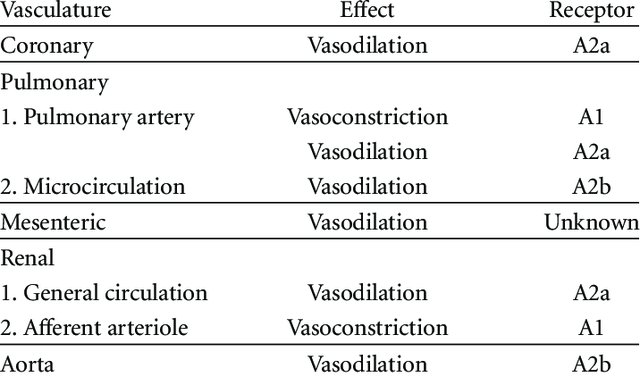
- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
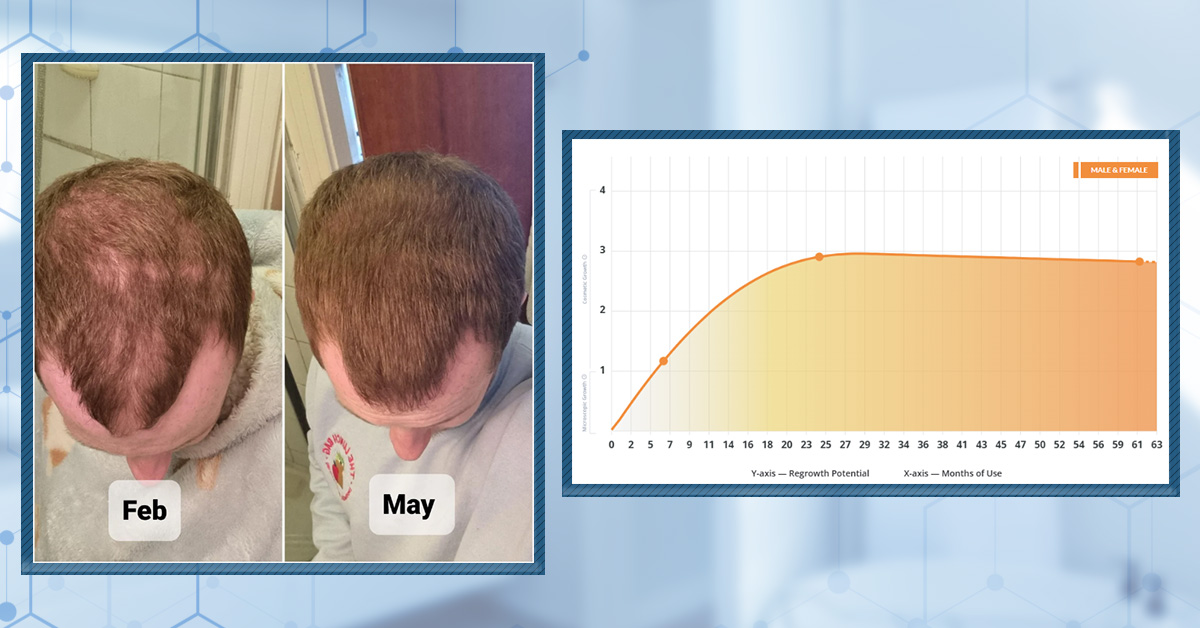
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
Articles
Minoxidil has been a cornerstone of hair-loss treatment for decades, and the options have expanded well beyond over-the-counter 2% and 5% topicals. Low-dose oral minoxidil has become increasingly common off-label, especially for people who struggle with topical routines or don’t respond well to them.
As a result, many people find themselves re-evaluating their approach and thinking about switching to oral minoxidil. We’ll break down how the oral and topical formulations compare and outline when a switch is likely to help.
Interested in Oral Minoxidil?
Low-dose oral minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

How Does Minoxidil Work?
Topical and oral minoxidil both work through the same basic mechanisms. At a biological level, minoxidil alters the hair cycle by shortening the resting (telogen) phase and promoting earlier entry into the growth (anagen) phase. This increases the proportion of follicles actively producing hair at any given time. Minoxidil also enlarges miniaturized hair follicles, resulting in thicker, longer hair shafts.[1]Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 150(2). 186–194. Available at: … Continue reading
Beyond hair-cycle effects, minoxidil influences several signaling and support pathways within the follicle. It increases local blood flow, activates the Wnt/β-catenin signaling pathway involved in follicular cell proliferation and differentiation, and appears to have cytoprotective and anti-inflammatory effects, in part by increasing prostaglandin E2 production.
What is the Difference Between Topical and Oral Minoxidil?
Where topical and oral minoxidil meaningfully diverge is not in what the drug does once active, but in how it becomes active and reaches the follicle.
Topical minoxidil must first penetrate the scalp barrier and then be converted into its active form within the hair follicle itself. This conversion depends on local sulfotransferase enzyme activity, which varies between individuals and even between scalp regions. As a result, exposure to active minoxidil can be highly variable, even when application is consistent.
Oral minoxidil, by contrast, is absorbed through the gastrointestinal tract and converted to its active form primarily in the liver, where sulfotransferase activity is abundant and consistent. The active drug is then delivered to hair follicles via the bloodstream, largely bypassing both the scalp barrier and variability in local enzymatic activation.
These differences in activation and delivery are what make switching between topical and oral formulations clinically meaningful.
Why Do People Switch Between Minoxidil Forms?
Switching is rarely about chasing a small theoretical advantage. Most switches happen for one of three reasons:
1. Convenience and Adherence
Topical minoxidil can work well in trials, but adherence is hard in real life. Many people struggle with twice-daily application for months, and discontinuation rates in real-world settings are high, with the most commonly cited reason being a lack of compliance (i.e., people couldn’t maintain their treatment regimen).[2]Shadi, Z. (2023). Compliance to topical minoxidil and reasons for discontinuation among patients with androgenetic alopecia. Dermatology and Therapy. 13(5). 1157–1169. Available at: … Continue reading
Topical minoxidil can also present cosmetic challenges that influence long-term adherence. Liquid solutions may leave the hair feeling greasy or stiff, create visible residue or flaking that can resemble dandruff, and interfere with hairstyling, particularly in people with longer hair or finer textures.
Oral minoxidil is simpler, usually a once-daily pill, and for many people, that alone changes outcomes.
2. Inconsistent Response to Topical Therapy
Some people use topical minoxidil correctly for long enough and still see little benefit. This can happen even when everything “looks right” on paper. The second most cited reason for discontinuing topical minoxidil was unsatisfactory results, and clinical data have shown that response rates are high in the first 3-6 months, but drop after a year or longer of use.[3]Olsen, E. A., Weiner, M. S., Amara, I. A., & DeLong, E. R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. *Journal of the American Academy of … Continue reading
3. Side Effects
The side effects most commonly associated with topical minoxidil are local rather than systemic, reflecting the fact that only a small fraction of the applied drug enters the bloodstream. The most frequently reported issues include scalp irritation, dryness, flaking, itching, and contact dermatitis. In many cases, these reactions are driven less by minoxidil itself and more by the vehicle used to deliver it, particularly formulations containing propylene glycol.[4]Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. Journal of the American Academy of Dermatology. 16(3). … Continue reading
Minoxidil is also toxic to pets, and even small exposures to topical or oral products can cause severe, sometimes fatal cardiovascular events. Because topical formulations can be harder to control, and need to be handled with care, some users may switch to oral formulations to decrease the risk of accidental exposure.[5]Tater, K. C., Gwaltney-Brant, S., & Wismer, T. (2021). Topical minoxidil exposures and toxicoses in dogs and cats: 211 cases (2001–2019). *Journal of the American Animal Hospital Association.* … Continue reading
Propylene glycol is included in many liquid formulations to enhance solubility and penetration, but it is a well-known irritant and contact sensitizer. In susceptible individuals, this can lead to erythema, scaling, burning, or eczematous dermatitis, particularly with twice-daily use.
Oral minoxidil produces a different side-effect profile because it is systemically absorbed and pharmacologically active throughout the body. At the low doses used for hair loss, it is generally well tolerated, but adverse effects occur more frequently than with topical formulations and tend to be dose-dependent.
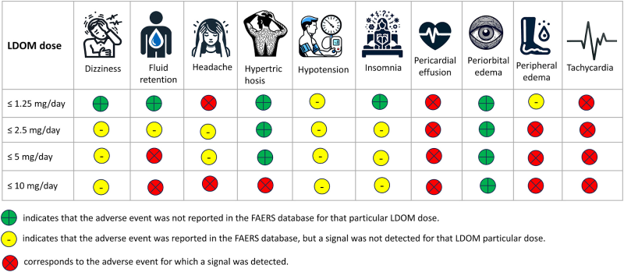
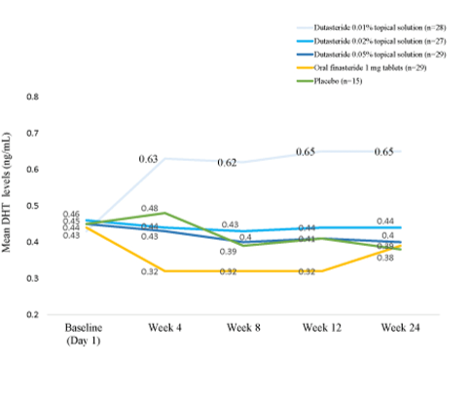
The most common side effect is hypertrichosis, or unwanted hair growth outside the scalp, including on the face. This was reported in 15% of 1404 users in a follow-up study.[6]Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair … Continue reading
Hypertrichosis is typically more of an issue for women taking minoxidil, for whom hair on the face and body may be off-putting.
Other common side effects reported in the follow-up study included lightheadedness (1.7%), fluid retention (1.3%), tachycardia (elevated heart rate, 0.9%), and headache (0.4%). Serious cardiovascular complications are rare at the low doses used for hair loss and have been reported primarily in higher-dose antihypertensive use or in individuals with pre-existing cardiovascular disease.
Figure 1. Occurrence of adverse events in individuals taking low-dose oral minoxidil (LDOM), as reported in the FDA Adverse Event Reporting System. Adapted from Figure 1.[7]Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse … Continue reading Image used under Creative Commons License.
If you’re concerned about minoxidil side effects, check out our article on 10 ways to reduce them.
Why Oral Minoxidil Might Work Better for Some
Topical minoxidil relies heavily on follicular sulfotransferase activity (notably SULT1A1). Lower activity is associated with weaker response.[8]Pietrauszka, K., & Bergler-Czop, B. (2022). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: … Continue reading
As such, minoxidil’s “ceiling” isn’t the same for everyone: people with lower SULT1A1 activity will generally not respond as well.
Similarly, the penetration of topical minoxidil through the scalp is essential for it to function. This can depend on a range of factors, including the type of formulation, scalp health, contact time, and application technique.
Oral minoxidil bypasses these barriers to absorption. It is absorbed through the gastrointestinal tract and converted into its active form primarily in the liver, where sulfotransferase activity is abundant. Systemic activation makes exposure more uniform across the scalp and less dependent on follicular enzyme variability.
Oral Minoxidil vs Topical Minoxidil
So what does the clinical evidence tell us about the efficacy of the two approaches?
Both topical and oral minoxidil are supported by robust clinical evidence demonstrating their effectiveness in treating hair loss, including androgenic alopecia (AGA).[9]Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: … Continue reading,[10]Panchaprateep, R., & Lueangarun, S. (2020). Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global … Continue reading,[11]Silva, M. N. E., Ramos, P. M., Silva, M. R., Silva, R. N. E., & Raposo, N. R. B. (2022). Randomized clinical trial of low-dose oral minoxidil for the treatment of female pattern hair loss: 0.25 … Continue reading,[12]Sinclair, R. D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. *International Journal of Dermatology.* 57(1). … Continue reading,[13]Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical … Continue reading,[14]Adil, A., & Godwin, M. (2017). The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. *Journal of the American Academy of Dermatology.* 77(1). … Continue reading
Direct head-to-head comparisons, however, are less common.
Direct Comparisons in Men
A 2024 randomized clinical trial compared 5 mg oral minoxidil once daily vs 5% topical minoxidil twice daily for 24 weeks in men with AGA. They found no significant difference in hair outcomes overall, but oral trended higher for density and showed significantly greater improvement in photographic assessment at the crown.[15]Penha, M. A., Miot, H. A., Kasprzak, M., & Ramos, P. M. (2024). Oral minoxidil vs topical minoxidil for male androgenetic alopecia: a randomized clinical trial. JAMA Dermatology. 160(6). … Continue reading
This matches the broader trend seen across male oral minoxidil studies at 2.5-5 mg daily: more consistent, dose-dependent results with cosmetic relevance.[16]Panchaprateep, R., & Lueangarun, S. (2020). Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global … Continue reading
Direct Comparisons in Women
As we’ve already noted, doses for women tend to be lower than for men, typically 1 mg daily versus 5 mg daily, so as to avoid hypertrichosis.
The first direct comparison in women comes from a 24-week randomized, open comparative study conducted in Brazil, which evaluated oral minoxidil 1 mg once daily versus topical minoxidil 5% solution applied once daily in women aged 18–65 with pattern hair loss.[17]Ramos, P. M., Melo, D. F., Radwanski, H., Cortez de Almeida, R. F., & Miot, H. A. (2023). Female-pattern hair loss: therapeutic update. Anais Brasileiros de Dermatologia. 98. 506–519. Available … Continue reading
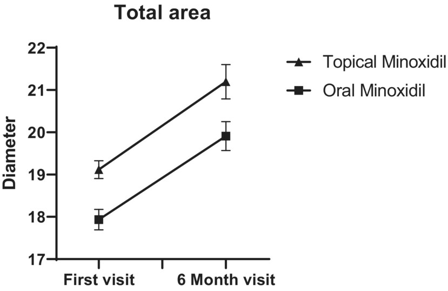
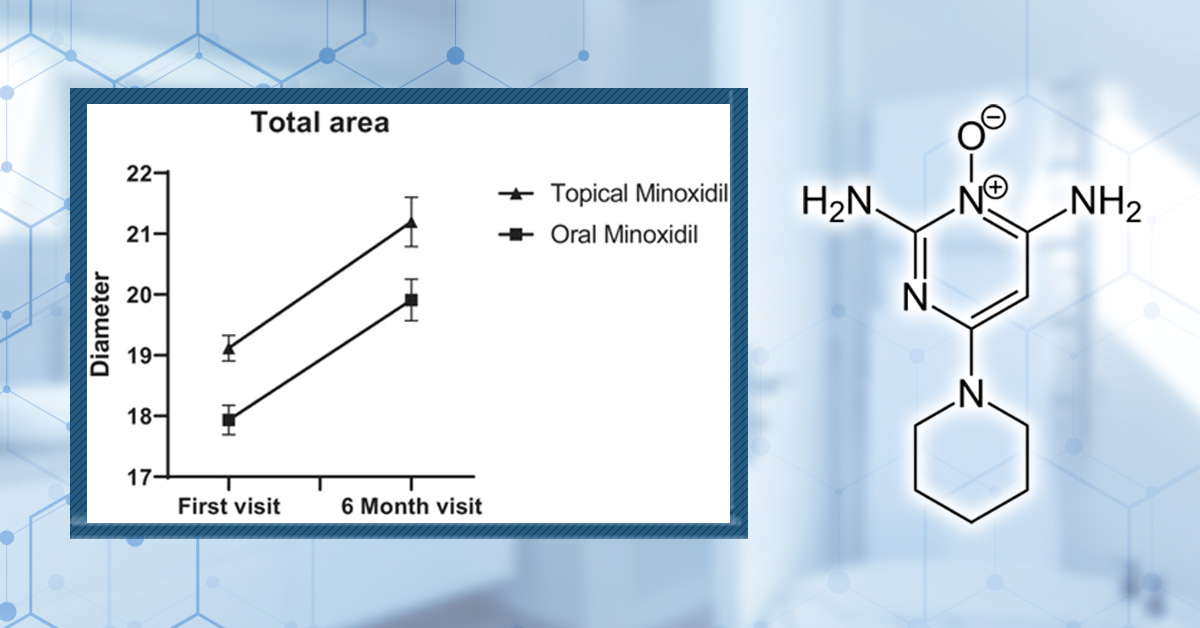
After 24 weeks, terminal hair density increased by 12% in the oral minoxidil group and 7.2% in the topical group. While this improvement favored oral therapy, the difference was not statistically significant.
Side-effect patterns differed in expected ways. Hypertrichosis was reported in 27% of women taking oral minoxidil, compared with 4% in the topical group.
A similar trial comparing 1mg oral minoxidil to 5% topical minoxidil also found no statistically significant differences between the two groups in hair diameter. They did, however, report improvement in photographic assessment in the topical group.[18]Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled … Continue reading
Lower doses of oral minoxidil for women may reduce its efficacy compared to topical treatments, which are typically applied at similar concentrations to men (5%).
Figure 2. Improvements in average hair diameter were comparable between 5% topical solution or 1 mg/day oral minoxidil over a six-month study period. Adapted from Figure 2.[19]Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled … Continue reading Image used under Creative Commons License.
What About Higher Concentrations of Topical Minoxidil?
As we’ve seen, most clinical research focuses on 5% topical minoxidil. However, in practice, many people use higher concentrations of minoxidil, with 7-8% being common, and sometimes concentrations as high as 10-15% are employed as a more aggressive treatment.
What’s more, minoxidil is often paired with tretinoin/retinoic acid, which enhances activation and penetration of the drug. While there is limited clinical data on such pairings, anecdotal evidence from our members suggests that this combination could help to optimize topical minoxidil’s efficacy.
We see that 7-8% minoxidil paired with 0.005-0.02% retinoic acid appears to outperform 5 mg oral minoxidil, even in cases of diffuse thinning.
For comprehensive information about using retinoic acid for hair loss, check out our ultimate guide.
Anecdotal evidence from our members also suggests that higher concentrations of topical minoxidil (10-15%) can generate similar results to 5 mg oral minoxidil.
Is there a clear winner?
In short, no. A 2025 meta-analysis comparing topical and oral minoxidil found no significant difference between approaches overall, which reflects how much outcomes depend on dose, population, and study design.[20]Fazal, F., Malik, B. H., Malik, H. M., Sabir, B., Mustafa, H., Ahmed, M., Abid, A., Adil, M. L., Shafi, U., & Saad, M. (2025). Can oral minoxidil be the game changer in androgenetic alopecia? A … Continue reading
It is important to note that adherence to a strict routine in trials is typically well enforced, and topical treatments are applied with consistent volumes at consistent times. This doesn’t necessarily reflect how people use topical minoxidil in the real world.
On the other hand, many users find that they can optimize a topical approach by increasing the strength or frequency of application, or by adding enhancer ingredients like tretinoin/retinoic acid. If you’re using such a combination therapy, you might, in fact, see worse results when switching to oral.
When Switching From Topical to Oral Makes the Most Sense
Switching to oral minoxidil is most rational when the limiting factor is activation or adherence, or when topical treatment isn’t delivering the results you expect.
Good Candidates for Switching to Oral Minoxidil
If systemic exposure is acceptable from a safety and tolerability standpoint, there are circumstances in which switching from an oral to a topical formulation makes sense.
Poor topical responders – If you’ve used topical minoxidil consistently for 6-12 months and can reasonably say adherence wasn’t the problem, oral is a logical next step because it bypasses follicular enzyme variability.[21]Pietrauszka, K., & Bergler-Czop, B. (2022). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: … Continue reading
Diffuse or Advanced Thinning – As larger areas of the scalp are affected, ensuring even coverage requires more time, higher product volumes, and careful technique, which many people struggle to sustain long term. Missed areas and uneven delivery are common and can limit results even when follicles remain responsive.
Oral minoxidil avoids these practical constraints by delivering the drug systemically, allowing uniform exposure across the entire scalp and making treatment easier to scale as hair loss progresses.
Convenience-driven adherence problems – When adherence is the primary issue, oral minoxidil often has a practical advantage. Topical therapy requires regular scalp application, drying time, and styling time, all of which can erode consistency over months or years of use. Missed applications are common and can reduce effectiveness. Oral minoxidil makes consistent long-term use more achievable for many people.
Scalp Inflammation – Inflammation of the scalp occurs in a high proportion of individuals with AGA, with seborrheic dermatitis being common. If you’re pairing minoxidil with inflammatory treatments like retinoic acid, this can exacerbate underlying chronic inflammation and prevent regrowth.
Scalp inflammation decreases the efficacy of topical minoxidil.[22]Whiting, D. A. (1993). Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. *Journal of the American Academy of Dermatology.* 28(5). … Continue reading Switching to oral minoxidil, or even removing the inflammatory component from your treatment, can help improve underlying inflammation.
People Who Should Be Cautious Switching
Anyone with cardiovascular disease, kidney issues, fluid retention tendencies, low blood pressure, or unexplained palpitations should be conservative and involve a clinician before starting or escalating oral minoxidil.[23]Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse … Continue reading
When Switching From Oral to Topical Makes the Most Sense
There are also times when switching from topical oral minoxidil might be a sensible decision. These will most commonly occur when side effects have made use difficult or intolerable.
You might also consider an optimized topical approach, incorporating enhancers such as tretinoin/retinoic acid or increasing topical dosage. Anecdotal experiences of our members suggest these topical combinations can outperform low-dose oral minoxidil.
If you’’re interested in topical minoxidil for, check out our articles for men and women on the best products currently available.
Hair Shedding: Should You Be Concerned?
When people start a new hair loss treatment, shedding events are common. This includes treatments that are working! ‘Treatment-induced telogen effluvium shedding’ is the term used to describe this phenomenon, and can actually be a good predictor of whether minoxidil treatment will work down the line.[24]Bi, L., Kan, H., Wang, J., Ding, Y., Huang, Y., Wang, C., & Fan, W. (2025). Whether the transient hair shedding phase exist after minoxidil treatment and does it predict treatment efficacy? A … Continue reading
However, it’s hard to tell if shedding when you switch treatments is a positive sign, or if it’s a indicator of decreased efficacy. These cases are referred to as a ‘treatment withdrawal telogen effluvium shed’, and won’t lead to improvement in the future.
Information from our members indicates that shedding after switching from topical to oral minoxodil tends to fall into the latter camp: shedding is a result of the treatment working less well.
Be aware of shedding after switching treatments, as it could be an indicator that your new treatment isn’t working as well.
You can read more about minoxidil shedding in our comprehensive article.
How to Switch
There’s no universally validated switching protocol supported by large controlled trials. But there are sensible principles that reduce avoidable problems.
-
Avoid Abrupt Stop and Start Changes
Minoxidil works by keeping follicles in a growth-supportive environment. Abrupt discontinuation can allow hairs that were being prolonged in anagen to shed as cycles normalize.
-
Expect a Transition Period
Whether switching to oral or topical, you’re changing local concentrations and the consistency of follicular exposure. That can mean a temporary shed or plateau before stabilization, so don’t judge too early.
-
Be Patient and Track Longterm Changes
As with all hair loss treatments, consistency is key. Because minoxidil primarily influences hair-cycle timing rather than creating new follicles, benefits accrue gradually and are best assessed over 6–12 months of consistent use. Tracking progress with standardized photographs, consistent lighting, and fixed time intervals can help distinguish true treatment effects from normal cycle-related variation.
If you experience severe adverse effects, you should discontinue treatment and speak to a clinician.
Final Thoughts
Switching between topical and oral minoxidil should be a deliberate and strategic decision guided by potential reasons your current approach isn’t working. The evidence consistently shows that both topical and oral minoxidil can be effective, but their real-world performance is shaped by adherence, activation, dose, side effects, and how well the treatment fits into your life.
For people with limited topical response, inconsistent application, or large areas of thinning, oral minoxidil can provide more uniform exposure and better long-term consistency. However, real-world experience and member data suggest that higher-strength topical formulations (7–8%), especially when paired with penetration or activation enhancers like retinoic acid, often produce stronger results.
In some cases, these optimized topical approaches appear to match or even outperform low-dose oral minoxidil. For people who tolerate topical therapy well and can maintain consistency, increasing topical strength may be a more effective next step than switching to oral treatment.
Ultimately, switching treatments always carries trade-offs and some risk. The safest path forward is deliberate, not reactive: avoid abrupt changes, allow sufficient time to assess outcomes, and monitor both hair and systemic responses carefully.
References[+]
References ↑1 Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 150(2). 186–194. Available at: https://doi.org/10.1111/j.1365-2133.2004.05785.x ↑2 Shadi, Z. (2023). Compliance to topical minoxidil and reasons for discontinuation among patients with androgenetic alopecia. Dermatology and Therapy. 13(5). 1157–1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑3 Olsen, E. A., Weiner, M. S., Amara, I. A., & DeLong, E. R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 22(4). 643–646. Available at: https://doi.org/10.1016/0190-9622(90)70089-Z ↑4 Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. Journal of the American Academy of Dermatology. 16(3). 688–695. Available at: https://doi.org/10.1016/S0190-9622(87)70089-9 ↑5 Tater, K. C., Gwaltney-Brant, S., & Wismer, T. (2021). Topical minoxidil exposures and toxicoses in dogs and cats: 211 cases (2001–2019). *Journal of the American Animal Hospital Association.* 57(5). 225–231. Available at: https://doi.org/10.5326/jaaha-ms-7154 ↑6 Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. Journal of the American Academy of Dermatology. 84(6). 1644–1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑7 Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse Event Reporting System (FAERS) with a focus on pericardial effusions. *Journal of Cosmetic Dermatology.* 24(1). e16574. Available at: https://doi.org/10.1111/jocd.16574 ↑8, ↑21 Pietrauszka, K., & Bergler-Czop, B. (2022). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. Advances in Dermatology and Allergology / Postępy Dermatologii i Alergologii. 39(3). 472–478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑9 Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: https://www.researchgate.net/profile/Suparuj-Lueangarun/publication/319006716_Efficacy_and_safety_of_oral_minoxidil_5_mg_daily_during_24-week_treatment_in_male_androgenetic_alopecia/links/5b46f1690f7e9b4637cde38b/Efficacy-and-safety-of-oral-minoxidil-5-mg-daily-during-24-week-treatment-in-male-androgenetic-alopecia.pdf ↑10 Panchaprateep, R., & Lueangarun, S. (2020). Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. *Dermatology and Therapy.* 10(6). 1345–1357. Available at: https://doi.org/10.1007/s13555-020-00448-x ↑11 Silva, M. N. E., Ramos, P. M., Silva, M. R., Silva, R. N. E., & Raposo, N. R. B. (2022). Randomized clinical trial of low-dose oral minoxidil for the treatment of female pattern hair loss: 0.25 mg versus 1 mg. 396–399. Available at: https://doi.org/10.1016/j.jaad.2022.01.017 ↑12 Sinclair, R. D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. *International Journal of Dermatology.* 57(1). 104–109. Available at: https://doi.org/10.1111/ijd.13838 ↑13 Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. *Journal of the American Academy of Dermatology.* 47(3). 377–385. Available at: https://doi.org/10.1067/mjd.2002.124088 ↑14 Adil, A., & Godwin, M. (2017). The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. *Journal of the American Academy of Dermatology.* 77(1). 136–141. Available at: https://doi.org/10.1016/j.jaad.2017.02.054 ↑15 Penha, M. A., Miot, H. A., Kasprzak, M., & Ramos, P. M. (2024). Oral minoxidil vs topical minoxidil for male androgenetic alopecia: a randomized clinical trial. JAMA Dermatology. 160(6). 600–605. Available at: https://doi:10.1001/jamadermatol.2024.0284 ↑16 Panchaprateep, R., & Lueangarun, S. (2020). Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. Dermatology and Therapy. 10(6). 1345–1357. Available at: https://doi.org/10.1007/s13555-020-00448-x ↑17 Ramos, P. M., Melo, D. F., Radwanski, H., Cortez de Almeida, R. F., & Miot, H. A. (2023). Female-pattern hair loss: therapeutic update. Anais Brasileiros de Dermatologia. 98. 506–519. Available at: https://doi.org/10.1016/j.abd.2022.09.006 ↑18 Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled trial. Journal of Cosmetic Dermatology. 23(3). 949–957. Available at: https://doi.org/10.1111/jocd.16086 ↑19 Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled trial. *Journal of Cosmetic Dermatology.* 23(3). 949–957. Available at: https://doi.org/10.1111/jocd.16086 ↑20 Fazal, F., Malik, B. H., Malik, H. M., Sabir, B., Mustafa, H., Ahmed, M., Abid, A., Adil, M. L., Shafi, U., & Saad, M. (2025). Can oral minoxidil be the game changer in androgenetic alopecia? A comprehensive review and meta-analysis comparing topical and oral minoxidil for treating androgenetic alopecia. Skin Health and Disease. vzaf009. Available at: https://doi.org/10.1093/skinhd/vzaf009 ↑22 Whiting, D. A. (1993). Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. *Journal of the American Academy of Dermatology.* 28(5). 755–763. Available at: https://doi.org/10.1016/0190-9622(93)70106-4 ↑23 Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse Event Reporting System (FAERS) with a focus on pericardial effusions. Journal of Cosmetic Dermatology. 24(1). e16574. Available at: https://doi.org/10.1111/jocd.16574 ↑24 Bi, L., Kan, H., Wang, J., Ding, Y., Huang, Y., Wang, C., & Fan, W. (2025). Whether the transient hair shedding phase exist after minoxidil treatment and does it predict treatment efficacy? A retrospective study in androgenetic alopecia patients. *Journal of Dermatological Treatment.* 36(1). 2480739. Available at: https://doi.org/10.1080/09546634.2025.2480739 Spironolactone is a potassium-sparing diuretic with anti-androgen properties that has become increasingly popular for treating hair loss, particularly female pattern hair loss. By blocking androgen receptors, spironolactone can slow hair shedding and promote regrowth in hormonally driven alopecia.
Despite its benefits, many patients worry about potential side effects, especially whether spironolactone leads to weight gain. Anecdotally, several patients have reported weight gain when they begin taking spironolactone. In this article, we examine that concern by reviewing typical dosing for hair loss, summarizing clinical evidence on weight changes, explaining possible biological mechanisms, and outlining practical strategies to minimize unwanted weight or fluid-related effects during treatment.
What is Spironolactone?
Spironolactone is a prescription medication classified as a potassium-sparing diuretic. In other words, it is a pill that helps the body get rid of excess fluid like water and salt by increasing urination, but it prevents excessive loss of the vital mineral potassium.
It mediates this function by acting as an aldosterone antagonist. Spironolactone blocks the binding of the hormone aldosterone to its receptors, an action that regulates the body’s sodium and potassium levels.
This is why spironolactone is commonly prescribed for hypertension (high blood pressure), heart failure, and edema (swelling). By blocking aldosterone, spironolactone reduces water and salt retention and helps lower blood pressure, strain on the heart, and swelling.[1]Carone, L., Oxberry, S.G., Twycross, R., Charlesworth, S., Mihalyo, M., Wilcock, A. (2017). Spironolactone. Journal of Pain and Symptom Management. 53(2). 288–292. Available at: … Continue reading
How Does Spironolactone Work For Hair Loss?
Aside from blocking aldosterone receptors, spironolactone is also capable of binding to androgen receptors. These receptors typically bind to male androgen hormones like testosterone and dihydrotestosterone (DHT).[2]Vargas-Mora, P., Morgado-Carrasco, D. (2020). Spironolactone in Dermatology: Uses in Acne, Hidradenitis Suppurativa, Female Pattern Hair Loss, and Hirsutism. Actas Dermo-Sifiliográficas (English … Continue reading
Levels of DHT above average are a common symptom in those with androgenic alopecia. In the body, the binding of DHT to androgen receptors stimulates a range of effects, including hair follicle miniaturization. That is, hairs become thinner, shorter, and weaker. This is because DHT reduces the length of the growth phase (the anagen phase) of hair follicles, while also increasing the length of the non-growing phase (the telogen phase).[3]Ustuner, E. T. (2013). Cause of Androgenic Alopecia: Crux of the Matter. Plast Reconstr Surg Glob Open. 1(7). e64. Available at: https://doi.org/10.1097/GOX.0000000000000005,[4]Sekhavat, H., Bar Yehuda, S., Asotra, S. (2025). Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss. Int J Mol Sci. 26(21). 10712. Available at: … Continue reading
Thus, by binding androgen receptors, spironolactone can prevent the binding of DHT and, in this way, effectively “blocks” DHT to prevent hair loss.[5]Vargas-Mora, P., Morgado-Carrasco, D. (2020). Spironolactone in Dermatology: Uses in Acne, Hidradenitis Suppurativa, Female Pattern Hair Loss, and Hirsutism. Actas Dermo-Sifiliográficas (English … Continue reading
Side effects
Because spironolactone blocks male hormones, it can lead to unwanted feminization in men, such as gynecomastia (enlarged breasts), reduced facial or body hair, and redistribution of body fat. It may also lead to a loss of libido and other sexual dysfunctions.[6]Carone, L., Oxberry, S.G., Twycross, R., Charlesworth, S., Mihalyo, M., Wilcock, A. (2017). Spironolactone. Journal of Pain and Symptom Management. 53(2). 288–292. Available at: … Continue reading,[7]Haynes, B.A., Mookadam, F. (2009). Male Gynecomastia. Mayo Clinic Proceedings. 84(8). 672. Available at: https://doi.org/10.4065/84.8.672
For this reason, it is usually prescribed to women but not to men for the purpose of treating non-life-threatening conditions, such as androgenic alopecia.
Women prescribed spironolactone may experience some other side effects. For example, 15-30% of women report irregular menstruation, while fewer than 5% report breast tenderness, reduced libido, nausea, headache, and fatigue.[8]Vargas-Mora, P., Morgado-Carrasco, D. (2020). Spironolactone in Dermatology: Uses in Acne, Hidradenitis Suppurativa, Female Pattern Hair Loss, and Hirsutism. Actas Dermo-Sifiliográficas (English … Continue reading
The Science: Hair Growth Benefits
Oral spironolactone as a hair loss treatment has been studied across concentrations from 25 mg to 200 mg per day. Collectively, these trials demonstrate that spironolactone can provide meaningful improvements in hair growth in those with androgenic alopecia; however, not all trials have the scientific robustness to make clear conclusions on its efficacy.
Low doses (25-50 mg/day)
A pilot study of 100 women taking 25 mg spironolactone with 0.25 mg minoxidil for 12 months nearly halved hair shedding and substantially improved hair loss severity. However, the absence of a placebo control group limits interpretation, meaning the true effectiveness of low-dose spironolactone within this combination remains uncertain.[9]Sinclair, R.D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. International Journal of Dermatology. 57(1). … Continue reading
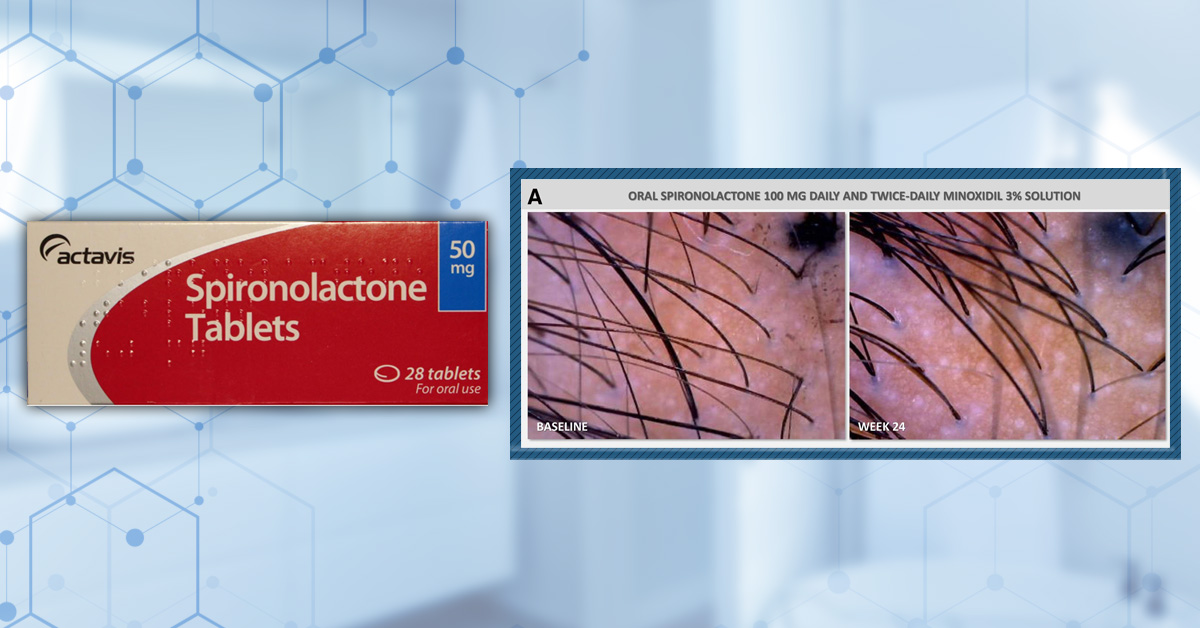
Medium doses (75-100 mg/day)
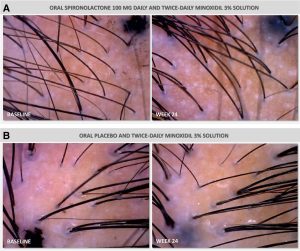
A 2025 randomized, placebo-controlled pilot study over 24 weeks evaluated 100 mg daily spironolactone plus 3% topical minoxidil versus placebo plus minoxidil in women with androgenic alopecia. Both groups experienced significant increases in hair density and diameter from baseline. The spironolactone group showed greater increases in terminal hairs and hair diameter than placebo, though this difference was not statistically significant.[10]Werachattawatchai, P., Khunkhet, S., Harnchoowong, S., Lertphanichkul, C. (2025). Efficacy and safety of oral spironolactone for female pattern hair loss in premenopausal women: a randomized, … Continue reading
Figure 2: Images of hair density at baseline and following 24 weeks of daily treatment with either 100 mg spironolactone and 3% topical minoxidil or a placebo and topical 3% minoxidil. Adapted from Figure 3.[11]Werachattawatchai, P., Khunkhet, S., Harnchoowong, S., Lertphanichkul, C. (2025). Efficacy and safety of oral spironolactone for female pattern hair loss in premenopausal women: a randomized, … Continue reading Image obtained in line with the Creative Commons License.
Earlier research from 1991 found that women treated with 75–100 mg spironolactone did not have a significant change in hair density after 12 months, while untreated women experienced decreases, suggesting spironolactone may prevent further loss rather than promote regrowth.[12]Rushton, D., Futterweit, W., Kingsley, D., Kingsley, P., Norris, M.J. (1990). Quantitative assessment of spironolactone treatment in women with diffuse androgen-dependent alopecia. Journal of the … Continue reading
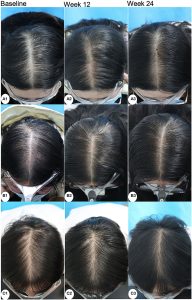
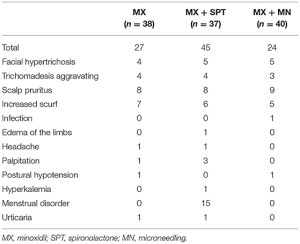
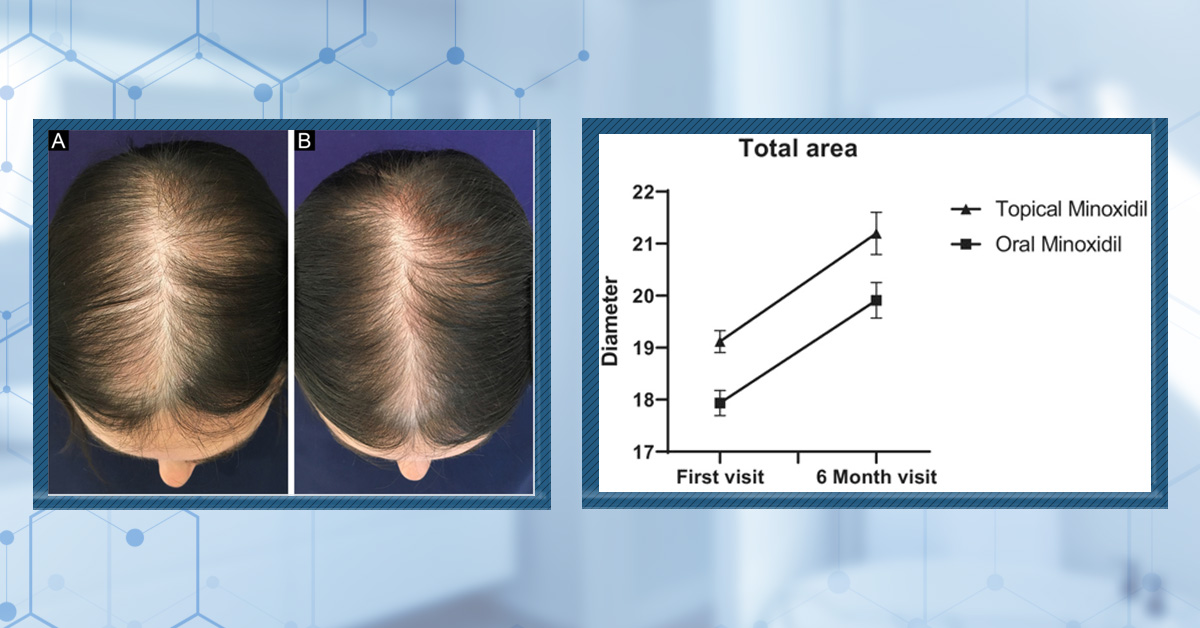
Combination therapies appear promising. A larger study comparing 5% minoxidil alone, minoxidil with 100 mg spironolactone, and minoxidil with microneedling found all groups had increased hair density at 24 weeks. The minoxidil plus spironolactone group had greater gains than minoxidil alone, though less than the microneedling group, indicating that adding spironolactone may enhance treatment effects.[13]Liang, X., Chang, Y., Wu, H., et al. (2022). Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A … Continue reading
Figure 4: Scalp images of patients treated with 5% minoxidil alone (A1-A3), 5% minoxidil and spironolactone (B1-B3), or 5% minoxidil and microneedling (C1-C3) at baseline, week 12, and week 24. Adapted from Figure 5.[14]Liang, X., Chang, Y., Wu, H., et al. (2022). Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A … Continue reading Image obtained in line with the Creative Commons License.
High doses (150-200 mg/day)
Early studies suggested high doses of spironolactone might benefit people with androgenic alopecia, but the supporting evidence is limited and inconsistent.
A very small trial reported large improvements in hair growth and reductions in hair loss after six months of 200 mg daily treatment; however, this study involved only four patients and lacked a control group, severely limiting its reliability.[15]Adamopoulos, D.A., Karamertzanis, M., Nicopoulou, S., Gregoriou, A. (1997). Beneficial effect of spironolactone on androgenic alopecia. Clinical Endocrinology. 47(6). 759–760. Available at: … Continue reading
Larger research has failed to confirm such strong effects. In a study of 80 women comparing spironolactone with another anti-androgen, no significant difference was found between treatments. Overall, only 44% of participants experienced regrowth, while the same proportion saw no clear change and 12% continued to lose hair.[16]Sinclair, R., Wewerinke, M., Jolley, D. (2005). Treatment of female pattern hair loss with oral antiandrogens. British Journal of Dermatology. 152(3). 466–473. Available at: … Continue reading
Indeed, case evidence suggests that spironolactone alone may not provide long-term benefits. A 53-year old woman with androgenic alopecia was treated with 200 mg spironolactone daily. Hair regrowth was evident after 12 months, but regrowth was not sustained after 24 months. Adding topical minoxidil alongside oral spironolactone to a treatment routine appeared to restore and maintain regrowth, indicating that combination therapy may be more effective than spironolactone on its own.[17]Hoedemaker, C., Van Egmond, S., Sinclair, R. (2007). Treatment of female pattern hair loss with a combination of spironolactone and minoxidil. Australasian Journal of Dermatology. 48(1). 43–45. … Continue reading
Is Spironolactone Effective for Hair Loss?
Evidence does not support spironolactone alone as an effective hair-loss treatment, especially at higher doses. Medium doses around 100 mg/day may slow further loss, but regrowth is poorly supported. Across all doses, spironolactone works best in combination therapies, particularly with minoxidil, where sustained regrowth is more consistently observed.
Does Spironolactone Have Any Mechanism That Could Cause Weight Gain?
There is no plausible biological mechanism by which spironolactone would cause true weight gain (i.e., fat accumulation). Typically, drugs that cause weight gain will cause one or more specific effects that can lead to fat-gain.[18]Verhaegen, A.A., Van Gaal, L.F. (2000). Drugs That Affect Body Weight, Body Fat Distribution, and Metabolism. Endotext. MDText.com, Inc. Available at: http://www.ncbi.nlm.nih.gov/books/NBK537590/ These include:
- Increased appetite
- Changed insulin resistance
- Slowdown of metabolism
- Increased cortisol
However, spironolactone has no known interactions that would lead to these effects. In fact, because spironolactone blocks aldosterone, it reduces sodium and water retention in the blood and can cause mild weight loss due to its diuretic effect.
Spironolactone and Weight Gain: What Do We See from Clinical Evidence?
Many clinical studies assessing the effectiveness of spironolactone for hair loss or other medical conditions also report side effects. As doses increase, more side effects are likely to occur, but is weight gain one of them?
Low doses (25-50 mg/day)
In the pilot study by Sinclair in 2018, side effects were reported in eight women, but they were not related to weight gain.[19]Sinclair, R.D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. International Journal of Dermatology. 57(1). … Continue reading
Medium doses (75-100 mg/day)
The trial by Werachattawatchai and colleagues in 2025 reported menstrual irregularities in 37.5% of women but no mention of weight gain.[20]Werachattawatchai, P., Khunkhet, S., Harnchoowong, S., Lertphanichkul, C. (2025). Efficacy and safety of oral spironolactone for female pattern hair loss in premenopausal women: a randomized, … Continue reading Similarly, in the study by Rushton and colleagues in 1991, some women reported changes to their menstrual cycles, but there was again no mention of weight gain.[21]Rushton, D., Futterweit, W., Kingsley, D., Kingsley, P., Norris, M.J. (1990). Quantitative assessment of spironolactone treatment in women with diffuse androgen-dependent alopecia. Journal of the … Continue reading
The trial by Liang and colleagues in 2022 also had no reports of weight gain, but the spironolactone group had the most adverse effects reported. Menstrual disorder, hyperkalemia, and edema of the limbs occurred only within groups receiving spironolactone treatment. While it may appear paradoxical as spironolactone is designed to treat edema, spironolactone can also cause edema because it promotes shifts in fluids from the bloodstream to the tissues. [22]Liang, X., Chang, Y., Wu, H., et al. (2022). Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A … Continue reading
Figure 5: Side effects recorded across patients receiving minoxidil (MN), minoxidil and spironolactone (SPT), or minoxidil and microneedling (MN) treatment. Adapted from Table 5.[23]Liang, X., Chang, Y., Wu, H., et al. (2022). Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A … Continue reading Image obtained in line with the Creative Commons License.
When used to treat hormonal disorders, a prospective clinical trial with women with polycystic ovary syndrome showed that intake of 100 mg spironolactone per day was not associated with weight gain.[24]Zulian, E., Sartorato, P., Benedini, S., et al. (2005). Spironolactone in the treatment of polycystic ovary syndrome: Effects on clinical features, insulin sensitivity and lipid profile. Journal of … Continue reading
High doses (150-200 mg/day)
Adamopoulos and colleagues reported that participants experienced no adverse side effects from taking 200 mg spironolactone per day.[25]Adamopoulos, D.A., Karamertzanis, M., Nicopoulou, S., Gregoriou, A. (1997). Beneficial effect of spironolactone on androgenic alopecia. Clinical Endocrinology. 47(6). 759–760. Available at: … Continue reading In the study by Sinclair in 2005 and case study by Hoedemaker and colleagues, adverse reactions, including weight gain, were not noted in the report, which suggests side effects were not measured.[26]Sinclair, R., Wewerinke, M., Jolley, D. (2005). Treatment of female pattern hair loss with oral antiandrogens. British Journal of Dermatology. 152(3). 466–473. Available at: … Continue reading,[27]Hoedemaker, C., Van Egmond, S., Sinclair, R. (2007). Treatment of female pattern hair loss with a combination of spironolactone and minoxidil. Australasian Journal of Dermatology. 48(1). 43–45. … Continue reading
In applications outside of hair loss, spironolactone has shown to actually cause weight loss. Administration of up to 200 mg spironolactone to patients with mineralocorticoid-induced hypertension resulted in a reversal of mineralocorticoid-induced abnormalities, including increased body weight.[28]Nicholls, M.G., Ramsay, L.E., Boddy, K., Fraser, R., Morton, J.J., Robertson, J.I.S. (1979). Mineralocorticoid-induced blood pressure, electrolyte, and hormone changes, and reversal with … Continue reading
Why Some People Think Spironolactone Causes Weight Gain
Even though spironolactone has no known mechanism that could cause weight gain, and has actually been shown to induce weight loss, some individuals taking spironolactone have reported weight gain anecdotally. Why could this be?
-
Water Retention
Spironolactone helps your body get rid of salt and water. It works by making the kidneys remove sodium and water from the bloodstream, which lowers the amount of fluid circulating in the vessels. That helps when edema is caused by too much circulating volume, like in heart failure or certain hormone disorders.
However, spironolactone can also relax veins and lower blood pressure. Edema can be caused by these exact symptoms. Thus, spironolactone can sometimes make fluid move into your tissues instead of staying in your bloodstream, causing swelling. This is why some studies report edema as a side effect.
Those in spironolactone may experience this water retention and swelling, causing them to feel that they have gained weight as a result of taking this medication.
-
Multiple Medications
It is likely that the individual is taking multiple medications. Spironolactone is best used in combination with other medications, and is safe to use with anti-depressants or birth control, both of which have clinically shown to cause weight gain.[29]Gafoor, R., Booth, H.P., Gulliford, M.C. (2018). Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. k1951. Available at: … Continue reading,[30]Lopez, L.M., Ramesh, S., Chen, M., et al. (2016). Progestin-only contraceptives: effects on weight. Cochrane Database of Systematic Reviews. 2016(8). CD008815. Available at: … Continue reading. Research summarising population characteristics of those prescribed spironolactone found that 22% had been using hormonal contraceptives, suggesting an alternative reason than spironolactone for reported weight gain.[31]Burns, L.J., Souza, B.D., Flynn, E., Hagigeorges, D., Senna, M.M. (2020). Spironolactone for treatment of female pattern hair loss. Journal of the American Academy of Dermatology. 83(1). 276–278. … Continue reading
-
Menopause
Weight gain is also one of the most common side effects of perimenopause, menopause, and postmenopause, affecting over 40% of women.[32]Knight, M.G., Anekwe, C., Washington, K., Akam, E.Y., Wang, E., Stanford, F.C. (2021). Weight Regulation in Menopause. Menopause. 28(8). 960–965. Available at: … Continue reading,[33]Davis, S.R., Castelo-Branco, C., Chedraui, P., et al. (2012). Understanding weight gain at menopause. Climacteric. 15(5). 419–429. Available at: https://doi.org/10.3109/13697137.2012.707385 Population characteristics of those prescribed spironolactone found that 51% were postmenopausal.[34]Burns, L.J., Souza, B.D., Flynn, E., Hagigeorges, D., Senna, M.M. (2020). Spironolactone for treatment of female pattern hair loss. Journal of the American Academy of Dermatology. 83(1). 276–278. … Continue reading Weight gain may therefore be related to life stage rather than spironolactone itself.
-
Other Side Effects
Many medications including spironolactone itself may cause symptoms such as fatigue. Those experiencing fatigue would be less inclined to stick to their active routines. Inactivity or changes to diet as a result could lead to weight gain.
-
Stress
We must also consider that external factors like stress can cause hormonal changes that influence weight. It is reasonable to assume that those prescribed spironolactone may be experiencing hypertension, heart failure, edema, or hair loss. Ongoing conditions like these could contribute to stress and hormonal changes that ultimately lead to weight gain.[35]Kyrou, I., Tsigos, C. (2009). Stress hormones: physiological stress and regulation of metabolism. Current Opinion in Pharmacology. 9(6). 787–793. Available at: … Continue reading
Managing Weight Fluctuations If They Occur
Because spironolactone has a diuretic effect, you may notice some temporary weight loss due to fluid reduction. This is not fat loss, but if you wish to maintain your weight, it’s important to monitor your body closely and make gradual lifestyle changes. The same can be advised if you are also experiencing weight gain for any reason.
To manage weight fluctuations if they occur, consider the following:
- Record keeping: Keep a consistent record of your weight to track change and distinguish between fluid-related changes and true weight changes
- Diet: Maintain a balanced diet rich in whole grains, lean proteins, healthy fats, fruits, and vegetables to stabilize weight.
- Hydration: Fluid shifts from diuretics can sometimes trigger dehydration, which may influence appetite and energy levels.
- Physical activity: Support muscle maintenance and overall metabolism by engaging in regular physical activity
- Take time: Weight changes from spironolactone are often temporary as your body gets used to the medication
If you feel your medication is causing your weight to change substantially, speak with your clinician, as it may indicate problems with your dosage, interactions with other medications, or additional health conditions.
Should I Take Spironolactone If I’m Concerned About Weight Gain?
There are some side effects of spironolactone, but weight gain is not one of them. Clinical evidence shows that daily usage of spironolactone at any dose is not known to cause weight gain.
If you’ve been prescribed spironolactone, take it with peace of mind that it will not cause permanent changes to your weight.
Final Verdict
Spironolactone isn’t a “fat-gain” drug, even though it can sometimes get that reputation online. Any early weight changes are usually from temporary fluid shifts, and many people actually notice weight loss over time due to the diuretic activity of this treatment.
Benefits to hair loss tend to appear at moderate doses like 50-100 mg per day and are most substantial when combined with common hair loss treatments like minoxidil. True weight gain has not been reported clinically, and anecdotal evidence from users is likely the result of other medications, age, activity level, or hormonal shifts. That being said, pairing treatment with sensible eating, hydration, and regular activity can help keep any weight changes in check, so the only thing you see growing is your hair.
References[+]
References ↑1, ↑6 Carone, L., Oxberry, S.G., Twycross, R., Charlesworth, S., Mihalyo, M., Wilcock, A. (2017). Spironolactone. Journal of Pain and Symptom Management. 53(2). 288–292. Available at: https://doi.org/10.1016/j.jpainsymman.2016.12.320 ↑2, ↑5, ↑8 Vargas-Mora, P., Morgado-Carrasco, D. (2020). Spironolactone in Dermatology: Uses in Acne, Hidradenitis Suppurativa, Female Pattern Hair Loss, and Hirsutism. Actas Dermo-Sifiliográficas (English Edition). 111(8). 639–649. Available at: https://doi.org/10.1016/j.adengl.2020.03.015 ↑3 Ustuner, E. T. (2013). Cause of Androgenic Alopecia: Crux of the Matter. Plast Reconstr Surg Glob Open. 1(7). e64. Available at: https://doi.org/10.1097/GOX.0000000000000005 ↑4 Sekhavat, H., Bar Yehuda, S., Asotra, S. (2025). Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss. Int J Mol Sci. 26(21). 10712. Available at: https://doi.org/10.3390/ijms262110712 ↑7 Haynes, B.A., Mookadam, F. (2009). Male Gynecomastia. Mayo Clinic Proceedings. 84(8). 672. Available at: https://doi.org/10.4065/84.8.672 ↑9, ↑19 Sinclair, R.D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. International Journal of Dermatology. 57(1). 104–109. Available at: https://doi.org/10.1111/ijd.13838 ↑10, ↑20 Werachattawatchai, P., Khunkhet, S., Harnchoowong, S., Lertphanichkul, C. (2025). Efficacy and safety of oral spironolactone for female pattern hair loss in premenopausal women: a randomized, double-blind, placebo-controlled, parallel-group pilot study. International Journal of Women’s Dermatology. 11(3). e227. Available at: https://doi.org/10.1097/JW9.0000000000000227 ↑11 Werachattawatchai, P., Khunkhet, S., Harnchoowong, S., Lertphanichkul, C. (2025). Efficacy and safety of oral spironolactone for female pattern hair loss in premenopausal women: a randomized, double-blind, placebo-controlled, parallel-group pilot study. International Journal of Women’s Dermatology. 11(3). e227. Available at: https://doi.org/10.1097/JW9.0000000000000227 ↑12, ↑21 Rushton, D., Futterweit, W., Kingsley, D., Kingsley, P., Norris, M.J. (1990). Quantitative assessment of spironolactone treatment in women with diffuse androgen-dependent alopecia. Journal of the Society of Cosmetic Chemists. 42. 317–325. Available at: https://www.researchgate.net/publication/285856638_Quantitative_assessment_of_spironolactone_treatment_in_women_with_diffuse_androgen-dependent_alopecia ↑13, ↑22 Liang, X., Chang, Y., Wu, H., et al. (2022). Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A Prospective, Single-Center, Parallel-Group, Evaluator Blinded, Randomized Trial. Frontiers in Medicine. 9. Article 905140. Available at: https://doi.org/10.3389/fmed.2022.905140 ↑14, ↑23 Liang, X., Chang, Y., Wu, H., et al. (2022). Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A Prospective, Single-Center, Parallel-Group, Evaluator Blinded, Randomized Trial. Frontiers in Medicine. 9. Article 905140. Available at: https://doi.org/10.3389/fmed.2022.905140 ↑15, ↑25 Adamopoulos, D.A., Karamertzanis, M., Nicopoulou, S., Gregoriou, A. (1997). Beneficial effect of spironolactone on androgenic alopecia. Clinical Endocrinology. 47(6). 759–760. Available at: https://doi.org/10.1046/j.1365-2265.1997.3761162.x ↑16, ↑26 Sinclair, R., Wewerinke, M., Jolley, D. (2005). Treatment of female pattern hair loss with oral antiandrogens. British Journal of Dermatology. 152(3). 466–473. Available at: https://doi.org/10.1111/j.1365-2133.2005.06218.x ↑17, ↑27 Hoedemaker, C., Van Egmond, S., Sinclair, R. (2007). Treatment of female pattern hair loss with a combination of spironolactone and minoxidil. Australasian Journal of Dermatology. 48(1). 43–45. Available at: https://doi.org/10.1111/j.1440-0960.2007.00332.x ↑18 Verhaegen, A.A., Van Gaal, L.F. (2000). Drugs That Affect Body Weight, Body Fat Distribution, and Metabolism. Endotext. MDText.com, Inc. Available at: http://www.ncbi.nlm.nih.gov/books/NBK537590/ ↑24 Zulian, E., Sartorato, P., Benedini, S., et al. (2005). Spironolactone in the treatment of polycystic ovary syndrome: Effects on clinical features, insulin sensitivity and lipid profile. Journal of Endocrinological Investigation. 28(3). 49–53. Available at: https://doi.org/10.1007/BF03345529 ↑28 Nicholls, M.G., Ramsay, L.E., Boddy, K., Fraser, R., Morton, J.J., Robertson, J.I.S. (1979). Mineralocorticoid-induced blood pressure, electrolyte, and hormone changes, and reversal with spironolactone, in healthy men. Metabolism. 28(5). 584–593. Available at: https://doi.org/10.1016/0026-0495(79)90201-4 ↑29 Gafoor, R., Booth, H.P., Gulliford, M.C. (2018). Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. k1951. Available at: https://doi.org/10.1136/bmj.k1951 ↑30 Lopez, L.M., Ramesh, S., Chen, M., et al. (2016). Progestin-only contraceptives: effects on weight. Cochrane Database of Systematic Reviews. 2016(8). CD008815. Available at: https://doi.org/10.1002/14651858.CD008815.pub4 ↑31 Burns, L.J., Souza, B.D., Flynn, E., Hagigeorges, D., Senna, M.M. (2020). Spironolactone for treatment of female pattern hair loss. Journal of the American Academy of Dermatology. 83(1). 276–278. Available at: https://doi.org/10.1016/j.jaad.2020.03.087 ↑32 Knight, M.G., Anekwe, C., Washington, K., Akam, E.Y., Wang, E., Stanford, F.C. (2021). Weight Regulation in Menopause. Menopause. 28(8). 960–965. Available at: https://doi.org/10.1097/GME.0000000000001792 ↑33 Davis, S.R., Castelo-Branco, C., Chedraui, P., et al. (2012). Understanding weight gain at menopause. Climacteric. 15(5). 419–429. Available at: https://doi.org/10.3109/13697137.2012.707385 ↑34 Burns, L.J., Souza, B.D., Flynn, E., Hagigeorges, D., Senna, M.M. (2020). Spironolactone for treatment of female pattern hair loss. Journal of the American Academy of Dermatology. 83(1). 276–278. Available at: https://doi.org/10.1016/j.jaad.2020.03.087 ↑35 Kyrou, I., Tsigos, C. (2009). Stress hormones: physiological stress and regulation of metabolism. Current Opinion in Pharmacology. 9(6). 787–793. Available at: https://doi.org/10.1016/j.coph.2009.08.007 Many people are interested in RU58841 after hearing anecdotes about it working well for hair regrowth. Even though RU58841 is classified as for “research purposes only,” you can find promotion of RU58841 on many YouTube channels. It is increasingly used as a preventive measure against hair loss, especially in the bodybuilding community and among individuals who cannot tolerate traditional treatments like finasteride due to side effects. Some people have demonstrated incredible results with no side effects. While others have reported no results and side effects ranging from brain fog to severe chest pain.
Despite its reputation, RU58841 is still a clinical mystery. No one knows why the human clinical trials were never published, and why the research chemical was ultimately abandoned. This article explores preclinical and clinical data on RU58841, presents our perspectives, and provides information we believe is rarely discussed about this research chemical and its potential issues.
What Is RU58841 and How Does It Work?
RU58841 is a topical androgen receptor antagonist developed in the mid-1990’s to combat androgenic alopecia by preventing DHT from having its effects.[1]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading
As an androgen receptor antagonist, RU58841 blocks the “landing pads” (i.e., androgen receptors) on cell surfaces for the hormone DHT, preventing DHT from exerting its effects. If DHT cannot bind to androgen receptors in balding-prone hair follicles, it cannot induce the cascade of events that leads to hair follicle miniaturization, which is the defining characteristic of androgenic alopecia.[2]Ho, C.H., Sood, T., Zito, P.M. (2024). Androgenetic Alopecia. In: StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430924/
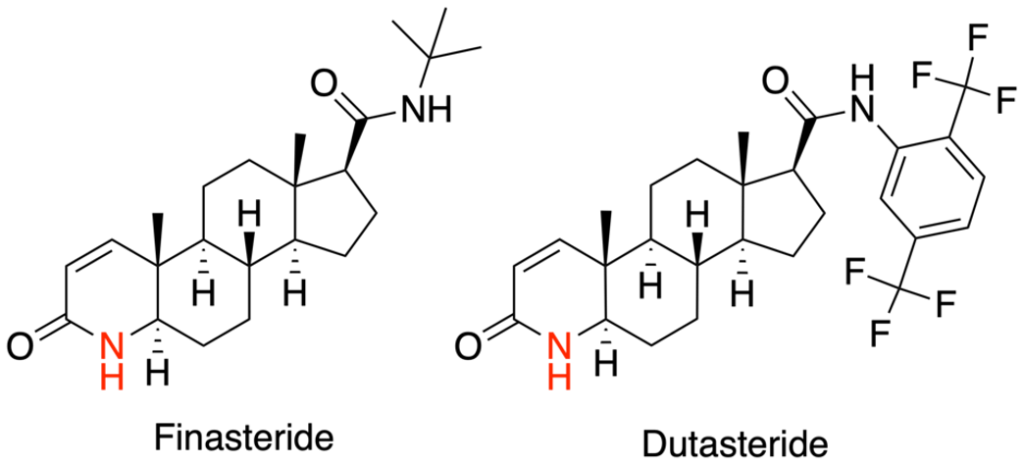
It’s important to note that androgen receptor antagonists like RU58841 work differently from drugs like finasteride or dutasteride that inhibit the enzyme that turns testosterone into DHT (reducing DHT levels overall). Androgen receptor antagonists block the “landing pads” on the cell surfaces, preventing DHT from binding. The body can still freely produce DHT, but in the presence of androgen receptor antagonists, DHT cannot bind to hair follicle sites and have its negative effects.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Follow this link to our article here to learn more about the side effects of finasteride.
The goal when developing RU58841 was to design a drug that blocked androgen receptors locally (i.e., in the scalp). With hopes of preventing its binding to androgen receptors everywhere else in the body (like with oral spironolactone), therefore preventing interference with androgen production in places like the testes, ovaries, or adrenal glands. In fact, one of the premises behind RU58841 was to simply avoid side effects that sometimes come with drugs like finasteride and dutasteride.
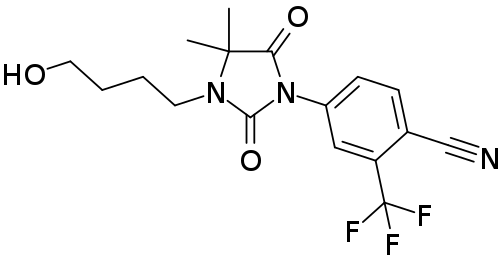
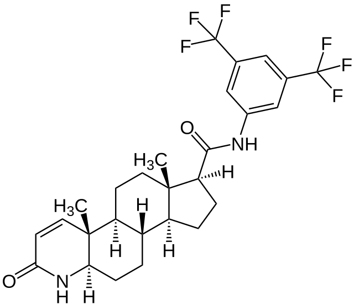
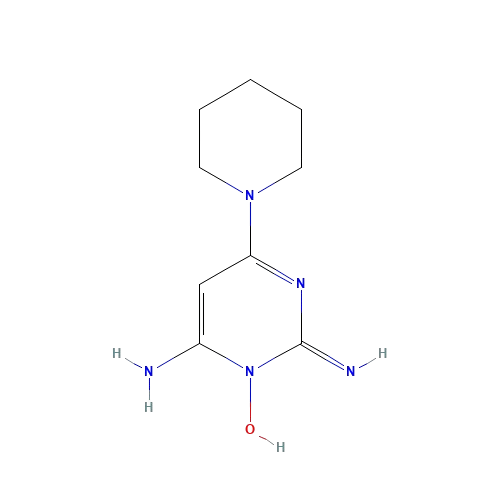
Figure 1. Structure of RU58841.[3]Wikipedia, (26 December 2018), RU-58841. Available at: https://en.wikipedia.org/wiki/RU-58841#/media/File:RU-58841_structure.svg (Accessed: 02 January 2026) Image in the Public Domain.
What the Evidence Actually Shows
Preclinical studies show promising results for RU58841. Yet despite tens of millions of dollars spent on RU58841’s human clinical trials, little information from those studies has been made public.[4]ISRCTN Registry, (no date), ISRCTN49873657. Available at: https://www.isrctn.com/ISRCTN49873657 (Accessed: 02 January 2026),[5]ISRCTN Registry, (no date), ISRCTN71083772. Available at: https://www.isrctn.com/ISRCTN71083772 (Accessed: 02 January 2026)
Soon after these clinical studies were completed, the drug’s research and development were discontinued, with financial concerns cited as the reason for halting RU58841’s progress. It was stated that with more money, development could continue. However, further development never occurred, and decades later (after the drug’s patents expired), companies began selling RU58841 for “research purposes only,” citing its initial, and small, clinical trials showing favorable results for male pattern hair loss.
Let’s break down what we actually know about RU58841 from preclinical and clinical research.
Evidence From Preclinical Data
Preclinical studies suggest RU58841 works as intended and may be effective for blocking androgen activity. But we must remember that they cannot confirm long-term human safety, and they do not guarantee purely local action in real-world conditions (where dose, formulation, frequency of application, and individual absorption vary).
How Strong Is RU58841 Compared to Natural Androgens?
RU58841 was designed as a nonsteroidal androgen-receptor antagonist and contains structural features (notably perfluoroalkyl and nitrile groups) that are associated with strong androgen-receptor binding.
Early receptor-binding experiments measuring equilibrium association constants (Ka) demonstrated that RU58841 binds the androgen receptor with affinity equal to or greater than testosterone in several species and skin-relevant tissues, including the hamster flank organ and the human receptor.[6]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading This is positive as it supports the premise that RU58841 can effectively compete with natural androgens at target sites.
When compared with other nonsteroidal antiandrogens such as flutamide, RU58841 was reported to have approximately 30x higher receptor affinity, a key reason it was pursued as a best-in-class topical antiandrogen candidate.[7]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading
But potency works both ways. Strong androgen receptor binding makes RU58841 mechanistically credible for scalp blockade. However, it also raises the stakes if any meaningful amount escapes the scalp, accumulates, or produces systemically active metabolites.
Does RU58841 Stay Local in the Skin?
In an early study using an intact hamster flank-organ model, topical RU58841 showed strong local antiandrogen activity with minimal evidence of systemic spillover at low doses.[8]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading When applied topically at doses up to 100 µg/animal, RU58841 reduced flank-organ area in a dose-dependent manner while showing no effect on the opposite flank organ, no meaningful change in serum testosterone, and no detectable antiandrogenic effects on sex organs (e.g., prostate/seminal vesicles).[9]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading These findings supported the premise that RU58841 can act locally within a certain exposure window.
Are the Effects of RU58841 Reversible?
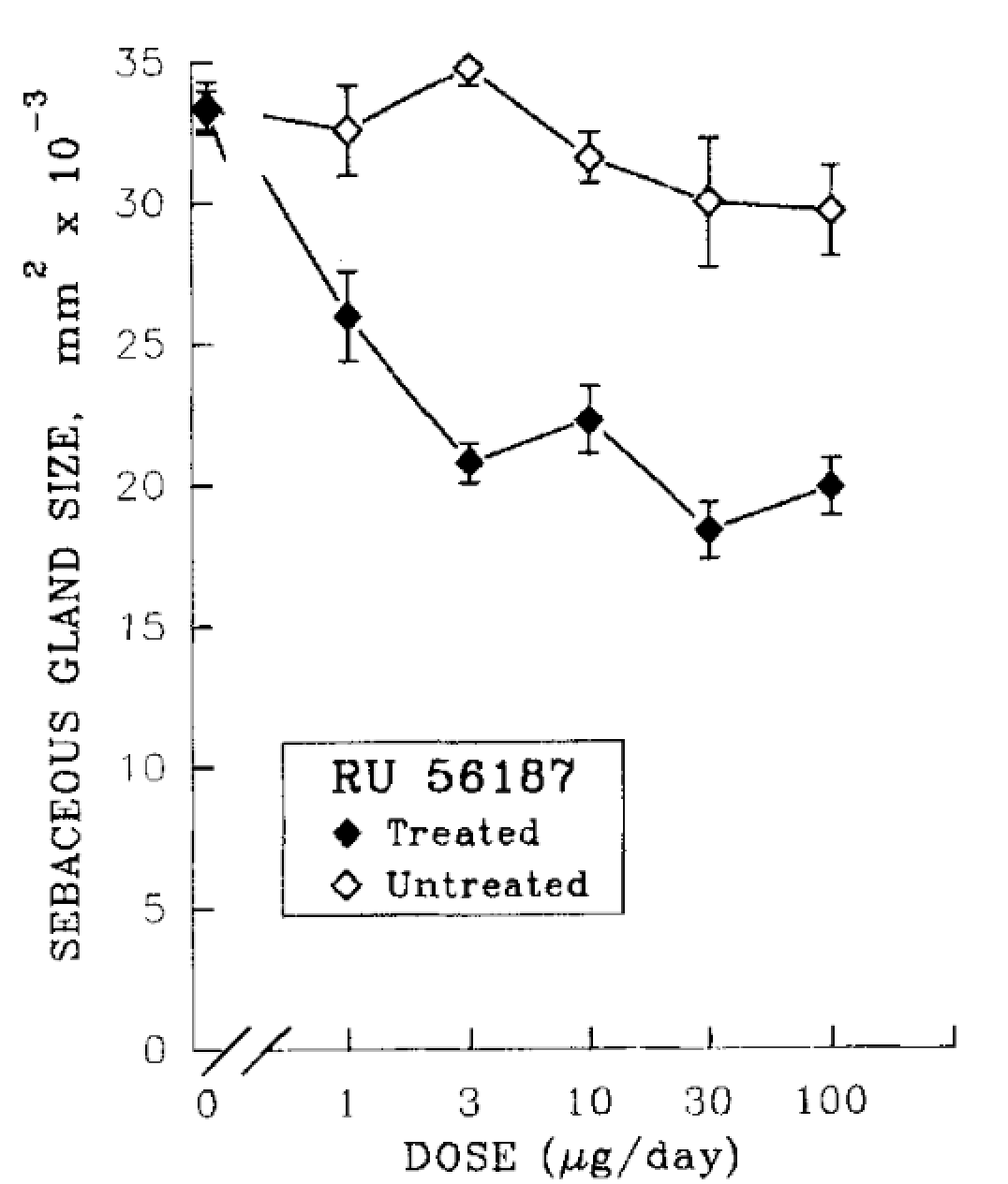
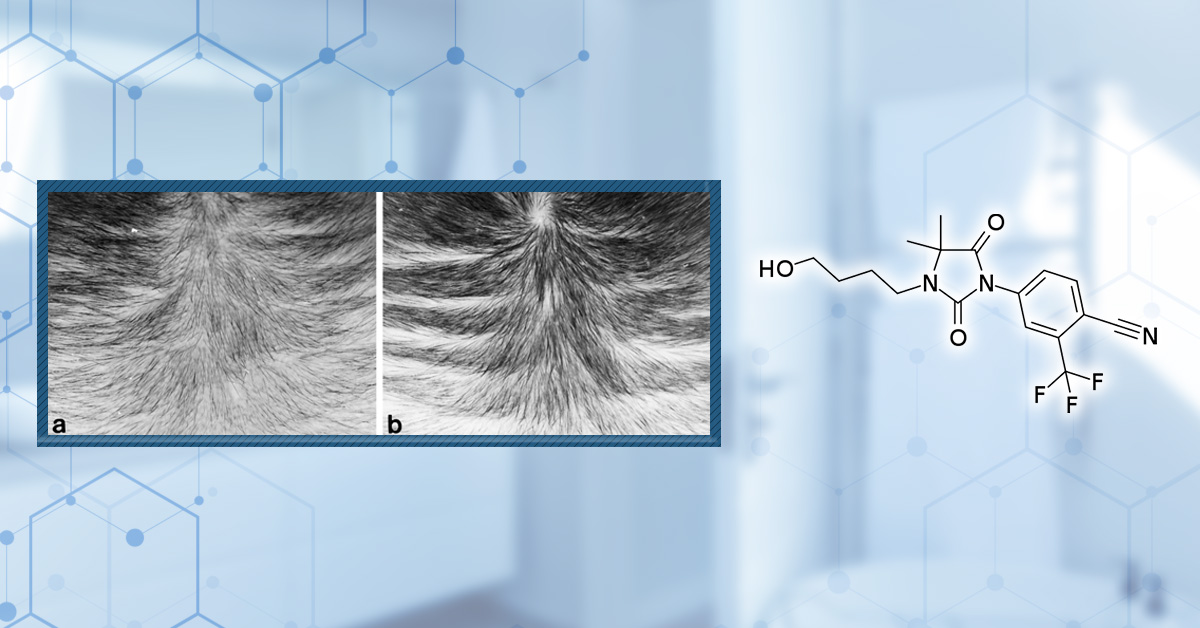
In a separate hamster ear model focused on sebaceous gland size, topical RU58841 (10 µg) reduced sebaceous gland volume by roughly 60% in the treated ear, with no measurable effect on the untreated ear.[10]Matias, J.R., Gaillard, M. (1995). Local Inhibition Of Sebaceous Gland Growth By Topically Applied RU 58841. Annals of the New York Academy of Sciences. 761. 56-65. Available at: … Continue reading Importantly, gland size returned to its baseline size within approximately four weeks after stopping treatment, showing that RU58841’s local antiandrogenic effects were reversible in this model. Dose-response experiments in this model revealed that more is not necessarily better. Around 3 µg/day was found to be as effective as 100 µg/day in reducing sebaceous gland size, indicating a pharmacologic limit.
Figure 2. RU58841 and its effects on sebaceous gland sizing: a dose-dependent study. Adapted from Figure 2.[11]Matias, J.R., Gaillard, M. (1995). Local Inhibition Of Sebaceous Gland Growth By Topically Applied RU 58841. Annals of the New York Academy of Sciences. 761. 56-65. Available at: … Continue reading
At What Point Does “Local” Become Systemic?
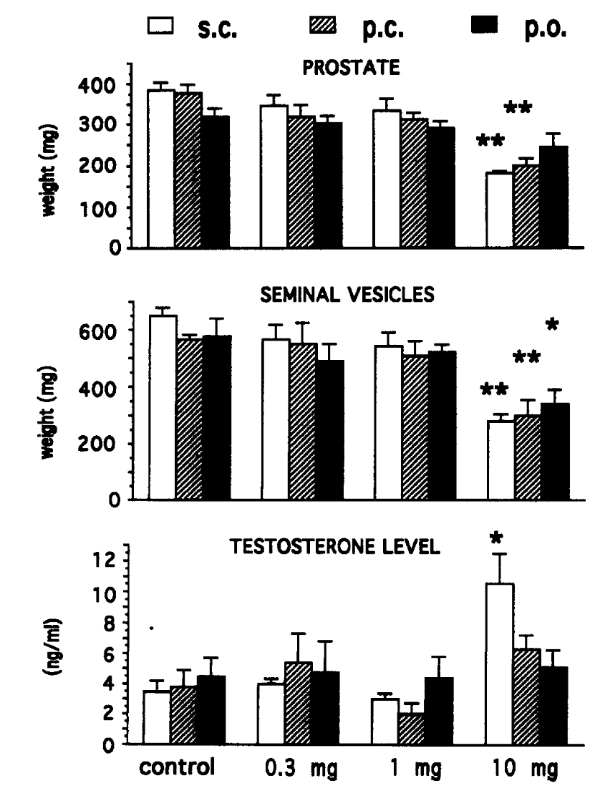
In the hamster flank-organ study, when RU58841 was given subcutaneously (to mimic complete systemic exposure), systemic antiandrogen signals emerged at higher doses.[12]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading At 300-1000 µg/animal, researchers observed reductions in prostate weight, indicating that systemic antiandrogenic effects can appear once exposure is high enough.
In intact rats, strong androgen-dependent effects were generally absent up to 1 mg/rat regardless of route of administration, but became apparent at 10 mg/rat. Testosterone increases were noted only after subcutaneous dosing at the highest dose. This suggests endocrine feedback can occur when systemic exposure is sufficiently high.
Figure 3. Antiandrogenic activity of RU5881 in the intact rat after subcutaneous (s.c.), percutaneous (p.c.) or oral (p.o.) treatment on sex organs and testosterone level. Adapted from Figure 3:[13]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading
Does RU58841 Regrow Hair in Primate Models?
The most hair-relevant preclinical data for RU58841 comes from studies conducted in stump-tailed macaques.[14]Obana, N., Chang, C., Uno, H. (1997). Inhibition Of Hair Growth By Testosterone In The Presence Of Dermal Papilla Cells From The Frontal Bald Scalp Of The Postpubertal Stumptailed Macaque. … Continue reading These primate species can develop an androgenic alopecia-like pattern with age, making it a useful, though still not perfect, translational model.
In these experiments, topical RU58841 was applied to alopecic scalp and produced concentration-dependent improvements in visible hair parameters. A lower concentration (around 0.5%) did not yield impressive results, while a higher concentration (around 5%) produced the strongest regrowth signal over months of treatment.
Reported benefits included increased hair density and hair length. Beyond surface-level changes, the primate work also reported findings that align with meaningful follicle biology improvements, including support for dermal papilla cell growth and evidence consistent with vellus-to-terminal hair conversion.
One particularly notable mechanistic detail from the macaque work is that RU58841 appeared to prevent testosterone-related androgen receptor effects in the dermal papilla. This suggests testosterone may also contribute to miniaturization through androgen receptor signaling, with the androgen receptor acting as a “lynchpin” for both testosterone and DHT in susceptible follicles.
Even so, the macaque evidence has clear constraints. Sample sizes were small, dosing equivalence to human scalp use is uncertain (vehicle, skin barrier, follicular penetration), and the overall pattern suggests benefits are linked to continued treatment rather than a permanent reversal of the underlying process.
Figure 4. Photographs showing the effects of topical RU58841 on hair growth in the bald frontal scalps of the stumptailed macaques. Increased density and length of hairs were observed in a scalp at 4 months after treatment with 5% RU58841 (b) compared with the scalp at pretreatment time (a). Adapted from Figure 3:[15]Obana, N., Chang, C., Uno, H. (1997). Inhibition Of Hair Growth By Testosterone In The Presence Of Dermal Papilla Cells From The Frontal Bald Scalp Of The Postpubertal Stumptailed Macaque. … Continue reading
Does Normal Blood Testosterone Mean It’s Truly Safe?
The primate experiments reported no meaningful change in circulating testosterone during topical RU58841 treatment. These results are often used as a surrogate for limited systemic exposure, particularly when compared with therapies designed to change systemic androgen levels.
However, unchanged serum hormones do not prove the drug is strictly confined to the scalp. Low-level systemic absorption can still occur without shifting hormone levels, and systemic androgen receptor modulation can theoretically happen even when circulating testosterone/DHT remains normal, especially if active metabolites are involved or if certain tissues are more sensitive. So while these findings are encouraging for safety, they are not definitive proof that effects are strictly confined to the application site.
Human Evidence: The Missing Link
Two human clinical trials of RU58841 (under the name PSK‑3841) were registered and marked as completed in the early 2000’s. But the interesting thing is that the results from both of these studies have never been published, leaving a critical gap between promising preclinical work and real-world clinical decision-making.
The unpublished PSK-3841 trials:
- Completion in 2002: A phase I study (ISRCTN49873657) tested a 5% PSK‑3841 solution twice daily for 4 weeks in about 30 men with androgenetic alopecia, with primary endpoints focused on safety, tolerability, endocrine profiles, plus pharmacokinetics.[16]ISRCTN Registry, (no date), ISRCTN49873657. Available at: https://www.isrctn.com/ISRCTN49873657 (Accessed: 02 January 2026)
- Completion in 2003: A larger, multi‑centre, double‑blind trial (ISRCTN71083772) then treated 120 men for 6 months with 2.5% or 5% PSK‑3841 once daily versus vehicle, measuring total and anagen hair counts, safety, tolerability, and pharmacokinetics.[17]ISRCTN Registry, (no date), ISRCTN71083772. Available at: https://www.isrctn.com/ISRCTN71083772 (Accessed: 02 January 2026)
The fact that both studies were completed, yet no peer-reviewed results or detailed reports have appeared, is a major red flag.
For a potentially first-in-class topical antiandrogen with completed phase I and II trials, companies are usually highly motivated to publish positive data. The fact that developers walked away after finishing a 6-month, 120-subject trial, without even a basic efficacy and safety paper, strongly suggests something in the data made the program unattractive to advance.
Why Unpublished Trials Change the Risk Calculation
Without access to the actual results from these clinical trials, it’s impossible to verify key parameters such as:
- How effective RU58841 was for human hair regrowth
- Types and frequency of any adverse events
- How many participants discontinued using RU58841, and the underlying reasons
- Changes in systemic hormones and sex organs
- Durability of any hair regrowth after stopping treatment
- Dose-response relationships
Missing the data from clinical studies forces a dangerous guessing game. Internet anecdotes cannot answer questions that only controlled trials can resolve, like whether rare but serious adverse events occurred, whether endocrine markers shifted subtly over months, or whether regrowth reversed after discontinuation.
Controlled trials also reveal patterns that never appear in forums, such as subgroup vulnerabilities, time-dependent toxicity, or dose thresholds where benefits collapse into harm. Without these insights, users are effectively self-experimenting with a research chemical that already failed to make it through the regulatory system.
Why Was RU58841 Abandoned?
The short answer is that nobody outside the original development team truly knows why RU58841 was abandoned.
But what we do know is that in pharmaceutical development, it is normal for early-stage compounds to fail.[18]Sun, D., Gao, W., Hu, H., Zhou, S. (2022). Why 90% Of Clinical Drug Development Fails And How To Improve It? Acta Pharmaceutica Sinica B. 12(7). 3049-3062. Available at: … Continue reading What is not normal is for companies to complete phase I and II trials and then allow all efficacy and safety data to vanish without any peer-reviewed publication, abstract, or regulatory disclosure.
Once a drug has reached a completed multi-centre, double-blind phase II study, the cost of publishing the results is trivial compared to the tens of millions already spent developing, patenting, manufacturing, and running the trials. Even negative results are typically published or disclosed.
Let’s examine the three most plausible explanations.
Hypothesis 1: Financial Reasons
The official explanation given by the companies involved was financial difficulty.
On the surface, this does sound plausible. Drug development is expensive. But when viewed in context, this explanation collapses. RU58841 had already cleared the most expensive hurdles, such as toxicology work, formulation development, and phase I and II human trials. Writing up and submitting those results would have cost a few thousand dollars at most.
It does not make sense for a company to invest tens of millions into patenting, testing, and completing two human trials, only to stop short of the final, cheapest step. For this reason, financial limitations are the weakest explanation.
Hypothesis 2: Lack of Efficacy
Another possibility is that RU58841 was not effective for hair regrowth in humans.
It is actually quite common for companies to bury disappointing studies while only publishing favorable ones. However, when it comes to RU58841, this reason is highly unlikely, mainly due to the consistent anecdotal reports across forums describing visible regrowth and stabilization with RU58841.
While anecdotes cannot replace clinical trials, the volume and consistency of regrowth reports make it unlikely that RU58841 was completely ineffective in humans. If efficacy were truly absent, the enthusiasm around this research chemical would almost certainly not have persisted throughout the past two decades.
Hypothesis 3: Safety Concerns
This leaves the most plausible explanation, something in the human data made RU58841 commercially or medically unattractive to advance.
While this hypothesis is speculative, it is supported by several warning signals. Follow-up research on RU58841 reported that at least one of its metabolites possessed strong antiandrogenic activity, undermining the original premise that the drug would only act locally in the scalp.[19]Cousty-Berlin, D., Bergaud, B., Bruyant, M.C., Battmann, T., Branche, C., Philibert, D. (1994). Preliminary Pharmacokinetics And Metabolism Of Novel Non-Steroidal Antiandrogens In The Rat: Relation … Continue reading If systemically active metabolites were forming in humans, this would fundamentally change the drug’s risk profile.
Preclinical work also raised more subtle molecular concerns that are rarely discussed online. Some androgen-receptor antagonists can exhibit something called antagonistic-agonism, where compounds designed to block the receptor can, under certain conditions, partially activate it as well.[20]Miyamoto, H., Yeh, S., Wilding, G., Chang, C. (1998). Promotion Of Agonist Activity Of Antiandrogens By The Androgen Receptor Coactivator, ARA70, In Human Prostate Cancer DU145 Cells. Proceedings of … Continue reading
This appears to involve androgen-receptor co-activators such as ARA70, which amplify androgen signaling once the receptor complex is transported into the nucleus. In experimental systems, RU58841 showed a mild but measurable ability to enhance androgen-receptor co-activation in wild-type receptors in a dose-dependent manner. [21]Miyamoto, H., Yeh, S., Wilding, G., Chang, C. (1998). Promotion Of Agonist Activity Of Antiandrogens By The Androgen Receptor Coactivator, ARA70, In Human Prostate Cancer DU145 Cells. Proceedings of … Continue reading While it remains unknown whether this effect occurs in humans or is clinically meaningful, it represents exactly the type of risk that would make long-term use of a topical antiandrogen far less predictable than originally hoped.
Additionally, it’s worth noting that some people trying RU58841 online have reported chest pain, brain fog, and other issues, such as sexual dysfunction. We have not found credible evidence that these effects are permanent, but their nature is consistent with unintended endocrine or cardiovascular involvement.
Together, the evidence is quite difficult to ignore. RU58841 may well have been effective for hair regrowth, but a combination of systemically active metabolites, loss of localization in the scalp, and early molecular signals suggesting complex androgen-receptor behavior may have made the drug too risky to advance.
If you’re a member and would like to find out more about these molecular safety mechanisms, click the link here for more details in our RU58841 Ultimate Guide
Final Thoughts
So is RU58841 legit for efficacy? Plausibly yes. Given the preclinical data and sheer volume of regrowth anecdotes, RU58841 does likely block androgen signaling in the scalp.
But is RU58841 legit as a recommended treatment? No. Its safety profile is still a clinical mystery. Its human trials were never published, and the drug was abandoned at a stage where most drugs either move forward or fail. The gap in clinical information changes the entire risk calculation.
In our opinion, evidence quality and long-term safety hold more weight than exciting anecdotes.
For individuals who cannot tolerate finasteride, there are topical antiandrogen options with more transparent development histories and human data, including topical fluridil, topical CB-03-03, topical spironolactone, and newer molecules like pyrilutamide that are currently progressing through regulated clinical trials. While none are perfect, they at least exist within a framework of published safety monitoring.
If you’d like to learn more about pyrilutamide, read our article here, which covers all of the publicly available data on pyrilutamide and evaluates its mechanisms of action, efficacy, and safety.
We recommend proceeding with extreme caution when using RU58841, and only use it if you understand and are willing to accept the absence of human safety and efficacy data.
The best practice is to prioritize therapies with published human trials and regulatory oversight. Ultimately, given the lack of safety information and regulatory oversight for RU58841, we recommend erring on the side of caution.
References[+]
References ↑1 Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, Androgenetic Alopecia and Hirsutism. J Steroid Biochem Mol Biol. 48(1). 55-60. Available at: https://doi.org/10.1016/0960-0760(94)90250-x ↑2 Ho, C.H., Sood, T., Zito, P.M. (2024). Androgenetic Alopecia. In: StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430924/ ↑3 Wikipedia, (26 December 2018), RU-58841. Available at: https://en.wikipedia.org/wiki/RU-58841#/media/File:RU-58841_structure.svg (Accessed: 02 January 2026) ↑4, ↑16 ISRCTN Registry, (no date), ISRCTN49873657. Available at: https://www.isrctn.com/ISRCTN49873657 (Accessed: 02 January 2026) ↑5, ↑17 ISRCTN Registry, (no date), ISRCTN71083772. Available at: https://www.isrctn.com/ISRCTN71083772 (Accessed: 02 January 2026) ↑6, ↑7, ↑8, ↑9, ↑12, ↑13 Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, Androgenetic Alopecia and Hirsutism. J Steroid Biochem Mol Biol. 48(1). 55–60. Available at: https://doi.org/10.1016/0960-0760(94)90250-x ↑10 Matias, J.R., Gaillard, M. (1995). Local Inhibition Of Sebaceous Gland Growth By Topically Applied RU 58841. Annals of the New York Academy of Sciences. 761. 56-65. Available at: https://doi.org/10.1111/j.1749-6632.1995.tb31369.x ↑11 Matias, J.R., Gaillard, M. (1995). Local Inhibition Of Sebaceous Gland Growth By Topically Applied RU 58841. Annals of the New York Academy of Sciences. 761. 56-65. Available at: https://doi.org/10.1111/j.1749-6632.1995.tb31369.x ↑14 Obana, N., Chang, C., Uno, H. (1997). Inhibition Of Hair Growth By Testosterone In The Presence Of Dermal Papilla Cells From The Frontal Bald Scalp Of The Postpubertal Stumptailed Macaque. Endocrinology. 138(1). 356-361. Available at: https://doi.org/10.1210/endo.138.1.4890 ↑15 Obana, N., Chang, C., Uno, H. (1997). Inhibition Of Hair Growth By Testosterone In The Presence Of Dermal Papilla Cells From The Frontal Bald Scalp Of The Postpubertal Stumptailed Macaque. Endocrinology. 138(1). 356-361. Available at: https://doi.org/10.1210/endo.138.1.4890 ↑18 Sun, D., Gao, W., Hu, H., Zhou, S. (2022). Why 90% Of Clinical Drug Development Fails And How To Improve It? Acta Pharmaceutica Sinica B. 12(7). 3049-3062. Available at: https://doi.org/10.1016/j.apsb.2022.02.002 ↑19 Cousty-Berlin, D., Bergaud, B., Bruyant, M.C., Battmann, T., Branche, C., Philibert, D. (1994). Preliminary Pharmacokinetics And Metabolism Of Novel Non-Steroidal Antiandrogens In The Rat: Relation Of Their Systemic Activity To The Formation Of A Common Metabolite. Journal of Steroid Biochemistry and Molecular Biology. 51(1-2). 47-55. Available at: https://doi.org/10.1016/0960-0760(94)90114-7 ↑20, ↑21 Miyamoto, H., Yeh, S., Wilding, G., Chang, C. (1998). Promotion Of Agonist Activity Of Antiandrogens By The Androgen Receptor Coactivator, ARA70, In Human Prostate Cancer DU145 Cells. Proceedings of the National Academy of Sciences of the United States of America. 95(13). 7379-7384. Available at: https://doi.org/10.1073/pnas.95.13.7379 Minoxidil has been a cornerstone of hair-loss treatment for decades, and there are now more options than ever for users. Alongside traditional topical solutions and foams, low-dose oral minoxidil has emerged as a popular off-label alternative.
Which raises the question: which formulation is the best choice for hair loss? And which option comes with the fewest side effects? In this article, we break down how oral and topical minoxidil compare across mechanisms, evidence, side effects, and real-world use, so you can make an informed decision about which approach fits your hair loss pattern, risk tolerance, and lifestyle.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

How Does Minoxidil Work?
Despite being used to treat hair loss for over 40 years, the mechanisms through which minoxidil promotes hair growth are only partially understood. We know that it alters the hair cycle, shortening the resting (telogen) phase so follicles enter the growth (anagen) phase earlier. This increases the number of growing hairs at any one time. It also increases follicle size, which translates into thicker, longer hair shafts.[1]Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. *British Journal of Dermatology.* 150(2). 186–194. Available at: … Continue reading
Minoxidil can increase blood flow to hair follicles, which increases the flow of oxygen and nutrients to support growth. The drug is also known to activate the Wnt/ꞵ-catenin signaling pathway, which supports the growth of cells in the follicle, and provides cytoprotective effects and regulates local inflammation.
Figure 1. Structure of minoxidil.[2]https://commons.wikimedia.org/wiki/File:Minoxidil_Structural_Formula_V1.svg) Image in the Public Domain.
In contrast to antiandrogenic drugs like finasteride and dutasteride, minoxidil doesn’t impact hormones.
Before we compare topical and oral treatments, we’ll look at the formulations separately to understand the options available for each.
Topical Minoxidil
Topical minoxidil is designed to work primarily at the scalp, not systemically. After you apply it, only a small fraction of the dose actually penetrates intact skin, and an even smaller fraction reaches the follicle structures where it matters most.
Where it does work is at (or near) the follicle, particularly around the outer root sheath and dermal papilla. That’s why topical minoxidil has a strong safety record: most of the drug never leaves the scalp in meaningful quantities.
Why Some People Respond Better to Topical Minoxidil
Minoxidil relies on activating enzymes to work, most notably sulfotransferase SULT1A1. SULT1A1 activity in the hair follicles is closely linked to how well topical minoxidil works, and people with lower levels of activity tend to see less benefit from treatment.[3]Pietrauszka, K., & Bergler-Czop, B. (2022). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: … Continue reading
Because minoxidil needs SULT1A1 to work, people with low levels of the enzyme in the hair follicle might not see much improvement from minoxidil (“enzyme low-responders”).
The effectiveness of topical minoxidil also depends on a range of other factors, including the type of formulation, scalp health, contact time, application technique, and the use of penetration enhancers. This is one reason why topical minoxidil performs reliably in clinical trials, but users find results more variable.
Typical Dosing: How Much and How Often?
Most over-the-counter minoxidil is sold in 2% or 5% formulations. 5% generally produces stronger average results and shows higher satisfaction in many studies. It also comes with a slightly higher irritation risk, especially in solution form. As such, 2% formulations are often used when scalp irritation from higher doses is too disruptive, or for milder cases.[4]Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical … Continue reading
Higher concentrations (7–15%) are also sometimes considered, but they offer diminishing returns for many users and a higher risk of irritation. Because of this, they’re usually reserved for people who don’t respond to standard options and can tolerate the vehicle.
Dosing frequency is about balancing efficacy with adherence: there’s no benefit to be gained from planning a treatment schedule that you can’t stick to.
Twice-daily use is the traditional standard for maximum effect, especially in people using solutions. Once-daily 5% is often good enough for many people, and can dramatically improve adherence, although studies have shown that switching to twice to once daily can slightly worsen outcomes. [5]Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). … Continue reading
Similarly, in women, once-daily 5% foam can perform similarly to twice-daily 2% solution while being easier to sustain. Foam formulations are often recommended for women because foam tends to stay localized on the scalp and drip down less onto the face than solutions. This reduces the chance of unwanted facial hair growing as a result of treatment. Evidence also suggests that foam formulations have lower systemic absorption into the bloodstream.[6]Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., & Garcia Bartels, N. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in … Continue reading,[7]Gogtay, J. A., & Panda, M. (2009). Minoxidil topical foam: a new kid on the block. *International Journal of Trichology.* 1(2). 142. Available at: https://doi.org/10.4103/0974-7753.58560
Limitations of Topical Minoxidil
Topical minoxidil works, but it requires consistency. Results typically require months of consistent use before they become apparent, and many people struggle to maintain the routine, especially with twice-daily application.
Absorption of topical solutions can also be inconsistent, and is impacted by scalp condition, contact time, and the type of vehicle used (propylene glycol-based solutions, foams, propylene glycol-free lotions, nanoemulsions, etc.). As such, even with consistent application, results can vary.
The main side effects associated with topical minoxidil are local rather than systemic. Common issues include itching, dryness, flaking, and contact dermatitis, which are often driven by the formulation rather than the drug itself. Many users also find the product cosmetically inconvenient due to a greasy feel, visible residue, or interference with hairstyling, particularly for those with longer hair.[8]Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). … Continue reading
Evidence for Topical Minoxidil
The use of topical minoxidil is backed up by substantial clinical data showing increased hair counts, density, and coverage in androgenic alopecia (AGA) and other alopecias, with response in a majority but not all patients. We’ll look at evidence from clinical trials involving men and women separately, as they are typically studied in different clinical trials.
Evidence in Men
The earliest significant clinical evidence supporting the use of minoxidil for hair loss is from a 1985 trial, which compared 2% and 3% solutions to placebo in 126 men with early male pattern baldness. They assessed changes in vellus hairs and terminal hairs, an important distinction as vellus hairs are fine, light colored hairs that don’t contribute to the overall appearance of hair thickness.[9]Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). … Continue reading
They found a significant increase in terminal hair counts in 2% and 3% minoxidil-treated participants, with a greater improvement in the 3% group, though this was not statistically significant.
The study methodology switched the placebo group to 3% minoxidil after 4 months and showed that the switch increased hair counts. Unfortunately, this means that it’s not possible to assess actual changes in hair count over the 12-month study. Notably, the placebo group also showed increases in hair count during the first 4 months, which might not be expected in men with pattern baldness or AGA and could cast doubt on the hair counts.
Larger trials have been conducted since. A 48 week study compared 2% and 5% solutions to placebo and found approximately 13% and 19% increases in nonvellus hairs respectively, although hair counts did appear to start to drop after week 16.[10]Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical … Continue reading
Similarly, a Japanese study compared 1% and 5% formulations, and found a 26.4% increase in nonvellus hair count in the 5% topical minoxidil group and 21.2% increase in the 1% group.[11]Tsuboi, R., Arano, O., Nishikawa, T., Yamada, H., & Katsuoka, K. (2009). Randomized clinical trial comparing 5% and 1% topical minoxidil for the treatment of androgenetic alopecia in Japanese … Continue reading
Notably, these trials tend to be relatively short, with many lasting only 16 weeks. Even in these short trials, there is a noticeable drop-off in hair counts after an initial growth boost. Data suggests that topical minoxidil response rates are high in the first 3-6 months, but drop after a year or longer of use. Real-world data show that 86–95% of users discontinue topical minoxidil within one year, most commonly because the results fall short of their expectations for visible cosmetic improvement.[12]Olsen, E. A., Weiner, M. S., Amara, I. A., & DeLong, E. R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. *Journal of the American Academy of … Continue reading,[13]Shadi, Z. (2023). Compliance to topical minoxidil and reasons for discontinuation among patients with androgenetic alopecia. *Dermatology and Therapy.* 13(5). 1157–1169. Available at: … Continue reading
If you’re interested in topical minoxidil for men, check out our article on the best products currently available.
Evidence in Women
Most clinical trials evaluating topical minoxidil in women have traditionally used a 2% solution applied twice daily. A meta-analysis, which combines the results from multiple trials found that 2% minoxidil produced an average increase of about 12 hairs per square centimeter compared with placebo after 24 weeks of treatment.[14]Adil, A., & Godwin, M. (2017). The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. *Journal of the American Academy of Dermatology.* 77(1). … Continue reading
Data also suggest that higher-strength formulations offer similar outcomes: both 5% minoxidil solution used twice daily and 5% minoxidil foam applied once daily have shown no significant difference in efficacy compared with twice-daily 2% solution use.[15]Lucky, A. W., Piacquadio, D. J., Ditre, C. M., Dunlap, F., Kantor, I., Pandya, A. G., Savin, R. C., & Tharp, M. D. (2004). A randomized, placebo-controlled trial of 5% and 2% topical minoxidil … Continue reading
As we’ve already noted, foams are often preferred by women as they can potentially limit off-target hair growth. A large, 24-week trial demonstrated that foam minoxidil formulation resulted in 9.1 hairs more per cm2 more than a placebo (foam without minoxidil), with no significant differences in irritation.[16]Lucky, A. W., Piacquadio, D. J., Ditre, C. M., Dunlap, F., Kantor, I., Pandya, A. G., Savin, R. C., & Tharp, M. D. (2004). A randomized, placebo-controlled trial of 5% and 2% topical minoxidil … Continue reading
Figure 2. Improvement in hair coverage in a woman with pattern hair loss following 6 months 5% topical minoxidil treatment. Adapted from Figure 1.[17]Ramos, P. M., Melo, D. F., Radwanski, H., Cortez de Almeida, R. F., & Miot, H. A. (2023). Female-pattern hair loss: therapeutic update. *Anais Brasileiros de Dermatologia.* 98. 506–519. … Continue reading Image used under Creative Commons License.
Oral Minoxidil
Unlike topical minoxidil, oral minoxidil is absorbed through the gastrointestinal tract and converted into its active form primarily in the liver, where sulfotransferase activity is abundant. This systemic activation largely bypasses one of the major limitations of topical therapy: variations in follicular enzyme activity. As a result, nearly all ingested minoxidil is activated and made bioavailable, which helps explain why oral formulations can work even in people who do not respond well to topical formulations.
Typical Dosing
Minoxidil was originally developed to treat hypertension, and it is still used for the condition today. Dosage for hair loss is much lower than that for hypertension, and most regimens fall within the low-dose range of 0.25 to 5 mg per day.
Within this range, efficacy appears to be dose-dependent. In men, doses between 2.5 and 5 mg daily are commonly used and tend to produce the most robust regrowth.[18]Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: … Continue reading,[19]Jimenez-Cauhe, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O. M., Fernandez-Nieto, D., & Vaño-Galvan, S. (2019). Effectiveness and safety of low-dose oral … Continue reading
However, lower doses may still be effective for slowing progression or improving density in milder cases. In women, lower doses (0.25 to 1.25 mg daily) are preferred, as they balance efficacy with a lower risk of unwanted body hair growth and fluid retention.[20]Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse … Continue reading
To find out more about minoxidil dosing, check out our article.
Oral Minoxidil Side Effects
Because oral minoxidil is systemically active, its side effects differ from those of topical formulations. Still, at the low doses used for hair loss, oral minoxidil is generally well tolerated, and most adverse effects are reversible with adjustment or discontinuation.
The most commonly reported side effect is unwanted hair growth outside of the scalp, including on the face and arms. While this effect is often mild and reversible, it is often more of an issue for women and becomes more likely as the dose increases.[21]Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair … Continue reading
Another relatively common effect is fluid retention, which may present as mild ankle or lower-leg swelling. This occurs because minoxidil is a vasodilator and can promote sodium and water retention. Some patients also report lightheadedness or dizziness, particularly when starting treatment or increasing the dose.
Less commonly, patients may experience palpitations or changes in heart rate. These symptoms are usually short-lasting and improve with dose reduction. Importantly, serious cardiovascular complications are rare at doses of 0.25-5 mg daily and have been reported primarily in higher-dose antihypertensive use or in individuals with underlying heart disease.[22]Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse … Continue reading
Figure 3. Occurrence of adverse events in individuals taking low-dose oral minoxidil (LDOM), as reported in the FDA Adverse Event Reporting System. Adapted from Figure 1.[23]Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse … Continue reading Image used under Creative Commons License.
If you’re concerned about minoxidil side effects, check out our article on 10 ways to reduce them.
Evidence in Men
Multiple studies in men with AGA show consistent increases in total hair count and visible thickening, with reported response rates ranging from roughly 60% at very low doses (0.25 mg) to 90–100% at doses between 2.5 and 5 mg daily over 6-12 months.
The 60% response at 0.25 mg comes from a 2020 retrospective study of 25 men, looking at the effect of very-low-dose minoxidil. While there was a 60% response rate, there wasn’t any meaningful increase in terminal hair count on average.[24]Pirmez, R., & Salas-Callo, C. I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: a study with quantitative trichoscopic documentation. *Journal of the American Academy of … Continue reading
At higher doses, response rates are higher, and hair growth is more predictable. A 24-week study using 5 mg minoxidil daily found a 19% increase in hair count. An open-label study using 5 mg minoxidil, without a placebo control, found increases in hair count and improvements in photographic assessment at 12 and 24 weeks. They also found a 19% improvement in hair counts compared to baseline.[25]Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: … Continue reading,[26]Panchaprateep, R., & Lueangarun, S. (2020). Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global … Continue reading
A retrospective study of only 41 men found a response rate of 90%, though changes were not well quantified.[27]Jimenez-Cauhe, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O. M., Fernandez-Nieto, D., & Vaño-Galvan, S. (2019). Effectiveness and safety of low-dose oral … Continue reading
In these studies, average gains included double-digit percentage increases in hair count and noticeable improvements on global photographic assessment, indicating that oral minoxidil can produce cosmetically relevant changes rather than purely statistical ones.
Evidence in Women
In female pattern hair loss (FPHL), oral minoxidil at doses between 0.25 and 1.25 mg daily has demonstrated efficacy comparable to 5% topical minoxidil, with the added benefit of avoiding scalp irritation. Combination approaches appear particularly promising: studies pairing low-dose oral minoxidil with spironolactone report earlier reductions in shedding and greater density gains than either agent alone, although direct head-to-head comparisons remain limited.[28]Silva, M. N. E., Ramos, P. M., Silva, M. R., Silva, R. N. E., & Raposo, N. R. B. (2022). Randomized clinical trial of low-dose oral minoxidil for the treatment of female pattern hair loss: 0.25 … Continue reading,[29]Sinclair, R. D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. *International Journal of Dermatology.* 57(1). … Continue reading
Oral Minoxidil in Special Populations
Evidence also supports oral minoxidil’s utility in several special populations. In people with diffuse thinning, including chronic telogen effluvium, low-dose oral minoxidil has been associated with reduced shedding and gradual improvements in density over 6–12 months.[30]Perera, E., & Sinclair, R. (2017). Treatment of chronic telogen effluvium with oral minoxidil: a retrospective study. *F1000Research.* 6. 1650. Available at: … Continue reading
While these studies are largely retrospective and lack placebo controls, the consistency of improvement across patients suggests a real biological effect, especially given minoxidil’s known ability to shorten telogen and promote anagen entry.
In alopecia areata, oral minoxidil alone produces modest response rates, but when added to immunomodulatory agents such as tofacitinib, it appears to substantially enhance regrowth without requiring higher doses of the primary drug.[31]Wambier, C. G., Craiglow, B. G., & King, B. A. (2021). Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. *Journal of the American Academy of Dermatology.* 85(3). … Continue reading
Oral vs. Topical Minoxidil: Direct Comparison
Due to differences in study design and the range of doses and formulations used, it is difficult to compare the results of studies on oral and topical minoxidil. Luckily, a few trials have compared the two treatment routes directly.
Comparison Study #1
The only direct comparison of the efficacy of topical and oral minoxidil in men comes from a 2024 randomized placebo-controlled trial comparing a 5 mg oral dose (once daily) and 5% topical formulation (twice daily) for 24 weeks. They found no significant difference between the two formulations in hair, though there was a 20% increase in hair density in the oral group compared to 7% increase in the topical group. Photographic assessment indicated a significantly greater improvement in the oral group at the crown.[32]Penha, M. A., Miot, H. A., Kasprzak, M., & Ramos, P. M. (2024). Oral minoxidil vs topical minoxidil for male androgenetic alopecia: a randomized clinical trial. *JAMA Dermatology.* 160(6). … Continue reading
There was a higher rate of adverse events in the oral treatment group, though this was largely due to increased hair growth in other parts of the body. Participants taking oral minoxidil experienced significantly more headaches, while the topical group experienced scalp irritation more frequently, as we would expect.
Comparison Study #2
There are more head-to-head comparisons of oral and topical formulations in women. The first direct comparison of oral and topical minoxidil for female-pattern hair loss comes from a 24-week randomized, open comparative study conducted in Brazil. The trial compared low-dose oral minoxidil (1 mg once daily) with topical minoxidil 5% solution applied once daily in women aged 18–65 years.[33]Ramos, P. M., Melo, D. F., Radwanski, H., Cortez de Almeida, R. F., & Miot, H. A. (2023). Female-pattern hair loss: therapeutic update. *Anais Brasileiros de Dermatologia.* 98. 506–519. … Continue reading
After 24 weeks, terminal hair density increased by 12% in the oral minoxidil group and by 7.2% in the topical group. This difference did not reach statistical significance . Secondary outcomes, including hair density, global photographic assessment by dermatologists, quality-of-life scores, and hair shedding, were also evaluated, with no significant between-group differences reported.
Hypertrichosis (hair growth on other parts of the body) was reported in 27% of the oral group, compared to 4% in the topical group. This lower incidence of adverse events in the oral group in this trial compared to the study #1 is likely due to lower doses used.
Comparison Study #3
In a 6-month trial, both treatment groups demonstrated a statistically significant increase in hair diameter. Again, no significant differences between topical and oral minoxidil were reported. Photographic assessment showed significant improvement in hair densityn the topical minoxidil group, whereas no significant improvement was detected in the oral minoxidil group; however, between-group differences were not statistically significant.[34]Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled … Continue reading
Patient satisfaction exceeded 60% in both groups, with no significant difference reported. Both treatments were well tolerated, and no major safety concerns were identified, though two patients in the oral group experienced hypertrichosis on their face and extremities.
Figure 4. Improvements in average hair diameter were comparable between 5% topical solution or 1 mg/day oral minoxidil over a six-month study period. Adapted from Figure 2.[35]Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled … Continue reading Image used under Creative Commons License.
Comparison Study #4
Our final study again showed no significant difference between the two groups. This study used lower doses than previously seen: 0.25 mg daily with a topical placebo or topical minoxidil 2% solution with an oral placebo for 9 months.[36]Vahabi-Amlashi, S., Layegh, P., Kiafar, B., Hoseininezhad, M., Abbaspour, M., Hajebi Khaniki, S., Forouzanfar, M., & Sabeti, V. (2021). A randomized clinical trial on therapeutic effects of 0.25 … Continue reading
In the oral minoxidil group, average hair density increased from 102/cm² to 115/cm² (1.12% increase), while in the topical minoxidil group, hair density increased from 107/cm² to 113/cm² (1.06%).
These improvements are modest compared to those we have seen previously, likely due to the lower doses used. This is also reflected in the very low incidence of side effects reported.
Meta-Analysis
Meta-analyses compile numerous studies together to see if there are consistent results across different research groups and trials. A recent meta-analysis comparing topical and oral minoxidil found that there was no significant difference between the two approaches.[37]Fazal, F., Malik, B. H., Malik, H. M., Sabir, B., Mustafa, H., Ahmed, M., Abid, A., Adil, M. L., Shafi, U., & Saad, M. (2025). Can oral minoxidil be the game changer in androgenetic alopecia? A … Continue reading
This finding is apparent in the studies we’ve looked at: there are some cases where oral treatment seems to provide better results, but this is not large or consistent enough to be significant when statistical analyses are performed.
Given the trend towards greater improvements in oral formulations, it is possible that larger, longer-term studies might find significant differences. However, on an individual basis, these small, population-scale differences are unlikely to be large enough to influence treatment decisions.
Is Topical Minoxidil Safer?
Both formulations are well tolerated across studies, with the most common reported side effect for topical formulations being scalp irritation. We have seen a higher rate of side effects in groups taking oral minoxidil, though this is most commonly due to increased hypertrichosis, which may be more of a concern for women.
More serious concerns around the use of oral minoxidil are related to cardiovascular events. A large-scale retrospective study assessed the safety of 1404 people taking oral minoxidil at a range of low doses appropriate for hair loss.[38]Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair … Continue reading
Apart from hypertrichosis, adverse events were rare, with lightheadedness reported in 1.7% of participants and tachycardia (fast heart rate) reported in 0.9%. No life‑threatening cardiovascular events were reported.
Therefore, while there is an increased risk of more serious side effects in oral formulations, at the low doses used for hair loss, there is minimal risk of adverse events.
Adherence and Practical Considerations
One of oral minoxidil’s most compelling advantages is its performance in people who do not respond adequately to topical therapy. Because topical minoxidil requires local scalp activation by the sulfotransferase enzyme SULT1A1, and because only a small fraction of applied drug penetrates the skin, many users see limited benefit despite correct use. Oral minoxidil bypasses both of these bottlenecks by being converted to its active sulfate form in the liver and delivered systemically, ensuring uniform exposure to hair follicles across the scalp.
As well as efficacy, there are some other practical considerations that should influence your decisions when comparing oral and topical minoxidil.
Ease of Use
From a day-to-day standpoint, oral and topical minoxidil place very different demands on the user. Oral minoxidil is taken as a pill, usually once daily, or split into morning and evening doses, which makes it largely invisible in daily life. There is no impact on hairstyling, no waiting period before washing, and no concern about residue, odor, or transfer to pillows, clothing, or pets. For many people, this simplicity is the primary reason oral minoxidil feels sustainable.
Topical minoxidil, by contrast, requires direct scalp application once or twice daily, ideally to a dry scalp, with sufficient contact time before washing. Proper use often means parting hair, targeting thinning areas precisely, and tolerating cosmetic downsides such as greasiness, stiffness, or flaking. While none of these steps are difficult in isolation, the cumulative friction can become a barrier over months and years of use.
Cost and Access
Topical minoxidil has a clear accessibility advantage: it is available over the counter in standard 2% and 5% formulations, allowing immediate initiation without a prescription. For many users, this makes topical therapy an easy first-line option. Basic formulations are relatively inexpensive on a monthly basis, especially when purchased in bulk.
Oral minoxidil is prescription-only and used off-label for hair loss. This introduces an additional step and sometimes variability in access depending on region, provider comfort, and insurance coverage. That said, once prescribed, low-dose oral minoxidil is often comparable in cost and, in some cases, less expensive over time than higher-strength or specialty topical formulations.
Who Should Choose What?
Choosing between oral and topical minoxidil isn’t really about which one is “stronger” on paper. Rather, it’s important to consider which one you’re most likely to tolerate and use consistently.
Who Topical Minoxidil Is Best For
Risk-averse individuals – Topical minoxidil remains the best first-line choice for many people because it offers meaningful benefit with minimal systemic exposure. If you want the lowest-risk long-term approach and are comfortable with scalp application, topical is usually the most conservative starting point.
Cardiovascular contraindications (or uncertainty) – Anyone with a history of cardiovascular disease, kidney issues, fluid retention, low blood pressure, or unexplained palpitations should be cautious with oral minoxidil and involve a clinician if considering it. Topical use is generally preferred in these cases because systemic absorption is far lower and systemic side effects are much less common.
Localized thinning – If hair loss is limited to smaller areas (early crown thinning, mild recession, small patches of miniaturization), topical minoxidil can be a good fit. It allows you to treat targeted regions without committing to systemic exposure, and it can work well when the application is straightforward and consistent.
Who Oral Minoxidil Is Best For
Poor topical responders – If you’ve used topical minoxidil correctly for 6-12 months and seen little to no improvement, oral minoxidil may be a logical next step. Topical therapy can fail even with perfect effort due to low follicular sulfotransferase activity or limited penetration through the scalp barrier. Oral minoxidil bypasses both issues by being activated systemically and delivered more uniformly to follicles.
Diffuse or advanced thinning – Oral minoxidil can be especially appealing when thinning is widespread across the scalp (rather than concentrated at the crown or hairline), because systemic delivery doesn’t depend on targeting specific areas. It also tends to be easier to scale for advanced loss, where topical application becomes time-consuming and difficult to do thoroughly.
Convenience-focused patients – If the routine topical treatment has been a major barrier, or if you experience issues with messiness, residue, or styling disruption, oral minoxidil is often more sustainable.
Final Thoughts
There isn’t a universally superior option between oral and topical minoxidil; it’s just a matter of different trade-offs. Oral minoxidil tends to produce more consistent regrowth for many users because it avoids the two biggest bottlenecks of topical therapy: variable absorption and variable enzyme activation. The cost of that consistency is higher systemic exposure, which increases the likelihood of side effects like hypertrichosis, fluid retention, dizziness, or palpitations, even if serious complications remain rare at low doses.
Topical minoxidil offers a safer long-term profile, wide accessibility, and decades of clinical data, but its outcomes are more variable in real life. The same factors that make it safe, local delivery, and limited systemic absorption, also limit predictability, especially when adherence slips or the scalp barrier and follicular activation aren’t favorable.
In the end, the best outcomes come from individualized treatment plans that match the patient’s biology, preferences, and ability to stay consistent. Minoxidil can be a powerful tool either way, and the version you can tolerate and actually use is the one most likely to work.
References[+]
References ↑1 Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. *British Journal of Dermatology.* 150(2). 186–194. Available at: https://doi.org/10.1111/j.1365-2133.2004.05785.x ↑2 https://commons.wikimedia.org/wiki/File:Minoxidil_Structural_Formula_V1.svg) ↑3 Pietrauszka, K., & Bergler-Czop, B. (2022). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. *Advances in Dermatology and Allergology / Postępy Dermatologii i Alergologii.* 39(3). 472–478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑4, ↑10 Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. *Journal of the American Academy of Dermatology.* 47(3). 377–385. Available at: https://doi.org/10.1067/mjd.2002.124088 ↑5 Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). 688–695. Available at: https://doi.org/10.1016/S0190-9622(87)70089-9 ↑6 Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., & Garcia Bartels, N. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. *Journal of the American Academy of Dermatology.* 65(6). 1126–1134. Available at: https://doi.org/10.1016/j.jaad.2010.09.724 ↑7 Gogtay, J. A., & Panda, M. (2009). Minoxidil topical foam: a new kid on the block. *International Journal of Trichology.* 1(2). 142. Available at: https://doi.org/10.4103/0974-7753.58560 ↑8, ↑9 Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). 688–695. Available at: https://doi.org/10.1016/S0190-9622(87)70089-9 ↑11 Tsuboi, R., Arano, O., Nishikawa, T., Yamada, H., & Katsuoka, K. (2009). Randomized clinical trial comparing 5% and 1% topical minoxidil for the treatment of androgenetic alopecia in Japanese men. *The Journal of Dermatology.* 36(8). 437–446. Available at: https://doi.org/10.1111/j.1346-8138.2009.00673.x ↑12 Olsen, E. A., Weiner, M. S., Amara, I. A., & DeLong, E. R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 22(4). 643–646. Available at: https://doi.org/10.1016/0190-9622(90)70089-Z ↑13 Shadi, Z. (2023). Compliance to topical minoxidil and reasons for discontinuation among patients with androgenetic alopecia. *Dermatology and Therapy.* 13(5). 1157–1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑14 Adil, A., & Godwin, M. (2017). The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. *Journal of the American Academy of Dermatology.* 77(1). 136–141. Available at: https://doi.org/10.1016/j.jaad.2017.02.054 ↑15 Lucky, A. W., Piacquadio, D. J., Ditre, C. M., Dunlap, F., Kantor, I., Pandya, A. G., Savin, R. C., & Tharp, M. D. (2004). A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. *Journal of the American Academy of Dermatology.* 50(4). 541–553. Available at: https://doi.org/10.1016/j.jaad.2003.06.014 ↑16 Lucky, A. W., Piacquadio, D. J., Ditre, C. M., Dunlap, F., Kantor, I., Pandya, A. G., Savin, R. C., & Tharp, M. D. (2004). A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. *Journal of the American Academy of Dermatology.* 50(4). 541–553. Available at: https://doi.org/10.1016/j.jaad.2003.06.014 ↑17, ↑33 Ramos, P. M., Melo, D. F., Radwanski, H., Cortez de Almeida, R. F., & Miot, H. A. (2023). Female-pattern hair loss: therapeutic update. *Anais Brasileiros de Dermatologia.* 98. 506–519. Available at: https://doi.org/10.1016/j.abd.2022.09.006 ↑18 Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: https://doi.org/10.1016/j.jaad.2015.02.466 ↑19, ↑27 Jimenez-Cauhe, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O. M., Fernandez-Nieto, D., & Vaño-Galvan, S. (2019). Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. *Journal of the American Academy of Dermatology.* 81(2). 648–649. Available at: https://doi.org/10.1016/j.jaad.2019.04.054 ↑20, ↑22 Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse Event Reporting System (FAERS) with a focus on pericardial effusions. *Journal of Cosmetic Dermatology.* 24(1). e16574. Available at: https://doi.org/10.1111/jocd.16574 ↑21, ↑38 Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. *Journal of the American Academy of Dermatology.* 84(6). 1644–1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑23 Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse Event Reporting System (FAERS) with a focus on pericardial effusions. *Journal of Cosmetic Dermatology.* 24(1). e16574. Available at: https://doi.org/10.1111/jocd.16574 ↑24 Pirmez, R., & Salas-Callo, C. I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: a study with quantitative trichoscopic documentation. *Journal of the American Academy of Dermatology.* 82(1). e21–e22. Available at: https://doi.org/10.1016/j.jaad.2019.08.084 ↑25 Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: https://www.researchgate.net/profile/Suparuj-Lueangarun/publication/319006716_Efficacy_and_safety_of_oral_minoxidil_5_mg_daily_during_24-week_treatment_in_male_androgenetic_alopecia/links/5b46f1690f7e9b4637cde38b/Efficacy-and-safety-of-oral-minoxidil-5-mg-daily-during-24-week-treatment-in-male-androgenetic-alopecia.pdf ↑26 Panchaprateep, R., & Lueangarun, S. (2020). Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. *Dermatology and Therapy.* 10(6). 1345–1357. Available at: https://doi.org/10.1007/s13555-020-00448-x ↑28 Silva, M. N. E., Ramos, P. M., Silva, M. R., Silva, R. N. E., & Raposo, N. R. B. (2022). Randomized clinical trial of low-dose oral minoxidil for the treatment of female pattern hair loss: 0.25 mg versus 1 mg. 396–399. Available at: https://doi.org/10.1016/j.jaad.2022.01.017 ↑29 Sinclair, R. D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. *International Journal of Dermatology.* 57(1). 104–109. Available at: https://doi.org/10.1111/ijd.13838 ↑30 Perera, E., & Sinclair, R. (2017). Treatment of chronic telogen effluvium with oral minoxidil: a retrospective study. *F1000Research.* 6. 1650. Available at: https://doi.org/10.12688/f1000research.11775.1 ↑31 Wambier, C. G., Craiglow, B. G., & King, B. A. (2021). Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. *Journal of the American Academy of Dermatology.* 85(3). 743–745. Available at: https://doi.org/10.1016/j.jaad.2019.08.080 ↑32 Penha, M. A., Miot, H. A., Kasprzak, M., & Ramos, P. M. (2024). Oral minoxidil vs topical minoxidil for male androgenetic alopecia: a randomized clinical trial. *JAMA Dermatology.* 160(6). 600–605. Available at: doi:10.1001/jamadermatol.2024.0284 ↑34, ↑35 Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled trial. *Journal of Cosmetic Dermatology.* 23(3). 949–957. Available at: https://doi.org/10.1111/jocd.16086 ↑36 Vahabi-Amlashi, S., Layegh, P., Kiafar, B., Hoseininezhad, M., Abbaspour, M., Hajebi Khaniki, S., Forouzanfar, M., & Sabeti, V. (2021). A randomized clinical trial on therapeutic effects of 0.25 mg oral minoxidil tablets on treatment of female pattern hair loss. *Dermatologic Therapy.* 34(6). e15131. Available at: https://doi.org/10.1111/dth.15131 ↑37 Fazal, F., Malik, B. H., Malik, H. M., Sabir, B., Mustafa, H., Ahmed, M., Abid, A., Adil, M. L., Shafi, U., & Saad, M. (2025). Can oral minoxidil be the game changer in androgenetic alopecia? A comprehensive review and meta-analysis comparing topical and oral minoxidil for treating androgenetic alopecia. *Skin Health and Disease.* vzaf009. Available at: https://doi.org/10.1093/skinhd/vzaf009 Finasteride and dutasteride are prescription medications that treat androgenic alopecia (AGA) by targeting the hormone pathway that drives follicle miniaturization. Because they share the same core mechanism and have similar effects on hair loss, understanding which treatment is the best choice for you is not straightforward.
In this article, we tackle the questions that come up most often when choosing between finasteride and dutasteride. We’ll break down how they differ biologically, what head-to-head studies really show about regrowth and side effects, and how oral versus topical use changes the equation, so you can make a more informed decision about which option makes sense for your hair loss and your goals.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What do Finasteride and Dutasteride Actually Do?
AGA is not a sudden shedding event but a slow remodeling of the hair follicle driven by androgen sensitivity. Across repeated hair cycles, susceptible follicles undergo progressive miniaturization: the growth phase (anagen) shortens with each cycle, while the rest phase (telogen) remains the same or lengthens.
Dihydrotestosterone (DHT) is the key androgen driving follicular miniaturization. An enzyme called 5α‑reductase (5AR) in the hair follicle converts testosterone to DHT. Because DHT is known to drive hair loss progression, many treatments for AGA target 5AR to reduce scalp DHT levels.[1]Asfour, L., Cranwell, W., & Sinclair, R. (2023). Male androgenetic alopecia. *Endotext [Internet].* Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/
Both finasteride and dutasteride can decrease levels of DHT in the body. They were originally created to treat a condition called benign prostate hyperplasia (BPH), but have been repurposed for AGA since their impact on hair growth was noticed. While finasteride is approved by the FDA for treating both BPH, dutasteride is approved only for BPH and is therefore used off-label for hair loss.
Type I vs Type II 5α-reductase
Finasteride is an inhibitor of Type II 5AR, which is highly expressed in hair follicles in androgen‑sensitive regions such as the scalp and prostate tissue. Type II is responsible for the conversion of testosterone to DHT inside miniaturizing scalp follicles, directly driving AGA‑related changes.[2]Makridakis, N., & Reichardt, J. K. V. (2005). Pharmacogenetic analysis of human steroid 5α-reductase type II: comparison of finasteride and dutasteride. *Journal of Molecular Endocrinology.* … Continue reading
Dutasteride is a dual 5AR inhibitor, which means that it blocks Type I 5AR as well as Type II. Type I 5AR plays a more supportive role and is mainly found in sebaceous glands and skin, so it has a less direct impact on scalp follicular miniaturization.[3]Frye, S. V. (2006). Discovery and clinical development of dutasteride, a potent dual 5α-reductase inhibitor. *Current Topics in Medicinal Chemistry.* 6(5). 405–421. Available at: … Continue reading
Because of this dual action, dutasteride may decrease levels of DHT to a greater extent than finasteride. In fact, studies have shown that dutasteride may decrease DHT by 50% more than finasteride.
Figure 1. The structures of finasteride and dutasteride.[4]Wikimedia Commons. (n.d.). Azasteroid Pharmaceuticals [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Azasteroid_Pharmaceuticals.png (Accessed: November 2025) Image in the Public Domain.
Does that mean that dutasteride is the better option? Not necessarily. Lower DHT doesn’t necessarily translate into improved hair growth. What’s more, side effects associated with decreased DHT, particularly sexual dysfunction, can affect other aspects of health and well-being.
To understand the potential benefits and side effects of finasteride and dutasteride, we’ll dive into the science and studies that can help us make choices about treatment options.
Formulations: Topical or Oral?
It’s important to recognize that there are many different ways both finasteride and dutasteride can be taken. This includes the drug’s concentration, the frequency of administration, and the formulation used.
Oral finasteride and later oral dutasteride were designed to suppress DHT throughout the body, and they remain the most thoroughly studied options for AGA. Oral finasteride is most commonly prescribed at 1mg daily for AGA, while oral dutasteride is typically taken at 0.5mg daily. Some studies have tested a wider range of concentrations to investigate if there are any changes in benefit or risk.
Topical formulations were introduced later to change the risk profile without affecting the mechanism. Instead of lowering DHT systemically by default, topical finasteride was designed to concentrate drug activity in the scalp while limiting how much reaches the bloodstream.
Topical dutasteride represents the newest and least mature category, but evidence is promising. Because dutasteride is more potent than finasteride, even very low topical concentrations (around 0.01-0.05%) can meaningfully suppress scalp DHT. Importantly, both finasteride and dutasteride can and do enter systemic circulation when applied to the scalp, so safety concerns still need to be addressed.
Which Improves Hair Growth Most?
Because there is a range of treatment options for both finasteride and dutasteride, there is also a web of comparisons we can make when trying to compare the two. First, we’ll compare oral formulations. These are best researched, and there are a number of head-to-head comparison studies we can use to assess their effectiveness.
Oral Finasteride vs Oral Dutasteride
Here is a summary of the clinical trials that compare oral finasteride and dutasteride treatment directly:
Study Design and Population Treatments Impact on DHT Hair Growth Comparison Study #1[5]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Randomized, placebo-controlled, double-blinded trial. 415 men with male pattern hair loss aged 21-45 years old; 24 weeks. 5 mg finasteride; 0.05, 0.1, 0.5, or 2.5 mg dutasteride; placebo. Serum DHT was significantly lower at 0.5 and 2.5 mg dutasteride than at 5 mg finasteride. 5mg finasteride decreased scalp DHT by 41%, while 0.5 and 2.5 mg dutasteride decreased it by 51% and 79% respectively. The proportion of participants with at least a 10% increase in hair counts was 41% for finasteride, and
48%, and 56% for 0.5 and 2.5 mg
dutasteride, respectively.
Study #2[6]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Randomized, placebo-controlled, double-blinded trial. 917 men with AGA aged 20-50; 24 weeks. 1 mg finasteride; 0.02, 0.1, or 0.5 mg dutasteride; placebo Not assessed. 0.5 mg dutasteride significantly improved hair growth compared to 1 mg finasteride. Study #3[7]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Open-label, randomized study; no placebo control. 90 men with AGA aged 18-40; 24 weeks. 1 mg finasteride; 0.5 mg dutasteride. Not assessed. Increase in total hair count was significantly higher in dutasteride compared to finasteride. Study #4[8]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading Retrospective chart review. 600 men over 18 with AGA. 1 mg finasteride; 0.5 mg dutasteride. Not assessed. Dutasteride improved BASP basic M classification of AGA significantly better than finasteride. These RCTs directly comparing finasteride and dutasteride provide the best insight into their comparative efficacy. We’ll focus on 1 mg finasteride vs. 0.5 mg as these are the most commonly used doses. While these studies differ in design and duration, their findings are consistent across several clinically meaningful outcomes.
Key Takeaways from Trials
- Across head-to-head trials lasting 24 weeks, dutasteride consistently outperforms finasteride in increasing hair counts within a predefined target scalp area.[9]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading[10]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
- Dutasteride also increases average hair shaft thickness more than finasteride 1 mg over 24 weeks.
- In trials that included investigator global photo assessment (GPA) or similar global assessments, dutasteride-treated participants were more likely to be rated as “improved” or “markedly improved” compared with those receiving finasteride.[11]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading
An important limitation to consider when interpreting these trials is the length of the studies. Most head-to-head RCTs run for 24 weeks, which may favor dutasteride if it is faster acting. Finasteride, by contrast, is known from longer studies to continue producing gains for 48 months, with hair counts typically plateauing only after around 2 years.[12]Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. … Continue reading
However, long-term data, though mostly retrospective rather than randomized, still suggest sustained superiority of dutasteride on global classification scales.[13]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading
How Big is the Difference in Reality?
On paper, dutasteride often looks clearly superior to finasteride. But translating statistical superiority from randomized trials into real-world expectations requires more nuance. The practical difference between these drugs depends less on averages and more on what kind of hair loss someone has, how fast it’s progressing, and how much regrowth is biologically possible.
Safety & Side Effects: Is Dutasteride Actually Riskier?
Blocking 5AR interferes with hormone activity and is known to cause some side effects. The most frequently discussed effects include:
- Sexual side effects – These include decreased libido, erectile dysfunction, and ejaculation disorders. Across randomized trials, these effects occur in a minority of users (generally in the single-digit percentages), appear most often early in treatment, and frequently resolve spontaneously, even while continuing therapy.[14]Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic … Continue reading
- Breast-related effects – Breast tenderness or enlargement has been reported, but rates are low across both drugs and typically comparable between finasteride and dutasteride.
- Other reported effects: Fatigue, mood changes, or nonspecific symptoms are occasionally reported, but these occur inconsistently, lack clear dose–response relationships, and are difficult to separate from background rates and expectation effects.
As we’ve already noted, the dual action of dutasteride could mean that it poses an increased risk of side effects. To see whether the evidence backs this up, we’ll take a look at what randomized trials and retrospective studies tell us about the comparative risks of each treatment. Below is a summary of the head-to-head studies to date.
Average incidence of side effects Decreased libido Ejaculation disorders Erectile dysfunction Breast enlargement and tenderness Study #1[15]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Finasteride 2.6% vs dutasteride 4.6% Finasteride – 5 mg: 4%. Dutasteride – 0.1 mg: 3%; 0.5 mg: 1%; 2.5 mg: 13%. Dutasteride – 0.1 mg: 4%; 0.5 mg: 1%; 2.5 mg: 13%. Finasteride – 5 mg: 1% Dutasteride – 0.1 mg: 0%; 0.5 mg: 0%; 2.5 mg: 0%. Finasteride – 5 mg: 1% Not assessed. Study #2[16]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Finasteride 10.0% vs dutasteride 10.0% Not assessed. Not assessed. Not assessed. Not assessed. Study #3[17]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Finasteride 13.4% vs dutasteride 11.5% Finasteride – 1 mg: 6.7%. Dutasteride – 0.02 mg: 8.1%; 0.1 mg: 6.9%; 0.5 mg: 3.3%. Finasteride – 1 mg: 3.9%. Dutasteride – 0.02 mg: 2.2%; 0.1 mg: 4.8%; 0.5 mg: 3.9%. Finasteride – 1 mg: 5.6%. Dutasteride – 0.02 mg: 4.3%; 0.1 mg: 3.7%; 0.5 mg: 5.6%. Finasteride – 1 mg: 0.6%. Dutasteride – 0.02 mg: 0.5%; 0.1 mg: 0.5%; 0.5 mg: 0.6%. Study #4[18]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading Finasteride 10.5% vs dutasteride 7.6% Finasteride 0.7% vs dutasteride 1.2% Not assessed Finasteride 0% vs dutasteride 0.4% Not assessed As we can see, across randomized and controlled studies comparing dutasteride and finasteride, the overall incidence of side effects is similar between the two drugs. When averaged across trials, total reported side effects occur at nearly identical rates (approximately 8-9% for both drugs).
In some studies, finasteride shows equal or higher rates of certain sexual side effects despite producing less DHT suppression. Importantly, these findings hold even when dutasteride reduces serum DHT by an additional 19-23% beyond finasteride and scalp DHT by significantly greater margins. The most closely scrutinized adverse effects, such as reduced libido, erectile dysfunction, and ejaculation disorders, do not follow a clear dose-response pattern with dutasteride.
Why Don’t We See More Side Effects With Dutasteride?
Given that dutasteride suppresses systemic and scalp DHT substantially more than finasteride, the lack of a clear increase in side effects may seem counterintuitive.
DHT reduction alone does not appear to be a reliable predictor of side-effect risk. This suggests that once DHT is reduced beyond a certain threshold, further suppression may not meaningfully increase the likelihood of side effects, particularly if compensatory mechanisms come into play. For example, lowering of DHT below a certain level might activate hormonal mechanisms to redress the balance. Long-term studies further support this idea, showing that side-effect incidence with dutasteride tends to decrease over time rather than accumulate.
Dutasteride’s size and tissue accessibility might also play a role. It has a much higher molecular weight (around 528 daltons) than finasteride (372 daltons), which likely limits its ability to cross the blood–brain barrier. Finasteride, by contrast, is small enough to enter brain tissue more readily. Because androgen metabolism in the brain is implicated in libido and sexual function, a drug that primarily exerts its effects in peripheral tissues may suppress DHT more aggressively without proportionally increasing neurological side effects.
Why do Incidence Estimates Vary?
Reported rates of side effects with finasteride and dutasteride vary widely across studies largely because incidence is highly dependent on study design. The makeup of the participant groups can impact how likely they are to experience side effects. How side effects are defined and recorded can also influence estimates. Some studies rely on spontaneous self-reporting, while others use targeted questionnaires or direct investigator questioning. These different approaches produce different reporting rates.
Psychological and selection factors further complicate interpretation. Participants who expect problems are more likely to notice and report them, even in placebo groups (this is called the nocebo effect). This means the information that participants receive can impact side effects.
How Long-Lasting are Side Effects?
Most randomized controlled trials are short (e.g., 24 weeks), enroll relatively healthy participants, and use structured adverse-event reporting. This means they tend to capture early, transient side effects, while missing longer-term patterns such as spontaneous resolution or adaptation. In contrast, observational and retrospective studies follow broader populations over longer periods but rely on less standardized reporting, which can inflate or obscure true incidence.
As a result, short-term trials may overestimate persistent risk, while long-term datasets may underrepresent early intolerance.
Where trials discuss the resolution of side effects, one trial reported that around half of affected participants taking dutasteride reported spontaneous resolution of symptoms while continuing treatment.[19]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading By contrast, finasteride-associated side effects in these trials were less likely to resolve during ongoing treatment, even though many resolved after stopping the drug.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Post-Finasteride Syndrome: What to Know
Post-Finasteride Syndrome (PFS) is a term used to describe persistent sexual, cognitive, or mood-related symptoms that continue for months after stopping finasteride. Its existence and prevalence remain highly debated among dermatologists, endocrinologists, and hair loss researchers.
While some clinicians argue that PFS represents a real, drug-induced condition affecting a very small subset of users, others contend that the available evidence is low quality, heavily confounded by psychological factors, media influence, and the high background rates of sexual dysfunction in adult men.[20]Traish, A. M. (2020). Post-finasteride syndrome: a surmountable challenge for clinicians. *Fertility and Sterility.* 113(1). 21–50. Available at: https://doi.org/10.1016/j.fertnstert.2019.11.030,[21]Trüeb, R. M., Régnier, A., Rezende, H. D., & Dias, M. F. R. G. (2019). Post-finasteride syndrome: an induced delusional disorder with the potential of a mass psychogenic illness?. *Skin … Continue reading
Critically, high-quality clinical trials of finasteride did not report persistent side effects after discontinuation, with symptoms resolving within three months in all affected participants.[22]Shapiro, J., & Kaufman, K. D. (2003). Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). *Journal of Investigative Dermatology Symposium Proceedings.* … Continue reading Claims of PFS largely arise from case reports, small observational studies, and self-reported data, all of which suffer from major limitations such as lack of controls and small sample sizes.
One important exception is finasteride-induced gynecomastia (enlarged breast tissue in males), which is a well-documented side effect and can persist in a minority of cases even after stopping the drug, demonstrating that long-lasting tissue changes are possible, albeit rare.[23]Kaplan, S. A., Chung, D. E., Lee, R. K., Scofield, S., & Te, A. E. (2012). A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has … Continue reading
If PFS is real, it is likely extremely rare, plausibly affecting fewer than 1 in 5,000 – 10,000 users. For users, the key takeaway is one of risk context: while persistent side effects cannot be ruled out entirely, their estimated risk appears very low when weighed against the well-established benefits of finasteride for AGA, especially when dosing strategies are used to minimize overall exposure.
Topical Formulations
After oral formulations, the next logical question is whether you can get the same DHT-blocking benefits where it matters most. That’s the core promise of topical finasteride and dutasteride formulations: to localize 5AR inhibition to the scalp and reduce the likelihood of systemic side effects.
The potential for using topical formulations opens up a host of new options for treating AGA. Like oral formulations, topicals can also come in a range of concentrations, but can also be delivered in a range of formulations and solutions, including:
- Solutions and Sprays – Alcohol‑ or hydroalcoholic‑based liquids applied with a dropper, spray, or pipette, sometimes combined with propylene glycol or similar penetration enhancers.
- Gels and Lotions – Semi‑solid preparations designed to increase contact time on the scalp and modulate absorption.
- Nanoparticle and Liposomal Carriers: Research formulations load finasteride or dutasteride into lipid or polymeric nanoparticles or liposomes to target follicular delivery and reduce systemic exposure.
Formulations can also be combined with physical methods, such as microneedling, or with other common pharmaceuticals, like minoxidil. This also means that the decisions we need to make when choosing the right hair loss treatment are more complicated. Again, we’ll focus on head-to-head trials that compare different topical and oral formulations, as well as what clinical studies can tell us about side effects.
Does Topical Finasteride Work?
Based on trials comparing topical dutasteride to other AGA treatments, the answer is a resounding yes. However, the challenge is that finasteride has a highly sensitive, logarithmic dose-response curve. In other words, you don’t need much finasteride in the bloodstream to create a big systemic DHT change. That’s why topical finasteride can still “leak” systemically, and why even small systemic absorption can defeat the original purpose of going topical.
To take a closer look, we’ll first see how topical formulations compare to oral.
Oral vs Topical Finasteride
Several trials directly comparing topical to oral finasteride show that topical treatment can improve target-area hair counts and other hair parameters similarly to oral dosing, while often producing less suppression of serum DHT and far lower measured systemic drug exposure.
In one Phase III trial, topical finasteride produced hair-count improvements comparable to oral finasteride. What’s more, the oral formulation reduced mean serum DHT by 55.6%, whereas topical finasteride reduced it by 34.5%, and sexual side effects were more associated with oral treatment.[24]Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic … Continue reading
Other randomized comparisons similarly report meaningful regrowth with topical finasteride, with most adverse events being local (irritation) and relatively few systemic complaints.[25]Hajheydari, Z., Akbari, J., Saeedi, M., & Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. *Indian Journal of … Continue reading
However, even at lower concentrations, topical finasteride can reduce serum DHT depending on how much you apply and how often you apply it. For example, one versus two daily applications of the same topical concentration can produce dramatically different serum DHT suppression, illustrating that frequency and volume can matter as much as concentration.[26]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
Figure 2. Multiple daily doses of topical finasteride can reduce serum DHT more than a single dose. Adapted from Figure 3.[27]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
What Determines Systemic Absorption?
There are a few key factors that impact absorption:
- Vehicle/carrier – Alcohol- and propylene glycol–based carriers tend to enhance penetration. Stronger penetration can improve scalp delivery, but it can also increase systemic leakage.
- Amount applied per use – This is often the most overlooked variable. Two people using the same concentration can have totally different systemic exposure if one applies 0.5 mL and the other applies 2 mL.
- Frequency of application – Twice-daily use can more than double systemic effects compared to once-daily use in certain protocols.
Does Topical Dutasteride Work?
Topical dutasteride research is newer and still developing, but early findings are encouraging. The current literature suggests that topical dutasteride can meaningfully improve hair density and shaft caliber while producing minimal changes in serum DHT.
A randomized, placebo-controlled trial in 34 men found that microneedling plus 0.01% topical dutasteride produced significant improvements in hair thickness, density, and the vellus-to-terminal ratio, compared with microneedling plus saline.[28]Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus … Continue reading
This is only a proof-of-concept study with a small sample size. However, it does demonstrate that topical dutasteride can drive clinically meaningful changes when follicular delivery is boostedby microneedling. But it also limits generalizability: microneedling itself is an active intervention, and it may not reflect what happens with topical dutasteride alone.
A newer Phase II trial tested 0.01%, 0.02%, and 0.05% topical dutasteride and reported dose-dependent improvements in total hair count and hair width versus placebo.[29]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading
The authors also reported that 0.05% appeared to outperform oral finasteride in total hair count. That’s an attention-grabbing claim, but it should be interpreted cautiously. Unusually high baseline counts and possible inconsistencies in target-area placement, which could inflate apparent differences, raise concerns about the methodology used.
Figure 3. Oral finasteride (yellow line) reduces serum DHT levels more than topical Dutaseride (blue lines). Adapted from Figure 3.[30]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading Image used under Creative Commons License
A common objection is that dutasteride is too large to penetrate the skin well. At around 528.5 Daltons, it sits slightly above the widely cited 500 Dalton rule, which suggests that larger molecules struggle to cross the stratum corneum. In practice, dutasteride appears capable of penetrating the scalp, as it can suppress DHT and promote hair growth.
Combination strategies: where topical regimens often perform best
Topical regimens tend to perform best when they’re used alongside other treatments, rather than as a single agent. DHT drives miniaturization, but follicular stimulation, blood-flow signaling, and wound-healing pathways all influence whether a follicle can thicken and re-enter robust growth.
Microneedling consistently appears in the literature as an accelerator of topical outcomes. Controlled micro-injury activates wound-healing signals that can independently improve density and shaft diameter, while also enhancing follicular penetration and local drug exposure.
Combination with other treatments, primarily minoxidil, can also improve outcomes. The underlying logic is straightforward: minoxidil boosts growth, while dual 5AR blockade increases the probability that follicles remain protected from hormonal changes.
One study showed that adding microneedling to topical finasteride therapy alongside minoxidil can increase hair density and shaft diameter more than minoxidil alone.[31]Chang, Y., Zhang, W., Zhou, J., Lv, L., He, Q., Chen, Y., Wang, P., & Zhai, Q. (2025). Clinical efficacy of microneedle combined with 5% minoxidil solution and finasteride in the treatment of … Continue reading
Similarly, combining dutasteride with microneedling and minoxidil has also demonstrated promising results. In a small study, all patients used topical minoxidil 5%, while adding dutasteride, either systemically or locally, significantly improved hair density and shaft caliber compared with minoxidil alone. The strongest overall results were seen with topical dutasteride 0.02% delivered via monthly microneedling, which outperformed oral dutasteride plus minoxidil on objective measures while maintaining a more favorable safety profile.[32]Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through … Continue reading
Overall, the evidence suggests that topical regimens may be most effective when they combine anti-androgen suppression, follicular stimulation, and delivery-enhancing strategies.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Dosing
Choosing the right dose depends on both the medication and the clinical context. Finasteride, whether oral or topical, is generally the best starting point for most men beginning pharmacologic treatment for androgenetic alopecia. Its pharmacokinetics are more forgiving, dose adjustments are easier, and when side effects occur, they are often manageable with modest reductions in dose or changes in dosing frequency.
Dutasteride, in contrast, is better suited for men with aggressive, fast-progressing AGA or for those who have not responded well to finasteride. Its greater potency comes with less flexibility, largely due to its long half-life and sustained enzyme binding. As a result, dosing with dutasteride should be conservative and deliberate, with careful consideration of long-term exposure and tolerability.
Oral Finasteride
The standard dose of oral finasteride for androgenetic alopecia is 1 mg once daily. This regimen has been used in the majority of randomized controlled trials and has consistently demonstrated improvements in key hair growth outcomes, including hair counts, hair shaft caliber, and investigator global photographic assessments.
A key pharmacologic feature of finasteride is its logarithmic dose–response curve. Most of the drug’s DHT-lowering effect occurs at relatively low doses, and by the time a daily dose of 1 mg is reached, inhibition of type II 5-alpha reductase is already close to maximal.
This explains why lower doses, such as 0.2–0.5 mg daily, can still meaningfully suppress both serum and scalp DHT, and why increasing the dose beyond 1 mg does not lead to proportionally greater hair regrowth. Instead, higher doses primarily increase systemic exposure and may raise the risk of side effects without offering additional cosmetic benefit.
Figure 4. The dose response of finasteride demonstrates that increasing the concentration of treatment doesn’t translate to increased DHT reduction.
From a practical standpoint, this dose–response relationship allows for flexibility in real-world use. Many individuals are able to maintain hair stabilization and regrowth with reduced dosing or alternate-day schedules, particularly after an initial period of stabilization.
When side effects do occur, they also tend to be dose-sensitive rather than all-or-nothing, meaning that lowering the dose or adjusting the dosing schedule is often sufficient to improve tolerability while preserving therapeutic benefit.
Oral Dutasteride
The standard oral dose of dutasteride for androgenetic alopecia is 0.5 mg once daily. This is the dose most consistently studied in clinical trials and the dose used in nearly all head-to-head comparisons with finasteride.
The reason dutasteride is prescribed at a lower milligram dose than finasteride comes down to its greater pharmacologic potency. Dutasteride’s pharmacokinetics are also different. The drug has a longer half-life (roughly 4–5 weeks), which means it accumulates in tissues over time.[33]Arif, T., Dorjay, K., Adil, M., & Sami, M. (2017). Dutasteride in androgenetic alopecia: an update. *Current Clinical Pharmacology.* 12(1). 31–35. Available at: … Continue reading
Because of this, missed doses tend to matter less for maintaining efficacy. However, the flip side is that if adverse effects occur, they may take weeks to months to fully resolve after discontinuation, as the drug slowly clears from the body.
These properties have important practical implications for dosing. Daily dosing is not always necessary to maintain clinical benefit, and some clinicians employ intermittent schedules, such as 0.5 mg taken two to three times per week, in an effort to balance efficacy with tolerability.
Topical Finasteride
Topical finasteride dosing is often misunderstood because the percentage concentration listed on the label is not the most important variable. What ultimately determines both efficacy and systemic risk is total daily finasteride exposure, measured in milligrams per day.
This total exposure is shaped by several interacting factors: the concentration of finasteride in the solution, the volume applied per use, how often it is applied (once versus twice daily), and the vehicle or carrier used, such as alcohol or propylene glycol, which influences skin penetration.
The key principle underlying topical finasteride dosing is that finasteride is extremely potent systemically. Only a very small fraction of the drug needs to enter circulation to meaningfully suppress serum DHT. As a result, topical formulations can unintentionally behave like oral finasteride if dosing is too aggressive, even when the product is intended to act locally on the scalp.
An evidence-informed dosing range is 1-2 mL of a 0.005%-0.02% topical finasteride solution applied once daily. This corresponds to roughly 0.1-0.2 mg of finasteride applied to the scalp per day, with estimated systemic exposure in common carriers of approximately 0.01-0.03 mg per day.
Generally, high-concentration formulations (≥0.25-1%) should be avoided, especially when combined with large application volumes or twice-daily use. These regimens can suppress serum DHT to a degree similar to 1 mg of oral finasteride, effectively defeating the purpose of choosing a topical formulation in the first place.
Topical Dutasteride
Topical dutasteride dosing is less standardized than topical finasteride, largely because the evidence base is newer and consists of fewer clinical trials. That said, several consistent patterns are beginning to emerge from the available data.
Most studies have evaluated topical dutasteride in a concentration range of 0.01% to 0.05%, typically applied once daily. In many protocols, topical dutasteride has been paired with microneedling to enhance follicular delivery, although some studies suggest that meaningful efficacy can be achieved even without microneedling.[34]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading
The practical takeaway is that low-concentration, low-volume topical dutasteride protocols appear to offer the best balance between efficacy and safety. Given the variability in formulations, vehicles, and individual skin permeability, optimal dosing is likely individualized, and careful titration remains essential as the clinical evidence continues to evolve.
Monitoring & Risk Management
Monitoring and risk management with finasteride or dutasteride are about using the minimum effective exposure while staying alert to tolerability over time. Ideally, treatment begins with a clear diagnosis of androgenetic alopecia, baseline photos, and, especially for topical or risk-averse users, optional baseline serum DHT testing to provide context for future changes.
Side effects, if they occur, should be addressed early with dose reduction, spacing doses, or switching delivery routes rather than abrupt discontinuation, since most are dose-sensitive and reversible. For topical users, repeat serum DHT testing after ~30 days can help confirm that systemic exposure remains low.
Ongoing monitoring should focus on objective progress via standardized photos every 3–6 months and general symptom awareness rather than day-to-day fluctuations.
Extra caution is warranted with dutasteride due to its long half-life and accumulation, making conservative dosing and deliberate adjustments especially important. When approached with planned checkpoints and proportional responses, both drugs can be used safely, predictably, and effectively over the long term.
Who Should Use Finasteride or Dutasteride?
Finasteride and dutasteride are among the most effective pharmacologic options for AGA, but they are not one-size-fits-all treatments. Choosing which drug to use depends on hair-loss pattern, rate of progression, prior treatment response, side-effect tolerance, and long-term goals.
Both drugs require consistent, long-term use to maintain results. Candidates should be comfortable with ongoing therapy and realistic timelines, as visible improvements often take 6-12 months, with maximal benefits closer to two years.
Consider Finasteride If…
You’re starting treatment, or your AGA is early-to-moderate. Oral finasteride is the usual baseline because it’s effective for most men and has a long track record. It’s also more forgiving: if you need to adjust dose, frequency, or route due to side effects, finasteride is generally easier to fine-tune.
If you’re risk-averse or simply want the most studied on-label option for hair loss, finasteride is usually the logical starting point, especially if you can commit to consistent use and track progress with photos over months.
Consider Dutasteride If…
Your hair loss is aggressive, or you’re losing ground despite finasteride. Dutasteride’s broader 5AR inhibition often translates into greater average gains in hair counts and thickness in shorter head-to-head trials. Clinically, it’s most often considered when progression is rapid, miniaturization is extensive, or finasteride hasn’t provided adequate stabilization after a consistent trial.
Who Should be Cautious (or Avoid)?
Men without androgenetic alopecia – Finasteride and dutasteride are ineffective for conditions such as alopecia areata, telogen effluvium, traction alopecia, or scarring alopecias. Using them in these contexts adds risk without meaningful benefit.
Those highly risk-averse to hormonal manipulation – While most users tolerate these medications well, anyone uncomfortable with altering systemic hormone levels, even at low risk, may prefer non-hormonal options or topical-only approaches with careful dosing.
Men who experienced significant adverse effects previously – Dutasteride may be more appropriate for men with rapid progression, extensive miniaturization, or those who continue to lose ground despite consistent finasteride use. Its stronger and broader DHT suppression can provide an additional margin of control in these cases.
Men trying to conceive in the near term – Although evidence of fertility harm at hair-loss doses is limited, many clinicians advise avoiding systemic 5AR inhibitors when actively trying to conceive, particularly dutasteride due to its long half-life. This decision should be individualized and discussed with a clinician.
Those unable to maintain consistency – Oral therapies require daily (or structured intermittent) dosing, while topical therapies demand regular application and attention to volume and frequency. If adherence is unlikely, expected benefits drop substantially.
Final Thoughts and Takeaways
Finasteride and dutasteride, in their different formulations, are not competing “good vs bad” options. Instead, they are different tools for different stages and severities of androgenetic alopecia.
The real decision isn’t which drug suppresses more DHT on paper, but how much suppression you need to control your hair loss, and how much systemic exposure you’re comfortable carrying long term.
Finasteride has the longest safety record, strong long-term efficacy, and a forgiving dose–response curve that allows real-world flexibility. For early-to-moderate AGA, or for men prioritizing reversibility and adjustability, finasteride, either oral or carefully dosed topical, covers the majority of clinical needs.
When hair loss is aggressive, rapidly progressive, or insufficiently controlled on finasteride, dutasteride’s broader 5AR inhibition offers a higher ceiling of protection. Head-to-head trials consistently show greater average gains, and it does not show a clearly higher overall incidence of side effects in controlled studies despite stronger systemic and scalp DHT reduction.
Topical vs oral delivery, total daily exposure, application volume, and frequency often have a larger impact on outcomes and tolerability than the finasteride-vs-dutasteride label alone. Hair loss therapy is a long game. The optimal choice is not the strongest drug you can tolerate for a few months, but the strategy you can follow consistently for years with acceptable trade-offs.
References[+]
References ↑1 Asfour, L., Cranwell, W., & Sinclair, R. (2023). Male androgenetic alopecia. *Endotext [Internet].* Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/ ↑2 Makridakis, N., & Reichardt, J. K. V. (2005). Pharmacogenetic analysis of human steroid 5α-reductase type II: comparison of finasteride and dutasteride. *Journal of Molecular Endocrinology.* 34(3). 617–623. Available at: https://doi.org/10.1677/jme.1.01725 ↑3 Frye, S. V. (2006). Discovery and clinical development of dutasteride, a potent dual 5α-reductase inhibitor. *Current Topics in Medicinal Chemistry.* 6(5). 405–421. Available at: https://doi.org/10.2174/156802606776743101 ↑4 Wikimedia Commons. (n.d.). Azasteroid Pharmaceuticals [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Azasteroid_Pharmaceuticals.png (Accessed: November 2025) ↑5, ↑15, ↑19 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑6, ↑10, ↑16 Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 70(3). 489–498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑7, ↑17 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑8, ↑11, ↑13, ↑18 Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. *Annals of Dermatology.* 34(5). 349. Available at: https://doi.org/10.5021/ad.22.027 ↑9 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑12 Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. *Journal of the American Academy of Dermatology.* 55(1). 71–74. Available at: https://doi.org/10.1016/j.jaad.2005.07.001 ↑14 Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. *The Journal of Clinical and Aesthetic Dermatology.* 9(7). 56–62. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5023004/ ↑20 Traish, A. M. (2020). Post-finasteride syndrome: a surmountable challenge for clinicians. *Fertility and Sterility.* 113(1). 21–50. Available at: https://doi.org/10.1016/j.fertnstert.2019.11.030 ↑21 Trüeb, R. M., Régnier, A., Rezende, H. D., & Dias, M. F. R. G. (2019). Post-finasteride syndrome: an induced delusional disorder with the potential of a mass psychogenic illness?. *Skin Appendage Disorders.* 5(5). 320–326. Available at: https://doi.org/10.1159/000497362 ↑22 Shapiro, J., & Kaufman, K. D. (2003). Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). *Journal of Investigative Dermatology Symposium Proceedings.* 8(1). 20–23. Available at: https://doi.org/10.1046/j.1523-1747.2003.12167.x ↑23 Kaplan, S. A., Chung, D. E., Lee, R. K., Scofield, S., & Te, A. E. (2012). A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. *International Journal of Clinical Practice.* 66(11). 1052–1055. Available at: https://doi.org/10.1111/j.1742-1241.2012.03010.x ↑24 Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. *Journal of the European Academy of Dermatology and Venereology.* 36(2). 286–294. Available at: https://doi.org/10.1111/jdv.17738 ↑25 Hajheydari, Z., Akbari, J., Saeedi, M., & Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. *Indian Journal of Dermatology, Venereology and Leprology.* 75. 47. Available at: https://doi.org/10.4103/0378-6323.45220 ↑26 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑27 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑28 Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. Journal of the European Academy of Dermatology & Venereology. 36(10). Available at: https://doi.org/10.1111/jdv.18285 ↑29 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑30 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. *Cureus.* 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑31 Chang, Y., Zhang, W., Zhou, J., Lv, L., He, Q., Chen, Y., Wang, P., & Zhai, Q. (2025). Clinical efficacy of microneedle combined with 5% minoxidil solution and finasteride in the treatment of androgenetic alopecia in males. *Archives of Dermatological Research.* 317(1). 428. Available at: https://doi.org/10.1007/s00403-025-03891-y ↑32 Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in treatment of androgenetic alopecia. *QJM: An International Journal of Medicine.* 117(Supplement_2). hcae175–207. Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑33 Arif, T., Dorjay, K., Adil, M., & Sami, M. (2017). Dutasteride in androgenetic alopecia: an update. *Current Clinical Pharmacology.* 12(1). 31–35. Available at: https://doi.org/10.2174/1574884712666170310111125 ↑34 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 Finasteride is one of the most effective and widely studied treatments for androgenic alopecia (AGA). It works by lowering dihydrotestosterone (DHT) levels at the scalp to slow or halt androgen-driven hair follicle miniaturization. While most men tolerate finasteride well and experience meaningful, long-term hair preservation, side effects can occur in a minority of users and are a legitimate concern for anyone considering or already using the medication.
Importantly, tolerance to finasteride varies from person to person. Factors such as baseline hormone levels, genetics, lifestyle, and overall health can influence how an individual responds. The goal of this article is to outline practical, evidence-informed strategies to help manage or reduce side effects, enabling thoughtful, safe decisions about finasteride use with appropriate medical guidance.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Common Finasteride Side Effects
Sexual side effects, such as decreased libido, erectile dysfunction, and ejaculation disorders, occur in a small minority of users. They are most commonly reported early in treatment and often improve or resolve on their own.[1]Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic … Continue reading
Breast tenderness and enlargement (gynecomastia) are sometimes reported. Gynecomastia in particular is well-documented and can persist in a minority of cases even after stopping the drug.[2]Kaplan, S. A., Chung, D. E., Lee, R. K., Scofield, S., & Te, A. E. (2012). A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has … Continue reading
Some men report cognitive symptoms while taking finasteride, most commonly described as “brain fog” or reduced mental clarity, but these complaints have not been observed at meaningful rates in controlled clinical trials. If such effects occur, they are likely subtle and gradual, which may make them difficult to quantify or consistently attribute to the medication
There is a small number of cases where sexual, cognitive, or mood-related symptoms have persisted, leading to the development of the term post-finasteride syndrome (PFS).
PFS remains controversial: some clinicians consider it a genuine but extremely rare drug-related condition, while others argue that existing evidence is low quality and heavily confounded by psychological factors, reporting bias, and the high baseline prevalence of sexual dysfunction in men.[3]Traish, A. M. (2020). Post-finasteride syndrome: a surmountable challenge for clinicians. *Fertility and Sterility.* 113(1). 21–50. Available at: https://doi.org/10.1016/j.fertnstert.2019.11.030,[4]Trüeb, R. M., Régnier, A., Rezende, H. D., & Dias, M. F. R. G. (2019). Post-finasteride syndrome: an induced delusional disorder with the potential of a mass psychogenic illness?. *Skin … Continue reading
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

How common are side effects?
It’s important to remember that the majority of men who use finasteride experience no noticeable side effects,
In the original large, randomized, placebo-controlled trial that led to FDA approval for AGA, sexual side effects were reported in about 4.2% of men taking finasteride versus 2.2% on placebo in the first year. This suggests a small absolute risk, and participants in the study who stopped taking finasteride due to side effects reported that they did not persist after the medication was discontinued.[5]Kaufman, K. D., Olsen, E. A., Whiting, D., Savin, R., DeVillez, R., Bergfeld, W., Price, V. H., et al. (1998). Finasteride in the treatment of men with androgenetic alopecia. *Journal of the American … Continue reading
In other trials, rates vary widely across studies depending on study design, definitions, and participant expectations.
Higher rates seen in some studies appear to be strongly influenced by the nocebo effect, where awareness of potential side effects increases reporting. More conservative analyses estimate that up to 10-15% of users may experience some form of sexual symptoms, most of which are mild, transient, and reversible.[6]Traish, A. M., Hassani, J., Guay, A. T., Zitzmann, M., & Hansen, M. L. (2011). Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and … Continue reading
The Most Important Rule: Speak to a Physician
If you get severe side effects, stop and speak to your primary care physician.
Early evaluation helps rule out other medical, hormonal, or psychological contributors, establishes whether symptoms are plausibly related to finasteride, and prevents unnecessary continuation of treatment when modification or discontinuation is appropriate.
Prompt medical guidance also ensures that side effects are documented and managed correctly, rather than being compounded by anxiety, misinformation, or unsupervised dose changes. The strategies outlined below apply only to mild or moderate symptoms, should be considered only under clinician supervision, and are not appropriate for severe, rapidly worsening, or distressing effects, which warrant immediate medical review.
With that in mind, we can now look at ways to reduce the side effects of finasteride.
1. Continue Treatment and Allow Time for Adaptation (When Symptoms Are Mild)
One of the most consistently observed patterns with finasteride and other 5α-Reductase (5AR) inhibitors is that many reported side effects improve or resolve with continued use, particularly when they are mild and occur early in treatment.
Clinical trials and follow-up data also suggest the side effects are most common in the first 12 months. This pattern supports the idea that early side effects often reflect a transient adjustment phase rather than a permanent intolerance.[7]Lowe, F. C., McConnell, J. D., Hudson, P. B., Romas, N. A., Boake, R., Lieber, M., Elhilali, M., et al. (2003). Long-term 6-year experience with finasteride in patients with benign prostatic … Continue reading,[8]Hudson, P. B., Boake, R., Trachtenberg, J., Romas, N. A., Rosenblatt, S., Narayan, P., Geller, J., et al. (1999). Efficacy of finasteride is maintained in patients with benign prostatic hyperplasia … Continue reading
Several biological mechanisms may explain this improvement. Androgen receptors and downstream hormone signaling can adapt to reduced DHT levels, central nervous system sensitivity to hormonal shifts may normalize, and early symptoms may be amplified by anxiety or expectancy effects that lessen with reassurance and time.
2. Reduce the Daily Dose
The standard dose of finasteride for AGA is 1 mg once daily, a regimen supported by large randomized controlled trials showing clear improvements in hair count, hair shaft thickness, and global photographic assessments.
However, a key pharmacologic feature of finasteride is its non-linear (logarithmic) dose–response curve, meaning that most of its DHT–lowering effect occurs at relatively low doses.
Figure 1. The dose response of finasteride demonstrates that increasing the concentration of treatment doesn’t translate to increased DHT reduction.
By the time a daily dose of 1 mg is reached, inhibition of 5AR is already near maximal. As a result, lower doses, such as 0.2–0.5 mg daily, can still meaningfully suppress serum and scalp DHT, often preserving much of finasteride’s hair-protective benefit. [9]Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W. P., Cotterill, P. C., Thiboutot, D. M., et al. (1999). The effects of finasteride on scalp skin and serum androgen levels in men with … Continue reading
Increasing the dose beyond 1 mg does not produce proportionally greater hair regrowth, but instead primarily increases systemic exposure, which may raise the likelihood of side effects without additional cosmetic gain.
From a practical standpoint, this dose–response relationship allows for flexibility in real-world use. Because finasteride side effects tend to be dose-sensitive rather than all-or-nothing, reducing the daily dose is often an effective first adjustment for improving tolerability while maintaining therapeutic efficacy.
3. Reduce Dosing Frequency
Finasteride’s pharmacology also allows for flexibility in dosing frequency. The drug binds tightly to 5AR, and suppression of DHT persists well beyond the drug’s short plasma half-life.[10]National Center for Biotechnology Information. (n.d.). Finasteride. *NCBI Bookshelf.* Available at: https://www.ncbi.nlm.nih.gov/books/NBK513329/ (Accessed: November 2025),[11]Steiner, J. F. (1996). Clinical pharmacokinetics and pharmacodynamics of finasteride. *Clinical Pharmacokinetics.* 30(1). 16–27. Available at: https://doi.org/10.2165/00003088-199630010-00002 In practical terms, this means that daily dosing is not strictly required to maintain meaningful inhibition of scalp DHT once steady-state suppression has been achieved.
Figure 2. Multiple doses of oral finasteride (square points) do not reduce serum DHT significantly more than a single dose over 36 hours. Adapted from Figure 3.[12]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
Common clinician-guided intermittent regimens include 1 mg three times per week or 0.5 mg every other day, both of which can significantly reduce cumulative drug exposure while preserving much of finasteride’s therapeutic effect. Because DHT levels recover gradually rather than immediately, these schedules often maintain adequate androgen suppression for hair stabilization.
Intermittent dosing may be especially helpful for men who develop mild side effects on daily therapy, those who are particularly sensitive to hormonal changes, or individuals transitioning into a long-term maintenance phase after initial hair loss control.
If you want to understand more about finasteride dosing, you can read our in-depth article here.
4. Switch to Topical Finasteride
Topical finasteride is designed to reduce DHT locally within the scalp while limiting systemic exposure, making it an attractive option for men who experience side effects with oral therapy. By delivering the drug directly to the scalp, topical formulations aim to suppress DHT activity in hair follicles without relying on sustained systemic circulation.
Randomized trials comparing topical and oral finasteride show that topical treatment can produce similar improvements in target-area hair counts and other hair growth parameters, while generally causing less suppression of serum DHT and lower measured systemic drug levels.[13]Hajheydari, Z., Akbari, J., Saeedi, M., & Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. *Indian Journal of … Continue reading
One trial demonstrated that topical finasteride reduced DHT in the blood by 34.5%, compared to 55.6% with oral finasteride. They also found that sexual side effects were more associated with oral treatment.[14]Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic … Continue reading
That said, finasteride’s highly sensitive, logarithmic dose–response curve means that even small amounts entering the bloodstream can suppress DHT. Total systemic exposure depends not only on concentration, but also on application volume, frequency, treated surface area, and formulation vehicle.
As such, topical treatments also require care when applying, and success with topical finasteride depends on consistent, measured application and adherence to the prescribed regimen.
When used thoughtfully, topical finasteride can preserve much of the hair benefit of oral therapy while reducing the risk of systemic side effects for many men.
Find out more about topical finasteride in our article.
5. Use Ultra–Low-Dose Topical Finasteride
As we’ve already seen, even low doses of finasteride can have a significant impact on hair growth. For men who are particularly sensitive to finasteride or who experience side effects even with standard topical formulations, ultra–low-dose topical finasteride may offer a further risk-reduction strategy.
These formulations typically use very low concentrations (e.g., 0.005%), aiming to provide localized scalp DHT suppression while minimizing systemic exposure as much as possible. Supporting this strategy, a small clinical study of 0.005% alcohol-based topical finasteride found appreciable changes in serum DHT levels, indicating little-to-no systemic absorption. Despite minimal systemic exposure, participants still experienced meaningful hair growth outcomes, suggesting that even ultra-low topical doses can be effective at the scalp level.[15]Mazzarella, G. F., Loconsole, G. F., Cammisa, G. A., Mastrolonardo, G. M., & Vena, G. A. (1997). Topical finasteride in the treatment of androgenic alopecia: preliminary evaluations after a … Continue reading
Ultra–low-dose topical finasteride has not been studied extensively in large randomized controlled trials. Observationally, many users report improved tolerability and stable hair outcomes, but responses are variable, and efficacy may be more modest than with higher doses.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

6. Use PDE5 Inhibitors to Address Sexual Side Effects
For men who develop erectile dysfunction while using finasteride, PDE5 inhibitors (such as sildenafil or tadalafil) can be an effective symptom-management option. These medications are well studied in urology, including in men treated with 5AR inhibitors for benign prostatic hyperplasia (BPH).[16]Casabé, A., Roehrborn, C. G., Da Pozzo, L. F., Zepeda, S., Henderson, R. J., Sorsaburu, S., Henneges, C., Wong, D. G., & Viktrup, L. (2014). Efficacy and safety of the coadministration of … Continue reading
In that setting, PDE5 inhibitors have consistently been shown to improve erectile performance and sexual confidence, even when the underlying hormonal environment is altered.
It’s important to be clear about what this strategy does (and doesn’t) do. PDE5 inhibitors do not reverse DHT suppression or directly address libido changes or ejaculatory volume in the same way they may improve erectile rigidity. However, for many men, symptom relief can meaningfully reduce distress and help prevent performance anxiety from amplifying the problem.
Because PDE5 inhibitors are prescription medications with cardiovascular considerations and potential drug interactions, they should be used only under clinician supervision, particularly in men with heart disease, low blood pressure, or those taking nitrates or certain alpha-blockers.[17]Lui, J. L., Shaw, N. M., Abbasi, B., Hakam, N., & Breyer, B. N. (2023). Adverse reactions of PDE5 inhibitors: an analysis of the World Health Organization pharmacovigilance database. *Andrology.* … Continue reading
7. Optimize Testosterone
Testosterone plays a vital role in sexual health and, like finasteride, is associated with a wide range of symptoms. Reduced libido, erectile changes, fatigue, or low mood overlap significantly with those of hypogonadism, thyroid dysfunction, or other hormonal imbalances.[18]National Center for Biotechnology Information. (n.d.). Dutasteride. *NCBI Bookshelf.* Available at: https://www.ncbi.nlm.nih.gov/books/NBK532933/ (Accessed: November 2025)
Improving total and free testosterone levels through lifestyle changes or medical treatment when indicated can therefore restore androgen balance and reduce the relative impact of DHT suppression in sensitive individuals.
In select cases, testosterone replacement therapy (TRT) may be considered, but only with careful specialist oversight. TRT can improve sexual function, energy, and quality of life, yet it may also accelerate AGA unless combined thoughtfully with hair-loss treatments.[19]Zhang, Y., Xu, J., Jing, J., Wu, X., & Lv, Z. (2018). Serum levels of androgen-associated hormones are correlated with curative effect in androgenic alopecia in young men. *Medical Science … Continue reading For these reasons, TRT should never be initiated solely to counter finasteride side effects without a clear medical diagnosis and a comprehensive discussion of risks, benefits, and long-term goals.
8. Review Diet, Lifestyle, and Environmental Factors
While lifestyle changes cannot reverse finasteride’s pharmacologic action on DHT, they can meaningfully improve erectile function, mood, energy levels, sleep quality, and overall androgen balance, all of which overlap with the most commonly reported finasteride-related complaints.
A wide body of evidence shows that diet, exercise, and weight management play a significant role in sexual function. Randomized trials demonstrate that intensive lifestyle programs can significantly improve erectile function, with a meaningful proportion of men regaining normal erectile performance over time.[20]Esposito, K., Giugliano, F., Di Palo, C., Giugliano, G., Marfella, R., D’Andrea, F., D’Armiento, M., & Giugliano, D. (2004). Effect of lifestyle changes on erectile dysfunction in obese men: … Continue reading,[21]Hehemann, M. C., & Kashanian, J. A. (2016). Can lifestyle modification affect men’s erectile function?. *Translational Andrology and Urology.* 5(2). 187. Available at: … Continue reading
Environmental and behavioral factors can also be reviewed. Excessive heat exposure (such as frequent sauna use), high alcohol intake, cannabis use, and certain medications (including antidepressants) can independently impair sexual function or mood and may confound the interpretation of finasteride-related symptoms.
Although no studies have looked specifically at lifestyle programs for finasteride side effects, strong evidence shows that improving fitness, diet, sleep, and stress can enhance the same areas (erectile function, mood, energy, and hormone balance) that many men find most affected.
9. Consider Experimental or Adjunctive Supplements (With Caution)
Some men explore supplements marketed for testosterone support or sexual function, such as tongkat ali, in an effort to counter finasteride-related symptoms. Small studies suggest that tongkat ali may modestly increase testosterone or improve libido in certain populations, but the evidence is limited, inconsistent, and highly variable between individuals.[22]Leisegang, K., Finelli, R., Sikka, S. C., & Selvam, M. K. P. (2022). Eurycoma longifolia (jack) improves serum total testosterone in men: a systematic review and meta-analysis of clinical trials. … Continue reading
It’s critical to remember these supplements are experimental, not first-line solutions. Product quality, dosing, and purity can vary widely, and robust data on long-term safety or interactions with finasteride are lacking. While some individuals report subjective benefit, others notice no effect at all.
For these reasons, supplements should only be considered after established strategies have been explored and ideally discussed with a clinician, rather than relied upon as a primary way to manage side effects.
10. Switch Treatments or Change Strategy Entirely
If side effects persist despite adjustments to dose, frequency, or formulation, it may be appropriate to change strategies altogether. Hair loss treatment is not all-or-nothing, and finasteride, while highly effective, is not the only option.
Many men choose to rely on non-hormonal treatments entirely. Options such as topical or oral minoxidil, microneedling, low-level laser therapy devices, platelet-rich plasma (PRP), or hair transplantation can provide meaningful benefits without altering androgen pathways.
You can read more about alternative treatments in our guides and in-depth articles on minoxidil, microneedling, low-level laser therapy devices, and platelet-rich plasma (PRP).
While these approaches may not match finasteride’s disease-modifying effect on DHT, they can still preserve or improve cosmetic density for many users.
If you’re using topical finasteride, you might consider switching to low-dose topical dutasteride. offer better scalp localization at very low application frequencies due to dutasteride’s stronger and longer-lasting binding to 5AR. When used carefully and infrequently, some patients tolerate topical dutasteride well while maintaining hair stabilization.
Final Thoughts
Finasteride side effects are often manageable with thoughtful adjustments to dose, dosing schedule, formulation, and supporting lifestyle factors. There is no single correct way to use finasteride. Personalization is key, and the right approach depends on individual biology, risk tolerance, and treatment goals.
Health should always come first. Any side effects warrant open discussion with a clinician, and treatment decisions should be made collaboratively rather than in isolation. With informed, flexible planning, many men are able to find a balance that protects both their hair and their overall well-being.
References[+]
References ↑1 Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. *The Journal of Clinical and Aesthetic Dermatology.* 9(7). 56–62. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5023004/ ↑2 Kaplan, S. A., Chung, D. E., Lee, R. K., Scofield, S., & Te, A. E. (2012). A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. *International Journal of Clinical Practice.* 66(11). 1052–1055. Available at: https://doi.org/10.1111/j.1742-1241.2012.03010.x ↑3 Traish, A. M. (2020). Post-finasteride syndrome: a surmountable challenge for clinicians. *Fertility and Sterility.* 113(1). 21–50. Available at: https://doi.org/10.1016/j.fertnstert.2019.11.030 ↑4 Trüeb, R. M., Régnier, A., Rezende, H. D., & Dias, M. F. R. G. (2019). Post-finasteride syndrome: an induced delusional disorder with the potential of a mass psychogenic illness?. *Skin Appendage Disorders.* 5(5). 320–326. Available at: https://doi.org/10.1159/000497362 ↑5 Kaufman, K. D., Olsen, E. A., Whiting, D., Savin, R., DeVillez, R., Bergfeld, W., Price, V. H., et al. (1998). Finasteride in the treatment of men with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 39(4). 578–589. Available at: https://doi.org/10.1016/S0190-9622(98)70007-6 ↑6 Traish, A. M., Hassani, J., Guay, A. T., Zitzmann, M., & Hansen, M. L. (2011). Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. *The Journal of Sexual Medicine.* 8(3). 872–884. Available at: https://doi.org/10.1111/j.1743-6109.2010.02157.x ↑7 Lowe, F. C., McConnell, J. D., Hudson, P. B., Romas, N. A., Boake, R., Lieber, M., Elhilali, M., et al. (2003). Long-term 6-year experience with finasteride in patients with benign prostatic hyperplasia. *Urology.* 61(4). 791–796. Available at: https://doi.org/10.1016/S0090-4295(02)02548-7 ↑8 Hudson, P. B., Boake, R., Trachtenberg, J., Romas, N. A., Rosenblatt, S., Narayan, P., Geller, J., et al. (1999). Efficacy of finasteride is maintained in patients with benign prostatic hyperplasia treated for 5 years. *Urology.* 53(4). 690–695. Available at: https://doi.org/10.1016/S0090-4295(98)00666-9 ↑9 Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W. P., Cotterill, P. C., Thiboutot, D. M., et al. (1999). The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 41(4). 550–554. Available at: https://doi.org/10.1016/S0190-9622(99)80051-6 ↑10 National Center for Biotechnology Information. (n.d.). Finasteride. *NCBI Bookshelf.* Available at: https://www.ncbi.nlm.nih.gov/books/NBK513329/ (Accessed: November 2025) ↑11 Steiner, J. F. (1996). Clinical pharmacokinetics and pharmacodynamics of finasteride. *Clinical Pharmacokinetics.* 30(1). 16–27. Available at: https://doi.org/10.2165/00003088-199630010-00002 ↑12 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑13 Hajheydari, Z., Akbari, J., Saeedi, M., & Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. *Indian Journal of Dermatology, Venereology and Leprology.* 75. 47. Available at: https://doi.org/10.4103/0378-6323.45220 ↑14 Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. *Journal of the European Academy of Dermatology and Venereology.* 36(2). 286–294. Available at: https://doi.org/10.1111/jdv.17738 ↑15 Mazzarella, G. F., Loconsole, G. F., Cammisa, G. A., Mastrolonardo, G. M., & Vena, G. A. (1997). Topical finasteride in the treatment of androgenic alopecia: preliminary evaluations after a 16-month therapy course. *Journal of Dermatological Treatment.* 8(3). 189–192. Available at: https://doi.org/10.3109/09546639709160517 ↑16 Casabé, A., Roehrborn, C. G., Da Pozzo, L. F., Zepeda, S., Henderson, R. J., Sorsaburu, S., Henneges, C., Wong, D. G., & Viktrup, L. (2014). Efficacy and safety of the coadministration of tadalafil once daily with finasteride for 6 months in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia. *The Journal of Urology.* 191(3). 727–733. Available at: https://doi.org/10.1016/j.juro.2013.09.059 ↑17 Lui, J. L., Shaw, N. M., Abbasi, B., Hakam, N., & Breyer, B. N. (2023). Adverse reactions of PDE5 inhibitors: an analysis of the World Health Organization pharmacovigilance database. *Andrology.* 11(7). 1408–1417. Available at: https://doi.org/10.1111/andr.13430 ↑18 National Center for Biotechnology Information. (n.d.). Dutasteride. *NCBI Bookshelf.* Available at: https://www.ncbi.nlm.nih.gov/books/NBK532933/ (Accessed: November 2025) ↑19 Zhang, Y., Xu, J., Jing, J., Wu, X., & Lv, Z. (2018). Serum levels of androgen-associated hormones are correlated with curative effect in androgenic alopecia in young men. *Medical Science Monitor.* 24. 7770. Available at: https://doi.org/10.12659/MSM.913116 ↑20 Esposito, K., Giugliano, F., Di Palo, C., Giugliano, G., Marfella, R., D’Andrea, F., D’Armiento, M., & Giugliano, D. (2004). Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. *JAMA.* 291(24). 2978–2984. Available at: https://doi.org/10.1001/jama.291.24.2978 ↑21 Hehemann, M. C., & Kashanian, J. A. (2016). Can lifestyle modification affect men’s erectile function?. *Translational Andrology and Urology.* 5(2). 187. Available at: https://doi.org/10.21037/tau.2016.02.05 ↑22 Leisegang, K., Finelli, R., Sikka, S. C., & Selvam, M. K. P. (2022). Eurycoma longifolia (jack) improves serum total testosterone in men: a systematic review and meta-analysis of clinical trials. *Medicina.* 58(8). 1047. Available at: https://doi.org/10.3390/medicina58081047 Dutasteride is one of the most powerful pharmacologic treatments for androgenic alopecia (AGA). Initially approved for benign prostatic hyperplasia, dutasteride is now commonly prescribed off-label for hair loss in men and, more recently, has gained attention in topical form to minimize systemic exposure. This has created an important clinical question: how do oral and topical dutasteride compare in terms of effectiveness, safety, and real-world practicality?
This article examines what the current scientific literature reveals about oral versus topical dutasteride, reviewing mechanisms of action, efficacy data, and safety profiles to help you make more informed treatment decisions.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Dutasteride Mechanism of Action and Formulations
AGA is driven by increases in dihydrotestosterone (DHT). DHT binds to androgen receptors in hair follicles, triggering miniaturization that leads to thinner, shorter hairs. Dutasteride works by inhibiting an enzyme called 5α-reductase (5AR), which converts testosterone into DHT. Dutasteride is a dual 5AR inhibitor, meaning it blocks both type I and type II 5AR, leading to decreased DHT levels and ultimately halting the progression of AGA and restoring thicker hair.[1]Gerst, C., Dalko, M., Pichaud, P., Galey, J. B., Buan, B., & Bernard, B. A. (2002). Type-1 steroid 5α-reductase is functionally active in the hair follicle as evidenced by new selective … Continue reading,[2]Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by … Continue reading
Figure 1. Structure of dutasteride, which is a dual 5AR inhibitor.[3]Wikimedia Commons. (n.d.). Dutasterid.svg [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Dutasterid.svg (Accessed: November 2025) Image used under Creative Commons License.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Oral Dutasteride – Mechanism and Formulations
Oral dutasteride is absorbed through the gastrointestinal tract into systemic circulation, leading to significant and sustained reductions in DHT in the blood (serum). This includes reductions in scalp DHT, since less DHT is available systemically to diffuse into hair follicles.[4]Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by … Continue reading,[5]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading
The flip side is that any symptoms tied to systemic DHT suppression, such as sexual side-effects, are driven by this same mechanism. Given the long half-life and accumulation, systemic exposure persists even if doses are missed or therapy is stopped, and any adverse effects may take weeks to resolve.[6]Tsai, T.-F., Choi, G. S., Kim, B. J., Kim, M.-B., Ng, C. F., Kochhar, P., Jasper, S., Brotherton, B., Orban, B., & Lulic, Z. (2018). Prospective randomized study of sexual function in men taking … Continue reading
Topical Dutasteride – Mechanism and Formulations
Topical dutasteride treatment might avoid some of these pitfalls: high exposure at the scalp and low exposure elsewhere can target delivery and avoid systemic issues. It is applied directly to the scalp in formulations such as:
- Simple solutions (often alcohol- or glycol-based)
- Gels or lotions
- Liposomal formulations
- Nanoemulsions and nanoemulgels
- Nanocrystalline and other particulate systems
These vehicles are designed to ensure that dutasteride can cross the stratum corneum (the outermost protective layer of the skin) and, ideally, remain localized within the scalp.
What is the Available Evidence for Oral Dutasteride?
The effect of dutasteride on hair loss is well documented: multiple randomized placebo-controlled studies have been conducted over the last 25 years. Dutasteride was first developed as a treatment for benign prostatic hyperplasia and taken as a tablet. Early studies into dutasteride for hair loss therefore used oral formulations, with topical treatments developed later.
Here, we’ll take a look at the evidence supporting the use of oral dutasteride for hair loss. Many studies compare dutasteride with other treatments, most notably finasteride, or use the drug in combination with other therapies. We’ll focus on data showing the effect of dutasteride alone compared to placebo, but will also touch on comparisons with other popular treatments.
Below is a summary of the findings to date on the safety and efficacy of oral dutasteride:
Study Design and Population Treatments and Concentrations (Daily) Hair Growth Outcomes Systemic Effects Study #1[7]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Randomized, placebo-controlled, double-blinded trial. 415 men with male pattern hair loss aged 21-45 years old; 24 weeks. 0.05, 0.1, 0.5, or 2.5 mg dutasteride; 5 mg finasteride. Dose-dependent increase in total hair count vs placebo. Improved hair count vs finasteride. Serum decrease in DHT level (92% at 0.5 mg). Study #2[8]Stough, D. (2007). Dutasteride improves male pattern hair loss in a randomized study in identical twins. *Journal of Cosmetic Dermatology.* 6(1). 9–13. Available at: … Continue reading Randomized, placebo-controlled, double-blinded trial. 17 pairs of identical twin males with AGA; 12 months.
0.5 mg dutasteride. Significant increase in total hair counts in dutasteride-treated twins. Not assessed. Study #3[9]Eun, H. C., Kwon, O. S., Yeon, J. H., Shin, H. S., Kim, B. Y., Ro, B. I., Cho, H. K., et al. (2010). Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male … Continue reading Randomized, placebo-controlled, double-blinded trial. 153 men with male pattern hair loss aged 18-49; 6 months 0.5 mg dutasteride. Significant increase in total hair count. Sexual dysfunction was reported in 4.1% of the treatment group and 2.7% of placebo (not statistically significant). Study #4[10]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Randomized, placebo-controlled, double-blinded trial. 917 men with AGA aged 20-50; 24 weeks. 0.02, 0.1, or 0.5 mg dutasteride; 1 mg finasteride Increase in terminal hair count and width vs placebo. Significant increase in hair width vs dutasteride. No significant changes in reported adverse events. Study #5[11]Tsunemi, Y., Irisawa, R., Yoshiie, H., Brotherton, B., Ito, H., Tsuboi, R., Kawashima, M., Manyak, M., & ARI114264 Study Group. (2016). Long-term safety and efficacy of dutasteride in the … Continue reading Open-label, prospective study, no control. 120 men with AGA aged 20-50; 52 weeks. 0.5 mg dutasteride. Increase in terminal hair count and hair width compared to baseline. 12% of men experienced libido decrease and impotence, of which 50% and 57%, respectively, were not resolved by the end of the study. Study #6[12]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Open-label, randomized study; no placebo control. 90 men with AGA aged 18-40; 24 weeks. 0.5 mg dutasteride or 1 mg finasteride. Increase in total hair count and width compared to baseline. Increase in total hair count was significantly higher in dutasteride than finasteride group. Erectile dysfunction and loss of libido reported in 3 and 4 patients respectively. Study #7[13]Tsai, T.-F., Choi, G. S., Kim, B. J., Kim, M.-B., Ng, C. F., Kochhar, P., Jasper, S., Brotherton, B., Orban, B., & Lulic, Z. (2018). Prospective randomized study of sexual function in men taking … Continue reading Randomized, placebo-controlled, double-blinded trial. 117 men with AGA aged 23-50; 24 weeks. 0.5 mg dutasteride. Not assessed. Incidence of sexual adverse events was approximately double in the dutasteride group (16% vs 8% in placebo). Study #8[14]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading Retrospective chart review. 600 men over 18 with AGA. 0.5 mg dutasteride or 1 mg finasteride. Greater improvements in BASP classifications of AGA compared to baseline were found in dutasteride vs finasteride. Incidence of adverse events was higher in finasteride group (10.5%) than in dutasteride group (7.6%). What is the Available Evidence for Topical Dutasteride?
While evidence for the efficacy of oral dutasteride is well established, research into topical dutasteride solutions started relatively recently. Below is an overview of the studies into the efficacy of topical dutasteride:
Study Design and Population Treatments and Concentrations (Daily) Hair Growth Outcomes Systemic Effects Study #1[15]Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus … Continue reading Randomized, placebo-controlled, double-blinded trial. 34 men with AGA aged 18-65; 20 weeks. Microneedling with 0.01% dutasteride solution. Placebo was microneedling with saline solution. Significant improvements in hair thickness, hair density, and vellous: terminal hair ratio. Not assessed. Study #2[16]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading Randomized, placebo-controlled, double-blinded trial. 135 adult males aged 20-60 with AGA; 24 weeks. 0.01%, 0.02%, or 0.05% topical dutasteride; 1mg oral dutasteride Dose-dependent increase in total hair count and hair width. 0.05% solution showed significant improvement over finasteride in total hair count. No change in serum DHT levels observed vs placebo. How Does Oral and Topical Dutasteride Stack Up Against Each Other?
When comparing oral and topical dutasteride, the most important point to note is that there are currently no published clinical trials directly comparing the two formulations alone head-to-head for AGA. All comparisons must be made indirectly, using separate studies with different designs, populations, timelines, and formulations.
What About the Quality of the Available Evidence?
Despite this limitation, the evidence available for each formulation allows us to evaluate its strength and predictability. The current evidence quality for each formulation is as follows:
- Oral dutasteride — 59/100
- Topical dutasteride — 55/100
By our metrics, this means that both treatments have similar evidence quality overall. Longer-term data support oral dutasteride, while topical dutasteride remains less validated, largely due to fewer studies and limited long-term follow-up.
We’ll take a closer look at the strength of the evidence supporting both formulations.
Oral Dutasteride Studies
Oral dutasteride can reduce serum DHT levels by up to 90% and scalp DHT levels by 51-79%.[17]Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by … Continue reading,[18]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Randomized controlled trials consistently demonstrate that dutasteride, particularly at the standard 0.5 mg daily dose, suppresses serum and scalp DHT and delivers some of the most robust regrowth outcomes documented for AGA.
A number of the studies outlined above include large sample sizes, often over 100 participants, with the largest including over 900.[19]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Importantly, these cohorts should be large enough to detect changes in the incidence of adverse events, even if they are relatively rare. The replication of results in multiple trials using similar endpoints is a strong indicator of the efficacy of the drug.
Unfortunately, the longest of these RCTs only lasted 6 months, which may mean that lasting impacts on growth are overlooked. Similarly, side effects associated with long-term use over many years may be missed.
We can turn to longer studies using finasteride for some indication of how sustained benefits may be, given the similar mechanism of action of the two treatments. A 3-year trials using 1 mg daily doses of finasteride showed sustained improvement in hair growth over the course of the study, while placebo groups saw decreased hair counts.[20]Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. … Continue reading
A 10-year follow-up study concluded that improvements in hair growth, as determined by expert analysis of hair photographs, from finasteride do not worsen over time, and in 21% of cases, hair growth got even better after 5 years of treatment.[21]Rossi, A., Cantisani, C., Scarnò, M., Trucchia, A., Fortuna, M. C., & Calvieri, S. (2011). Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year … Continue reading
While we can’t draw any firm conclusions about the long-term efficacy of dutasteride from studies using finasteride, these findings can indicate the impact of long-term inhibition of 5AR, which is also seen in dutasteride treatment.
Topical Dutasteride Studies
Evidence for topical dutasteride is promising but remains less mature. Early clinical research indicates that topical formulations can meaningfully improve hair density, hair shaft diameter, and overall investigator-assessed regrowth, with efficacy in trials approaching that of oral therapy.
One small study with only 34 participants showed significant improvements in hair growth and thickness when combined with microneedling, compared to microneedling alone. Another study combined dutasteride formulations with minoxidil and also found that topical dutasteride delivered via microneedling produced hair density and thickness improvements similar to oral dutasteride, with fewer systemic side effects.[22]Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus … Continue reading,[23]Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through … Continue reading
A recently published Phase II trial, the first to test topical dutasteride without microneedling, reported that all concentrations improved hair growth versus placebo, produced minimal changes in serum DHT, and that 1 ml daily of 0.05% appeared to outperform oral finasteride. However, concerns about unusually high baseline hair counts and inconsistencies in the placement and size of the 1 cm² target areas used for hair measurements raise questions about the reliability of these results.[24]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading
Figure 2. Oral dutasteride (yellow line) reduces serum DHT levels more than topical Dutaseride (blue lines). Adapted from Figure 3.[25]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading Image used under Creative Commons License.
Real-world outcomes from low-dose topical dutasteride users (0.01–0.02% applied daily or near-daily) suggest more modest benefits, with good scalp localization and stable serum DHT noted as positives, rather than regrowth exceeding that with oral finasteride.
There have been some concerns raised about the ability of dutasteride to penetrate into the scalp. Dutasteride is a relatively large molecule, with one molecule weighing 528.5 Daltons (Da). There is a commonly cited rule, called the 500 Dalton Rule, that states that molecules should be under 500 Da in order to penetrate the skin. However, dutasteride has been demonstrated to be effective in topical formulations and can measurably lower DHT concentrations. What’s more, a small, unpublished study has also shown that the drug does penetrate into both the scalp and serum, even at low concentrations. As such, in this case, dutasteride does seem to penetrate the skin in the scalp, without the need for microneedling or injection.
How Safe are Dutasteride Formulations?
Because dutasteride produces sustained suppression of DHT, safety considerations, particularly regarding hormonal and sexual side effects, are central to treatment selection. Most clinical trials record adverse events, while some studies are focused specifically on side-effects.[26]Tsai, T.-F., Choi, G. S., Kim, B. J., Kim, M.-B., Ng, C. F., Kochhar, P., Jasper, S., Brotherton, B., Orban, B., & Lulic, Z. (2018). Prospective randomized study of sexual function in men taking … Continue reading
Oral Dutasteride
Across randomized trials and long-term observational studies, the most consistently reported adverse effects of oral dutasteride involve sexual function, including:
- Decreased libido
- Erectile dysfunction
- Ejaculation disorders
- Gynecomastia and breast tenderness (rare)
Trials report overall total adverse event rates between 4 and 12%, with sexual adverse events such as decreased libido as high as 13%, although this was reported in higher doses (2.5mg daily).[27]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading At the standard 0.5mg daily dose, sexual side effects typically occur at a rate of between 1 and 5%. In the context of other popular treatments, comparisons with finasteride show no consistent increase in sexual side effects with dutasteride, despite dutasteride lowering serum DHT by an additional 20-30% beyond finasteride.[28]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
Most controlled studies show that the majority of sexual side effects resolve either during treatment or after discontinuation. However, due to dutasteride’s long half-life (4-5 weeks) and high tissue binding, systemic effects can persist. At standard 0.5mg daily dosing, serum DHT generally returns to 80% of baseline within 6 months of discontinuation.
Topical Dutasteride
Topical dutasteride is generally well tolerated, and reported adverse events are usually mild and localized, including:
- Scalp irritation
- Erythema
- Contact dermatitis
- Pruritus or dryness
These occur inconsistently and are formulation-dependent, influenced by solvent systems, penetration enhancers, and application volume.
The primary safety advantage of topical dutasteride is reduced systemic exposure. In trials, topical regimens in the 0.01–0.05% range produce minimal or no significant changes in serum DHT or testosterone.[29]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading When decreases in serum DHT are observed with topical use, they are generally smaller than those seen with oral dosing.[30]Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through … Continue reading
Adherence and Practical Considerations
Dutasteride, whether oral or topical, is primarily used for AGA in men, though its use for hair loss remains off-label in the United States. Oral dutasteride is formally FDA-approved only for benign prostatic hyperplasia, while topical formulations exist solely through compounding pharmacies.
Adherence and Route of Application
Oral dutasteride offers the simplest adherence burden: one capsule daily (or a modified intermittent schedule). This ease is a major factor behind its consistent outcomes. Topical dutasteride, while potentially safer from a systemic standpoint, requires regular scalp application and attention to dosing volume.
The effectiveness of topical formulations is heavily influenced by factors such as vehicle, scalp contact time, treated surface area, and individual skin permeability. These practical considerations can meaningfully shape both efficacy and systemic absorption.
Advanced vehicles such as nanoemulsions or nanoemulgels can increase scalp penetration by up to 1.5-fold compared to conventional solutions.[31]Ali, M. S., Alam, M. S., Alam, N., & Siddiqui, M. R. (2014). Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. … Continue reading However, this may also increase systemic exposure. Application frequency can be adjusted to balance efficacy and hormonal safety. Because dutasteride binds to 5AR for prolonged periods, daily application is not always necessary.
Time on the scalp also matters. The longer a topical formulation remains in contact with the skin, the more dutasteride can penetrate the follicle. Most protocols recommend allowing at least four hours before washing the hair. Rinsing too soon can reduce efficacy, while prolonged contact increases both potency and the possibility of systemic absorption.
The size of the application area affects systemic exposure. Treating a small region, such as the crown, introduces less drug into circulation than applying the same concentration across the entire scalp. Patients with diffuse thinning often benefit from using lower volumes or concentrations to counterbalance the increased surface area.
As we can see, there is a wide range of factors to consider when making a treatment plan with topical dutasteride, which may make consistent application a challenge.
Use in Women
Off-label use of dutasteride in women is limited and generally reserved for postmenopausal women or women with documented hyperandrogenism or a strong family history of AGA. Due to the systemic androgenic impacts of the drug, it’s typically only used in cases where other therapies (minoxidil, spironolactone) are inadequate.
Clinical Interpretation and Decision-Making
The key trade-off between oral and topical formulations is systemic potency versus localized targeting, balanced against differences in side-effect risk, convenience, and cost. Oral therapy is simpler and less variable; topical therapy requires consistent application but offers greater hormonal safety for risk-averse patients.
Combination therapy, most commonly topical dutasteride with minoxidil, can enhance outcomes through complementary mechanisms. Oral dutasteride may also be paired with topical agents in some cases.
Significant research gaps remain, including the absence of direct head-to-head oral-versus-topical trials, limited long-term safety data for topical dutasteride, and the lack of standardized dosing guidelines for topical formulations.
Final Thoughts
Dutasteride, whether oral or topical, represents one of the most powerful tools currently available for treating AGA. Oral dutasteride offers the most consistent and robust regrowth outcomes, supported by the strongest body of clinical evidence, but with predictable systemic hormonal suppression and long persistence after discontinuation.
Topical dutasteride, while still supported by a smaller and newer evidence base, offers a compelling alternative for patients seeking meaningful scalp-level efficacy with reduced systemic exposure. Its ultimate success depends heavily on formulation quality, dosing strategy, and adherence.
There is no universally “best” option: only the best option for specific circumstances. Treatment selection should be guided by hair-loss severity, prior treatment response, risk tolerance, reproductive plans, and long-term adherence capacity. As research continues to evolve, clinical guidance will become more precise. For now, optimal results come from choosing the formulation that best aligns with your goals and comfort with risk.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

References[+]
References ↑1 Gerst, C., Dalko, M., Pichaud, P., Galey, J. B., Buan, B., & Bernard, B. A. (2002). Type-1 steroid 5α-reductase is functionally active in the hair follicle as evidenced by new selective inhibitors of either type-1 or type-2 human steroid 5α-reductase. *Experimental Dermatology.* 11(1). 52–58. Available at: https://doi.org/10.1034/j.1600-0625.2002.110106.x ↑2, ↑4, ↑17 Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α-reductase inhibitor. *The Journal of Clinical Endocrinology & Metabolism.* 89(5). 2179–2184. Available at: https://doi.org/10.1210/jc.2003-030330 ↑3 Wikimedia Commons. (n.d.). Dutasterid.svg [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Dutasterid.svg (Accessed: November 2025) ↑5, ↑18 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑6, ↑13, ↑26 Tsai, T.-F., Choi, G. S., Kim, B. J., Kim, M.-B., Ng, C. F., Kochhar, P., Jasper, S., Brotherton, B., Orban, B., & Lulic, Z. (2018). Prospective randomized study of sexual function in men taking dutasteride for the treatment of androgenetic alopecia. *The Journal of Dermatology.* 45(7). 799–804. Available at: https://doi.org/10.1111/1346-8138.14329 ↑7, ↑27 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑8 Stough, D. (2007). Dutasteride improves male pattern hair loss in a randomized study in identical twins. *Journal of Cosmetic Dermatology.* 6(1). 9–13. Available at: https://doi.org/10.1111/j.1473-2165.2007.00297.x ↑9 Eun, H. C., Kwon, O. S., Yeon, J. H., Shin, H. S., Kim, B. Y., Ro, B. I., Cho, H. K., et al. (2010). Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. *Journal of the American Academy of Dermatology.* 63(2). 252–258. Available at: https://doi.org/10.1016/j.jaad.2009.09.018 ↑10, ↑19, ↑28 Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 70(3). 489–498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑11 Tsunemi, Y., Irisawa, R., Yoshiie, H., Brotherton, B., Ito, H., Tsuboi, R., Kawashima, M., Manyak, M., & ARI114264 Study Group. (2016). Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. *The Journal of Dermatology.* 43(9). 1051–1058. Available at: https://doi.org/10.1111/1346-8138.13310 ↑12 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑14 Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. *Annals of Dermatology.* 34(5). 349. Available at: https://doi.org/10.5021/ad.22.027 ↑15, ↑22 Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. *Journal of the European Academy of Dermatology & Venereology.* 36(10).. Available at: https://doi.org/10.1111/jdv.18285 ↑16, ↑25, ↑29 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. *Cureus.* 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑20 Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. *Journal of the American Academy of Dermatology.* 55(1). 71–74. Available at: https://doi.org/10.1016/j.jaad.2005.07.001 ↑21 Rossi, A., Cantisani, C., Scarnò, M., Trucchia, A., Fortuna, M. C., & Calvieri, S. (2011). Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. *Dermatologic Therapy.* 24(4). 455–461. Available at: https://doi.org/10.1111/j.1529-8019.2011.01441.x ↑23, ↑30 Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in treatment of androgenetic alopecia. *QJM: An International Journal of Medicine.* 117(Supplement_2). hcae175–207. Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑24 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. *Cureus.* 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑31 Ali, M. S., Alam, M. S., Alam, N., & Siddiqui, M. R. (2014). Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. *Iranian Journal of Pharmaceutical Research (IJPR).* 13(4). 1125. Available at: https://doi.org/10.3390/pharmaceutics14061152 Minoxidil is a medication that began as a treatment for hypertension (high blood pressure). However, after observing excess hair growth during the testing stages of the drug, minoxidil was repurposed as a treatment for hair loss. Topical minoxidil soon received FDA approval for the treatment of both male and female pattern hair loss, while oral minoxidil is also used as an off-label treatment for hair loss.[1]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug design, development and therapy. 2777-2786. Available at: … Continue reading
So, if minoxidil is a treatment for hair loss, why does it cause an increase in hair loss when you start using it? Yes, you did read that right – minoxidil can actually cause an increase in hair loss as part of what is often called the ‘dread shed.’ This phenomenon was demonstrated in a retrospective study of 435 patients with androgenic alopecia (AGA) who were prescribed low-dose oral minoxidil [≤5 mg per day] by the same clinic. Self-reported adverse events were recorded for each of the users and, of the 435 patients, 32% experienced increased hair shedding.[2]Sanabria, B., de Nardo Vanzela, T., Miot, H. A., & Ramos, P. M. (2021). Adverse effects of low-dose oral minoxidil for androgenetic alopecia in 435 patients. Journal of the American Academy of … Continue reading
This is evidently cause for concern – if you have just started minoxidil treatment to prevent hair loss, a sudden increase in hair loss is possibly the last thing you would hope and expect to experience. So what is it about minoxidil that causes this to happen, how long does it usually last, and is it actually a good thing? In this article, we will explore the hair cycle and minoxidil in detail to provide answers to each of these questions.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

The Hair Cycle
To understand why minoxidil can increase hair shedding, let’s first take a refresher on the hair cycle. Our hair is constantly going through a cycle of growing (anagen), regression and transition (catagen), resting (telogen), and shedding (exogen), which it repeats continuously.
Healthy hairs grow for anywhere between 2 and 8 years, and there is a correlation between the length and strength of a hair and the time spent in anagen. Catagen is the transition from anagen to telogen, a period of approximately 2 weeks during which the follicle regresses from the hair shaft and disconnects it from the blood supply, preventing any further growth. Telogen follows and lasts for 2-3 months, with a new hair shaft beginning to develop at the base of the follicle underneath the now resting hair shaft. Exogen then represents the transition from telogen to anagen, with the growing hair shaft pushing out the old hair shaft.[3]Natarelli, N., Gahoonia, N., & Sivamani, R. K. (2023). Integrative and mechanistic approach to the hair growth cycle and hair loss. Journal of clinical medicine. 12(3). 893. Available at: … Continue reading
In the healthy scalp, the percentage of hairs in each of the hair cycle stages is thought to remain fairly consistent. At any one time, evidence suggests that approximately 9% of the hairs on a healthy scalp are in the telogen phase (although there are some suggestions that this figure may actually be too high).[4]Natarelli, N., Gahoonia, N., & Sivamani, R. K. (2023). Integrative and mechanistic approach to the hair growth cycle and hair loss. Journal of clinical medicine. 12(3). 893. Available at: … Continue reading These hairs exist in a largely asynchronous fashion, with hair follicles progressing through the hair cycle according to their own unique pattern. Hair follicles undergo between 10 and 30 full cycles, and it is normal for up to 150 hairs to fall out per day without there being an underlying hair loss problem, as the hairs are constantly being replaced.[5]Bergfeld, W. (2009). Diffuse hair loss: its triggers and management. Cleve Clin J Med. 76(6). 361-370. Available at: https://doi.org/10.3949/ccjm.76a.08080 This keeps hair fall relatively consistent, preventing periods of significant hair shedding.
What Happens to the Hair Cycle during AGA?
AGA, the hair loss condition for which topical minoxidil is an approved treatment, is caused by damage to the hair follicle that contributes to its miniaturization. This is when individual strands of hair become smaller and smaller over time, eventually becoming vellus hairs that are shorter, thinner, and more white, which makes them difficult to see. We have a previous article that explores AGA-induced hair loss in great detail, but let’s summarize the key characteristics below:
- Perifollicular fibrosis – collagen deposition in the spaces around the hair follicles restricts the room within which hair shafts are able to grow, acting as a physical barrier to hair growth.
- Increased telogen:anagen ratio – healthy scalps typically have 1 telogen hair for every 12 anagen hairs (1:12 telogen:anagen), but people with AGA may exhibit ratios that are as high as 1:4 or even 2:5. The telogen phase also lengthens, with hairs spending longer in a state of non-growth.
- Shortened anagen phase – as previously mentioned, the length of an anagen phase directly corresponds to the length of the eventual hair shaft, and people with AGA typically exhibit anagen phases that become shorter with every hair cycle.
Although the exact mechanisms underlying AGA are yet to be fully understood, it is evident that AGA is a progressive and cumulative process that occurs due to harmful factors damaging the hair follicle and shortening the anagen phase.
How Minoxidil Works
Chemical Structure of Minoxidil. Adapted from:[6]Pubchem (no date). Minoxidil (Compound). Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Minoxidil#section=2D-Structure (Accessed: June 2025
Minoxidil is also yet to be fully understood, but several mechanisms have been suggested that could explain how it reduces hair loss:
- Vasodilation – Minoxidil started out as a treatment for hypertension, so its function as a vasodilator is well documented. Studies have shown that minoxidil increases blood flow by opening potassium channels, reducing high blood pressure. However, an unintended benefit of this function is increased blood flow to the hair follicles, providing them with nutrients and oxygen that help to prolong the anagen phase.[7]Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British journal of dermatology. 150(2). 186-194. Available at: … Continue reading
- Prostaglandin mediation – Minoxidil has been shown to increase the production of prostaglandin E2 (PGE2) in cultured human dermal papilla fibroblasts, the specialized cells that sit at the base of the hair follicle. It is thought that PGE2 may protect hair follicles from damage.[8]Michelet, J. F., Commo, S., Billoni, N., Mahé, Y. F., & Bernard, B. A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair … Continue reading
- Growth factor mediation – Studies have shown that minoxidil has the potential to upregulate the expression of several growth factors that support hair growth, including vascular endothelial growth factor in human dermal papilla cells.[9]Lachgar, Charveron, Gall, & Bonafe. (1998). Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. British Journal of Dermatology. 138(3). … Continue reading
It is likely that minoxidil reduces hair loss through a combination of several mechanisms, including those noted above and perhaps others that are yet to be discovered.
How Might Minoxidil Cause the Dread Shed?
So, we know the basics of the hair cycle, some of the mechanisms that contribute to pattern hair loss, and some of the mechanisms by which minoxidil may reduce hair loss. But what does all this have to do with the ‘dread shed ’? Fortunately, observing the effects of minoxidil is more straightforward than trying to understand how it works, and there is one key effect which is believed to contribute to the increase in shedding: shortening of the telogen phase.
As we previously discussed, a key characteristic of AGA is lengthening of the telogen phase, which causes an abnormal amount of scalp hair to be in a state of arrested growth at the same time. It is widely believed that minoxidil directly addresses this issue by both shortening the telogen phase and accelerating the telogen to anagen transition. In one study, application of topical minoxidil to rats caused a dramatic shortening of the telogen phase, falling from 20 days to just 1-2 days.[10]Mori, O., & Uno, H. (1990). The effect of topical minoxidil on hair follicular cycles of rats. The Journal of dermatology. 17(5). 276-281. Available at: … Continue reading In a separate study that was also conducted in rats, topical minoxidil caused a significant switch from the telogen to anagen phase as quickly as 10 days after beginning treatment (Figure 2).[11]Shatalebi, M. A., & Rafiei, Y. (2014). Preparation and evaluation of minoxidil foamable emu oil emulsion. Research in pharmaceutical sciences. 9(2). 123-133. Available at: … Continue reading
Figure 1: The percentage of rat hair follicles in the anagen and telogen phases following treatment with minoxidil. Control rats were not given any treatment, market formulation refers to standard topical minoxidil [5%], main formulation is minoxidil in a foamable emulsion, and blank formulation is the foamable emulsion without the minoxidil.[12]Shatalebi, M. A., & Rafiei, Y. (2014). Preparation and evaluation of minoxidil foamable emu oil emulsion. Research in pharmaceutical sciences. 9(2). 123-133. Available at: … Continue reading
We could only find one clinical study that has investigated the telogen-anagen shift in humans at an early stage after beginning minoxidil use. They conducted a 24-week trial in which men with AGA either applied topical minoxidil [5%] or topical cetirizine for the first 16 weeks, then stopped use for 8 weeks. Although the results were not statistically significant, they showed that minoxidil caused an increase in the percentage of anagen hair and a decrease in the percentage of telogen hair, supporting the idea that minoxidil rapidly induces shortening of the telogen phase.[13]Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a … Continue reading
In accelerating the telogen to anagen transition, minoxidil also causes “hair follicle synchronization” or synchronization of the hair cycle. As we highlighted earlier, healthy scalps are somewhat ‘protected’ from significant hair shedding events due to the asynchronous nature of the hairs and their individual hair cycles. However, due to the increased density of telogen follicles in the AGA-affected scalp, minoxidil causes a greater-than-normal percentage of hairs to enter anagen at the same time. This syncing of the hair cycles then results in more hairs being pushed out at the same time.
So, to summarize the process that is believed to be the key factor behind the dread shed:
- In AGA, a greater number of hair follicles are in telogen
- Minoxidil treatment accelerates the transition of these hair follicles from telogen to anagen and causes many follicular units to become synchronized
- The transition leads to initiation of the shedding phase (exogen)
- Increased hair shedding occurs
Is The ‘Dread Shed’ Real?
Until very recently, evidence of minoxidil-induced hair shedding was either anecdotal or provided by studies of minoxidil in which increased hair shedding was noted as an adverse event. However, a newly published study sought to investigate the shedding phase in detail.
In this 2025 study, 49 patients with AGA used topical minoxidil [2% or 5%] for 24 weeks. Total hair shedding was quantified daily by the participants, who self-assessed their hair fall after combing, after washing, and on the pillow after sleeping. This was then averaged every 4 weeks and compared to the level of hair shedding prior to starting treatment. They found that the participants who used 5% minoxidil exhibited increased hair shedding (relative to pre-treatment) for 4-8 weeks, while the participants who used 2% minoxidil exhibited increased shedding for 8-12 weeks (Figure 2).[14]Bi, L., Kan, H., Wang, J., Ding, Y., Huang, Y., Wang, C., Du, Y., Lu, C., Zhao, M., Sun, W. & Su, T. (2025). Whether the transient hair shedding phase exist after minoxidil treatment and does it … Continue reading
Figure 2: Relative hair loss in the 24 weeks after starting treatment with minoxidil. *p < 0.05, **p < 0.01, ***p < 0.001. (A) Hair loss across all patients. (B) Hair loss in patients using 2% topical minoxidil. (C) Hair loss in patients using 5% topical minoxidil.[15]Bi, L., Kan, H., Wang, J., Ding, Y., Huang, Y., Wang, C., Du, Y., Lu, C., Zhao, M., Sun, W. & Su, T. (2025). Whether the transient hair shedding phase exist after minoxidil treatment and does it … Continue reading
This study provides definitive evidence that minoxidil does cause an initial shedding phase. However, importantly, hair shedding eventually fell below baseline levels in both groups, indicating that the initial shedding phase is temporary and that minoxidil did begin to reduce hair loss.
Is Initial Hair Loss Actually a Good Sign?
The same authors investigating the minoxidil shedding phase also sought to determine whether the amount of shedding had any association with treatment efficacy. They compared peak relative hair shedding (within the first 12 weeks) to changes in AGA severity using the Basic and Specific classification (BASP), which is a universal hair loss classification system that is used to assess the distribution and severity of hair loss in men and women of all races. They also compared peak relative hair shedding to several trichoscopy measurements, including hair density, hair diameter, and terminal hair proportion.[16]Bi, L., Kan, H., Wang, J., Ding, Y., Huang, Y., Wang, C., Du, Y., Lu, C., Zhao, M., Sun, W. & Su, T. (2025). Whether the transient hair shedding phase exist after minoxidil treatment and does it … Continue reading
Interestingly, in the 5% minoxidil group, a significant association was found between the amount of initial hair shedding and hair density, hair diameter, and the proportion of terminal hairs. In other words, people who lost more hair in the ‘dread shed’ actually experienced greater outcomes from minoxidil treatment. Furthermore, participants who initially shed the most hair in both the 2% and 5% minoxidil groups demonstrated the greatest improvements in AGA severity by week 24.[17]Bi, L., Kan, H., Wang, J., Ding, Y., Huang, Y., Wang, C., Du, Y., Lu, C., Zhao, M., Sun, W. & Su, T. (2025). Whether the transient hair shedding phase exist after minoxidil treatment and does it … Continue reading
These results are very interesting – not only does the initial shedding phase indicate that the minoxidil is working, but more shedding may even predict better treatment outcomes! So, if you’ve just started treatment, don’t fear the shed!
Final Thoughts
Minoxidil is an FDA-approved treatment for AGA but, in some cases, it can cause increased hair shedding in the early stages of use. Known as the ‘dread shed,’ this phase is believed to be caused by minoxidil shortening the telogen phase of the hair cycle, causing old hairs to fall out. This shedding is even more pronounced due to the increased density of telogen hairs present in the scalps of people with AGA. However, this shedding is only temporary, typically lasting between 4 and 8 weeks. Moreover, people who experience more shedding in this initial phase may actually experience greater overall outcomes from their minoxidil treatment. So, if you have just started minoxidil and are noticing increased shedding, don’t panic – it is most likely a sign that the minoxidil is working.
References[+]
References ↑1 Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug design, development and therapy. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑2 Sanabria, B., de Nardo Vanzela, T., Miot, H. A., & Ramos, P. M. (2021). Adverse effects of low-dose oral minoxidil for androgenetic alopecia in 435 patients. Journal of the American Academy of Dermatology. 84(4). 1175-1178. Available at: https://doi.org/10.1016/j.jaad.2020.11.035 ↑3 Natarelli, N., Gahoonia, N., & Sivamani, R. K. (2023). Integrative and mechanistic approach to the hair growth cycle and hair loss. Journal of clinical medicine. 12(3). 893. Available at: https://doi.org/10.3390/jcm12030893 ↑4 Natarelli, N., Gahoonia, N., & Sivamani, R. K. (2023). Integrative and mechanistic approach to the hair growth cycle and hair loss. Journal of clinical medicine. 12(3). 893. Available at: https://doi.org/10.3390/jcm12030893 ↑5 Bergfeld, W. (2009). Diffuse hair loss: its triggers and management. Cleve Clin J Med. 76(6). 361-370. Available at: https://doi.org/10.3949/ccjm.76a.08080 ↑6 Pubchem (no date). Minoxidil (Compound). Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Minoxidil#section=2D-Structure (Accessed: June 2025 ↑7 Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. British journal of dermatology. 150(2). 186-194. Available at: https://doi.org/10.1111/j.1365-2133.2004.05785.x ↑8 Michelet, J. F., Commo, S., Billoni, N., Mahé, Y. F., & Bernard, B. A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. Journal of investigative dermatology. 108(2). 205-209. Available at: https://doi.org/10.1111/1523-1747.ep12334249 ↑9 Lachgar, Charveron, Gall, & Bonafe. (1998). Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. British Journal of Dermatology. 138(3). 407-411. Available at: https://doi.org/10.1046/j.1365-2133.1998.02115.x ↑10 Mori, O., & Uno, H. (1990). The effect of topical minoxidil on hair follicular cycles of rats. The Journal of dermatology. 17(5). 276-281. Available at: https://doi.org/10.1111/j.1346-8138.1990.tb01641.x ↑11, ↑12 Shatalebi, M. A., & Rafiei, Y. (2014). Preparation and evaluation of minoxidil foamable emu oil emulsion. Research in pharmaceutical sciences. 9(2). 123-133. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4311290/ ↑13 Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a randomized, single-blind controlled study. Journal of Pharmacy & Pharmaceutical Sciences. 24. 191-199. Available at: https://doi.org/10.18433/jpps31456 ↑14, ↑16, ↑17 Bi, L., Kan, H., Wang, J., Ding, Y., Huang, Y., Wang, C., Du, Y., Lu, C., Zhao, M., Sun, W. & Su, T. (2025). Whether the transient hair shedding phase exist after minoxidil treatment and does it predict treatment efficacy? A retrospective study in androgenetic alopecia patients. Journal of Dermatological Treatment. 36(1). 2480739. Available at: https://doi.org/10.1080/09546634.2025.2480739 ↑15 Bi, L., Kan, H., Wang, J., Ding, Y., Huang, Y., Wang, C., Du, Y., Lu, C., Zhao, M., Sun, W. & Su, T. (2025). Whether the transient hair shedding phase exist after minoxidil treatment and does it predict treatment efficacy? A retrospective study in androgenetic alopecia patients. Journal of Dermatological Treatment. 36(1). 2480739. Available at: https://doi.org/10.1080/09546634.2025.2480739 Caffeine & Hair Growth: A Scientific Deep-Dive
In recent years, there’s been an explosion of interest in caffeine shampoos / topicals and their potential to improve pattern hair loss (androgenic alopecia). The hope: that applying caffeine to our scalps might stimulate growth factors, improve blood flow, and maybe even reverse hair follicle miniaturization.
At first glance, caffeine use might look like a viable, natural intervention. But what does the research actually say?
In other words, does caffeine work? Is it a viable alternative to minoxidil? Is caffeine better if ingested, applied topically, or used as a shampoo? How much hair regrowth can we expect? And are there any longterm side effects?
This article dives in the science (and answers).
We’ll dispel a lot of common knowledge about caffeine’s efficacy for hair growth. We’ll also comb through the evidence, set realistic expectations, and reveal how to best use caffeine to maximize your chances of hair recovery.
Long-story short: caffeine isn’t a miracle cure. But it might not be completely useless, either.
Topical caffeine: highlights
- Effort. Low – daily topical application, shampoo use, or ingestion are all easy to implement
- Expectations. According to studies, hair improvements are typically observed by 6+ months
- Response rate: 75%
- Regrowth rate: 0-5%
- Cost. $10-25 per month
- Problems. Low-grade clinical evidence; studies measure outcomes like shedding rates and anagen:telogen ratios, not hair counts; lacking impressive before-after photosets; likely less effective than minoxidil; must be combined with other treatments or ingredients like azaleic acid for maximized benefit.
All-Natural Hair Supplement + Topical
The top natural ingredients for hair growth, all in one serum & supplement.
Take the next step in your hair growth journey with a world-class natural serum & supplement. Ingredients, doses, & concentrations built by science.

Key takeaways
Topical caffeine is clinically shown to reduce hair shedding rates and improve anagen:telogen ratios in men with androgenic alopecia. Unfortunately, it’s still unclear just how effective caffeine-based topicals and shampoos are for improving pattern hair loss.
Caffeine shampoos/topicals fall under an intervention umbrella of “low risk, low reward”. In other words, caffeine’s risk of significant side effects is minimal, as is the amount of hair growth it may initiate.
Having said that, not all caffeine is created equally. While topical caffeine products have been shown to improve shedding rates and anagen:telogen ratios, oral caffeine might actually increase hair loss in those who have insulin resistance or are hypothyroid.
Of all clinical studies on topical caffeine for pattern hair loss, the best results seem to occur when topical caffeine is combined with ingredients like azelaic acid or drugs like minoxidil.
In any case, a 0.2% caffeine dilution for topical solutions and a 1% caffeine dilution for shampoo formulations seem to be the best studied (and most promising), so look for brands that meet these criteria.
If you’re going to use caffeine as a potential hair loss intervention, please understand that this stimulant is only clinically tested on androgenic alopecia, and that it’s likely not effective as a standalone treatment.
More information on the science behind caffeine – its mechanisms, as well as the optimal delivery methods, dilution, and more for hair recovery – can be found below.
What is caffeine?
Caffeine is a stimulant derived from plants – namely, coffee and tea. It’s the most popular stimulant on the planet.
Caffeine structure
As a stimulant, caffeine has a variety of effects on the human body – from better focus to improvements in endurance. But interestingly, the magnitude of these effect often vary per person, and as a result of differences in our genetic constitution, food consumption, and even past caffeine exposure.
What popularized caffeine as a potential hair loss treatment?
In general, caffeine has been studied for its effects on:
- The cardiovascular system – i.e., increased heart rate and possible vasodilation and/or vasoconstriction
- Longevity – i.e., muscular development, cellular metabolism
- Hormones – i.e., cortisol and thyroid hormones (these may play a role in caffeine’s “energizing” effects)
And interestingly, vasodilation, cellular metabolism, cortisol, and thyroid hormones have all been studied as potential treatments to different hair loss disorders.
For instance, hair loss drugs like minoxidil improve hair growth by increasing vasodilation; thyroid drugs like levothyroxine help to improve hypothyroid-related hair loss by restoring thyroid functionality.
This begs the question: what sort of impact might caffeine have on our hair follicles?
Can caffeine – ingested orally or applied topically – mimic the mechanisms of hair loss interventions? And if so, are the effects of caffeine strong enough to actually improve hair loss outcomes?
These connective points are what prompted scientists to start studying caffeine as a potential hair loss intervention. And taking a deeper look, there is some mechanistic overlap in how this stimulant might improve hair loss outcomes.
Caffeine is one of the most popular stimulants. It’s well-studied in terms of its effects on vasodilation, cellular metabolism, and hormonal health. And interestingly, these research avenues have left scientists wondering if caffeine can also be reoriented as a hair loss solution.
How might caffeine help with hair regrowth?
It’s hard to say. On the one hand, caffeine does have some hair-promoting effects. On the other hand, caffeine also has some issues that may actually contribute to hair loss. All in all, the way it will effect you will boil down to (1) the dose and (2) the ingestion type (oral or topical), and (3) your genetic constitution.
Here are a few effects that caffeine has – both positive and negative – in regard to hair health.
1. Topical caffeine may improve vasodilation (good)
Caffeine’s effects on blood flow vary depending on the mode of ingestion (i.e., topical versus oral) and the actual tissue being measured. Just see this chart demonstrating how oral caffeine impacts blood flow across body tissues.
Caffeine’s location-dependent effects on blood flow
(source)
Interestingly, both topical caffeine and oral caffeine seem to improve blood flow in microcapillary networks – the blood vessel networks that supply our peripheral tissues (i.e., skin) – and the same blood vessel networks that help support the growth of our hair follicles.
This is because in vascular smooth muscle cells, caffeine acts as a phosphodiesterase inhibitor. In other words, caffeine helps to block the enzyme phosphodiesterase.
This enzyme helps inactivate a molecule called cyclic adenosine monophosphate – a biological messenger molecule that regulates vasolidation (i.e., blood flow) in smooth muscle cells. In the absence of phosphodiesterase, more cyclic adenosine monophosphate accumulates, thus expanding vasodilation in smooth muscle tissues.
This is also why phosphodiesterase inhibitors are often prescribed for a variety of blood flow-related health conditions – i.e., erectile dysfunction, hypertension, and even vascular disease. They all help promote blood flow.
Caffeine happens to be one of these phosphodiesterase inhibitors. And while it’s a weak inhibitor, it still has an effect on these capillary networks.
But, there’s one caveat here. While it’s true that a defining characteristic of androgenic alopecia (AGA) is reduced blood flow, it’s still debated whether blood flow is a cause or consequence to hair follicle miniaturization. So, we don’t yet know if caffeine’s vasodilation effects will really have any impact to our hair.
2. Caffeine may increase cellular metabolism (good)
Caffeine doesn’t just inhibit phosphodiesterase. It also inhibits adenosine receptors – a type of neural receptor that helps to regulate cellular metabolism and our own sense of “wakefulness”.
In the absence of caffeine, a molecule called adenosine normally binds to an adenosine receptor in our brain. When adenosine binds to an adenosine receptor, our brain’s neural activity begins to quiet. The end-result: we feel a bit sleepier.
Caffeine is an adenosine receptor antagonist. That means that when it’s ingested, caffeine blocks adenosine receptors so that adenosine cannot bind to them. This prevents the “quieting” of neural activity – and thus promotes longer periods of wakefulness.
Interestingly, there’s also evidence that caffeine’s inhibition of both phosphodiesterase and adenosine receptors may promote cellular metabolism. To put it more bluntly, caffeine ingestion might help to improve (1) energy utilization in the body, and (2) the mobilization of free fatty acids for energy usage.
This may have pro-hair effects, as many genes that are upregulating in balding scalp tissues tend to have an association with impaired cellular metabolism. But again, we just don’t know for sure.
3. High-dose oral caffeine may increase insulin resistance (bad)
Unfortunately, not all effects from caffeine are pro-hair. While improving cellular metabolism may help support the growth stage of our hair follicles, there are also consequences to the way in which caffeine improves cellular metabolism that may negatively impact our hair.
For instance, one study found that oral caffeine consumption decreased insulin sensitivity by 15% in healthy adults. That’s not good – especially for young men and women who are balding, as insulin resistance is almost always a commonly confounding factor in early-onset AGA.
Moreover, there’s also evidence that at high dosages, oral caffeine’s “liberation” of free fatty acids also promotes hyperglycemia and insulin resistance in peripheral tissues (i.e., our skin) – possibly as a result of increased stress hormones like cortisol. Which brings us to our second concern…
4. Oral caffeine increases cortisol levels, and may impair thyroid function and skin quality.
Evidence strongly implicates oral caffeine consumption and an increase in cortisol levels. Unfortunately, the hormone cortisol, when chronically elevated, can negatively impact hair-related bodily functions and in two major ways:
- It can lead to skin degradation around the follicle and disrupt the hair cycle
- It can reduce thyroid functionality, which can increase hair loss for those susceptible to hypothyroidism.
Another noteworthy mention is that coffee can also impair the absorption of thyroxine. So, if you are taking this medication for a thyroid disorder, it’s likely in your best interest to avoid consuming coffee around the same time. But, it’s unclear whether this effect is a result of the caffeine content or other compounds found in coffee.
Again, we don’t yet have any data correlating oral caffeine consumption to pattern hair loss. But these concerns are worth noting for anyone who’s balding and has had a history of hyperglycemia, insulin resistance, hypothyroidism, or adrenal dysregulation.
What about topical caffeine?
In contrast to high dose oral caffeine, it doesn’t seem like topical caffeine elicits the exact same anti-hair effects. Rather, topical caffeine (as a lotion or shampoo) might have some therapeutic benefit to our scalp hair.
For reference, in vitro studies in humans and in vivo studies in mice suggest that caffeine’s effects on (1) phosphodiesterase inhibition and (2) adenosine receptor binding probably will improve hair growth, and through a variety of means.
Specifically, topical caffeine might…
- Prolong the growth phase of the hair cycle (i.e., the anagen phase)
- Stimulate matrix keratinocyte proliferation – or encourage the growth of new hair fibers
- Increase IGF-1 – a signaling protein that helps regulate hair growth
- Inhibit apoptosis – or the “death” of cells responsible for regulating hair growth
- Promote blood flow – which is historically much lower in balding versus non-balding scalps
So, overall, it seems like mechanistic evidence supports at least the use of topical caffeine as a potential hair loss intervention. And this conclusion is why so many researchers have bothered studying caffeine lotions and shampoos for the improvement of AGA.
Caffeine is a (1) phosphodiesterase inhibitor and (2) an adenosine receptor antagonist. While its effects vary on a tissue-by-tissue basis, oral and topical caffeine seem to improve microcapillary networks in periphery tissues (where our hair follicles reside). Moreover, caffeine can help improve cellular metabolism by liberating free fatty acids for energy use. Improvements to both (1) vasodilation and (2) cellular metabolism should theoretically benefit our hair.
At the same time, oral caffeine seems to also increase insulin resistance in peripheral tissues. This is problematic – as reduced insulin sensitivity may interfere with the growth cycles of our hair follicles. Moreover, oral caffeine consumption can increase cortisol levels and decrease thyroid functionality – which may also negatively impact hair growth cycles.
Despite concerns of oral caffeine use for hair, evidence does support the use of topical caffeine for hair growth – at least from a mechanistic standpoint. In vitro research suggests that, in human hair follicles, topical caffeine helps to prolong anagen duration, increase IGF-1, inhibit cell death, and improve blood flow. While this doesn’t mean that these effects will translate in vivo, it does give credence to the idea that topical caffeine is worth testing as a hair loss intervention.
This all brings us to our next question: what does the clinical data say about topical caffeine and its use as a hair growth stimulant?
Is topical caffeine effective for hair loss?
This is harder to answer than it may seem.
At face-value, the answer is yes. This is because there are a lot of studies showing that caffeine in a topical or shampoo (or caffeine in conjunction with minoxidil and/or azelaic acid) can improve hair loss outcomes.
For instance, this recent literature review on topical caffeine dives into over a dozen clinical studies, many of which report:
- “…Statistically significantly better results” when used in conjunction with minoxidil versus minoxidil alone
- Higher patient satisfaction when used with minoxidil compared to minoxidil alone
- Decrease in hair loss after washing when using caffeine + minoxidil + azelaic acid, with similar results to a minoxidil solution after 36 weeks
- Decrease in hairs lost in a hair pull test after 6 months of using a caffeine shampoo (no placebo) and 4 months of using a caffeine-containing lotion.
Reading these conclusions, it’s no wonder why caffeine topical sales have spiked in the last few years.
However, taking a closer look, these studies might not be as encouraging as their conclusions imply. Here’s why.
Quality analysis: how do caffeine-hair loss studies stack up?
When it comes to research, not all peer-reviewed papers are created equally.
Some studies are published in predatory journals that circumvent the peer review process; others are published in low-ranking journals; others simply have major methodological concerns that draw the findings of those studies into question.
When it comes to the studies on caffeine and hair regrowth, it’s that last issue that’s most prevalent. That’s not to say that we should dismiss caffeine’s effects entirely. But, there are several concerns worth highlighting.
1. Methodological concerns
In the above literature review, most of the feature studies don’t measure hair count increases. Rather, they measure endpoints like patient self-satisfaction surveys, changes to anagen:telogen ratios, and a reduction in hair fall during “wash tests” or “tug tests”.
These measurements are difficult to standardize and are notoriously unreliable, meaning it can be hard to determine the true effectiveness of caffeine from any study designed this way.
In fact, it’s my belief that a lot of industry-funded research purposefully chooses these measurement endpoints because of their unreliability. For reference, these types of endpoints are why so many low-level laser therapy studies will report almost unbelievable hair improvement – i.e., “200% hair diameter increases” or “80% hair density improvements” in their clinical trials – while paradoxically, having no visual improvements to show subjects’ photographic assessments.
The bottom line: these measurement endpoints aren’t very strong, and some investigators who choose these endpoints may be doing so to deliberately skew caffeine’s perception of efficacy. But no matter what, the weaker the hair measurement endpoints, the less reliable the results.
2. Topical caffeine is rarely measured as a standalone treatment
Most of these studies measure topical caffeine alongside enhancer ingredients – like azelaic acid, minoxidil, or both – and not caffeine as a standalone treatment. This makes it hard to evaluate whether caffeine by itself is very effective.
In fact, there’s just one study that we could find that measured topical caffeine as a standalone treatment. Unfortunately, it measures topical caffeine versus minoxidil, not a placebo. That study’s takeaway? That topical caffeine is similarly effective to 5% minoxidil… at least when we compare weak measurement endpoints (see #1).
3. Conflicts of interest
Of all the clinical research done on caffeine and hair growth, most of it is industry-funded.
At face-value, this isn’t necessarily a problem. After all, a significant portion of hair loss studies comes from industry-funded research teams. Where there’s financial incentive for a treatment, there will be attempts at peer-reviewed research to prove efficacy.
Having said that, this does become a problem when the studies are typically designed with poor measurement outcomes – such that the odds of achieving “favorable” results increases dramatically. Nearly all of the topical caffeine studies on AGA have this very problem. Compile that with the issue of almost never measuring caffeine alone, and you have even more problems (see #1 and #2).
4. Manufacturers publish research on topical caffeine, but sell you shampoo-based caffeine
One of the most frustrating aspects of hair loss products are that manufacturers will publish a study showing their product demonstrating benefit, but then sell you a product that’s different from the one studied.
This happens all the time with LLLT devices, and it seemingly also happens with caffeine products for hair loss.
Case in point: Alpecin’s study on a topical caffeine solution. The findings showed that this topical did improve hair loss outcomes. But ironically, Alpecin doesn’t sell this topical; it sells a caffeine shampoo. Topicals are leave-in for hours, whereas shampoos may only come into contact with the scalp for 60 seconds. It was this issue in addition to #1-#3 that got Alpecin banned in certain countries from saying their “shampoos” could reduce hair loss.
It was a combination of these issues that led researchers in the above literature review to conclude that while caffeine might help improve aspects of our hair, there isn’t yet enough evidence to support most claims being made by manufacturers.
Topical caffeine does have clinical research supporting its use for hair loss. However, in literature reviews of the dozen or so studies on topical caffeine, concerns of endpoint measurements, lacking study as a standalone treatment, conflicts of interest, and discrepancies in what’s studied versus sold to consumers raises red flags as to caffeine being a truly viable long-term solution.
Despite all of this, there is evidence that topical caffeine might help our hair
Circling back to that literature review, there is accumulating evidence that caffeine can help reduce hair shedding from androgenic alopecia and even improve anagen:telogen ratios. It’s just that if we’re going to use it, we shouldn’t set our expectations at regrowth; we should set our expectations at a slowing of hair loss.
This leaves us with an interesting dilemma: if we want to leverage caffeine as a hair growth promoter, we need to do so in topical or shampoo form. And that means we need to know:
- What’s the best caffeine formulation: topical or shampoo?
- Is there a difference in regrowth reported between 0.2% and 1% caffeine dilutions?
- What’s the optimal frequency of application? Once a week, once a day, etc.?
- Does the effectiveness of caffeine wane over time – like minoxidil and other hair loss drugs?
Sifting through the literature review, there are studies that answer these questions. But again, they’re all subject to significant bias.
Even still, we can use these studies to guide some caffeine best practices – at least for those who want to make the investment and try it out.
Using caffeine for hair growth: best practices
The best caffeine formulation is likely within a shampoo or topical
Oral caffeine likely doesn’t accumulate in the scalp at a high enough degree to elicit adverse or beneficial effects. However, it may have peripheral action through increased cortisol levels, and this may indirectly impede hair growth for those with insulin resistance and/or hypothyroidism.
Knowing this, the safest and most effective way to use caffeine for hair growth is through topical means, either through some sort of lotion or shampoo.
Considering one study found that caffeine solutions penetrate the hair follicle after 2 minutes and peak at 2 hours, a leave-on caffeine solution may be optimal over a shampoo formulation.
The best caffeine dilution is 0.2% for topicals and 1% for shampoos (as far as we can tell)
But to be clear: the answer likely depends on whatever other ingredients are also in the topical (i.e., azelaic acid, minoxidil, etc.). Here’s why.
- This study, the only study measuring the efficacy of caffeine alone, found that a 0.2% caffeine topical (Alpecin) was just as effective as 5% minoxidil at improving anagen hair percentages.
- Problems: does not measure total hair count changes, thus improvements to anagen:telogen ratio can also be achieved by simply just shedding more telogen hairs for either treatment group; study only went for 6 months and results (i.e., improvements to anagen:telogen ratio for both caffeine topical and minoxidil) are within range for what we’d expect from seasonality
- Pazoki-Toroudi et al. (2013) assessed the combination of caffeine 1%, minoxidil 5%, and azelaic acid 1.5% versus minoxidil 5%. The combination therapy was more effective than minoxidil alone.
- Problems: does not measure caffeine alone, so we can’t really say.
- Bussoletti et al. (2010, 2011) found that a 1% caffeine shampoo alone and caffeine lotion alone both resulted in less hair lost to a pull test after 6 and 4 months, respectively.
- Problems: no concentration specified for the lotion; no control group used to assess true efficacy.
- Golpur et al. (2013) found that caffeine + 2.5% minoxidil was more effective than 2.5% minoxidil alone.
- Problems: no percentage of caffeine was indicated.
- Sisto et al. (2013) analyzed the effects of a caffeine-containing shampoo vs a caffeine-free shampoo and found that the active treatment was superior to the placebo.
- Problems: no concentration specified.
So, from what we can garner from the limited studies available, a 0.2% solution for topical application seems to be somewhat effective. However, keep in mind that this is only for a leave-on treatment, not a shampoo.
We don’t really have much information on optimal shampoo dilution. A 1% dilution was the only concentration reported for caffeine shampoo alone, but this treatment wasn’t compared against a placebo and, so, results aren’t super applicable. Even still, 1% seems to be comparably effective to minoxidil.
A 1% caffeine, minoxidil 5%, and 1.5% azelaic acid topical was considered more effective than minoxidil alone, but we can’t extrapolate this 1% dilution to a caffeine-only shampoo.
0.2% for topical solutions and 1% for shampoo formulations is all we can really extract from the current body of evidence, but it should be noted that these recommendations aren’t necessarily reliable given the minimal evidence.
What is the optimal frequency of use?
Like concentration, data on the frequency of use is also sparse. In the highest quality study, subjects used a 0.2% caffeine topical twice a day, every day. Another study instructed subjects to apply a caffeine lotion (unspecified concentration) once daily.
Other studies likely using shampoos most likely involved subjects using the treatment product however often they would normally shampoo their hair. So, it can be difficult to quantify just how often is optimal because use wasn’t standardized, as far as we can see.
With the knowledge we have, daily use of a topical seems to be optimal. Conversely, using a shampoo daily may be drying to the hair and the scalp, so it’s safe to say that using a caffeine-containing shampoo however often you would normally shampoo is probably best.
Does caffeine’s efficacy wane over time?
While no studies measure caffeine’s effectiveness for long enough to see if regrowth is sustained, we can assume – like nearly all topicals – that its hair-promoting effects will likely lessen over time.
The reasons why will be explained in a future article – one comparing the long-term outcomes of antiandrogenic versus non-antiandrogenic hair loss interventions. But the short answer is that we can liken any sort of topical formulation for hair loss – even minoxidil – as a bandaid that won’t necessarily fully address the underlying roots of the problem.
Summary
Topical caffeine may help to elongate the anagen phase of the hair cycle, improve anagen:telogen ratios, decrease hair shedding, and slow the progression of androgenic alopecia (AGA). But it’s by no means a miracle cure, and evidence so far suggests that this topical isn’t any better than 5% minoxidil.
The limited evidence we do have on topical caffeine is relatively biased, poorly designed, and has only been studied for androgenic alopecia. Until studies with better designs are published, it’s hard to say with certainty just how much of an effect topical caffeine will have on our hair.
Nevertheless, topical caffeine leave-on treatments have more quality evidence to support their use (as opposed to shampoos). 0.2% dilutions for topicals and 1% dilutions for shampoos seem to be the most promising. Moreover, the best way to utilize caffeine seems to be in conjunction with minoxidil and, possibly, azelaic acid.
Paradoxically, oral caffeine consumption may have an adverse effect on hair growth through increased cortisol release, increased hyperglycemia in periphery tissues, and potentially decreased thyroid functionality. For these reasons, anyone with a history of insulin resistance or hypothyroidism is probably better off avoiding oral caffeine altogether – particularly for hair health.
If you do decide to implement caffeine into your regrowth regimen, the first thing you’ll notice is how difficult it is to field the market. Almost all companies that have any merit in this space don’t quantify the caffeine dilution in their products. Considering most caffeine treatments are upwards of $35 USD, you may want to call around and see if their customer service representatives can give you more information.
Let us know if you have personal experience or testament in using (or excluding) caffeine. If you start using caffeine, report your progress here for others to see! Any questions or comments? Please reach out in the comments section.
The Ultimate Guide To Platelet-Rich Plasma Therapy (PRP)
In the last decade, thousands of dermatologists have started offering platelet-rich plasma (PRP) therapy as a treatment for hair loss. At first glance, PRP seems like an enticing therapy: a hands-free, drug-free approach to improve our hair thinning…
…but with a $1,000+ price tag, is the therapy worth it? Is PRP right for all hair loss sufferers? And if platelet-rich plasma therapy does work, how much hair can we expect to regrow?
This ultimate guide to platelet-rich plasma therapy uncovers the answers. Here we’ll reveal how platelet-rich plasma therapy works, how it compares to similar therapies, and what most dermatologists don’t tell you about their PRP “before-after” photos.
We’ll also reveal how hair regrowth from PRP depends largely on your form of hair loss, whether you combine PRP with other treatments, and the type of PRP your dermatologist provides (Acell, etc.).
If you’re considering PRP as a hair loss treatment, this guide will help you determine if the costs make sense for your situation and, if so, how to select the right provider.
PRP Therapy: Highlights
- Effort. Medium (requires a few dermatology appointments, but is otherwise a hands-free therapy)
- Expectations. According to studies, regrowth can occur in as little as 3 months.
- Response rate: 80%+
- Regrowth rate: this varies depending on if PRP is done alone or alongside other therapies.
- PRP (alone): 25-30%
- PRP + finasteride: +1%
- PRP + microneedling: +5%
- PRP + minoxidil: +5%
- PRP + microneedling + minoxidil: +5-10%
- PRP + ACell: no studies (yet), but anecdotes suggest better results than PRP alone
- PRP (alone): 25-30%
- Cost. $1,000-$4,000+
- Problems. Expensive; real-world results often don’t match those of studies; similar mechanisms targeted with massaging and microneedling; results vary wildly depending on the clinic; results contingent upon continued sessions
All-Natural Hair Supplement + Topical
The top natural ingredients for hair growth, all in one serum & supplement.
Take the next step in your hair growth journey with a world-class natural serum & supplement. Ingredients, doses, & concentrations built by science.

Key takeaways
PRP is effective for androgenic alopecia and alopecia areata, but it’s expensive and requires ongoing injections to maintain results. It works best as an adjunct treatment alongside other hair loss therapies, and while it has helped both men and women, evidence suggests that it’s less effective for females overall. If you’re going to try PRP, don’t just go to any clinic; rather, vet your cosmetic surgeon by asking them a list of questions we’ve suggested (below).
What is platelet-rich plasma therapy (PRP)?
Platelet-rich plasma therapy (PRP) is an injection-based therapy. It’s the injection of a modified version of our own blood into a tissue site – with the goal to accelerate healing, reduce scarring, and improve injury outcomes.
PRP has been used for dentistry, facial reconstruction surgery, orthopedics, sports injuries, acne scarring, and fat grafting. But in the last decade, it’s been given serious attention as a potential treatment for thinning hair.
How does PRP work?
PRP therapy is a multi-step process that involves drawing a sample of our blood, separating out its platelets, concentrating those platelets, and then re-injecting those platelets into a targeted location (like our scalp).
If you’re considering PRP, the procedure usually takes around an 30-90 minutes, and the process looks something like this:
- Obtain blood from the patient
- Centrifuge once – under a low speed – to separate out platelet-rich plasma from the blood
- Remove anything that isn’t plasma
- Centrifuge again
- Repeat step 3
- Mix the solution to make it uniform
Why focus on platelets?
Our blood volume contains roughly 55% plasma, 40-45% red blood cells, 6% platelets, and 1% white blood cells. Whenever our tissues incur a wound – for example, a paper cut – an inflammatory reaction begins, and our bodies will send blood to our injury site to initiate repair.
Interestingly, our platelets – which constitute just 6% of our blood volume – are responsible for a huge part of the entire repair process. Specifically, platelets do two things:
- Platelets help clot a wound to prevent excessive bleeding
- Platelets carry with them dozens of growth factors, all of which help to coordinate the entire healing process.
This begs the question: what if we could concentrate our platelets so that instead of sending only 6% of platelets to a wound tissue, we could send a much higher percentage? Would we see better injury outcomes? Would we see less scarring?
Well, this is exactly what platelet-rich plasma therapy does. In fact, recent advents in “centrifugation” – or the swirling, mixing, and separation of platelets from our blood – have enabled dermatologists to achieve blood platelet concentrations higher than 94%+. That’s a huge jump from the 6% typically carried within our normal blood volume.
And as of today, it seems like platelet concentrations do improve injury outcomes and scarring. Decades of studies show that, on average, if we concentrate high levels of plasma and send that plasma to an injury site, we can improve injuries, reduce scar tissue, and in doing so, maybe even regrow some hair.
How, exactly, does PRP improve hair loss?
There are several growth factors carried within plasma linked to hair growth, most notably:
- Platelet-derived growth factor
- Transforming growth factor
- Vascular endothelial growth factor
- Epidermal growth factor
- Fibroblast growth factor
- Connective tissue growth factor
- Insulin-like growth factor (IGF-1)
And when injected into balding scalp tissues, the arrival of these growth factors can do a few things:
- They reactivate dormant hair follicles and encourage them to reenter the anagen phase, thus increasing hair counts.
- They prevent hair follicles from entering the catagen phase or undergoing cell apoptosis (programmed cell death).
- They help to resolve longstanding inflammation and reduce scar tissue surrounding miniaturizing hair follicles, thereby increasing the hair thickness of miniaturized hairs.
- The promote blood vessel growth around the hair follicles to aid in hair regeneration.
Isn’t this similar to how massaging and microneedling work?
Yes. PRP’s mechanisms overlap with one of the ways by which massaging and microneedling improve hair loss: they both increase the number of growth factors in balding scalp regions. But, they do it in slightly different ways.
Massaging and microneedling first generate acute inflammation (i.e., micro-wounding), which then increases growth factors, which then helps to promote hair recovery. The order of operations is as follows:
Massaging / microneedling >> evokes micro-inflammation >> evokes platelets / growth factors >> decreases scarring proteins / increases angiogenesis >> reduces perifollicular fibrosis >> improves blood flow to miniaturizing hair follicles / increases follicle growth space >> increases hair growth
But there’s a key difference between PRP and these two therapies. With massaging and microneedling, you need to first evoke inflammation to increase growth factors to a wound site. With platelet-rich plasma, you essentially skip that first step, and instead, you simply inject platelets directly into the tissue of your choosing.
When it comes to balding scalp tissues, we can think of the mechanistic difference between these therapies as this:
Now, there is some wounding involved in PRP procedures. But that wounding / acute inflammation is a consequence to the injection of the platelets. In this way, PRP is sort of like a supercharged microneedling or massage session – only with many more platelets present.
Is platelet-rich plasma therapy effective?
This is a tricky question to answer.
If forced to give a one-word answer, then yes. Most studies on PRP show positive outcomes for hair loss. But if you’re going to invest thousands of dollars into the therapy, there are caveats of which you should be made aware.
The reality is that PRP’s effectiveness for regrowth depends on the study you reference and how you define the term, “effective”. Moreover, PRP efficacy varies greatly by:
- Hair loss type (i.e., androgenic alopecia, alopecia areata, etc.)
- Whether we combine PRP with other treatments (i.e., minoxidil, finasteride, microneedling)
- The type of PRP procedure (i.e., PRP alone, PRP + Acell, etc.).
We’ll cover all of this below. First, we’ll start with PRP’s issues. Then, we’ll dive into PRP’s benefits (and its effects on our hair).
Cons
Problem #1: most PRP studies have a high risk of bias
Most PRP studies are conducted by dermatologists who offer PRP procedures at their clinics. That creates an incentive to achieve positive results – because those positive results might encourage patients to do the procedure at their specific clinic.
However, this problem isn’t necessarily game-ending. In fact, nearly all hair loss research contains some level of bias. For instance, despite our efforts to control for bias in our own study on the massages, technically you could argue that because this site conducted it, our results are at risk of bias, too.
In any case, there are plenty of well-controlled studies on platelet-rich plasma and hair loss. We’ve filtered for these. But if you go digging through the literature and find a PRP study with crazy results, just know that if it wasn’t included in our analysis, there’s probably a good reason why.
Problem #2: most platelet-rich plasma studies aren’t standardized
Nearly every PRP study has a different patient profile (i.e., ages and hair loss severities), methodology (i.e., injection methods, rounds, treatment regions), trial duration (i.e., three months versus two years), and hair assessment method.
For instance, here are just a few ways PRP studies have measured hair loss “improvements” (ranked from worst to best).
- Hair tug tests (i.e., when an investigator yanks at a subject’s hair before and after treatment, then counts the number of hairs that fell out)
- Reduction in hair fall (i.e., hair shedding collected in the shower, by patients, pre- and post- treatment)
- Patient satisfaction scores (i.e., when a patient fills out a survey saying whether they’re happy with the treatment)
- Independent visual assessments (i.e., when a dermatologist eyeballs photos to give their input on hair improvements)
- Follical unit survival rates (for PRP + hair transplant studies) (i.e., how many transplanted hairs survived the transfer)
- Hair thickness (i.e., the change in the diameter of hair shafts in a specific scalp region)
- Terminal/vellus hair ratio (i.e., the number of thick and healthy versus thin and wispy hairs in a specific region)
- Hair counts (i.e., when a region is marked – usually with a temporary tattoo – to count hairs before and after treatment)
To be fair, this isn’t just a problem with PRP; it’s a problem with all of hair loss research. It’s why literature reviews have a hard time drawing conclusions about most treatments – because there are rarely apples-to-apples comparisons.
But again, we’ve sorted through all the PRP studies we could find to standardize the research (as best we can) and give you ballpark assessments of regrowth rates (i.e., increases to hair count in balding regions).
Problem #3: platelet-rich plasma therapy likely requires ongoing treatments
When you look into the research on minoxidil or finasteride, studies show that within 3-12 months of quitting either drug, your hair loss will return to what it was prior to the intervention. So, how long will results hold for platelet-rich plasma after quitting?
Well, it’s unclear how long results will last after you stop doing PRP treatments, but evidence suggests that a percentage of people will start seeing their hair return to baseline after a year.
Out of all PRP studies, the one with the longest follow-up period (two years) included 20 patients. Interestingly, four of them experienced a relapse in hair loss one-year post-PRP. In fact, their androgenic alopecia progressed beyond their pre-trial hair counts by the 16-month mark. This suggests that for about 20% of people, PRP’s effects start to wane 12-18 months after the treatment.
This wouldn’t be such an issue if the procedure were cheap, but it isn’t: several therapeutic rounds of PRP cost $1,000-$4,000+. So, if you’re going to give this procedure a try, make sure you’re financially comfortable with the investment.
Problem #4: PRP might not work for every person with hair loss
Out of all the research on PRP, only two studies found that PRP was an ineffective treatment option. One study was on females with androgenic alopecia. The other study was on men with advanced androgenic alopecia (Norwood gradients 4+).
If you were to ask me why the first study failed to produce results, I would say that it was probably because (like most women with hair loss) the females in that study likely had other undiagnosed hair loss types (like telogen effluvium / hair loss related to a chronic condition).
And as far as the study on men with advanced androgenic alopecia (AGA) – we need to keep in mind that the investigation team only did two rounds of PRP injections. For what it’s worth, in all of the PRP studies which saw improvement, a minimum of three PRP injection rounds were performed. So, it’s likely that either this study didn’t do enough injections to see results, or that men with advanced AGA needed several more injections before PRP begins to repeat significant benefit.
Problem #5: most PRP studies don’t measure PRP by itself
In fact, the overwhelming majority of studies measure PRP alongside other hair loss treatments – like minoxidil, finasteride, or even a hair transplant. So, it’s important to delineate between the studies you reference when evaluating whether PRP is right for you (we’ve done this below).
This also brings up another problem: dermatologists showcasing their PRP results online often don’t tell you something important: that they’re showing you PRP results alongside drugs like minoxidil and finasteride.
This is incredibly disingenuous, and I suggest that if you’re shopping around for a PRP clinic, you call ahead and ask the doctor if the results they showcase on their website are from PRP alone. If they are, great. If they’re not, but they’re labeled to make it seem as such, then that means these dermatologists are intentionally misleading prospective patients, and they should lose your business (and their license to practice).
Pros
While we might’ve just painted a problematic picture for PRP, this isn’t the whole story. In fact, PRP is an incredibly effective treatment for hair loss under the right circumstances. This is all covered below.
Alopecia areata
PRP has shown great promise for the hair loss disorder alopecia areata. This is an autoimmune condition that leads to hair loss in patchy spots throughout the head. In some cases, it can advance to complete baldness (alopecia universalis).
In fact, studies show that PRP is very effective in treating at least 70% of alopecia areata cases. Here are some of the really promising photos (source):
Androgenic alopecia
PRP alone (no other treatments)
When looking at PRP as a standalone treatment, most studies measuring hair counts suggest that the average patient will regrow 15 hairs per square centimeter (i.e., half the size of a penny). That’s about a 25-30% regrowth rate at 3-6 month follow-ups.
Quantitatively, that’s pretty impressive. For a benchmark, most studies on finasteride show just a 10% increase in hair count over two years.
Across studies, some of the less quantitative outcomes for PRP alone (at least at the 3-6 month mark) are:
- Patients report about a 75% increase in hair quality and thickness, and a decrease in the rate of hair loss.
- Patients usually perform better on the hair pull test, meaning that when they pull on a clump of the patient’s hair, fewer hairs came out compared to control groups.
- Patients report somewhat high satisfaction with the procedure at month three of follow up
Finally, a common trend mentioned among researchers is that PRP treatment seems to be more effective for patients with less severe forms of AGA. So, if you’re in the early stages of hair loss, PRP might be a great option for you.
PRP + hair transplantation
In one study examining hair transplantation, two areas with 50 grafts each(not a lot to measure, especially for hair transplantation) were compared with or without PRP injections. The area with PRP had, on average, 46.75 units that survived compared to the non-PRP which had, on average, 41 units that survived.
While this isn’t that drastic of an increase – we have to keep in mind that transplant procedures are incredibly costly… and that means that every hair follicle unit counts.
So, if you’re considering a hair transplant, you’ve spent the finances to secure a skilled surgeon, and you still have some extra spending money you’d like to throw into improving your results – do it alongside PRP. Chances are your hair transplant survival rates will improve, as will your overall hair count.
PRP + microneedling
In this study, 30 male participants received 6 PRP injections following microneedling sessions. At the six month follow-up, the average patient had a hair density increase of about 30%. This study also noted that the most significant improvement was seen in patients with less severe AGA.
PRP + minoxidil or PRP + finasteride
In this study comparing the efficacy of PRP + minoxidil and PRP + finasteride, while both outcomes were deemed effective, the PRP + minoxidil treatment actually achieved significantly better results than the PRP + finasteride group. In fact, the PRP + minoxidil group showed five-fold better hair increases versus the PRP + finasteride group.
Now, you might read these results and think that makes no sense. Finasteride is clinically more effective than minoxidil. So, why would PRP + minoxidil outperform PRP + finasteride?
Well, the devil is in the details. For one, the sample size of each PRP subgroup was less than 15 people. So, it’s possible these differences might’ve been due to statistical noise which would’ve canceled itself out with subgroups of 150+ people.
And secondly, while a five-fold improvement might sound drastic, we’re actually dealing with the law of small numbers here. Yes, PRP + finasteride saw an additional hair count lift of 1% versus 5% for the PRP + minoxidil group. And yes, that is technically a five-fold improvement. But in all reality, that’s just a few percentage points better.
PRP + minoxidil + microneedling
In this study comparing PRP + minoxidil + microneedling versus minoxidil alone, the earlier treatment proved to be much more effective than the latter, although exact numbers were not given in this study to show this. However, we can assume that PRP + minoxidil + microneedling is probably better than PRP + minoxidil, and that PRP + minoxidil is probably better than PRP alone.
PRP + Acell
PRP + Acell is a relatively new procedure that a lot of PRP practitioners are offering now. Acell is a protein matrix derived from pig bladder (you read that right) that creates a “scaffold” for new hairs. Acell essentially offers a platform by which all of our growth factors (and hair) can cling to. It also helps to stimulate stem cell activity.
[Note: since ACell is made from pig, people allergic to pig products should notify their physicians about their allergy prior to the treatment.]
There doesn’t seem to be any studies measuring the results of PRP + Acell compared to PRP alone, so it’s hard to objectively say whether it increases the effectiveness of PRP.
Are there any risks to PRP?
At least so far, there haven’t been any severe risks reported. However, some milder symptoms have been noted during and shortly after the procedure:
- Headaches
- Pain
- Itching
- Drowsiness
- Infection (rarer)
- Scarring or calcification of the injection site (for those with a propensity toward keloid scarring) (rarer)
- Blood vessel or nerve injury (rarer)
Who’s a good candidate for PRP? Who’s a bad candidate?
Androgenic alopecia treatments vary depending on your (1) finances, (2) willingness to invest time into a therapy, and (3) comfortability with FDA-approved drugs. Compared to some of the other AGA specific treatments, like standardized scalp massages and microneedling, PRP is a pricey option. At the same time, PRP is a lot less time consuming than microneedling or massaging because you may only go into a clinic for a handful of injection rounds before you start seeing results.
You are a great candidate for PRP if you…
- Are comfortable with the financial investment and ongoing treatments
- Want to stack PRP alongside other treatments in your hair loss regimen – like microneedling, massaging, minoxidil, or finasteride
- Are going to get a hair transplant and want to optimize your outcome of the procedure
- Have early-stage to mild AGA (and relatively small areas of hair loss)
- Have alopecia areata
- Are a poor candidate / do not want to try traditional hair loss medications
- Are physically healthy in general
You are not a great candidate for PRP if you…
- Do not want to spend thousands of dollars on the procedure
- Have advanced AGA and aren’t willing to commit to several rounds (and months-to-years) of injections
- Are a female with androgenic alopecia alongside conditions like hypothyroidism (treat that first!)
- Do not want to keep reinvesting money into a procedure that might not prove effective after a year.
- Have a bleeding disorder with clotting factor deficiencies or low platelet counts
- Use blood thinners
- Have a liver disorder
- Have a recent cancer history or sepsis (blood infection)
- Are a heavy smoker, alcoholic, or illicit drug user
- Have low blood pressure or chronic user of steroids
Do PRP study results hold true in the real world?
When evaluating any hair loss therapy, it’s important to note that, sometimes, study results don’t match up to real-world results. On hair loss blogs and forums, there’s sometimes an inkling that this might be the case with PRP.
For starters, while there are positive patient stories with PRP, there are also many anecdotes of patients who tried PRP without success. This video is a perfect example. And if you dig deeper, you’ll probably find more negative than positive anecdotes.
This can be confusing – as most of the literature tends to describe PRP as seemingly beneficial. And even more troublesome, it’s also worth noting that I’ve spoken with dozens of readers who’ve tried PRP… and most of them have also reported negligible improvements.
This begs the question: is PRP that effective? And regardless of the answer, why might there be a discrepancy between studies’ results and patient reports?
What could explain the discrepancies between PRP studies and real-world results?
For starters, it’s actually unclear if there are clinical versus real-world discrepancies for PRP. For instance, it’s possible that PRP treatments might just suffer from the “Yelp effect”. This is when someone with a negative experience is far more likely to leave a public review versus someone with a positive experience.
So, PRP might just be one of those treatments that have collected negative reviews over a period of years – much like finasteride and its reports of sexual side effects.
Secondly (and this is the more important point to make), clinical research does not always depict reality. For instance, while a 25-40% increase in hair count from PRP looks great on paper, it doesn’t always translate to cosmetic results.
This tends to be a problem with even the “best” FDA-approved treatments. Just take a look at these five men who did combination treatments of minoxidil, finasteride, laser combs, and even hair transplants – and their results after one year.
I’ll save you the suspense: their final “after” photos are darkened to obscure just how minimal their hair changes are. And, for the hundreds (to thousands) of dollars each of the men spent, their hair seems more-or-less cosmetically unchanged.
(Note: that video is just one of the reasons why, for most AGA sufferers, I recommend approaches like massaging / microneedling as a baseline for any regimen. Not only do these therapies enhance other hair loss treatments, but also without their inclusion, you’re statistically likely to see no cosmetic improvements from your other treatments).
Thirdly, we just learned that without follow-up sessions, PRP results will fade for 20% of patients starting 12-18 months after their last round of injections. So, if you’re reading a review of someone who got PRP once four years ago and never saw results (or is experiencing continued hair loss) – you’ll know that it’s probably because they didn’t do enough injection rounds and they didn’t keep up with the therapy.
In any case, it’s worth noting that of the readers with whom I’ve communicated, the ones who tried PRP + Acell all reported positive results. While there aren’t yet studies validating this combination therapy, it seems to be the most promising from an anecdotal standpoint (if that means anything to you).
How to choose the right PRP practitioner
If you’re going to invest $1,000+ into platelet-rich plasma, don’t give your money to the first PRP clinic you find.
Instead, find a few clinics near you that offer the procedure and make sure they have a proven track record. That means they should have a website with PRP before-after photos.
Make a list of these clinics. Then, call each clinic and ask if their online photos are of PRP alone or of PRP + finasteride / minoxidil.
If the photos are of PRP alone, great! If they’re of PRP + multi-therapies but advertised to represent only PRP… then hang up, cross them off your list, and consider reporting them the clinic to the Better Business Bureau.
Doing this should eliminate 60-70% of providers.
Next, call the remaining providers and ask about their PRP techniques. You’ll want to find a clinic that offers 1-4+ months between injection rounds. If someone offers you a package of 10 PRP sessions spaced out as one injection round per week, that goes against the literature’s recommendations (and our understanding of wound-healing timelines) – and you should probably find another provider.
Moreover, the actual PRP product that a clinic uses will vary by volume, number of injection rounds, color, platelet count, leukocyte count, and protein content. The best PRP providers will offer double-spin centrifugation preparation with an activator like thrombin or calcium chloride. While some evidence suggests that “platelet activators” are not necessary, they also don’t seem to hurt the procedure. In any case, it’ll be good to speak with your provider about all of this – so you can get a feel of whether they can actually answer these questions. If they can’t, then cross them off your list.
Lastly, ask your remaining clinics if they offer PRP + Acell. If they do (and the costs aren’t prohibitive), then this might be a better option versus PRP alone. If they don’t, it’s not the end of the world – and chances are you’ll still see benefit from the procedure.
Following this process should leave you a few great PRP clinic options. And, as long as you’re willing to commit to injections every 4-6 months, you should see a considerable lift in hair count… especially if you’re combining platelet-rich plasma with other therapies.
Summary
Platelet-rich plasma therapy is effective for both androgenic alopecia and alopecia areata, but the procedure is cost-prohibitive and requires repeated clinic visits to see sustained results. These factors are big turnoffs for most hair loss sufferers considering PRP as a treatment option.
PRP works best as an adjunct treatment alongside other hair loss therapies. So, don’t just try PRP as the only thing to help your hair. We recommend combining it with massaging and/or microneedling, and (if you’re comfortable), FDA-approved drugs (especially minoxidil) to maximize results. And, if you can, try to find a clinic that provides PRP + Acell.
While PRP can help both male and female hair loss sufferers, preliminary evidence suggests that it’s just not as effective for females. However, this might be because of the added complexities of female AGA – and the fact that women are so frequently misdiagnosed with AGA but instead actually have hair shedding disorders related to underlying chronic conditions.
Be careful about clinic selection for PRP. For instance, the photos you’ll see on most dermatology websites offering PRP are from patients doing PRP + finasteride / minoxidil (rather than PRP alone). The equivalent would be if I advertised a before-after photo of someone doing massaging + finasteride, but decided to position the photo as if the results were only from massaging. It’s just bad business. So, be aware of this. Call all clinics to confirm the regimens of their highlighted patients.
If you’re seeing improvements from PRP, chances are you’ve already navigated through this mess to find a good provider. So, steer the course and keep us posted with your progress!
Saw palmetto for hair growth: does it stack up to finasteride?
Saw palmetto is often touted as nature’s finasteride. This herbal extract reduces levels of the hormone dihydrotestosterone (DHT). For this reason, it’s clinically demonstrated to improve hair growth in men with androgenic alopecia (AGA).
But is saw palmetto as effective as finasteride?
Not quite. In fact, a closer look into the research on saw palmetto reveals that taking this supplement also comes with risks.
That’s not to say that saw palmetto is ineffective. But if you’re going to opt for saw palmetto over finasteride, you’re going to want to weigh its benefits and risks. Specifically, you’re going to want to know how saw palmetto compares to finasteride in terms of its (1) ability to reduce DHT, (2) ability to regrow hair, and (3) reported side effects.
This Quick Win dives into the details (and answers). The bottom line: saw palmetto isn’t as powerful as finasteride, but it also comes with some upsides that may make it a better option, at leaast for certain hair loss sufferers.
Note: Quick Wins are short articles focused on answering one question about hair loss. Given their specificity, these articles are written in a more scientific tone. If you’re new to hair loss research, start with our long-form articles.
All-Natural Hair Supplement + Topical
The top natural ingredients for hair growth, all in one serum & supplement.
Take the next step in your hair growth journey with a world-class natural serum & supplement. Ingredients, doses, & concentrations built by science.

What is saw palmetto?
Saw palmetto is a palm plant native to warm humid climates (Florida).
Saw palmetto plant
Thirty years ago, researchers discovered that certain polyphenol and lipid extracts of saw palmetto could reduce the activity of type II 5-alpha reductase – an enzyme our bodies use to make the hormone dihydrotestosterone (DHT).
DHT is the main hormone implicated in pattern hair loss, also called androgenic alopecia (AGA). And interestingly, the popular hair loss drug finasteride (Propecia®) reduces that same enzyme – type II 5-alpha reductase – to lower DHT levels and improve pattern hair loss outcomes in ~80% of men trying the drug.
Unfortunately, finasteride use is also associated with sexual side effects. This scares a lot of men away from trying the drug. It also leads many of them to wonder…
“If I use saw palmetto instead of finasteride, can I still regrow hair while also reducing my risk of side effects? After all, saw palmetto is natural, and natural often means safer.”
For starters, anything branded as “natural” isn’t always safer. Many natural supplements sold through Amazon and tested by third parties have been found to contain dangerous levels of heavy metals. Moreover, many popular natural extracts – i.e., green tea extract – have been associated with hepatic failure due to the metabolic demands these extracts can place on our livers. (For more information, see my master class).
In any case, if we’re to answer the question, “Is saw palmetto as effective as finasteride?”, we’ll need to evaluate saw palmetto versus finasteride in terms of its (1) expected hair regrowth, and (2) risk of side effects.
The rest of this Quick Win does just this.
Saw palmetto vs finasteride: hair regrowth
“Hair regrowth” is a non-specific term, and most clinical studies on pattern hair loss define this term differently:
- Reductions to hair shedding
- Improvements to anagen:telogen ratios
- Increases in non-vellus hair counts
- Global photography assessments
…the list goes on.
That’s why, whenever we’re diving in studies on hair loss, we standardize the term “hair regrowth” into two categories:
- Response Rate. This is the percentage of people receiving treatment who see a slowing, stopping, or reversal in their hair loss. After all, simply slowing down the progression of AGA is a win for most hair loss sufferers.
- Regrowth Rate. This is the percent change in terminal (i.e., non-vellus) hair counts before and after treatment. We use this metric because it’s best out there in terms of translation to visual improvements. Increases to vellus hair counts (i.e., small, wispy, white hairs) just don’t show up in photographs, so we don’t count those.
So, if we summarize the data on saw palmetto versus finasteride, how do these two interventions compare? (1) (2)
Saw Palmetto (320mg)
Finasteride (1mg)
Response Rate 60%; dependent on the dose and delivery (supplement or topical) 80-90% Regrowth Rate 0-10%; potentially higher if used alongside other therapies 10%, alongside thickening of miniaturizing hair The key takeaway: compared to finasteride, saw palmetto has a lower response rate and regrowth rate. And when we look at the individual studies that constitute these aggregated estimations, things look a little bleak.
To date, there’s only one clinical study directly comparing saw palmetto versus finasteride for the treatment of androgenic alopecia. The investigation team randomized 100 men with androgenic alopecia into two groups. They gave one group 320mg of saw palmetto and the other group 1mg of finasteride – every day, for two years. (3)
The results? After 24 months, 68% of finasteride users saw hair regrowth, while only 38% of men taking saw palmetto saw hair regrowth. Moreover, investigators noted that in the saw palmetto group, hair regrowth only occurred in the crown (i.e., vertex), and that the magnitude of regrowth was significantly less than finasteride.
In other words, saw palmetto achieves half the response rate of finasteride, and that if regrowth does occur, it’s not nearly as impressive as what finasteride achieves.
Having said that, it’s not all bad news. That same study also demonstrated that 45/50 men in the saw palmetto group saw a stop in hair loss over the two years that they took it. So, in this one study, saw palmetto showed a response rate of 90%.
This means it’s not a complete stretch to say that most men taking 320mg of saw palmetto daily should see an improvement in their pattern hair loss. It’s just that this improvement won’t be anywhere near on-par what is achievable with finasteride.
Why isn’t saw palmetto as effective as finasteride?
This likely has to do with the ways in which saw palmetto reduces DHT, and the amount of DHT that saw palmetto reduces.
Finasteride is a synthetic azosteroid. It reduces DHT by competing with (and binding to) a coenzyme that our bodies use to make type II 5-alpha reductase – the enzyme that converts free testosterone into DHT.
Conversely, saw palmetto competitively and non-competitively inhibiting type II 5-alpha reductase, reducing the binding of DHT to androgen receptors, and increasing the conversion of DHT to a weaker metabolite called androstanediol (4).
The long-story short is that these factors, along with differences in the half lives of both finasteride and saw palmetto, lead to differences in their abilities to lower DHT.
For reference, see this graph on the DHT-reducing capabilities of both saw palmetto and finasteride, as organized by different tissue sites.
The bottom line: saw palmetto is about half as effective as finasteride because it just doesn’t reduce as much DHT.
What about side effects?
At this point, we’ve really only evaluated half of the question: is saw palmetto as effective as finasteride?
In terms of response rates and regrowth rates, the answer is no. But can saw palmetto make up for its lower efficacy by being a safer long-term supplement for hair loss sufferers?
Maybe.
Saw palmetto: risk of adverse events
Long-term clinical studies show that saw palmetto’s overall rate of side effects is just 2%. Moreover, if side effects do occur, they’re relegated more so to gastrointestinal distress than to sexual dysfunction. Even better, many studies on saw palmetto show no change in libido; some studies show improvement to sexual health (at least for men with enlarged prostates). (5) (6) (7)
Finasteride: risk of adverse events
The “Yelp effect” is a phenomenon where patrons of a business are far more likely to leave a review if their experience was negative rather than positive. Finasteride is a drug that suffers from the Yelp effect, meaning that its sexual side effects are often overstated and amplified online.
Having said that, side effects do occur. Moreover, the self-assessment questionnaires filled out by participants in large-scale clinical trials for finasteride were worded in such a way where under-reporting certain side effects were more likely than not (but that’s for another article).
In any case, finasteride use does come with a heightened risk of side effects. Depending on which study you cite, between 1% and 25% of finasteride users will report issues ranging from brain fog to depression to sexual dysfunction. (8) (9) Moreover, when side effects are reported, they seem to be of higher magnitude versus saw palmetto.
The bottom line:
- Finasteride’s side effects are likely overstated online and understated in the literature
- Compared to saw palmetto, finasteride likely leads to side effects that are (1) of higher incidence, and (2) more severe
Saw palmetto or finasteride: which one is right for you?
It depends on your risk tolerance for side effects, and whether you plan on combining saw palmetto with other treatments, therapies, or procedures to make up for its lower response rates and regrowth rates.
There’s evidence that supplemental + topical saw palmetto, alongside other ingredients, might lead to better hair loss outcomes than just supplemental saw palmetto. Moreover, combining saw palmetto with massaging, microneedling, platelet-rich plasma therapy, or other interventions might help mitigate its lower efficacy.
Having said that, making these choices will depend entirely on someone’s needs, preferences, and unique hair loss situation.
Anything else to know?
Unlike finasteride, saw palmetto isn’t standardized. Serenoa repens growing conditions, extraction methods, and manufacturing practices can all impact the composition, bioavailability, and absorption of each saw palmetto supplement. In fact, these differences might explain the variances in response rates and regrowth rates seen across saw palmetto studies.
So, if you’re looking for a more comprehensive guide on how to use saw palmetto – including recommendations for dosages, extraction practices, and combinations therapies – see our saw palmetto ultimate guide here.
The bottom line
In order to evaluate whether saw palmetto is as effective as finasteride, we need to understand how the supplement compares in terms of (1) response rates, (2) regrowth rates, and (3) risk of side effects.
Saw palmetto isn’t as effective as finasteride in terms of its response rates or regrowth rates, but it also seems to cause fewer (and less severe) side effects. Because of this, making the choice to use saw palmetto over finasteride depends entirely on someone’s risk tolerance for side effects, as well as whether they plan on combining the supplement with other treatments, therapies, or procedures to make up for its less-impressive efficacy.
Saw Palmetto (320 mg)
Finasteride (1 mg)
Response Rate 60%; dependent on the dose and delivery (supplement or topical) 80-90% Regrowth Rate 0-10%; potentially higher if used alongside other therapies 10%, alongside thickening of miniaturizing hair Side Effects 2%; more relegated to gastrointestinal distress than sexual side effects 1-25%; partly psychosomatic, but more severe than saw palmetto Otherwise, if you have any questions, please feel free to leave them in the comments section.
References
- https://perfecthairhealth.com/saw-palmetto-for-hair-growth/
- https://my.perfecthairhealth.com/courses/finasteride/
- Rossi A, Mari E, Scarno M, et al. Comparitive effectiveness of finasteride vs Serenoa repensin male androgenetic alopecia: a two-year study. Int J Immunopathol Pharmacol. 2012;25:1167–73.
- Suzuki, M., Ito, Y., Fujino, T. et al.Pharmacological effects of saw palmetto extract in the lower urinary tract. Acta Pharmacol Sin 30, 271–281 (2009) doi:10.1038/aps.2009.1
- McPartland J, Pruitt P. Benign prostatic hyperplasia treated with saw palmetto: a literature search and an experimental case study. J Am Osteopath Assoc2000;100(2):89–96.
- Carraro, J.‐C., Raynaud, J.‐P., Koch, G., Chisholm, G.D., Di Silverio, F., Teillac, P., Da Silva, F.C., Cauquil, J., Chopin, D.K., Hamdy, F.C., Hanus, M., Hauri, D., Kalinteris, A., Marencak, J., Perier, A. and Perrin, P. (1996), Comparison of phytotherapy (Permixon®) with finasteride in the treatment of benign prostate hyperplasia: A randomized international study of 1,098 patients. Prostate, 29: 231-240. doi:1002/(SICI)1097-0045(199610)29:4<231::AID-PROS4>3.0.CO;2-E
- Sinescu I, Geavlete P, Multescu R, Gangu C, Miclea F, Coman I, Ioiart I, Ambert V, Constantin T, Petrut B, Feciche B: Long-Term Efficacy of Serenoa repens Treatment in Patients with Mild and Moderate Symptomatic Benign Prostatic Hyperplasia. Urol Int 2011;86:284-289. doi: 10.1159/000322645
- https://www.rxlist.com/propecia-drug.htm#side_effects
- http://www.drproctor.com/propecia/propecia.pdf
Latanoprost is a medication that was originally developed to treat glaucoma. Over the last two decades, research groups have also started testing it as a treatment for hair loss – including alopecia areata, androgenic alopecia, and telogen effluvium.
In this ultimate guide, we’ll explore the evidence on latanoprost, dive into the mechanisms and clinical data regarding hair growth, and identify who might be a good candidate for this experimental intervention. We’ll also specify which formulations, dilutions, and usage frequencies are reaping the best results in terms of both efficacy and safety.
Finally, we’ll uncover critical caveats with its use – including lesser-discussed (but significant) safety risks – and where to get latanoprost (should you choose to incorporate it into your regrowth regimen).
Latanoprost: Key Takeaways
- Drug. Latanoprost is a drug that changes the activity of substances called prostaglandins. It was originally used to treat glaucoma, but after patients began reporting thicker eyelash hairs following its use, it has since been repurposed as a topical and tested on hair loss sufferers.
- Clinical data. Two clinical studies testing topical latanoprost for androgenic alopecia suggest that 0.1 mL x 0.1% latanoprost and 1-2 mL x 0.005% latanoprost improve hair counts, with visual improvements occurring in 30-50% of people over 6-8 months. While both formulations equate to ~0.1 mg of latanoprost exposure daily, preliminary data suggest that lower concentrations (i.e., 0.005%) are much more tolerable for patients, at least in terms of scalp irritation. For alopecia areata of the eyelashes, 3 clinical studies suggest that 0.005% latanoprost has no effect. For alopecia areata of the scalp, one clinical study suggests that 0.005% latanoprost may improve hair growth in ~25% of patients, but that treatments such as 0.05% betamethasone diproprionate reap superior efficacy.
- Safety. Clinical data on topical latanoprost indicate a strong safety profile when used at 1-2 mL x 0.005% to 0.01% daily. At higher concentrations and lower volumes (i.e., 0.1 mL x 0.1% latanoprost), one study found that 50% of patients reported symptoms of scalp irritation – suggesting that these formulations may evoke skin sensitivities in a relatively large number of people. Finally, none of the clinical studies on topical latanoprost for hair growth ran longer than 6-8 month. This is a problem, because long-term safety data are not yet established despite well-defined long-term risks, like pigmentation changes to the skin, eyes, and hair. Long-term clinical studies on latanoprost for glaucoma show that up to 10% of patients experience a darkening of their irises, with the effect beginning after one year of treatment. For these reasons, we have significant reservations about the long-term viability of latanoprost when used on the scalp – particularly for people who have lighter colors of skin / hair and who would prefer to not experience any hypopigmentation.
- Best practices. While topical latanoprost is a popular treatment amongst dermatologists and certain online hair loss circles, we have significant reservations about its long-term viability – mainly because of the unknown risks (but high potential) for hypopigmentation in the skin, hair, and (perhaps) eyes – depending on systemic leakage. If you decide to try topical latanoprost, it seems to be most appropriate for those with androgenic alopecia, and at dilutions of 1-2 mL x 0.005% latanoprost daily.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is latanoprost?
Latanoprost is a drug developed to treat disorders of the eye – specifically, glaucoma. Originally formulated for delivery as eye drops, some users began reporting the elongation and/or regrowth of eyelash hairs.[1]https://jamanetwork.com/journals/jamaophthalmology/fullarticle/413134 Resultantly, researchers started investigating whether latanoprost – when formulated as a topical – might also enhance hair regrowth in other areas of the body, such as the scalp.
How does latanoprost work?
Latanoprost modulates levels of prostaglandin F2 – a substance our bodies produce that is derived from essential fatty acids (i.e., omega 6 fatty acids). More specifically, latanoprost stimulates the activity of the prostaglandin F2 alpha receptor. This allows for prostaglandin F2 levels to increase in cell sites, and when this occurs in eye tissues, it can reduce (or alleviate) ocular pressure and thereby improve cases of glaucoma.[2]https://www.ncbi.nlm.nih.gov/books/NBK540978/
How might latanoprost regrow hair?
There’s evidence that the eyelash hair regrowth reported by many latanoprost users isn’t just happenstance. For instance, as prostaglandin F2 activity increases…
- Microvascular networks tend to widen, thereby improving blood, oxygen, and nutrient transport to hair follicles affected by hair loss.
- Mitosis (i.e., cellular division and proliferation) increases. When more hair follicle related cells divide, more cells can contribute to hair follicle growth overall. [3]https://pubmed.ncbi.nlm.nih.gov/15854125/
- Prostaglandin D2 activity may simultaneously decrease – because precursor molecules for prostaglandins start shifting toward prostaglandin F2 alpha production and away from prostaglandin D2 production.[4]https://www.frontiersin.org/articles/10.3389/fphys.2020.594313/full This is relevant because some evidence implicates prostaglandin D2 as a contributor to the balding process, so lowering its activity may help improve hair growth.[5]https://pubmed.ncbi.nlm.nih.gov/33854354/
Interestingly, many of the above mechanisms (i.e., improved vasodilation and prostaglandin modification) overlap with the suspected mechanisms of the FDA-approved hair loss drug, topical minoxidil. As such, it’s no surprise that in a case series on 300+ patients using eye drops of latanoprost to treat glaucoma, 77% saw hypertrichosis (i.e., additional hair growth) in the areas surrounding the eyes. [6]https://www.jaad.org/article/S0190-9622(01)70206-X/fulltext
With all of these cases, it’s also not out-of-the-question that latanoprost might also help regrow some scalp hair if reformulated as a topical.
In fact, this was corroborated by a 2002 study on primates (i.e., stump-tailed macaques) who happen to be one of the only other species to also suffer from pattern hair loss, or androgenic alopecia. Over an 8-month study, monkeys receiving a topical application of 0.5 ml x 0.005% to 0.05% latanoprost daily showed improvements to hair loss and cosmetic degrees of hair regrowth.
Fig. 1.Frontal bald scalp of a 10-year-old, female, macaque, showing sparce short vellus hairs in the bald scalp at pretreatment time (a). After 5 months of treatment with latanoprost 50 mg/ml, thickness and density of hair increased in upper central and lower lateral regions (b). After 3 months of latanoprost 500 ug/ml thickness and density of hair significantly increased in mid and lower lateral region.
Fig. 2.Sequential changes of average scores of categorical grades of hair growth in latanoprost and vehicle-treated monkeys.
The anecdotes on eyelash hair growth in glaucoma patients – and the preliminary evidence of hair regrowth on balding monkeys – certainly provides a strong enough signal to further investigate topical latanoprost as a hair loss treatment in humans.
So, over the last 20 years, what does the clinical evidence on latanoprost show?
Human evidence: topical latanoprost for the treatment of hair loss
While the data are limited (so far), clinical studies on latanoprost for hair loss have been conducted on both androgenic alopecia, telogen effluvium, and alopecia areata.
Clinical study on androgenic alopecia (2011)
In its first-ever randomized, double-blinded, placebo-controlled clinical trial, researchers tested topical latanoprost on 16 men with early-stage androgenic alopecia.
Each participant received two drops of 0.1% latanoprost daily – one drop per receding temple region – equating to 0.1 mL of solution applied daily. In other words, each man applied 0.1 mg of latanoprost per day.[7]https://pubmed.ncbi.nlm.nih.gov/21875758/
Fig 1. Male subject with androgenetic alopecia (Norwood Hamilton scale grade III). Marked areas indicate minizone position for topical application of latanoprost and placebo solutions.
2011 clinical results
Over the course of 24 weeks, daily application of 0.1 mg of latanoprost…
- Improved terminal hair counts and hair density versus placebo
- Improved the ratio of anagen:telogen hairs versus placebo (i.e., growing vs. shedding hairs)
- Did not cause any serious adverse events
The results were reflected statistically through phototrichogram data (i.e. the gold-standard for clinical research on hair loss disorders).
Fig 3. Number and percentage of anagen and telogen hairs/cm2 at baseline and 24 weeks. [8]https://pubmed.ncbi.nlm.nih.gov/21875758/
While the data seem encouraging, it’s important to point out three caveats. Across all latanoprost users:
- 8 of 16 men did report skin irritation-like effects – such as dermatitis. So, the risk of mild side effects were relatively high (i.e., 50%).
- 50% of men demonstrated what investigators called a “clinical improvement”. In other words, they saw improvements in at least two criteria points on the phototrichogram: increased hair density, increased hair counts, etc.
- Of the remaining men, 44% demonstrated no response, and 6% demonstrated a “poor” response – meaning things got worse for them versus placebo.
In other words, latanoprost seemed to work well in half of participants, with the other half of participants showing hair loss stabilization or no response at all.
For what it’s worth, this is a common trait among hair loss drugs that target factors like histamine responses and/or prostaglandins – for instance, minoxidil and cetirizine. [9]https://perfecthairhealth.com/histamine-and-hair-loss-is-there-a-use-for-antihistamines/ For some, these drugs work wonders. For others, they see no effect. Why?
In our estimation, the dichotomies in responses are likely related to the following:
- The degree of inflammation in the scalp – as studies have shown inflamed scalps respond more poorly to topicals such as minoxidil[10]https://pubmed.ncbi.nlm.nih.gov/8496421/
- The level of histamine activity surrounding hair follicles – as this problem may contribute to some cases of androgenic alopecia, but not all[11]https://perfecthairhealth.com/histamine-and-hair-loss-is-there-a-use-for-antihistamines/
- The activation of these compounds – in the case of minoxidil, its sulfation (which can be improved with retinoic acid, microneedling, and/or switching to oral minoxidil)[12]https://my.perfecthairhealth.com/courses/minoxidil/topical-minoxidil/
Therefore, this initial (albeit, small) clinical study on topical latanoprost suggests that it might have a place in treating some cases of hair loss, but not all.
Clinical study on androgenic alopecia and telogen effluvium (2018)
In 2018, another research group investigated the use of topical latanoprost by itself (and combined with topical minoxidil) in the treatment of men and women with androgenic alopecia or telogen effluvium.[13]http://www.surgicalcosmetic.org.br/details/618/en-US/latanoprost-and-minoxidil–comparative-double-blind–placebo-controlled-study-for-the-treatment-of-hair-loss
First, the researchers split 123 participants into six groups, with each group testing one of the following:
- G1: Placebo (i.e., nothing)
- G2: 5% minoxidil
- G3: 5% minoxidil + 0.005% latanoprost
- G4: 0.005% latanoprost
- G5: 5% minoxidil + 0.01% latanoprost
- G6: 0.01% latanoprost
Unfortunately, the investigators didn’t specify how much solution each participant applied once daily. Based on the photos of participants featured inside their study, many participants had moderate levels of hair loss and beyond, which would likely require at least 1-2 mL of solution to cover all balding regions. So, we can only make rough estimations as to how much daily exposure of these drugs each participant received.
Note: the absence of daily drug exposure volume is just one of the many flaws in this study. We’ll uncover more issues soon.
2018 clinical results
Over the course of 180-240 days, 25 of the 123 people withdrew from the study for reasons that the investigators state were “not related to the research” – though they don’t specify what those reasons were. Withdrawal rates were mostly equivalent across all six groups, which leaves us with 98 people and 16-18 participants per group for statistical comparisons.
Among those who remained, here are the key findings:
- No side effects were reported, with no significant signs of dermatitis or scalp irritation for any participant across all treatment groups.
- These groups saw statistically significant hair count improvements versus placebo:
- G2: 5% minoxidil
- G3: 5% minoxidil + 0.005% latanoprost
- G4: 0.005% latanoprost
- G5: 5% minoxidil + 0.01% latanoprost
- These groups did not see hair count improvements versus placebo:
- G6: 0.01% latanoprost
- The response rates (i.e., the percent of people seeing visually cosmetic regrowth) were:
- G2: 5% minoxidil – 35%
- G3: 5% minoxidil + 0.005% latanoprost – 36%
- G4: 0.005% latanoprost – 19%
- G5: 5% minoxidil + 0.01% latanoprost – 6%
- G6: 0.01% latanoprost – 0%
Here are some photos the investigators featured of participants using 5% minoxidil and 0.005% latanoprost – albeit from an extension period that ran up to 240 days:
Figure 1: Macro image of a female participant at the start and end periods (treatment with 5% minoxidil + 0.005% latanoprost)
Figure 2: Macro image of a male participant at the start and end periods (treatment with 5% minoxidil + 0.005% latanoprost)
And here is data regarding anagen hair counts (i.e., the number of “growing” hairs) across all groups and time periods (day 2, day 92, and day 182):
Graph 2: Number of anagen hairs by period and by treatment
What can we glean from this 2018 study?
First, let’s start off by interpreting a good signal from this study.
Topical formulations of latanoprost that are of higher volume and lower concentration might greatly minimize the risk of side effects.
If we recall from the 2011 study on topical latanoprost, two drops of 0.1% latanoprost totaling 0.1 mL of daily use led to skin irritation-related side effects in 50% of participants. But in this study on 0.005% to 0.01% latanoprost applied daily at 1-2 mL of volume, no adverse events were reported.
It might interest you to know that both of these studies likely exposed participants to the exact same total amount of latanoprost each day. It’s just that the 2011 study used a higher concentration (i.e., 0.1%) but with less volume of topical (i.e., 0.1 mL), whereas the 2018 study used a 10-20x lower concentration (i.e., 0.005% to 0.01%) but with 10-20x more volume (i.e., 1-2 mL).
Yet mathematically, both studies exposed their participants to ~0.1mg of latanoprost daily.
This is important, because it suggests that 0.1mg of latanoprost may become a lot more tolerable for hair loss patients when it is spread out over a larger area. So, if nothing else comes from this study, we’re at least encouraged by this signal.
So what else can we surmise?
1-2 mL daily of 0.005% latanoprost may improve hair counts for a portion of people with androgenic alopecia and/or telogen effluvium… particularly if combined with 5% minoxidil
According to the data presented by the research team, 0.005% latanoprost by itself was effective at improving hair parameters, as was 0.005% latanoprost + 5% minoxidil.
However, we need to keep in mind the response rates here: just 35% to 36% of participants in these groups saw visual regrowth. That’s not a lot, and it gives credence to the belief that while latanoprost might work for some people, it’s probably not appropriate for all hair loss sufferers.
What shouldn’t we glean from this 2018 study?
Looking at all of the data from this study can be confusing. After all, we have 6 different groups statistically measured across one another, with varying methodologies applied for some groups (like extension time periods for those using 0.005% latanoprost + 5% minoxidil), and with poor clarity in writing regarding both results and discussion organizations.
Nonetheless, we’ve read online about some people presuming that this study gives us enough insights to include the following. So, before adopting similar opinions, please read our counterarguments below:
Fallacy #1: this study suggests that 0.01% latanoprost might be less effective than 0.005% latanoprost
It’s not abnormal to make this assumption given the data presented. After all, the groups using 0.005% latanoprost saw a 35% and 36% response rate, whereas the groups using 0.01% latanoprost saw a 6% and 0% response rate.
Moreover, it’s not unusual to see dose-dependent effects in medicine – where some drugs have a strong therapeutic benefit at lower concentrations but can have opposing or toxic effects at higher concentrations. We discuss these relationships in our guide on flutamide.
Nonetheless, there are significant methodological flaws in this study that do not allow us to jump to this conclusion. Here are a few:
- Participant dropout rates were nearly 20%. This increases the likelihood of survivorship bias, whereby those who are left at the end of the study are only there because they actually are seeing an effect… while those who were seeing no benefit quit. This phenomenon artificially enhances “response rates”, and while it can be controlled for statistically using Bayesian modeling, the investigators did not do this.
- There was an unknown number of telogen effluvium participants. Telogen effluvium is a type of hair loss that differs from androgenic alopecia. It’s often caused by stress, micronutrient imbalances, and/or chronic conditions like hypothyroidism. It also self-resolves given enough time away from a stressor, or after the micronutrient imbalance or chronic condition is corrected. This study includes an unknown number of telogen effluvium patients, and no data on how many were in each group. With group sizes of just 16-18 people per treatment, keep in mind that all it would take would be 5-6 people “recovering” from telogen effluvium (which is likely not treatable through the mechanisms suspected from latanoprost) to hit a 35% “response rate”. That’s a huge problem… especially because the investigators don’t specify their method of randomization – which means there could be a disproportionate number of telogen effluvium sufferers in one of these groups – either artificially stuffing down or inflating their response rates.
- Participants were included if they had quit other hair loss treatments only 4+ weeks prior. Multiple studies definitively show that abruptly quitting hair loss topicals, such as minoxidil, results in a telogen effluvium-based shed that can take upwards of 6+ months from which to fully recover back to baseline. That’s the entire duration of this study. As such, any study that includes participants who just quit other treatments or topicals only 4+ weeks ago could be capturing a huge percentage of participants who happen to be entering into a telogen effluvium shed due to quitting those treatments… which would obfuscate any hair count results from introducing a new treatment during the same period.
As such, we need to be incredibly careful about drawing any efficacy or comparison conclusions from this study. It’s entirely possible that 0.005% and 0.01% latanoprost are equally effective… but that the groups receiving higher dilutions of latanoprost had poorer results because of participant sampling. Without better data, we just don’t know.
As an aside, when discussing this study across our own team, we were even apprehensive to mention the positive safety signal – as this 2018 study is so poorly designed that any conclusions whatsoever are potentially problematic and/or at-odds with the real-world experiences of latanoprost users.
Nonetheless, we have to work with the data currently available, and these two studies on topical latanoprost are all that we’ve got (so far) for androgenic alopecia.
So, what about other hair loss disorders, such as alopecia areata?
Clinical studies: topical latanoprost for the treatment of alopecia areata
To date, there have been five publications (to which we could find) assessing topical latanoprost for alopecia areata. Here are their quick summaries and results:
Alopecia areata of the eyelashes and eyebrows
A 2003 case report details the experience of an 11-year old female who experienced alopecia areata of the eyelashes after recovering from a viral illness. After multiple failed treatments, doctors eventually tested topical latanoprost – at an unknown dilution – which was applied daily for a period of six months. During that time, photographic assessments show a near full recovery of all lost eyelashes.[14]https://pubmed.ncbi.nlm.nih.gov/12724722/
A 2009 clinical study tested 0.005% latanoprost – at an unknown volume – on a total of 26 men and women with alopecia areata of the eyebrows and eyelashes. Participants applied latanoprost to just one side of the face, with the other side acting as an intra-patient “control”. After four months, researchers saw partial hair recovery in just one patient, and concluded that topical latanoprost was no more effective than applying nothing at all. Aside from a transient headache fro one patient, no adverse events were reported.[15]https://pubmed.ncbi.nlm.nih.gov/19620039/
Interestingly, these results were mirrored by prior clinical studies done on similar dilutions of topical latanoprost for eyelash-related alopecia areata from 2005 and 2008.[16]https://pubmed.ncbi.nlm.nih.gov/16310083/[17]http://www.iranjd.ir/article_98048_64fda5d6552e3e9d714cc2162d5686a4.pdf As such, it appears that the data (so far) suggest topical latanoprost does not improve this specific hair loss disorder for the overwhelming majority of people trying it.
Alopecia areata of the scalp
Finally, a 2021 randomized clinical study tested daily applications of 0.005% latanoprost versus 0.05% betamethasone diproprionate (an autoimmune-related medication) for the treatment of scalp-related alopecia areata in 50 patients.[18]https://ijdvl.com/a-randomized-comparative-study-of-the-efficacy-of-topical-latanoprost-versus-topical-betamethasone-diproprionate-lotion-in-the-treatment-of-localized-alopecia-areata/
Unfortunately, the researchers did not specify the amount (in mL) of topical applied daily. As such, we cannot estimate daily exposure volumes.
Over a 16-week period, 24% of patients using 0.005% latanoprost saw improvements, whereas 56% of patients using 0.05% betamethasone diproprionate saw improvements. In fact, patients in the 0.05% betamethasone diproprionate group also tended to see a faster recovery and a more dramatic reduction of alopecia areata lesions.
Therefore, the researchers concluded that 0.05% betamethasone diproprionate was superior to 0.005% latanoprost in the treatment of scalp alopecia areata.
Topical latanoprost for hair loss: summaries of the clinical studies
From what we can glean across all clinical studies on topical latanoprost for hair loss:
- Androgenic alopecia / pattern hair loss: When applied daily at a total exposure volume of 0.1 mg, topical latanoprost seems to improve 30-50% of androgenic alopecia cases over a six-month period. Concentrations of 1-2 mL x 0.005% latanoprost are preferred, particularly when combined with 5% minoxidil. Moreover, 1-2 mL x 0.005%-0.01% latanoprost seems to be a safer formulation than 0.1 mL x 0.1% latanoprost – even though both formulations equate to a 0.1 mg daily exposure volume.
- Alopecia areata of the eyelashes: Currently, the totality of evidence suggests that 0.005% latanoprost at undisclosed daily usage volumes does not work, minus one or two case reports.
- Alopecia areata of the scalp: One clinical study shows that 0.005% latanoprost improves alopecia areata of the scalp in ~25% of participants, but that its response rate is just half that of 0.05% betamethasone diproprionate (another autoimmune treatment), and that patients using 0.05% betamethasone diproprionate respond more quickly versus 0.005% latanoprost.
What about safety?
It’s easy to look at these studies and note that dilutions of 1-2 mL daily of 0.005% to 0.01% were well-tolerated for nearly all participants, with only a handful of minor, transient adverse events reported across all patient cohorts testing this formulation. As such, it’s easy to presume that topical latanoprost must be safe.
We have significant reservations with that conclusion.
After all, these topical latanoprost studies for hair growth ran – at most – 8 months. While no serious adverse events were reported during these short timeframes, there is a well-known phenomenon that occurs from long-term latanoprost use, and one that does not typically show up until after one full year of treatment: pigment changes.
In glaucoma patients, pigment changes of the iris begin to occur after one year of daily latanoprost use, and they worsen over time – affecting up to 10% of glaucoma patients.[19]https://www.ncbi.nlm.nih.gov/books/NBK540978/ Other studies suggest an incidence as high as 70%, depending on the eye color & duration of use.[20]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1771317/
This is because regular latanoprost use increases melanin activity (i.e., the activity of cells that produce pigmentation in our skin, eyes, etc.). The effects, over time, can be quite dramatic. Just see the results from this patient, who was treated for glaucoma in their right eye with latanoprost (picture A) but not in their left eye (picture B). It’s as if we are looking at two different eyes. [21]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1771317/
As such, a critical question remains: does latanoprost applied topically produce the same effects in the skin, hair, and (potentially) through the eyes via systemic absorption?
Unfortunately, we have no way of knowing… because the clinical studies have not yet been conducted. Moreover, none of the studies on topical latanoprost for hair loss ran even remotely long enough to measure if this effect would also apply to the scalp.
In cases of treatments producing serious, cosmetically-unwanted adverse events – like a darkening of scalp skin – we tend to err on the side of caution and say that without more data, most people should be seriously cautious about long-term use of topical latanoprost. Perhaps better data will demonstrate this is not a concern, but until we have that data, we don’t feel comfortable recommending topical latanoprost as a guinea pig experiment to members here… even despite its popularity online as an adjuvant hair loss treatment.
For more contraindications and side effects from latanoprost, please visit this resource.[22]https://www.ncbi.nlm.nih.gov/books/NBK540978/
Latanoprost: best and worst candidates
You are likely a candidate for latanoprost if:
- You have androgenic alopecia
- You are planning to use latanoprost alongside better-studied treatments (and not as a standalone treatment)
- You are not concerned about the unknown risks of pigmentation changes that tend to start presenting after 1+ years of latanoprost use
You are likely not a candidate for latanoprost if:
- You don’t have androgenic alopecia
- You are concerned about the unknown risks of pigmentation changes that tend to start presenting after 1+ years of latanoprost use
Summary
Latanoprost has some limited clinical data supporting its use for androgenic alopecia, but with response rates of 30-50%, it’s unlikely that this treatment is right for most hair loss sufferers. For these reasons, we recommend people use other prostaglandin analogues instead – such as minoxidil – and only resort to latanoprost if they’re using it as an add-on to their regimen and if they’re comfortable with the unknowns about its long-term safety profile.
For alopecia areata of the eyelashes, latanoprost is unlikely to help. For alopecia areata of the scalp, one small study found that topical latanoprost may help up to 25% of people. However, that same study also found that 0.05% betamethasone diproprionate was twice as effective at producing a response rate versus latanoprost, and that it also worked faster than latanoprost.
References[+]
References ↑1 https://jamanetwork.com/journals/jamaophthalmology/fullarticle/413134 ↑2, ↑19, ↑22 https://www.ncbi.nlm.nih.gov/books/NBK540978/ ↑3 https://pubmed.ncbi.nlm.nih.gov/15854125/ ↑4 https://www.frontiersin.org/articles/10.3389/fphys.2020.594313/full ↑5 https://pubmed.ncbi.nlm.nih.gov/33854354/ ↑6 https://www.jaad.org/article/S0190-9622(01)70206-X/fulltext ↑7, ↑8 https://pubmed.ncbi.nlm.nih.gov/21875758/ ↑9, ↑11 https://perfecthairhealth.com/histamine-and-hair-loss-is-there-a-use-for-antihistamines/ ↑10 https://pubmed.ncbi.nlm.nih.gov/8496421/ ↑12 https://my.perfecthairhealth.com/courses/minoxidil/topical-minoxidil/ ↑13 http://www.surgicalcosmetic.org.br/details/618/en-US/latanoprost-and-minoxidil–comparative-double-blind–placebo-controlled-study-for-the-treatment-of-hair-loss ↑14 https://pubmed.ncbi.nlm.nih.gov/12724722/ ↑15 https://pubmed.ncbi.nlm.nih.gov/19620039/ ↑16 https://pubmed.ncbi.nlm.nih.gov/16310083/ ↑17 http://www.iranjd.ir/article_98048_64fda5d6552e3e9d714cc2162d5686a4.pdf ↑18 https://ijdvl.com/a-randomized-comparative-study-of-the-efficacy-of-topical-latanoprost-versus-topical-betamethasone-diproprionate-lotion-in-the-treatment-of-localized-alopecia-areata/ ↑20, ↑21 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1771317/ IngredientsPharmaceuticalsNatural RemediesCompany ReviewsBy Perfect Hair Health TeamJan 16, 2026Oral Finasteride Before and After: Real Case Studies
Oral finasteride remains the gold-standard treatment for male pattern hair loss, with decades of data showing consistent improvement in density and prevention of further thinning. However, dramatic online regrowth photos can lead to unrealistic expectations. In your regrowth journey, it’s important ...By Perfect Hair Health TeamJan 16, 2026Best Topical Dutasteride: 8 Choices for 2026
Topical dutasteride is emerging as a powerful next-generation option for treating hair loss. This expert-reviewed guide ranks the 8 best topical dutasteride treatments, comparing strength, safety, and customization, to help you find the most effective DHT-blocking solution for your needs. From Ulo’s...By Catherine Kennedy, PhDJan 12, 2026Does Finasteride Lower Testosterone?
Finasteride’s reputation for affecting hormones has led many to assume it suppresses testosterone, but the biology is far more nuanced. In this article, we unpack what happens to testosterone when DHT is inhibited, why levels often rise instead of fall, and how this squares with the sexual side effe...By Cassie Hopton, PhDJan 12, 2026Saw Palmetto Shampoo: Is It Effective for Hair Loss?
Saw palmetto is hailed as a natural hair loss treatment, promising hair growth and strength. It is incorporated into all manner of hair loss products, including shampoos. But is the hype backed by science? This article dissects the science of saw palmetto as a hair growth ingredient, as well as sham...By Michael Williams, PhDJan 12, 2026Switching From Topical Minoxidil To Oral Minoxidil
Thinking about switching from topical to oral minoxidil, or back again? Changing your approach to hair loss can significantly improve your results, but changing treatment can also be a gamble that sets back progress. We break down what the evidence actually shows, when you might want to consider swi...By Cassie Hopton, PhDJan 12, 2026Does Spironolactone Cause Weight Gain?
Spironolactone is a popular off-label treatment for female pattern hair loss, but many users worry about gaining weight while taking it. This article breaks down how the medication works, what the research actually shows about weight changes, typical dosing for hair growth, and simple strategies to ...By Sophie Grice, PhDJan 9, 2026RU58841 for Hair Loss: Is It Legit?
RU58841 is one of the most talked-about research chemicals in hair loss forums, and the anecdotes are often surprisingly positive. Mechanistically, it makes sense: block androgen receptors in the scalp so dihydrotestosterone (DHT) can’t trigger follicle miniaturization. But the problem is that the h...By Michael Williams, PhDJan 9, 2026Oral vs. Topical Minoxidil: What’s Better for Hair Loss?
Oral vs topical minoxidil for hair loss: which works better, and at what cost? We break down the evidence on effectiveness, safety, dosing, and real-world tradeoffs to help you choose the right option.
By Michael Williams, PhDDec 31, 2025Finasteride vs. Dutasteride: The Ultimate Guide
Finasteride and dutasteride are the two most powerful medications available for androgenetic alopecia, but choosing between them isn’t straightforward. We break down how these drugs truly compare across efficacy, side effects, oral versus topical use, and real-world decision-making, so you can deter...By Michael Williams, PhDDec 31, 202510 Ways to Reduce the Side Effects of Finasteride
Finasteride is one of the most effective treatments for male pattern hair loss, but side effects can happen. This evidence-based guide outlines 10 practical strategies to reduce or manage finasteride side effects, from dose and formulation adjustments to lifestyle considerations, while emphasizing w... - Mission Statement
 Scroll Down
Scroll Down