- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
7 Best Oils for Hair Growth
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesSwitching From Finasteride to Dutasteride
First Published Jan 27 2026Last Updated Jan 27 2026Pharmaceutical Researched & Written By:Sophie Grice, PhD
Researched & Written By:Sophie Grice, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Switching from finasteride to dutasteride is often framed as a simple upgrade or downgrade. In reality, each switch changes not only DHT suppression but also exposure patterns, timelines for results, washout dynamics, and evidence quality. This article breaks down realistic switching scenarios, oral to oral, topical to topical, and cross-route, using the best available human data, plus practical guidance on how to minimize trade-offs.
Full Article
Here’s a story we see all the time. You’ve been using finasteride, saw some initial improvement or stabilization, but now your hair regrowth is plateauing, you’re still noticing miniaturization, and wondering whether it’s time to switch to dutasteride.
On the surface, the logic is simple. Dutasteride suppresses DHT more than finasteride, and in head-to-head studies, it usually improves hair counts and thickness more. But switching doesn’t just change potential results. It changes how long the drug continues to suppress DHT after discontinuation, how quickly you can evaluate whether it’s helping (or causing side effects), and how easily you can adjust or exit the treatment if needed.
Many people think of this switch as changing one variable. In reality, switching from finasteride to dutasteride can involve changing multiple variables at once: oral versus topical delivery, dose equivalence, absorption variability, drug persistence (half-life), transition strategy, and expectations for timelines. Each of these can alter both outcomes and tolerability.
This article breaks down the real-world trade-offs of switching from finasteride to dutasteride, compares the most common transition scenarios (oral-to-oral, topical-to-topical, and cross-route switches), explains what to expect, and offers our perspective on which decisions are evidence-based and which require a little more skepticism.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

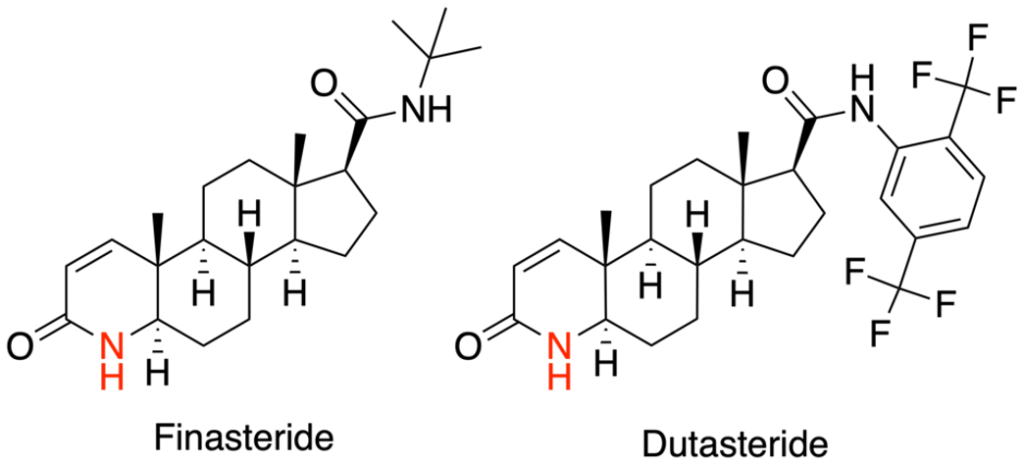
Biology of Finasteride and Dutasteride
DHT is the key androgen driving follicular miniaturization in androgenic alopecia (AGA). An enzyme called 5α‑reductase (5AR) in the hair follicle converts testosterone to DHT. Because DHT is known to drive hair loss progression, many treatments for AGA target 5AR to reduce scalp DHT levels.[1]Asfour, L., Cranwell, W., & Sinclair, R. (2023). Male androgenetic alopecia. *Endotext [Internet].* Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/ Dutasteride blocks both Type I and Type II 5AR, leading to more DHT suppression than finasteride, which only targets Type II.
Oral finasteride is most commonly prescribed at 1 mg daily for AGA, while oral dutasteride is typically taken at 0.5 mg daily. Topical formulations were developed later to change the risk profile without affecting the mechanism. Instead of lowering DHT systemically by default, topical finasteride was designed to concentrate drug activity in the scalp while limiting how much reaches the bloodstream. Topical dutasteride represents the newest and least mature category, but evidence is promising. Because dutasteride is more potent than finasteride, even very low topical concentrations (around 0.01-0.05%) can meaningfully suppress scalp DHT.
Figure 1. The structures of finasteride and dutasteride.[2]Wikimedia Commons. (n.d.). Azasteroid Pharmaceuticals [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Azasteroid_Pharmaceuticals.png (Accessed: November 2025) Image in the Public Domain.
Switching Scenarios
There are many treatment options for both dutasteride and finasteride, each with different trade-offs. We’ll compare the available clinical evidence across formulations and break down the most common switching scenarios, highlighting four core considerations: expected efficacy, side-effect profile, commitment and washout dynamics, and strength of evidence.
Key Takeaways:
- Oral finasteride to oral dutasteride: the most evidence-backed upgrade.
- Oral finasteride to topical dutasteride: one study suggests improvement, but we’re skeptical about the data.
- Topical finasteride to topical dutasteride: largely guesswork, with no direct comparative trials.
- Topical finasteride to oral dutasteride: no comparative studies, but surrogate evidence suggests a gain in efficacy.
- Oral dutasteride to topical finasteride: no direct studies, but surrogate data suggests a likely loss in efficacy.
These answers can all change when you account not just for drug switches or formulation switches, but also the dose.
Oral Finasteride to Oral Dutasteride
This is the cleanest and most evidence-supported switch. Four major comparative randomized controlled trials (RCTs) provide direct comparisons between oral finasteride and oral dutasteride at commonly used doses.
Efficacy
Here is a summary of the clinical trials that compare oral finasteride and dutasteride treatment directly:
Study Study Design and Population Treatments Study #1[3]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Randomized, placebo-controlled, double-blinded trial. 415 men with male pattern hair loss aged 21-45 years old; 24 weeks. 5 mg oral finasteride; 0.05, 0.1, 0.5, or 2.5 mg oral dutasteride; placebo. Study #2[4]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Randomized, placebo-controlled, double-blinded trial. 917 men with AGA aged 20-50; 24 weeks. 1 mg oral finasteride; 0.02, 0.1, or 0.5 mg oral dutasteride; placebo Study #3[5]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Open-label, randomized study; no placebo control. 90 men with AGA aged 18-40; 24 weeks. 1 mg oral finasteride; 0.5 mg oral dutasteride. Study #4[6]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading Retrospective chart review. 600 men over 18 with AGA. 1 mg oral finasteride; 0.5 mg oral dutasteride. Across these studies, dutasteride consistently outperformed finasteride on hair parameters:
- Target-area hair counts: In Study #1 the proportion of participants with at least a 10% increase in hair counts was 41% for finasteride (5 mg), 48% for dutasteride (0.5 mg) and 56% for dutasteride (2.5 mg).[7]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading In Study #3, dutasteride (0.5 mg) significantly increased total hair count and decreased thin hair count per cm2 compared to finasteride (1 mg).[8]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Notably, Study #2 showed that dutasteride (0.5 mg) significantly increased terminal hair count compared to finasteride (1 mg).[9]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
- Hair shaft diameter: Study #2 demonstrated that dutasteride (0.5 mg) was statistically superior to finasteride (1 mg) in increasing hair width.[10]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
- Global photographic assessment: Across studies, investigators were more likely to rate dutasteride (0.5 mg) users as “improved” or “markedly improved” compared to those receiving finasteride (1 mg).[11]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading
- DHT suppression: In Study #1, serum DHT levels were significantly lower with dutasteride (0.5 mg and 2.5 mg) than with finasteride (5 mg). Additionally, finasteride (5mg) decreased scalp DHT by 41%, while dutasteride (0.5 mg) decreased it by 51%, and dutasteride (2.5 mg) decreased it by 79%.[12]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading
Important caveat: Most RCTs lasted only 24 weeks. Finasteride continues to produce gains for up to 48 months in longer studies, so some of dutasteride’s apparent advantage may reflect faster onset rather than an infinite ceiling of benefit. However, longer-term observational data suggest that dutasteride maintains superiority on global classification scales.
Side-Effects
The most frequently discussed side effects of finasteride and dutasteride include:
- Sexual side effects – These include decreased libido, erectile dysfunction, and ejaculation disorders. Across randomized trials, these effects occur in a minority of users (generally in the single-digit percentages), appear most often early in treatment, and frequently resolve spontaneously, even while continuing therapy.[13]Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic … Continue reading
- Breast-related effects – Breast tenderness or enlargement has been reported, but rates are low across both drugs and typically comparable between finasteride and dutasteride.
- Other reported effects: Fatigue, mood changes, or nonspecific symptoms are occasionally reported, but these occur inconsistently, lack clear dose–response relationships, and are difficult to separate from background rates and expectation effects.
Across clinical trials, overall side-effect rates were similar for finasteride and dutasteride, averaging approximately 8-9% for both drugs. Notably, a 2019 systematic review and meta-analysis found no significant difference in adverse event rates between the two treatments, including for sexual side effects such as altered libido, erectile dysfunction, and ejaculation disorders.[14]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The Efficacy And Safety Of Dutasteride Compared With Finasteride In Treating Men With Androgenetic Alopecia: A Systematic Review And … Continue reading
The key difference may not be how often side effects occur, but how long drug-induced hormonal changes persist after stopping treatment. Because dutasteride remains biologically active far longer than finasteride, any changes may take longer to unwind after stopping.
Commitment & Washout Dynamics
In this article, when we discuss commitment or washout dynamics, we are referring to how long a drug continues to suppress DHT after it is stopped, not how long hair regrowth or side effects persist. This reflects how quickly the body can clear the medication and return toward baseline hormone activity once therapy is discontinued.
Dutasteride involves a higher level of commitment than finasteride because it remains biologically active for far longer. Oral dutasteride (0.5 mg daily) has a half-life of around 4-5 weeks, meaning drug activity can persist for months after discontinuation, whereas oral finasteride has a half-life of roughly 6-8 hours.
This means that dose changes or discontinuation of dutasteride lead to much slower changes in systemic DHT suppression compared with finasteride. Dutasteride is not a “try it and see how you feel in two weeks” medication. Its pharmacologic effects unwind gradually, requiring a substantially higher level of decision commitment.
Evidence Strength: Strong
Overall, the oral finasteride to oral dutasteride switch is the most evidence-supported pathway in terms of both efficacy and safety. This consistent superiority in hair outcomes is why oral dutasteride is often considered when finasteride results plateau.
If you’re a member and would like to learn more about oral dutasteride, read our ultimate guide here.
Oral Finasteride to Topical Dutasteride
At the end of 2025, the first RCT involving 135 men with AGA comparing oral finasteride (1 mg) to topical dutasteride (0.01-0.05%) over 24 weeks was published.[15]Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled … Continue reading
The study suggested that topical dutasteride (0.05%) outperformed oral finasteride, which would represent a major shift in how we think about treatment escalation.
But, after we took a closer look at the paper, we think the findings should be interpreted with caution due to multiple concerns with their methodology.
Efficacy
In the study, several hair parameters appeared to improve more in the topical dutasteride (0.05%) group than in those receiving oral finasteride (1 mg). The authors reported an average increase of +75 hairs per cm² with topical dutasteride compared to +41 hairs per cm² with oral finasteride. Additionally, 69% of participants using topical dutasteride were rated as “improved” on global photographic assessment, compared with 21% in the oral finasteride group.
However, we think these results are likely inflated. Here are two of the major problems with this study:
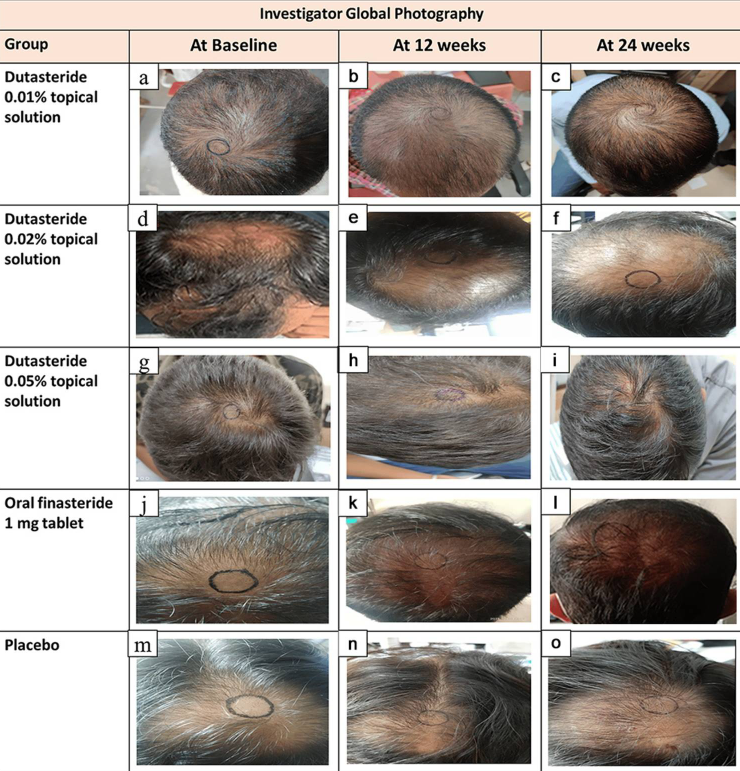
#1 Baseline hair counts defy biology
The study reports baseline densities of 300+ hairs per cm² in balding men. For context, healthy, non-balding scalps have around 100-250 hairs per cm², whereas men with Norwood III-V AGA typically have around 25-100 hairs per cm² in the vertex.
This suggests they likely counted vellus hairs, mis-measured the sampling area, or used unreliable manual counting methods. This could significantly inflate improvements.
#2 The measurement circle moves
In the published images, the target circles change location, size, and shape, they appear hand-drawn, and they clearly do not track the same exact scalp area over time.
A 1-2 mm shift can change hair counts by 50%+. The study’s reported gains (10-30%) fall well within the error range of sloppy circle placement, meaning the entire efficacy signal could be measurement noise.
Figure 2. Representative hair growth images. Adapted from Figure 2.[16]Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled … Continue reading Image used under Creative Commons License.
Side-Effects
Despite large hair-growth claims, systemic hormone changes were minimal. Serum DHT change for oral finasteride was between -11% to -27%, and -9% to -11% for topical dutasteride (0.05%). Plasma dutasteride levels were mostly near the quantification limit, but some samples spiked to 2,555 pg/mL, and dutasteride remained detectable at day 168 while participants were still receiving treatment. This suggests that average exposure was low, but absorption may be unpredictable in some users.
Commitment & Washout Dynamics
While participants were actively using topical dutasteride, most had very low drug levels in the blood, but a small number showed noticeable absorption. This means that applying dutasteride to the scalp does not completely prevent it from entering the bloodstream, and individual responses can vary.
Because the study did not measure drug levels after treatment was stopped, it does not tell us how long topical dutasteride continues to have effects once discontinued. So although average exposure appears lower than with oral finasteride, the length of time its effects persist after stopping remains uncertain.
Evidence Strength: Moderate (but controversial)
This is the first RCT that compared oral finasteride directly with topical dutasteride alone, but it had major pitfalls, including implausible baseline hair counts, unreliable target area placement, and results that contradict years of real-world outcomes. These flaws reduce our confidence in this study’s efficacy claims
If you’d like to read more about our interpretation of the 2025 study comparing oral finasteride to topical dutasteride, read our article here.
Topical Finasteride to Topical Dutasteride
Currently, there are no head-to-head studies comparing topical finasteride with topical dutasteride. That means outcomes are highly dependent on formulation choices, and most conclusions in this switch category are informed speculation rather than solid evidence.
Efficacy
Because there are no comparative trials, we cannot say with confidence that topical dutasteride is more effective than topical finasteride. Any perceived improvement would depend on:
- Drug concentration
- Vehicle (alcohol, liposomal, foam, etc.)
- Volume applied per use
- Application frequency
- Individual scalp absorption
In other words, this switch should be viewed as an experiment, not an upgrade supported by clinical data.
Side-Effects
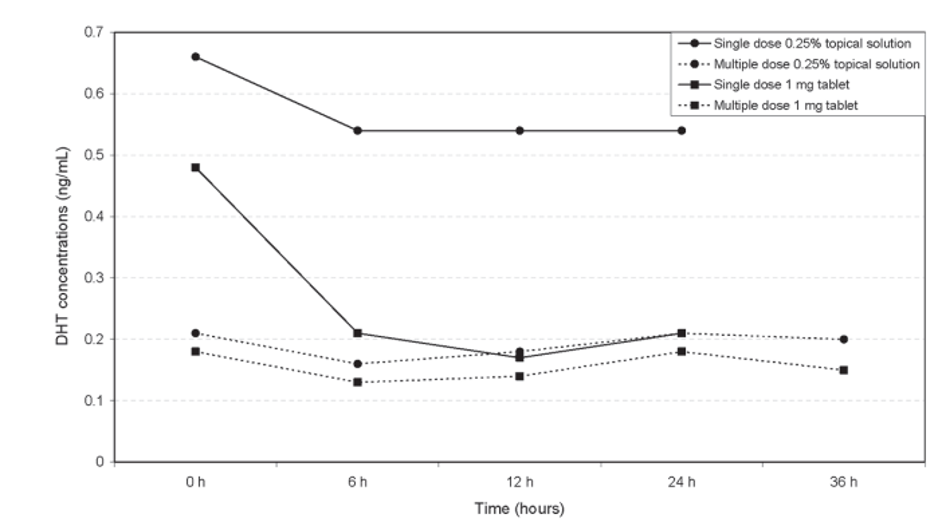
Topical application does not guarantee that finasteride or dutasteride will remain confined to the scalp. Both agents can suppress serum DHT depending on formulation, dose, vehicle, and application frequency. As a result, systemic effects are still possible, particularly when higher concentrations or larger application volumes are used.[17]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
Figure 3. Multiple daily doses of topical finasteride can reduce serum DHT more than a single dose. Adapted from Figure 3.[18]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
Commitment & Washout Dynamics
Topical finasteride generally leads to lower overall drug exposure than oral finasteride, with smaller reductions in serum DHT, suggesting a shorter washout window and greater flexibility with dose adjustments.[19]Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic … Continue reading
Topical dutasteride still uses a drug with a long half-life and strong binding to 5AR. However, in the trial comparing oral finasteride (1 mg) to topical dutasteride (0.05%), systemic exposure and serum DHT suppression were modest, suggesting that at these doses most of its effect is likely occurring in the scalp rather than through prolonged whole-body exposure.[20]Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled … Continue reading
This implies that topical dutasteride (0.05%) is less committing than oral dutasteride (0.5 mg daily), but may still be more committing than low-dose topical finasteride, especially at higher concentrations or with frequent application, because of dutasteride’s pharmacology. That said, this remains an inference rather than a proven conclusion.
Evidence Strength: Weak
Due to the lack of clinical studies comparing topical finasteride with topical dutasteride, the evidence base for making the switch between these two topical therapies is limited. Any decision to make the switch should be treated as a dose-dependent experiment rather than an evidence-backed upgrade.
Topical Finasteride to Oral Dutasteride
There are no direct comparative studies for this switch. Still, using surrogate data from Study #1-4 comparing oral finasteride to oral dutasteride, we can reasonably expect a gain in efficacy for many users. This transition moves from a variable, dose-dependent topical regimen to a potent and consistently studied systemic DHT-suppression strategy.
While this switch is likely to improve hair outcomes, it also represents a meaningful change in drug exposure. It involves moving from a formulation that often limits systemic absorption to one that produces sustained, whole-body DHT suppression with a drug that remains biologically active for weeks. As a result, dose adjustments and discontinuation lead to slower changes in hormone suppression, and any unwanted effects may take longer to resolve after stopping.
Oral Dutasteride to Topical Finasteride
There are also no head-to-head studies for changing from oral dutasteride to topical finasteride. But we can infer that switching from oral dutasteride to topical finasteride will have the opposite effect to what was seen in Study #1-4 comparing oral finasteride to oral dutasteride.
Switching from oral dutasteride to topical finasteride will likely result in a loss of efficacy for many people, as there will be both a reduction in pharmacologic potency and a move from systemic exposure to a less predictable topical delivery system.
From an exposure standpoint, this switch may generally reduce overall systemic drug levels and shorten the washout window. Topical finasteride typically lowers serum DHT less than oral dutasteride and clears the body more quickly, meaning that changes in dosing or discontinuation tend to result in faster shifts in biological activity.
How To Make the Decision to Switch
Step 1: Are you stable and satisfied using finasteride?
Yes: Don’t fix what isn’t broken. If your hair is stable or slowly improving, the safest move is often staying the course and reassessing with standardized photos every 3-6 months.
No: Go to Step 2.
Step 2: Is the priority more efficacy, or minimizing downside risk?
Efficacy priority: The most evidence-backed switch is oral finasteride to oral dutasteride. This switch is best suited for those with aggressive or rapidly progressing AGA, extensive miniaturization, or those who have clearly plateaued after a consistent finasteride trial.
Risk priority: Consider optimizing finasteride (dose/frequency) or adding adjuncts (minoxidil/microneedling). Finasteride remains the more forgiving option due to its shorter half-life and easier dose adjustment.
Step 3: Do you tolerate oral 5AR inhibitors?
Yes: Oral dutasteride is the cleaner evidence path for improved efficacy.
No: Topical options can work, but treat them as dose-dependent experiments rather than guaranteed upgrades.
If you’d like more information on oral versus topical dutasteride, take a look at our article here.
Step 4: Set the evaluation window before you switch.
Don’t judge a switch in 6-8 weeks. Commit to 6-12 months and take standardized photographs to track your progress.
Avoid switching (or be extra cautious) if:
- You’re trying to conceive soon.
- You’ve previously experienced severe side effects when using 5AR inhibitors.
- You prefer treatments that leave your system quickly if something goes wrong.
Our Final Thoughts
Both finasteride and dutasteride offer real benefits, but each comes with distinct trade-offs. If you’re considering switching, it’s critical to focus on what the evidence actually supports. Currently, the only switch backed by high-quality comparative data is oral finasteride to oral dutasteride, making it the most evidence-based upgrade.
That said, this path comes with a higher level of commitment. Because dutasteride persists in the body for weeks, drug-induced hormonal changes unwind more slowly, which may delay how quickly unwanted effects fade after stopping. Other switching scenarios currently don’t have robust clinical evidence, so they should be approached with caution and treated as informed experiments rather than proven upgrades.
If you’d like a deeper breakdown of finasteride versus dutasteride, read our ultimate guide here.
References[+]
References ↑1 Asfour, L., Cranwell, W., & Sinclair, R. (2023). Male androgenetic alopecia. *Endotext [Internet].* Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/ ↑2 Wikimedia Commons. (n.d.). Azasteroid Pharmaceuticals [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Azasteroid_Pharmaceuticals.png (Accessed: November 2025) ↑3 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑4, ↑9, ↑10 Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 70(3). 489–498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑5 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑6, ↑11 Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. *Annals of Dermatology.* 34(5). 349. Available at: https://doi.org/10.5021/ad.22.027 ↑7, ↑12 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑8 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑13 Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. *The Journal of Clinical and Aesthetic Dermatology.* 9(7). 56–62. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5023004/ ↑14 Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., Cui, Y. (2019). The Efficacy And Safety Of Dutasteride Compared With Finasteride In Treating Men With Androgenetic Alopecia: A Systematic Review And Meta-Analysis. Clinical Interventions in Aging. 14. 399-406. Available at: https://doi.org/10.2147/CIA.S192435 ↑15 Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects With Androgenetic Alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑16, ↑20 Panuganti, V.K., Kumar Madala, P., Ramalingayya Grandhi, V., Varma Alluri, C., Mohammad, J., Rao, K.S.S.V.V., Reddy Dundigalla, M., (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects With Androgenetic Alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑17 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑18 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑19 Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. *Journal of the European Academy of Dermatology and Venereology.* 36(2). 286–294. Available at: https://doi.org/10.1111/jdv.17738 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sophie Grice, PhD
Sophie completed a BSc in Pharmacology before earning a PhD in Immunopharmacology at the University of Liverpool. Her doctoral research examined drug hypersensitivity reactions in patients treated with immune checkpoint inhibitors. She later pursued postdoctoral research focused on T cell mediated immune responses, with an emphasis on the immunogenicity of gene therapies.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down