- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesTopical Finasteride Side Effects: What to Know
First Published Dec 4 2025Last Updated Dec 4 2025Pharmaceutical Researched & Written By:Sarah King, PhD
Researched & Written By:Sarah King, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Topical finasteride is marketed as a “safer, localized” alternative to the oral pill, but how true is that? In this article, we break down what the science actually shows about systemic absorption, sexual and mood-related side effects, real-world user reports, and why responses vary so widely. Learn how to minimize risk, choose better formulations, and determine whether topical finasteride is the right fit for your hair-loss plan.
Full Article
Topical finasteride has emerged to meet demand for hair loss treatments that promise strong results with fewer systemic side effects than oral finasteride by concentrating action at the scalp while reducing overall drug exposure. Early studies suggest it can improve hair growth and lower scalp dihydrotestosterone (DHT) with less systemic absorption, but side effects are not eliminated, and long-term head-to-head data versus oral finasteride remain limited.
This article will cut through the “same results, fewer side effects” marketing narrative and set realistic expectations by focusing on what current clinical evidence and real-world user patterns actually support.
How Does Finasteride Work?
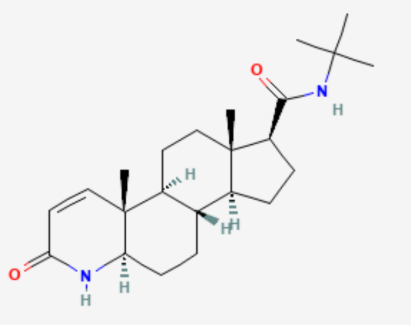
Finasteride is a 5-alpha reductase inhibitor, meaning that it blocks this enzyme from converting testosterone into DHT, the more potent androgen that drives miniaturization of sensitive scalp follicles in androgenic alopecia (AGA). The enzyme catalyzes this conversion in the scalp, skin, liver, and prostate. By inhibiting it, finasteride lowers both local (scalp/prostate) and circulating DHT.[1]Zito PM, Bistas KG, Patel P, et al. Finasteride. [Updated 2024 Feb 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: … Continue reading
Finasteride structure
Why Oral Finasteride is Systemic
A standard 1 mg oral dose reduces DHT by roughly 60-70%, with some studies reporting around 70% suppression at steady state, and intraprostatic DHT falls by about 90%.[2]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading,[3]Smith, A.B., Carson, C.C. (2009). Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Therapeutics and Clinical Risk Management. 5. 535-545. Available at: … Continue reading Because 5 alpha-reductase is expressed in multiple organs, this systemic inhibition affects DHT signaling throughout the body, not only in the scalp.
Why Side Effects Occur
Sexual and mood-related side effects are not purely “in the mind”; they reflect altered androgen physiology in a subset of men whose tissues are more sensitive to a DHT drop, even though testosterone usually stays within the normal range or rises modestly. Reduced DHT can influence libido, erectile function, ejaculate volume, and, in some individuals, mood or energy, consistent with the hormone’s broader role beyond hair follicles.[4]Traish, A.M. (2017). Negative Impact of Testosterone Deficiency and 5ɑ-Reductase Inhibitors Therapy on Metabolic and Sexual Function in Men. Advances in experimental medicine and biology. 1043. … Continue reading
Topical Targeting vs. Spillover
Topical finasteride aims to concentrate drug action in the scalp by delivering finasteride directly to hair follicles, potentially lowering local DHT while limiting how much drug reaches the bloodstream. However, percutaneous absorption still occurs, and pharmacokinetic studies do show measurable reductions in serum DHT with topical formulations, meaning systemic exposure is reduced compared to oral dosing, but not eliminated.[5]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading
What the Research Says About Topical Finasteride Side Effects
The evidence on topical finasteride’s side effect profile shows a nuanced picture – measurable systemic exposure, lower but not zero adverse event rates, and substantial variability between formulations and individuals.
Systemic Absorption
The 2021 Piraccini trial, one of the largest randomized double-blind topical finasteride studies to date, tested a 0.25% finasteride spray applied once daily over 24 weeks in 458 men.[6]Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E., Topical Finasteride Study Group. (2022). Efficacy and safety … Continue reading Plasma finasteride concentrations were over 100-fold lower than with oral finasteride. Serum DHT fell by 34.5% with topical use compared to 55.6% with a 1 mg oral tablet.
Caserini found that lower volumes (100-200 μL of 0.25% solution) reduced serum DHT by 24-26%, while higher volumes (300-400 μL) dropped it by 44-48%.[7]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading Even at the lowest tested doses, scalp DHT declined by roughly 47-52%, demonstrating effective local action with more limited systemic spillover.
Other studies report a range of serum DHT suppression with topical formulations, but all confirm that absorption is lower than oral administration, not absent. One hydroxyl-propyl chitosan formulation was designed specifically to retain finasteride in the reticular dermis near hair bulbs; repeated-dose rat experiments showed no detectable plasma finasteride and no accumulation in skin, although human data remain sparse.[8]Monti. D., Tampucci, S., Burgalassi, S., Chetoni, P., Lenzi, C., Pirone, A., Mailland, F. (2014). Topical formulations containing finasteride. Part I: in vitro permeation/penetration study and in … Continue reading
So to summarize, topical finasteride’s systemic exposure is markedly reduced compared to oral dosing, yet reductions in circulating DHT do still occur across most formulations (in human studies), meaning that some degree of systemic exposure is virtually unavoidable.
Reported Side Effect Rates in Studies
Clinical trials have monitored sexual dysfunction, dermatological reactions, and less common systemic complaints, though sample sizes and durations limit the ability to detect rare or delayed adverse events.
Sexual Side Effects
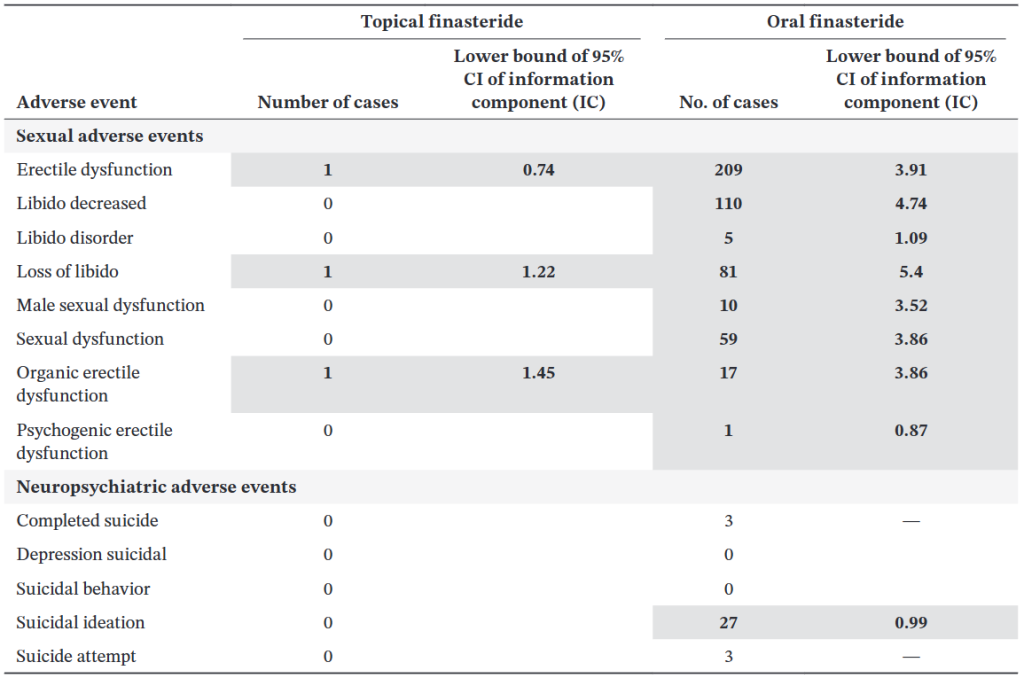
In the 2021 Piraccini study, 2.8% of topical finasteride users reported sexual adverse events (decreased libido, erectile dysfunction, and ejaculatory dysfunction) versus 4.8% on oral finasteride and 3.3% on placebo.
The similarity between the topical and placebo groups led some investigators to suggest that many reports may not be causally linked to the drug. However, a 2025 FDA pharmacovigilance analysis of adverse event reports (2019-2024) identified 32 cases involving topical finasteride formulations, most of which were compounded products, with complaints of erectile dysfunction, anxiety, brain fog, depression, fatigue, insomnia, decreased libido, and testicular pain.[9]US Food and Drug Administration. (2025). FDA alerts health care providers, compounders and consumers of potential risks associated with compounded topical finasteride products. FDA. Available at: … Continue reading What’s more, many of these symptoms reportedly persisted after discontinuation.
A separate analysis found that topical finasteride generated fewer signals for post-finasteride syndrome (PFS) – like events than oral finasteride, but erectile dysfunction remained the most consistently reported adverse event for both routes.[10]Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated With Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study With Data From … Continue reading
Figure 1: Association between finasteride use (topical vs. oral) and occurrence of adverse events (sexual and neuropsychiatric) between 2006and 2011 (inclusive) and across men.[11]Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated With Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study With Data From … Continue reading
Scalp Irritation and Contact Dermatitis
Local reactions are the most common topical-specific side effects. A 2018 systematic review noted reports of erythema, contact dermatitis, and scalp irritation in several studies, though serious cutaneous adverse events were absent.[12]Lee, S.W., Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in … Continue reading
Rates of contact dermatitis ranged from 12-24% in some combination formulations (finasteride + minoxidil), though these were generally mild and did not lead to treatment discontinuation. The irritation effect likely derives from vehicle components (alcohol, propylene glycol) as well as the active drug.
Headaches, Fatigue, and Other Systemic Complaints
Less frequently, trials have documented headaches, feeling about to faint, testicular pain, increased liver enzymes, and even bed-wetting in isolated cases. The 2025 FDA alert highlighted fatigue and insomnia among the systemic complaints associated with topical formulations, reinforcing that absorption can produce effects beyond the scalp.
Trial Limitations: Duration and Sample Size
Most published trials run 12-24 weeks with cohorts of 30-135 participants per arm, which may under-detect rare events (incidence <1%) or adverse effects that emerge only after prolonged exposure. Only a handful of studies have extended beyond six months, and none approach the multi-year observation periods needed to assess phenomena such as PFS.[13]Pereira, A.F.J.R., Coehlo, T, O. de A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001 Recruitment through specialist dermatology centres may also select for patients more tolerant of medication, introducing survivorship bias.
How do the Side Effects Compare with Oral Finasteride?
Head-to-head data have shown that topical finasteride has a numerically lower, but not zero, incidence of systemic adverse events. In Piraccini’s trial, sexual side effects occurred in 2.8% of topical users versus 4.8% of oral users, representing an approximate halving of risk.
There are two important points often overlooked in clinical summaries, however:
- Population averages do not predict individual susceptibility: Even if 97% of topical finasteride users report no sexual side effects in a 24-week trial, that statistic offers little reassurance to someone who is biochemically sensitive to modest DHT reductions. Genetic polymorphisms in 5ɑ-reductase isoforms, androgen receptor sensitivity, and neurosteroid metabolism all vary widely, meaning that a 25% serum DHT drop can be innocuous for one man and symptomatic for another.[14]Pereira, A.F.J.R., Coehlo, T, O. de A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001
- Short trials may miss long-latency or persistent events: Most published studies conclude at 6 months, yet reports to the FDA and patient registries describe symptoms that onset gradually or persist long after stopping treatment. The cases flagged in the FDA alert involved adverse events that continued to persist after product discontinuation, suggesting that systemic absorption, however modest, can trigger durable changes in a subset of users. However, the exact cause of symptom persistence is not yet known.
Why Do Some People Still Get Side Effects From Topical Finasteride?
Despite the reduced systemic exposure, some people still experience sexual, mood, and systemic adverse effects. Let’s take a look at the mechanisms why.
Skin Permeability Varies Significantly Between Individuals
Human skin is not a uniform barrier. Stratum corneum thickness, lipid composition, hydration status, pH, and blood flow differ among individuals and even within scalp regions of a single person.
In vitro permeation studies using human cadaver scalp skin show that finasteride flux ranges of 1.1-20.1 μg/cm2/h, depending on vehicle choice, a roughly 18-fold variation. Live scalp skin likely exhibits even greater heterogeneity due to sebum content, follicle density, and individual differences in dermal blood flow, all of which influence how much drug reaches the bloodstream versus remaining confined to follicular tissue.[15]Farah, A.H., Brown, M.B., McAuley, W.J. (2020). Enhanced Follicular Delivery of Finasteride to Human Scalp Skin Using Heat and Chemical Penetration Enhancers. Pharmaceutical Research. 37(6). 112. … Continue reading
For topical finasteride to minimize systemic exposure, it needs to stay within the area of the hair follicle. Variability in penetration means some individuals absorb more systemically than others, despite using the same concentration and volume.
Vehicle Formulation Dramatically Alters Absorption
The formulation of a topical finasteride product isn’t just a minor detail; it’s one of the biggest determinants of how much of the medication actually enters the bloodstream. Not all vehicles behave the same way, and the differences can be dramatic. For example, in vitro research comparing common topical solvents on human scalp skin shows that propylene glycol (PG) and isopropyl alcohol (IPA) formulations allow far more drug to pass through the skin: roughly 62–81 µg/cm² over 24 hours. In contrast, dimethyl isosorbide (DMI), often marketed as a “low-penetration” carrier, delivered just 17.8 µg/cm² in the same timeframe.[16]Farah, A.H., Brown, M.B., McAuley, W.J. (2020). Enhanced Follicular Delivery of Finasteride to Human Scalp Skin Using Heat and Chemical Penetration Enhancers. Pharmaceutical Research. 37(6). 112. … Continue reading That’s a 4.5-fold reduction in systemic permeation, purely from changing the solvent.
More sophisticated delivery systems behave differently still. Liposomal and niosomal gels, for example, are designed to deposit the medication deeper into hair follicles while limiting diffusion into the bloodstream. And in controlled experiments, these vesicular carriers did exactly that: liquid-state liposomes delivered 2.1–2.3% of the applied dose into the skin, compared to only 0.76% from a standard hydroalcoholic solution.[17]Tabbakhian, M., Tavakoli, N., Jaafari, M.R., Daneshamouz, S. (2006). Enhancement of follicular delivery of finasteride by liposomes and niosomes: 1. In vitro permeation and in vivo deposition studies … Continue reading
In other words, depending on the vehicle, the same dose of finasteride can behave like a local scalp medication or something much closer to an oral drug.
At the opposite end of the spectrum are ethosomes, ethanol-rich vesicles that dramatically increase drug penetration. In one study, finasteride ethosomes produced 7.4-times higher transdermal flux than the same drug in an aqueous solution, reaching all the way into the dermis.[18]Rao, Y., Zheng, F., Zhang, X., Gao, J., Liang, W. (2008). In vitro Percutaneous Permeation and Skin Accumulation of Finasteride Using Vesicular Ethosomal Carriers. 9(3). 860-865. Available at: … Continue reading
This is the kind of penetration behavior that significantly raises the likelihood of systemic absorption.
The bottom line: two topical finasteride products with the same concentration can have completely different side-effect profiles, simply because the vehicles modulate how much drug gets through the skin. Hydroalcoholic sprays tend to penetrate more aggressively; liposomal or niosomal gels are usually more conservative; and individual biology, skin barrier integrity, inflammation, and sebaceous output add yet another layer of variability. This is why real-world responses to topical finasteride are so mixed, and why formulation needs to be taken as seriously as the dose itself.
Concentration and Application Volume Compounds Absorption
Higher concentrations and larger volumes increase both local scalp DHT reduction and systemic spillover. As mentioned above, Caserini’s dose-response study found that applying 100-200 μL of 0.25% finasteride reduced serum DHT by 24-26%, whereas 300-400 μL volumes reduced it by 44-48%, approaching the efficacy of oral dosing at the higher end.[19]Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men … Continue reading
Users who apply larger volumes, cover a wider scalp area, or use higher-concentration formulations absorb more drug systemically, shifting the risk-benefit profile toward greater potential for side effects.
Finasteride’s Half-Life Enables Accumulation
Finasteride has a terminal half-life of 5-6 hours in young men and 8 hours in older men.[20]Mysore, V. (2012). Finasteride and sexual side effects. Indian Dermatology Online Journal. 3(1). 62-65. Available at: https://doi.org/10.4103/2229-5178.93496 Although short, daily topical application leads to steady-state accumulation in plasma and skin tissues.
With topical dosing, measurable finasteride concentrations appear in plasma within hours and remain detectable for a full day; repeated daily application amplifies cumulative levels, particularly if absorption is higher than intended due to vehicle choice or individual skin permeability.[21]Tai, Z., Cui, Z., Shi, X., Li, H., Chai, R., Huang, Y., Fang, Y., Jia, D., Zhu, Q., Chen, Z. (2025). The Pharmacokinetics of Topical Finasteride 0.25% Spray in Chinese Adult Male Volunteers with … Continue reading
DHT Sensitivity Varies Widely Between Individuals
Even when two people experience the same level of DHT reduction, their bodies may not respond the same way. This is where individual biology plays a much bigger role than most clinical studies acknowledge. Variations in genes tied to 5α-reductase activity, androgen receptor sensitivity (including CAG repeat length), and enzymes involved in neurosteroid production, such as 3α-HSD, can all influence how strongly someone reacts to changes in DHT.[22]Cecchin, E., De Mattia, E., Toffoli, G., Mazzon, G., Cauci, S., Trombetta, C. (2014). A Pharmacogenetic Survey of Androgen Receptor (CAG)N and (GGN)N Polymorphisms in Patients Experiencing … Continue reading,[23]Rahimi-Ardabili, B., Pourandarjani, R., Habibollahi, P., Mualeki, A. (2006). Finasteride-induced depression: a prospective study. BMC Clinical Pharmacology. 6(7). Available at: … Continue reading
For one man, a 30% drop in circulating DHT might feel completely benign. For another, that exact same reduction might coincide with noticeable changes in libido, erectile quality, or overall sexual function.
Layered on top of genetics are factors like baseline hormone levels, underlying hypogonadism, and even a person’s psychophysiological sensitivity to small hormonal shifts. Together, these variables help explain why side effects remain so individualized and difficult to predict, and why two people using an identical topical finasteride formulation can walk away with very different experiences. This isn’t meant to alarm anyone, only to highlight that DHT suppression is not a one-size-fits-all phenomenon, and that understanding your own sensitivity is often just as important as choosing the right dose or vehicle.
How Can I Minimize the Risk of Topical Finasteride Side Effects?
Target the Lowest Effective Exposure
- Understand exposure = concentration × volume. The systemic finasteride dose delivered through the scalp depends on both how strong the solution is and how much is applied. A low-concentration solution applied in large volume can expose the body to as much finasteride as a small volume of a stronger solution. Both variables must be considered together when aiming to reduce side effects.
- Start with the minimum viable dose. Evidence suggests that daily exposure to around 0.1 mg (for example, 0.005% solution applied at 2 mL per day) can still achieve measurable scalp DHT reduction with negligible systemic suppression.
- 0.25% remains the most studied concentration. Clinical trials show that 0.25% topical finasteride effectively treats androgenetic alopecia with hair density gains similar to oral finasteride yet significantly lower serum DHT reduction. For most users, starting at or below this level helps balance efficacy and safety.
- Higher isn’t always better. Increasing concentration or volume each raises systemic absorption, but hair-growth benefits tend to plateau. Pushing beyond the optimal concentration can shift the risk-to-benefit ratio unfavorably.
- Consider titrating upward. If highly sensitive to hormonal side effects, begin at lower exposure (e.g., 0.025–0.1% at 1 mL daily), then increase concentration or frequency only if well-tolerated and results plateau. Serum DHT measurements can help assess systemic exposure over time.
Adjust Volume and Frequency to Limit Systemic Load
- Keep volume conservative. Systemic absorption scales with total dose applied. Around 1 mL per application is a common clinical standard; using more typically provides diminishing returns for hair outcomes but notably raises systemic uptake.
- Use the lowest frequency that maintains results. Because twice-daily 0.25% use can suppress serum DHT by up to 70%—similar to oral dosing—while once-daily reduces it by only 20–35%, finding a balance between efficacy and exposure is key. For many users, once-daily application of a lower-strength solution achieves a good safety–efficacy trade-off.
Choose Vehicles Carefully
- Hydroalcoholic vs. liposomal: Standard hydroalcoholic (alcohol-based) solutions enhance drug penetration, which can lead to greater systemic absorption. In contrast, advanced vehicles like liposomal or other nanoparticle-based formulations are designed to target drug delivery to the hair follicle and reduce transdermal flux into the bloodstream.
Implement Smart Application Strategies
- Apply to a dry scalp: Applying topicals to wet skin can enhance absorption. Ensure the scalp is completely dry to create a more robust barrier.
- Target affected areas only: Use the applicator to apply the solution precisely to the areas of thinning hair, not the entire scalp, to limit the total surface area of absorption.
- Avoid compromised barriers: Do not apply the solution immediately after microneedling, on sunburnt skin, or on a scalp with cuts, inflammation, or dermatitis, as a compromised skin barrier significantly increases uptake.
- Wash hands thoroughly: Always wash hands with soap and water after application to prevent accidental transfer to other body parts or individuals.
Important: If you experience any side effects, we recommend first stopping and speaking to your primary care physician.
Who Should Avoid or Be Cautious with Topical Finasteride?
While topical finasteride carries a lower risk profile than its oral counterpart, some people may still need to exercise caution or avoid the drug entirely.
Groups That Should Exercise Caution
- Those with a prior history of persistent sexual dysfunction from oral finasteride.
- People with underlying endocrine disorders or baseline sexual dysfunction.
- Those sensitive to propylene glycol or alcohol-based carrier agents.
It should be mentioned that a history of side effects from oral finasteride does not mean that topical finasteride won’t work well for you; however, it does warrant a personalized, cautious approach. This approach might include starting with lower doses, extended monitoring, and communication with the prescribing physician about any potential symptoms.
Groups That Should Avoid Topical Finasteride
- Women who are pregnant or attempting to conceive.
- People with severe scalp dermatitis or barrier dysfunction.
- Individuals with known allergy/hypersensitivity to finasteride or formulation components.
If I Can’t Tolerate Topical Finasteride, What Should I Do?
For users unable to tolerate topical finasteride due to side effects or scalp irritation, several evidence-based alternatives exist.
Pharmacological Alternatives
Low Dose Oral Minoxidil
Oral minoxidil is a hair growth stimulant that bypasses the hormonal pathway entirely. Studies show high efficacy (e.g., 43% of men achieving excellent results with 5 mg), but it carries systemic risks such as hypertrichosis (excess body hair), fluid retention, and cardiovascular effects like tachycardia.[24]Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., … Continue reading
Alternative Topical Anti-Androgens
Fluridil is a topical anti-androgen designed to degrade in water (i.e., blood), minimizing systemic exposure. Clinical data are limited but suggest efficacy without affecting serum testosterone or sexual function.[25]Sovak, M., Seligson, A.L., Kucerova, R., Bienova, M., Hajduch, M., Bucek, M. (2002). Fluridil, a rationally designed topical agent for androgenetic alopecia: first clinical experience. Dermatologic … Continue reading
Clascoterone (17ɑ-proprionate) is an androgen receptor antagonist that competes with DHT at the follicle rather than reducing DHT production. It offers a different mechanism but is still under investigation for hair loss.[26]Devjani, S., Ezemma, O., Kelley, K.J., Stratton, S., Senna, M. (2023). Androgenetic Alopecia: Therapy Update. Drugs. 83(8). 701-715. Available at: PMID: 37166619
Pyrilutamide is a non-steroidal anti-androgen that binds to the androgen receptor with high affinity. Like fluridil, it is designed to metabolize into an inactive form upon entering systemic circulation, theoretically offering a safety advantage over finasteride.[27]Biospace. (2023). Kintor Pharma’s KX-826 and GT20029 for Treatment of Androgenetic Alopecia (AGA) and Acne Presented at AAD 2023. Biospace. Available at: … Continue reading
Non-Pharmaceutical Options
Microneedling creates micro-injuries in the scalp and stimulates growth factors (PDGF, VEGF) and activates Wnt/ꞵ-catening signaling, promoting hair regeneration even without concurrent drug use.[28]Dhurat, R., Sukesh, M.S., Ayhad, G., Dandale, A., Pal, A., Pund, P. (2013). A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study. International … Continue reading
Low-level laser therapy (LLLT) devices use red light (650-655 nm) to stimulate mitochondrial activity in hair follicles, prolonging anagen and improving density with an excellent safety profile.[29]Egger, A., Resnik, S.R., Aickara, D., Maranda, E., Kaiser, M., Wikramanayake, T.C. (2020). Examining the Safety and Efficacy of Low-Level Laser Therapy for Male and Female Pattern Hair Loss: A … Continue reading
Some botanical supplements have some 5ɑ-reductase inhibitory properties. Saw palmetto and pumpkin seed oil have been shown to have mild 5ɑ-reductase inhibition.[30]Cho, Y.H., Lee, S.Y., Jeong, D.W., Choi, E.J., Kim, Y.J., Lee, J.G., Yi, H.Y., Cha, H.S. (2014). Effect of Pumpkin Seed Oil on Hair Growth in Men with Androgenetic Alopecia: A Randomized, … Continue reading,[31]Pilar, P., 2010. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Advances in Therapy. 27(8). 555-563. Available at: … Continue reading While less potent than finasteride (reducing DHT by ~30-40% vs. >60%), they may provide a viable “middle ground” for those seeking modest stabilization.
Final Thoughts
Topical finasteride offers a compelling middle path for those seeking the hair-growth benefits of 5-α-reductase inhibition with a lower likelihood of systemic side effects. But “lower” does not mean “none,” and responses vary widely depending on formulation, dose, skin permeability, and individual DHT sensitivity. The current research, while promising, remains short-term, and real-world patterns remind us that systemic exposure is still possible.
For many people, topical finasteride can be a safe, effective component of a broader hair-loss program. The key is approaching it strategically: start low, personalize dosing, choose the right vehicle, and monitor closely for changes. For others, especially those prone to hormonal side effects, alternatives ranging from anti-androgen topicals to non-pharmaceutical options may offer a better balance of efficacy and tolerability.
As with any AGA therapy, the best results come from matching the treatment to the individual, not the other way around.
References[+]
References ↑1 Zito PM, Bistas KG, Patel P, et al. Finasteride. [Updated 2024 Feb 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513329/ (Accessed: December 2025) ↑2 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. International Journal of Clinical Pharmacology and Therapeutics. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑3 Smith, A.B., Carson, C.C. (2009). Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Therapeutics and Clinical Risk Management. 5. 535-545. Available at: https://doi.org/10.2147/tcrm.s6195 ↑4 Traish, A.M. (2017). Negative Impact of Testosterone Deficiency and 5ɑ-Reductase Inhibitors Therapy on Metabolic and Sexual Function in Men. Advances in experimental medicine and biology. 1043. 473-526. Available at: https://doi.org/10.1007/978-3-319-70178-3_22 ↑5 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. International Journal of Clinical Pharmacology and Therapeutics. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑6 Piraccini, B.M., Blume-Peytavi, U., Scarci, F., Jansat, J.M., Falques, M., Otero, R., Tamarit, M.L., Galvan, J., Tebbs, V., Massana, E., Topical Finasteride Study Group. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑7, ↑19 Caserini, M., Radicioni, M., Leuratti, C., Terragni, E., Iorizzo, M., Palmieri, R. (2016). Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. International Journal of Clinical Pharmacology and Therapeutics. 54(1). 19-27. Available at: https://doi.org/10.5414/CP202467 ↑8 Monti. D., Tampucci, S., Burgalassi, S., Chetoni, P., Lenzi, C., Pirone, A., Mailland, F. (2014). Topical formulations containing finasteride. Part I: in vitro permeation/penetration study and in vivo pharmacokinetics in hairless rat. Journal of Pharmaceutical Sciences. 103(8). 2307-2314. Available at: https://doi.org/10.1002/jps.24028 ↑9 US Food and Drug Administration. (2025). FDA alerts health care providers, compounders and consumers of potential risks associated with compounded topical finasteride products. FDA. Available at: https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-providers-compounders-and-consumers-potential-risks-associated-compounded (Accessed: December 2025) ↑10 Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated With Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study With Data From the United States Food and Drug Administration Adverse Event Reporting System. International Journal of Dermatology. Available at: https://doi.org/10.1111/ijd.17957 ↑11 Gupta, A.K., Talukder, M., Keene, S.A., Bamimore, M.A. (2025). Is the Safety of Finasteride Correlated With Its Route of Administration: Topical Versus Oral? A Pharmacovigilance Study With Data From the United States Food and Drug Administration Adverse Event Reporting System. International Journal of Dermatology. Available at: https://doi.org/10.1111/ijd.17957 ↑12 Lee, S.W., Juhasz, M., Mobasher, P., Ekelem, C., Mesinkovska, N.A. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of Drugs in Dermatology. 17(4). 457-463. Available at: PMID: 29601622 ↑13, ↑14 Pereira, A.F.J.R., Coehlo, T, O. de A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001 ↑15, ↑16 Farah, A.H., Brown, M.B., McAuley, W.J. (2020). Enhanced Follicular Delivery of Finasteride to Human Scalp Skin Using Heat and Chemical Penetration Enhancers. Pharmaceutical Research. 37(6). 112. Available at: https://doi.org/10.1007/s11095-020-02822-y ↑17 Tabbakhian, M., Tavakoli, N., Jaafari, M.R., Daneshamouz, S. (2006). Enhancement of follicular delivery of finasteride by liposomes and niosomes: 1. In vitro permeation and in vivo deposition studies using hamster flank and ear models. International Journal of Pharmaceutics. 1-2(323). 1-10. Available at: https://doi.org/10.1016/j.ijpharm.2006.05.041 ↑18 Rao, Y., Zheng, F., Zhang, X., Gao, J., Liang, W. (2008). In vitro Percutaneous Permeation and Skin Accumulation of Finasteride Using Vesicular Ethosomal Carriers. 9(3). 860-865. Available at: https://doi.org/10.1208/s12249-008-9124-y ↑20 Mysore, V. (2012). Finasteride and sexual side effects. Indian Dermatology Online Journal. 3(1). 62-65. Available at: https://doi.org/10.4103/2229-5178.93496 ↑21 Tai, Z., Cui, Z., Shi, X., Li, H., Chai, R., Huang, Y., Fang, Y., Jia, D., Zhu, Q., Chen, Z. (2025). The Pharmacokinetics of Topical Finasteride 0.25% Spray in Chinese Adult Male Volunteers with Androgenic Alopecia: A Phase I Study. Advances in Therapy. 42(3). 1494-1505. Available at: https://doi.org/10.1007/s12325-025-03106-w. ↑22 Cecchin, E., De Mattia, E., Toffoli, G., Mazzon, G., Cauci, S., Trombetta, C. (2014). A Pharmacogenetic Survey of Androgen Receptor (CAG)N and (GGN)N Polymorphisms in Patients Experiencing Long-Term Side Effects after Finasteride Discontinuation. The International Journal of Biological Markers. 29(4). 310-316. Available at: https://doi.org/10.5301/jbm.500095 ↑23 Rahimi-Ardabili, B., Pourandarjani, R., Habibollahi, P., Mualeki, A. (2006). Finasteride-induced depression: a prospective study. BMC Clinical Pharmacology. 6(7). Available at: https://doi.org/10.1186/1472-6904-6-7 ↑24 Vano-Galvan, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, O.M., Saceda-Corralo, D., Rodrigues-Barata, R., Jiminez-Cauhe, J., Koh, W.L., Poa, J.E., Jerjen, R., de Carvalho, L.T., John, J.M., Salas-Callo, C.I., Vincenzi, C., Yin, L., Lo-Sicco, K., Waskiel-Burnat, A., Starace, M., Zamorano, J.L., Jaen-Olasolo, P., Piraccini, B.M., Rudnicka, L., Shapiro, J., Tosti, A., Sinclair, R., Bhoyrul, B. (2021). Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. Journal of the American Academy of Dermatology. 84(6). 1644-1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑25 Sovak, M., Seligson, A.L., Kucerova, R., Bienova, M., Hajduch, M., Bucek, M. (2002). Fluridil, a rationally designed topical agent for androgenetic alopecia: first clinical experience. Dermatologic Surgery. 28(8). 678-685. Available at: https://doi.org/10.1046/j.1524-4725.2002.02017.x ↑26 Devjani, S., Ezemma, O., Kelley, K.J., Stratton, S., Senna, M. (2023). Androgenetic Alopecia: Therapy Update. Drugs. 83(8). 701-715. Available at: PMID: 37166619 ↑27 Biospace. (2023). Kintor Pharma’s KX-826 and GT20029 for Treatment of Androgenetic Alopecia (AGA) and Acne Presented at AAD 2023. Biospace. Available at: https://www.biospace.com/kintor-pharma-s-kx-826-and-gt20029-for-treatment-of-androgenetic-alopecia-aga-and-acne-presented-at-aad-2023 (Accessed: December 2025) ↑28 Dhurat, R., Sukesh, M.S., Ayhad, G., Dandale, A., Pal, A., Pund, P. (2013). A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study. International Journal of Trichology. 5(1). 6-11. Available at: https://doi.org/10.4103/0974-7753.114700 ↑29 Egger, A., Resnik, S.R., Aickara, D., Maranda, E., Kaiser, M., Wikramanayake, T.C. (2020). Examining the Safety and Efficacy of Low-Level Laser Therapy for Male and Female Pattern Hair Loss: A Review of the Literature. Skin Appendage Disorders. 6(5). 259-267. Available at: https://doi.org/10.1159/000509001 ↑30 Cho, Y.H., Lee, S.Y., Jeong, D.W., Choi, E.J., Kim, Y.J., Lee, J.G., Yi, H.Y., Cha, H.S. (2014). Effect of Pumpkin Seed Oil on Hair Growth in Men with Androgenetic Alopecia: A Randomized, Double-Blind, Placebo-Controlled Trial. Evidence-Based Complementary and Alternative Medicine. 23. 549721. Available at: https://doi.org/10.1155/2014/549721 ↑31 Pilar, P., 2010. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Advances in Therapy. 27(8). 555-563. Available at: https://doi.org/10.1007/s12325-010-0041-6 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sarah King, PhD
Dr. Sarah King is a researcher & writer who holds a BSc in Medical Biology, an MSc in Forensic Biology, and a Ph.D. in Molecular and Cellular Biology. While at university, Dr. King’s research focused on cellular aging and senescence through NAD-dependent signaling – along with research into prostaglandins and their role in hair loss. She is a co-author on several upcoming manuscripts with the Perfect Hair Health team.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down