- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
OS-01 Hair Review: Does It Live Up to the Hype?
-
Stretching The Truth: 3 Misrepresented Claims From Hair Loss Studies
-
Minoxidil Shedding – What to Expect & When it Stops
-
Does Minoxidil Cause Skin Aging?
-
Thermus Thermophilus Extract Does Not Increase Hair Density By 96.88%, Despite Dermatology Times’ Claims.
-
Does Retinoic Acid (Tretinoin) Improve Hair Growth From Minoxidil?
-
Topical Cetirizine: An Anti-Histamine That Regrows Hair? (New Evidence)
-
Scalp Psoriasis: Symptoms, Causes, and Effects on Hair Loss
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesMinoxidil Side Effects: Everything You Need To Know
First Published Feb 6 2025Last Updated Feb 28 2025PharmaceuticalResearched & Written By:Sarah King, PhDReviewed By:Rob English, Medical EditorWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Minoxidil is an FDA-approved treatment for androgenic alopecia, but its use can come with side effects – from mild scalp irritation to cardiovascular risks. This comprehensive guide assess common and rare side effects from topical minoxidil and oral minoxidil – along with mitigation strategies.
Full Article
Minoxidil is a widely used medication for treating androgenic alopecia (AGA) in both men and women. Originally developed as a treatment for high blood pressure, minoxidil was found to improve hair growth outcomes as a side effect, making its topical form one of only two (the other being finasteride) FDA-approved treatments for AGA.
Minoxidil is primarily available in two forms: topical and oral. The topical form, which includes both solutions and foams, is more commonly used and can be purchased over the counter. Oral minoxidil, however, is prescription only and is typically given at doses of 2.5 mg or 5 mg for hair loss treatment.
While minoxidil is generally considered safe, it’s important to understand its potential side effects and who might be more at risk from them. In this article, we will examine the side effects of topical and oral minoxidil and discuss how you can adjust your treatment regimen to mitigate these.
How Does Minoxidil Work?
Minoxidil was originally developed as an antihypertensive medication. However, when treated participants started experiencing hypertrichosis (excessive hair growth), studies were conducted to find out if it could improve hair regrowth outcomes in humans (spoiler alert – it could!).[1]Bryan, J. (2011). How minoxidil was transformed from an antihypertensive to hair-loss drug. The Pharmaceutical Journal. Available at: … Continue reading
Minoxidil’s mechanism of action is not fully understood, but it is thought to work through two main mechanisms:
- Vasodilatory effects: It activates vascular endothelial growth factor (VEGF), increasing blood flow, oxygen, and growth factor delivery to the hair follicle.[2]Alhayaza, G., Hakami, A., AlMarzouk, L.H., Al Qurashi, A.A., Alghamdi, G., Alharithy, R. (2023). Topical minoxidil reported hair discoloration: a cross-sectional study. Dermatology Reports. 16(1). … Continue reading
- Potassium channel activation: This prolongs the growth (anagen) phase and shortens the resting (telogen) phase.
Research also shows that minoxidil can act on androgenic receptors, suppressing the expression of the androgen receptor and CYP17A1 and boosting the activity of CYP19A1. This decreases the formation and binding of dihydrotestosterone and enhances the production of estradiol, which may also benefit those with AGA.[3]Shen, Y., Zhu, Y., Zhang, L., Sun, J., Xie, B., Zhang, H., Song, X. (2023). New Target for Minoxidil in the Treatment of Androgenetic Alopecia. Drug Design, Development and Therapy. 17. 2537-2547. … Continue reading
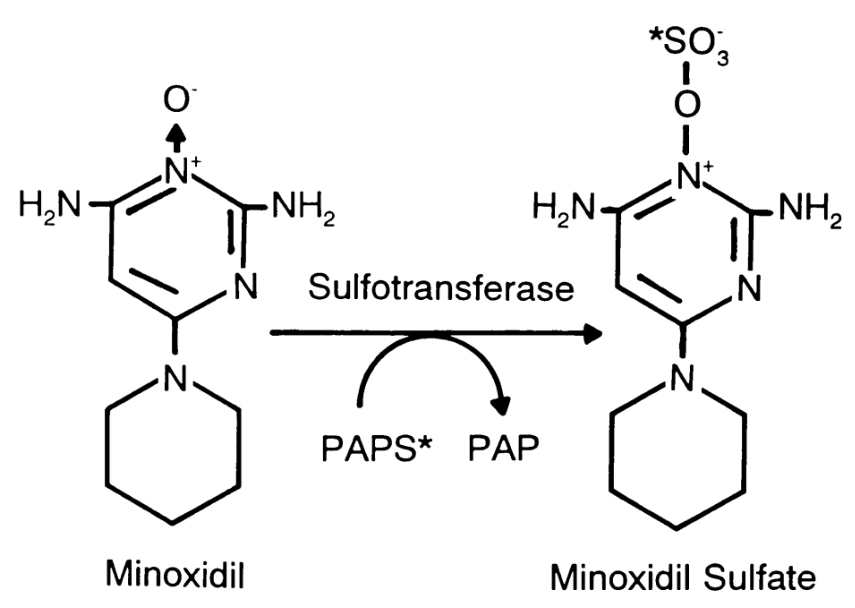
How is Minoxidil Activated in the Body?
Minoxidil needs to be converted into its active form, minoxidil sulfate, by sulfotransferase enzymes before it can effectively stimulate hair growth.[4]Dhurat, R., Daruwalla, S., Pai, S., Kovacevic, M., McCoy, J., Shapiro, J., Sinclair, R., Vano-Galvan, S., Goren, A. (2021). SULT1A1 (Minoxidil Sulfotransferase) enzyme booster significantly improves … Continue reading This conversion primarily occurs in the scalp for topical minoxidil and in the liver for oral minoxidil.
Figure 1: The conversion of minoxidil to minoxidil sulfate by sulfotransferase.[5]Anderson, R.J., Kudlacek, P.E., Clemens, D.L. (1998). Sulfation of minoxidil by multiple human cytosolic sulfotransferases. Chemico-Biological Interactions. 109. 53-67. Available at: … Continue reading
However, enzyme activity varies significantly among individuals, meaning that some people naturally produce higher levels of sulfotransferase, allowing for better activation and increased effectiveness of the drug, while others have lower enzyme activity, which can limit its impact. You can read our article on how your genetics can influence sulfotransferase activity here.
Topical Minoxidil Vs. Oral Minoxidil: Activation Pathways
Topical minoxidil and oral minoxidil differ in application and effectiveness. Topical minoxidil is applied directly to the scalp, while oral minoxidil is taken as a pill.
The efficacy of these two treatment routes differs because of their distinct metabolic pathways.
Topical Minoxidil
Topical minoxidil is applied directly to the scalp, presenting a challenge for drug activation. The drug requires conversion to its active form, minoxidil sulfate, by sulfotransferase enzymes in the scalp. However, research has shown that 40-60% of people may lack sufficient enzyme levels to effectively activate the medication. This enzymatic variability means that for many users, topical minoxidil may not be 100% effective.
Around 1.4% of topical minoxidil is systemically absorbed, further limiting minoxidil efficacy. To counteract this limitation, researchers have explored combining minoxidil with treatments like microneedling or retinoic acid. It was found that with these combinations, minoxidil efficacy can significantly increase.[6]Lama, S.B.C., Pérez-González, L.A., Kosoglu, M.A., Dennis, R., Ortega-Quijano, D. (2024). Physical Treatments and Therapies for Androgenetic Alopecia. Journal of Clinical Medicine. 13(15). 4534. … Continue reading
Oral Minoxidil
Oral minoxidil, however, is metabolized in the liver, where sulfotransferase enzymes are abundant. The activated minoxidil sulfate then enters the bloodstream, where it reaches the hair follicle. Studies consistently demonstrate higher response rates for oral minoxidil compared to topical.[7]Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: … Continue reading However, because oral minoxidil involves systemic absorption, it comes with a broader potential for side effects.
What Are the Side Effects of Topical Minoxidil?
Common Side Effects
While topical minoxidil typically has a great safety profile, there are some side effects that people can experience.
Common side effects include:
- Scalp Irritation and Contact Dermatitis: Redness, itching, flaking, and burning sensations.
These are the most frequently reported side effects from minoxidil. One retrospective study found that 6.4% of men reported mainly irritant and allergic reactions to minoxidil.[8]Shadi, Z. (2023). Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy (Heidelb). 13(5). 1157-1169. Available at: … Continue reading These effects are typically due to an allergic reaction to propylene glycol rather than the minoxidil itself.[9]Lessmann, H., Schnuch, A., Geier, J., Uter, W. (2005). Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 53(5). 247-259. Available at: … Continue reading
To mitigate the negative skin effects of topical minoxidil, you can do several things. Starting with a lower concentration, such as 2% instead of 5%, can reduce the risk of irritation while still providing benefits. Additionally, reducing application frequency from twice to once daily can limit exposure. Switching to a foam-based minoxidil product can be particularly effective, as these formulations typically don’t contain propylene glycol, which is often responsible for uncomfortable side effects like irritation, redness, and scalp burning. Furthermore, incorporating a moisturizer into your scalp care routine can help keep the skin hydrated and comfortable, further alleviating potential irritation.
- Shedding Phase: Temporary increase in hair loss when starting treatment.
One study reported that 55% of topical minoxidil users experience minoxidil shedding.[10]Ghonemy, S., Bessar, H., Alarawi, A. (2019). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic … Continue reading This is considered a normal side effect and usually indicates that the treatment is working. The shedding phase typically begins 2 to 4 weeks after starting treatment and subsides within 6 to 8 weeks as the hair cycle normalizes. After a few months of continuous use, most users should start seeing visible new hair growth.[11]Kaiser, M., Abdin, R., Gaumond, S.I., Issa, T. N., Jiminez, J.J. (2023). Treatment of androgenetic alopecia: current guidance and unmet needs. Clinical Cosmetic and Investigational Dermatology. 16. … Continue reading
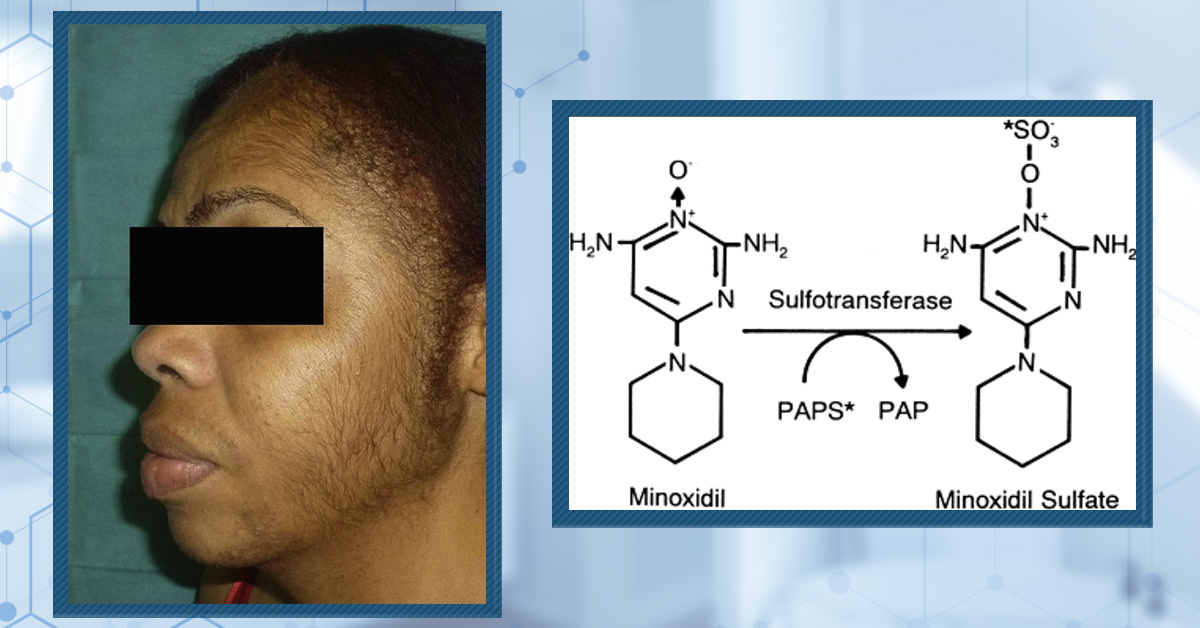
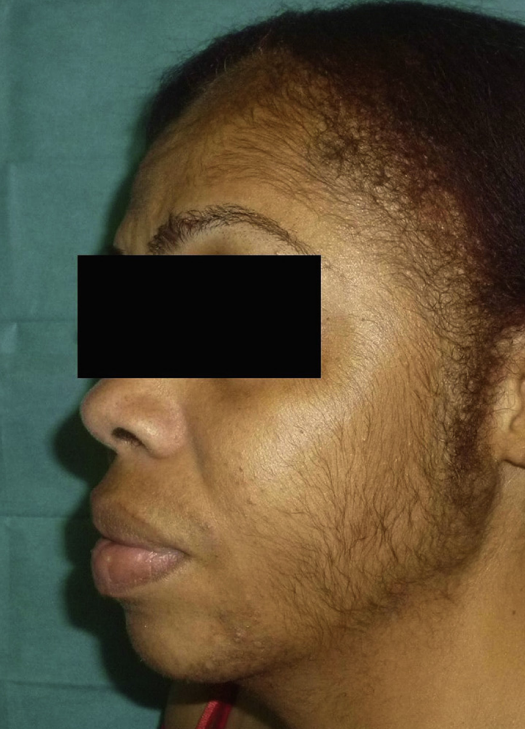
- Facial Hypertrichosis: Unwanted excess facial hair.
Women, especially those over 50 or with pre-existing facial hair, seem to be at higher risk of developing hypertrichosis from topical minoxidil use, and as with other side effects, it is more common when using the 5% than the 2% concentration.[12]Dawber, R.P.R, Rundegren, J. (2003). Hypertrichosis in females applying minoxidil topical solution and in normal controls. Journal of the European Academy of Dermatology and Venereology. 17(3). … Continue reading
Figure 2: A 42 year old woman with generalized hypertrichosis after using 5% topical minoxidil for two weeks.[13]Gargallo V, Gutierrez C, Vanaclocha F, Guerra-Tapia A. Hipertricosis generalizada secundaria a minoxidil tópico. Actas Dermosifiliogr. 2015;106:599–600. Available at: … Continue reading
There are several theories for why hypertrichosis occurs in people using topical minoxidil.
- Product application: The product wasn’t applied carefully enough or was not completely dried before bed, leading to product transfer from the pillow to the face. However, this doesn’t account for hypertrichosis seen away from the site of application and is unlikely to cause the effects seen in case studies.
- Percutaneous absorption: Although topical minoxidil is primarily intended for local effects, a small amount can be absorbed systemically through the skin. This can lead to circulating minoxidil reaching hair follicles in distant areas of the body.
- Follicular hypersensitivity: Some people may have hair follicles that are exceptionally responsive to minoxidil. In these cases, even minimal systemic exposure might be sufficient to stimulate hair growth in distant follicles. This hypersensitivity appears to occur randomly, depending on the person.
- Dose-dependent response: Higher concentrations are more likely to cause hypertrichosis.
While hypertrichosis can occur, it is generally reversible once minoxidil treatment is stopped and typically resolves within 3-4 months.
There are a number of ways that hypertrichosis can be avoided or treated once it occurs.
Starting with a lower concentration can reduce the risk, especially for women and those with a history of excess facial hair. Careful application to the scalp only, allowing proper drying time, and adhering to the recommended dosage can also minimize systemic absorption and unintended spread.
Switching to a foam version may help those experiencing side effects. If you are experiencing mild hypertrichosis and don’t want to stop using minoxidil, you could use hair removal methods while continuing treatment.
For more severe cases, spironolactone (~25 mg daily) ando/or low-dose bicalutamide (~10 mg daily) have shown promise in managing minoxidil-induced hypertrichosis. However, these medications should be used under medical supervision.[14]Darendeliler, F., Bas, F., Balaban, S., Bundak, R., Demirkol, D., Saka, N., Gunoz, H. (1996). Spironolactone therapy in hypertrichosis. European Journal of Endocrinology. 135(5).604-608. Available … Continue reading,[15]Moussa, A., Kazmi, A., Bakhari, L., Sinclair, R.D. (2022). Bicalutamide improves minoxidil-induced hypertrichosis in female pattern hair loss: a retrospective review of 35 patients. Journal of the … Continue reading
- Headaches
Some people also experience headaches after using topical minoxidil. One study found that in users applying 2% minoxidil solution, 0.6% reported headaches, compared to 3% of participants using 5% minoxidil solution.[16]Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading
Some people may simply experience headaches due to the smell of the product they are using, in which case, switching to an alcohol-free or unscented alternative may help. Others may be particularly sensitive to minoxidil and its vasodilatory effects, which might contribute to headaches. In this case, switching to a lower concentration or consulting with a healthcare provider might be preferable.
Some of our members have also switched to nanoxidil, an analogue of minoxidil that may offer a better safety profile (you can read more about the research quality of nanoxidil here), and found that their headaches resolved.
The above side effects are more often seen in the 5% than 2% solutions and are typically considered to be non-serious.[17]Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of … Continue reading
Some of the rarer side effects include:
- Water Retention
While less common than with oral minoxidil, topical minoxidil application can lead to water retention. Some topical minoxidil users have reported under-eye bags, which may be due to increased water retention near the application areas. There are also anecdotal reports of “puffy face” from topical minoxidil application, suggesting localized fluid retention.[18]Gungor, S., Kocaturk, E., Topal, I.O. (2015). Frontal Edema Due to Topical Application of %5 Minoxidil Solution Following Mesotherapy Injections. International Journal of Trichology. 7(2). 86-87. … Continue reading
Anecdotally, there have also been reports of swollen feet and weight gain from using topical minoxidil; however, we couldn’t find these reports reflected in peer-reviewed literature. These effects may also stop with continued use of the drug, so some people keep an eye on the symptoms and wait to see if they go away.
However, you can try reducing the concentration or frequency of usage if the symptoms continue longer than you are comfortable with. Furthermore, excessive salt intake can exacerbate symptoms of edema. Limiting salt intake may help you resolve the edema without having to stop using minoxidil.[19]Patel, P., Nessel, T.A., Kumar, D. (2023). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (Accessed: … Continue reading
- Cardiovascular Issues
Some people also experience cardiovascular side effects. One case study found that applying large amounts of 2% topical minoxidil led to hypotension (low blood pressure) and feelings of faintness.[20]Ponomareva, M.A., Romanova, M.A., Shapshnikova, A.A., Piavchenko, G.A. (2024). Topical Minoxidil Overdose in a Young Man with Androgenetic Alopecia: A Case Report. Cureus. 16(6). E62382. Available … Continue reading Another case also documented low blood pressure and fainting after applying 12.5% topical minoxidil daily.[21]Dubrey, S.W., vanGriethuysen, J., Edwards, C.M.B. A hairy fall: syncope resulting from topical application of minoxidil. BMJ Case Reports. 1-2. Available at: https://doi.org/10.1136/bcr-2015-210945
Other people may experience heart palpitations, feelings of the heart beating rapidly or “skipping a beat”. While this is rare, one study found that 3.5% of women developed heart palpitations or a rapid heart rate after usage of 2% topical minoxidil solution compared to 1.8% who were using a 5% foam.[22]Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the … Continue reading
Therefore, we would recommend seeking medical advice and potentially finding a new treatment for hair loss if you experience these side effects.
So we’ve covered the side effects of topical minoxidil, but what about oral?
What Are the Side Effects of Oral Minoxidil?
Oral minoxidil side effects are similar to those of topical minoxidil, and both forms exhibit dose-dependent effects. However, oral administration leads to significantly greater systemic exposure to the drug than topical application. As a result, oral minoxidil typically produces more pronounced hair regrowth and more pronounced systemic side effects. It should be noted that use of oral minoxidil to improve hair regrowth is an off-label use of the drug and it is not FDA-approved for this indication.
Common Side Effects
- Hair Shedding
Like with topical minoxidil, you may experience increased hair shedding when you start taking minoxidil. This is considered to be a normal part of the hair cycle remodeling process, typically beginning two to four weeks after starting treatment and subsiding within six to eight weeks as the hair cycle normalizes.
- Hypertrichosis
While rare with topical minoxidil use, one of the most frequently reported side effects of oral minoxidil is hypertrichosis, which involves excessive hair growth on various parts of the body. This effect is dose-dependent, with some studies showing that increasing the dosage of oral minoxidil by just 1 mg daily is associated with a 17.6% increased risk of hypertrichosis.[23]Gupta, A.K., Hall, D.C., Talukder, M., Bamimore, M.A. (2022). There is a Positive Dose-Dependent Association between Low-Dose Oral Minoxidil and Its Efficacy for Androgenetic Alopecia: Findings from … Continue reading
Hypertrichosis frequently occurs on the face (sideburns, temples, upper lip, and chin), with rarer cases of generalized hypertrichosis occurring over the whole body.[24]Desai, D.D., Nohria, A., Brinks, A., Needle, C., Shapiro, J., Lo Sicco, K.I. (2024). Minoxidil-induced hypertrichosis: Pathophysiology, clinical implications, and therapeutic strategies. JAAD … Continue reading
- Fluid Retention
Fluid retention is another common side effect of oral minoxidil, occurring in about 1.3% of patients.[25]Trueb, R.M., Caballero-Uribe, N., Luu, N.N.C., Dmitriev, A. (2022). Serious complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 30. 97-98. Available at: … Continue reading This can manifest as mild swelling in the face, hands, or feet. In some cases, it may lead to rapid weight gain.
This weight gain can be significant and sudden, with some patients experiencing up to 20 pounds of weight gain (as water weight) in as little as one month.[26]Patel.K., Omar, J. (2023). Low dose oral minoxidil causing peripheral edema and rapid weight gain. Journal of General Internal Medicine. 38(Suppl 3). S592. Available at: … Continue reading
Fluid retention can manifest in several ways:
- Peripheral edema: Swelling in the extremities, particularly in the legs, ankles, and feet.
- Facial swelling: Puffiness or swelling in the face.
- Overall weight gain: A sudden increase in body weight, often 5 pounds or more.
This rapid retention can be concerning for patients and may lead to additional health risks if left unmanaged. Excess fluid in the body can potentially lead to congestive heart failure if not properly addressed.
To mitigate these side effects, there are a number of options:
- Use of diuretics: Doctors can prescribe a diuretic (water pill) alongside minoxidil to help counteract fluid retention. This is typically a low-dose loop diuretic.
- Sodium restriction: Limiting sodium intake can help reduce fluid retention. Patients are often not advised about this when starting minoxidil, which can exacerbate the problem.
- Dose adjustment: If fluid retention becomes severe, temporarily discontinuing minoxidil or significantly reducing the dose may be necessary. This allows the edema to resolve naturally before restarting at a lower dose.
- Regular monitoring: Daily weight monitoring can help identify fluid retention early, making it easier to manage.
- Gradual titration: Slowly increasing the dose of minoxidil over time, rather than starting with 5 mg, can help minimize side effects.
If these symptoms don’t resolve when trying these strategies, then it’s recommended to visit your doctor and potentially stop taking minoxidil.
- Lightheadedness, Tachycardia (high heart rate), Headache and Insomnia
Oral minoxidil can cause several cardiovascular and neurological side effects, including lightheadedness, tachycardia, headache, and insomnia.[27]Trueb, R.M., Caballero-Uribe, N., Luu, N.N.C., Dmitriev, A. Serious complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 30. 97-98. Available at: … Continue reading These effects are generally dose-dependent and more common at higher doses.
Lightheadedness has been reported to occur in 1.7% of patients, tachycardia in 0.9%, headache in 0.4%, and insomnia in 0.2% of patients. These can all be symptoms of decreased blood pressure due to minoxidil’s vasodilatory properties.
Headaches and insomnia are reported in around 0.4% and 0.2% of patients, respectively, using low-dose oral minoxidil. While the exact mechanism of these side effects is not clear, it is thought that it could be related to the vasodilatory effects of the drug.
What Are The Cardiovascular Concerns of Oral Minoxidil Usage?
The severity and frequency of cardiovascular side effects are closely tied to the dosage of oral minoxidil. A meta-regression analysis found a positive dose-dependent correlation between low-dose oral minoxidil and the risk of cardiovascular adverse events.
Low Dose (0.25-1.25 mg/day)
At lower doses, oral minoxidil is generally well-tolerated. Women typically start with doses ≤ 1 mg, which minimizes the risk of significant side effects. Lower doses are considered to be a safer starting point for most patients, and even very low doses (0.25 mg/day) have shown efficacy in some studies.[28]Ramírez-Marín, H.A., Tosti, A. (2022). Role of oral minoxidil in patterned hair loss. Indian Dermatology Online Journal. 13(6). 729-733. Available at: https://doi.org/10.4103/idoj.idoj_246_22 If you are experiencing side effects at higher doses, you can reduce your dose at home using a pill cutter.
Moderate Dose (2.5-5 mg/day)
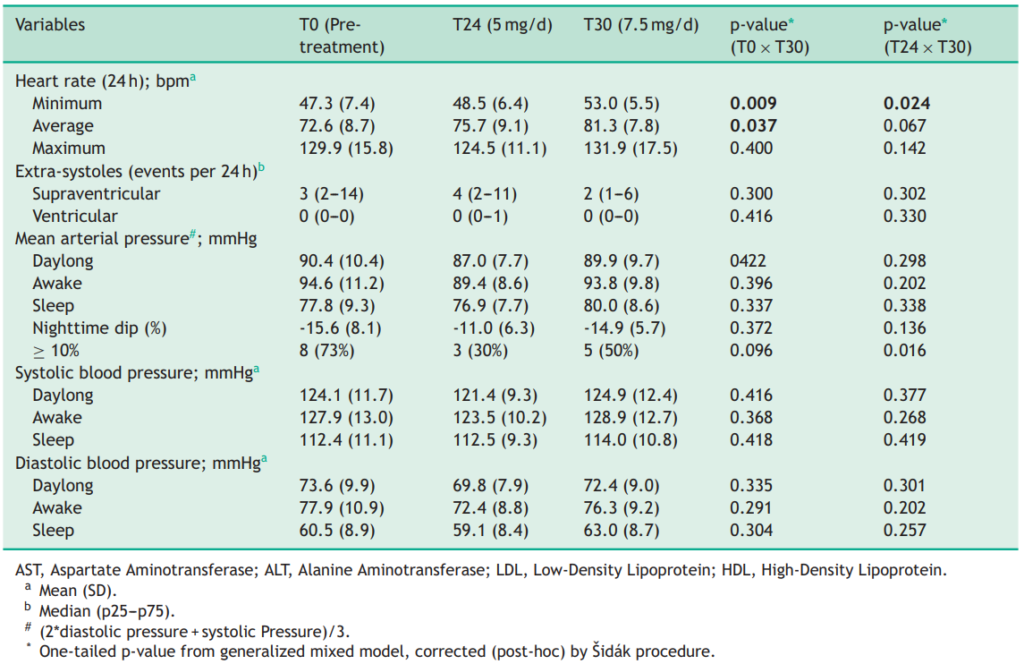
Men may be prescribed up to 5 mg of minoxidil daily, which can increase the likelihood of side effects such as dizziness and fluid retention. A recent study examined the effects of 7.5 mg/day oral minoxidil in patients with normal blood pressure and AGA.[29]Sanabria, B.D., Perdomo, Y.C., Miot, H.A., Ramos, P.M. (2024). Oral minoxidil 7.5 mg for hair loss increases heart rate with no change in blood pressure in 24 h ambulatory blood pressure monitoring. … Continue reading The results showed a mild increase in heart rate but no significant changes in blood pressure, suggesting that doses slightly higher than the typical 5 mg can be tolerated. However, this should only be considered under medical supervision.
Figure 3: Heart rate and blood pressure monitoring of 11 adult males with AGA after 24 weeks (T24) of treatment with 5 mg/day of oral minoxidil and after 6 weeks (T30) of treatment with 7.5 mg/day of oral minoxidil.[30]Sanabria, B.D., Perdomo, Y.C., Miot, H.A., Ramos, P.M. (2024). Oral minoxidil 7.5 mg for hair loss increases heart rate with no change in blood pressure in 24 h ambulatory blood pressure monitoring. … Continue reading
High Dose (10 mg/day and above)
Doses above 10 mg daily are associated with a higher risk of serious cardiac events and are typically not recommended for hair loss treatment. The hypotensive effect of oral minoxidil becomes more significant at these higher doses and is often prescribed alongside beta blockers and diuretics to manage the side effects.
How Can I Mitigate Oral Minoxidil Side Effects?
We have covered a number of ways to mitigate oral minoxidil side effects, but there are some further ways that you can adjust the use of minoxidil to reduce your risk.
Split-Dosing
Splitting the daily dosage of oral minoxidil into two administrations, one in the morning and one in the evening, can potentially optimize its efficacy while minimizing side effects. This approach is based on the pharmacokinetics of oral minoxidil, which has a relatively short half-life of approximately 3-4 hours.[31]Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., … Continue reading
By dividing the total daily dose, you can maintain more consistent blood levels of minoxidil throughout the day, potentially leading to more stable hair growth stimulation. For example, if 5 mg is prescribed daily, taking 2.5 mg in the morning and 2.5 mg in the evening may be more beneficial than a single 5 mg dose.
Sublingual Minoxidil
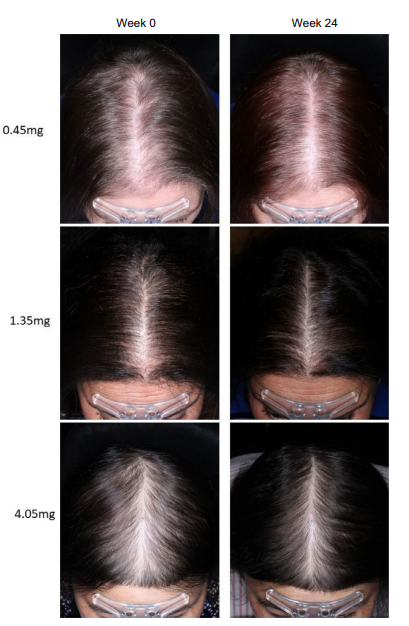
Some recommend using sublingual minoxidil as an alternative to traditional oral minoxidil. Sublingual administration involves a tablet that dissolves under the tongue. One 2021 randomized, double-blind, placebo-controlled phase 1b clinical trial investigated this delivery method.[32]Bokhari, L., Jones, L.N., Sinclair, R.D. (2021). Sublingual minoxidil for the treatment of male and female pattern hair loss: a randomized, double-blind, placebo-controlled, phase 1B clinical trial. … Continue reading The study tested daily doses of 0.45 mg to 4.05 mg of sublingual minoxidil.
This method offers several advantages:
- Bypasses first-pass metabolism in the liver.
- It allows for direct entry into the bloodstream, which can travel inactivated to the scalp and be activated by sulfotransferase at the site.
- Reduces systemic side effects.
- Improves bioavailability.
Key findings from the study included a dose-dependent improvement in hair parameters, with reduced side effects compared to oral minoxidil and no significant effect on blood pressure. In the blood, peak serum concentrations of minoxidil were only 10% of those seen with typical oral minoxidil.
Figure 4: Effect of different doses of sub-lingual minoxidil on hair regrowth outcomes after 24 weeks.[33]Bokhari, L., Jones, L.N., Sinclair, R.D. (2021). Sublingual minoxidil for the treatment of male and female pattern hair loss: a randomized, double-blind, placebo-controlled, phase 1B clinical trial. … Continue reading
At the 24-week follow-up, approximately 45% of patients in the 0.45 mg sublingual minoxidil group experienced improvements in frontal hair density, and 55% showed vertex improvement. Higher doses (4.05 mg) led to further results, with nearly 67% of patients experiencing improvements in both frontal and vertex hair density.
Sublingual minoxidil appears to be particularly beneficial for people concerned about the side effects of oral minoxidil. The medication was undetectable in plasma after 24 hours, and the mean peak minoxidil plasma concentration was significantly below the threshold associated with changes in blood pressure.
While the results are promising, it should be noted that this is the only study using sublingual minoxidil for AGA, and further studies with larger patient numbers are needed.
Switch To Topical Minoxidil
There is a chance that none of these options will work out for you, so you can try to switch to topical minoxidil. The side effects are more manageable for topical treatments, meaning that you can increase the dose and try to pair them with other treatments like microneedling or retinoic acid to further improve hair growth outcomes.
Can I Use Minoxidil If I Am Planning a Family?
Medical professionals generally advise against using both topical and oral minoxidil for both men and women when planning a family and for women during pregnancy and while breastfeeding.
While there is limited data on human pregnancies, animal studies have shown potential risks, including evidence of increased fetal resorption at high doses, one case report of fetal malformation associated with topical minoxidil use, and neonatal hypertrichosis reported following exposure during pregnancy.[34]Drugs. (2023). Minoxidil pregnancy and breastfeeding warnings. Drugs.com. Available at: https://www.drugs.com/pregnancy/minoxidil.html (Accessed: February 2025),[35]Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth defects research. Part A, Clinical and … Continue reading
There is a significant lack of well-controlled studies on minoxidil use during pregnancy and lactation. However, given the animal studies, medical professionals typically recommend avoiding minoxidil use when planning pregnancy, during pregnancy, and while breastfeeding, using adequate contraception if taking minoxidil, and discontinuing minoxidil use before attempting to conceive.[36]National Institute of Health and Care Excellence. (2021). Topical minoxidil. NICE. Available at: … Continue reading
Does Minoxidil Affect Male Fertility?
Current research suggests that minoxidil has minimal to no direct impact on male fertility. However, some research has linked minoxidil with oxidative stress and morphological changes to the testicles, which could indicate a potential negative impact.[37]Santana, F.F.V., Lozi, A.A., Goncalves, R.V., Silva, J.D., Matta, S.L.P.D. (2023). Comparative effects of finasteride and minoxidil on the male reproductive organs: A systematic review of in vitro … Continue reading
If you’re thinking about trying minoxidil and you are trying to conceive or have a pregnant partner, then it is advisable to talk to a medical professional before starting any treatment.
What if I’m Still Experiencing Side Effects?
Fortunately, minoxidil is just one of several treatment options available. If you’ve tried all of them and still experience side effects, you might consider exploring alternative therapies.
We have a wealth of information available so you can weigh your options and find out exactly how each treatment works and what your regrowth roadmap might look like. If you have any questions, reach out in the dedicated discussion thread below.
Final Thoughts
While minoxidil remains one of the most widely used treatments for AGA, both topical and oral formulations present unique challenges. The choice of which to use should be weighed carefully against the side effects, varying from mild scalp irritation to more significant cardiovascular effects. Ultimately, while the research supports minoxidil’s efficacy, it is not the only option out there, and if it isn’t working for you, then it is important to find the right one.
References[+]
References ↑1 Bryan, J. (2011). How minoxidil was transformed from an antihypertensive to hair-loss drug. The Pharmaceutical Journal. Available at: https://pharmaceutical-journal.com/article/news/how-minoxidil-was-transformed-from-an-antihypertensive-to-hair-loss-drug#:~:text=DAMN%2DO%20was%20effective%20in,seen%20in%20canine%20toxicity%20studies.&text=Despite%20the%20adverse%20effects%2C%20demand,week%20limit%20on%20treatment%20duration.&text=Owing%20to%20the%20drug’s%20effectiveness,of%20hypertrichosis%20began%20to%20emerge. (Accessed: February 2025) ↑2 Alhayaza, G., Hakami, A., AlMarzouk, L.H., Al Qurashi, A.A., Alghamdi, G., Alharithy, R. (2023). Topical minoxidil reported hair discoloration: a cross-sectional study. Dermatology Reports. 16(1). 9745. Available at: https://doi.org/10.4081/dr.2023.9745 ↑3 Shen, Y., Zhu, Y., Zhang, L., Sun, J., Xie, B., Zhang, H., Song, X. (2023). New Target for Minoxidil in the Treatment of Androgenetic Alopecia. Drug Design, Development and Therapy. 17. 2537-2547. Available at: https://doi.org/10.2147/DDDT.S427612 ↑4 Dhurat, R., Daruwalla, S., Pai, S., Kovacevic, M., McCoy, J., Shapiro, J., Sinclair, R., Vano-Galvan, S., Goren, A. (2021). SULT1A1 (Minoxidil Sulfotransferase) enzyme booster significantly improves response to topical minoxidil for hair growth. 21(1). 343-346. Available at: https://doi.org/10.1111/jocd.14299 ↑5 Anderson, R.J., Kudlacek, P.E., Clemens, D.L. (1998). Sulfation of minoxidil by multiple human cytosolic sulfotransferases. Chemico-Biological Interactions. 109. 53-67. Available at: https://doi.org/10.1016/S0009-2797(97)00120-8 ↑6 Lama, S.B.C., Pérez-González, L.A., Kosoglu, M.A., Dennis, R., Ortega-Quijano, D. (2024). Physical Treatments and Therapies for Androgenetic Alopecia. Journal of Clinical Medicine. 13(15). 4534. Available at: https://doi.org/10.3390/jcm13154534 ↑7 Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: https://doi.org/10.1080/09546634.2021.1945527 ↑8 Shadi, Z. (2023). Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy (Heidelb). 13(5). 1157-1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑9 Lessmann, H., Schnuch, A., Geier, J., Uter, W. (2005). Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 53(5). 247-259. Available at: https://doi.org/10.1111/j.0105-1873.2005.00693.x. ↑10 Ghonemy, S., Bessar, H., Alarawi, A. (2019). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. Journal of Dermatological Treatment. 32(2). 236-241. Available at: https://doi.org/10.1080/09546634.2019.1654070 ↑11 Kaiser, M., Abdin, R., Gaumond, S.I., Issa, T. N., Jiminez, J.J. (2023). Treatment of androgenetic alopecia: current guidance and unmet needs. Clinical Cosmetic and Investigational Dermatology. 16. 1387-1406. Available at: https://doi.org/10.2147/CCID.S385861 ↑12 Dawber, R.P.R, Rundegren, J. (2003). Hypertrichosis in females applying minoxidil topical solution and in normal controls. Journal of the European Academy of Dermatology and Venereology. 17(3). 271-275. Available at: https://doi.org/10.1046/j.1468-3083.2003.00621.x. ↑13 Gargallo V, Gutierrez C, Vanaclocha F, Guerra-Tapia A. Hipertricosis generalizada secundaria a minoxidil tópico. Actas Dermosifiliogr. 2015;106:599–600. Available at: https://doi.org/10.1016/j.adengl.2015.06.019 ↑14 Darendeliler, F., Bas, F., Balaban, S., Bundak, R., Demirkol, D., Saka, N., Gunoz, H. (1996). Spironolactone therapy in hypertrichosis. European Journal of Endocrinology. 135(5).604-608. Available at: https://doi.org/10.1530/eje.01350604 ↑15 Moussa, A., Kazmi, A., Bakhari, L., Sinclair, R.D. (2022). Bicalutamide improves minoxidil-induced hypertrichosis in female pattern hair loss: a retrospective review of 35 patients. Journal of the American Acadamy of Dermatology. 87(2). 488-490. Available at: https://doi.org/10.1016/j.jaad.2021.10.048 ↑16 Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑17 Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of Cosmetic Dermatology. 20. 3759-3781. Available at: https://doi.org/10.1111/jocd.14537 ↑18 Gungor, S., Kocaturk, E., Topal, I.O. (2015). Frontal Edema Due to Topical Application of %5 Minoxidil Solution Following Mesotherapy Injections. International Journal of Trichology. 7(2). 86-87. Available at: https://doi.org/10.4103/0974-7753.160124 ↑19 Patel, P., Nessel, T.A., Kumar, D. (2023). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (Accessed: February 2025) ↑20 Ponomareva, M.A., Romanova, M.A., Shapshnikova, A.A., Piavchenko, G.A. (2024). Topical Minoxidil Overdose in a Young Man with Androgenetic Alopecia: A Case Report. Cureus. 16(6). E62382. Available at: https://doi.org/10.7759/cureus.62382 ↑21 Dubrey, S.W., vanGriethuysen, J., Edwards, C.M.B. A hairy fall: syncope resulting from topical application of minoxidil. BMJ Case Reports. 1-2. Available at: https://doi.org/10.1136/bcr-2015-210945 ↑22 Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. Journal of the American Academy of Dermatology. 65(6). 1126-1134. Available at: https://doi.org/10.1016/j.jaas.2010.09.724 ↑23 Gupta, A.K., Hall, D.C., Talukder, M., Bamimore, M.A. (2022). There is a Positive Dose-Dependent Association between Low-Dose Oral Minoxidil and Its Efficacy for Androgenetic Alopecia: Findings from a Systematic Review with Meta-Regression Analyses. Skin Appendage Disorders. 8(5). 355-361. Available at: https://doi.org/10.1159/000525137 ↑24 Desai, D.D., Nohria, A., Brinks, A., Needle, C., Shapiro, J., Lo Sicco, K.I. (2024). Minoxidil-induced hypertrichosis: Pathophysiology, clinical implications, and therapeutic strategies. JAAD Reviews. 2. 41-49. Available at: https://doi.org/10.1016/j.jdrv.2024.08.002 ↑25 Trueb, R.M., Caballero-Uribe, N., Luu, N.N.C., Dmitriev, A. (2022). Serious complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 30. 97-98. Available at: https://doi.org/10.1016/j.jdcr.2022.09.035 ↑26 Patel.K., Omar, J. (2023). Low dose oral minoxidil causing peripheral edema and rapid weight gain. Journal of General Internal Medicine. 38(Suppl 3). S592. Available at: https://scholarlycommons.henryford.com/internalmedicine_mtgabstracts/162/ (Accessed: February 2024 ↑27 Trueb, R.M., Caballero-Uribe, N., Luu, N.N.C., Dmitriev, A. Serious complication of low-dose oral minoxidil for hair loss. JAAD Case Reports. 30. 97-98. Available at: https://doi.org/10.1016/j.jdcr.2022.09.035 ↑28 Ramírez-Marín, H.A., Tosti, A. (2022). Role of oral minoxidil in patterned hair loss. Indian Dermatology Online Journal. 13(6). 729-733. Available at: https://doi.org/10.4103/idoj.idoj_246_22 ↑29 Sanabria, B.D., Perdomo, Y.C., Miot, H.A., Ramos, P.M. (2024). Oral minoxidil 7.5 mg for hair loss increases heart rate with no change in blood pressure in 24 h ambulatory blood pressure monitoring. Anais Brasileiros de Dermatologia. 99(5). 734-736. Available at: https://doi.org/10.1016/j.abd.2023.08.016 ↑30 Sanabria, B.D., Perdomo, Y.C., Miot, H.A., Ramos, P.M. (2024). Oral minoxidil 7.5 mg for hair loss increases heart rate with no change in blood pressure in 24 h ambulatory blood pressure monitoring. Anais Brasileiros de Dermatologia. 99(5). 734-736. Available at: https://doi.org/10.1016/j.abd.2023.08.016 ↑31 Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., Salas-Callo, C.I., Vincenzi, C., Yin, L., Lo-Sicco, K., Waskiel-Burnat, A., Starace, M., Zamorano, J.L., Jaen-Olasolo, P., Piraccini, B.M., Rudnicka, L., Shapiro, J., Tosti, A., Sinclair, R., Bhoyrul, B. (2021). Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. Journal of the American Academy of Dermatology. 84(6). 1644-1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑32 Bokhari, L., Jones, L.N., Sinclair, R.D. (2021). Sublingual minoxidil for the treatment of male and female pattern hair loss: a randomized, double-blind, placebo-controlled, phase 1B clinical trial. Journal of the European Academy of Dermatology and Venereology. (36)1. E62-e66. Available at: https://doi.org/10.1111/jdv.17623 ↑33 Bokhari, L., Jones, L.N., Sinclair, R.D. (2021). Sublingual minoxidil for the treatment of male and female pattern hair loss: a randomized, double-blind, placebo-controlled, phase 1B clinical trial. Journal of the European Academy of Dermatology and Venereology. (36)1. E62-e66. Available at: https://doi.org/10.1111/jdv.17623 ↑34 Drugs. (2023). Minoxidil pregnancy and breastfeeding warnings. Drugs.com. Available at: https://www.drugs.com/pregnancy/minoxidil.html (Accessed: February 2025) ↑35 Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth defects research. Part A, Clinical and molecular teratology. 67(12). 997-1001. Available at: https://doi.org/10.1002/bdra.10095 ↑36 National Institute of Health and Care Excellence. (2021). Topical minoxidil. NICE. Available at: https://cks.nice.org.uk/topics/female-pattern-hair-loss-female-androgenetic-alopecia/prescribing-information/topical-minoxidil/ (Accessed: February 2025) ↑37 Santana, F.F.V., Lozi, A.A., Goncalves, R.V., Silva, J.D., Matta, S.L.P.D. (2023). Comparative effects of finasteride and minoxidil on the male reproductive organs: A systematic review of in vitro and in vivo evidence. Toxicology and Applied Pharmacology. 478. 11670. Available at: https://doi.org/10.1016/j.taap.2023.116710. Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreSarah King, PhD
Dr. Sarah King is a researcher & writer who holds a BSc in Medical Biology, an MSc in Forensic Biology, and a Ph.D. in Molecular and Cellular Biology. While at university, Dr. King’s research focused on cellular aging and senescence through NAD-dependent signaling – along with research into prostaglandins and their role in hair loss. She is a co-author on several upcoming manuscripts with the Perfect Hair Health team.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down