- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesTopical Minoxidil for Hair Growth: Efficacy, Safety, and How to Enhance Results
First Published Jul 16 2025Last Updated Sep 19 2025Pharmaceutical Researched & Written By:Sarah King, PhD
Researched & Written By:Sarah King, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Topical minoxidil is the most established over-the-counter treatment for hair loss, with decades of use behind its FDA approval. But how does it actually work, and what determines whether it will work for you? In this article, we explore the science behind topical minoxidil, its strengths and limitations, key factors that influence its success, how to enhance results with add-ons like microneedling or retinoids, and what to expect over the course of months and years of use.
Full Article
Topical minoxidil is one of the most widely used treatments for hair loss, known for its accessibility, relatively low risk profile, and ability to slow or partially reverse androgenetic alopecia in both men and women. First developed as a blood pressure medication, it gained FDA approval in the 1980s after researchers observed its unexpected side effect, stimulating hair growth when applied to the scalp.
In this article, we examine the mechanisms of action, formulation differences, dosing strategies, long-term efficacy, side effects, and methods to enhance results through combination therapies, such as microneedling or retinoids. Whether you’re just starting your hair restoration journey or reassessing your regimen, this guide provides an evidence-based foundation to help you make informed decisions.
Key Takeaways
- What is it? Topical minoxidil is an over-the-counter, FDA-approved liquid or foam. It is usually supplied as 2% or 5% as standard (although it can be offered at higher concentrations). It acts locally and is converted to minoxidil sulfate by scalp SULT1A1.
- Clinical Data. Topical minoxidil has been demonstrated in multiple randomized controlled trials to increase hair count, prolong the anagen phase, and enhance patient satisfaction in the short term, particularly at a 5% concentration. However, long-term data reveal diminishing efficacy over time, with many users reporting waning results or plateauing benefits after 12–18 months, particularly when used without adjunctive therapies like microneedling or anti-androgens.
- Safety. Topical minoxidil is generally safe, with a low incidence of systemic side effects due to minimal absorption. The most common adverse reactions are local, including scalp irritation, itching, and flaking, which are primarily attributed to the presence of propylene glycol in liquid formulations. More severe side effects like hypertrichosis or cardiovascular symptoms are rare and typically linked to misuse or high-sensitivity individuals. It is highly toxic to cats and should be handled with care by pet owners.
- Evidence Quality. Topical minoxidil scored 97/100 for evidence quality by our metrics.
- Best Practices. Apply topical minoxidil to a dry scalp once or twice daily, using 1 mL of solution or half a capful of foam per session. Gently massage to enhance penetration and avoid washing the scalp for at least two hours after application. Foam formulations are often better tolerated due to their lack of propylene glycol. For enhanced results, consider combining this treatment with microneedling, topical retinoids to boost SULT1A1 activity, or anti-androgenic therapies to counteract ongoing miniaturization.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Topical Minoxidil?
Topical minoxidil formulations were originally developed in the early 1980s after an interesting side effect was discovered when it was used as an oral hypertensive agent. This side effect was hypertrichosis, or increased hair growth. The U.S. FDA approved topical minoxidil for male pattern hair loss in 1988 and for female pattern hair loss in 1991.[1]Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: … Continue reading
Minoxidil is a pro-drug: it requires enzymatic activation to exert its pharmacological effect on hair follicles. The key enzyme involved in this is sulfotransferase SULT1A1, which is highly expressed in the outer root sheath (ORS) of hair follicles.[2]Bacqueville, D., Jacques, C., Duprat, L., Jamin, E.L., Guiraud, B., Perdu, E., Bessou-Touya, S., Zalko, D., Duplan, H. (2017). Characterization of xenobiotic metabolizing enzymes of a reconstructed … Continue reading The level of SULT1A1 activity in the hair follicle correlates strongly with clinical response to topical minoxidil; individuals with low SULT1A1 activity are less likely to benefit from treatment.[3]Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a … Continue reading
Topical minoxidil typically comes in two main formulations:
- Solution: The original and most widely used form. Some use carrier agents like propylene glycol to improve solubility and scalp penetration, but it is a common cause of local irritation (itching, redness, flaking).[4]Epstein, G.K., Epstein, J., Cohen, J. (2019). Hair Loss in Men and Women: Medical and Surgical Therapies. 2(1). 161-176. Available at: https://doi.org/10.1016/j.yacs.2019.02.006
- Foam: Developed to address PG sensitivity. The foam formulation is propylene glycol-free, reducing the risk of irritation and allergic reactions. It is easier to apply, dries quickly, and is FDA-approved at 5% strength.[5]Lupatini, R., Sidhu, R., Patel, H., Bichar, K. (2021). Stability Evaluation of Minoxidil in FOAMIL Foam Base with Bracketing Study Design. International Journal of Pharmaceutical Compounding. 25(3) … Continue reading
Some options also offer propylene glycol-free solutions and gels. Some newer products use water-based or liposomal vehicles to cater to users with sensitive skin or allergies.
Why Might I Consider Topical Minoxidil Over Oral?
Many people opt for topical minoxidil over oral formulations due to key differences in safety, accessibility, and side effect profiles.
Over-the-Counter Access & fewer Barriers
Topical minoxidil is available over the counter, making it easy to obtain and start treatment promptly. Oral minoxidil, however, requires a prescription and is typically used off-label for hair loss, which can delay access and introduce hurdles within the healthcare system.
Minimal Systemic Exposure
Applying minoxidil directly to the scalp results in minimal absorption into the bloodstream. This localized delivery reduces the risk of systemic side effects that are more common with oral minoxidil, which circulates throughout the body.[6]Ashique, S., Sandhu, N.K., Haque, S.N., Koley, K. (2020). A Systemic Review on Topical Marketed Formulations, Natural Products, and Oral Supplements to Prevent Androgenic Alopecia: A Review. Natural … Continue reading
Lower Risk of Hypertrichosis, Edema, and Cardiac Events
Oral minoxidil demonstrates a high incidence of hypertrichosis, whereas in topical minoxidil, it is usually localized and linked to misuse.[7]Jiminez-Cauhe, J., Sicco, K.I.L., Shapiro, J., Hermosa-Gelbard, A., Burgos-Blasco, P., Melian-Olivera, A., Ortega-Quijano, D., Pindado-Ortega, C., Buendia-Castano, D., Asz-Sigall, D., Vano-Galvan, S. … Continue reading
Similarly, edema (fluid retention/swelling) and cardiac side effects (such as tachycardia, pericardial effusion, and exacerbation of angina) are rare with topical use but do have the potential to cause issues for those with preexisting cardiovascular, renal, or hepatic conditions.[8]do Nascimento, I.J.B., Harries, M., Rocha, V.B., Thompson, J.Y., Wong, C.H., Varkaneh, H.K., Guimaraes, N.S., Arantes, A. J. R., Marcolini, M.S. (2020). Effect of Oral Minoxidil for Alopecia: … Continue reading
How Does Topical Minoxidil Work?
Topical minoxidil stimulates hair growth through a variety of interconnected biological mechanisms, many of which have been elucidated in peer-reviewed research.
Local Vasodilation and VEGF Up-Regulation
Minoxidil acts as a potent vasodilator by opening potassium channels in vascular smooth muscle, leading to increased blood flow around hair follicles. This enhanced microcirculation delivers more oxygen and nutrients to the follicular environment, creating conditions favorable for hair growth.
Additionally, minoxidil upregulates the expression of vascular endothelial growth factor (VEGF) in dermal papilla cells. VEGF is a key mediator of angiogenesis, supporting the development and maintenance of the follicular blood supply necessary for robust hair growth.[9]Zeltzer, A.A., Keren, A., Paus, R., Gilhar, A. (2024). Topical minoxidil rejuvenates hair follicles from men with androgenetic alopecia in vivo. Acta Dermato Venereologica. 104(24213). Available at: … Continue reading
Anagen Induction and Telogen Shortening
One of the hallmark effects of topical minoxidil is its ability to induce the anagen (growth) phase of the hair cycle and shorten the telogen (resting) phase.[10]Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study … Continue reading By prompting resting hair follicles to re-enter the growth phase more rapidly, minoxidil effectively “resets” the hair cycle, leading to increased hair density and thickness over time. This is a central reason for its clinical efficacy in treating AGA and other hair loss disorders.
Wnt/ꞵ-Catenin Activation in Dermal Papilla
Recent studies have shown that minoxidil activates the Wnt/ꞵ-catenin signaling pathway within dermal papilla cells. This pathway is essential for hair follicle development, regeneration, and maintenance. Activation of ꞵ-catenin dermal papilla cells prolongs the anagen phase and supports the proliferation and differentiation of follicular cells, further enhancing hair growth.[11]Kwack, M.H., Kang, B.M., Kim, M.K., Kim, J.C., Sung, Y.K. (2011). Minoxidil activates ꞵ-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. … Continue reading
Prostaglandin Modulation (PGE2)
Minoxidil has been found to increase the production of prostaglandin E2 (PGE2) by activating prostaglandin synthase-1 (PGHS-1) in dermal papilla fibroblasts. Elevated PGE2 levels are associated with hair growth promotion, possibly by providing cytoprotective effects and modulating local inflammation within the follicle environment.[12]Michelet, J.F., Commo, S., Billoni, N., Mahe, Y.F., Bernard, B.A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating … Continue reading This prostaglandin modulation may also counteract the inhibitory effects of certain nonsteroidal anti-inflammatory drugs (NSAIDs).
Penetration Factors: Vehicle, Skin Barrier, and Enzymes
The effectiveness of topical minoxidil depends significantly on its ability to penetrate the scalp and reach the hair follicle. Only about 1.4% of applied minoxidil is typically absorbed through intact skin.[13]Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: … Continue reading
The formulation’s vehicle (e.g., alcohol-based solution, foam, or novel delivery systems like cetosomes), the integrity of the skin barrier (stratum corneum), and the presence of activating enzymes (notably sulfotransferase SULT1A1 in the outer root sheath) all influence absorption and efficacy.[14]Sattur, S. Talathi, A., Shetty, G., Arsiwala, S., Pereira, R., Dhoot, D. (2023). Comparative Clinical Study Evaluating the Efficacy and Safety of Topical 5% Cetosomal Minoxidil and Topical 5% … Continue reading
Dosing and Application Logistics
Once vs Twice Daily – What the Evidence Shows
Clinical studies have demonstrated that once-daily application of 5% topical minoxidil achieves hair count improvements comparable to twice-daily use of the 2% solution in men with AGA.[15]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading In long-term studies, men who switched from twice daily to once-daily minoxidil maintained most of their hair gains, though there was a slightly greater mean loss in those on the once-daily regimen compared to those who remained on twice-daily dosing.[16]Olsen, E.A., DeLong, E.R., Weiner, M.S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. Journal of the American Academy of Dermatology. 16(3 Pt 2). … Continue reading
Importantly, using 5% minoxidil twice daily can provide an incremental benefit, approximately 10-15% greater hair regrowth, over once-daily 5% application or twice-daily 2% solution, though this comes with a higher risk of local irritation.[17]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
For women with FPHL, randomized clinical trials have shown that once-daily application of 5% minoxidil foam is non-inferior to twice-daily use of the 2% minoxidil solution in terms of hair regrowth and target area hair counts.[18]Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the … Continue reading Both regimens lead to similar improvements, but the once-daily 5% foam offers a significant compliance advantage, as it is easier to incorporate into daily routines and is associated with fewer reports of scalp irritation.[19]Ramos, P.M., Melo, D.F., Radwanski, H., de Almeida, R.F.C., Miot, H.A. (2023). Female-pattern hair loss: therapeutic update. Anais Brasileiros de Dermatologica. 98(4). 506-519. Available at: … Continue reading This practical benefit makes once-daily 5% foam a preferred initial therapy for many women.
Choosing a Topical Minoxidil Concentration
Selecting the right minoxidil concentration depends on your goals, tolerance, and clinical context.
2% Minoxidil
- Profile: Traditionally labeled for women, especially those with sensitive scalps or mild hair loss.
- Efficacy: Offers modest improvement in hair counts and density; superior to the placebo but less effective than higher strengths.[20]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
- Tolerability: Minimal irritation and side effects; preferred for those prone to dermatitis or with a low threshold for adverse reactions.
- Best for: Mild AGA, sensitive skin, or those who prioritize safety over maximal regrowth.
5% Minoxidil
- Profile: The most widely studied and FDA-approved concentration for both men and women.
- Efficacy: Consistently outperforms 2% for both hair counts and patient satisfaction; considered the best balance between response rate and tolerability.[21]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
- Tolerability: Slightly higher risk of local irritation (itching, redness, flaking) than 2%, but generally well-tolerated, especially in foam (propylene glycol-free) formulations.
- Best for: Most patients with AGA seeking robust, evidence-based results.
7% Minoxidil
- Profile: Custom-compounded, not commercially available or FDA-approved; sometimes used for those with low follicular sulfotransferase activity (“enzyme low-responders”).
- Efficacy: May offer incremental benefit for select non-responders to 5%, though supporting data are limited and mixed.[22]Singh, S., Patil, A., Kianfar, N., Waskiel-Burnat, A., Rudnicka, L., Sinclair, R., Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit? … Continue reading
- Tolerability: Higher risk of contact dermatitis due to increased propylene glycol and alcohol content; requires careful monitoring for irritation.
- Best for: Patients who do not respond to 5% and have confirmed low enzyme activity, under clinical supervision.
10-15% Minoxidil
- Profile: Compounded, not FDA-approved; considered a last-line topical option before transitioning to oral minoxidil.
- Efficacy: Evidence is conflicting; some studies suggest no added benefit over 5%, while others report modest gains in select cases. Side effects increase substantially with higher concentrations.[23]Vasantha, K.L. (2025). Efficiency and Safety of 10% Minoxidil in the Treatment of Alopecia Areata: A Randomised Controlled Trial. Journal of Population Therapeutics and Clinical Pharmacology. 32(4). … Continue reading,[24]Ghonemy, S., Alarawi, A., Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic … Continue reading
- Risks: Marked increase in scalp irritation, dermatitis, and cost. Formulations typically require more propylene glycol and alcohol for solubility, compounding the risk of adverse reactions.
- Best for: Refractory cases under specialist care, when all lower strengths have failed, and before considering oral therapy.
Vehicle and Formulation Tweaks
There are a number of options you can take in terms of formulation or additional treatments to improve the efficacy of topical minoxidil.
Foam vs. Liquid: Propylene Glycol, Irritation, and Drying Time
Liquid minoxidil formulations can contain propylene glycol, which improves drug solubility and skin penetration.[25]Grice, J.E., Ciotti, S., Weiner, N., Lockwood, P., Cross, S.E., Roberts, M.S. (2010). Relative uptake of minoxidil into appendages and stratum corneum and permeation through human skin in vitro. … Continue reading However, propylene glycol is a frequent cause of scalp irritation; itching, redness, and flaking are common complaints, especially among users with sensitive skin.[26]Patel, K., Palmer, A., Nixon, R. (2023). Allergic contact dermatitis from propylene glycol: A case series from Australia. Contact Dermatitis. 89(2). 79-84. Available at: … Continue reading
Foam minoxidil was specifically developed to avoid propylene glycol usage and minimize irritation. As a result, foam is generally much better tolerated, especially for individuals prone to dermatitis or scalp discomfort.[27]Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of … Continue reading Additionally, the foam dries faster than the traditional liquid, making it more convenient for daily use.
Efficacy: Most comparative studies and clinical experience suggest the 5% foam and 2% liquid are equivalent in hair regrowth when used appropriately; the choice often comes down to scalp tolerance and application preference.[28]Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once-daily versus 2% minoxidil solution twice daily in the … Continue reading
Propylene Glycol Free, Fast-Drying, Glycerin-Based Recipes.
For patients unable to tolerate propylene glycol or those seeking even less greasy, faster-drying options, newer propylene glycol-free formulations have been developed. These often use other solvents or humectants, with in vitro and clinical studies showing similar efficacy to products containing propylene glycol.[29]Barbareschi, M., Vescovi, V., Starace, M., Piraccini, B.M., Milani, M. (2020). Propylene glycol free 5% minoxidil lotion formulation: cosmetic acceptability, local tolerability, clinical efficacy and … Continue reading
Addition of Retinol/Tretinoin (0.01%-0.025%) to Up-Regulate SULT1A1
As mentioned above, SULT1A1 is the key enzyme in the hair follicle that activates minoxidil by converting it to minoxidil sulfate, its effective form. Retinoids, notably tretinoin (0.01-0.025%), can be added to topical regimens to upregulate SULT1A1 in the outer root sheath, enhancing the local activation of minoxidil.[30]Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvin, S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in … Continue reading
The mechanism appears to involve both increased skin permeability and direct modulation of SULT1A1 gene expression, making this combination particularly useful for patients with known or suspected low enzyme activity.
Topical Minoxidil Efficacy
When assessing the benefits of topical minoxidil, it’s critical to consider how response rates, regrowth rates, and especially months of use influence real-world outcomes for individuals with AGA. While many early studies highlight minoxidil’s promise, longer-term data reveal a more nuanced reality.
Short-Term Response
Within 3-6 months, minoxidil demonstrates high response rates. Surveys and clinical studies consistently show that roughly 60% of men perceive a meaningful response (slowing, stopping, or reversal of hair loss) in this window (as per self-assessment and investigator evaluations).[31]Asilian, A., Farmani, A., Saber, M. (2023). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: A randomized controlled trial. … Continue reading
Long-Term Response
After one year or longer, response rates drop dramatically. Multiple studies report rates below 30%, and even lower in five-year follow-up populations, with only about 20-30% of users satisfied with the results.[32]Olsen, E.A., Weiner, M.S., Amara, I.A., DeLong, E.R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. Journal of American Academy of Dermatology. 22(4). … Continue reading
Real-world data indicate discontinuation rates of 86–95% by the one-year mark. The leading reason cited by users for stopping? “Low effect,” or disappointment with the cosmetic improvement offered by the drug.[33]Shadi, Z. (2023) Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy. 13(5). 1157-1169. Available at: … Continue reading
Regrowth Rate: Short-Lived Gains
In one pivotal trial, men using 5% minoxidil twice daily saw a nearly 60% increase in hair “weight” (total mass of regrown/thickened hair) at 6 months. By 96 weeks, gains had diminished to just 25% above the baseline, a significant drop from the initial response.[34]Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study … Continue reading
Over 48 weeks, users displayed a 12% rise in hair count compared with 3% for placebo (statistically significant). Still, these increases in hair number did not always correspond to better visible scalp coverage when judged by experts, indicating that raw hair counts may overstate clinical benefit.
Why Does Efficacy Diminish With Time?
Lack of DHT Targeting and Ongoing Miniaturization
Minoxidil’s primary action is to stimulate hair follicles into a growth (anagen) phase; however, it does not block the effects of DHT, the androgen responsible for follicle miniaturization in genetic hair loss.[35][Updated 2023 Feb 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482378/ Accessed: July 2025 Therefore, hair shafts may still become thinner despite greater numbers, diminishing real-world cosmetic impact.
Fibrosis: The Hidden Limitation
Emerging evidence points to fibrosis, the gradual formation of restrictive, scar-like tissue around miniaturizing follicles, as a rate-limiting factor in AGA.[36]Trueb, R.M., Dias, M.F.R.G., Rezende, H.D. (2021). Comment on Follicular Inflammation and Fibrosis in Pattern Hair Loss. Skin Appendage Disorders. 7(2). 159-160. Available at: … Continue reading Minoxidil does not directly address this fibrotic process, so even if follicles are nudged back into growth, their ability to produce robust hair continues to decline as surrounding tissue stiffens and shrinks.
The Limits of Hair Count as a Metric
Statistical increases in hair count do not guarantee clinical satisfaction. Studies repeatedly show that even impressive numeric gains in hair counts (or surrogate measures, such as hair mass index) are not always matched by noticeable differences in scalp coverage or user satisfaction.[37]Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study … Continue reading Patient dissatisfaction and discontinuation remain high in the absence of true visual improvement, underscoring the practical limits of relying on surrogate endpoints.
Combining Minoxidil with Microneedling
Recent clinical trials have shown that adding microneedling to minoxidil treatment can significantly enhance both response and regrowth rates. One landmark study reported a nearly fourfold greater increase in hair count (approximately a 40% gain) compared to minoxidil alone at 12 weeks, with participants experiencing actual visible improvements in density.[38]Ahmed, K.M.A., Kozaa, Y.A., Abuawwad, M.T., Al-Najdawi, A.I.A., Mahmoud, Y.W., Ahmed, A.M., Taha, M.J.J., Fadhli, T., Giannopoulou, A. (2025). Evaluating the efficacy and safety of combined … Continue reading
A six-month trial showed an 85% effective rate and a significant increase in hair count for the combination approach.[39]Zhang, Y., Sheng, Y., Zeng, Y., Hu, R., Zhao, J., Wang, W., Yang, Q. (2022). Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair … Continue reading
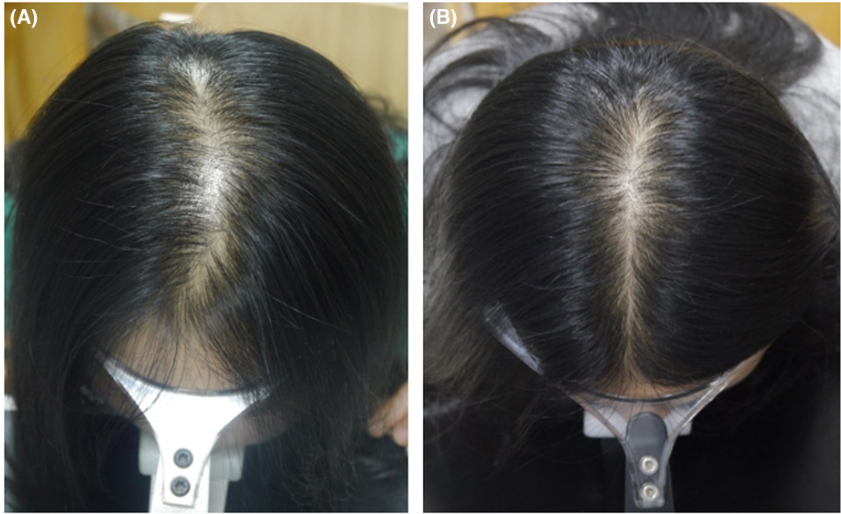
Figure 1: A 35-year-old female patient with 5 years of FPHL in group 2 (combination treatment) at baseline and endline.[40]Zhang, Y., Sheng, Y., Zeng, Y., Hu, R., Zhao, J., Wang, W., Yang, Q. (2022). Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair … Continue reading
Why Does Microneedling Work So Well?
- Improved drug absorption: Microneedling transiently disrupts the scalp’s barrier, allowing minoxidil to penetrate more effectively.
- Enzyme upregulation: The procedure may increase the local activity of sulfotransferase enzymes needed for minoxidil activation, potentially overcoming a key bottleneck for some non-responders.[41]Sharma, A., Surve, R., Dhurat, R., Sinclair, R., Tan, T., Zou, Y., Ramos, M.P., Wambier, C., Vernier, I., Kovacevic, M., Goren, A. (2020). Microneedling improves minoxidil response in androgenetic … Continue reading
- Counteracting fibrosis: Early research suggests that microneedling may help remodel fibrotic tissue, making the scalp environment more conducive to robust hair regrowth.
Non-Responder Pathway (SULT1A1-Guided)
For people who don’t respond adequately to topical minoxidil, several strategies can help overcome this resistance and optimize hair growth.
Increase Topical Minoxidil Concentration (7-10%)
Raising the concentration of topical minoxidil beyond 5%, to compounded strengths such as 7% or 10%, is a common clinical approach for patients identified as poor responders. The rationale is that higher drug levels may compensate, at least partially, for suboptimal enzymatic conversion of minoxidil to its active sulfate form in the outer root sheath.
Add Topical Retinoic Acid (0.01-0.05%)
Topical retinoic acid (such as tretinoin) can be combined with minoxidil to upregulate SULT1A1 activity, thereby enhancing the local activation of minoxidil in the hair follicle. Peer-reviewed research indicates that applying topical tretinoin upregulates follicle sulfotransferase enzymes, thereby enhancing the response to minoxidil.[42]Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvan, S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in … Continue reading
Incorporate Weekly Microneedling (0.5-1.5 mm)
Microneedling is an increasingly popular adjunctive treatment for hair loss, particularly in individuals who do not respond to minoxidil. Weekly sessions using devices with needles 0.5–1.5 mm in length create controlled micro-injuries in the scalp, stimulating growth factors, activating dermal papilla cell signaling (such as Wnt/β-catenin), and boosting SULT1A1 enzyme expression.[43]Dhurat, R., Sukesh, M.S., Avhad, G., Dandale, A., Pal, A., Pund, P. (2013). A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study. International … Continue reading,[44]Kumar, K.M., Inamadar, A.C., Palit, A. (2018). A Randomized Controlled, Single-Observer Blinded Study to Determine the Efficacy of Topical Minoxidil plus Microneedling versus Topical Minoxidil Alone … Continue reading,[45]Zhang, Y., Sheng, Y., Zeng, Y., Hu, R., Zhao, J., Wang, W., Yang, Q. (2022). Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair … Continue reading
Switch to or Add Low-Dose Oral Minoxidil
Low-dose oral minoxidil, typically 0.25–5 mg daily, offers systemic activation, as minoxidil is converted to its active sulfate in the liver (where SULT1A1 levels are abundant).[46]Ramirez-Marin, H.A., Tosti, A. (2022). Role of Oral Minoxidil in Patterned Hair Loss. Indian Dermatology Online Journal. 13(6). 729-733. Available at: https://doi.org/10.4103/idoj.idoj_246_22 For individuals whose follicular SULT1A1 is insufficient for topical efficacy, oral minoxidil can bypass the need for high local enzyme activity. Recent comprehensive reviews and clinical experience suggest that low-dose oral minoxidil is effective for hair regrowth in male and female pattern hair loss, with a favorable safety profile when started at low dosages.[47]Gupta, A.K., Talukder, M., Shemer, A., Piraccini, B.M., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: … Continue reading
Explore Compounded Minoxidil Sulfate (Direct Active Form)
Compounding pharmacies can prepare topical formulations containing minoxidil sulfate, the direct, active metabolite, bypassing the need for SULT1A1 conversion. Though theoretically attractive for SULT1A1 nonresponders, minoxidil sulfate is chemically less stable than parent minoxidil and is also more expensive.[48]Chemical Book. (no date). Minoxidil Sulphate. ChemBook. Available at: https://www.chemicalbook.com/Price/Minoxidil-sulphate.htm Accessed: July 2025,[49]Shan, Y., Xu, C., Guo, Y., Wen, L., Zhou, S., Fang, L., Xu, J., Zhen, H. (2025). Liposomes enhance the hair follicle delivery of minoxidil sulfate with improved treatment of androgenetic alopecia. … Continue reading Its shelf-life, risk of degradation, and limited availability currently constrain its widespread clinical use. Efficacy and safety data are lacking beyond case reports and experimental settings, and this approach remains investigational.
What To Expect Time-Line Wise
Understanding the typical milestones and changes during minoxidil treatment helps set realistic expectations, avoids premature discontinuation, and guides regimen adjustments for optimal hair regrowth. Here’s a week-by-week and month-by-month breakdown based on clinical evidence and peer-reviewed research.
Weeks 4-6: The Shedding Spike (“Telogen Synch”)
What happens: Many users notice an initial increase in hair shedding after starting minoxidil, usually peaking around weeks 4–6.
Why: This effect, known as telogen effluvium, occurs as minoxidil accelerates the transition of hair follicles from the resting (telogen) to the active (anagen) growth phase. Older, miniaturized hairs are shed to make way for new ones.
What to do: This shedding is temporary and generally indicates that the treatment is starting to take effect. Stopping minoxidil because of early shedding is unnecessary and may forfeit later gains.
Month 3: Early Vellus Thickening and the First Cosmetic Hints
What happens: Fine, soft “vellus” hairs begin to thicken. With combination treatments (such as minoxidil plus microneedling), these changes may be more pronounced and start to become cosmetically noticeable.
Why: Follicles kickstart new anagen cycles, and some can already be seen transitioning to more pigmented, visible hairs.[50]Rafi, A.W., Katz, R.M. (2011). Pilot study of 15 patients receiving a new treatment regimen for androgenic alopecia: the effects of atopy on AGA. ISRN Dermatology. 11. 241953. Available at: … Continue reading
Expectations: While cosmetic gains are modest at this stage using monotherapy, up to 60–74% of users report improvement in density and coverage when asked at 3–4 months.[51]Rundegren, J. (2004). Rapid onset of action of minoxidil 5% topical solution in a 4-month German observational study on both patients and physicians. Journal of the American Academy of Dermatology. … Continue reading
Months 6 – 8: Peak Visible Gains (Most Noticeable Results) and Plateau
What happens: The majority of the visible thickening and coverage improvements emerge by 6-8 months. Around this time, many people also see gains plateau and further visible regrowth slows considerably
Clinical evidence: At this point, studies show peak increases in terminal hair count and density (e.g., a 12–15% rise for standard 5% twice-daily solutions in men).[52]Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and … Continue reading
What to do: Regimen reevaluation is appropriate here; ensure ongoing compliance, assess satisfaction, and consider any adjuncts or boosters if progress is suboptimal. If additional improvement is desired, this is the window to explore adjunctive approaches, such as adding microneedling, retinoids, or anti-androgen therapies, to enhance or sustain growth.
Month 9 +: Gradual Waning Without Additional Support
What happens: Many users see diminishing improvements after 9 months; some may even experience slow retreating benefits despite continued use.
Why: Minoxidil alone does not address the underlying drivers of androgenetic alopecia, namely, DHT-mediated follicle miniaturization and progressive perifollicular fibrosis. Without combining minoxidil with anti-DHT agents or regenerative wounding therapies, natural progression often limits sustained gains.
Long-term evidence: Only ~30% of users report being satisfied after several years; discontinuation is common, with the lack of a visible effect being the primary reason.[53]Shadi, Z. (2023) Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy. 13(5). 1157-1169. Available at: … Continue reading
Safety and Side Effects
Dermatitis (2-6%): Mainly Propylene Glycol
- Incidence: Irritant or allergic contact dermatitis occurs in ~2-6% of users.
- Primary Cause: Most cases are induced by propylene glycol, a key solvent in liquid minoxidil solutions, rather than by minoxidil itself.[54]Jadeed, H.B., Almudimeegh, A.M., Alomran, S.A., Alshathry, A.H. (2021). A Case of Contact Allergic Dermatitis to Topical Minoxidil. Cureus. 13(1). E12510. Available at: … Continue reading
- Symptoms: Itching, redness, scaling, and sometimes localized swelling.
- Management: Switching to propylene glycol-free foam formulations or compounded solutions using alternative solvents (like glycerol or butylene glycol) can resolve symptoms for sensitive users. Those with confirmed allergy to minoxidil itself should avoid all forms.
Scalp Dryness and Flaking
- Presentation: Dryness and flaking of the scalp are common, primarily due to the alcohol and propylene glycol content in the formulation.
- Management:
- Use gentle, sulfate-free shampoos.
- Prefer shorter contact times and allow the product to dry completely.
- Consider propylene glycol or alcohol-free versions when persistent dryness occurs.
Facial Hypertrichosis
- Incidence: Mild, reversible facial hair growth (hypertrichosis) may develop, especially with high doses or accidental application beyond the intended area, such as the scalp.
- Course: Usually appears within 2-5 months of starting minoxidil and typically resolves within one to five months after stopping or reducing use.
- Mechanism: Thought to result from inadvertent transfer or high follicular sensitivity, not systemic toxicity.[55]Chellini, P.R., Pirmez, R., Raso, P., Sodre, C.T. (2015). Generalized hypertrichosis induced by topical minoxidil in an adult woman. International Journal of Trichology. 7(4). 182-183. Available at: … Continue reading
Toxicity to cats (prevalence: low if you’re careful; high if you’re reckless)
- Cats lack the enzyme required to metabolize minoxidil, much like dogs lack the enzymes required to quickly metabolize substances within chocolate. Resultantly, minoxidil is highly toxic to cats.[56]DeClementi, C., Bailey, K.L., Goldstein, S.C., Orser, M.S. (2004). Suspected toxicosis after topical administration of minoxidil in 2 cats. Veterinary Emergency & Critical Care. 14(4). 287-292. … Continue reading From 2001 to 2014, there were four reported feline deaths from minoxidil exposure.
- This doesn’t mean that if you have cats, you can’t use minoxidil. It simply means that you need to take extra precautions during storage, application, and drying, and ensure your cats don’t come into contact with the drug (even through their fur).
Heart palpitations (prevalence: very low)
- There is some systemic absorption from the use of topical minoxidil. As such, a small percentage of users have reported blood pressure-related effects ranging from dizziness to heart palpitations.[57]Leenen, F.H., Smith, D.L., Unger, W.P. (1988). Topical minoxidil: cardiac effects in bald man. British Journal of Clinical Pharmacology. 26(4). 481-485. Available at: … Continue reading
Stopping Topical Minoxidil
Discontinuing topical minoxidil is a significant decision, as virtually all clinical evidence and real-world experience indicate that hair gains made during treatment will be lost, usually within 3 to 12 months. This process is driven by the underlying physiology of follicles and the progressive nature of AGA.
The Physiology Behind Minoxidil Withdrawal
- Follicular Cycling: When minoxidil is stopped, hair follicles revert to their pre-treatment anagen (growth phase) timing within approximately three months. This leads to a synchronized shedding phase and a temporary drop in hair density, often falling below pre-treatment baseline before returning to the genetic trajectory over the subsequent months.
- Rebound Shedding: After ceasing minoxidil, the majority of users experience significant shedding, which can sometimes be more aggressive than before they began treatment. Typically, this shedding lasts for 3–6 months, but can persist for up to a year in some cases. The extent of the shed varies from person to person, but those who respond best to the drug are usually most affected, simply because they maintain or regrow more hair than they lose.
Evidence in Clinical Studies
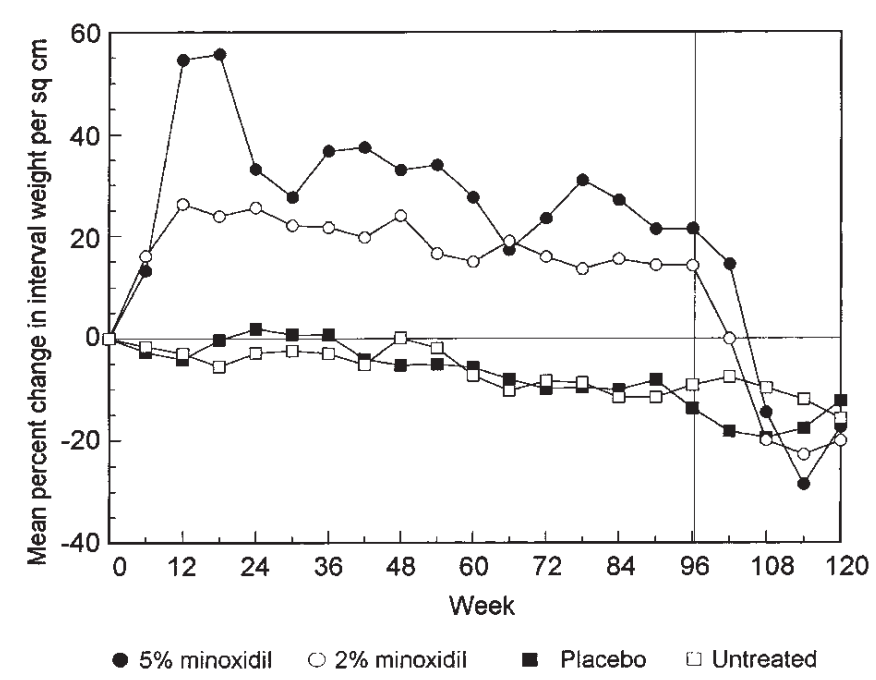
One 96-week study on both 2% and 5% topical minoxidil found that after treatment was halted, hair counts and weight fell below both baseline and placebo levels within 3 months.[58]Price, V.H., Menefee, E., Strauss, P.C. (1999). Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. … Continue reading By six months post-withdrawal, most subjects’ hair counts rebounded to baseline, indicating the temporary and reversible effects of minoxidil therapy in AGA.
Figure 2: Comparison of mean percentage change in interval weight per square centimeter for 4 treatment groups. Vertical line at 96 weeks marks cessation of treatment.[59]Price, V.H., Menefee, E., Strauss, P.C. (1999). Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. … Continue reading
Tapering Off: Protocol to Blunt Rebound Shedding
Abruptly ceasing minoxidil use (“cold turkey”) tends to induce more severe and acute shedding. To mitigate this shock to the hair cycle and scalp, expert opinion and clinical guidelines recommend a gradual taper, similar to protocols used for oral minoxidil or other hair loss medications.
A 6-month Tapering Schedule could look like this.
Weeks Protocol Description 1-2 Alternate once-daily and twice-daily applications 3-4 Three days once-daily, one day twice-daily 5-6 Once daily, Monday-Friday, twice on weekends 7-9 Once daily 10-12 Once daily on weekdays, off weekends 13-15 Once daily, Monday-Thursday, off Friday-Sunday 16-18 Apply every other day 19 Every third day 20 Every fourth day 21 Every fifth day 22 Every sixth day 23 Once weekly, then discontinue Bridging Strategies During the Transition
No protocol can eliminate post-minoxidil shedding, but supporting the scalp with additional therapies may enhance retention and cushion the transition.
- Microneedling: Weekly sessions (with or without ongoing minoxidil) can upregulate growth factors and SULT1A1, thereby enhancing follicle health, and have been shown to help some users retain density for several months after discontinuing minoxidil.
- Anti-Androgens: Initiating topical or oral anti-androgens (e.g., finasteride, spironolactone) before and during withdrawal can help offset DHT-driven miniaturization—a process that is not alleviated by minoxidil alone.
- Low-Level Laser Therapy (LLLT): LLLT devices supply scalp stimulation and may decrease shedding when minoxidil is reduced or stopped.
- Alternative Topicals: Some transitioners try diluted rosemary oil (2–5%) or similar topicals for additional support, though strong clinical evidence for these alternatives is limited.
You can read our article on what happens when you quit taking minoxidil here.
Best Practices
- Apply only to a dry scalp: Before treatment, ensure both hair and scalp are completely dry. This maximizes absorption and efficacy.
- Dosage: For solution, use exactly 1 mL (measure with the supplied dropper); for foam, use half a capful per application.
- Massage in: Gently massage the product into thinning areas for approximately 30 seconds to improve penetration.
- Wait before washing: Allow at least 2 hours after application before shampooing or rinsing your scalp. This ensures adequate drug absorption.
Who Is an Ideal Candidate for Topical Minoxidil?
You’re a good candidate if you are:
- An adult man or woman with AGA: This is the main, FDA-approved indication for topical minoxidil. It has the strongest evidence in these groups.
- Able to commit to at least 6 months of consistent use: Appreciable improvements are most common with twice-daily application and become noticeable after 3–6 months. Stopping before this period makes it hard to judge the benefit.
- Ready for ongoing, indefinite use: All gains from minoxidil are lost within 3–6 months of discontinuation, so lifelong adherence is necessary for persistent effects.
- Prepared for manageable, mostly mild side effects: Most users tolerate minoxidil well, but 2–6% experience skin irritation, mainly from propylene glycol in liquid formulas. Switching to propylene glycol-free foams or other vehicle-adjusted products usually solves these issues.
- Willing to pair with additional therapies (optional, but recommended): Combining minoxidil with weekly microneedling or oral/topical finasteride increases efficacy by two- to four-fold, with up to 70%+ response rates and greater visual gains. Microneedling notably enhances regrowth and can prolong the effectiveness of minoxidil.
- Financially able to sustain $15–$30/month long-term: Over the years of use, the accumulated cost is a practical consideration.
- Comfortable following a simple scalp-care routine: Best practices include applying the product to a dry scalp, massaging for at least 30 seconds, allowing it to absorb for 2 hours, and treating any scalp inflammation first with anti-inflammatory shampoos (like ketoconazole or zinc pyrithione).
Who May Want to Reconsider?
- Catowners unable to limit pet exposure: Cats are highly sensitive to minoxidil and can suffer fatal toxicity from even minute exposure. Strict precautions are required to keep pets safe.
- People with severe or persistent skin sensitivity/allergy: If unable to tolerate any formulation or if allergic to both minoxidil and carrier ingredients, other options may be preferable.
- Individuals seeking a permanent or “cure-all” solution: Minoxidil does not address androgenic miniaturization (DHT effect) or scalp fibrosis. On its own, efficacy usually plateaus or wanes, making it mostly a maintenance or adjunct therapy.
- Those seeking visible, dramatic regrowth from monotherapy alone: Statistical hair count increases may not always correspond to better scalp coverage or visually satisfying restoration.
FAQs
Will minoxidil lower my libido? No, minoxidil is not known to lower libido, as it does not affect hormones and has shown no significant link to sexual side effects in clinical studies or post-marketing data. For more information, refer to the following resources: Minoxidil: Getting Started (Interactive Guide).
Can I dilute topical minoxidil without losing its efficacy? You can dilute topical minoxidil or mix it with other topicals, but doing so may reduce its efficacy if it alters absorption, concentration, or stability, so it’s best to either separate applications (morning vs. evening) or use professionally compounded combinations to ensure reliable results. For more information, watch this video.
Can I use topical minoxidil alongside other topicals? Yes, you can use topical minoxidil alongside other topicals, but to avoid interference with absorption, it’s best to either apply them at different times of day (e.g., morning vs. evening) or combine them into a single formulation, ideally through a compounding pharmacy or by carefully preparing them at home following best practices.
How long should I leave minoxidil on my scalp? You should leave minoxidil on your scalp for at least one hour, but ideally for four hours or more, to ensure optimal absorption, especially if you’re applying it only once a day. Most of the drug is absorbed within the first few hours, so extending contact time helps maximize its effectiveness.
Does minoxidil accelerate skin aging? Minoxidil is unlikely to accelerate skin aging; reported changes in facial skin quality are more likely due to mild allergic reactions to propylene glycol or temporary water retention, both of which are typically reversible and manageable with formulation changes or improved application practices.
Final Thoughts
Topical minoxidil remains the cornerstone over‑the‑counter therapy for androgenetic alopecia, valued for its solid safety record, ease of access, and decades of clinical data supporting meaningful, if often modest, improvements in hair density and retention. For many men and women, especially those early in their hair-loss journey or those averse to systemic medications, it offers a practical first line of defense that can be further amplified with adjuncts such as microneedling, retinoids, or anti-androgens.
Yet its benefits are not limitless: results plateau without complementary treatments, lifelong use is required to maintain gains, and irritation or simple user fatigue remain common reasons for discontinuation. Approached realistically, committing to at least six months of consistent, correctly dosed application, monitoring scalp health, and integrating synergistic therapies where appropriate, topical minoxidil can serve as a reliable, evidence‑based pillar in a comprehensive hair‑restoration plan.
References[+]
References ↑1 Suchonwanit, P., Thammarucha, S., Leerunyakul, K. (2019). Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 13. 2777-2786. Available at: https://doi.org/10.2147/DDDT.S214907 ↑2 Bacqueville, D., Jacques, C., Duprat, L., Jamin, E.L., Guiraud, B., Perdu, E., Bessou-Touya, S., Zalko, D., Duplan, H. (2017). Characterization of xenobiotic metabolizing enzymes of a reconstructed human epidermal model from adult hair follicles. Toxicology and Applied Pharmacology. 15(329). 190-201. Available at: https://doi.org/10.1016/j.taap.2017.05.040 ↑3 Pietrauszka, K., Bergler-Czop, B. (2020). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. Advances in Dermatology and Allergology. 39(3). 472-478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑4 Epstein, G.K., Epstein, J., Cohen, J. (2019). Hair Loss in Men and Women: Medical and Surgical Therapies. 2(1). 161-176. Available at: https://doi.org/10.1016/j.yacs.2019.02.006 ↑5 Lupatini, R., Sidhu, R., Patel, H., Bichar, K. (2021). Stability Evaluation of Minoxidil in FOAMIL Foam Base with Bracketing Study Design. International Journal of Pharmaceutical Compounding. 25(3) 236-240. Available at: PMID: 34125714 ↑6 Ashique, S., Sandhu, N.K., Haque, S.N., Koley, K. (2020). A Systemic Review on Topical Marketed Formulations, Natural Products, and Oral Supplements to Prevent Androgenic Alopecia: A Review. Natural Products and Bioprospecting. 10(6). 345-365. Available at: https://doi.org/10.1007/s13659-020-00267-9 ↑7 Jiminez-Cauhe, J., Sicco, K.I.L., Shapiro, J., Hermosa-Gelbard, A., Burgos-Blasco, P., Melian-Olivera, A., Ortega-Quijano, D., Pindado-Ortega, C., Buendia-Castano, D., Asz-Sigall, D., Vano-Galvan, S. (2025). Characterization and Management of Adverse Events of Low-Dose Oral Minoxidil Treatment for Alopecia: A Narrative Review. Journal of Clinical Medicine. 14(6). 1805. Available at: https://doi.org/10.3390/jcm14061805 ↑8 do Nascimento, I.J.B., Harries, M., Rocha, V.B., Thompson, J.Y., Wong, C.H., Varkaneh, H.K., Guimaraes, N.S., Arantes, A. J. R., Marcolini, M.S. (2020). Effect of Oral Minoxidil for Alopecia: Systematic Review. International Journal of Trichology. 12(4). 147-155. Available at: https://doi.org/10.4103/ijt.ijt_19_20 ↑9 Zeltzer, A.A., Keren, A., Paus, R., Gilhar, A. (2024). Topical minoxidil rejuvenates hair follicles from men with androgenetic alopecia in vivo. Acta Dermato Venereologica. 104(24213). Available at: https://doi.org/10.2340/actadv.v104.24213 ↑10 Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study protocol. Skin Research and Technology. 26(4). 542-557. Available at: https://doi.org/10.1111/srt.12827 ↑11 Kwack, M.H., Kang, B.M., Kim, M.K., Kim, J.C., Sung, Y.K. (2011). Minoxidil activates ꞵ-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. Journal of Dermatological Science. 154-159. Available at: https://doi.org/10.1016/j.jdermsci.2011.01.013 ↑12 Michelet, J.F., Commo, S., Billoni, N., Mahe, Y.F., Bernard, B.A. (1997). Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. Journal of Investigative Dermatology. 108(2). 205-209. Available at: https://doi.org/10.1111/1523-1747.ep12334249 ↑13 Gupta, A.K., Talukder, M., Venkataraman, M., Bamimore, M.A. (2022). Minoxidil: a comprehensive review. Journal of Dermatological Treatment. 33(4). 1896-1906. Available at: https://doi.org/10.1080/09546634.2021.1945527 ↑14 Sattur, S. Talathi, A., Shetty, G., Arsiwala, S., Pereira, R., Dhoot, D. (2023). Comparative Clinical Study Evaluating the Efficacy and Safety of Topical 5% Cetosomal Minoxidil and Topical 5% Alcohol-Based Minoxidil Solutions for the Treatment of Androgenetic Alopecia in Indian Men. Cureus. 15(10). E46568. Available at: https://doi.org/10.7759/cureus.46568 ↑15, ↑17, ↑20, ↑21, ↑52 Olsen, E.A., Dunlap, F.E., Funicella, T., Koperski, J.A., Swinehart, J.M., Tschen, E.H., Trancik, R.J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. Journal of the American Academy of Dermatology. 47(3). 377-385. Available at: https://doi.org/10.1067/mjd.2002.124088 ↑16 Olsen, E.A., DeLong, E.R., Weiner, M.S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. Journal of the American Academy of Dermatology. 16(3 Pt 2). 688-695. Available at: https://doi.org/10.1016/s0190=9622(87)70089-9 ↑18 Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. Journal of the American Academy of Dermatology. 65(6). 1126-1134. Available at: https://doi.org/10.1016/j.jaad.2010.09.724 ↑19 Ramos, P.M., Melo, D.F., Radwanski, H., de Almeida, R.F.C., Miot, H.A. (2023). Female-pattern hair loss: therapeutic update. Anais Brasileiros de Dermatologica. 98(4). 506-519. Available at: https://doi.org/10.1016/j.abd.2022.09.006 ↑22 Singh, S., Patil, A., Kianfar, N., Waskiel-Burnat, A., Rudnicka, L., Sinclair, R., Goldust, M. (2022). Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit? Clinical and Experimental Dermatology. 47(11). 1951-1955. Available at: https://doi.org/10.1111/ced.15338 ↑23 Vasantha, K.L. (2025). Efficiency and Safety of 10% Minoxidil in the Treatment of Alopecia Areata: A Randomised Controlled Trial. Journal of Population Therapeutics and Clinical Pharmacology. 32(4). 724-730. Available at: https://doi.org/10.53555/56ggpq88 ↑24 Ghonemy, S., Alarawi, A., Bessar, H. (2021). Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. Journal of Dermatological Treatment. 32(2). 236-241. Available at: https://doi.org/10.1080/09546634.2019.1654070 ↑25 Grice, J.E., Ciotti, S., Weiner, N., Lockwood, P., Cross, S.E., Roberts, M.S. (2010). Relative uptake of minoxidil into appendages and stratum corneum and permeation through human skin in vitro. Journal of Pharmaceutical Science. 99(2). 712-718. Available at: https://doi.org/10.1002/jps.21856 ↑26 Patel, K., Palmer, A., Nixon, R. (2023). Allergic contact dermatitis from propylene glycol: A case series from Australia. Contact Dermatitis. 89(2). 79-84. Available at: https://doi.org/10.1111/cod.14325 ↑27 Nestor, M.S., Ablon, G., Gade, A., Han, H., Fischer, D.L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. Journal of Cosmetic Dermatology. 20(12). 3759-3781. Available at: https://doi.org/10.1111/jocd.14537 ↑28 Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., Bartels, N.G. (2011). A randomized, single-blind trial of 5% minoxidil foam once-daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. Journal of the American Academy of Dermatology. 65(6). 1126-1134. Available at: https://doi.org/10.1016/j.jaad.2010.09.724 ↑29 Barbareschi, M., Vescovi, V., Starace, M., Piraccini, B.M., Milani, M. (2020). Propylene glycol free 5% minoxidil lotion formulation: cosmetic acceptability, local tolerability, clinical efficacy and in-vitro skin absorption evaluations. Edizioni Minerva Medica. 155(3). 341-345. Available at: https://doi.org/10.23736/S0392-0488.20.06554-2 ↑30 Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvin, S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatologic Therapy. 32(3). 12915. Available at: https://doi.org/10.1111/dth.12915 ↑31 Asilian, A., Farmani, A., Saber, M. (2023). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: A randomized controlled trial. Journal of Cosmetic Dermatology. 23(3). 949-957. Available at: https://doi.org/10.1111/jocd.16086 ↑32 Olsen, E.A., Weiner, M.S., Amara, I.A., DeLong, E.R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. Journal of American Academy of Dermatology. 22(4). 643-646. Available at: https://doi.org/10.1016/0190-9622(90)70089-z ↑33, ↑53 Shadi, Z. (2023) Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatology and Therapy. 13(5). 1157-1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑34, ↑37 Van Neste, D. (2020). Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study protocol. Skin Research & Technology. 26(4). 542-557. Available at: https://doi.org/10.1111/srt.12827 ↑35 [Updated 2023 Feb 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482378/ Accessed: July 2025 ↑36 Trueb, R.M., Dias, M.F.R.G., Rezende, H.D. (2021). Comment on Follicular Inflammation and Fibrosis in Pattern Hair Loss. Skin Appendage Disorders. 7(2). 159-160. Available at: https://doi.org/10.1159/000513089 ↑38 Ahmed, K.M.A., Kozaa, Y.A., Abuawwad, M.T., Al-Najdawi, A.I.A., Mahmoud, Y.W., Ahmed, A.M., Taha, M.J.J., Fadhli, T., Giannopoulou, A. (2025). Evaluating the efficacy and safety of combined microneedling therapy versus topical Minoxidil in androgenetic alopecia: a systematic review and meta-analysis. Archives of Dermatological Research. 317(1). 528. Available at: https://doi.org/10.1007/s00403-025-04032-1 ↑39, ↑40, ↑45 Zhang, Y., Sheng, Y., Zeng, Y., Hu, R., Zhao, J., Wang, W., Yang, Q. (2022). Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair loss in a Chinese population. Journal of Cosmetic Dermatology. 21(12). 6985-6991. Available at: https://doi.org/10.1111/jocd.15424 ↑41 Sharma, A., Surve, R., Dhurat, R., Sinclair, R., Tan, T., Zou, Y., Ramos, M.P., Wambier, C., Vernier, I., Kovacevic, M., Goren, A. (2020). Microneedling improves minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Journal of Biological Regulators and Homeostatic Agents. 34(2). 659-661. Available at: https://doi.org/10.23812/19-385-L-51 ↑42 Sharma, A., Goren, A., Dhurat, R., Agrawal, S., Sinclair, R., Trueb, R.M., Vano-Galvan, S., Chen, G., Tan, Y., Kovacevic, M., Situm, M., McCoy, J. (2019). Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatologic Therapy. 32(3). E12915. Available at: https://doi.org/10.1111/dth.12915 ↑43 Dhurat, R., Sukesh, M.S., Avhad, G., Dandale, A., Pal, A., Pund, P. (2013). A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study. International Journal of Trichology. 5(1). 6-11. Available at: https://doi.org/10.4103/0974-7753.114700 ↑44 Kumar, K.M., Inamadar, A.C., Palit, A. (2018). A Randomized Controlled, Single-Observer Blinded Study to Determine the Efficacy of Topical Minoxidil plus Microneedling versus Topical Minoxidil Alone in the Treatment of Androgenetic Alopecia. Journal of Cutaneous and Aesthetic Surgery. 11(4). 211-216. Available at: https://doi/org/10.4103/JCAS.JCAS_130_17 ↑46 Ramirez-Marin, H.A., Tosti, A. (2022). Role of Oral Minoxidil in Patterned Hair Loss. Indian Dermatology Online Journal. 13(6). 729-733. Available at: https://doi.org/10.4103/idoj.idoj_246_22 ↑47 Gupta, A.K., Talukder, M., Shemer, A., Piraccini, B.M., Tosti, A. (2023). Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review. Skin Appendage Disorders. 9(6). 423-437. Available at: https://doi.org/10.1159/000531890 ↑48 Chemical Book. (no date). Minoxidil Sulphate. ChemBook. Available at: https://www.chemicalbook.com/Price/Minoxidil-sulphate.htm Accessed: July 2025 ↑49 Shan, Y., Xu, C., Guo, Y., Wen, L., Zhou, S., Fang, L., Xu, J., Zhen, H. (2025). Liposomes enhance the hair follicle delivery of minoxidil sulfate with improved treatment of androgenetic alopecia. 677. 125642. Available at: https://doi.org/10.1016/j.ijpharm.2025.125642 ↑50 Rafi, A.W., Katz, R.M. (2011). Pilot study of 15 patients receiving a new treatment regimen for androgenic alopecia: the effects of atopy on AGA. ISRN Dermatology. 11. 241953. Available at: https://doi.org/10.5402/2011/241953 ↑51 Rundegren, J. (2004). Rapid onset of action of minoxidil 5% topical solution in a 4-month German observational study on both patients and physicians. Journal of the American Academy of Dermatology. 50(3). 91. Available at https://doi.org/10.1016/j.jaad.2003.10.290 ↑54 Jadeed, H.B., Almudimeegh, A.M., Alomran, S.A., Alshathry, A.H. (2021). A Case of Contact Allergic Dermatitis to Topical Minoxidil. Cureus. 13(1). E12510. Available at: https://doi.org/10.7759/cureus.12510 ↑55 Chellini, P.R., Pirmez, R., Raso, P., Sodre, C.T. (2015). Generalized hypertrichosis induced by topical minoxidil in an adult woman. International Journal of Trichology. 7(4). 182-183. Available at: https://doi.org/10.4103/0974-7753.171587 ↑56 DeClementi, C., Bailey, K.L., Goldstein, S.C., Orser, M.S. (2004). Suspected toxicosis after topical administration of minoxidil in 2 cats. Veterinary Emergency & Critical Care. 14(4). 287-292. Available at: https://doi.org/10.1111/j.1476-4431.2004.04014.x ↑57 Leenen, F.H., Smith, D.L., Unger, W.P. (1988). Topical minoxidil: cardiac effects in bald man. British Journal of Clinical Pharmacology. 26(4). 481-485. Available at: https://doi.org/10.1111/j.1365-2125.1988.tb03410.x ↑58, ↑59 Price, V.H., Menefee, E., Strauss, P.C. (1999). Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. Journal of the American Academy of Dermatology. 41(5). 717-721. https://doi.org/10.1016/S0190-9622(99)70006-X Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sarah King, PhD
Dr. Sarah King is a researcher & writer who holds a BSc in Medical Biology, an MSc in Forensic Biology, and a Ph.D. in Molecular and Cellular Biology. While at university, Dr. King’s research focused on cellular aging and senescence through NAD-dependent signaling – along with research into prostaglandins and their role in hair loss. She is a co-author on several upcoming manuscripts with the Perfect Hair Health team.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down