- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
7 Best Oils for Hair Growth
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesTopical & Mesotherapy Dutasteride for Hair Loss: Evidence, Dosing, Safety
First Published Nov 17 2025Last Updated Dec 20 2025Pharmaceutical Researched & Written By:Perfect Hair Health Team
Researched & Written By:Perfect Hair Health Team Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor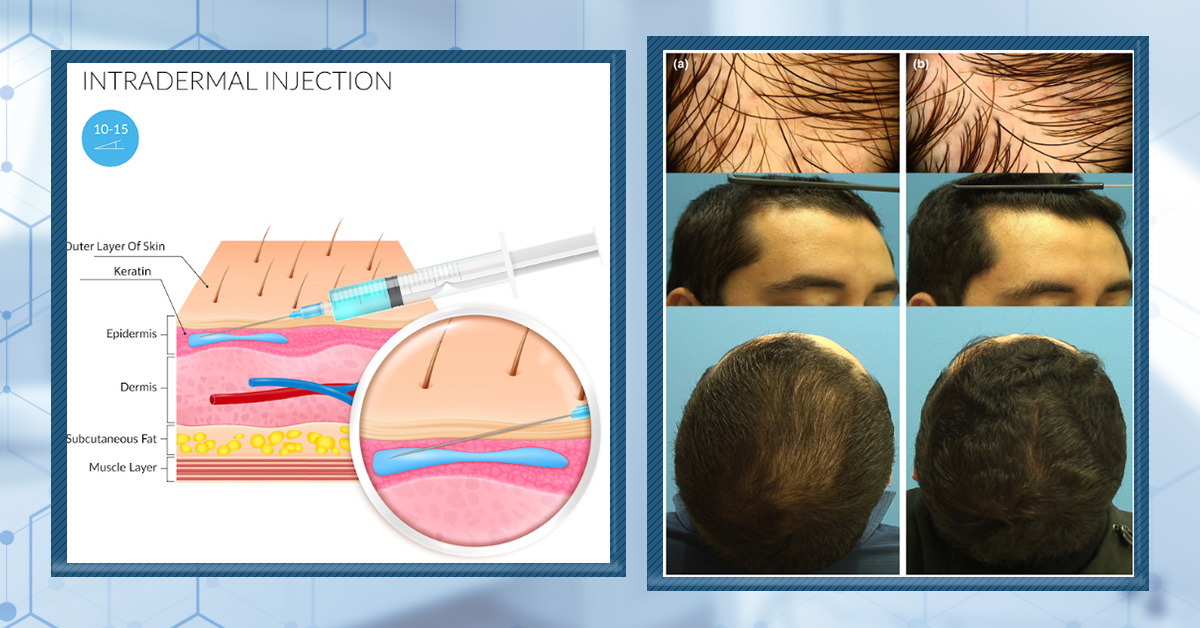
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Dutasteride isn’t just oral anymore; emerging research shows that topical and mesotherapy dutasteride can significantly improve androgenic alopecia while keeping most of the action localized to the scalp. This guide breaks down the science, realistic regrowth expectations, dosing ranges, and how these options compare to oral finasteride and dutasteride, plus practical tips on working with a clinician (and why DIY injections are risky). If you’re curious about maximizing hair regrowth with minimal systemic DHT suppression, this is a must-read before you start experimenting.
Full Article
Dutasteride is one of the strongest off-label treatments for androgenic alopecia. In this guide, we’ll explore the evidence supporting two delivery methods for dutasteride: topical dutasteride and mesotherapy dutasteride. We’ll also provide clear step-by-step instructions for anyone looking to try these therapies at home or find clinicians willing to do the procedures.
Overview: Topical / Mesotherapy Dutasteride
- Effort: Low (once weekly-to-monthly applications, depending on topical or mesotherapy delivery).
- Expectations: Hair improvements within 4-6 months.
- Response rate (i.e., the percent of people who experience a slowing, stopping, or partial reversal of AGA):
- Topical dutasteride: 70-90%
- Topical dutasteride + microneedling: 60-80%
- Mesotherapy dutasteride: 60-80%
- Regrowth rates (i.e., the percent change to hair counts, hair diameters, and hair density (i.e., ∆hair counts x ∆hair diameters)):
- Topical dutasteride: +23-25% increase in hair counts (mean +75 hairs/cm² vs baseline in the 0.05% group over 24 weeks).
- Topical dutasteride + microneedling: 10-15% increase in hair counts, 15-20% increase in hair density (sometimes more)
- Mesotherapy dutasteride: 10-15% increase in hair counts, 15-20% increase in hair density (sometimes more)
- Advantages: Easy to administer; works for the majority of people; minimizes DHT reductions elsewhere in the body; likely confers with a lower side effect profile.
- Disadvantages: Clinical studies are of lower quality; no standardization across mesotherapy needle depths or dutasteride exposure volumes per session; high variability between patients regarding systemic absorptions puts the onus on each patient to test serum DHT levels before/during treatment; expensive.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Key Takeaways
- The Good News:
- Topical / mesotherapy dutasteride works. While the evidence is limited and of lower quality, multiple studies from different research groups all suggest that topical dutasteride and mesotherapy dutasteride improve hair loss outcomes for men and women with androgenic alopecia.
- Topical / mesotherapy dutasteride may minimize unwanted declines in serum DHT. Multiple studies (while not perfectly designed) suggest, when dosing is done properly, that topical dutasteride and/or mesotherapy dutasteride can improve hair parameters while only modestly reducing serum DHT levels (a marker for systemic absorption). This has been confirmed by members here who’ve done serum DHT tests before/after treatment and seen only 10-15% declines in DHT. Moreover, absorption studies testing 1 mL of 0.025% topical dutasteride (i.e., 0.25 mg) applied to the scalp show, after 12 hours, that ~7% of the drug enters the bloodstream while 25% of the drug remains within the dermis of the scalp. This means that within proper exposure volumes, we can maximize DHT reduction in the scalp while minimizing the effects of DHT reduction elsewhere in the body.
- No sexual side effects (yet) reported in the clinical data. While the currently available studies have small sample sizes, all investigations on topical / mesotherapy dutasteride have yet to have a participant report sexual side effects. This experience is echoed by members here who’ve adhered to the usage ad frequency parameters of both topical and mesotherapy dutasteride.
- Critical Unknowns:
- How does topical dutasteride and mesotherapy dutasteride compare to each other (and other therapies)? Due to lacking head-to-head clinical studies, we don’t yet have an answer. However, directional hair count data across studies suggest that topical / mesotherapy dutasteride is probably less powerful than its oral counterpart. (This comparison is unscientific, but it’s all we can currently glean.)
- What’s the best dosage, frequency of application, and carrier agents? While low-exposure volumes of topical / mesotherapy dutasteride show minimal effects on serum DHT, we don’t yet know the perfect dosing schedule to maximize hair regrowth while minimizing serum DHT reductions. It’s likely that leakage from the scalp and systemic absorption vary depending on the individual and the treatment methodologies: age, genetics, mode of delivery, dosage per application, application frequency, and application volume are just a few of the dozens of factors impacting the answers to these questions.
- How does topical dutasteride perform as a standalone therapy? Early data suggest that low-dose topical dutasteride (including 0.05%) can improve hair parameters and may, in some trials, appear comparable or even superior to oral finasteride, but methodological limitations and conflicting real-world experiences mean these findings should be interpreted cautiously until independently replicated.
- Best Practices & Recommendations
- See below.
Key Recommendations
Topical dutasteride and mesotherapy dutasteride are probably most appropriate for people who:
- Have androgenic alopecia
- Don’t want to use oral finasteride or oral dutasteride, but want to leverage a pharmaceutical 5-alpha reductase inhibitor
- Want to better localize the effects of dutasteride to scalp
- Are comfortable experimenting with delivery methods that are lesser explored and have lower levels of clinical support
- Are comfortable investing $50-$400 per month into treatment, depending on the mode of delivery (topical or mesotherapy)
- Don’t have metabolic syndrome (as one study suggested that mesotherapy dutasteride was ineffective for these patients)
Step-By-Step Guide
Topical dutasteride
If trying topical dutasteride:
- Limit topical exposure of dutasteride to 0.1-0.5mg weekly.
- This can be achieved in many ways. Consider 1 mL formulas more appropriate for localized hair loss, and 2 mL formulas more appropriate for diffuse hair loss (for more spread of liquid). Examples:
- 1 mL of 0.01% to 0.05% dutasteride, once weekly. Exposure = 0.1-0.5 mg
- 1 mL of 0.025% dutasteride, twice weekly. Exposure = 0.5 mg
- 2 mL of 0.005% to 0.025% dutasteride, once weekly. Exposure = 0.1-0.5 mg
- 2 mL of 0.005% to 0.0125% dutasteride, twice weekly. Exposure = 0.2-0.5 mg
- This can be achieved in many ways. Consider 1 mL formulas more appropriate for localized hair loss, and 2 mL formulas more appropriate for diffuse hair loss (for more spread of liquid). Examples:
- Leave in scalp for at least 4 hours, and preferably 8-12 hours
- This will allow 25% of the dutasteride applied to penetrate into the percutaneous tissues, so it can have an effect.
- This will minimize the amount of dutasteride that enters the bloodstream, thus reducing systemic absorption.
- The easiest way to do this is to apply topical dutasteride before bed and then wash it out in the morning.
- Consider combining with microneedling
- If combining with microneedling, do not apply topical dutasteride the day of microneedling
- Consider testing serum DHT levels before/during treatment
- This will help track if topical dutasteride is remaining localized to the scalp.
- In tracking DHT levels, you can increase or decrease dutasteride dosing depending on your serum DHT readouts.
- Product recommendations
- MinoxidilMax: 0.1% topical dutasteride, 60 mL.
- You can use this solvent to dilute the formula down to 0.005% to 0.02%. If you’d like help with this, ask us. We’re currently building out a “how to” video for diluting topicals that will make this process simple. But if you want to get a jumpstart on this, please don’t hesitate to ask for our help.
- MinoxidilMax: 0.1% topical dutasteride, 60 mL.
Mesotherapy dutasteride
If trying mesotherapy dutasteride:
- Start with mesotherapy injections once monthly.
- After 6+ months, move to one session every 1-3 months.
- If once monthly injections are infeasible, opt for injections once every three months.
- Limit dutasteride exposure to 0.1-0.2 mg per session.
- This is equivalent to 1-2mL of 0.01% dutasteride
- Consider testing serum DHT levels before/during treatment
- This will help track if topical dutasteride is remaining localized to the scalp.
- In tracking DHT levels, you can increase or decrease dutasteride dosing depending on your serum DHT readouts.
How do I track down a clinician who can provide mesotherapy dutasteride?
It’s not easy, but it can be done.
Step #1: Build a list of practitioners offering mesotherapy in your locale
Run a google search for dermatologists and/or medical spas in your area. When checking out their websites, make sure they offer mesotherapy. It’s okay if the mesotherapy they offer is not related to hair loss. All that matters is that they offer mesotherapy, because that means they will have a mesotherapy gun in their office.
Track down their names and contact information via contact forms and emails listed on their sites. Try to build a list of 5-10 clinicians within travel distance.
Step #2: Send each practitioner this personalized email
Here’s an email script that has given us some success. Please personalize as necessary:
Dear [CLINICIAN],
My name is [YOUR NAME]. I’m reaching out because I’m interested in trying mesotherapy dutasteride for the treatment of androgenic alopecia. The procedures are typically done once monthly for 6+ months. I wanted to see if your offices would be willing to provide the therapy to me.
I’ve been encouraged by the results of this mini-review, suggesting that repeated mesotherapy dutasteride may improve hair loss outcomes for men with androgenic alopecia, and without significantly altering serum DHT levels.[1]Herz-Ruelas, M.E., Alvarez-Villalobos, N.A., Millan-Alanis, J.M., de Leon-Gutierrez, H., Ocampo-Garza, S.S., Grimalt, R. (2020). Efficacy of Intralesional and Oral Dutasteride in the Treatment of … Continue reading I’m planning on tracking serum DHT before/during treatment, and I’d be happy to share the blood work with you (especially as interest in this therapy is growing, it might be nice to have the data).
I’m happy to source the mesotherapy dutasteride serum. All I need is a clinician trained in mesotherapy to administer the procedure. Are you interested? If so, I can come to offices as soon as next week.
All my best,
[YOUR NAME]
[PHONE NUMBER]Step #3: Secure a practitioner
Typically, for every 5-10 clinicians who receive the above email, one will respond to say that they’re interested. Meet with these clinicians, print out and share with them the studies, and show them the methodologies (below) to standardize the procedure, thus making it as easy as possible for the doctor to administer.[2]Herz-Ruelas, M.E., Alvarez-Villalobos, N.A., Millan-Alanis, J.M., de Leon-Gutierrez, H., Ocampo-Garza, S.S., Grimalt, R. (2020). Efficacy of Intralesional and Oral Dutasteride in the Treatment of … Continue reading
Methods For Mesotherapy Dutasteride (With A Clinician):
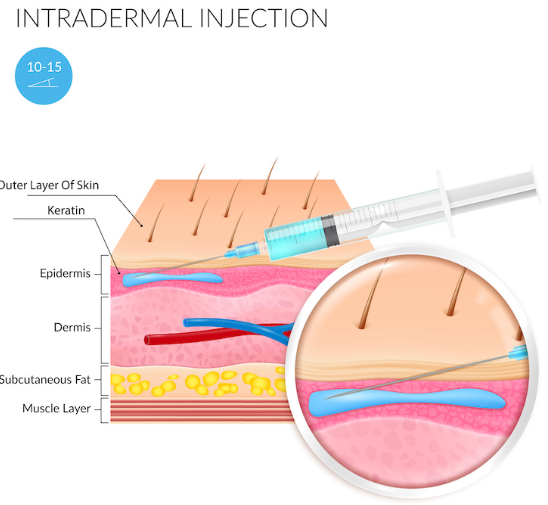
- Injection depth: intradermal (i.e., between the epidermal and dermal skin layers, roughly 0.5-0.75 mm deep)
- Mesotherapy formulation: 0.01% dutasteride in a 10 mL vial (provider here)
- Injection volume per session: 1-2 mL (i.e., 0.1-0.2 mg of dutasteride)
- Injection frequency: once monthly for six months, then once every 1-3 months thereafter
Can I try mesotherapy at home?
To be clear, we DO NOT recommend taking a DIY approach to mesotherapy. If you cannot find a provider, we recommend trying topical dutasteride + microneedling. Topical dutasteride is far more accessible, and it’s also easily administered at home. It’s also likely that the combination produces similar results to mesotherapy dutasteride.
Under the circumstances that you decide not to heed our warnings, here are DIY instructions for mesotherapy dutasteride.
Methods for Mesotherapy Dutasteride (At-Home):
- Buy this mesotherapy device. Use the 9-needle cartridges and adjust the needle depth to 0.5mm when operating.
- Buy mesotherapy dutasteride from this provider (we lab tested their products; they meet quality and purity standards).
- Follow the instructions from the manufacturer to deliver mesotherapy to your scalp once per month.
- Consider tracking serum DHT levels before/during treatment.
Below is a scientific deep-dive on topical / mesotherapy dutasteride.
What Is Topical Dutasteride and How Does It Work?
Topical dutasteride is topical version of the oral drug, dutasteride. Whereas oral dutasteride tends to have hormonal effects all throughout the body, topical dutasteride is designed to selectively target just the scalp skin – allowing people to reap all the hair-growing benefits of dutasteride, and without the risk of systemic side effects.
At least, that’s the hope.
Specifically, dutasteride lowers the hormone dihydrotestosterone (DHT), which is causally linked to androgenic alopecia. It does so by inhibiting the enzyme that converts testosterone into DHT: 5-alpha reductase.
Dutasteride is one of the most powerful 5-alpha reductase inhibitors available. Whereas hair loss drugs like finasteride inhibit 5-alpha reductase and lower DHT levels by ~70%, dutasteride can lower DHT levels by 95%.
This is because there are three known isoforms of 5-alpha reductase: type I, II, and III. Compared to finasteride, dutasteride is more effective at inhibiting them. In fact, dutasteride is able to target the type I isoform 100x better than finasteride, and the type II isoform 3x better than finasteride.[3]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue reading
How Does Oral Dutasteride Compare To Oral Finasteride for Androgenic Alopecia?
Dutasteride’s larger effects on DHT reduction also confer better hair regrowth outcomes.
A 2019 meta-analysis by Zhou et al. found that oral dutasteride regrew more hair than oral finasteride – even at dosage ranges lower than 0.5 mg daily.[4]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue reading And while the studies in this review were relatively short (i.e., 6 months) and had sample sizes of less than 500 participants, it’s hard to ignore the preliminary evidence suggesting that oral dutasteride packs a big punch for hair regrowth – even at dosages as low as 0.1mg daily.
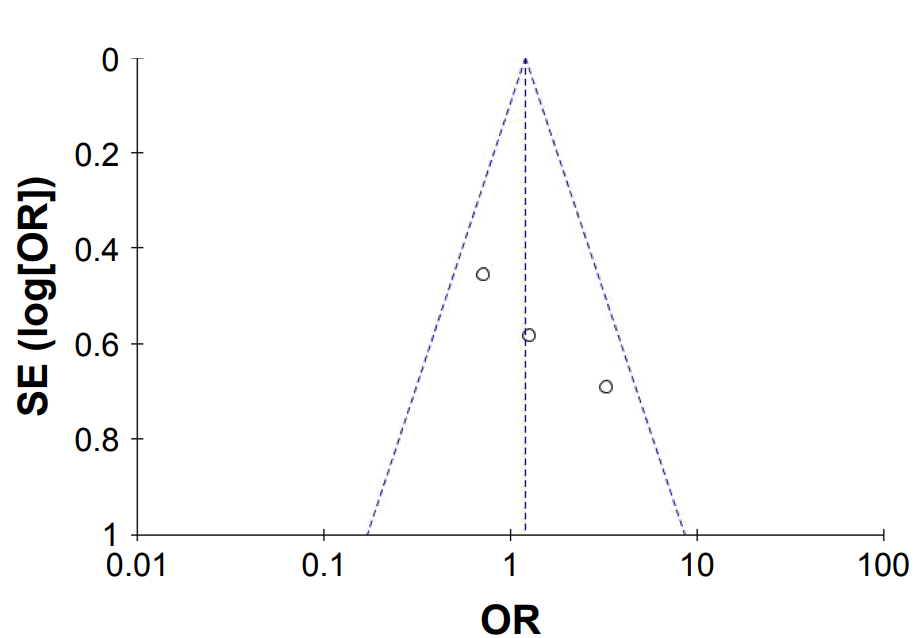
Figure 1: The studies cited maintain a low risk of bias, but because of the low sample size, it’s difficult to say with confidence that dutasteride is superior to finasteride.[5]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue reading
What About Safety?
Despite reducing more DHT and regrowing more hair, both finasteride and dutasteride appear to carry the same risk of side effects.
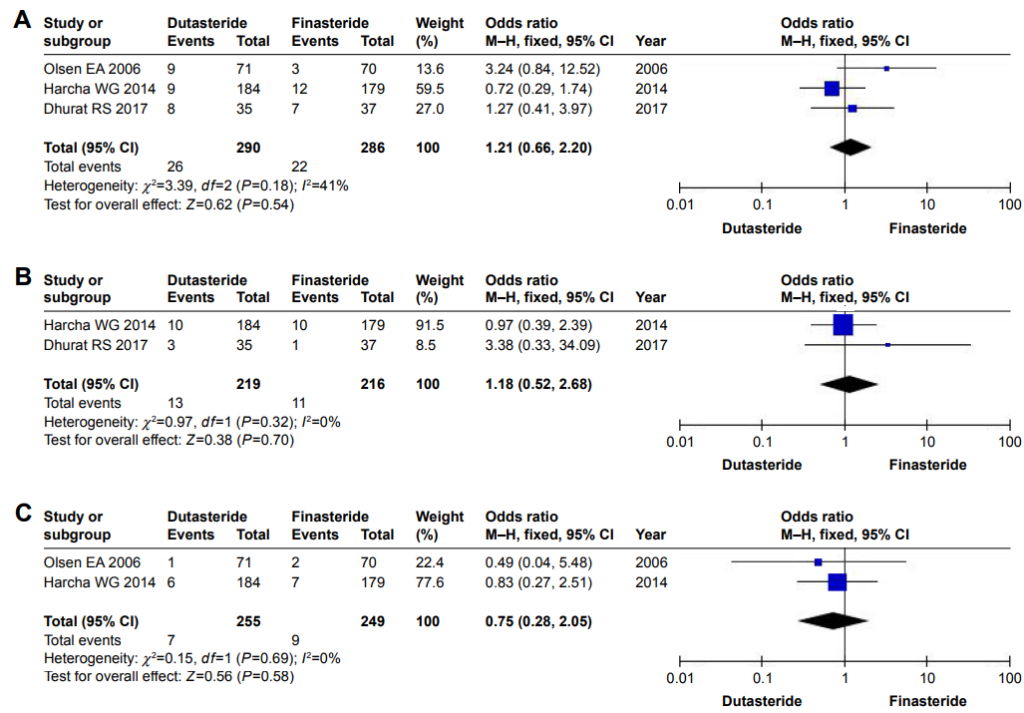
Figure 2: Forest plots showing changes in (A) altered libido, (B) erectile dysfunction, and (C) ejaculation disorders.
Abbreviation: M–H, Mantel–Haenszel.[6]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue readingWhy is this? Wouldn’t we expect dutasteride to carry a higher risk of side effects, since it reduces DHT levels more?
Generally, yes. But that’s not currently reflected in the data. Regarding why, there are many hypotheses:
- Biases in sample sizes and study durations. Perhaps dutasteride does carry a larger risk of side effects, as it has been shown to do so in some patient populations (for instance, dutasteride carries a higher risk versus finasteride for gynecomastia in patients with enlarged prostates). But perhaps the sample sizes in these hair loss studies were too small in these studies to pick up the differences. Or, perhaps the studies did not run long enough to capture all risks.[7]Hagberg, K.W., Divan, H.A., Fang, S.C., Nickel, J.C., Jick, S.S. (2017). Risk of gynecomastia and breast cancer associated with the use of 5-alpha reductase inhibitors for benign prostatic … Continue reading
- Differences in isoform side effect attribution. Perhaps the type II 5-AR enzyme (i.e., the one predominantly targeted by finasteride) is the one isoform predominantly responsible for most of the side effects associated with finasteride and dutasteride. (We’ve heard this argument before, but we don’t necessarily believe it.[8]Rahimi-Ardabili, B., Pourandarjani, R., Habibollahi, P., Mualeki, A. (2006). Finasteride induced depression: a prospective study. BMC Clinical Pharmacology. 6(7). Available at: … Continue reading
- Tissue-dependent concentration differences of isoforms. For example, in the prostate and testes, the type II isoform is expressed far more than type I. The opposite is true in brain tissue, where type I 5-AR is predominantly found. Perhaps the side effects of 5-AR inhibitors depend on how much 5-AR is inhibited in specific tissues, and at the dosages administered for dutasteride and finasteride, there just isn’t enough difference in DHT reduction to make one drug more likely to produce sexual side effects than the other.[9]Wang, K., Fan, D-D., Jin, S., Xing, N-Z., Niu, Y-N. (2014). Differential expression of 5-alpha reductase isozymes in the prostate and its clinical implications. Asian Journal of Andrology. 16(2). … Continue reading
Even still, there remains no consensus among clinicians for why this is the case.
Pharmacokinetics
How Does Dutasteride Behave In Our Bodies?
Pharmacokinetics is a term used to describe the behavior of drugs once they enter the body: how quickly they are transported to tissues, how quickly they are metabolized (i.e., their half-life), and more.
Dutasteride and finasteride, despite being structurally similar, differ dramatically in their half-lives. For instance, finasteride has a half-life of 5-8 hours. That means that after ingesting finasteride, it takes 5-8 hours before 50% of that finasteride leaves our bloodstream.
Dutasteride, on the other hand, has a half-life that can last 5+ weeks. In fact, dutasteride’s half-life depends on a lot of factors: age, genetics, and, most importantly, the amount of dutasteride given to an individual at one time.
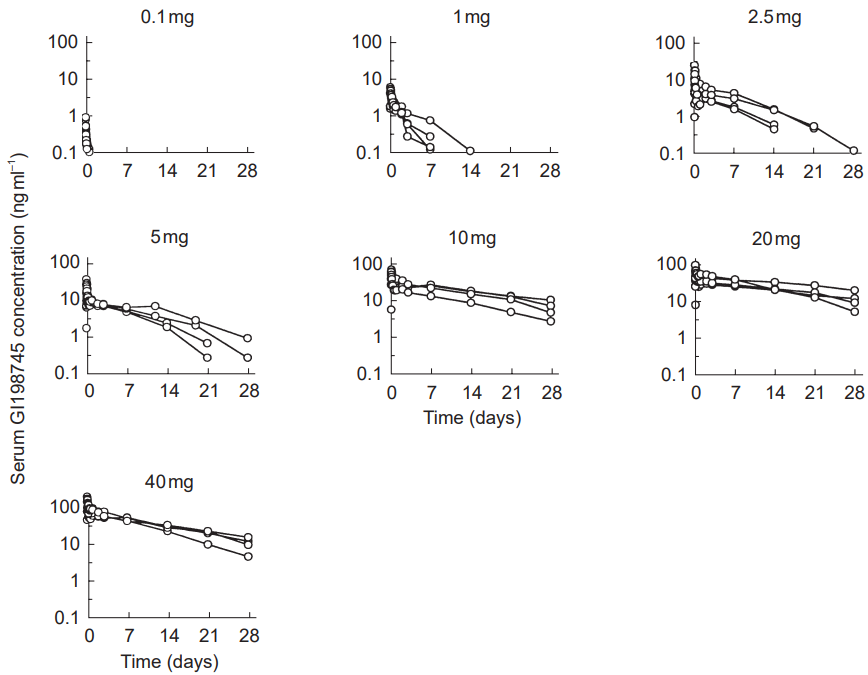
This was investigated by Gislekog et al. (1998) by giving a dose of dutasteride ranging from 0.01mg to 40mg to 32 healthy males. The findings showed a number of distinctions:
- The half-life of the drug increases with increasing dose.
- Serum concentrations of dutasteride can last for quite a while before it is undetectable.
This is presented in the following table:
Dose Serum Concentration Duration (up to 1ng mL-1) 0.1mg Approximately one day. 1.0mg Averaging around seven days, but can be up to 14 days. 2.5mg Averaging approximately 14-21 days, but can be as long as 28 days. 5.0mg Approximately 21-28 days. 10.0mg Greater than 28 days. 20.0mg Greater than 28 days. 40.0mg Greater than 28 days. And here are the graphs from the study:Concentration of dutasteride in serum is dependent on how large a dose is used. This means that beyond half-life, the general concentration of the drug can last for quite a while when using greater than 2.5mg doses.[10]Gisleskog, P.O., Hermann, D., Hammarlund-Udenaes, M., Karlsson, M.O. (1999). The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. … Continue reading
Can Topical Dutasteride Lower Scalp DHT While Minimally Affecting Hormones Elsewhere In The Body?
This is not only possible, but plausible… provided that we play with the dosages of dutasteride and the frequencies of application.
Since dutasteride rapidly distributes across tissues and has a dose-dependent half-life, the key is to find an exposure volume of topical dutasteride that…
- Is large enough to effectively saturate the epidermal and dermal layers of the scalp (so that we can locally lower DHT)
- Is small enough such that the amount of dutasteride that leaks daily into the bloodstream is rapidly metabolized (so that DHT levels elsewhere are minimally impacted)
Encouragingly, we have some data to suggest there are pharmacokinetic “sweet spots” to achieving this.
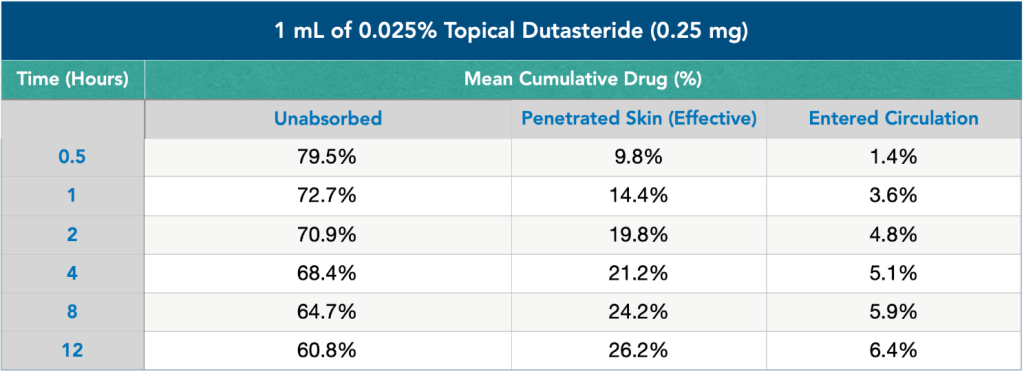
A recent analysis measured how much of a 1 mL solution of 0.025% dutasteride (i.e., 0.25mg) applied to the scalp (1) stayed on the skin, (2) penetrated into the dermis, and (3) travelled systemically into the bloodstream.
Across 12 hours of study, here’s what the investigators discovered:
Figure 4: Effect of topical dutasteride on skin absorption and circulation entry.
If the above chart feels overwhelming, don’t worry. For now, just know that 12 hours after applying topical dutasteride:
- 60.8% of the dutasteride remained on the surface of the scalp and was unabsorbed (i.e., 0.152 mg)
- 26.2% of the dutasteride penetrated into the scalp (i.e, 0.06 mg).
- 6.4% of the dutasteride entered into the bloodstream (i.e., 0.016 mg)
This is actually good news. Why? Because 12 hours after applying 1 mL of 0.025% topical dutasteride, 0.06 mg of that dutasteride permeated into balding tissues, while only 0.016 mg entered into the bloodstream.
In balding skin tissue, 0.06 mg of dutasteride is probably:
- Enough drug to appreciably lower scalp DHT levels, and…
- Enough drug to increase the elimination half-life to > 1 day, so that the scalp DHT suppression can last for multiple days.
Simultaneously, with the amount of dutasteride reaching the bloodstream, 0.016 mg of dutasteride is probably:
- Not enough drug to appreciably lower serum DHT levels, and…
- Not enough drug to increase the elimination half-life to > 1 day. As such, any serum DHT suppression will likely be short-lived.
In other words, one application of 1 mL of 0.025% topical dutasteride might lead to impressive reductions to scalp DHT, while having minimal impact on serum DHT (and thereby DHT levels elsewhere in the body).
So, Should We All Apply 1 mL of 0.025% Topical Dutasteride, Once Or Twice Daily?
We wish it were that simple.
The problem is that the above analysis only measures the effects from one application of topical dutasteride. If we apply this dosage daily, dutasteride levels in the scalp skin will accumulate, as will the amount of dutasteride entering the bloodstream.
Additionally, after that 12-hour mark, a portion of the ~25% of dutasteride already absorbed into the skin will continue to leak into the bloodstream. The above study does not capture these effects. So, while the analysis is insightful, it unfortunately does not run long enough to reveal the full extent of systemic absorption from a single 1 mL dose of 0.025% topical dutasteride. That means total bloodstream exposure from 1 mL of 0.025% topical dutasteride is probably a bit higher than the 0.016 mg suggested by the study.
Finally, as blood levels of dutasteride increase, so too will the drug’s half-life. Remember: the more dutasteride present, the longer the half-life. So, after a few weeks of daily application, it’s not unreasonable to expect serum drug exposure to accumulate and thereby serum DHT levels to substantially decrease. This is exactly what most people trying topical dutasteride want to avoid!
Fortunately, we don’t have to rely exclusively on mathematical modeling to figure this out. There are clinical studies testing topical dutasteride for the treatment of androgenic alopecia – some of which measured not only hair growth, but also changes to serum DHT levels before and after treatment.
Topical Dutasteride: Clinical Studies (Hair Counts, Serum DHT, & More)
Nada et al. (2018): Microneedling + Topical Dutasteride vs. Microneedling Alone
In a 2018 clinical study, researchers sought to determine how topical dutasteride + microneedling fared versus microneedling alone.[11]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. … Continue reading
So, they recruited 30 males with androgenic alopecia, randomized them into two groups, and tracked hair parameter changes of a six-month period.
Using a Dermapen with a 12-gauge needle cartridge and a 1.5mm needle length, both groups received 13 microneedling sessions over six months, and using the following schedule:
- Once per week (weeks 0-7)
- Once every two weeks (weeks 9 and 11)
- Once every four weeks (weeks 15, 19, and 23)
However, during each microneedling session, the topical dutasteride group also received up to 2 mL of 0.02% topical dutasteride (i.e., 0.4mg) applied to the scalp.
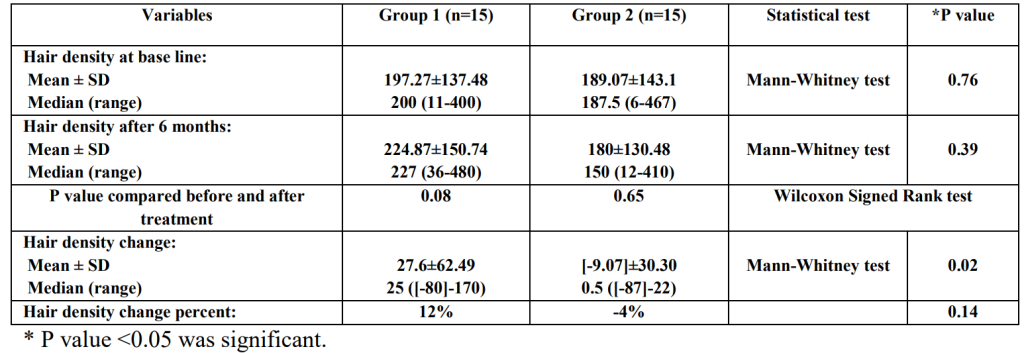
After six months, the topical dutasteride + microneedling group saw greater improvements in hair density versus the microneedling-only group. This equated to a 12% increase in hair density.
Figure 5: Changes of hair density in the study groups before and after treatment.[12]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. … Continue reading
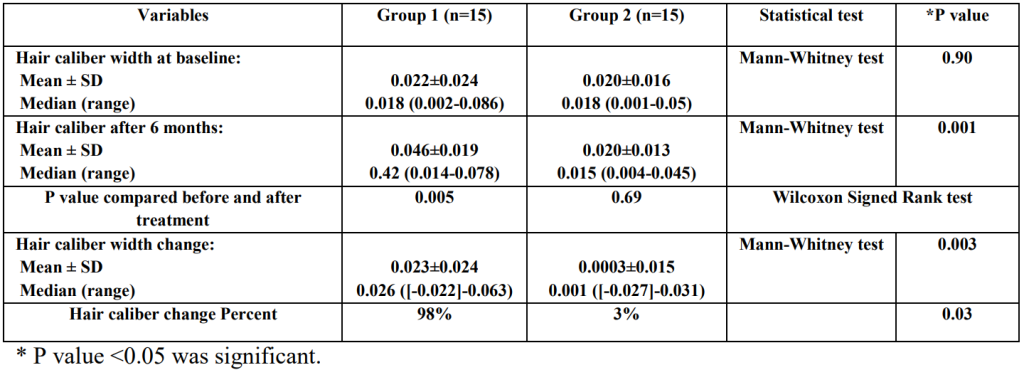
Additionally, the topical dutasteride + microneedling group also saw hair thickness improve by nearly 100%.
Figure 6: Changes in hair caliber width in the study groups before and after treatment.[13]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. … Continue reading
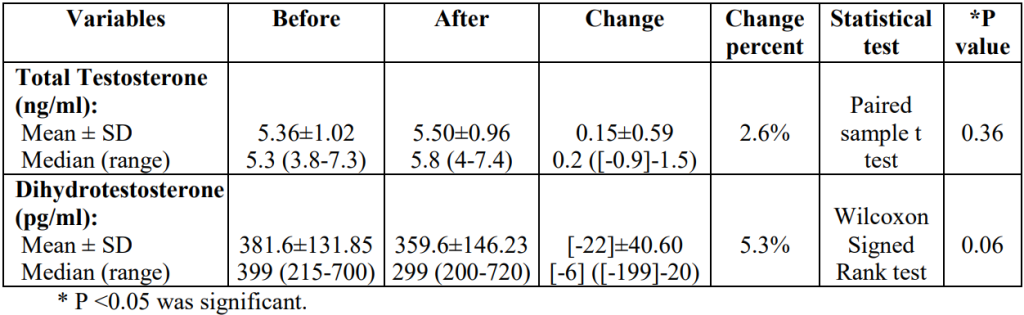
Finally, hormonal testing revealed that the topical dutasteride + microneedling group did experience a 5.3% reduction in serum levels of DHT. However, this level of DHT reduction is considered biologically insignificant. Keep in mind that serum DHT levels can fluctuate as much as 20-40% daily, depending on if the measurements were taken during the morning or night. So a ~5% change in DHT isn’t very concerning.
Figure 7: Hormone levels before and after treatment.[14]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. … Continue reading
Our Analysis
While these results are encouraging, they’re also problematic. Specifically, the results are obfuscated by a methodological concern: staggered microneedling + topical dutasteride sessions, and no measurements for serum DHT during shorter durations between dutasteride applications.
Throughout the study, time gaps between topical dutasteride applications grew. For the first 8 weeks, topical dutasteride was applied once weekly. But for the last 12 weeks, it was applied once monthly. The investigators only reported changes to serum DHT before/after the study concluded. Specifically, they recorded serum DHT before the study began, and one week after their final dutasteride application. So, are these results really representative of serum DHT levels throughout the study… or were serum DHT levels much lower in the first few months when application frequency was 4x higher?
Unfortunately, the investigators do not give us enough information to know the answers to these questions. We’re reaching out and hope to have answers for you soon.
In the interim, we’ve had several members emulate the methodologies of this study, test serum DHT levels before/during treatment, and adhere to a once-weekly topical dutasteride application rate (at up to 2 mL of 0.025% dutasteride, that’s up to 0.4mg of weekly dutasteride exposure).
The good news is that all of these members reported only 10-15% decreases in serum DHT. While those decreases are likely partly due to serum dutasteride, they’re still within the realm of biologically insignificant. And encouragingly, our members’ results not only align with the ballpark results of this study, but also the previous dosing analysis – at least in terms of expectations for systemic leakage.
In our eyes, these are all positive signs. They’re preliminary signals (validated by some members) that at weekly dosages of up to 2 mL x 0.02% topical dutasteride (i.e., 0.4 mg per week), we can mostly preserve serum DHT levels – even when adding in microneedling.
Encouragingly, a 2022 study also validated this approach – at least in terms of efficacy.
Sanchez et al. (2022): Microneedling + Topical Dutasteride vs. Microneedling Alone
In 2022, investigators conducted another study on topical dutasteride + microneedling, albeit without serum DHT and with slightly different methodologies.[15]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
These investigators randomized 34 men into two groups. One received microneedling + 1 mL of 0.01% topical dutasteride (i.e., 0.1 mg). The other received microneedling + 1 mL of saline solution (i.e., water).
Both groups were treated once every four weeks for a total of three sessions. In both groups, the investigators used a Dr. Pen A6 Ultimate microneedling device and set the needle depth to 2.5mm.
Hair parameters were assessed 4-8 weeks later, thus bringing the study duration to 20 weeks (despite each group only having received three microneedling + topical treatments).
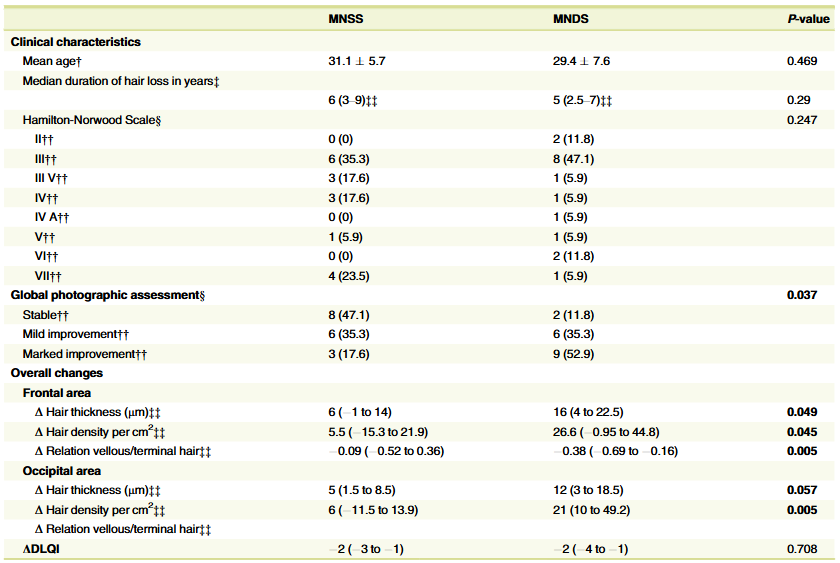
Encouragingly, over 50% of people in the microneedling + topical dutasteride group observed “marked improvement” in their hair loss. Hair thickness, hair density, and the ratio of vellus:terminal hairs also improved versus the microneedling-only group.
Figure 8: Results from a 2022 study on microneedling + dutasteride (left column) versus microneedling alone (right column).[16]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
While serum DHT levels were not measured, there were no reports of sexual side effects from participants.
Our Analysis
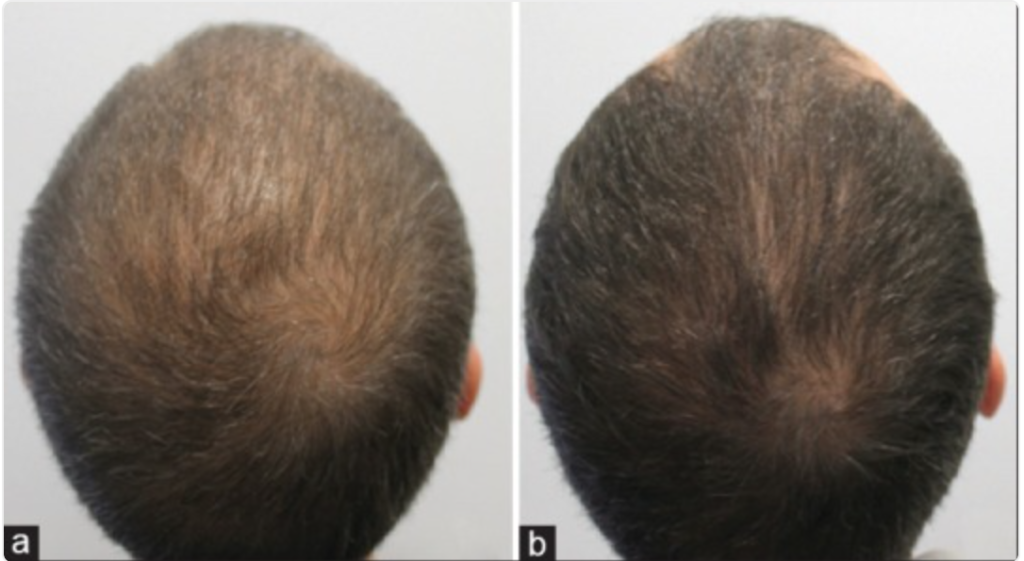
This is another positive (albeit preliminary) signal that topical dutasteride can produce results and reduce our risk of adverse events (and drug exposure) – especially when paired with microneedling. Just see these before-and-after photos from one study participant:
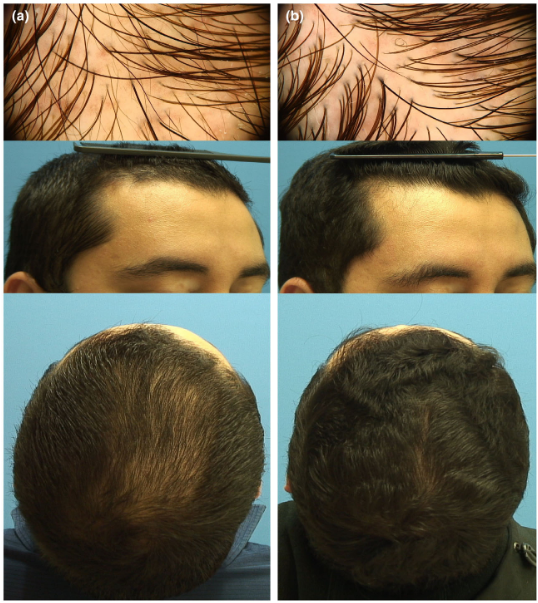
Figure 9: (a). Vertex area trichoscopy and clinical photograph of a patient with androgenetic alopecia (Hamilton-Norwood Scale III) at baseline visit. (b). Frontal area trichoscopy and clinical photograph showing marked improvement (HamiltonNorwood Scale II) at week 16 after 3 sessions with microneedling plus topical 0.01% solution of dutasteride in monotherapy.[17]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
Another encouraging component of this study is that the total dutasteride dosage was lower than the 2018 study, yet still produced results. Whereas the 2018 study used dosages as high as 0.4mg weekly (at least for the first 8 weeks), this 2022 study used dosages equating to 0.1mg once every four weeks. If we are to take the minimal serum DHT changes from the 2018 study at face-value, then it’s even more unlikely that the dutasteride volumes used in this 2022 study led to appreciable changes to serum DHT.
Another study published this year (2025) also gave some insight into low topical dosages of dutasteride in people with AGA.
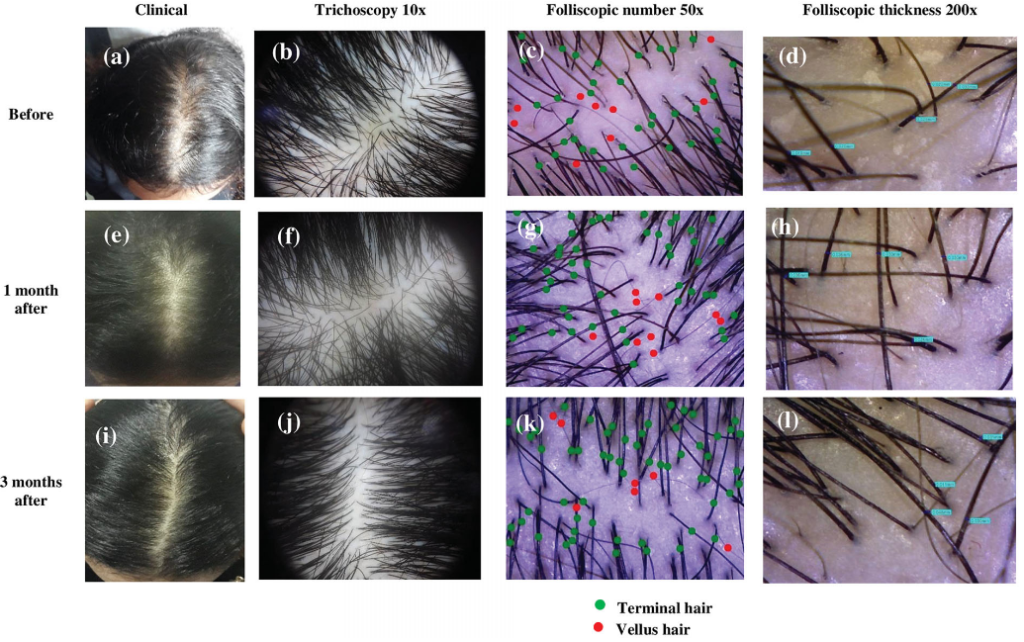
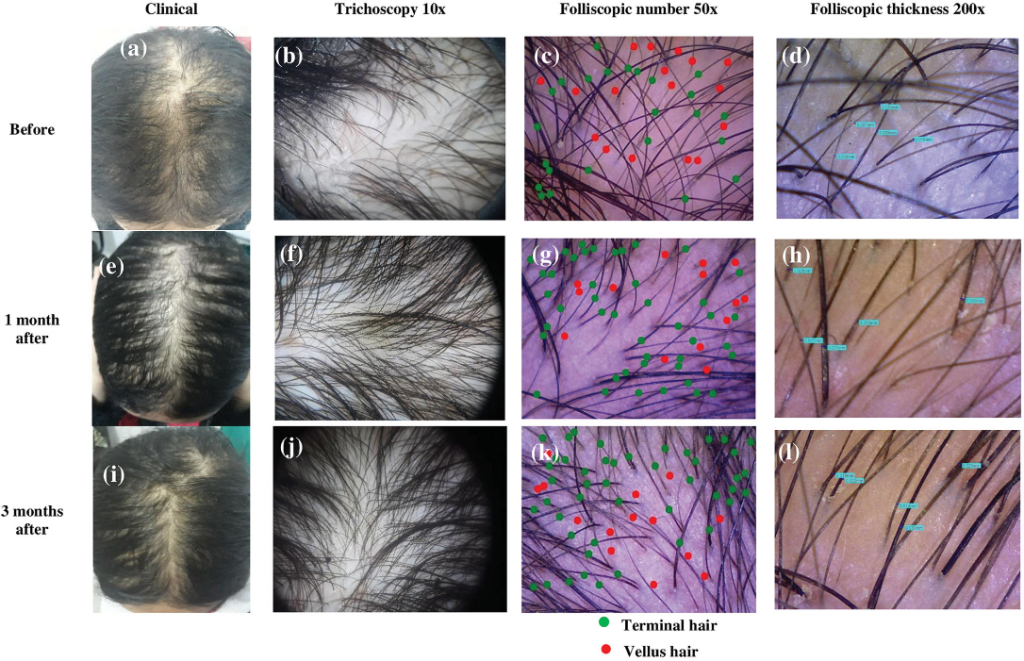
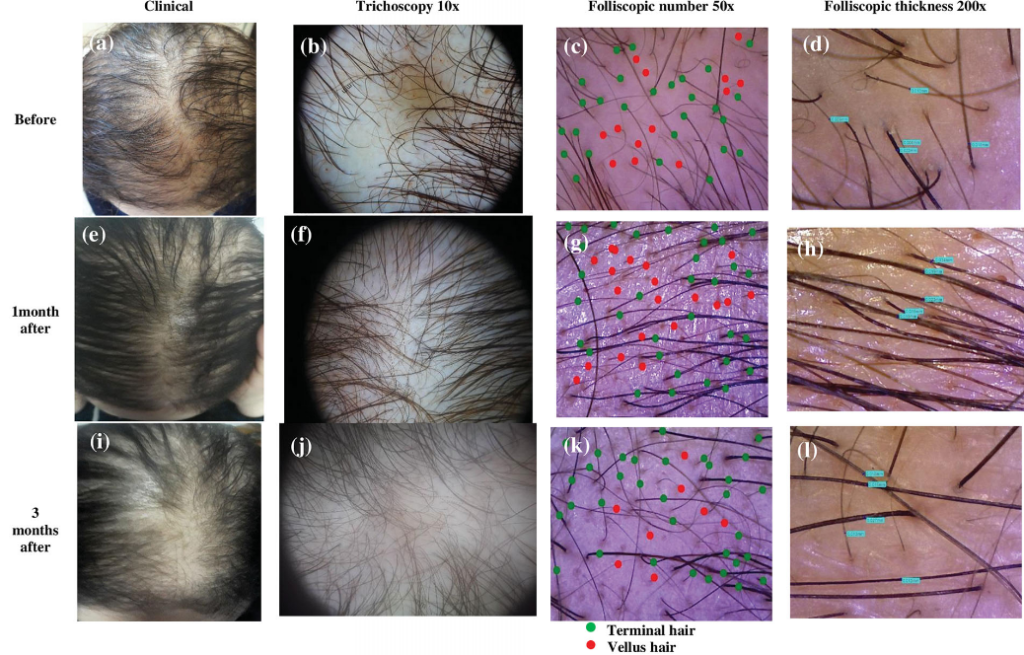
Panuganti et al, 2025: Topical Dutasteride at 0.01%, 0.02%, and 0.05% w/v
The investigators randomized 135 men with AGA into five groups: 0.01%, 0.02%, or 0.05% topical dutasteride (1 mL once daily), 1 mg oral finasteride daily, or a topical placebo. Treatments were continued for 24 weeks, with the topical solutions applied to a marked vertex target area.[18]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate … Continue reading
Baseline characteristics were balanced across groups. Efficacy was assessed via macrophotography and target area hair counts (TAHC), global photographic assessment, and patient satisfaction questionnaires.
At 24 weeks:
- The 0.05% topical dutasteride group showed the largest mean increase in TAHC, reported as +75.5 hairs/cm² vs baseline (p=0.0001), compared with +41.2 hairs/cm² in the oral finasteride group (p=0.0001) and essentially no change in the placebo group (+0.07 hairs/cm², p=0.9957).
- The 0.01% and 0.02% topical groups improved versus placebo, but did not outperform oral finasteride.
- Hair shaft width improved in a dose-dependent manner; 0.05% topical dutasteride increased hair width by 11.6 µm vs placebo at week 24 (p=0.0185).
- Patient satisfaction and investigator global assessments were highest in the 0.05% group.
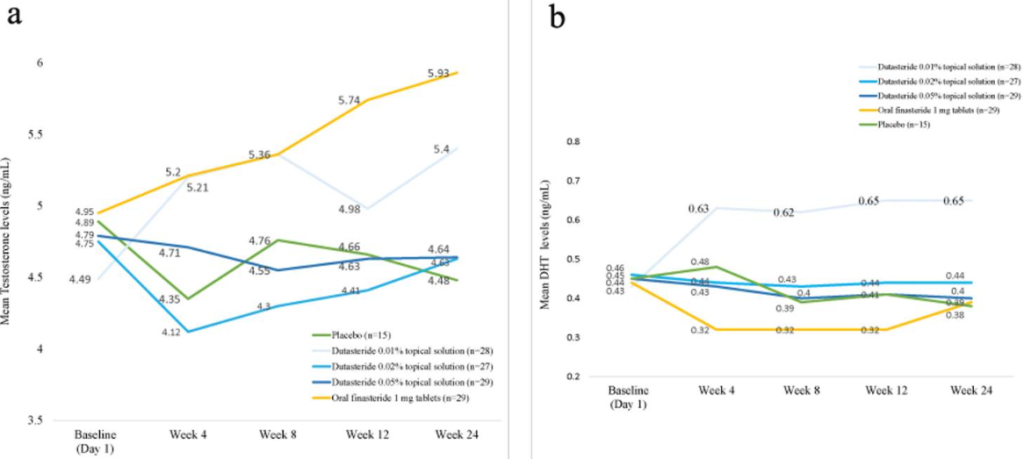
- The study also evaluated serum testosterone and DHT. Reported changes with topical dutasteride were modest and dose-dependent. In the 0.05% group, serum DHT decreased by 8.9% at week 12 and 11% at week 24, while testosterone changes were described as minor. In contrast, oral finasteride was associated with a 27% DHT reduction at week 12 and 11% at week 24, alongside a gradual increase in serum testosterone (up to ~20% by week 24).
- Across all topical dutasteride arms, the authors state there were no statistically significant changes in serum DHT or testosterone, low systemic exposure, and no sexual side effects or clinically relevant lab abnormalities. The 0.05% group delivered the greatest reported efficacy while appearing to avoid the hormonal shifts seen with oral finasteride.
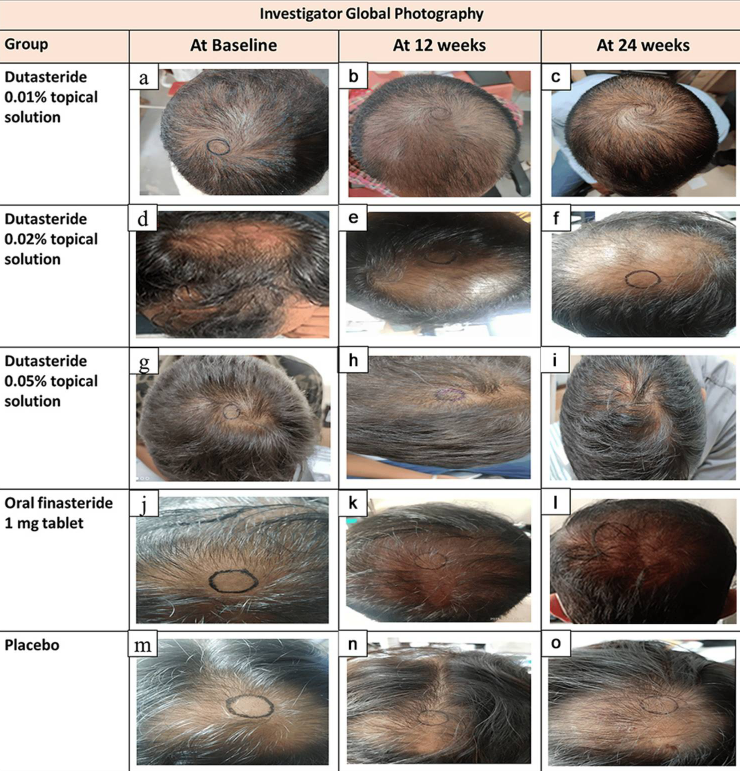
Figure 10: Representative images of hair growth in male-pattern androgenetic alopecia after treatment with 0.01% dutasteride topical solution at 12 week (b) and 24 week (c) vs baseline (a); 0.02% dutasteride topical solution at 12 week (e) and 24 week (f) vs baseline (d); 0.05% dutasteride topical solution at 12 week (h) and 24 week (i) vs baseline (g); oral finasteride 1 mg tablets at week 12 (k) and week 24 (l) vs baseline (j); placebo at week 12 (n) and week 24 (o) vs baseline (m).[19]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate … Continue reading
Our Analysis
On the surface, the Panuganti trial is extremely attractive: it suggests that low-dose topical dutasteride (0.05%) can outperform 1 mg oral finasteride in hair regrowth while producing only minimal average changes in serum DHT. However, several issues with the design and reporting warrant caution, and in our view, the topline conclusion should not be taken at face value.
Sponsorship and authorship
The trial was sponsored by Shilpa Medicare Limited, and all authors were company employees. Industry sponsorship does not invalidate results, but it does increase the importance of methodological transparency and independent replication—especially when findings are strikingly positive and out of step with real-world experience.
Hair counts that don’t match typical scalp biology
The reported baseline hair counts (≈300–330 hairs/cm²) in men with Norwood III–V vertex thinning are difficult to reconcile with established data. In non-balding adults, typical terminal hair densities are ~100–250 hairs/cm², and they fall substantially with moderate-to-severe AGA. Yet the study presents baseline photos of visibly thinned vertex regions alongside hair counts that exceed even full-density scalps.
The most likely explanations are that:
- Vellus hairs were included in the counts without a clear diameter cut-off (e.g., ≤40 µm), and/or
- The counted area was effectively larger than 1 cm², or hair counts were performed manually rather than via standardized software.
Any of these will inflate absolute hair counts and complicate the interpretation of changes over time.
Questionable consistency of the hair count target area
Accurate hair counting depends on revisiting the exact same 1 cm² area at each time point, typically anchored to a tattoo or permanent scalp mark. In the figures provided, the circular “counting zones” appear to shift in position, shape, and size between baseline, week 12, and week 24 for the same participant.
Even a 1–2 mm shift in the center of a 1 cm² circle can change measured hair counts by 50% or more in patchy thinning regions. Since the study reports changes on the order of ~10–15%, small misalignments in the counting area could fully account for the apparent “regrowth,” particularly in groups with smaller gains.
Limited insight into systemic exposure
Serum DHT and testosterone were reported only as group mean percentage changes, with no measures of variability (e.g., standard deviations) or individual pharmacokinetic correlations. This means:
- A reported 11% mean DHT decrease could still conceal substantial outliers with much larger reductions.
- We can’t assess whether men with the best regrowth had proportionally greater systemic exposure.
For a drug like dutasteride, where individual absorption and metabolism vary widely, this lack of granularity limits the conclusions we can draw about “safety” at the individual level.
Conflict with real-world data and dose–response expectations
Over several years, our community has tracked people using low-dose topical dutasteride (e.g., 0.01–0.02%, 1–2 mL daily, sometimes higher weekly exposure than in the 0.05% arm of this study). Broadly:
- Serum DHT typically changes by ~10–15%, which aligns with the idea of good scalp localization.
- Clinical outcomes are generally hair loss stabilization, with modest regrowth in some cases, not the large average gains suggested to outperform oral finasteride.
- More pronounced regrowth tends to appear only at much higher weekly exposure (e.g., around 8–10+ mg/week or with added microneedling), and at that point, systemic side effects become more common.
Taken together, this creates a tension: the study’s claim that 1 mL of 0.05% topical dutasteride daily can outperform 1 mg oral finasteride on average, without meaningful systemic effects, does not match what we see in practice or what we would expect based on dutasteride pharmacology.
Figure 11: Changes from baseline to week 24 in (a) testosterone levels and (b) DHT levels.[20]Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate … Continue reading
Overall, the Panuganti et al. trial supports that low-dose topical dutasteride (0.01–0.05%) has real biologic activity, can improve hair parameters versus placebo, and may keep average serum DHT changes relatively modest. However, issues with baseline hair counts, apparent inconsistencies in how target areas were measured, limited systemic exposure data, and a headline result that conflicts with prior studies and real-world experience mean we don’t think this study justifies expecting low-dose topical dutasteride to reliably outperform oral finasteride. For now, it’s more reasonable to view low-dose topical dutasteride (≤0.05% with limited weekly exposure) as a localized, hair-loss–stabilizing option with modest regrowth and relatively mild systemic impact, while higher-dose topical regimens are more likely to produce cosmetically meaningful gains but also to behave more like oral dutasteride in terms of systemic DHT suppression and potential side effects.
Yet another study came out in 2025, combining skin patting and iontophoresis (a medical procedure using a mild electrical current) with dutasteride gel in male and menopausal female AGA.
Cedirian et al., 2025: 6% Dutasteride Gel, Skin Patting and Iontophoresis
In this single-center pilot study, researchers enrolled 20 adults (10 men, 10 postmenopausal women) with AGA who had failed at least 12 months of standard therapy (topical minoxidil and/or oral finasteride).[21]Cedirian, S., Pampaloni, F., Quadrelli, F., Rapparini, L., Bruni, F., Martelli, G., Piraccini, B.M., Starace, M. (2025). Efficacy of Skin Patting and Iontophoresis with Dutasteride Gel in Male and … Continue reading Participants underwent monthly sessions of a compounded 6% dutasteride gel delivered using a device that combines skin patting (SPi), microneedling, iontophoresis, electrostimulation, and red LED light over a standardized frontal-vertex treatment field for four months.
Hair density and shaft diameter were assessed by trichoscopy at baseline and again 8 weeks after the final session (i.e., roughly 6 months from baseline), alongside a hair pull test and patient-reported satisfaction scores. By the end of follow-up, frontal and vertex hair density increased significantly (p<0.001), hair shaft diameter improved in both regions (vertex p<0.001; frontal p=0.046), and pull test counts nearly halved (p<0.001), pointing to both regrowth and reduced shedding.
Clinically, investigators rated mean global improvement at +1.9 on a 7-point scale, corresponding to moderate regrowth and less shedding. Subjective outcomes were similarly positive: about 70% of participants perceived the treatment as moderately to very effective, mean cosmetic satisfaction was 3.4/4, and 85% described the procedure as pleasant. No adverse events or tolerability concerns were reported during treatment or follow-up.
Our Analysis
On paper, this is an encouraging signal: the participants experienced measurable gains in density, diameter, and shedding. The high satisfaction scores and absence of side effects also support the idea that this approach is, at minimum, well-tolerated.
However, the design makes it difficult to isolate what’s actually driving the benefit. Because the protocol combines several modalities, we can’t separate the relative contribution of dutasteride from the other treatments. Outcome measures were also partly subjective and non-validated, and all assessments were unblinded, which opens the door to expectation and observer bias.
A second major limitation is the complete absence of systemic safety data. Unlike the other studies, a high dosage of topical dutasteride is used (6%), but we don’t know the effect of this on systemic/serum DHT levels.
While lab work is really the only way to get a grip on things, this study suggests that topical dosages as low as 1 mL x 0.01%, and as infrequently applied as once every four weeks, can still produce results for more than 50% of men with androgenic alopecia.
This begs the question: just how low can we go for dosing? While we don’t yet have the answer, studies on a different delivery vehicle for dutasteride – mesotherapy – suggest we can go even lower.
Mesotherapy Dutasteride: Clinical Studies (Hair Counts, Serum DHT, & More)
Mesotherapy dutasteride is also known as intradermal dutasteride. This is different from topical dutasteride.
Whereas topical dutasteride is applied directly to the scalp, mesotherapy dutasteride is when dutasteride is injected directly into the scalp skin – between the epidermis and dermis (i.e., 0.5-0.7mm deep into the scalp).
Whereas only 25% of topical dutasteride is absorbed into the scalp dermis over 12 hours, 100% of mesotherapy dutasteride is absorbed into the scalp dermis, and immediately upon injection. After all, the drug is injected directly into the scalp, leaving no material to evaporate or get wiped away by sweat or during a shower.
That means, across equal dosages, that mesotherapy dutasteride will better saturate the scalp skin, but also lead to more systemic absorption.
The question then becomes: how do the pharmacokinetics of mesotherapy dutasteride change the equation for how much (and how frequently) we should be exposing our scalps to this medication in order to maximize hair gains while minimizing systemic DHT reductions?
While we don’t yet have perfect answers, there are a few studies that suggest the dose is even lower than topical dutasteride.
Corralo et. al (2017): Mesotherapy Dutasteride
A 2017 study by Corralo et al. investigated the effects of mesotherapy dutasteride on people with androgenic alopecia.[22]Saceda-Corralo, D., Rodrigues-Barata, A.R., Vano-Galvan, S., Jaen-Olasolo, P. (2017). Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia. International Journal of Trichology. … Continue reading The researchers selected six participants to receive 1 mL of 0.01% dutasteride (i.e., 0.1 mg of dutasteride) once every three months, totaling three injection sessions at months 0, 3, and 6.
The investigation group also took serum DHT measurements before starting treatment, and one month after the final mesotherapy injection (according to personal email correspondences with the authors).
Three months after the last mesotherapy injection, the investigators measured hair parameter improvements. Encouragingly, 1 mL of 0.01% mesotherapy dutasteride once every three months led to statistically significant hair improvements for all participants. Moreover, no changes to serum hormonal profiles were observed.
Here are the before-and-after photos of a featured patient:
Figure 13: Androgenetic alopecia in a 33-year-old man before (a) and after (b) treatment with dutasteride injections through 6 months with a one-session treatment every 3 months.[23]Saceda-Corralo, D., Rodrigues-Barata, A.R., Vano-Galvan, S., Jaen-Olasolo, P. (2017). Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia. International Journal of Trichology. … Continue reading
Our Analysis
The sample size of this study is incredibly small. With that said, the results are impressive, and they add to a growing number of signals suggesting that mesotherapy dutasteride (and topical dutasteride) improve hair growth for men and women with androgenic alopecia.
Moreover, this study suggests that a dosing schedule as infrequent as 1 mL of 0.01% dutasteride (i.e., 0.1 mg) once every three months can appreciably improve hair parameters, and without altering systemic levels of hormones.
However, just as with the last study measuring serum DHT levels, this investigation group waited a full month after a mesotherapy dutasteride session to read serum levels of DHT. While the results showed no effect on serum hormones, the reality is that serum DHT levels could have dropped during the days and weeks following an injection, only to rebound to baseline by the time these investigators decided to test.
On the other hand, it’s also possible that mesotherapy dutasteride changes the pharmacokinetics of dutasteride itself – perhaps by increasing tissue saturation time and decreasing systemic leakage rates. While this may sound absurd, other studies have demonstrated mesotherapy to exhibit this behavioral shift with other drugs and compounds.[24]Mammucari, M., Maggiori, E., Russo, D., Giorgio, C., Ronconi, G., Ferrara, P.E., Cazona, F., Antonaci, L., Violo, B., Velluci, R., Mediati, D.R., Migliore, A., Massafra, U., Bifarini, B., Gori, F., … Continue reading Therefore, it’s not unreasonable to assume these effects may also carry over to mesotherapy dutasteride, and that for unknown reasons, the drug has longer staying power in the scalp when injected (and less systemic absorption).
Based on the results of these serum DHT tests, preliminary evidence points toward mesotherapy dutasteride perhaps lasting longer in the scalp and conferring slower systemic absorption. But be forewarned: there is likely an upper limit for how much mesotherapy dutasteride we can inject before serum DHT levels start to decline.
Member Experience: Too Much Mesotherapy Dutasteride?
We once had a member test 2 mL of 0.05% dutasteride (i.e., 1.0 mg of dutasteride, or 10x the dosage used in the 2017 study) during his first mesotherapy session. He did a great job tracking serum DHT changes before treatment and 4 days, 4 weeks, and 8 weeks after the injections.
His results? Serum DHT decreases shortly after treatment, which continued to decline up through month one. At that point, the member started experiencing cognitive-related side effects. By the second month, serum DHT levels began to rebound and all side effects went away.
Serum DHT Reported side effects Pre-treatment 51 ng/dL (reference: 30-90 ng/dl) No Post-treatment (4 days) 35 ng/dL No Post-treatment (4 weeks) 25 ng/dL Yes Post-treatment (8 weeks) 31 ng/dL No With that said, there are a few things worth noting about this member’s experience:
- First, these effects occurred at 10x the dosage of the 2017 study by Saceda. So, it’s unlikely that if you follow the study parameters, the same thing will happen to you.
- Secondly, the delayed decline in serum DHT puzzled us, because it didn’t necessarily fit with the pharmacokinetics expected from mesotherapy or the better-studied pharmacokinetic expectations of oral dutasteride. Keep in mind that 1mg of oral dutasteride rapidly distributes across tissues and has a half-life of 7-14 days. With mesotherapy, much of the drug remains in the scalp skin and a lesser amount gets absorbed into the serum. Therefore, even if mesotherapy had delayed the systemic absorption of dutasteride, we shouldn’t expect the drop at 4 weeks to be more dramatic than the drop at 4 days – much less significantly lower at 4 weeks versus 4 days.
- Thirdly, after having a conversation with this member, we learned they also started using an over-the-counter allergy medication at some point after the mesotherapy sessions. Studies suggest that this allergy medication can lower testosterone, which can inadvertently lower DHT. As such, we’re not certain enough to attribute 100% of these serum DHT reductions exclusively to mesotherapy dutasteride – even if we believe the therapy to be responsible for the majority of the drops observed.
- Lastly, regardless of the drop in DHT and side effects, this member did report better hair growth after just one session.
So, by following lower dosage guidelines, we’re confident you can avoid a similar experience. In fact, a 2013 study on mesotherapy dutasteride echoes the experience from our member and shows why mesotherapy dosages, volumes, and frequencies matter a lot.
Sobhy et al. (2013): Low-Dose Vs. High-Dose Mesotherapy Dutasteride
In 2013, a research team in Egypt enrolled 90 men with androgenic alopecia to test mesotherapy dutasteride at varying dosages, with the higher dosage also containing other potential hair growth-promoting ingredients. They randomized the men into three groups, and then set them up with the following treatment:
- Group A: 1.5-2.0 mL of 0.005% mesotherapy dutasteride (i.e., 0.075-0.1 mg of dutasteride per session).
- Group B: 1.5-2.0 mL of 0.05% mesotherapy dutasteride (i.e., 0.75-1.0 mg of dutasteride per session; 10x more than Group A). The 10 mL solution also contained 500 mg of dexapanthenol, 20 mg of biotin 20 mg, and 200 mg of pyridoxine.
- Group C: 1.5-2.0 mL of saline solution (i.e., water; this is a placebo group).
Then, the investigators performed 9 mesotherapy sessions over a span of 19 weeks. The sessions were as follows:
- Once per week (weeks 0-3)
- Once every two weeks (weeks 5 and 7)
- Once every four weeks (weeks 11, 15, 19
Given this dosing schedule, here’s how much dutasteride each group was exposed to over 19 weeks:
- Group A: 0.675-0.9 mg
- Group B: 6.75-9.0 mg (i.e., 10x more than Group A)
- Group C: 0 mg (they’re the placebo group)
Results
After 19 weeks, here were the changes to hair parameters and serum DHT levels:
- Hair Parameters:
- Unsurprisingly, both Group A and Group B saw hair parameter improvements. However, Group B saw more robust improvements relative to Group A. This is probably because they received 10x the dosage of dutasteride.
- Serum DHT:
- Group A saw a significant increase in serum DHT levels before and after the study ended. The authors attributed this change to the preservation of diurnal DHT fluctuations. This suggests that at dosages of as low as 0.9 mg over 19 weeks, serum DHT levels are basically preserved.
- Group B saw a significant decrease in serum DHT levels. While this would be expected given what we’ve just learned about the dosing and the pharmacokinetics of both mesotherapy and dutasteride, the investigation group was also puzzled by the fact that the placebo group also saw a decline in serum DHT levels. Again, the researchers chalked it up to normal diurnal fluctuations to DHT. However, based on members’ experiences and the additional studies published since this 2013 investigation, our best bet is that Group B saw serum DHT levels decline mainly because of higher exposure rates to dutasteride.
The bottom line: stick to mesotherapy dutasteride dosages of 0.1-0.2 mg per month. That’s probably close to the “sweet spot” for maximizing hair gains while minimizing unwanted systemic hormonal effects.
Ultimately, a number of factors determine how much of a substance will be absorbed systemically and how much of that substance will remain saturated in a tissue to exert its maximal effect. These factors include:
- Drug pKa. This is a measurement of the acidity of a drug.
- Drug polarity. this affects the hydrophilic-hydrophobic ratio of a compound in reference to its location within the dermis.
- Location of the mesodermal application. Keep in mind the scalp dermis is distinctly different from the facial dermal layer.
- The diffusion coefficient of a compound.[25]Souto, E.B., Fangueiro, J.F., Fernandes, A.R., Cano, A., Sanchez-Lopez, E., Garcia, M.L., Severino, P., Paganelli, M.O., Chaud, M.V., Silva, A.M. (2022). Physicochemical and biopharmaceutical aspects … Continue reading This is a measurement for how quickly and widely a compound can diffuse in the surrounding tissue.
The above factors are influenced by drug preservatives, carrier agents, temperatures, injection needle depths, aqueous secretions from sweat glands, and even apocrine secretions from sebaceous glands (just to name a few). We can tell you right now: none of the studies on mesotherapy dutasteride made an effort to standardize these factors, or even mention them in their methodologies. In fact, we couldn’t even find standards for just how deep the mesotherapy needles were injected for any study. These are all big limitations for data quality.
(Side note: we’re planning on investigating these factors in early 2023, so that we can develop a better protocol for mesotherapy dutasteride – one that is optimized based on human data across multiple injection frequencies, exposure volumes, carrier agents, and needle depths. Expect more details about this toward the end of 2022.)
Are There Any Other Studies On Mesotherapy Dutasteride?
Yes. However, they mostly repeat the results we’ve discussed above. Moreover, to our knowledge, none of the other studies measured serum DHT levels, so they don’t help us get closer to answering our core question: “What’s the best protocol for mesotherapy dutasteride to maximize hair gains while minimizing systemic hormonal effects?”
With that said, there is one final study that provides some nuanced insights – particularly related to mesotherapy dutasteride and its variability in hair regrowth, depending on whether someone has metabolic syndrome.
Mofta et al. (2021): Mesotherapy Dutasteride In Females With Metabolic Syndrome
In this 2021 study, researchers sought to determine the effects of mesotherapy dutasteride on females with pattern hair loss (androgenic alopecia), and how hair growth outcomes changed depending on whether participants had metabolic syndrome.[26]Moftah, N., Mubarak, R., Abdelghani, R. (2021). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection … Continue reading
The researchers identified 51 females with pattern hair loss. Then, they determined which women also had metabolic syndrome. For reference, the investigators defined metabolic syndrome as meeting at least three of the following criteria:
- Raised waist circumference (population- and country-specific definitions), i.e.,> 92.3 cm in Egyptian women
- Fasting blood glucose ≥ 100 mg/dL or on diabetes treatment
- Blood pressure ≥ 130/85 mm Hg, or on antihypertensive treatment
- Triglycerides ≥150 mg/dL or on treatment
- High-density lipoprotein-cholesterol < 50 mg/dL, or on treatment
This left 26 and 25 females with and without metabolic syndrome, respectively.
Next, the investigators administered mesotherapy dutasteride to all participants. This included 1 mL of 0.02% mesotherapy dutasteride (i.e., 0.2 mg of dutasteride) per session, with eight sessions spanning a period of ~6 months. A few months later, hair parameters were assessed to gauge results.
Results
Surprisingly, females with metabolic syndrome experienced an initial improvement in hair growth at month one, followed by an acceleration of hair loss by month three. Contrastingly, females without metabolic syndrome saw their hair growth increasingly improve throughout each month of treatment.
These results were not only reflected in phototrichograms (i.e., hair counts, hair diameters, and anagen:telogen ratios) but also in global photographs.
Just see these before-and-after photos of two females without metabolic syndrome:
Figure 14: Before and after of woman one without metabolic syndrome.[27]Moftah, N., Mubarak, R., Abdelghani, R. (2019). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection … Continue reading
Figure 15: Before and after of woman two without metabolic syndrome.[28]Moftah, N., Mubarak, R., Abdelghani, R. (2019). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection … Continue reading
Now see this before-and-after photoset of a female with metabolic syndrome:
Figure 16: Before-and-after images of a woman with metabolic syndrome.[29]Moftah, N., Mubarak, R., Abdelghani, R. (2019). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection … Continue reading
On a positive note, no females in either group reported any side effects – sexual or otherwise. But the discrepancy in regrowth between those affected and unaffected by metabolic syndrome was, to put it simply, puzzling.
Why Did Female With Metabolic Syndrome See A Worsening Of Hair Loss With Mesotherapy Dutasteride?
The researchers did not have a definitive answer. However, some studies suggest an association between metabolic syndrome and androgenic alopecia, with mechanistic data suggesting that insulin insensitivity can enhance androgen production in certain tissues – like the ovaries in women and, perhaps, other organs like the skin.
Oral dutasteride is known to have a mildly negative effect on insulin sensitivity. For most healthy people, this isn’t problematic. But the data are less clear for people who are already afflicted with metabolic syndrome and then start taking dutasteride.
Here’s the rationale the authors used to explain their results:
“These findings could be explained by the study conducted by Upreti et al. [59] who confirmed that dual inhibition of 5α reductase I and II by dutasteride lead to modulation of insulin sensitivity in the peripheral tissues. Accordingly, dutasteride induced reduction of insulin sensitivity that is already impaired in both MetS [55, 60] and FPHL [61-63] could be a possible mechanism of deterioration in participants with MetS. This possibility was recently supported by Sadgrove [64] who suggested that insulin resistance could enhance microinflammation in androgenetic alopecia both directly and indirectly by increased microbial colonization. Moreover, an in vitro study carried out by Philpott et al. [39] revealed that removal of insulin from the culture medium of human hair follicles could lead to follicular transformation into catagen state.“
The bottom line: if you’re already insulin resistant, perhaps it is worth getting those levels within normal ranges before trying dutasteride altogether– including via mesotherapy delivery vehicles.
Where Can We Buy Mesotherapy Dutasteride?
Here are a few resources.
- SuperHumanStore (worldwide). We recently lab-tested this product. It passed all tests for purity and sterility. Unfortunately, the store no longer carries that product. So, we’re currently lab-testing their new product. We’ll update this guide when we have the results.
- FUE Clinic (U.S.). This company sells 0.01% mesotherapy dutasteride alongside biotin, pyridoxine, and a blend of “cellular matrix growth factors”. Don’t be fooled by those fancy words; we can assure you that cellular matrix growth factors are currently more so buzzwords than they are clinically-proven hair growth stimulants.
Where Can We Buy Topical Dutasteride?
Here are a few resources.
- MinoxidilMax (worldwide): 0.1% topical dutasteride, 60 mL. On the one hand, MinoxidilMax definitely sells prescription-grade hair loss drugs without requiring a prescription, which we’re 99% sure is illegal. On the other hand, when we lab-tested their products, they contained what they claimed – which is a good sign. We like MinoxidilMax because (1) they ship worldwide, and (2) they sell solvents to pair with their products – so you can dilute their topicals down to lower dosages: 0.005%, 0.01%, 0.02%, etc.
- Happy Head (U.S.). Their products contain topical finasteride, minoxidil and retinoic acid. But they allow customization by including topical dutasteride with or in place of finasteride.
- Regrowth Labs, D57α (U.S.). This is a topical solution containing dutasteride in a 0.5% concentration. Their suggested dose is 1mL which would give a 0.5mg dose topically. However, the company suggests applying this volume twice a day, daily. The accompanying ingredients include azelaic acid, vitamins, phytonutrients and polar carriers to help with absorption.
- CFS Pharmacy (U.S.). This also sells topical dutasteride as a prescription medication. The topical solution comes in a gel. While CFS Pharmacy promotes themselves as capable of making any dosage of topical dutasteride, we believe they require a prescription. So, before you exercise this option, be sure to find a doctor who will write you a prescription for the dutasteride concentration you’d like.
- Bauman Medical, 82D (U.S.). This is a topical dutasteride + minoxidil solution. The amount of dutasteride contained in the product is a 0.75% concentration. There is no mention of what the additional ingredients or carrier solutions are.
References[+]
References ↑1, ↑2 Herz-Ruelas, M.E., Alvarez-Villalobos, N.A., Millan-Alanis, J.M., de Leon-Gutierrez, H., Ocampo-Garza, S.S., Grimalt, R. (2020). Efficacy of Intralesional and Oral Dutasteride in the Treatment of Androgenetic Alopecia: A Systematic Review. Skin Appendage Disorders. 6(6). 338-345. Available at: https://doi.org/10.1159/000510697 ↑3, ↑4, ↑5, ↑6 Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis. Clinical Interventions in Aging. 14. 399-406. Available at: PMID 30863034 ↑7 Hagberg, K.W., Divan, H.A., Fang, S.C., Nickel, J.C., Jick, S.S. (2017). Risk of gynecomastia and breast cancer associated with the use of 5-alpha reductase inhibitors for benign prostatic hyperplasia. Clinical Epidemiology. 9. 83-91. Available at: https://doi.org/10.2147/CLEP.S124674 ↑8 Rahimi-Ardabili, B., Pourandarjani, R., Habibollahi, P., Mualeki, A. (2006). Finasteride induced depression: a prospective study. BMC Clinical Pharmacology. 6(7). Available at: https://doi.org/10.1186/1472-6904-6-7 ↑9 Wang, K., Fan, D-D., Jin, S., Xing, N-Z., Niu, Y-N. (2014). Differential expression of 5-alpha reductase isozymes in the prostate and its clinical implications. Asian Journal of Andrology. 16(2). 274-279. Available at: https://doi.org/10.4103/1008-682X.123664 ↑10 Gisleskog, P.O., Hermann, D., Hammarlund-Udenaes, M., Karlsson, M.O. (1999). The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. British Journal of Clinical Pharmacology. 47. 53-58. Available at: https://doi.org/10.1046/j.1365-2125.1999.00843.x ↑11 Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. Available at: https://smj.journals.ekb.eg/article_42083_24b61cbba4be9982db23c318414034c0.pdf (Accessed: Novemeber 2025) ↑12, ↑13, ↑14 Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmagd, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-401. Available at: https://smj.journals.ekb.eg/article_42083_24b61cbba4be9982db23c318414034c0.pdf (Accessed: November 2025) ↑15, ↑16, ↑17 Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. JEADV. 36. c806-c808. Available at: https://doi.org/10.1111/jdv.18285 ↑18, ↑19, ↑20 Panuganti, V.K., Madala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., KSSVV, S.R., Dundigalla, M.R. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects With Androgenetic Alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑21 Cedirian, S., Pampaloni, F., Quadrelli, F., Rapparini, L., Bruni, F., Martelli, G., Piraccini, B.M., Starace, M. (2025). Efficacy of Skin Patting and Iontophoresis with Dutasteride Gel in Male and Menopausal Female Androgenetic Alopecia: A Pilot Study. Dermatologic Therapy. 15(11). 3419-3424. Available at: https://doi.org/10.1007/s13555-025-01532-w. ↑22, ↑23 Saceda-Corralo, D., Rodrigues-Barata, A.R., Vano-Galvan, S., Jaen-Olasolo, P. (2017). Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia. International Journal of Trichology. 9(3). 143-145. Available at: https://doi.org/10.4103/ijt.ijt_73_16 ↑24 Mammucari, M., Maggiori, E., Russo, D., Giorgio, C., Ronconi, G., Ferrara, P.E., Cazona, F., Antonaci, L., Violo, B., Velluci, R., Mediati, D.R., Migliore, A., Massafra, U., Bifarini, B., Gori, F., di Carlo, M., Brauneis, S., Paolucci, T., Rocchi, P., Cugutti, A., Di Marzo, R., Bomprezzi, A., Santini, S., Giardini, M., Catizzone, A.R., Troili, F., Dorato, D., Gallo, A., Guglielmo, C., Natoli, S. (2020). Mesotherapy: From Historical Notes to Scientific Evidence and Future Prospects. ScientificWorldJournal. 3542848. Available at: https://doi.org/10.1155/2020/3542848 ↑25 Souto, E.B., Fangueiro, J.F., Fernandes, A.R., Cano, A., Sanchez-Lopez, E., Garcia, M.L., Severino, P., Paganelli, M.O., Chaud, M.V., Silva, A.M. (2022). Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon. 8(2). e08938. Available at: https://doi.org/10.1016/j.heliyon.2022.e08938 ↑26 Moftah, N., Mubarak, R., Abdelghani, R. (2021). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection in patients with female pattern hair loss: a prospective cohort study. 32(7). 827-836. Available at: https://doi.org/10.1080/09546634.2019.1708849 ↑27, ↑28, ↑29 Moftah, N., Mubarak, R., Abdelghani, R. (2019). Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection in patients with female pattern hair loss: a prospective cohort study. Journal of Dermatological Treatment. 32(7). Available at: https://doi.org/10.1080/09546634.2019.1708849 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Perfect Hair Health Team
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down