: a randomized controlled trial. Dermatology Reports. 16(3). 9945. Available at: https://doi.org/10.4081/dr.2024.9945 ↑20 Rafi, A.W., Katz, R.M. (2011). Pilot Study of 15 Patients Receiving a New Treatment Regimen for Androgenic Alopecia: The Effects of Atopy on AGA. ISRN Dermatology. 241953. Available at: https://doi.org/10.5402/2011/241953 Topical minoxidil is a first-line treatment for female pattern hair loss (FPHL) and other conditions such as telogen effluvium and traction alopecia. Many women of reproductive age use minoxidil and may wonder about its safety when trying to conceive or in early pregnancy.
While most reproductive safety discussions focus on oral or systemic medications, even topically applied drugs can raise questions about potential effects on fertility or early fetal development. Although systemic absorption from topical formulations is low, uncertainty persists regarding potential hormonal or embryonic effects.
This article examines available evidence to evaluate whether topical minoxidil poses any risk to female fertility or conception. By reviewing preclinical studies and clinical and pharmacological data, we aim to clarify whether there is any risk associated with topical minoxidil when trying to conceive.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Topical Minoxidil: Mechanism and Pharmacology
Minoxidil may promote hair growth through several complementary mechanisms. After topical application, the drug is enzymatically converted by sulfotransferase (SULT1A1) into minoxidil sulfate, its active form. This metabolite enhances potassium channel activity within follicular cells, promoting cell proliferation and prolonging the anagen (growth) phase of the hair cycle. Additionally, minoxidil increases vascularity around the follicle by stimulating vascular endothelial growth factor (VEGF), improving oxygen and nutrient delivery to support stronger, thicker hair growth.[1]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading
There are important pharmacological distinctions between topical and oral formulations. Topical minoxidil acts locally on the scalp with minimal systemic absorption, which significantly reduces the risk of adverse effects. In contrast, oral minoxidil can cause systemic side effects, such as fluid retention, weight gain, increased blood pressure (hypertension), and other cardiovascular changes.[2]StatPearls Editorial Team. (no date). Minoxidil. In: StatPearls [Internet]. StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (Accessed: October 2025)
Female Fertility: What Matters When Trying to Conceive?
Female fertility relies on coordinated hormonal cycles, healthy egg development, and a receptive uterine environment. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) regulate ovulation, while estrogen and progesterone prepare the uterus for implantation and support early pregnancy.[3]Thiyagarajan, D. K., Basit, H., & Jeanmonod, R. (2024 Jan 1). Physiology, menstrual cycle. In: StatPearls [Internet]. StatPearls Publishing. Available at: … Continue reading In addition to hormonal balance, healthy reproductive anatomy is important: clear fallopian tubes, a healthy uterus, and a cervix free of significant abnormalities are required for fertilization and embryo implantation.[4]Mayo Clinic Staff. (no date). Female infertility – Symptoms & causes. Available at: https://www.mayoclinic.org/diseases-conditions/female-infertility/symptoms-causes/syc-20354308/ (Accessed: … Continue reading
Of course, women, or individuals who are female at birth, looking to conceive also need to consider the impact of medications following conception. The safe use of medications is essential to support maternal health and healthy fetal development while minimizing risks of complications and birth defects (teratogenic effects).
Systemic medications can interfere with fertility and pregnancy by disrupting hormonal balance or exerting teratogenic effects. For example, antiandrogens such as spironolactone, cyproterone acetate, and finasteride, sometimes prescribed for female hair loss, are contraindicated when trying to conceive due to their potential impact on fetal development.[5]BinJadeed, H. F., & Alajlan, A. (2021). Pregnancy and neonatal outcome with maternal exposure to finasteride: a case series. Journal of Dermatology and Dermatologic Surgery. 25(2). 84-86. … Continue reading,[6]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading Animal models suggest these agents interfere with androgen signaling, which is essential for normal sexual differentiation, particularly in male fetuses.[7]Conley, J. M., Lambright, C. S., Evans, N., Cardon, M., Furr, J., Wilson, V. S., & Gray Jr, L. E. (2018). Mixed “antiandrogenic” chemicals at low individual doses produce reproductive tract … Continue reading In contrast, nutritional supplements like iron, vitamin D, and biotin are generally safe and can support overall reproductive health.
Systemic Absorption of Topical Minoxidil: How Much Gets In?
Topical minoxidil is formulated to act locally on the scalp, with only a small proportion entering systemic circulation – typically around 1–2%.[8]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading Pharmacokinetic studies show that this absorption rate can vary depending on several factors, including formulation strength (2% vs. 5%), frequency of application, and the integrity of the scalp barrier. Plasma minoxidil levels in individuals using topical formulations generally remain well below those associated with systemic pharmacologic or reproductive effects observed in animal studies.[9]Singh, S., Patil, A., Kianfar, N., Waśkiel-Burnat, A., Rudnicka, L., Sinclair, R., & Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical … Continue reading,[10]Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: … Continue reading
By contrast, oral minoxidil is almost completely absorbed, leading to significantly higher plasma concentrations and systemic exposure. This explains why oral therapy, though often more potent, also carries a greater risk of systemic side effects, including edema, tachycardia, and generalized hypertrichosis.[11]Randolph, M., & Tosti, A. (2021). Oral minoxidil treatment for hair loss: a review of efficacy and safety. Journal of the American Academy of Dermatology. 84(3). 737-746. Available at: … Continue reading
Does Topical Minoxidil Affect Female Fertility, Conception, or Pregnancy?
When considering the impact of medications on women’s fertility and ability to conceive, it is also essential to review the evidence regarding potential effects during pregnancy, as treatments will inevitably continue to be taken into early pregnancy, and systemic effects may be long-lasting. Here, we review preclinical and clinical evidence on whether topical minoxidil affects female fertility and pregnancy.
Preclinical Evidence
Minoxidil is classified as a non-hormonal treatment, suggesting it is unlikely to impact hormonal cycles that impact fertility. However, due to the relationship between hair growth and hormonal pathways, some preclinical research has focused on how minoxidil affects androgen pathways.
One cell-based study suggests that minoxidil can directly interact with and weaken androgen receptor signaling. However, this study used higher concentrations of minoxidil than would be used in hair loss treatment, and for practical purposes, topical minoxidil remains classified as a non-hormonal treatment.[12]Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: … Continue reading
However, in an animal model using golden Syrian hamsters, 5% topical minoxidil did not prevent the androgen-dependent growth of pigmented spots, suggesting that minoxidil does not have any effect on androgen response.[13]Nuck, B. A., Fogelson, S. L., & Lucky, A. W. (1987). Topical minoxidil does not act as an antiandrogen in the flank organ of the golden Syrian hamster. Archives of Dermatology. 123(1). 59-61. … Continue reading
There is a significant lack of research using preclinical animal models investigating the impact of topical minoxidil on female fertility and during pregnancy. However, one small-scale study did examine the effect of 5% topical minoxidil on embryonic mortality in rats. They found no impact of daily minoxidil application over the course of 20 days of pregnancy compared to the control group. However, the sample size was relatively small (20 rats in total), and no molecular or cellular analysis was performed to look for potential mechanisms that could cause embryonic lethality.[14]Turkina, V. A., Chemodurova, N. Y., Pryzyglei, H. V., & Kuzminov, Y. B. (2021). Potential embryotoxic effect study of minoxidil-containing lotion in experiment with female rats. Світ … Continue reading
Clinical Evidence
Unfortunately, a lack of research into the impact of medications on women and fetuses during pregnancy is a systemic issue within medicine. Pregnant women are generally excluded from clinical trials due to safety concerns, as well as the confounding impact of widespread hormonal changes associated with pregnancy.[15]Stock, S. J., & Norman, J. E. (2019). Medicines in pregnancy. F1000Research. 8(F1000Faculty Rev):911. Available at: https://doi.org/10.12688/f1000research.17535.1 These issues extend to women trying to conceive, and most clinical trials have explicit protocols that remove individuals from clinical trials after they become pregnant.[16]Phelan, A. L., Kunselman, A. R., Chuang, C. H., Raja-Khan, N. T., & Legro, R. S. (2016). Exclusion of women of childbearing potential in clinical trials of type 2 diabetes medications: a review … Continue reading
The safety of medications for pregnant women and their children is typically established after drugs have come to market, through observational studies and pharmacovigilance, which looks for cases where problems have arisen. While such processes are good at spotting signals of high risk, these forms of evidence are less powerful than clinical trials and cannot detect less impactful effects.
As such, determining the safety of drugs for pregnant women is often done using scarce information. As a result, many medications are contraindicated (their use is advised against) for pregnant women due to a lack of evidence, not because of evidence.[17]Thomas, S. H., & Yates, L. M. (2012). Prescribing without evidence – pregnancy. British Journal of Clinical Pharmacology. 74(4). 691-697. Available at: … Continue reading
Multiple reviews of the safety of dermatological treatments in pregnant women advise against the use of monoxidil.[18]Koh, Y. P., Tian, E. A., & Oon, H. H. (2019). New changes in pregnancy and lactation labelling: Review of dermatologic drugs. International Journal of Women’s Dermatology. 5(4). 216-226. … Continue reading,[19]Truong, T. M., Yaghi, M., & Murase, J. (2023). Dermatologic Drug Safety in Pregnancy. Journal of Dermatology for Physician Assistants. 17(3). 1-12. Available at: … Continue reading,[20]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777–2786. Available at: … Continue reading However, this advice appears to be based on two case studies where topical minoxidil was theorized as the cause of the birth defects. Case studies present a single individual (or group of individuals) where an event or phenomenon has occurred. They are a weak form of evidence and are typically used as the basis for larger studies.
In one of these case studies, the pregnancy of a 28-year-old woman who was applying topical minoxidil was terminated due to the presence of fetal malformation. Topical minoxidil was suggested as a possible cause of teratogenesis.[21]Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., & Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth Defects Research Part A: Clinical … Continue reading The other study noted a case of a rare condition called caudal regression syndrome in a fetus where the mother had been using topical minoxidil, though other causes were also proposed.[22]Rojansky, N., Fasouliotis, S. J., Ariel, I., & Nadjari, M. (2002). Extreme caudal agenesis. Possible drug-related etiology?. Journal of the American Academy of Dermatology. 47(3). 241-245. … Continue reading These cases were published in 2002 and 2003, and both advised that further studies were warranted.
In a more systematic approach, one study analysed FDA Adverse Event Reporting System (FAERS) data, which collects evidence of adverse effects related to medications.[23]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading The study used data collected between 2004 and 2014. Over the ten years analyzed, 2 cases of induced abortion and 2 cases of spontaneous abortion were reported in women using minoxidil. This represents around 2% of cases reported. For comparison, induced abortion represented around 18% of reported adverse effects related to finasteride, for which it was the most commonly reported issue. No cases related to sexual dysfunction or loss of libido were reported.
Figure 1: Comparison between adverse events reported by female alopecia areata patients exposed to finasteride and minoxidil. Adapted from Table 4.[24]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading Image used in accordance with the PMC Creative Commons Licence.
It is due to the lack of evidence and the potential issues that these case studies raise that minoxidil is typically contraindicated for women who are trying to conceive or are pregnant. There is no evidence to suggest that minoxidil has any impact on female fertility. Larger observational and controlled studies would be required to make evidence-based conclusions regarding the safety of minoxidil for women trying to conceive.
Summary Consensus
There is no direct evidence linking topical minoxidil to reduced fertility. The use of topical minoxidil is advised against for women trying to conceive due to a small number of reported cases where topical minoxidil was associated with fetal abnormalities.
Counseling and Clinical Recommendations
The Food and Drug Administration (FDA) classifies topical minoxidil as a pregnancy category C drug. This means studies have shown a risk to the fetus, but there are no adequate, controlled studies in pregnant women. Such drugs are generally only prescribed if there is a significant benefit to the mother that outweighs potential risks. As such, a review published by the American Academy of Dermatology in 2025 advises against using minoxidil while pregnant.[25]Olsen, E. A., Sinclair, R., Hordinsky, M., Mesinkovska, N. A., Sadick, N., Shapiro, J., & Bergfeld, W. (2025). Summation and recommendations for the safe and effective use of topical and oral … Continue reading In the UK, the National Institute for Health and Care Excellence (NICE) also advises against the use of topical minoxidil when pregnant or breastfeeding.[26]National Institute for Health and Care Excellence. (no date). Female-pattern hair loss (female androgenetic alopecia) — Prescribing information: Topical minoxidil. Available at: … Continue reading
Does topical minoxidil cause infertility?
There is no evidence to suggest that topical minoxidil can impact fertility in women. There are a very limited number of cases reported where minoxidil has been associated with impotence in men.[27]Rietschel, R. L., & Duncan, S. H. (1987). Safety and efficacy of topical minoxidil in the management of androgenetic alopecia. Journal of the American Academy of Dermatology. 16(3). 677-685. … Continue reading
Does minoxidil interfere with female hormones or ovulation?
There is limited research into the effect of minoxidil on female hormones or ovulation. However, minoxidil is not a hormonal drug, and there is limited evidence to suggest that it interferes significantly with hormones at levels associated with topical treatment.
Should women stop minoxidil months before trying to conceive?
While there is no evidence that minoxidil can impact women’s ability to conceive, it is not advised to use the treatment while pregnant. Therefore, it is recommended to stop using minoxidil while trying to conceive.[28]Olsen, E. A., Sinclair, R., Hordinsky, M., Mesinkovska, N. A., Sadick, N., Shapiro, J., & Bergfeld, W. (2025). Summation and recommendations for the safe and effective use of topical and oral … Continue reading
Can minoxidil exposure cause miscarriage or birth defects?
There are limited case reports that associate topical minoxidil with birth defects and fetal malformation, leading to termination. These findings have not been expanded on by larger trials or observational studies.[29]Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., & Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth Defects Research Part A: Clinical … Continue reading,[30]Rojansky, N., Fasouliotis, S. J., Ariel, I., & Nadjari, M. (2002). Extreme caudal agenesis. Possible drug-related etiology?. Journal of the American Academy of Dermatology. 47(3). 241-245. … Continue reading
Do all hair loss drugs have the same reproductive risks?
Different hair loss treatments have fundamentally different mechanisms. Finasteride and dutasteride are hormonally active and may impact male fertility and fetal development.[31]BinJadeed, H. F., & Alajlan, A. (2021). Pregnancy and neonatal outcome with maternal exposure to finasteride: a case series. Journal of Dermatology and Dermatologic Surgery. 25(2). 84-86. … Continue reading,[32]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading Risks associated with minoxidil are significantly less well established, and adverse reproductive events have been shown to be significantly lower than finasteride.[33]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading
Latest Research and Knowledge Gaps
There is a significant gap in our knowledge of whether minoxidil affects pregnancy and the mechanisms by which it might do so. Data from preclinical models has focused on hair cycle dynamics rather than reproductive parameters, with only limited research on its impact on androgenic hormonal pathways.
Both of the case studies that associated topical minoxidil with adverse pregnancy outcomes are over 20 years old, with no follow-up studies to better understand these reports. Clinical trials or observational studies would be necessary to make evidence-based assessments on the safety of minoxidil during pregnancy and its impact on female fertility.
Final Thoughts
Current evidence does not show that topical minoxidil impairs fertility or conception. However, because pregnancy safety data remain incomplete, discontinuation is recommended when planning conception – a precautionary measure rather than a proven necessity.
For women experiencing distressing hair loss, treatment decisions should balance cosmetic benefit with potential but unproven reproductive risk, ideally in consultation with a dermatologist or reproductive health specialist.
References[+]
References ↑1, ↑8 Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑2 StatPearls Editorial Team. (no date). Minoxidil. In: StatPearls [Internet]. StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (Accessed: October 2025) ↑3 Thiyagarajan, D. K., Basit, H., & Jeanmonod, R. (2024 Jan 1). Physiology, menstrual cycle. In: StatPearls [Internet]. StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK500020/ (Accessed: October 2025) ↑4 Mayo Clinic Staff. (no date). Female infertility – Symptoms & causes. Available at: https://www.mayoclinic.org/diseases-conditions/female-infertility/symptoms-causes/syc-20354308/ (Accessed: October 2025) ↑5, ↑31 BinJadeed, H. F., & Alajlan, A. (2021). Pregnancy and neonatal outcome with maternal exposure to finasteride: a case series. Journal of Dermatology and Dermatologic Surgery. 25(2). 84-86. Available at: https://doi.org/10.4103/jdds.jdds_33_21 ↑6, ↑23, ↑24, ↑33 Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. Available at: https://doi.org/10.18632/oncotarget.12617 ↑7 Conley, J. M., Lambright, C. S., Evans, N., Cardon, M., Furr, J., Wilson, V. S., & Gray Jr, L. E. (2018). Mixed “antiandrogenic” chemicals at low individual doses produce reproductive tract malformations in the male rat. Toxicological Sciences. 164(1). 166-178. Available at: https://doi.org/10.1093/toxsci/kfy069 ↑9 Singh, S., Patil, A., Kianfar, N., Waśkiel-Burnat, A., Rudnicka, L., Sinclair, R., & Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit?. Clinical and Experimental Dermatology. 47(11). 1951-1955. Available at: https://doi.org/10.1111/ced.15338 ↑10, ↑12 Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: /https://doi.org/10.18632/oncotarget.1886 ↑11 Randolph, M., & Tosti, A. (2021). Oral minoxidil treatment for hair loss: a review of efficacy and safety. Journal of the American Academy of Dermatology. 84(3). 737-746. Available at: https://doi.org/10.1016/j.jaad.2020.06.1009 ↑13 Nuck, B. A., Fogelson, S. L., & Lucky, A. W. (1987). Topical minoxidil does not act as an antiandrogen in the flank organ of the golden Syrian hamster. Archives of Dermatology. 123(1). 59-61. Available at: https://doi.org/10.1001/archderm.1987.01660250065019 ↑14 Turkina, V. A., Chemodurova, N. Y., Pryzyglei, H. V., & Kuzminov, Y. B. (2021). Potential embryotoxic effect study of minoxidil-containing lotion in experiment with female rats. Світ медицини та біології. (2). 248-251. Available at: https://doi.org/10.26724/2079-8334-2021-2-76-248-251 ↑15 Stock, S. J., & Norman, J. E. (2019). Medicines in pregnancy. F1000Research. 8(F1000Faculty Rev):911. Available at: https://doi.org/10.12688/f1000research.17535.1 ↑16 Phelan, A. L., Kunselman, A. R., Chuang, C. H., Raja-Khan, N. T., & Legro, R. S. (2016). Exclusion of women of childbearing potential in clinical trials of type 2 diabetes medications: a review of protocol-based barriers to enrollment. Diabetes Care. 39(6). 1004-1009. Available at: https://doi.org/10.2337/dc15-2723 ↑17 Thomas, S. H., & Yates, L. M. (2012). Prescribing without evidence – pregnancy. British Journal of Clinical Pharmacology. 74(4). 691-697. Available at: https://doi.org/10.1111/j.1365-2125.2012.04332.x ↑18 Koh, Y. P., Tian, E. A., & Oon, H. H. (2019). New changes in pregnancy and lactation labelling: Review of dermatologic drugs. International Journal of Women’s Dermatology. 5(4). 216-226. Available at: https://doi.org/10.1016/j.ijwd.2019.05.002 ↑19 Truong, T. M., Yaghi, M., & Murase, J. (2023). Dermatologic Drug Safety in Pregnancy. Journal of Dermatology for Physician Assistants. 17(3). 1-12. Available at: https://doi.org/10.58744/001c.88954 ↑20 Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777–2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑21, ↑29 Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., & Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth Defects Research Part A: Clinical and Molecular Teratology. 67(12). 997-1001. Available at: https://doi.org/10.1002/bdra.10095 ↑22, ↑30 Rojansky, N., Fasouliotis, S. J., Ariel, I., & Nadjari, M. (2002). Extreme caudal agenesis. Possible drug-related etiology?. Journal of the American Academy of Dermatology. 47(3). 241-245. Available at: https://pubmed.ncbi.nlm.nih.gov/11933692/ ↑25, ↑28 Olsen, E. A., Sinclair, R., Hordinsky, M., Mesinkovska, N. A., Sadick, N., Shapiro, J., & Bergfeld, W. (2025). Summation and recommendations for the safe and effective use of topical and oral minoxidil. Journal of the American Academy of Dermatology. 93(2). 457-465. Available at: https://doi.org/10.1016/j.jaad.2025.04.016 ↑26 National Institute for Health and Care Excellence. (no date). Female-pattern hair loss (female androgenetic alopecia) — Prescribing information: Topical minoxidil. Available at: https://cks.nice.org.uk/topics/female-pattern-hair-loss-female-androgenetic-alopecia/prescribing-information/topical-minoxidil/ (Accessed: October 2025) ↑27 Rietschel, R. L., & Duncan, S. H. (1987). Safety and efficacy of topical minoxidil in the management of androgenetic alopecia. Journal of the American Academy of Dermatology. 16(3). 677-685. Available at: https://doi.org/10.1016/S0190-9622(87)70087-5 ↑32 Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. Available at: https://doi.org/10.18632/oncotarget.12617 Topical minoxidil is one of the most widely used treatments for androgenic alopecia (AGA) in both men and women. While its efficacy and safety as a hair growth stimulant are well established, some men express concern about whether continuing hair loss treatments is safe when trying to conceive. These concerns often stem from known reproductive warnings associated with other medications, particularly finasteride, which directly influences hormonal pathways. Minoxidil, however, works through entirely different mechanisms, enhancing blood flow, prolonging the hair growth phase, and stimulating follicular activity.
This article examines the available scientific evidence to determine whether topical minoxidil has any measurable effect on male fertility. By reviewing preclinical studies, clinical trials, and pharmacologic data, we aim to clarify whether there is any credible link between minoxidil use and changes in reproductive hormones, sperm quality, or conception outcomes.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

How Does Minoxidil Work?
Topical minoxidil stimulates hair growth primarily by increasing blood flow and nutrient delivery to hair follicles. Once applied to the scalp, it’s converted by the enzyme sulfotransferase (SULT1A1) into its active metabolite, minoxidil sulfate, which promotes follicular cell proliferation and prolongs the anagen (growth) phase of the hair cycle. It also upregulates vascular endothelial growth factor (VEGF) and activates potassium channels, both of which create a more favorable microenvironment for hair growth.[1]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading
Many people choose topical minoxidil over oral formulations because it offers similar local benefits with minimal systemic absorption. This greatly reduces the risk of side effects such as water retention, hypertrichosis (excess body hair), or cardiovascular symptoms sometimes seen with oral minoxidil. As an over-the-counter option with a well-established safety profile, topical minoxidil remains the most accessible first-line treatment for AGA.
Male Fertility: What Matters When Trying to Conceive?
Male fertility is defined as the ability to produce viable sperm capable of fertilizing an egg, and is impacted by a wide range of factors. These include hormonal regulation of sperm production, urogenital abnormalities, genetic factors, and environmental factors such as diet, obesity, and cigarette smoking. Semen quality is the best single snapshot of male reproductive potential. Sperm count influences the statistical odds that enough sperm reach the egg; sperm motility determines whether sperm can traverse cervical mucus, the uterus, and the fallopian tube; and morphology reflects the proportion of intact sperm likely to bind and penetrate the egg. These parameters work together, so clinicians interpret them as a profile rather than in isolation.[2]StatPearls; Male Infertility. Available at: https://www.ncbi.nlm.nih.gov/books/NBK562258/ (Accessed: October 2025)
Importantly, medications or drugs can influence many aspects of sperm production and function. Certain prescription drugs, such as anabolic steroids, testosterone supplements, finasteride, spironolactone, and some antidepressants or antihypertensives, can suppress the hormonal pathways that regulate spermatogenesis, reducing sperm count or motility. Others may interfere with sperm maturation, DNA integrity, or seminal fluid composition, leading to poorer fertilization potential. Even topical or seemingly localized medications can, in rare cases, exert mild systemic effects that alter reproductive hormones or oxidative balance.[3]Semet, M., Paci, M., Saïas‐Magnan, J., Metzler‐Guillemain, C., Boissier, R., Lejeune, H., & Perrin, J. (2017). The impact of drugs on male fertility: a review. Andrology. 5(4). 640-663. … Continue reading
For this reason, men actively trying to conceive are often advised to review all current medications and supplements with a clinician to assess potential reproductive impacts and, if necessary, explore safer alternatives or temporary adjustments.
Systemic Absorption of Topical Minoxidil: How Much Gets In?
Topical minoxidil is designed to act locally on the scalp, with only a small fraction of the drug entering the bloodstream. Studies estimate that around 1-2% of applied minoxidil is systemically absorbed through intact skin, though this can vary depending on factors such as scalp condition, formulation type, and frequency of use.
For instance, alcohol-based liquid solutions may enhance penetration slightly more than foam preparations, while damaged or inflamed skin can increase absorption. The scalp’s stratum corneum serves as the main barrier, limiting systemic exposure and ensuring that most of the drug remains localized to hair follicles.[4]Gupta, A. K., Talukder, M., Venkataraman, M., & Bamimore, M. A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: … Continue reading
In contrast, oral minoxidil is fully absorbed into systemic circulation and metabolized in the liver, resulting in significantly higher plasma concentrations of active minoxidil sulfate. This explains why oral therapy, though often more potent, also carries a greater risk of systemic side effects, including edema, tachycardia, and generalized hypertrichosis.[5]Randolph, M., & Tosti, A. (2021). Oral minoxidil treatment for hair loss: a review of efficacy and safety. Journal of the American Academy of Dermatology. 84(3). 737-746. Available at: … Continue reading Topical use, by comparison, delivers similar follicular benefits with a much lower risk profile, making it the preferred option for those seeking targeted hair regrowth.
Does Topical Minoxidil Affect Male Fertility?
Topical minoxidil might raise concerns for men trying to conceive, as its activity and impact are related to hormonally sensitive pathways. To explore this question properly, we can look at the evidence from laboratory models, human studies, and systematic analyses to understand any potential risks.
Preclinical Evidence
Other hair loss treatments, such as finasteride, act on hormonal pathways to alter the activity of androgens like testosterone. They may therefore pose a risk to male fertility. As such, it’s important to establish the impact of minoxidil on hormonal factors such as androgen response.
Animal models, particularly those involving rodents and primates, have primarily examined hair cycle dynamics rather than reproductive parameters. These studies consistently show that minoxidil promotes the transition of hair follicles from the telogen (resting) to anagen (growth) phase, enlarges follicular structures, and increases local blood flow.
One study using golden Syrian hamsters showed that 5% topical minoxidil did not prevent the androgen-dependent growth of pigmented spots, suggesting that minoxidil does not have any effect on androgen response.[6]Nuck, B. A., Fogelson, S. L., & Lucky, A. W. (1987). Topical minoxidil does not act as an antiandrogen in the flank organ of the golden Syrian hamster. Archives of Dermatology. 123(1). 59-61. … Continue reading
In contrast, a more recent, small-scale study using rats investigated the impact of topical minoxidil on sperm health. They found that the experimental group, receiving 10% minoxidil, had a 12-13% decline in testicular weight compared to the controls, as well as increases in markers related to oxidative stress within the testicles.[7]Turkina, V., Chemodurova, N., Hrushka, O., & Pryzyhlei, H. (2020). Topical effect of minoxidil-containing lotion on morphofunctional indicators of male rats’ reproductive system. … Continue reading Unfortunately, the authors do not specify the volume of 10% minoxidil used, making it impossible to make a concrete comparison to normal dosing in humans. This makes the translatability of these toxicology studies more challenging.
Research using cell-based models, including human hair dermal papilla cells, suggests that minoxidil can directly interact with and weaken androgen receptor signaling. However, this study used higher concentrations of minoxidil than would be used in hair loss treatment, and for practical purposes, topical minoxidil remains classified as a non-hormonal treatment.[8]Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: … Continue reading
Figure 1: In vitro studies show a potential effect of minoxidil on androgen receptor activity. Adapted from:[9]Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: … Continue reading Image used in accordance with the Creative Commons Attribution Licence.
Clinical Evidence
While preclinical studies can provide insights into minoxidil activity, evidence for its effect and potential risks should be derived primarily from studies in humans. In clinical settings, topical minoxidil has been studied extensively for AGA, with trials ranging from short-term safety assessments to large randomized controlled trials. Across numerous studies, using 2%, 3%, 5%, and even 10% topical concentrations, the most commonly reported adverse events have been mild scalp irritation, contact dermatitis, or unwanted hair growth on non-target areas.[10]Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical … Continue reading,[11]Hasanzadeh, H., Nasrollahi, S. A., Halavati, N., Saberi, M., & Firooz, A. (2016). Efficacy and safety of 5% minoxidil topical foam in male pattern hair loss treatment and patient satisfaction. … Continue reading,[12]Ghonemy, S., Alarawi, A., & Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a … Continue reading
One randomized trial of 149 subjects reported two cases of impotence during the study, which disappeared following discontinuation of minoxidil treatment.[13]Rietschel, R. L., & Duncan, S. H. (1987). Safety and efficacy of topical minoxidil in the management of androgenetic alopecia. Journal of the American Academy of Dermatology. 16(3). 677-685. … Continue reading This was the only case of sexual side effects we could find, and it seems to represent a rare complaint. Notably, however, none of these trials have reported reproductive or hormonal disturbances.
One randomized, controlled study that compared the impact of topical minoxidil alone to a solution containing 0.25% finasteride and 3% minoxidil explicitly monitored sexual side effects. The trial assessed levels of dihydrotestosterone (DHT), a marker for androgen suppression that may be relevant to male fertility. The report found a modest decrease in DHT only in the group who also applied finasteride, suggesting that minoxidil did not impact androgen activity.[14]Suchonwanit, P., Srisuwanwattana, P., Chalermroj, N., & Khunkhet, S. (2018). A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride … Continue reading
The largest comparative analysis to date, using the FDA Adverse Event Reporting System (FAERS) data, reinforces these findings. Over ten years of reporting revealed that finasteride was associated with markedly higher rates of erectile dysfunction, decreased libido, and ejaculatory disorders. In contrast, minoxidil, both topical and oral, was not associated with reproductive toxicity in either men or women. Dermatologic reactions such as irritation or dermatitis were the only consistent signals. This post-marketing evidence, covering thousands of patients, supports the conclusion that minoxidil does not interfere with male sexual function or fertility.[15]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading
It is worth noting that most clinical studies do not measure semen parameters, testosterone, or dihydrotestosterone (DHT) levels in participants, so while there is no evidence of harm, data on direct fertility endpoints remain limited. However, trials that have assessed hormonal changes indirectly, such as topical finasteride-minoxidil combinations, report only minimal and clinically insignificant reductions in DHT, well within physiologic ranges and unlikely to impact spermatogenesis. Collectively, this body of evidence supports that topical minoxidil, when used at therapeutic concentrations, exerts its effects locally on hair follicles without meaningful systemic hormonal consequences.
Comparison to Other Hair Loss Treatments
Compared with other medical treatments for hair loss, topical minoxidil has a distinctly favorable reproductive safety profile. Finasteride and dutasteride, both 5-α-reductase inhibitors, work by suppressing the conversion of testosterone to DHT, a potent androgen involved in follicular miniaturization. While effective, this mechanism directly influences sex hormone pathways and has been associated in some users with reduced libido, erectile dysfunction, decreased semen volume, and abnormal sperm morphology.[16]Gupta, A. K., Venkataraman, M., Talukder, M., & Bamimore, M. A. (2022). Finasteride for hair loss: a review. Journal of Dermatological Treatment. 33(4). 1938-1946. Available at: … Continue reading,[17]Estill, M. C., Ford, A., Omeira, R., & Rodman, M. (2023). Finasteride and dutasteride for the treatment of male androgenetic alopecia: a review of efficacy and reproductive adverse effects. … Continue reading
Topical minoxidil, in contrast, exerts no effect on androgen synthesis or receptor binding, acts locally, and is absorbed systemically at rates of roughly 1-2%. Clinical and pharmacologic studies have not detected significant changes in serum hormones, testicular function, or sperm parameters associated with its use. For men attempting to conceive, topical minoxidil is therefore considered the safer option when pharmacologic hair restoration is desired.
Summary Consensus
No systematic reviews or meta-analyses have found an association between topical minoxidil and male reproductive dysfunction. Across clinical trials and pharmacovigilance datasets (such as FAERS), the consensus remains that topical minoxidil poses minimal to no risk to male fertility. Its non-hormonal mechanism stands in contrast to antiandrogenic drugs like finasteride or dutasteride, which are known to suppress DHT levels and can cause sexual side effects in some users.
The absence of evidence of reproductive toxicity for topical minoxidil is notable given its widespread use over several decades. Still, it is important to acknowledge that the absence of evidence is not definitive proof of absence, and few studies have directly measured sperm quality or fertility outcomes, leaving a modest evidence gap for future research.
Counseling and Clinical Recommendations
Professional society guidelines, including those from the American Academy of Dermatology (AAD), European Academy of Dermatology and Venereology (EADV), and National Institute for Health and Care Excellence (NICE), all recognize topical minoxidil as a first-line treatment for AGA. None include warnings about male fertility or recommend discontinuing treatment when trying to conceive. Recommendations against use in pregnancy and breastfeeding are directed exclusively at women due to potential fetal exposure, not paternal risk.[18]Olsen, E. A., Sinclair, R., Hordinsky, M., Mesinkovska, N. A., Sadick, N., Shapiro, J., & Bergfeld, W. (2025). Summation and recommendations for the safe and effective use of topical and oral … Continue reading
In clinical practice, dermatologists generally reassure male patients that topical minoxidil can be safely continued when planning conception. They may, however, emphasize good application hygiene, using the prescribed dose, applying only to the scalp, washing hands afterward, and allowing the product to dry fully before physical contact to prevent unintended transfer to partners or infants. Because systemic absorption is minimal, these precautions are primarily to avoid topical irritation rather than systemic effects.
Myths and Misconceptions About Topical Minoxidil and Fertility
Given the links between minoxidil activity and hormonally sensitive pathways related to hair loss, some men may have concerns about using the medication when trying to conceive. Let’s look at some of the potential misconceptions about topical minoxidil and fertility.
Topical minoxidil causes infertility
There is some limited evidence from animal studies suggesting that topical minoxidil may affect sperm quality.[19]Turkina, V., Chemodurova, N., Hrushka, O., & Pryzyhlei, H. (2020). Topical effect of minoxidil-containing lotion on morphofunctional indicators of male rats’ reproductive system. … Continue reading However, there is no evidence that topical minoxidil reduces sperm count, motility, or morphology. Clinical trials of 3%, 5%, and 10% minoxidil report vanishingly few reproductive or hormonal side effects. The drug acts locally on the scalp, with systemic absorption of only about 1–2%, far below levels that could influence reproductive physiology. Neither animal studies nor human trials have identified any fertility-related toxicity associated with topical minoxidil use.
Topical minoxidil alters male hormone levels
Studies suggest that minoxidil does not interfere with androgen production, metabolism, or receptor binding at doses relevant for hair loss. It promotes hair growth through vascular and cellular mechanisms, such as increasing VEGF expression and opening potassium channels, not by modifying testosterone or dihydrotestosterone (DHT) pathways.
However, some research using cell-based models has suggested that minoxidil might impact androgen receptor activity at high concentrations.[20]Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: … Continue reading
Topical minoxidil causes erectile dysfunction
Sexual side effects are very rarely reported in any major clinical trials or pharmacovigilance data concerning topical minoxidil, with only two cases of impotence associated with topical minoxidil reported in the literature. Erectile dysfunction, decreased libido, and ejaculatory changes are associated with oral 5-α-reductase inhibitors (such as finasteride), not with topical minoxidil.[21]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading
Fertility risks are the same for all hair loss drugs
Different hair loss treatments have fundamentally different mechanisms. Finasteride and dutasteride lower DHT and can influence sexual function and semen parameters in some users.[22]Estill, M. C., Ford, A., Omeira, R., & Rodman, M. (2023). Finasteride and dutasteride for the treatment of male androgenetic alopecia: a review of efficacy and reproductive adverse effects. … Continue reading Minoxidil, on the other hand, does not interact with the androgen pathway and has no known reproductive risks. Therefore, the fertility profile of topical minoxidil is significantly safer than that of antiandrogenic agents.
Final Thoughts
Topical minoxidil remains one of the safest and most accessible treatments for male pattern hair loss, with decades of research supporting its efficacy and tolerability. Unlike hormone-modulating therapies such as finasteride, minoxidil works through non-androgenic pathways and shows no evidence of affecting male fertility, hormone levels, or sexual function. While future studies could further clarify long-term reproductive outcomes, the current consensus is clear: topical minoxidil is a fertility-neutral, first-line option for men looking to manage hair loss safely.
References[+]
References ↑1 Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑2 StatPearls; Male Infertility. Available at: https://www.ncbi.nlm.nih.gov/books/NBK562258/ (Accessed: October 2025) ↑3 Semet, M., Paci, M., Saïas‐Magnan, J., Metzler‐Guillemain, C., Boissier, R., Lejeune, H., & Perrin, J. (2017). The impact of drugs on male fertility: a review. Andrology. 5(4). 640-663. Available at: https://doi.org/10.1111/andr.12366 ↑4 Gupta, A. K., Talukder, M., Venkataraman, M., & Bamimore, M. A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: https://doi.org/10.1080/09546634.2021.1945527 ↑5 Randolph, M., & Tosti, A. (2021). Oral minoxidil treatment for hair loss: a review of efficacy and safety. Journal of the American Academy of Dermatology. 84(3). 737-746. Available at: https://doi.org/10.1016/j.jaad.2020.06.1009 ↑6 Nuck, B. A., Fogelson, S. L., & Lucky, A. W. (1987). Topical minoxidil does not act as an antiandrogen in the flank organ of the golden Syrian hamster. Archives of Dermatology. 123(1). 59-61. Available at: https://doi.org/10.1001/archderm.1987.01660250065019 ↑7, ↑19 Turkina, V., Chemodurova, N., Hrushka, O., & Pryzyhlei, H. (2020). Topical effect of minoxidil-containing lotion on morphofunctional indicators of male rats’ reproductive system. Український журнал сучасних проблем токсикології. (2). 27-31. Available at: https://protox.medved.kiev.ua/index.php/en/issues/2020/2/item/633-topical-effect-of-minoxidil-containing-lotion-on-morphofunctional-indicators-of-male-rats-reproductive-system ↑8 Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: /https://doi.org/10.18632/oncotarget.1886 ↑9, ↑20 Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: /https://doi.org/10.18632/oncotarget.1886 ↑10 Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. Journal of the American Academy of Dermatology. 47(3). 377-385. Available at: https://doi.org/10.1067/mjd.2002.124088 ↑11 Hasanzadeh, H., Nasrollahi, S. A., Halavati, N., Saberi, M., & Firooz, A. (2016). Efficacy and safety of 5% minoxidil topical foam in male pattern hair loss treatment and patient satisfaction. Acta Dermatovenerol Alp Pannonica Adriat. 25(3). 41-44. Available at: https://doi.org/10.15570/actaapa.2016.12 ↑12 Ghonemy, S., Alarawi, A., & Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. Journal of Dermatological Treatment. 32(2). 236-241. Available at: https://doi.org/10.1080/09546634.2019.1654070 ↑13 Rietschel, R. L., & Duncan, S. H. (1987). Safety and efficacy of topical minoxidil in the management of androgenetic alopecia. Journal of the American Academy of Dermatology. 16(3). 677-685. Available at: https://doi.org/10.1016/S0190-9622(87)70087-5 ↑14 Suchonwanit, P., Srisuwanwattana, P., Chalermroj, N., & Khunkhet, S. (2018). A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. Journal of the European Academy of Dermatology and Venereology. 32(12). 2257-2263. Available at: https://doi.org/10.1111/jdv.15171 ↑15, ↑21 Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. Available at: https://doi.org/10.18632/oncotarget.12617 ↑16 Gupta, A. K., Venkataraman, M., Talukder, M., & Bamimore, M. A. (2022). Finasteride for hair loss: a review. Journal of Dermatological Treatment. 33(4). 1938-1946. Available at: https://doi.org/10.1080/09546634.2021.1959506 ↑17 Estill, M. C., Ford, A., Omeira, R., & Rodman, M. (2023). Finasteride and dutasteride for the treatment of male androgenetic alopecia: a review of efficacy and reproductive adverse effects. Georgetown Medical Review. 7(1). Available at: https://doi.org/10.52504/001c.88531 ↑18 Olsen, E. A., Sinclair, R., Hordinsky, M., Mesinkovska, N. A., Sadick, N., Shapiro, J., & Bergfeld, W. (2025). Summation and recommendations for the safe and effective use of topical and oral minoxidil. Journal of the American Academy of Dermatology. 93(2). 457-465. Available at: https://doi.org/10.1016/j.jaad.2025.04.016 ↑22 Estill, M. C., Ford, A., Omeira, R., & Rodman, M. (2023). Finasteride and dutasteride for the treatment of male androgenetic alopecia: a review of efficacy and reproductive adverse effects. Georgetown Medical Review. 7(1). Available at: https://doi.org/10.52504/001c.88531 Finasteride is widely used for androgenic alopecia (AGA) and benign prostate hyperplasia (BPH). It’s generally well tolerated, with most users experiencing no significant adverse effects. However, clinicians in eye clinics have reported occurrences of dry eye that could be related to the use of finasteride.[1]NZ Optics. (2022). Finasteride and dry eye disease. Available at: https://www.nzoptics.co.nz/live-articles/finasteride-and-dry-eye-disease/ (Accessed: October 2025)
Dry eye disease (DED) is a common eye disorder characterized by insufficient tear production or poor tear quality. This can cause inadequate lubrication of the eye’s surface, leading to discomfort, irritation, and potentially damage to the eye. The condition is multifactorial, meaning it can be caused by a number of different factors. One important cause is increased tear evaporation, leading to evaporative dry eye. The quality and structure of tears are regulated by hormonal pathways, meaning that the anti-androgenic activity of finasteride could drive the development of the condition.[2]Golden, M. I., Meyer, J. J., Zeppieri, M., & Patel, B. C. (2024). Dry Eye Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: … Continue reading
In this article, we will examine the research that links finasteride to dry eye disease and explore the potential mechanisms by which it may be contributing to the condition.
Interested in Oral Finasteride?
Oral finasteride & minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Finasteride and the Eye
Finasteride is a 5α-reductase inhibitor and works by reducing the conversion of testosterone to dihydrotestosterone (DHT). DHT drives the shrinking and weakening of hair follicles in AGA, meaning that finasteride activity can counteract these effects by decreasing the amount of DHT. Finasteride is typically administered orally, meaning it can have systemic effects on multiple parts of the body. This is particularly important in tissues that are sensitive to androgens like DHT.[3]Zito, P. M., Bistas, K. G., Patel, P., & Syed, K. (2024). Finasteride. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: … Continue reading
Figure 1. 5α-reductase converts testosterone into dihydrotestosterone. Finasteride is a 5α-reductase inhibitor, meaning it prevents or reduces this activity. Image adapted from Figure 1.[4]Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti 5-alpha-reductase activity: A prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: … Continue reading Image used under Creative Commons License.
The meibomian glands are one such tissue. They are special sebaceous (oil-producing) glands located within the upper and lower eyelids, with each eye containing 20 – 40 of these glands. They produce meibum, a lipid-rich secretion that forms the outermost layer of the tear film and plays a critical role in maintaining ocular surface health. With every blink, meibum is expressed from the gland orifices, forming a thin, uniform layer over the eye. Meibum’s main function is to prevent evaporation of the tear film by creating a stable oil barrier, ensuring long-lasting lubrication of the eye’s surface.[5]Sabeti, S., Kheirkhah, A., Yin, J., & Dana, R. (2020). Management of meibomian gland dysfunction: a review. Survey of Ophthalmology. 65(2). 205-217. Available at: … Continue reading
Figure 2. The meibomian glands are arranged in parallel rows within the upper and lower eyelids. They produce meibum, which forms the lipid layer of the tear that coats the surface of the eye. Image adapted from Figure 3.[6]Dhamdhere, K., & Badawi, D. (2021). A blink-assisted, cornea-sparing wearable eyelid device for the effective penetration of therapeutic thermal energy into the meibomian glands for the treatment … Continue reading Image used under Creative Commons License.
Meibomian glands are directly regulated by androgens, via receptors present in their epithelial cells. Androgens, such as DHT, can bind to these receptors, directly influencing the synthesis of lipids essential for tear formation. Importantly, DHT is also synthesized in meibomian glands by 5α-reductase. This is where the connection to finasteride comes in: inhibition of 5α-reductase by the drug could stop DHT activity in the meibomian glands, stopping the production of lipids required to prevent tear evaporation. As such, while finasteride doesn’t target the eye, its impact on androgen pathways can have indirect effects.[7]Traish, A. M. (2020). Health risks associated with long-term finasteride and dutasteride use: it’s time to sound the alarm. The World Journal of Men’s Health. 38(3). 323-337. Available at: … Continue reading
Even partial androgen suppression, like oral finasteride for hair, could be enough to impact tear production, particularly in someone who has other risk factors associated with DED, such as older age, prolonged screen time, certain medications (antihistamines, antidepressants, diuretics, beta-blockers), autoimmune or connective tissue diseases, and environmental exposures such as wind, dry climates, or smoke.
Do DHT-Reducing Therapies Lead to Dry Eye?
A role for androgens in regulating meibomian gland function was proposed in a paper published in 2000.[8]Krenzer, K. L., Reza Dana, M., Ullman, M. D., Cermak, J. M., Tolls, D. B., Evans, J. E., & Sullivan, D. A. (2000). Effect of androgen deficiency on the human meibomian gland and ocular surface. … Continue reading Patients who were taking antiandrogen drugs for issues related to the prostate were assessed on symtpoms of DED and the contents of their meibomian gland secretions. They were compared to a control group that was on such medications. They found that antiandrogen therapy changes the composition of lipids in tears, while also decreasing the quality of tears and increasing participants’ susceptibility to light sensitivity, painful eyes, and blurred vision. This study laid the foundation for our current understanding of how DHT-lowering therapies might cause DED.
Preclinical Data
Animal models have been developed to understand the mechanisms that lead to DED, including a potential role for DHT-lowering therapies as suggested by these clinical findings. A 2014 study utilized a rat model to investigate the impact of finasteride on tear production.[9]Singh, S., Moksha, L., Sharma, N., Titiyal, J. S., Biswas, N. R., & Velpandian, T. (2014). Development and evaluation of animal models for sex steroid deficient dry eye. Journal of … Continue reading They found that oral administration of 1.6 mg/kg finasteride every day significantly decreased tear flow and increased the speed at which tears break up after 10 days in both male and female rats.
This study was supported by further research published in 2017, which used the same concentration of finasteride in rats for 40 days.[10]Li, K., Zhang, C., Yang, Z., Wang, Y., & Si, H. (2017). Evaluation of a novel dry eye model induced by oral administration of finasteride. Molecular Medicine Reports. 16(6). 8763-8770. Available … Continue reading They also found that tear production was reduced in finasteride-treated rats, and makers for tear break-up were increased. The impact of finasteride on tear production in rats is so reliable that it has been used to induce DED in models investigating potential treatments for the condition.[11]Zhang, C., Li, K., Yang, Z., Wang, Y., & Si, H. (2016). The effect of the aqueous extract of Bidens pilosa L. on androgen deficiency dry eye in rats. Cellular Physiology and Biochemistry. 39(1). … Continue reading
Figure 3. Finasteride treatment (Fin – right-hand group) increases fluorescein staining in the tears of rats, an indicator of tear break-up. Adapted from Figure 1.[12]Li, K., Zhang, C., Yang, Z., Wang, Y., & Si, H. (2017). Evaluation of a novel dry eye model induced by oral administration of finasteride. Molecular Medicine Reports. 16(6). 8763-8770. Available … Continue reading Image used under Creative Commons License.
Notably, these animal models use considerably higher concentrations of finasteride than would be recommended for hair loss – an equivalent dose for the average American man would be around 126 mg per day. In comparison, the standard regimen for AGA is only 1 mg per day. However, they do demonstrate the capacity for finasteride to impact tear production.
Clinical Data
Preclinical data, as well as a deeper understanding of the androgen-associated causes of DED, led to some concern about the potential adverse effects of finasteride.[13]Traish, A. M. (2020). Health risks associated with long-term finasteride and dutasteride use: it’s time to sound the alarm. The World Journal of Men’s Health. 38(3). 323-337. Available at: … Continue reading
Recently, clinical researchers have further explored this link. A retrospective case series looked at 116 patients at an eye clinic with a history of DED who were taking finasteride.[14]Nguyen, B. J., Meer, E., Gupta, A. S., Jinpeng, G., Ying, G. S., Bunya, V. Y., Macchi, I., & Massaro-Giordano, M. (2022). The effect of finasteride on dry eye disease. Investigative Ophthalmology … Continue reading
They found that a significantly greater proportion of patients had MGD, conjunctival abnormalities, and corneal abnormalities during follow-up examinations compared to their initial assessment, a period of four and a half years on average.
They also found a trend toward increased disease severity in patients on a higher dose of finasteride, although these findings were not statistically significant. It is hard to draw any conclusions from this research, as it only looked at individuals who both took finasteride and had DED. A retrospective study or clinical trial that included individuals who did not take finasteride would provide significantly greater insights.
Anecdotal reports from an eye clinic in New Zealand have indicated that increasingly, patients presenting with DED are also taking finasteride.[15]NZ Optics. (2022). Finasteride and dry eye disease. Available at: https://www.nzoptics.co.nz/live-articles/finasteride-and-dry-eye-disease/ (Accessed: October 2025) Data on the impact of finasteride on DED is, therefore, lacking, and robust clinical studies are required to better understand the potential link.
Some conclusions can also be drawn from clinical studies investigating other antiandrogenic drugs. Such medications are often used against prostate cancer, which depends on androgens such as testosterone and DHT for its growth and survival. A retrospective study compared a group of 1791 patients with prostate cancer who were taking a range of androgen deprivation therapies with those who weren’t.[16]Chien, H. W., Lin, C. W., Lee, C. Y., Huang, J. Y., Yang, S. F., & Wang, K. (2022). The use of androgen deprivation therapy for prostate cancer and its effect on the subsequent dry eye disease: a … Continue reading They found no significant difference in the development of DED.
A similar study compared a smaller group of prostate cancer patients on antiandrogens (31 in total) to those not on such medications. However, it investigated tear formation in greater depth.[17]Kurna, S. A., Hacisalihoglu, A. O., Altun, A., Ozel, N. O., Uruc, F., Kanar, H. S., & Arsan, A. K. (2022). Effects of systemic anti-androgen drugs on the ocular surface. Journal Français … Continue reading
They found a significant increase in markers for tear break-up and complaints of dry eye. Importantly, neither of these studies distinguishes between forms of antiandrogen therapy, nor do they specifically investigate the role of finasteride.
Summary Consensus
Data from animal models provide a clear mechanism by which finasteride could impact tear production and cause MGD and DED. However, the limited availability of clinical data makes it challenging to draw evidence-based conclusions about the potential adverse effects of finasteride. Clinical studies using a wide range of antiandrogen therapies in a different cohort (prostate cancer patients) also provide conflicting information. It’s important to remember that the absence of evidence doesn’t mean definitive proof of absence, and you should still consider the potential effects on your eyes when taking finasteride.
How Might Finasteride Cause Dry Eye?
- Finasteride reduces DHT: Finasteride blocks 5α-reductase, lowering dihydrotestosterone (DHT) levels in the body.
- Decreased androgen activity in eyelid glands: Lower DHT means less stimulation of the meibomian glands, which rely on androgens to produce the oils that keep tears in place.
- Reduced meibum quality and quantity: With reduced androgen influence, these glands produce less oil, thereby decreasing the stability of the tear film.
- Gland obstruction and dysfunction: Poor-quality meibum can clog gland openings, leading to meibomian gland dysfunction and reduced tear protection.
- Evaporative tear loss and dry eye symptoms: Tears evaporate more quickly, causing dryness, irritation, and blurred vision.
Secondary effects – Inflammation and surface damage: Ongoing tear film instability can trigger inflammation, discomfort, and damage to the eye’s surface over time.
Who Might Be Most At Risk of DED?
If you’re concerned about the risk of developing DED while taking finasteride, it’s important to consider other risk factors that could have an additive effect.[18]Britten-Jones, A. C., Wang, M. T., Samuels, I., Jennings, C., Stapleton, F., & Craig, J. P. (2024). Epidemiology and risk factors of dry eye disease: considerations for clinical management. … Continue reading Those at risk include:
Peri-and Post Menopausal Women – The hormonal changes of menopause lead to reduced secretion and quality in both lacrimal and meibomian glands, the core tear-producing tissues
Older People – The risk sharply increases after age 40, with prevalence rising further with each decade due to reduced tear production and gland function.
Contact Lens Wearers – Long-term use is linked to higher DED risk due to tear film disruption.
Screen Users – Reduced blink rate and incomplete blinks from device use elevate risk, especially in professional and urban populations.
Individuals on Certain Medications – Antihistamines, antidepressants, hormone therapies, and diuretics reduce tear secretion or alter tear composition.
People Exposed to Dry, Polluted, or Urban Environments – Low ambient humidity, air conditioning, pollution, and smoke contribute to increased evaporation and instability of the tear film.
How Can I Manage or Prevent Dry Eye While on Finasteride?
Dry eye can often be managed through a combination of lifestyle changes, environmental adjustments, proper eye care, and medical support.[19]Donthineni, P. R., Shanbhag, S. S., & Basu, S. (2021). An evidence-based strategic approach to prevention and treatment of dry eye disease, a modern global epidemic. Healthcare. 9(1). 89. … Continue reading If you’re taking finasteride and notice eye discomfort or dryness, these steps may help minimize symptoms and protect long-term eye health.
- Avoid smoky environments and consider quitting smoking. Both active and passive smoke can worsen dryness and irritation.
- Take regular eye breaks. Blinking more often and positioning screens slightly below eye level can also help reduce tear evaporation.
- Clean your eyelids daily using a warm compress followed by gentle cleansing with a mild, non-irritating lid cleanser. This can help keep oil glands functioning correctly, especially if you have blepharitis or meibomian gland dysfunction (MGD).
- Apply lubricating eye drops to keep the eyes moist and comfortable.
- Boost your intake of omega-3s through foods such as salmon, mackerel, walnuts, and flaxseed, or consider supplements under your doctor’s guidance. These healthy fats support meibomian gland function and tear quality.
- Protect your eyes from UV rays and dust by wearing sunglasses outdoors. Avoid rubbing your eyes, get sufficient sleep, and maintain a balanced diet rich in vitamins A, C, and E.
- If dryness, irritation, or visual changes continue, see an ophthalmologist or optometrist. They can assess tear quality and meibomian gland health, and may recommend advanced treatments such as prescription eye drops.
What’s Still Unknown
Although preclinical and clinical evidence suggest a possible link between finasteride and DED, many questions remain unanswered. Animal models suggest a causal connection but cannot replicate human physiology, and typically administer much higher doses than those that would be administered in humans. Large, controlled trials investigating the use of finasteride at the 1 mg hair-loss dose are required to draw evidence-based conclusions about the role of finasteride in DED.
Final Thoughts
Finasteride is an effective, well-established, and typically well-tolerated treatment for hair. However, concerns about links to meibomian gland dysfunction and DED are rising. The eye is a hormonally responsive organ, and systemic medications can have subtle but meaningful effects on ocular health. While animal studies and hormonal mechanisms support a potential link to dry eye disease, human data remain limited and inconclusive, especially at the low doses used for androgenic alopecia. For now, users should monitor for symptoms like dryness or irritation and seek ophthalmologic advice if they occur.
References[+]
References ↑1, ↑15 NZ Optics. (2022). Finasteride and dry eye disease. Available at: https://www.nzoptics.co.nz/live-articles/finasteride-and-dry-eye-disease/ (Accessed: October 2025) ↑2 Golden, M. I., Meyer, J. J., Zeppieri, M., & Patel, B. C. (2024). Dry Eye Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK470411/ (Accessed: October 2025) ↑3 Zito, P. M., Bistas, K. G., Patel, P., & Syed, K. (2024). Finasteride. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK513329/ (Accessed: October 2025) ↑4 Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti 5-alpha-reductase activity: A prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: https://doi.org/10.12688/f1000research.51066.3 ↑5 Sabeti, S., Kheirkhah, A., Yin, J., & Dana, R. (2020). Management of meibomian gland dysfunction: a review. Survey of Ophthalmology. 65(2). 205-217. Available at: https://doi.org/10.1016/j.survophthal.2019.08.007 ↑6 Dhamdhere, K., & Badawi, D. (2021). A blink-assisted, cornea-sparing wearable eyelid device for the effective penetration of therapeutic thermal energy into the meibomian glands for the treatment of dry eye disease. Journal of Clinical & Experimental Ophthalmology. 12 (S12):003. Available at: https://www.longdom.org/open-access/a-blinkassisted-corneasparing-wearable-eyelid-device-for-the-effectivepenetration-of-therapeutic-thermal-energy-into-the.pdf (Accessed: October 2025) ↑7, ↑13 Traish, A. M. (2020). Health risks associated with long-term finasteride and dutasteride use: it’s time to sound the alarm. The World Journal of Men’s Health. 38(3). 323-337. Available at: https://doi.org/10.5534/wjmh.200012 ↑8 Krenzer, K. L., Reza Dana, M., Ullman, M. D., Cermak, J. M., Tolls, D. B., Evans, J. E., & Sullivan, D. A. (2000). Effect of androgen deficiency on the human meibomian gland and ocular surface. The Journal of Clinical Endocrinology & Metabolism. 85(12). 4874-4882. Available at: https://doi.org/10.1210/jcem.85.12.7072 ↑9 Singh, S., Moksha, L., Sharma, N., Titiyal, J. S., Biswas, N. R., & Velpandian, T. (2014). Development and evaluation of animal models for sex steroid deficient dry eye. Journal of Pharmacological and Toxicological Methods. 70(1). 29-34. Available at: https://doi.org/10.1016/j.vascn.2014.03.004 ↑10 Li, K., Zhang, C., Yang, Z., Wang, Y., & Si, H. (2017). Evaluation of a novel dry eye model induced by oral administration of finasteride. Molecular Medicine Reports. 16(6). 8763-8770. Available at: https://doi.org/10.3892/mmr.2017.7754 ↑11 Zhang, C., Li, K., Yang, Z., Wang, Y., & Si, H. (2016). The effect of the aqueous extract of Bidens pilosa L. on androgen deficiency dry eye in rats. Cellular Physiology and Biochemistry. 39(1). 266-277. Available at: https://doi.org/10.1159/000445622 ↑12 Li, K., Zhang, C., Yang, Z., Wang, Y., & Si, H. (2017). Evaluation of a novel dry eye model induced by oral administration of finasteride. Molecular Medicine Reports. 16(6). 8763-8770. Available at: https://doi.org/10.3892/mmr.2017.7754 ↑14 Nguyen, B. J., Meer, E., Gupta, A. S., Jinpeng, G., Ying, G. S., Bunya, V. Y., Macchi, I., & Massaro-Giordano, M. (2022). The effect of finasteride on dry eye disease. Investigative Ophthalmology & Visual Science. 63(7). 427. Available at: https://doi.org/10.1167/iovs.63.7.427 ↑16 Chien, H. W., Lin, C. W., Lee, C. Y., Huang, J. Y., Yang, S. F., & Wang, K. (2022). The use of androgen deprivation therapy for prostate cancer and its effect on the subsequent dry eye disease: a population-based cohort study. International Journal of Medical Sciences. 19(7). 1103-1109. Available at: https://doi.org/10.7150/ijms.73417 ↑17 Kurna, S. A., Hacisalihoglu, A. O., Altun, A., Ozel, N. O., Uruc, F., Kanar, H. S., & Arsan, A. K. (2022). Effects of systemic anti-androgen drugs on the ocular surface. Journal Français d’Ophtalmologie. 45(6). 619-627. Available at: https://doi.org/10.1016/j.jfo.2021.06.007 ↑18 Britten-Jones, A. C., Wang, M. T., Samuels, I., Jennings, C., Stapleton, F., & Craig, J. P. (2024). Epidemiology and risk factors of dry eye disease: considerations for clinical management. Medicina. 60(9). 1458. Available at: https://doi.org/10.3390/medicina60091458 ↑19 Donthineni, P. R., Shanbhag, S. S., & Basu, S. (2021). An evidence-based strategic approach to prevention and treatment of dry eye disease, a modern global epidemic. Healthcare. 9(1). 89. Available at: https://doi.org/10.3390/healthcare9010089 Testosterone replacement therapy (TRT) can be a life-changing treatment for many men, restoring healthy hormone levels and renewing vitality after declines associated with low testosterone. However, many encounter a harsh irony: the same treatment that revitalizes them may also lead to accelerated hair loss, known as androgenetic alopecia (AGA). This frustrating dilemma can compel some men to choose between their appearance and their health.
Why do TRT patients often experience hair thinning just months into treatment? The culprit is testosterone’s conversion to DHT, the hormone that drives male pattern hair loss. Fortunately, new developments in comprehensive care address the testosterone-to-hair-loss pathway, allowing men to enjoy both hormone optimization and healthy hair. These clinically rigorous, customizable, and medically supervised treatments eliminate the either-or paradox.
Perfect Hair Health’s PhD experts have teamed up with Ulo, a leader in men’s wellness and hair care, to share five evidence-based techniques for preserving hair while on TRT. With Ulo’s unique expertise at the intersection of hormone optimization and hair restoration, patients gain access to solutions that are both proven and personalized.
Read on to learn how Ulo can help you maintain both hormonal health and hair density.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

The Science of Testosterone and Hair Loss
Before exploring solutions, we will briefly explain the science behind TRT and hair loss, including relevant medical research that supports prevention-based strategies for preserving hair during TRT.
Testosterone Metabolism and DHT Formation
To understand the link between TRT and hair loss, we turn to the molecular process that converts testosterone into dihydrotestosterone (DHT), the hormone that causes follicular degradation and balding. Two types of 5α-reductase enzymes are responsible for deriving DHT from testosterone: Type I (primarily located in the skin and sebaceous glands) and Type II (mainly located in the hair follicles of the crown and temporal regions).[1]Owecka, B., Tomaszewska, A., Dobrzeniecki, K., Owecki, M. (2024). The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines. 12(3). 513. Available at: … Continue reading
Androgenetic alopecia (AGA) can occur naturally in susceptible individuals, whose hair follicles have high concentrations of androgen receptors and 5α-reductase enzymes. However, adding exogenous T through TRT can expedite this process. TRT’s elevation of testosterone levels can raise DHT to two or three times higher than starting levels, depending on the dosage and mode of application. The conversion of T to DHT is especially prevalent in the crown and temporal areas of the scalp, where it causes hair thinning and loss.[2]Owecka, B., Tomaszewska, A., Dobrzeniecki, K., Owecki, M. (2024). The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines. 12(3). 513. Available at: … Continue reading,[3]Stocks, B., Asempa, O. (2025). Does testosterone replacement therapy cause hair loss? Baylor College of Medicine. Available at: … Continue reading
Genetic predisposition plays a significant role, as variations in androgen receptor genes determine individual sensitivity to DHT. This explains why patients on identical TRT regimens can experience varying levels of hair loss, with some experiencing dramatic loss and others experiencing little or no effect. In those with hereditary androgen sensitivity, the hair follicles respond poorly to even slight elevations in local DHT levels on the scalp.[4]Owecka, B., Tomaszewska, A., Dobrzeniecki, K., Owecki, M. (2024). The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines. 12(3). 513. Available at: … Continue reading
Why TRT Accelerates Pattern Hair Loss
As previously noted, TRT increases testosterone and leads to higher levels of its derivative hormone, DHT, which binds to hair follicles and causes hair thinning and loss in genetically susceptible men. Spikes in DHT and associated hair loss can occur even with moderate TRT, as raising testosterone into the normal range can represent a significant increase from severe deficiency.[5]Borst, S.E., Shuster, J.J., Zou, B., Ye, F., Jia, H., Wokhlu, A., Yarrow, J.F. (2014). Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systemic review … Continue reading
Episodic TRT protocols like weekly injections can create testosterone peaks over 1,200 ng/dL for up to 48 hours after injection, leading to surges in scalp DHT production during these windows. Such DHT spikes can damage the follicles and contribute to rapid hair loss.[6]Stocks, B., Asempa, O. (2025). Does testosterone replacement therapy cause hair loss? Baylor College of Medicine. Available at: … Continue reading,[7]Shoskes, J.J., Wilson, M.K., Spinner, M.L. (2016). Pharmacology of testosterone replacement therapy preparations. Translational Andrology and Urology. 5(6). 834-843. Available at: … Continue reading
Disruption of hair follicle cycles can begin in the first 3-6 weeks of TRT. However, noticeable hair loss typically doesn’t emerge for several months, as early damage is concealed by hair growth cycles. Studies suggest higher TRT doses correlate with more rapid hair loss in susceptible patients.[8]Saad, F., Aversa, A., Isidori, A.M., Zafalon, L., Zitzmann, M., Gooren, L. (2011). Onset of effects of testosterone treatment and time span until maximum effects are achieved. European Journal of … Continue reading,[9]Fu, D., Huang, J., Li, K., Chen, Y., He, Y., Sun, Y., Guo, Y., Du, L., Qu, Q., Miao, Y., Hu, Z. (2021). Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 … Continue reading
Balancing Hormonal Benefits with Hair Protection
Although DHT can harm the hair follicles, it plays a vital role in various biological processes, including sexual function, bone mineralization, and muscle development. Therefore, the aim of hair loss treatment during TRT is not to eliminate the hormone. Rather, the therapeutic goal is to lower DHT to a level that minimizes follicular damage while preserving systemic functions. Striking this balance in individuals often requires sophisticated strategies that exceed standardized DHT suppression protocols.[10]Owecka, B., Tomaszewska, A., Dobrzeniecki, K., Owecki, M. (2024). The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines. 12(3). 513. Available at: … Continue reading
While some patients may be tempted to terminate TRT to protect their hair, research findings support a more measured approach that takes TRT’s broader benefits into account. Patients who elect to stop taking testosterone for hair preservation may experience a return of detrimental symptoms: sexual dysfunction, depression, fatigue, muscle loss, and more. The psychological and physiological impacts of low T can outweigh cosmetic concerns.
Given these considerations, practitioners advise strategic early intervention over reactionary care, as hair restoration becomes much more challenging after considerable follicular shrinkage. Early discussion with healthcare providers about hair preservation strategies helps maintain hair health throughout the hormone optimization process.[11]Owecka, B., Tomaszewska, A., Dobrzeniecki, K., Owecki, M. (2024). The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines. 12(3). 513. Available at: … Continue reading
With the science behind TRT and hair loss clear, let’s look at the latest, evidence-based ways patients can protect their hair.
Expert Solutions for TRT-Related Hair Loss
Hair loss associated with TRT often has distinct features, like episodic spikes in testosterone and DHT conversion rates. Although conventional hair loss interventions can be useful, protocols may benefit from personalization according to TRT regimens.
Ulo is a unique provider due to its innovative hair loss treatments and dual expertise in both hair restoration and hormone optimization. This combined clinical experience enables them to tailor treatments to individual patient needs, such as timing factors and treatment responses specific to TRT. All Ulo treatment protocols are physician-crafted and include close follow-up care to monitor for side effects and make adjustments as needed.
Treatment Options Overview
This overview outlines Ulo’s comprehensive strategy for hair loss intervention, from advanced prescription formulas to targeted nutritional support. All offerings are transparently priced with customizable features to account for TRT-related factors and individual patient considerations.
Product Category Mechanism of Action Delivery Method Formula Details Price Oral Medications Systemic 5α-reductase inhibition and vasodilation Oral capsule, tablet, or softgel Finasteride, dutasteride, and minoxidil for systemic DHT blocking and growth stimulation $24-65/month Topical Finasteride Localized Type II 5α-reductase inhibition with minimal systemic exposure Solution Concentration range (0.005%-0.2%); customizable adjuvants; irritant-free $64+/month Topical Dutasteride Localized dual enzyme (Types I & II) inhibition for maximum scalp DHT suppression Solution Concentration range (0.02%-0.2%); customizable adjuvants; irritant-free $84+/month Growth Booster Topicals Vasodilation, anti-inflammatory action without DHT manipulation Solution Customizable combinations of minoxidil, tretinoin, caffeine, melatonin, and cetirizine $54/month OTC Support Products Nutritional optimization, scalp environment enhancement, and daily follicular support Serum, supplements, shampoo, and conditioner Evidence-based supplements targeting TRT-specific deficiencies; irritant-free hair care products $29+/month Method #1: Oral Medications for Systemic Effects
Why Choose Oral Treatment?
This systemic approach provides several key advantages for men on testosterone therapy:
Benefit: Details: ✓ Comprehensive treatment Systemic DHT blocking plus vasodilation for growth stimulation ✓ Maximum potency Up to 93% DHT reduction with dutasteride ✓ Convenience Once-daily dosing ✓ Customizable Combination options with growth stimulators How Do Oral Medications Work to Treat Hair Loss?
DHT blockers like finasteride and dutasteride treat hair loss by suppressing the 5α-reductase enzymes that metabolize testosterone into DHT. Oral preparations have systemic effects and therefore offer extensive treatment. They can be used in conjunction with oral growth stimulators, such as minoxidil, to minimize hair loss associated with TRT.
Finasteride inhibits the Type II 5α-reductase enzyme to reduce DHT levels by up to 70%. In contrast, dutasteride is a more potent intervention as a dual inhibitor of both Type I and Type II 5α-reductase enzymes, achieving up to 93% lower DHT levels. This thorough DHT suppression can make dutasteride useful for advanced hair loss cases as well as high-dose TRT patients who may not respond to topical treatments.[12]Arif, T., Dorjay, K., Adil, M., Sami, M. (2017). Dutasteride in Androgenetic Alopecia: An Update. Current Clinical Pharmacology. 12(1). 31-35. Available at: … Continue reading
Studies indicate that dutasteride’s therapeutic effects may continue to build beyond the 12 months at which many finasteride users experience a plateau, further establishing dutasteride as the most potent treatment option. While oral DHT blockers offer powerful treatment, their systemic effects contribute to a greater risk of serious side effects, so strict medical oversight is required.[13]Harcha, W.G., Martinez, J.B., Tsai, T-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy … Continue reading
Ulo’s Oral Medication Approach
Ulo’s oral treatments offer effective systemic intervention through various formulations designed for optimal bioavailability, featuring customizable options. Their oral Dutasteride Rx delivers a 0.5 mg dose of dual enzyme-blocking treatment in a softgel format designed to enhance absorption, overcoming the poor bioavailability of powder formulas touted by competitors.
Their gentler oral Finasteride Rx also offers systemic DHT suppression in a once-daily capsule, while an oral Minoxidil Rx (1.25 mg or 2.5 mg) tablet formula increases systemic circulation and upregulates hair growth factors to treat hair loss as an alternative to DHT-blocking medications.
Ulo also offers oral preparations that synergistically combine DHT blockers with minoxidil for effects that are shown to exceed those of monotherapies: Dutasteride + Minoxidil Rx and Finasteride + Minoxidil Rx, which come with optional adjuvants like vitamin D and folic acid to address deficiencies. Rather than combining oral dutasteride and minoxidil into a single capsule, as competitors do, Ulo maintains a separate soft gel and powder format for each, enabling proper absorption.
Key Takeaway: Oral medications such as finasteride, dutasteride, and minoxidil provide the strongest systemic protection against hair loss during TRT, but their broad effects mean they require careful medical supervision to manage potential side effects.
Method #2: Topical Finasteride for Precision DHT Blocking
Why Choose Topical Finasteride?
Topical finasteride offers these strategic benefits for TRT patients:
Benefit: Details: ✓ Targeted action Scalp-focused DHT reduction ✓ Flexible dosing Low-dose (0.005%) and full-strength (0.2%) options ✓ Safer alternative to oral Comparable efficacy to oral without systemic exposure side effects ✓ Customizable Optional minoxidil and adjuvant combinations How Does Topical Finasteride Work?
Topical finasteride delivers targeted DHT suppression directly to the scalp, minimizing effects on the rest of the body. Studies show that topical finasteride can reduce scalp DHT to a therapeutic effect with little impact on serum DHT levels. In contrast, oral finasteride reduces DHT levels throughout the entire system. For men on TRT, maintaining systemic DHT is essential for bone, muscle, and sexual health; therefore, topical finasteride is typically the preferred option.[14]Kinter KJ, Amraei R, Anekar AA. Biochemistry, Dihydrotestosterone. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: … Continue reading,[15]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray … Continue reading
Topical formulations also allow more precise dosing with flexible concentrations that can be tailored to patient needs and responses. Low-dose formulas (0.005%) may be more appropriate for early-stage or moderate TRT patients, while higher-strength options (0.2%) can offer more aggressive treatment for substantial hair loss or intensive TRT protocols. Topical solutions can easily incorporate elective adjuvants, such as absorption enhancers and anti-inflammatories, for synergistic benefits.[16]Kinter KJ, Amraei R, Anekar AA. Biochemistry, Dihydrotestosterone. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: … Continue reading
Ulo’s Topical Finasteride System
Ulo’s topical finasteride solution provides targeted scalp treatment with an optimized delivery base that is free from potential irritants, such as propylene glycol. Their fully customizable Finasteride + Minoxidil Plus Rx is available in finasteride doses of 0.005% for gentler therapy and 0.2% for stronger intervention.
The robust formulation includes a wide range of synergistic add-on choices that address multiple hair loss pathways: up to 7% minoxidil for growth stimulation, tretinoin for better absorption, and multiple antioxidants for comprehensive support. This adaptability enables treatment tailored to patient needs rather than fixed protocols. Physician-crafted and supervised topical protocols are considered safe and effective for prolonged use to complement TRT journeys.
Key Takeaway: Topical finasteride allows TRT patients to lower scalp DHT while preserving systemic androgen function, making it a preferred option for balancing hair health with hormonal benefits.
Method #3: Topical Dutasteride for Comprehensive Scalp Protection
Why Choose Topical Dutasteride?
This powerful topical approach delivers distinct advantages:
Benefit: Details: ✓ Dual enzyme blocking Inhibits both Type I and Type II 5α-reductase ✓ Max scalp DHT reduction Superior enzyme inhibition for aggressive protocol ✓ Safe and targeted treatment Potent effects without systemic exposure ✓ Concentration control Low-dose (0.02%) to full-strength (0.2%) options ✓ Personalized add-ons Adjuvants can be tailored to individual needs How Does Topical Dutasteride Work?
Topical dutasteride leverages the dual enzyme inhibition and localized efficacy discussed in Methods 1 and 2 to powerfully suppress scalp DHT levels while maintaining systemic DHT. This approach can be beneficial for patients on intensive TRT protocols that don’t respond to single-enzyme-blocking treatments.
The superior targeted DHT suppression of topical dutasteride makes it an excellent choice for severe hair loss and TRT scenarios. It is the most potent topical DHT blocker available. Like topical finasteride, its concentration flexibility and easy incorporation of adjuvants enable more highly tailored and adaptable treatment compared to oral administration.[17]Arif, T., Dorjay, K., Adil, M., Sami, M. (2017). Dutasteride in Androgenetic Alopecia: An Update. Current Clinical Pharmacology. 12(1). 31-35. Available at: … Continue reading,[18]Harcha, W.G., Martinez, J.B., Tsai, T-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy … Continue reading,[19]Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in … Continue reading
Ulo’s Topical Dutasteride Excellence
Ulo’s topical dutasteride offerings include both low-dose (0.02%) and full-strength (0.2%) versions of their Dutasteride + Minoxidil Plus Rx solution, delivering potent dual enzyme inhibition for precision scalp treatment. While the lower concentration may suit maintenance or preventative protocols, the highly concentrated formula may benefit severe hair loss patients and aggressive TRT regimens.
Like Ulo’s topical finasteride products, their dutasteride selections are fully customizable with synergistic add-ons that include minoxidil (up to 7%), caffeine, tretinoin, cetirizine, and melatonin. The clinically backed formulas are designed to deeply penetrate the scalp and follicles without the use of irritants, corticosteroids, or unproven ingredients seen in competing premium brands.
The platform’s Build Your Own Topical feature offers complete customization across the full range of topical electives, including a choice of finasteride or dutasteride with individualized adjuvant combinations.
Key Takeaway: Topical dutasteride delivers the most powerful scalp-targeted DHT reduction available and is well-suited for men with significant hair loss or intensive TRT regimens that do not respond to other approaches.
Method #4: Growth Booster Topicals for Non-Hormonal Treatment
Why Choose Growth Booster Topicals?
Non-hormonal growth stimulation provides these unique benefits:
Benefit: Details: ✓ Hormone-free treatment Growth stimulation without hormonal manipulation ✓ Anti-inflammatory Addresses scalp inflammation contributing to hair loss ✓ High-strength formulas 7% minoxidil with absorption enhancers ✓ Versatile use Standalone or in combination with DHT blockers How Do Growth Booster Topicals Work?
Growth stimulators are useful for comprehensive hair restoration during TRT to address non-hormonal hair loss pathways outside of DHT’s purview. Minoxidil is one of the most commonly used medications, acting as a vasodilator to boost scalp circulation while upregulating growth factors to extend the anagen phase of each hair cycle.[20]Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in … Continue reading,[21]Koralewicz, M.M., Szatkowska, O.A. (2024). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 71-78. Available at: … Continue reading
Calming agents like cetirizine, melatonin, and caffeine address scalp inflammation, another key contributor to follicular damage and hair loss. Caffeine also enhances follicular nutrient supply to act as a growth stimulant, while melatonin’s antioxidant protection reduces shedding.[22]Koralewicz, M.M., Szatkowska, O.A. (2024). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 71-78. Available at: … Continue reading
The powerful combination of growth stimulation with anti-inflammatory action can provide standalone benefits or be applied as a complementary therapy with DHT blockers. Studies show that minoxidil is effectively combined with DHT blockers, offering greater outcomes than single-agent therapies. Growth boosters are therefore vital to the success of TRT hair loss prevention protocols.[23]Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in … Continue reading,[24]Koralewicz, M.M., Szatkowska, O.A. (2024). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 71-78. Available at: … Continue reading
Ulo’s Growth Booster Solutions
Ulo offers two customizable growth stimulation topicals that can be used alone or in conjunction with DHT blockers, depending on the prescribed protocol. Their Minoxidil + Tretinoin Plus Rx solution features a high 7% minoxidil in combination with tretinoin for optimal absorption. Adjuvants like cetirizine, melatonin, and caffeine may be added for anti-inflammatory support, antioxidant protection, and enhanced microcirculation.
For patients interested in calming the scalp without actives like minoxidil, the Cetirizine Plus Rx solution delivers a proven dose of 1% cetirizine. This potent anti-inflammatory also has anti-histamine properties to support scalp health and hair growth. Caffeine and melatonin may be added for well-rounded, non-hormonal treatment.
Like all Ulo topicals, these selections offer superior scalp penetration without irritating carriers or excipients, making them ideal for extended daily use in patients with sensitive skin or long-term combination therapy needs.
Key Takeaway: Growth booster topicals like minoxidil, caffeine, and melatonin support follicle strength through circulation, inflammation control, and antioxidant activity, and they can be used alone or combined with DHT blockers for greater results.
Method #5: OTC Support Products for Foundational Care
Why Choose Premium OTC Products?
Quality over-the-counter support offers these foundational advantages:
Benefit: Details: ✓ Nutritional support Addresses deficiencies contributing to hair loss ✓ Scalp optimization Creates an ideal environment for treatment absorption ✓ Natural ingredients Active botanicals and peptides ✓ Complete system Serum, supplements, shampoo, and conditioner How Do OTC Products Work?
High-quality, non-prescription products can also help target underlying hair health factors without hormonal suppression. These include nutritional support formulas that help address common deficiencies linked to hair loss. Such formulas may contain active botanical agents with anti-inflammatory or growth-promoting effects, providing gentle systemic support.[25]Koralewicz, M.M., Szatkowska, O.A. (2024). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 71-78. Available at: … Continue reading
Specialty hair care products, such as shampoos, conditioners, and serums, nourish and balance the scalp, providing an optimal environment for prescription medications to take hold and support overall treatment objectives. Non-prescription hair growth serums may contain botanical extracts and peptide-based therapeutics that soothe and strengthen the follicles as a complement to medical protocols.
Ulo’s OTC Support Range
Ulo’s four non-prescription haircare products support foundational hair health in synergy with the brand’s extensive prescription range. Their Hair Growth Serum contains three proprietary peptide blends that demonstrated comparable efficacy to minoxidil in a medically supervised study. Natural active ingredients such as saw palmetto, rosemary oil, and adenosine provide gentle DHT suppression, enhanced circulation, and growth stimulation, respectively.
The nutritional Hair Growth Supplement provides extensive hair and scalp nourishment with proven ingredients like vitamin D, biotin, hydrolyzed fish collagen, and saw palmetto. Antioxidants such as chlorophyll and astaxanthin combat oxidative stress from within.
Lastly, the Thickening Shampoo and Conditioner provide gentle daily scalp optimization with natural actives like caffeine to boost circulation, keratin to strengthen strands, and saw palmetto to suppress DHT levels. The PhD scientist-crafted formulas are free of known irritants and artificial fragrances, made instead with nourishing oils and amino acids that encourage natural hair growth and work to improve prescription treatment response.
Key Takeaway: Over-the-counter serums, supplements, and hair care products build the foundation for long-term hair preservation by supporting scalp health, addressing nutritional needs, and enhancing the effects of prescription treatments.
Why Ulo Stands Out for TRT Patients
Managing hair health during TRT requires clinical expertise to address unique hormonal challenges that generic treatments often miss. Ulo’s multidisciplinary care, personalized protocols, advanced formulations, and high-quality standards make it a leader in TRT-focused hair restoration telehealth. Below is a summary of what sets Ulo apart.
✓ Comprehensive Medical Oversight
Ulo partners exclusively with licensed and board-certified physicians to provide safe and effective care that meets the highest regulatory and safety standards. Full medical consultations and evaluations at onboarding enable Ulo’s qualified doctors to craft tailored treatment plans, while ongoing monitoring protocols establish positive outcomes.
✓ Dual Expertise in TRT and Hair Loss
Ulo operates as a leading telehealth provider with state-of-the-art services in both hair restoration and TRT, employing physicians with experience in both areas. The specialists at Ulo are uniquely qualified to address TRT-related hair loss with practical insights and actionable solutions.
✓ True Customization for TRT Needs
While most competitors tout fixed, one-size-fits-all formulations, Ulo, in contrast, carries truly customizable treatment options with adjustable doses and elective add-ons to meet individual patient needs. This is an important feature for TRT patients, who may require medications that vary from standard formulas and can be adjusted to changing responses over time.
✓ Pharmaceutical-Grade Quality Standards
Ulo ensures product quality by sourcing all medications from certified domestic pharmacies. Formulations are designed for peak bioavailability with innovative delivery systems, free of harsh additives like propylene glycol, which can be found in many hair loss products.
✓ Transparent Pricing and Customer Support
Ulo’s fixed-rate monthly subscription pricing avoids the rising costs and hidden fees that are common among telemedicine competitors. This allows patients to budget for long-term integrated care. Subscriptions cover all essential services, including physician consultations, follow-ups, prescription deliveries, and more. The platform’s responsive customer service ensures uninterrupted treatment and full support throughout the process.
Ulo vs. Standard Hair Loss Providers
The chart below illustrates the main differences between Ulo’s expert model and standard telehealth hair loss providers, showcasing how Ulo is uniquely equipped to meet the hair restoration needs of TRT patients.
Feature Ulo Standard Providers Medical Oversight Board-certified physicians with TRT and hair loss expertise Generic telehealth consultations Customization True concentration flexibility (0.005%-0.2%); adjuvant options Fixed formulations; limited options Formulation Quality Pharmaceutical-grade; soft gel dutasteride for optimal bioavailability Standard compounding; limited bioavailability Ingredient Safety Irritant-free; no propylene glycol or corticosteroids Often contains propylene glycol and harsh excipients TRT Knowledge Dual expertise in hormone optimization and hair restoration Hair loss focus only; limited TRT understanding Pricing Model Transparent flat-rate monthly subscriptions Hidden fees and escalating costs are common Quality Assurance Certificates of analysis; FDA-approved pharmacy sourcing Limited transparency; variable quality standards Combination Options Extensive adjuvant library (tretinoin, caffeine, melatonin, cetirizine) Basic combinations; limited synergistic ingredients Follow-up Care Unlimited physician access; regular monitoring protocols Limited follow-up; basic customer service Treatment Coordination Can coordinate with existing TRT protocols No hormone therapy coordination Safety Considerations
Hair restoration therapies, especially hormonal medications like DHT blockers, can interact with TRT and require strict medical oversight to monitor for adverse reactions. Among the different treatment methods discussed, safety profiles vary. Here is a brief overview of their safety findings. All medical concerns should ultimately be discussed with a qualified clinician.
Prescription treatments display differing safety profiles based on the delivery method, whether oral or topical. Oral DHT blockers have systemic exposure and therefore carry the risk of systemic side effects like sexual dysfunction, mood disorders, and gynecomastia; all of which require prompt medical evaluation. Oral minoxidil requires cardiovascular monitoring due to its circulatory effects.
Topical DHT blockers have limited systemic exposure and therefore milder associated side effects, often no more than minor localized scalp irritation that tends to resolve within 2-4 weeks. Regular follow-up exams are required every 3-6 months during the first year of prescribed hair loss treatment.
Over-the-counter products, such as shampoos and nutritional supplements, carry minimal risk; however, patients with allergies or sensitivities should carefully read the ingredients before use. OTC treatments can sometimes interact with medications or exacerbate underlying conditions, so it’s best to consult a healthcare professional for guidance.
Contraindications for prescription DHT blockers include severe cardiovascular conditions, active scalp infections, and liver disease. Women who are pregnant or plan to have a future pregnancy should avoid DHT blockers due to possible birth defects.
Consult your doctor for a complete list of all possible side effects, contraindications, and risk mitigation strategies.
Conclusion: Battling the Hair vs. Health Trade-Off
Accelerated hair loss can be one of the most frustrating and disruptive elements of TRT, calling for sophisticated approaches that go beyond conventional hair loss treatment. TRT creates unique challenges that demand advanced intervention strategies.
Thanks to the wide-ranging methods discussed in this guide, men no longer have to choose between hair preservation and hormone optimization on TRT. With dual expertise, qualified medical oversight, and tailored formulations that meet leading quality standards, Ulo rises to meet the varied needs of TRT patients where generic providers fall short.
Don’t allow hair loss to interfere with your journey to hormonal health and restored vitality. Ulo’s scientific approach and clinical experience make them the clear choice for men seeking to maintain their hair while optimizing their hormone therapy. Consult with a licensed Ulo physician today to craft a personalized treatment plan.
Discover your unique hair preservation strategy with Ulo.
Frequently Asked Questions
Does Ulo offer testosterone replacement therapy?
Yes, Ulo has testosterone replacement therapy offerings that include injections, topicals, and oral preparations. Like Ulo’s hair restoration services, their TRT program is medically rigorous and tailored to individual needs. Ulo’s multidisciplinary expertise gives them unique insight, enabling them to properly address both sides of the hormone equation when treating TRT-related hair loss.
Should I discuss hair loss concerns and treatment options with my doctor when starting TRT?
Yes, all hair concerns should be discussed with your clinician before starting TRT to enable proactive treatment planning and fully informed care decisions. Your doctor will assess your health profile and treatment goals to decide whether hair loss intervention is an appropriate choice and, if so, which method is best for you.
Which hair loss treatments require a prescription?
Prescriptions are required for DHT blockers like dutasteride and finasteride, growth stimulators like minoxidil, and some customized topicals. All require proper diagnostic and follow-up care protocols that meet clinical safety standards. Options that don’t require a prescription include over-the-counter nutritional supplements, serums, shampoos, and conditioners with unrestricted active ingredients like botanical extracts and peptides.
Can I combine hair loss treatment methods?
Yes, some hair loss treatments can be combined for synergistic effects. Popular combinations include minoxidil with DHT blockers (dutasteride or finasteride), alongside nutritional support. Treatments should only be combined under close medical supervision to avoid harmful interactions.
When do most hair loss treatments show results?
Although results differ depending on the patient and treatment type, most users experience initial effects within the first 3-6 months and more substantial benefits over 6-18 months. Consistent daily administration is required to successfully achieve and maintain hair loss treatment benefits.
What are common hair loss treatment side effects?
Potential side effects vary based on the type of treatment and are usually controllable with the right medical care. Topical treatments can produce mild and temporary irritation at the application site. Although rare, oral DHT-blocking medications can lead to systemic side effects like sexual dysfunction and mood disturbances requiring immediate medical attention. Thoroughly discuss all potential side effects and suitable monitoring protocols with your provider.
How much do Ulo’s hair loss treatments typically cost?
Ulo’s price points vary by treatment type and level of customization. Pricing reflects flat-rate monthly subscription fees that not only cover the cost of the medication but also include essential telehealth services like doctor consultations, follow-ups, and prescription deliveries. Their prescription oral treatments range from $24 to $65 per month, while topicals range from about $49 to $94 per month. OTC support products start at $29 per month.
What should I expect during my first consultation with Ulo?
The Ulo onboarding process starts with a thorough medical intake form that covers your health profile, treatment goals, and current medications. You will be partnered with one of Ulo’s licensed physicians, who, after reviewing your information, will collaborate with you to design a treatment plan tailored to your specific needs. During the initial consultation, your doctor will thoroughly discuss treatment options, realistic expectations, safety, and follow-up protocols to guarantee safe and effective care.
References[+]
References ↑1, ↑10, ↑11 Owecka, B., Tomaszewska, A., Dobrzeniecki, K., Owecki, M. (2024). The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines. 12(3). 513. Available at: https://doi.org/10.3390/biomedicines12030513 ↑2, ↑4 Owecka, B., Tomaszewska, A., Dobrzeniecki, K., Owecki, M. (2024). The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines. 12(3). 513. Available at: https://doi.org/10.3390/biomedicines12030513 ↑3, ↑6 Stocks, B., Asempa, O. (2025). Does testosterone replacement therapy cause hair loss? Baylor College of Medicine. Available at: https://blogs.bcm.edu/2025/08/11/does-testosterone-replacement-therapy-cause-hair-loss/ (Accessed: November 2025) ↑5 Borst, S.E., Shuster, J.J., Zou, B., Ye, F., Jia, H., Wokhlu, A., Yarrow, J.F. (2014). Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systemic review and meta-analysis. BMC Medicine. 12. 211. Available at: https://doi.org/10.1186/s12916-014-0211-5 ↑7 Shoskes, J.J., Wilson, M.K., Spinner, M.L. (2016). Pharmacology of testosterone replacement therapy preparations. Translational Andrology and Urology. 5(6). 834-843. Available at: https://doi.org/10.21037/tau.2016.07.10 ↑8 Saad, F., Aversa, A., Isidori, A.M., Zafalon, L., Zitzmann, M., Gooren, L. (2011). Onset of effects of testosterone treatment and time span until maximum effects are achieved. European Journal of Endocrinology. 165(5). 675-685. Available at: https://doi.org/10.1530/EJE-11-0221 ↑9 Fu, D., Huang, J., Li, K., Chen, Y., He, Y., Sun, Y., Guo, Y., Du, L., Qu, Q., Miao, Y., Hu, Z. (2021). Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomedicine & Pharmacology. 137. 111247. Available at: https://doi.org/10.1016/j.biopha.2021.111247 ↑12 Arif, T., Dorjay, K., Adil, M., Sami, M. (2017). Dutasteride in Androgenetic Alopecia: An Update. Current Clinical Pharmacology. 12(1). 31-35. Available at: https://doi.org/10.2174/1574884712666170310111125 ↑13 Harcha, W.G., Martinez, J.B., Tsai, T-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. Journal of the American Academy of Dermatology. 70(3). 489-498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑14, ↑16 Kinter KJ, Amraei R, Anekar AA. Biochemistry, Dihydrotestosterone. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557634/ (Accessed: November 2025) ↑15 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E. (2021). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology & Venereology. 36(2). Available at: https://doi.org/10.1111/jdv.17738 ↑17 Arif, T., Dorjay, K., Adil, M., Sami, M. (2017). Dutasteride in Androgenetic Alopecia: An Update. Current Clinical Pharmacology. 12(1). 31-35. Available at: https://doi.org/10.2174/1574884712666170310111125 ↑18 Harcha, W.G., Martinez, J.B., Tsai, T-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. Journal of the American Academy of Dermatology. 70(3). 489-498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑19 Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in treatment of androgenetic alopecia. QJM: An International Journal of Medicine, 117(Supplement 1), hcae175.207. Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑20, ↑23 Obeid, M., Fattah, N., Elfangary, M., & Husseni, R. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in treatment of androgenetic alopecia. QJM: An International Journal of Medicine, 117(Supplement 1), hcae175.207. Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑21, ↑22, ↑24, ↑25 Koralewicz, M.M., Szatkowska, O.A. (2024). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 71-78. Available at: https://doi.org/10.5603/fd.101208 IngredientsPharmaceuticalsNatural RemediesCompany ReviewsBy Michael Williams, PhDDec 17, 2025Oral vs. Topical Dutasteride: What Studies Show
Oral dutasteride is one of the most effective treatments for androgenic alopecia, but its systemic hormone suppression raises safety concerns. Topical dutasteride is often promoted as a way to deliver similar scalp-level benefits with fewer side effects, but does the evidence support that claim? In ...By Ben Fletcher, PhDDec 17, 2025Minoxidil Shedding – What to Expect & When it Stops
Starting minoxidil and seeing more hair fall out? You’re not alone. This article breaks down the phenomenon known as the “dread shed”—a temporary spike in hair loss that can happen when beginning treatment. We explore why this shedding occurs, what it means for your hair cycle, and whether it’s actu...By Perfect Hair Health TeamDec 17, 2025Peppermint Oil for Hair Growth: Better Than Minoxidil?
In 2014, a study on mice found that peppermint oil improved hair growth outcomes better than minoxidil. In 2015, a study on men with androgenic alopecia showed that topical rosemary oil (similar to peppermint) improved hair counts, and as effectively as minoxidil. While these studies appear promisin...By Perfect Hair Health TeamDec 17, 2025Caffeine For Hair Loss (AGA): Evidence & Recommendations
Caffeine’s role in hair loss (and hair growth) is hotly debated. On the one hand, oral caffeine may indirectly exacerbate hair loss in those who are hyperglycemic or hypothyroid. On the other hand, topical caffeine does have efficacy as a potential hair loss intervention – at least when combin...By Perfect Hair Health TeamDec 16, 2025Microneedling For Hair Loss: 5 Strategies to Reduce Pain
Microneedling is an experimental therapy for androgenic alopecia and other hair loss disorders. One of its downsides is that it is painful to administer. However, there are simple and effective strategies to reduce pain levels while microneedling across the scalp:(1) opting for devices that ensure n...By Perfect Hair Health TeamDec 16, 2025Saw Palmetto: Is It As Effective As Finasteride?
Saw palmetto is less effective than finasteride for treating pattern hair loss – with clinical studies showing lower response rates and regrowth rates in men taking 320mg of saw palmetto versus 1mg of finasteride daily. However, saw palmetto is also associated with fewer sexual side effects. This me...By Perfect Hair Health TeamDec 16, 2025Platelet-Rich Plasma (PRP): Does It Regrow Hair? Maybe, Maybe Not.
PRP can increase hair counts by 25-40% for those with androgenic alopecia. However, it’s expensive and requires ongoing injections to maintain results. It works best as an adjunct treatment, and it might be less effective for women. Before you invest in PRP, make sure to vet your dermatologist with ...By Perfect Hair Health TeamDec 16, 2025The Misleading Results Of The Pumpkin Seed Oil-Hair Loss Study
People looking for natural solutions to hair loss often stumble upon pumpkin seed oil. After all, many articles claim that a 2016 study showed this supplement can regrow hair. But these articles are missing a key detail: that 2016 study wasn’t on pumpkin seed oil; it was on an herbal blend. Wh...By Perfect Hair Health TeamDec 15, 2025Topical Latanoprost For Hair Loss: Results, Doses, & Side Effect Concerns
For androgenic alopecia, clinical studies suggest that ~0.1 mg daily of topical latanoprost can improve hair counts, with visual improvements occurring in 30-50% of hair loss patients over 6-8 months. Telehealth brands are prescribin doses 10-20 times higher than what was clinically studied, thus po...By Richard Clayton, PhDDec 8, 2025Finasteride For Women: What’s The Perfect Dose?
Oral finasteride is an effective treatment for male pattern hair loss, also known as androgenic alopecia. However, the drug’s benefits to female pattern hair loss are less clear. Some studies show that females taking between 1.0 to 5.0 mg of finasteride daily improve their hair loss, while oth... - Mission Statement
 Scroll Down
Scroll Down