- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesTopical Cetirizine: An Anti-Histamine That Regrows Hair? (New Evidence)
First Published Apr 29 2025Last Updated Sep 19 2025PharmaceuticalResearched & Written By:Ben Fletcher, PhDReviewed By:Rob English, Medical EditorWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Curious if an allergy medication could help with hair loss? Cetirizine, a second-generation antihistamine, is best known for treating allergies, but emerging research suggests it might also promote hair growth when applied topically. From reducing inflammation and mast cell activity to lowering levels of PGD2, cetirizine could offer new hope for those with androgenetic alopecia. But how strong is the evidence, and is it safe long-term? Here’s what we know so far.
Full Article
Cetirizine is a second-generation antihistamine that is approved by the FDA for the treatment of allergies. It works by blocking the H1 receptor and preventing the function of histamine, the biological compound that is responsible for causing allergic symptoms.[1]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008
In this article, we will explore the way in which cetirizine has been proven to work and look at some additional functions that may influence hair health. We will also look to see if there are any clinical studies that show cetirizine can promote hair growth, as well as any safety data that indicates whether it is safe to use.
Key Takeaways
- What is it? Cetirizine is a second-generation antihistamine that is approved for use as an oral medication to treat allergies. It primarily works by binding the H1 receptor, preventing histamine from binding and causing allergic symptoms. Studies have also shown that cetirizine has anti-inflammatory effects, inhibiting the migration of inflammatory mediators. Moreover, it has been shown to reduce PGD2 production, a molecule with strong links to hair loss.
- Clinical Data. In clinical studies, the topical application of cetirizine has been shown to increase hair density, hair thickness, hair regrowth, and the proportion of hairs in the anagen (growing) phase. This indicates that topical cetirizine may promote hair growth, but there are also several limitations that are discussed in detail below.
- Safety. Oral cetirizine is FDA-approved and has been shown to cause limited side effects, none of which are considered serious. No conclusive safety data for topical cetirizine exists, but it is likely to be safe based on the safety profile of oral cetirizine. That said, people who are breastfeeding or have kidney failure may not be able to use cetirizine.
- Evidence Quality. Cetirizine scored 57/100 for evidence quality by our metrics.
- Best Practices. Cetirizine is approved for use at the following dosages: 10mg within a 24-hour period in adults, 5mg twice daily in children aged 6-11 years, 2.5mg twice daily in children aged 2-5 years. No recommended dosages exist for the use of topical cetirizine, but 1% cetirizine formulations applied at 1-2 mL daily have shown benefit in several clinical studies.
Interested in Topical Cetirizine?
Support your hair with full-strength topical cetirizine, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What is Cetirizine?
Cetirizine is a second-generation antihistamine that is widely used for its anti-allergic properties, which makes it particularly effective for treating the symptoms of seasonal allergic rhinitis (otherwise known as hayfever) and chronic idiopathic urticaria (hives).[2]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008 Such is the importance of cetirizine that it is included in the World Health Organizations List of Essential Medicines.[3]World Health Organization. (2023). WHO Model List of Essential Medicines – 23rd list, 2023. WHO/MHP/HPS/EML/2023.02. Available at: … Continue reading
Origin and Structure
Cetirizine, a second-generation antihistamine, is derived from the metabolism of hydroxyzine, a first-generation antihistamine. A common feature of second-generation antihistamines, including cetirizine, is limited crossing of the blood-brain barrier and the rapid efflux of any molecules that do enter the brain.[4]Pagliara, A., Testa, B., Carrupt, P.A., Jolliet, P., Morin, C., Morin, D., Urien, S., Tillement, J.P. and Rihoux, J.P. (1998). Molecular properties and pharmacokinetic behavior of cetirizine, a … Continue reading This reduced uptake limits the effects on the central nervous system, thus lowering the potential for side effects such as drowsiness which were common in the first-generation.[5]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008
How Does Cetirizine Work?
When used for treating the symptoms of allergies, cetirizine is delivered orally as a liquid or tablet. Cetirizine has been used to treat allergies since the 1980s and its mechanism of action, as well as its therapeutic effects, are very well documented. We’ll start by summarizing these effects, before taking a closer look at more recent research that has explored the potential benefits of topical cetirizine in the treatment AGA.
H1-Receptor Antagonism
Cetirizine primarily functions as a potent and selective antagonist of the histamine H1 receptors. Hypersensitive reactions, which manifest as allergies, are initiated when allergens trigger the release of histamine from cells of the immune system. The released histamine binds to H1 receptors, which is the mechanism that is responsible for producing the classic symptoms of allergies.[6]Ghosh, S., Debnath, I., Bhunia, S., Hazra, S., Nandi, S., & Mandal, S. K. (2025). A Review on Mechanism of Histamine Mediated Allergic Reactions: Therapeutic Role, Safety, and Clinical Efficacy … Continue reading Cetirizine blocks this mechanism by binding to the H1 receptors and blocking the histamine, preventing it from activating the allergic response.[7]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008
Anti-inflammatory Properties
Beyond its role as an antihistamine, cetirizine exhibits significant anti-inflammatory effects that contribute to its therapeutic value. This is particularly beneficial because allergic reactions are characterized by inflammation and the infiltration of immune cells into the different tissues of the body.[8]Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008
In an animal study, rats were orally administered cetirizine (900µg/kg; equivalent to the standard human dose of 10mg/kg) or a control substance. Acute inflammation was then induced by injecting the pro-inflammatory substance carrageenin into the paw. After 3 hours, the volume of edema (swelling) in the paw was quantified and compared between the treated and untreated rats. This revealed that there was significantly less swelling (-41.93%) in the cetirizine-treated rats compared to the untreated rats.[9]Vardhamane, S. H., Santoshkumar, J., & Vardhamane, S. H. (2013). Anti-inflammatory activity of H1 receptor antagonist Cetirizine in animal models. Journal of Evolution of Medical and Dental … Continue reading
The same authors also tested cetirizine in rats with chronic inflammation. Once again, rats were provided with orally administered cetirizine (900µg/kg; equivalent to the standard human dose of 10mg/kg) or a control substance. Inflammation was induced by inserting rexin pellets underneath skin and the rats monitored for 7 days, receiving a daily dose of cetirizine. After 7 days, the granuloma (area of tissue indicative of inflammation) was excised from the rats and weighed. They showed that treatment with cetirizine reduced the granuloma weight (-50.05%), indicating that cetirizine inhibited inflammation.[10]Vardhamane, S. H., Santoshkumar, J., & Vardhamane, S. H. (2013). Anti-inflammatory activity of H1 receptor antagonist Cetirizine in animal models. Journal of Evolution of Medical and Dental … Continue reading
There are also several clinical trials that have shown its anti-inflammatory properties. In one such study, a total of 12 patients faced an allergen-specific conjunctival challenge, where a known allergen was dropped into the eye. These patients were randomly assigned into one of two groups – 10 mg cetirizine or placebo, both twice daily. Although the number of inflammatory cells increased following the allergen challenge, their levels were still significantly lower in the cetirizine group than the untreated controls.[11]Ciprandi, G., Buscaglia, S., Pesce, G., Passalacqua, G., Rihoux, J. P., Bagnasco, M., & Canonica, G. W. (1995). Cetirizine reduces inflammatory cell recruitment and ICAM-1 (or CD54) expression on … Continue reading
A double-blinded, placebo-controlled study was also conducted in 14 patients with atopic dermatitis (eczema) and 16 healthy subjects. Participants either received cetirizine (10mg in the morning, 20mg in the evening) or the placebo, and they consumed their respective product every day for 3 days. They took blood samples after Day 2 and then tested the ability of monocytes, key cells of the immune system, to migrate towards a chemical stimulus. The migration of these cells forms a key part of their ability to induce inflammation, so a reduction in the ability to migrate would suggest a reduced inflammatory response. Their results showed that treatment with cetirizine reduced or even abolished ex vivo monocyte migration in both the healthy subjects and the patients with atopic dermatitis.[12]Jinquan, T., Reimert, C. M., Deleuran, B., Zachariae, C., Simonsen, C., Thestrup-Pedersen, K., & May, R. (1995). Cetirizine inhibits the in vitro and ex vivo chemotactic response of T lymphocytes … Continue reading
Therefore, the literature strongly suggests that cetirizine exhibits anti-inflammatory properties.
Mast Cell Stabilization
Studies also suggest that some of the effects of cetirizine may be mediated by its regulation of mast cells. These white blood cells are found throughout the body and they form part of the immune response, particularly the allergic response. This is because they contain inflammatory mediators (including histamine). When mast cells are stimulated by an allergen, they degranulate and release their inflammatory compounds, driving inflammation and the classic signs of allergy.[13]Amin, K. (2012). The role of mast cells in allergic inflammation. Respiratory medicine. 106(1). 9-14. Available at: https://doi.org/10.1016/j.rmed.2011.09.007
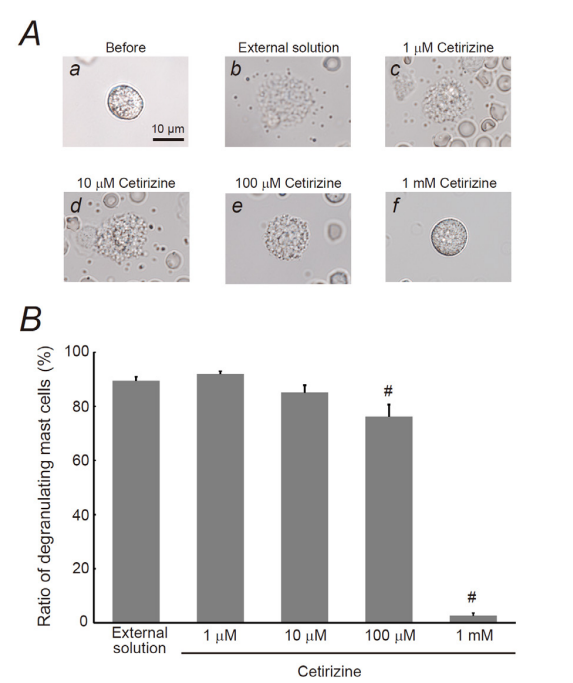
The effect of cetirizine on mast cells has been demonstrated in vitro. Mast cells were isolated from rats and then treated with increasing concentrations of cetirizine, before exocytosis (degranulation) was induced using a chemical compound. The authors observed a significant decrease in degranulation at a cetirizine concentration of 100µM, while the highest concentration of 1mM induced near-total suppression of degranulation (Figure 1). Furthermore, cetirizine was also shown to significantly outperform a first-generation antihistamine (diphenhydramine) when comparing the relative reduction of degranulation.[14]Fujimura, R., Asada, A., Aizawa, M., & Kazama, I. (2022). Cetirizine more potently exerts mast cell-stabilizing property than diphenhydramine. Drug Discoveries & Therapeutics. 16(5). 245-250. … Continue reading Thus, this study provides evidence that cetirizine may exert its anti-allergic and anti-inflammatory effects via the mediation of mast cell activity.
Figure 1: Cetirizine reduces rat mast cell degranulation in vitro. (A) Microscopic images showing the effects of cetirizine on the mast cells, with visibly reduced degranulation. (B) Cetirizine at 100µM significantly reduces degranulation, while 1mM of cetirizine causes almost total suppression.[15]Fujimura, R., Asada, A., Aizawa, M., & Kazama, I. (2022). Cetirizine more potently exerts mast cell-stabilizing property than diphenhydramine. Drug Discoveries & Therapeutics. 16(5). 245-250. … Continue reading
Histamine and Hair Loss: Why It Matters
What is Histamine?
Histamine is a biologically active compound that is derived from the amino acid histidine. As previously noted, histamine is stored in the mast cells that are found throughout the body, but it can also be found in basophils (another type of white blood cell). Histamine may also be found in the neurons of the brain, but the white blood cells are its primary store.[16]Carthy, E., & Ellender, T. (2021). Histamine, neuroinflammation and neurodevelopment: a review. Frontiers in neuroscience. 15. 680214. Available at: https://doi.org/10.3389/fnins.2021.680214
Histamine primarily functions by binding to the H1, H2, H3, and H4 receptors that are found in the brain, blood vessels, heart, neurons, and many other tissues throughout the body. Depending on the receptor it binds to, histamine can exert effects that include initiating inflammation, changing the blood flow, or making the blood vessels more permeable which facilitates the leakage of fluid into surrounding tissues (causing swelling).[17]Branco, A. C. C. C., Yoshikawa, F. S. Y., Pietrobon, A. J., & Sato, M. N. (2018). Role of histamine in modulating the immune response and inflammation. Mediators of inflammation. 2018(1). … Continue reading
This wide range of functions means that histamine can exert influence over many bodily processes, so it is not surprising that histamine has been linked to hair loss.
Histamine’s Role in Hair Biology
Although the evidence is rather limited, there are a number of studies that have suggested histamine could be contributing to hair loss.
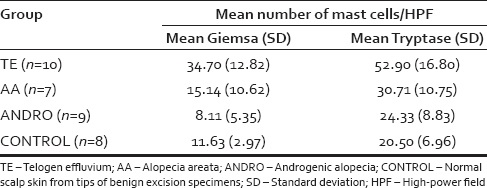
Scalp biopsies from patients with telogen effluvium (TE; 10 patients), alopecia areata (AA; 7 patients), androgenetic alopecia (AGA or ANDRO; 9 patients) were compared to those of healthy volunteers (8 patients). They stained each of these biopsies to count the number of mast cells and determine if there was a difference between conditions. Sure enough, they found that the TE biopsies contained a significantly greater number of mast cell granules (indicated by Giemsa staining) and a greater abundance of the enzyme tryptase (Figure 2).[18]Grace, S. A., Sutton, A. M., Abraham, N., Armbrecht, E. S., & Vidal, C. I. (2017). Presence of mast cells and mast cell degranulation in scalp biopsies of telogen effluvium. International journal … Continue reading
These results are indicative of an increased number of mast cells and greater mast cell activation in the scalp of people with TE. It has been suggested by this and a number of other studies that mast cells play an important role in hair loss, involved in a system that is triggered by stress and characterized by a switch to a pro-inflammatory phenotype.[19]Arck, P. C., Handjiski, B., Hagen, E., Joachim, R., Klapp, B. F., & Paus, R. (2001). Indications for a brain‐hair follicle axis: inhibition of keratinocyte proliferation and up‐regulation of … Continue reading
Figure 2: Table showing the mast cell count (indicated by the Giemsa stain) and tryptase abundance in the scalp biopsies of patients with telogen effluvium, alopecia areata, androgenetic alopecia, and healthy controls.[20]Grace, S. A., Sutton, A. M., Abraham, N., Armbrecht, E. S., & Vidal, C. I. (2017). Presence of mast cells and mast cell degranulation in scalp biopsies of telogen effluvium. International journal … Continue reading
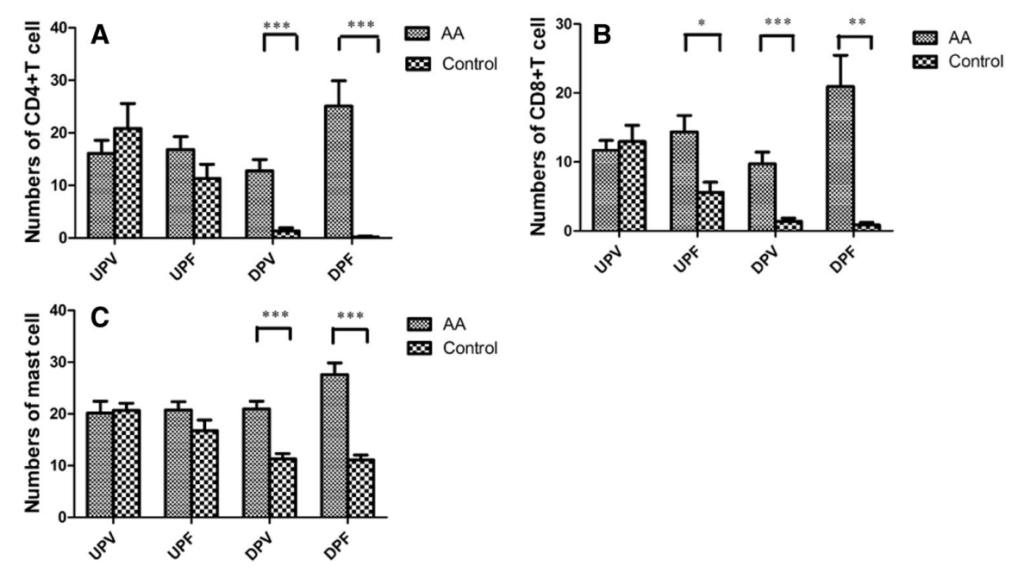
A separate study investigated scalp biopsies from 55 patients with AA and quantified the levels of immune cells and inflammatory mediators, comparing the results to healthy controls. They showed increased CD4+ T cells, CD8+ T cells, and mast cells in the deep perifollicular and deep perivascular areas of the scalp (Figure 3). They also observed increased expression of nerve growth factor, which is potentially indicative of inflammation within the scalp.[21]Zhang, X., Zhao, Y., Ye, Y., Li, S., Qi, S., Yang, Y., Cao, H., Yang, J. and Zhang, X. (2015). Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia … Continue reading
This provides further evidence that suggests mast cells, and therefore histamine, may play a role in hair loss. Indeed, histamine release from mast cells can trigger the release of T cells, so it is possible that the increase in T cells was driven by increased histamine.[22]Jutel, M., Watanabe, T., Klunker, S., Akdis, M., Thomet, O.A., Malolepszy, J., Zak-Nejmark, T., Koga, R., Kobayashi, T., Blaser, K. and Akdis, C.A. (2001). Histamine regulates T-cell and antibody … Continue reading
Figure 3: The abundance of CD4+ T cells, CD8+ T cells, and mast cells in the scalp of patients with AA. Control samples were compared to AA. *P < 0.05, **P < 0.01, ***P < 0.001.[23]Zhang, X., Zhao, Y., Ye, Y., Li, S., Qi, S., Yang, Y., Cao, H., Yang, J. and Zhang, X. (2015). Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia … Continue reading
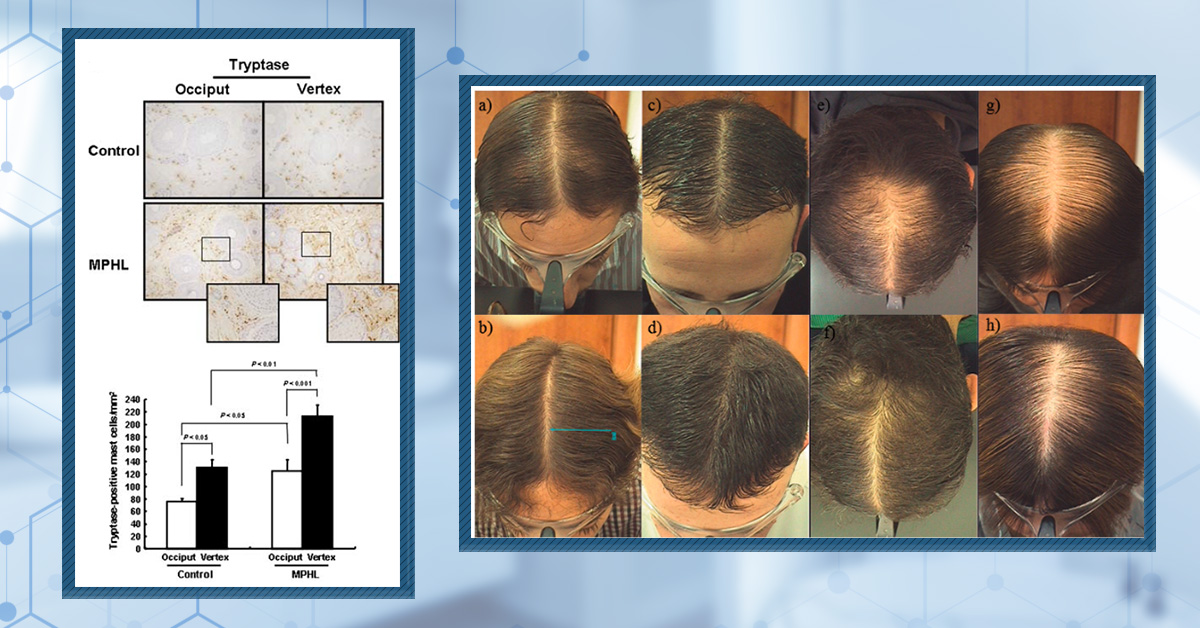
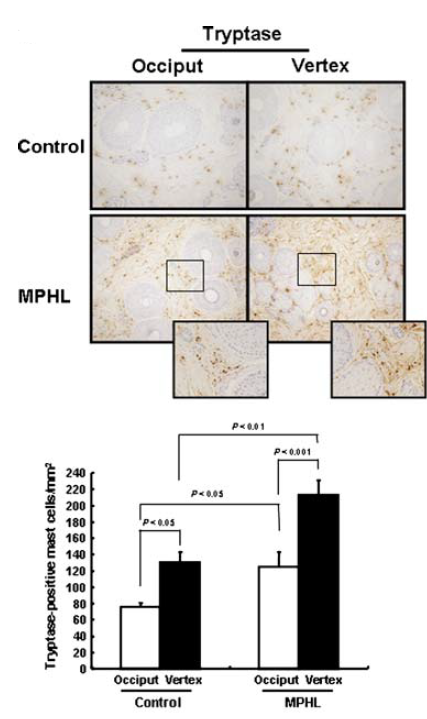
There is also evidence that mast cells may contribute to the extracellular matrix remodelling that is seen in patients with AGA. Biopsies were taken from the balding (vertex) and non-balding (occipital) areas of 10 males with AGA, as well as biopsies from the same regions of 5 healthy control subjects. Using tryptase quantification, they showed that active mast cell counts were almost 2-fold greater in the scalps of AGA patients relative to the healthy controls (Figure 4). Furthermore, they showed an increase in the number of collagen bundles and elastic fibres within the balding regions of the scalp, and a positive correlation between the number of active mast cells and the number of elastic fibres.[24]Won, C.H., Kwon, O.S., Kim, Y.K., Kang, Y.J., Kim, B.J., Choi, C.W., Eun, H.C. and Cho, K.H. (2008). Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Archives of … Continue reading
It should be noted that the small study size is a limiting factor. However, the results suggest that mast cells may contribute to the remodelling that is observed in AGA. This is supported by studies that have linked tryptase to fibroblast proliferation, collagen production, and elastin production.
Figure 4: Tryptase-positive mast cells in the vertex and occipital regions of the scalp. (A) Microscopic images of the scalp, including the tryptase-positive mast cells. (B) Quantification of the tryptase-positive mast cells in the vertex and occipital regions.[25]Won, C.H., Kwon, O.S., Kim, Y.K., Kang, Y.J., Kim, B.J., Choi, C.W., Eun, H.C. and Cho, K.H. (2008). Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Archives of … Continue reading
It is possible that this recruitment and activation of mast cells (and, potentially, histamine) is caused by changes to the skin microbiome. A study was conducted on hairless mice to compare mast cell activation against control mice with hair. They found that the number of activated mast cells was significantly greater in the hairless mice. Additionally, the patchy sections of hair had fewer mast cells than the hairless sections, but more than the control mice (with a full coat of hair). This difference was associated with a greater amount of gram-positive bacteria on the skin of the hairless mice. Interestingly, when the TLR2 receptor (that recognizes the bacteria) was deleted in hairless mice, the number of mast cells reduced.[26]Wu, C. C., Kim, J. N., Wang, Z., Chang, Y. L., Zengler, K., & Di Nardo, A. (2019). Mast cell recruitment is modulated by the hairless skin microbiome. Journal of Allergy and Clinical Immunology. … Continue reading
Collectively, these studies suggest that mast cell activation may contribute to hair loss. As an effector of mast cell activity, this might suggest that histamine is also contributing to hair loss. Therefore, this raises the question of whether antihistamines could be used to prevent hair loss.
How Does Cetirizine Have an Impact on Hair Follicles?
To answer the following question, we checked the literature for studies that have investigated the use of antihistamines in hair loss treatment. Despite the limited research, cetirizine has been tested in a number of different studies and generated some interesting results. Let’s start by looking at the mechanistic effects of cetirizine that suggest it could influence hair health.
A double-blind, placebo-controlled crossover study was conducted in 10 people with ragweed allergies. The patients were given oral cetirizine (20mg) or placebo once a day, for two days, before their skin was exposed to the allergen. The exposed portion of the skin was then biopsied for testing. Surprisingly, although erythema (reddening) was reduced, cetirizine did not inhibit the release of histamine. However, the authors found that cetirizine treatment did significantly reduce the production of prostaglandin D2 (PGD2), and there was also a significant reduction in the migration of inflammatory cells white blood cells (eosinophils and neutrophils) to the exposed site.[27]Charlesworth, E. N., Kagey-Sobotka, A., Norman, P. S., & Lichtenstein, L. M. (1989). Effect of cetirizine on mast cell-mediator release and cellular traffic during the cutaneous late-phase … Continue reading
These results are interesting when it comes to hair health. PGD2 has a previously documented role in promoting hair loss, particularly in those with AGA. PGD2 is thought to block key receptors within hair follicles and prevent hairs from entering the anagen (growth phase). This consequently prevents hair growth and contributes to hair follicle miniaturization. Furthermore, suppression of PGD2 has been shown to have beneficial effects, suggesting that it may be a therapeutic target.[28]Shin, D. W. (2022). The physiological and pharmacological roles of prostaglandins in hair growth. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological … Continue reading
However, it should be noted that recent studies using PGD2 inhibitors are yet to show improvement in hair loss outcomes. You can read more about this in our GPR44 article.
Studies have also shown that inflammation plays a role in driving hair loss. Numerous studies have shown a significant inflammatory response in the scalp of people suffering with hair loss, driven by infiltration of inflammatory mediators (including mast cells) into the hair follicles. This then disrupts the hair cycle and impairs hair growth.[29]Peyravian, N., Deo, S., Daunert, S., & Jimenez, J. J. (2020). The inflammatory aspect of male and female pattern hair loss. Journal of inflammation research. 879-881. Available at: … Continue reading
Therefore, it is plausible that cetirizine could be beneficial for hair loss.
Clinical Evidence
Thankfully, a number of clinical studies have been conducted to test the efficacy of cetirizine as a hair loss treatment.
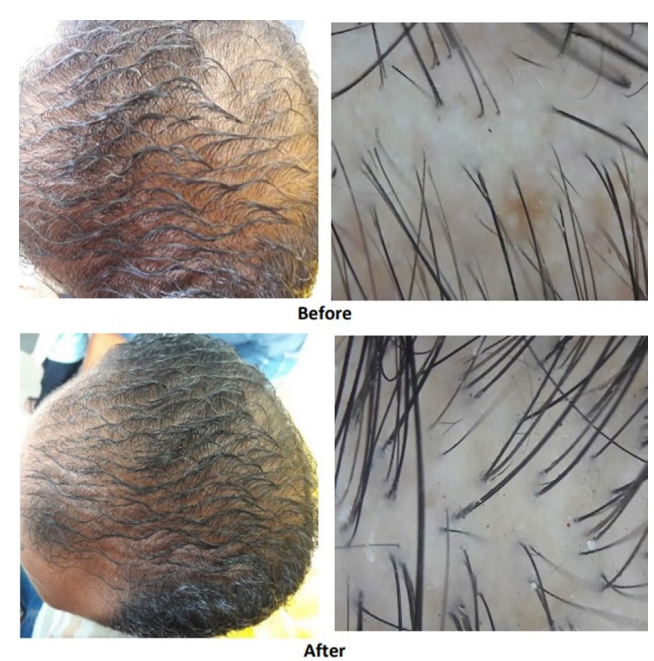
One study was conducted on 60 males with AGA between the ages of 22 and 55. The study was double-blinded and the participants were randomized into the placebo or cetirizine group. Those in the cetirizine group were required to apply 1mL of a topical lotion (1% cetirizine) to their scalp every day, while the placebo group followed the same procedure with their respective product. At the beginning and end of the study, assessments of hair regrowth were performed by a dermatologist, photographs were assessed by dermatologists to provide a Hamilton-Norwood classification, and subjective assessments were provided by the participants in the form of questionnaires. The change in each parameter relative to the start of the study was then compared between groups.[30]Zaky, M. S., Abo Khodeir, H., Ahmed, H. A., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: a case‐controlled study. … Continue reading
Dermatological assessment determined hair regrowth to be significantly greater in the cetirizine group, with 43.3% of participants exhibiting hair regrowth compared to 0% of the placebo group. Similarly, photographic assessment determined that 43.3% of the cetirizine group exhibited improvements in Hamilton-Norwood classification, compared to 0% of the placebo group, which was also a significant difference. Finally, self-assessment by questionnaire revealed significantly greater satisfaction in the cetirizine group, with 43.3% stating that their hair growth had shown an improvement compared to 0% of the placebo group.[31]Zaky, M. S., Abo Khodeir, H., Ahmed, H. A., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: a case‐controlled study. … Continue reading
Figure 5: Photographic and dermoscopic comparison of the hair and scalp before and after 6 months of treatment with topical cetirizine (1%).[32]Zaky, M. S., Abo Khodeir, H., Ahmed, H. A., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: a case‐controlled study. … Continue reading
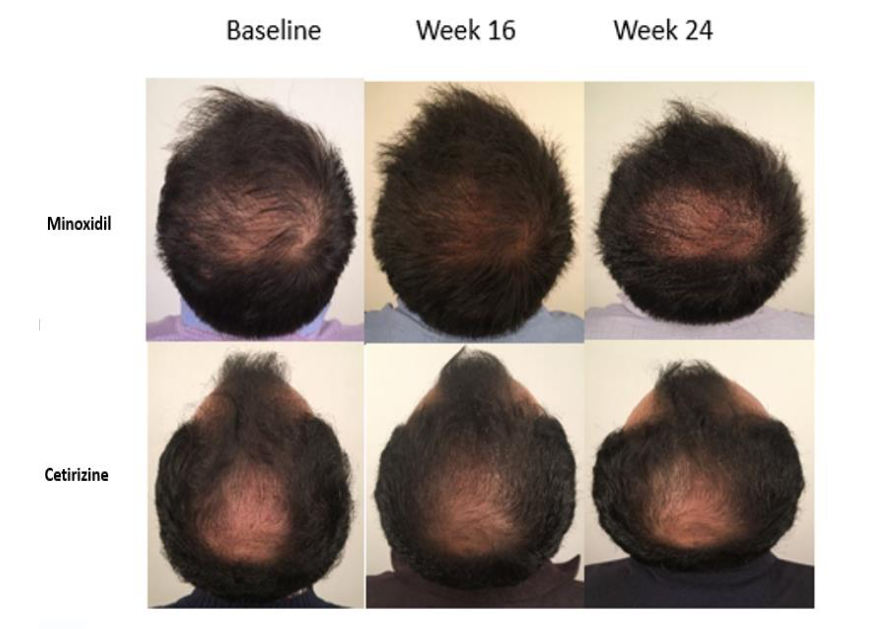
In another study, 40 males between the ages of 18 and 49 were recruited to compare topical cetirizine (1%) and minoxidil (5%), and FDA approved therapy for hair loss. Participants in this single-blind study were randomized into one of the two groups and then applied 1mL of solution per day to the balding area of their head. After 16 weeks, every participant was switched to a placebo product for a further 8 weeks.[33]Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a … Continue reading
Using Trichoscan to conduct objective assessments of the hair, they found that both cetirizine and minoxidil caused a significant increase in total hair density and vellus hair density after 16 weeks, with minoxidil outperforming cetirizine. The percentage of hair in anagen phase increased in both groups after 16 weeks, then subsequently diminished after placebo use. Assessments completed by physicians suggested that a comparable percentage of participants in each group showed improvement in hair density, although 17% of the cetirizine group were determined to have exhibited a reduction compared to 0% of the minoxidil group. Furthermore, the participants in the minoxidil group reported greater satisfaction with their treatment.[34]Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a … Continue reading
Figure 6: Scalp photographs of people with AGA after 16 weeks of treatment with topical minoxidil or cetirizine, followed by 8 weeks of a placebo.[35]Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a … Continue reading
A third study recruited 85 males and females between the ages of 20 and 65 with AGA. 67 participants were placed in the treatment group and used 1mL of topical cetirizine (1%) daily for 6 months, with 18 participants using a placebo product for the same length of time. Using macrophotographs and Trichoscan, they found that total hair density and terminal hair density were both significantly increased in the cetirizine treatment group.[36]Rossi, A., Campo, D., Fortuna, M.C., Garelli, V., Pranteda, G., De Vita, G., Sorriso-Valvo, L., Di Nunno, D. and Carlesimo, M., 2018. A preliminary study on topical cetirizine in the therapeutic … Continue reading
Figure 7: Global photography of people with AGA before (top) and after (bottom) treatment with topical cetirizine (1%) for 6 months.[37]Rossi, A., Campo, D., Fortuna, M.C., Garelli, V., Pranteda, G., De Vita, G., Sorriso-Valvo, L., Di Nunno, D. and Carlesimo, M., 2018. A preliminary study on topical cetirizine in the therapeutic … Continue reading
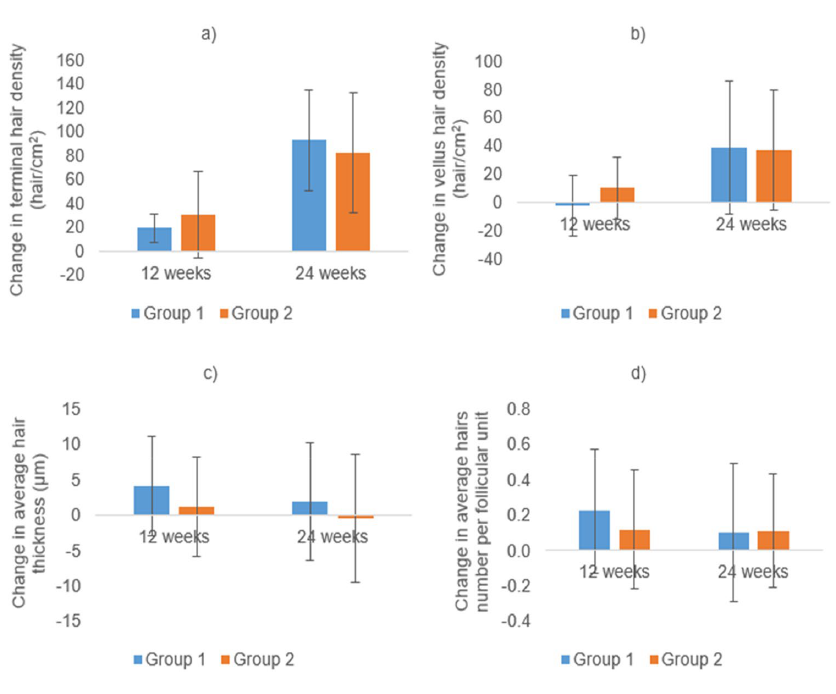
One last study recruited 60 female patients, with AGA, between the ages of 20 and 50. The study was double-blinded and randomized. Both groups applied 1mL topical minoxidil (5%) every morning for 6 months; one of these groups also applied 1mL topical cetirizine (1%) in the evening, while the other group applied a placebo. Phototrichscopy was used to show that both groups exhibited a significant increase in frontal and vertex terminal and vellus hair density. Interestingly, the minoxidil+cetirizine group also showed a significant increase in hair thickness and number of hairs per follicular unit, which was not present in the minoxidil only group. However, it should be noted that change relative to baseline in both groups was comparable across all parameters. Participants in the minoxidil+cetirizine group also scored better in the self-assessment of new hairs, hair growth, bald areas, and satisfaction with the treatment.[38]Bassiouny, E. A., El-Samanoudy, S. I., Abbassi, M. M., Nada, H. R., & Farid, S. F. (2023). Comparison between topical cetirizine with minoxidil versus topical placebo with minoxidil in female … Continue reading
Figure 8: Changes in hair-related parameters relative to baseline in participants that took minoxidil (5%) and cetirizine (1%) in combination (Group 1) and participants that took minoxidil (5%) alone (Group 2).[39]Bassiouny, E. A., El-Samanoudy, S. I., Abbassi, M. M., Nada, H. R., & Farid, S. F. (2023). Comparison between topical cetirizine with minoxidil versus topical placebo with minoxidil in female … Continue reading
Collectively, these studies provide evidence that suggests topical cetirizine may be beneficial in the treatment of hair loss. However, there are limitations with the studies that have been described. For one, it would be good to assess the efficacy of cetirizine over a longer period of time (more than 6 months), to determine whether its beneficial effects improve further, plateau, or even reverse. Larger studies would be highly beneficial, particularly with the inclusion of more females. All of these studies were also limited to the inclusion of AGA patients which, while useful, means that the efficacy of cetirizine in treating other hair loss conditions remains unknown. Some of the studies also lack placebo controls, which means definitive conclusions on the effectiveness of cetirizine cannot be drawn from their results. That said, the results are promising, and seemingly suggest that cetirizine may provide some beneficial effects.
Safety and Side Effects
Oral cetirizine has received FDA approval for treating allergies and is considered safe at the recommended dose (10mg within a 24-hour period in adults, 5mg twice daily in children aged 6-11 years, 2.5mg twice daily in children aged 2-5 years).[40]NICE. (No date). Cetirizine hydrochloride. National Institute for Health and Care Excellence. Available at: https://bnf.nice.org.uk/drugs/cetirizine-hydrochloride/ (Accessed: 29 April 2025)
Common side effects include feeling of sleepiness and tiredness, as well as headaches, dizziness, dry mouth, diarrhea, sore throat, and sneezing. However, it has been reported that small amounts of cetirizine can get into breast milk, so those who are breastfeeding should use cetirizine with caution. Additionally, people with kidney failure may be unable to use cetirizine.[41]NHS. (2025). Side effects of cetirizine. National Health Service. Available at: https://www.nhs.uk/medicines/cetirizine/side-effects-of-cetirizine/ (Accessed: 29 April 2025)
In the three clinical studies that used topical cetirizine (1%) alone, no notable adverse events or side effects were reported. In the study that used minoxidil (5%) together with cetirizine (1%), itching, dry hair, initial hair loss, and dandruff were reported. However, these are known side effects of minoxidil, and there were no differences in the number of adverse events between the two groups. Although there is no conclusive safety data related to topical cetirizine, it is safe to use (up to the recommended dosage) as an oral therapeutic. Given that oral therapeutics are more likely to trigger systemic effects, this suggests that topical cetirizine may be safe for use, though some side effects may occur in relation to its application on the skin. However, please note that there is no recommended dosage for topical cetirizine.
Is Cetirizine For Me?
You may want to experiment with topical cetirizine if:
- You are happy to use a product with limited evidence of efficacy.
- You are happy to use a product with no evidence of efficacy beyond 6 months of use.
- You have not seen beneficial effects from treatment with minoxidil and would like to try something else.
- You want to try a combined treatment with other products like minoxidil.
Final Thoughts
Cetirizine is a second-generation antihistamine that has been FDA approved (as an oral medication) for the treatment of allergies since the 1990s. It primarily works by binding the H1 receptor and preventing histamine from binding there, the molecule that is responsible for allergic symptoms. Alongside its well documented function, oral cetirizine has been shown to both reduce inflammation and inhibit the production of PGD2, the activity of which is strongly linked to hair loss. Several clinical studies have been conducted in AGA patients who used topical cetirizine as a treatment, with the results showing improvements in hair growth, hair density, and hair thickness. Furthermore, no side effects were associated with the topical use of cetirizine, and the oral version of the therapeutic is considered safe for use. Although there is a lack of large, long-term studies that have investigated topical cetirizine, the results suggest that it may provide help to improve hair loss.
References[+]
References ↑1, ↑2, ↑5, ↑7, ↑8 Curran, M. P., Scott, L. J., & Perry, C. M. (2004). Cetirizine: a review of its use in allergic disorders. Drugs. 64. 523-561. Available at: https://doi.org/10.2165/00003495-200464050-00008 ↑3 World Health Organization. (2023). WHO Model List of Essential Medicines – 23rd list, 2023. WHO/MHP/HPS/EML/2023.02. Available at: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02 (Accessed: 24 April 2025) ↑4 Pagliara, A., Testa, B., Carrupt, P.A., Jolliet, P., Morin, C., Morin, D., Urien, S., Tillement, J.P. and Rihoux, J.P. (1998). Molecular properties and pharmacokinetic behavior of cetirizine, a zwitterionic H1-receptor antagonist. Journal of medicinal chemistry. 41(6). 853-863. Available at: https://doi.org/10.1021/jm9704311 ↑6 Ghosh, S., Debnath, I., Bhunia, S., Hazra, S., Nandi, S., & Mandal, S. K. (2025). A Review on Mechanism of Histamine Mediated Allergic Reactions: Therapeutic Role, Safety, and Clinical Efficacy of Cetirizine in Modern Allergy and Other Diseases Management. Biomedical and Pharmacology Journal. 18(1). 411-429. Available at: https://dx.doi.org/10.13005/bpj/3097 ↑9, ↑10 Vardhamane, S. H., Santoshkumar, J., & Vardhamane, S. H. (2013). Anti-inflammatory activity of H1 receptor antagonist Cetirizine in animal models. Journal of Evolution of Medical and Dental Sciences. 2(13). 2050-2060. Available at: https://doi.org/10.14260/JEMDS/499 ↑11 Ciprandi, G., Buscaglia, S., Pesce, G., Passalacqua, G., Rihoux, J. P., Bagnasco, M., & Canonica, G. W. (1995). Cetirizine reduces inflammatory cell recruitment and ICAM-1 (or CD54) expression on conjunctival epithelium in both early-and late-phase reactions after allergen-specific challenge. Journal of allergy and clinical immunology. 95(2). 612-621. Available at: https://doi.org/10.1016/S0091-6749(95)70324-1 ↑12 Jinquan, T., Reimert, C. M., Deleuran, B., Zachariae, C., Simonsen, C., Thestrup-Pedersen, K., & May, R. (1995). Cetirizine inhibits the in vitro and ex vivo chemotactic response of T lymphocytes and monocytes. Journal of allergy and clinical immunology. 95(5). 979-986. Available at: https://doi.org/10.1016/S0091-6749(95)70098-6 ↑13 Amin, K. (2012). The role of mast cells in allergic inflammation. Respiratory medicine. 106(1). 9-14. Available at: https://doi.org/10.1016/j.rmed.2011.09.007 ↑14, ↑15 Fujimura, R., Asada, A., Aizawa, M., & Kazama, I. (2022). Cetirizine more potently exerts mast cell-stabilizing property than diphenhydramine. Drug Discoveries & Therapeutics. 16(5). 245-250. Available at: https://doi.org/10.5582/ddt.2022.01067 ↑16 Carthy, E., & Ellender, T. (2021). Histamine, neuroinflammation and neurodevelopment: a review. Frontiers in neuroscience. 15. 680214. Available at: https://doi.org/10.3389/fnins.2021.680214 ↑17 Branco, A. C. C. C., Yoshikawa, F. S. Y., Pietrobon, A. J., & Sato, M. N. (2018). Role of histamine in modulating the immune response and inflammation. Mediators of inflammation. 2018(1). 9524075. Available at: https://doi.org/10.1155/2018/9524075 ↑18, ↑20 Grace, S. A., Sutton, A. M., Abraham, N., Armbrecht, E. S., & Vidal, C. I. (2017). Presence of mast cells and mast cell degranulation in scalp biopsies of telogen effluvium. International journal of trichology. 9(1). 25-29. Available at: https://doi.org/10.4103/ijt.ijt_43_16 ↑19 Arck, P. C., Handjiski, B., Hagen, E., Joachim, R., Klapp, B. F., & Paus, R. (2001). Indications for a brain‐hair follicle axis: inhibition of keratinocyte proliferation and up‐regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. The FASEB Journal. 15(13). 2536-2538. Available at: https://doi.org/10.1096/fj.00-0699fje ↑21, ↑23 Zhang, X., Zhao, Y., Ye, Y., Li, S., Qi, S., Yang, Y., Cao, H., Yang, J. and Zhang, X. (2015). Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Archives of dermatological research. 307. 319-331. Available at: https://doi.org/10.1007/s00403-015-1539-1 ↑22 Jutel, M., Watanabe, T., Klunker, S., Akdis, M., Thomet, O.A., Malolepszy, J., Zak-Nejmark, T., Koga, R., Kobayashi, T., Blaser, K. and Akdis, C.A. (2001). Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 413(6854). 420-425. Available at: https://doi.org/10.1038/35096564 ↑24, ↑25 Won, C.H., Kwon, O.S., Kim, Y.K., Kang, Y.J., Kim, B.J., Choi, C.W., Eun, H.C. and Cho, K.H. (2008). Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Archives of dermatological research. 300. 147-152. Available at: https://doi.org/10.1007/s00403-007-0826-x ↑26 Wu, C. C., Kim, J. N., Wang, Z., Chang, Y. L., Zengler, K., & Di Nardo, A. (2019). Mast cell recruitment is modulated by the hairless skin microbiome. Journal of Allergy and Clinical Immunology. 144(1). 330-333. Available at: https://doi.org/10.1016/j.jaci.2019.02.033 ↑27 Charlesworth, E. N., Kagey-Sobotka, A., Norman, P. S., & Lichtenstein, L. M. (1989). Effect of cetirizine on mast cell-mediator release and cellular traffic during the cutaneous late-phase reaction. Journal of allergy and clinical immunology. 83(5). 905-912. Available at: https://doi.org/10.1016/0091-6749(89)90104-8 ↑28 Shin, D. W. (2022). The physiological and pharmacological roles of prostaglandins in hair growth. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological Society and the Korean Society of Pharmacology. 26(6). 405-413. Available at: https://doi.org/10.4196/kjpp.2022.26.6.405 ↑29 Peyravian, N., Deo, S., Daunert, S., & Jimenez, J. J. (2020). The inflammatory aspect of male and female pattern hair loss. Journal of inflammation research. 879-881. Available at: https://doi.org/10.2147/JIR.S275785 ↑30, ↑31, ↑32 Zaky, M. S., Abo Khodeir, H., Ahmed, H. A., & Elsaie, M. L. (2021). Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: a case‐controlled study. Journal of Cosmetic Dermatology. 20(4). 1154-1159. Available at: https://doi.org/10.1111/jocd.13940 ↑33, ↑34, ↑35 Mostafa, D. H., Samadi, A., Niknam, S., Nasrollahi, S. A., Guishard, A., & Firooz, A. (2021). Efficacy of cetirizine 1% versus minoxidil 5% topical solution in the treatment of male alopecia: a randomized, single-blind controlled study. Journal of Pharmacy & Pharmaceutical Sciences. 24. 191-199. Available at: https://doi.org/10.18433/jpps31456 ↑36, ↑37 Rossi, A., Campo, D., Fortuna, M.C., Garelli, V., Pranteda, G., De Vita, G., Sorriso-Valvo, L., Di Nunno, D. and Carlesimo, M., 2018. A preliminary study on topical cetirizine in the therapeutic management of androgenetic alopecia. Journal of Dermatological Treatment. 29(2). 149-151. Available at: https://doi.org/10.1080/09546634.2017.1341610 ↑38, ↑39 Bassiouny, E. A., El-Samanoudy, S. I., Abbassi, M. M., Nada, H. R., & Farid, S. F. (2023). Comparison between topical cetirizine with minoxidil versus topical placebo with minoxidil in female androgenetic alopecia: a randomized, double-blind, placebo-controlled study. Archives of Dermatological Research. 315(5). 1293-1304. Available at: https://doi.org/10.1007/s00403-022-02512-2 ↑40 NICE. (No date). Cetirizine hydrochloride. National Institute for Health and Care Excellence. Available at: https://bnf.nice.org.uk/drugs/cetirizine-hydrochloride/ (Accessed: 29 April 2025) ↑41 NHS. (2025). Side effects of cetirizine. National Health Service. Available at: https://www.nhs.uk/medicines/cetirizine/side-effects-of-cetirizine/ (Accessed: 29 April 2025) Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreBen Fletcher, PhD
Benjamin Fletcher is a researcher & writer who holds a BSc in Biological Sciences and an MSc in Genes, Drugs & Stem Cells. Benjamin is currently pursuing a Ph.D. in Molecular Biology & Genetics, conducting research to better understand the regulatory mechanisms that drive muscle atrophy in disease, with a particular focus on the influence of microRNAs.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down