- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesWomen: Is Topical Minoxidil Safe While Trying to Conceive?
First Published Nov 12 2025Last Updated Nov 18 2025Pharmaceutical Researched & Written By:Michael Williams, PhD
Researched & Written By:Michael Williams, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Topical minoxidil is the most widely used treatment for female hair loss, but is it safe when trying to conceive? Here’s what current research says about fertility, pregnancy, and reproductive risks.
Full Article
Topical minoxidil is a first-line treatment for female pattern hair loss (FPHL) and other conditions such as telogen effluvium and traction alopecia. Many women of reproductive age use minoxidil and may wonder about its safety when trying to conceive or in early pregnancy.
While most reproductive safety discussions focus on oral or systemic medications, even topically applied drugs can raise questions about potential effects on fertility or early fetal development. Although systemic absorption from topical formulations is low, uncertainty persists regarding potential hormonal or embryonic effects.
This article examines available evidence to evaluate whether topical minoxidil poses any risk to female fertility or conception. By reviewing preclinical studies and clinical and pharmacological data, we aim to clarify whether there is any risk associated with topical minoxidil when trying to conceive.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Topical Minoxidil: Mechanism and Pharmacology
Minoxidil may promote hair growth through several complementary mechanisms. After topical application, the drug is enzymatically converted by sulfotransferase (SULT1A1) into minoxidil sulfate, its active form. This metabolite enhances potassium channel activity within follicular cells, promoting cell proliferation and prolonging the anagen (growth) phase of the hair cycle. Additionally, minoxidil increases vascularity around the follicle by stimulating vascular endothelial growth factor (VEGF), improving oxygen and nutrient delivery to support stronger, thicker hair growth.[1]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading
There are important pharmacological distinctions between topical and oral formulations. Topical minoxidil acts locally on the scalp with minimal systemic absorption, which significantly reduces the risk of adverse effects. In contrast, oral minoxidil can cause systemic side effects, such as fluid retention, weight gain, increased blood pressure (hypertension), and other cardiovascular changes.[2]StatPearls Editorial Team. (no date). Minoxidil. In: StatPearls [Internet]. StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (Accessed: October 2025)
Female Fertility: What Matters When Trying to Conceive?
Female fertility relies on coordinated hormonal cycles, healthy egg development, and a receptive uterine environment. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) regulate ovulation, while estrogen and progesterone prepare the uterus for implantation and support early pregnancy.[3]Thiyagarajan, D. K., Basit, H., & Jeanmonod, R. (2024 Jan 1). Physiology, menstrual cycle. In: StatPearls [Internet]. StatPearls Publishing. Available at: … Continue reading In addition to hormonal balance, healthy reproductive anatomy is important: clear fallopian tubes, a healthy uterus, and a cervix free of significant abnormalities are required for fertilization and embryo implantation.[4]Mayo Clinic Staff. (no date). Female infertility – Symptoms & causes. Available at: https://www.mayoclinic.org/diseases-conditions/female-infertility/symptoms-causes/syc-20354308/ (Accessed: … Continue reading
Of course, women, or individuals who are female at birth, looking to conceive also need to consider the impact of medications following conception. The safe use of medications is essential to support maternal health and healthy fetal development while minimizing risks of complications and birth defects (teratogenic effects).
Systemic medications can interfere with fertility and pregnancy by disrupting hormonal balance or exerting teratogenic effects. For example, antiandrogens such as spironolactone, cyproterone acetate, and finasteride, sometimes prescribed for female hair loss, are contraindicated when trying to conceive due to their potential impact on fetal development.[5]BinJadeed, H. F., & Alajlan, A. (2021). Pregnancy and neonatal outcome with maternal exposure to finasteride: a case series. Journal of Dermatology and Dermatologic Surgery. 25(2). 84-86. … Continue reading,[6]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading Animal models suggest these agents interfere with androgen signaling, which is essential for normal sexual differentiation, particularly in male fetuses.[7]Conley, J. M., Lambright, C. S., Evans, N., Cardon, M., Furr, J., Wilson, V. S., & Gray Jr, L. E. (2018). Mixed “antiandrogenic” chemicals at low individual doses produce reproductive tract … Continue reading In contrast, nutritional supplements like iron, vitamin D, and biotin are generally safe and can support overall reproductive health.
Systemic Absorption of Topical Minoxidil: How Much Gets In?
Topical minoxidil is formulated to act locally on the scalp, with only a small proportion entering systemic circulation – typically around 1–2%.[8]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading Pharmacokinetic studies show that this absorption rate can vary depending on several factors, including formulation strength (2% vs. 5%), frequency of application, and the integrity of the scalp barrier. Plasma minoxidil levels in individuals using topical formulations generally remain well below those associated with systemic pharmacologic or reproductive effects observed in animal studies.[9]Singh, S., Patil, A., Kianfar, N., Waśkiel-Burnat, A., Rudnicka, L., Sinclair, R., & Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical … Continue reading,[10]Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: … Continue reading
By contrast, oral minoxidil is almost completely absorbed, leading to significantly higher plasma concentrations and systemic exposure. This explains why oral therapy, though often more potent, also carries a greater risk of systemic side effects, including edema, tachycardia, and generalized hypertrichosis.[11]Randolph, M., & Tosti, A. (2021). Oral minoxidil treatment for hair loss: a review of efficacy and safety. Journal of the American Academy of Dermatology. 84(3). 737-746. Available at: … Continue reading
Does Topical Minoxidil Affect Female Fertility, Conception, or Pregnancy?
When considering the impact of medications on women’s fertility and ability to conceive, it is also essential to review the evidence regarding potential effects during pregnancy, as treatments will inevitably continue to be taken into early pregnancy, and systemic effects may be long-lasting. Here, we review preclinical and clinical evidence on whether topical minoxidil affects female fertility and pregnancy.
Preclinical Evidence
Minoxidil is classified as a non-hormonal treatment, suggesting it is unlikely to impact hormonal cycles that impact fertility. However, due to the relationship between hair growth and hormonal pathways, some preclinical research has focused on how minoxidil affects androgen pathways.
One cell-based study suggests that minoxidil can directly interact with and weaken androgen receptor signaling. However, this study used higher concentrations of minoxidil than would be used in hair loss treatment, and for practical purposes, topical minoxidil remains classified as a non-hormonal treatment.[12]Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: … Continue reading
However, in an animal model using golden Syrian hamsters, 5% topical minoxidil did not prevent the androgen-dependent growth of pigmented spots, suggesting that minoxidil does not have any effect on androgen response.[13]Nuck, B. A., Fogelson, S. L., & Lucky, A. W. (1987). Topical minoxidil does not act as an antiandrogen in the flank organ of the golden Syrian hamster. Archives of Dermatology. 123(1). 59-61. … Continue reading
There is a significant lack of research using preclinical animal models investigating the impact of topical minoxidil on female fertility and during pregnancy. However, one small-scale study did examine the effect of 5% topical minoxidil on embryonic mortality in rats. They found no impact of daily minoxidil application over the course of 20 days of pregnancy compared to the control group. However, the sample size was relatively small (20 rats in total), and no molecular or cellular analysis was performed to look for potential mechanisms that could cause embryonic lethality.[14]Turkina, V. A., Chemodurova, N. Y., Pryzyglei, H. V., & Kuzminov, Y. B. (2021). Potential embryotoxic effect study of minoxidil-containing lotion in experiment with female rats. Світ … Continue reading
Clinical Evidence
Unfortunately, a lack of research into the impact of medications on women and fetuses during pregnancy is a systemic issue within medicine. Pregnant women are generally excluded from clinical trials due to safety concerns, as well as the confounding impact of widespread hormonal changes associated with pregnancy.[15]Stock, S. J., & Norman, J. E. (2019). Medicines in pregnancy. F1000Research. 8(F1000Faculty Rev):911. Available at: https://doi.org/10.12688/f1000research.17535.1 These issues extend to women trying to conceive, and most clinical trials have explicit protocols that remove individuals from clinical trials after they become pregnant.[16]Phelan, A. L., Kunselman, A. R., Chuang, C. H., Raja-Khan, N. T., & Legro, R. S. (2016). Exclusion of women of childbearing potential in clinical trials of type 2 diabetes medications: a review … Continue reading
The safety of medications for pregnant women and their children is typically established after drugs have come to market, through observational studies and pharmacovigilance, which looks for cases where problems have arisen. While such processes are good at spotting signals of high risk, these forms of evidence are less powerful than clinical trials and cannot detect less impactful effects.
As such, determining the safety of drugs for pregnant women is often done using scarce information. As a result, many medications are contraindicated (their use is advised against) for pregnant women due to a lack of evidence, not because of evidence.[17]Thomas, S. H., & Yates, L. M. (2012). Prescribing without evidence – pregnancy. British Journal of Clinical Pharmacology. 74(4). 691-697. Available at: … Continue reading
Multiple reviews of the safety of dermatological treatments in pregnant women advise against the use of monoxidil.[18]Koh, Y. P., Tian, E. A., & Oon, H. H. (2019). New changes in pregnancy and lactation labelling: Review of dermatologic drugs. International Journal of Women’s Dermatology. 5(4). 216-226. … Continue reading,[19]Truong, T. M., Yaghi, M., & Murase, J. (2023). Dermatologic Drug Safety in Pregnancy. Journal of Dermatology for Physician Assistants. 17(3). 1-12. Available at: … Continue reading,[20]Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777–2786. Available at: … Continue reading However, this advice appears to be based on two case studies where topical minoxidil was theorized as the cause of the birth defects. Case studies present a single individual (or group of individuals) where an event or phenomenon has occurred. They are a weak form of evidence and are typically used as the basis for larger studies.
In one of these case studies, the pregnancy of a 28-year-old woman who was applying topical minoxidil was terminated due to the presence of fetal malformation. Topical minoxidil was suggested as a possible cause of teratogenesis.[21]Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., & Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth Defects Research Part A: Clinical … Continue reading The other study noted a case of a rare condition called caudal regression syndrome in a fetus where the mother had been using topical minoxidil, though other causes were also proposed.[22]Rojansky, N., Fasouliotis, S. J., Ariel, I., & Nadjari, M. (2002). Extreme caudal agenesis. Possible drug-related etiology?. Journal of the American Academy of Dermatology. 47(3). 241-245. … Continue reading These cases were published in 2002 and 2003, and both advised that further studies were warranted.
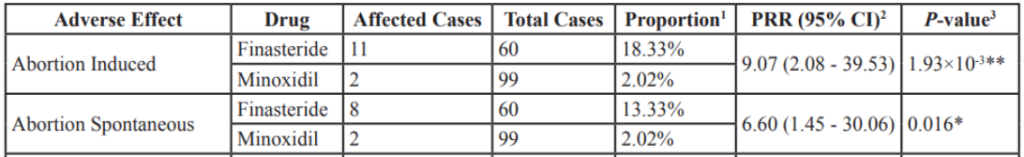
In a more systematic approach, one study analysed FDA Adverse Event Reporting System (FAERS) data, which collects evidence of adverse effects related to medications.[23]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading The study used data collected between 2004 and 2014. Over the ten years analyzed, 2 cases of induced abortion and 2 cases of spontaneous abortion were reported in women using minoxidil. This represents around 2% of cases reported. For comparison, induced abortion represented around 18% of reported adverse effects related to finasteride, for which it was the most commonly reported issue. No cases related to sexual dysfunction or loss of libido were reported.
Figure 1: Comparison between adverse events reported by female alopecia areata patients exposed to finasteride and minoxidil. Adapted from Table 4.[24]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading Image used in accordance with the PMC Creative Commons Licence.
It is due to the lack of evidence and the potential issues that these case studies raise that minoxidil is typically contraindicated for women who are trying to conceive or are pregnant. There is no evidence to suggest that minoxidil has any impact on female fertility. Larger observational and controlled studies would be required to make evidence-based conclusions regarding the safety of minoxidil for women trying to conceive.
Summary Consensus
There is no direct evidence linking topical minoxidil to reduced fertility. The use of topical minoxidil is advised against for women trying to conceive due to a small number of reported cases where topical minoxidil was associated with fetal abnormalities.
Counseling and Clinical Recommendations
The Food and Drug Administration (FDA) classifies topical minoxidil as a pregnancy category C drug. This means studies have shown a risk to the fetus, but there are no adequate, controlled studies in pregnant women. Such drugs are generally only prescribed if there is a significant benefit to the mother that outweighs potential risks. As such, a review published by the American Academy of Dermatology in 2025 advises against using minoxidil while pregnant.[25]Olsen, E. A., Sinclair, R., Hordinsky, M., Mesinkovska, N. A., Sadick, N., Shapiro, J., & Bergfeld, W. (2025). Summation and recommendations for the safe and effective use of topical and oral … Continue reading In the UK, the National Institute for Health and Care Excellence (NICE) also advises against the use of topical minoxidil when pregnant or breastfeeding.[26]National Institute for Health and Care Excellence. (no date). Female-pattern hair loss (female androgenetic alopecia) — Prescribing information: Topical minoxidil. Available at: … Continue reading
Does topical minoxidil cause infertility?
There is no evidence to suggest that topical minoxidil can impact fertility in women. There are a very limited number of cases reported where minoxidil has been associated with impotence in men.[27]Rietschel, R. L., & Duncan, S. H. (1987). Safety and efficacy of topical minoxidil in the management of androgenetic alopecia. Journal of the American Academy of Dermatology. 16(3). 677-685. … Continue reading
Does minoxidil interfere with female hormones or ovulation?
There is limited research into the effect of minoxidil on female hormones or ovulation. However, minoxidil is not a hormonal drug, and there is limited evidence to suggest that it interferes significantly with hormones at levels associated with topical treatment.
Should women stop minoxidil months before trying to conceive?
While there is no evidence that minoxidil can impact women’s ability to conceive, it is not advised to use the treatment while pregnant. Therefore, it is recommended to stop using minoxidil while trying to conceive.[28]Olsen, E. A., Sinclair, R., Hordinsky, M., Mesinkovska, N. A., Sadick, N., Shapiro, J., & Bergfeld, W. (2025). Summation and recommendations for the safe and effective use of topical and oral … Continue reading
Can minoxidil exposure cause miscarriage or birth defects?
There are limited case reports that associate topical minoxidil with birth defects and fetal malformation, leading to termination. These findings have not been expanded on by larger trials or observational studies.[29]Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., & Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth Defects Research Part A: Clinical … Continue reading,[30]Rojansky, N., Fasouliotis, S. J., Ariel, I., & Nadjari, M. (2002). Extreme caudal agenesis. Possible drug-related etiology?. Journal of the American Academy of Dermatology. 47(3). 241-245. … Continue reading
Do all hair loss drugs have the same reproductive risks?
Different hair loss treatments have fundamentally different mechanisms. Finasteride and dutasteride are hormonally active and may impact male fertility and fetal development.[31]BinJadeed, H. F., & Alajlan, A. (2021). Pregnancy and neonatal outcome with maternal exposure to finasteride: a case series. Journal of Dermatology and Dermatologic Surgery. 25(2). 84-86. … Continue reading,[32]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading Risks associated with minoxidil are significantly less well established, and adverse reproductive events have been shown to be significantly lower than finasteride.[33]Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. … Continue reading
Latest Research and Knowledge Gaps
There is a significant gap in our knowledge of whether minoxidil affects pregnancy and the mechanisms by which it might do so. Data from preclinical models has focused on hair cycle dynamics rather than reproductive parameters, with only limited research on its impact on androgenic hormonal pathways.
Both of the case studies that associated topical minoxidil with adverse pregnancy outcomes are over 20 years old, with no follow-up studies to better understand these reports. Clinical trials or observational studies would be necessary to make evidence-based assessments on the safety of minoxidil during pregnancy and its impact on female fertility.
Final Thoughts
Current evidence does not show that topical minoxidil impairs fertility or conception. However, because pregnancy safety data remain incomplete, discontinuation is recommended when planning conception – a precautionary measure rather than a proven necessity.
For women experiencing distressing hair loss, treatment decisions should balance cosmetic benefit with potential but unproven reproductive risk, ideally in consultation with a dermatologist or reproductive health specialist.
References[+]
References ↑1, ↑8 Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑2 StatPearls Editorial Team. (no date). Minoxidil. In: StatPearls [Internet]. StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (Accessed: October 2025) ↑3 Thiyagarajan, D. K., Basit, H., & Jeanmonod, R. (2024 Jan 1). Physiology, menstrual cycle. In: StatPearls [Internet]. StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK500020/ (Accessed: October 2025) ↑4 Mayo Clinic Staff. (no date). Female infertility – Symptoms & causes. Available at: https://www.mayoclinic.org/diseases-conditions/female-infertility/symptoms-causes/syc-20354308/ (Accessed: October 2025) ↑5, ↑31 BinJadeed, H. F., & Alajlan, A. (2021). Pregnancy and neonatal outcome with maternal exposure to finasteride: a case series. Journal of Dermatology and Dermatologic Surgery. 25(2). 84-86. Available at: https://doi.org/10.4103/jdds.jdds_33_21 ↑6, ↑23, ↑24, ↑33 Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. Available at: https://doi.org/10.18632/oncotarget.12617 ↑7 Conley, J. M., Lambright, C. S., Evans, N., Cardon, M., Furr, J., Wilson, V. S., & Gray Jr, L. E. (2018). Mixed “antiandrogenic” chemicals at low individual doses produce reproductive tract malformations in the male rat. Toxicological Sciences. 164(1). 166-178. Available at: https://doi.org/10.1093/toxsci/kfy069 ↑9 Singh, S., Patil, A., Kianfar, N., Waśkiel-Burnat, A., Rudnicka, L., Sinclair, R., & Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit?. Clinical and Experimental Dermatology. 47(11). 1951-1955. Available at: https://doi.org/10.1111/ced.15338 ↑10, ↑12 Hsu, C. L., Liu, J. S., Lin, A. C., Yang, C. H., Chung, W. H., & Wu, W. G. (2014). Minoxidil may suppress androgen receptor‐related functions. Oncotarget. 5(8). 2187-2197. Available at: /https://doi.org/10.18632/oncotarget.1886 ↑11 Randolph, M., & Tosti, A. (2021). Oral minoxidil treatment for hair loss: a review of efficacy and safety. Journal of the American Academy of Dermatology. 84(3). 737-746. Available at: https://doi.org/10.1016/j.jaad.2020.06.1009 ↑13 Nuck, B. A., Fogelson, S. L., & Lucky, A. W. (1987). Topical minoxidil does not act as an antiandrogen in the flank organ of the golden Syrian hamster. Archives of Dermatology. 123(1). 59-61. Available at: https://doi.org/10.1001/archderm.1987.01660250065019 ↑14 Turkina, V. A., Chemodurova, N. Y., Pryzyglei, H. V., & Kuzminov, Y. B. (2021). Potential embryotoxic effect study of minoxidil-containing lotion in experiment with female rats. Світ медицини та біології. (2). 248-251. Available at: https://doi.org/10.26724/2079-8334-2021-2-76-248-251 ↑15 Stock, S. J., & Norman, J. E. (2019). Medicines in pregnancy. F1000Research. 8(F1000Faculty Rev):911. Available at: https://doi.org/10.12688/f1000research.17535.1 ↑16 Phelan, A. L., Kunselman, A. R., Chuang, C. H., Raja-Khan, N. T., & Legro, R. S. (2016). Exclusion of women of childbearing potential in clinical trials of type 2 diabetes medications: a review of protocol-based barriers to enrollment. Diabetes Care. 39(6). 1004-1009. Available at: https://doi.org/10.2337/dc15-2723 ↑17 Thomas, S. H., & Yates, L. M. (2012). Prescribing without evidence – pregnancy. British Journal of Clinical Pharmacology. 74(4). 691-697. Available at: https://doi.org/10.1111/j.1365-2125.2012.04332.x ↑18 Koh, Y. P., Tian, E. A., & Oon, H. H. (2019). New changes in pregnancy and lactation labelling: Review of dermatologic drugs. International Journal of Women’s Dermatology. 5(4). 216-226. Available at: https://doi.org/10.1016/j.ijwd.2019.05.002 ↑19 Truong, T. M., Yaghi, M., & Murase, J. (2023). Dermatologic Drug Safety in Pregnancy. Journal of Dermatology for Physician Assistants. 17(3). 1-12. Available at: https://doi.org/10.58744/001c.88954 ↑20 Suchonwanit, P., Thammarucha, S., & Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777–2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑21, ↑29 Smorlesi, C., Caldarella, A., Caramelli, L., Di Lollo, S., & Moroni, F. (2003). Topically applied minoxidil may cause fetal malformation: a case report. Birth Defects Research Part A: Clinical and Molecular Teratology. 67(12). 997-1001. Available at: https://doi.org/10.1002/bdra.10095 ↑22, ↑30 Rojansky, N., Fasouliotis, S. J., Ariel, I., & Nadjari, M. (2002). Extreme caudal agenesis. Possible drug-related etiology?. Journal of the American Academy of Dermatology. 47(3). 241-245. Available at: https://pubmed.ncbi.nlm.nih.gov/11933692/ ↑25, ↑28 Olsen, E. A., Sinclair, R., Hordinsky, M., Mesinkovska, N. A., Sadick, N., Shapiro, J., & Bergfeld, W. (2025). Summation and recommendations for the safe and effective use of topical and oral minoxidil. Journal of the American Academy of Dermatology. 93(2). 457-465. Available at: https://doi.org/10.1016/j.jaad.2025.04.016 ↑26 National Institute for Health and Care Excellence. (no date). Female-pattern hair loss (female androgenetic alopecia) — Prescribing information: Topical minoxidil. Available at: https://cks.nice.org.uk/topics/female-pattern-hair-loss-female-androgenetic-alopecia/prescribing-information/topical-minoxidil/ (Accessed: October 2025) ↑27 Rietschel, R. L., & Duncan, S. H. (1987). Safety and efficacy of topical minoxidil in the management of androgenetic alopecia. Journal of the American Academy of Dermatology. 16(3). 677-685. Available at: https://doi.org/10.1016/S0190-9622(87)70087-5 ↑32 Wu, M., Yu, Q., & Li, Q. (2016). Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 7(50). 82074-82084. Available at: https://doi.org/10.18632/oncotarget.12617 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Michael Williams, PhD
Michael is a researcher and writer who holds a BSc in Bioscience, an MSc in Regenerative Medicine, and a PhD in Translational Biomedicine. He undertook his PhD research at Houston Methodist Research Institute, Texas, focusing on cell signaling in the ovarian cancer tumor microenvironment. He conducted postdoctoral research at Barts Cancer Institute in London, exploring cellular metabolism in acute myeloid leukemia. He has published work in a range of fields, including oncology, nanomedicine, and cell-based therapeutics.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down