- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesRU58841 for Hair Loss: Is It Legit?
First Published Jan 9 2026Last Updated Jan 12 2026Pharmaceutical Researched & Written By:Sophie Grice, PhD
Researched & Written By:Sophie Grice, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor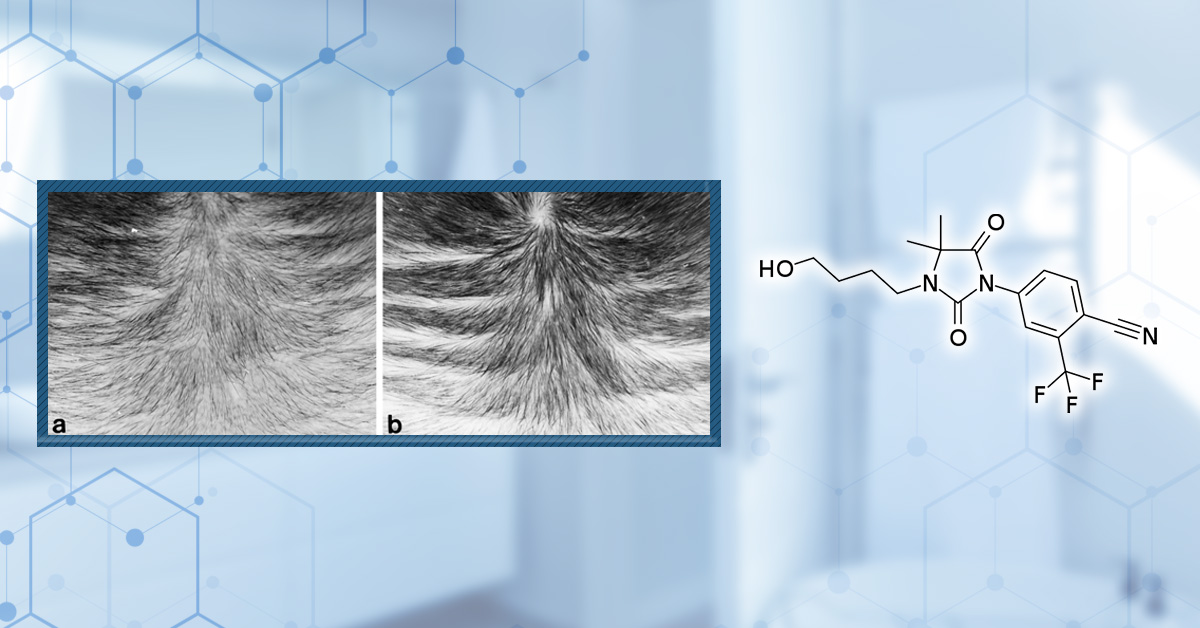
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
RU58841 is one of the most talked-about research chemicals in hair loss forums, and the anecdotes are often surprisingly positive. Mechanistically, it makes sense: block androgen receptors in the scalp so dihydrotestosterone (DHT) can’t trigger follicle miniaturization. But the problem is that the human clinical trials were never published, and the compound was abandoned by its own inventors. In this article, we explore what RU58841 is, the preclinical data, the missing human data, and the under-discussed safety concerns that keep RU58841 in the “potentially effective, but risky” category.
Full Article
Many people are interested in RU58841 after hearing anecdotes about it working well for hair regrowth. Even though RU58841 is classified as for “research purposes only,” you can find promotion of RU58841 on many YouTube channels. It is increasingly used as a preventive measure against hair loss, especially in the bodybuilding community and among individuals who cannot tolerate traditional treatments like finasteride due to side effects. Some people have demonstrated incredible results with no side effects. While others have reported no results and side effects ranging from brain fog to severe chest pain.
Despite its reputation, RU58841 is still a clinical mystery. No one knows why the human clinical trials were never published, and why the research chemical was ultimately abandoned. This article explores preclinical and clinical data on RU58841, presents our perspectives, and provides information we believe is rarely discussed about this research chemical and its potential issues.
What Is RU58841 and How Does It Work?
RU58841 is a topical androgen receptor antagonist developed in the mid-1990’s to combat androgenic alopecia by preventing DHT from having its effects.[1]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading
As an androgen receptor antagonist, RU58841 blocks the “landing pads” (i.e., androgen receptors) on cell surfaces for the hormone DHT, preventing DHT from exerting its effects. If DHT cannot bind to androgen receptors in balding-prone hair follicles, it cannot induce the cascade of events that leads to hair follicle miniaturization, which is the defining characteristic of androgenic alopecia.[2]Ho, C.H., Sood, T., Zito, P.M. (2024). Androgenetic Alopecia. In: StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430924/
It’s important to note that androgen receptor antagonists like RU58841 work differently from drugs like finasteride or dutasteride that inhibit the enzyme that turns testosterone into DHT (reducing DHT levels overall). Androgen receptor antagonists block the “landing pads” on the cell surfaces, preventing DHT from binding. The body can still freely produce DHT, but in the presence of androgen receptor antagonists, DHT cannot bind to hair follicle sites and have its negative effects.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Follow this link to our article here to learn more about the side effects of finasteride.
The goal when developing RU58841 was to design a drug that blocked androgen receptors locally (i.e., in the scalp). With hopes of preventing its binding to androgen receptors everywhere else in the body (like with oral spironolactone), therefore preventing interference with androgen production in places like the testes, ovaries, or adrenal glands. In fact, one of the premises behind RU58841 was to simply avoid side effects that sometimes come with drugs like finasteride and dutasteride.
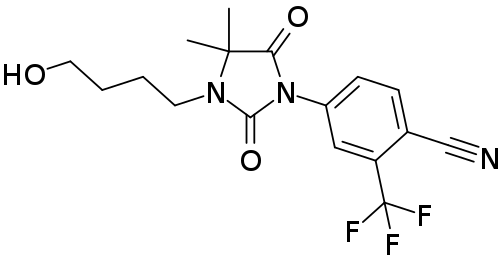
Figure 1. Structure of RU58841.[3]Wikipedia, (26 December 2018), RU-58841. Available at: https://en.wikipedia.org/wiki/RU-58841#/media/File:RU-58841_structure.svg (Accessed: 02 January 2026) Image in the Public Domain.
What the Evidence Actually Shows
Preclinical studies show promising results for RU58841. Yet despite tens of millions of dollars spent on RU58841’s human clinical trials, little information from those studies has been made public.[4]ISRCTN Registry, (no date), ISRCTN49873657. Available at: https://www.isrctn.com/ISRCTN49873657 (Accessed: 02 January 2026),[5]ISRCTN Registry, (no date), ISRCTN71083772. Available at: https://www.isrctn.com/ISRCTN71083772 (Accessed: 02 January 2026)
Soon after these clinical studies were completed, the drug’s research and development were discontinued, with financial concerns cited as the reason for halting RU58841’s progress. It was stated that with more money, development could continue. However, further development never occurred, and decades later (after the drug’s patents expired), companies began selling RU58841 for “research purposes only,” citing its initial, and small, clinical trials showing favorable results for male pattern hair loss.
Let’s break down what we actually know about RU58841 from preclinical and clinical research.
Evidence From Preclinical Data
Preclinical studies suggest RU58841 works as intended and may be effective for blocking androgen activity. But we must remember that they cannot confirm long-term human safety, and they do not guarantee purely local action in real-world conditions (where dose, formulation, frequency of application, and individual absorption vary).
How Strong Is RU58841 Compared to Natural Androgens?
RU58841 was designed as a nonsteroidal androgen-receptor antagonist and contains structural features (notably perfluoroalkyl and nitrile groups) that are associated with strong androgen-receptor binding.
Early receptor-binding experiments measuring equilibrium association constants (Ka) demonstrated that RU58841 binds the androgen receptor with affinity equal to or greater than testosterone in several species and skin-relevant tissues, including the hamster flank organ and the human receptor.[6]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading This is positive as it supports the premise that RU58841 can effectively compete with natural androgens at target sites.
When compared with other nonsteroidal antiandrogens such as flutamide, RU58841 was reported to have approximately 30x higher receptor affinity, a key reason it was pursued as a best-in-class topical antiandrogen candidate.[7]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading
But potency works both ways. Strong androgen receptor binding makes RU58841 mechanistically credible for scalp blockade. However, it also raises the stakes if any meaningful amount escapes the scalp, accumulates, or produces systemically active metabolites.
Does RU58841 Stay Local in the Skin?
In an early study using an intact hamster flank-organ model, topical RU58841 showed strong local antiandrogen activity with minimal evidence of systemic spillover at low doses.[8]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading When applied topically at doses up to 100 µg/animal, RU58841 reduced flank-organ area in a dose-dependent manner while showing no effect on the opposite flank organ, no meaningful change in serum testosterone, and no detectable antiandrogenic effects on sex organs (e.g., prostate/seminal vesicles).[9]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading These findings supported the premise that RU58841 can act locally within a certain exposure window.
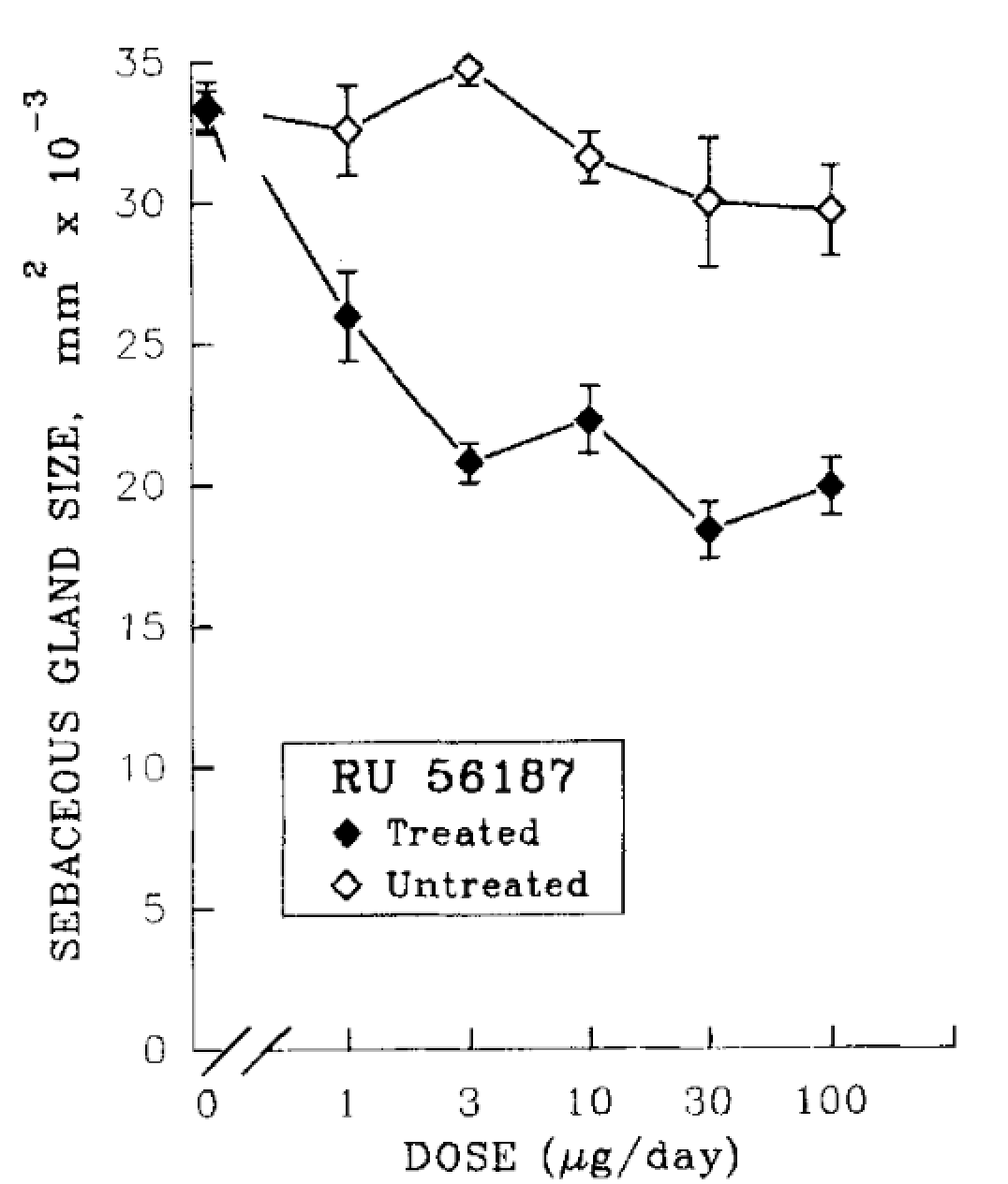
Are the Effects of RU58841 Reversible?
In a separate hamster ear model focused on sebaceous gland size, topical RU58841 (10 µg) reduced sebaceous gland volume by roughly 60% in the treated ear, with no measurable effect on the untreated ear.[10]Matias, J.R., Gaillard, M. (1995). Local Inhibition Of Sebaceous Gland Growth By Topically Applied RU 58841. Annals of the New York Academy of Sciences. 761. 56-65. Available at: … Continue reading Importantly, gland size returned to its baseline size within approximately four weeks after stopping treatment, showing that RU58841’s local antiandrogenic effects were reversible in this model. Dose-response experiments in this model revealed that more is not necessarily better. Around 3 µg/day was found to be as effective as 100 µg/day in reducing sebaceous gland size, indicating a pharmacologic limit.
Figure 2. RU58841 and its effects on sebaceous gland sizing: a dose-dependent study. Adapted from Figure 2.[11]Matias, J.R., Gaillard, M. (1995). Local Inhibition Of Sebaceous Gland Growth By Topically Applied RU 58841. Annals of the New York Academy of Sciences. 761. 56-65. Available at: … Continue reading
At What Point Does “Local” Become Systemic?
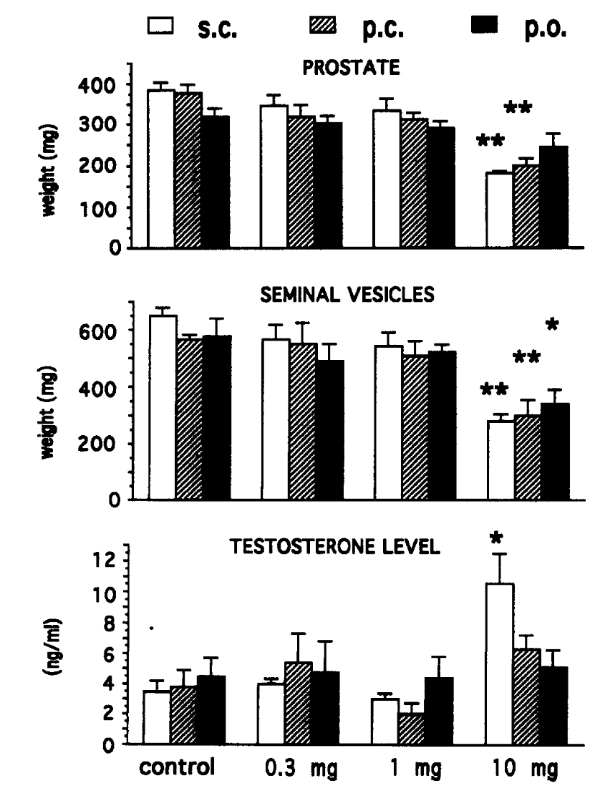
In the hamster flank-organ study, when RU58841 was given subcutaneously (to mimic complete systemic exposure), systemic antiandrogen signals emerged at higher doses.[12]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading At 300-1000 µg/animal, researchers observed reductions in prostate weight, indicating that systemic antiandrogenic effects can appear once exposure is high enough.
In intact rats, strong androgen-dependent effects were generally absent up to 1 mg/rat regardless of route of administration, but became apparent at 10 mg/rat. Testosterone increases were noted only after subcutaneous dosing at the highest dose. This suggests endocrine feedback can occur when systemic exposure is sufficiently high.
Figure 3. Antiandrogenic activity of RU5881 in the intact rat after subcutaneous (s.c.), percutaneous (p.c.) or oral (p.o.) treatment on sex organs and testosterone level. Adapted from Figure 3:[13]Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, … Continue reading
Does RU58841 Regrow Hair in Primate Models?
The most hair-relevant preclinical data for RU58841 comes from studies conducted in stump-tailed macaques.[14]Obana, N., Chang, C., Uno, H. (1997). Inhibition Of Hair Growth By Testosterone In The Presence Of Dermal Papilla Cells From The Frontal Bald Scalp Of The Postpubertal Stumptailed Macaque. … Continue reading These primate species can develop an androgenic alopecia-like pattern with age, making it a useful, though still not perfect, translational model.
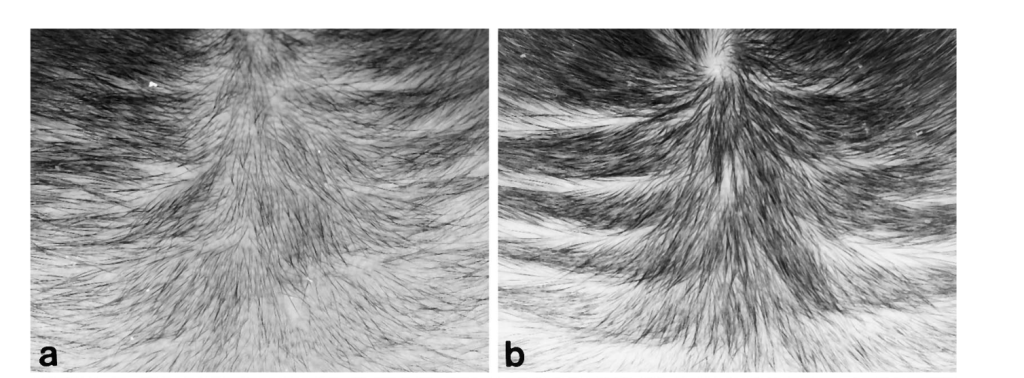
In these experiments, topical RU58841 was applied to alopecic scalp and produced concentration-dependent improvements in visible hair parameters. A lower concentration (around 0.5%) did not yield impressive results, while a higher concentration (around 5%) produced the strongest regrowth signal over months of treatment.
Reported benefits included increased hair density and hair length. Beyond surface-level changes, the primate work also reported findings that align with meaningful follicle biology improvements, including support for dermal papilla cell growth and evidence consistent with vellus-to-terminal hair conversion.
One particularly notable mechanistic detail from the macaque work is that RU58841 appeared to prevent testosterone-related androgen receptor effects in the dermal papilla. This suggests testosterone may also contribute to miniaturization through androgen receptor signaling, with the androgen receptor acting as a “lynchpin” for both testosterone and DHT in susceptible follicles.
Even so, the macaque evidence has clear constraints. Sample sizes were small, dosing equivalence to human scalp use is uncertain (vehicle, skin barrier, follicular penetration), and the overall pattern suggests benefits are linked to continued treatment rather than a permanent reversal of the underlying process.
Figure 4. Photographs showing the effects of topical RU58841 on hair growth in the bald frontal scalps of the stumptailed macaques. Increased density and length of hairs were observed in a scalp at 4 months after treatment with 5% RU58841 (b) compared with the scalp at pretreatment time (a). Adapted from Figure 3:[15]Obana, N., Chang, C., Uno, H. (1997). Inhibition Of Hair Growth By Testosterone In The Presence Of Dermal Papilla Cells From The Frontal Bald Scalp Of The Postpubertal Stumptailed Macaque. … Continue reading
Does Normal Blood Testosterone Mean It’s Truly Safe?
The primate experiments reported no meaningful change in circulating testosterone during topical RU58841 treatment. These results are often used as a surrogate for limited systemic exposure, particularly when compared with therapies designed to change systemic androgen levels.
However, unchanged serum hormones do not prove the drug is strictly confined to the scalp. Low-level systemic absorption can still occur without shifting hormone levels, and systemic androgen receptor modulation can theoretically happen even when circulating testosterone/DHT remains normal, especially if active metabolites are involved or if certain tissues are more sensitive. So while these findings are encouraging for safety, they are not definitive proof that effects are strictly confined to the application site.
Human Evidence: The Missing Link
Two human clinical trials of RU58841 (under the name PSK‑3841) were registered and marked as completed in the early 2000’s. But the interesting thing is that the results from both of these studies have never been published, leaving a critical gap between promising preclinical work and real-world clinical decision-making.
The unpublished PSK-3841 trials:
- Completion in 2002: A phase I study (ISRCTN49873657) tested a 5% PSK‑3841 solution twice daily for 4 weeks in about 30 men with androgenetic alopecia, with primary endpoints focused on safety, tolerability, endocrine profiles, plus pharmacokinetics.[16]ISRCTN Registry, (no date), ISRCTN49873657. Available at: https://www.isrctn.com/ISRCTN49873657 (Accessed: 02 January 2026)
- Completion in 2003: A larger, multi‑centre, double‑blind trial (ISRCTN71083772) then treated 120 men for 6 months with 2.5% or 5% PSK‑3841 once daily versus vehicle, measuring total and anagen hair counts, safety, tolerability, and pharmacokinetics.[17]ISRCTN Registry, (no date), ISRCTN71083772. Available at: https://www.isrctn.com/ISRCTN71083772 (Accessed: 02 January 2026)
The fact that both studies were completed, yet no peer-reviewed results or detailed reports have appeared, is a major red flag.
For a potentially first-in-class topical antiandrogen with completed phase I and II trials, companies are usually highly motivated to publish positive data. The fact that developers walked away after finishing a 6-month, 120-subject trial, without even a basic efficacy and safety paper, strongly suggests something in the data made the program unattractive to advance.
Why Unpublished Trials Change the Risk Calculation
Without access to the actual results from these clinical trials, it’s impossible to verify key parameters such as:
- How effective RU58841 was for human hair regrowth
- Types and frequency of any adverse events
- How many participants discontinued using RU58841, and the underlying reasons
- Changes in systemic hormones and sex organs
- Durability of any hair regrowth after stopping treatment
- Dose-response relationships
Missing the data from clinical studies forces a dangerous guessing game. Internet anecdotes cannot answer questions that only controlled trials can resolve, like whether rare but serious adverse events occurred, whether endocrine markers shifted subtly over months, or whether regrowth reversed after discontinuation.
Controlled trials also reveal patterns that never appear in forums, such as subgroup vulnerabilities, time-dependent toxicity, or dose thresholds where benefits collapse into harm. Without these insights, users are effectively self-experimenting with a research chemical that already failed to make it through the regulatory system.
Why Was RU58841 Abandoned?
The short answer is that nobody outside the original development team truly knows why RU58841 was abandoned.
But what we do know is that in pharmaceutical development, it is normal for early-stage compounds to fail.[18]Sun, D., Gao, W., Hu, H., Zhou, S. (2022). Why 90% Of Clinical Drug Development Fails And How To Improve It? Acta Pharmaceutica Sinica B. 12(7). 3049-3062. Available at: … Continue reading What is not normal is for companies to complete phase I and II trials and then allow all efficacy and safety data to vanish without any peer-reviewed publication, abstract, or regulatory disclosure.
Once a drug has reached a completed multi-centre, double-blind phase II study, the cost of publishing the results is trivial compared to the tens of millions already spent developing, patenting, manufacturing, and running the trials. Even negative results are typically published or disclosed.
Let’s examine the three most plausible explanations.
Hypothesis 1: Financial Reasons
The official explanation given by the companies involved was financial difficulty.
On the surface, this does sound plausible. Drug development is expensive. But when viewed in context, this explanation collapses. RU58841 had already cleared the most expensive hurdles, such as toxicology work, formulation development, and phase I and II human trials. Writing up and submitting those results would have cost a few thousand dollars at most.
It does not make sense for a company to invest tens of millions into patenting, testing, and completing two human trials, only to stop short of the final, cheapest step. For this reason, financial limitations are the weakest explanation.
Hypothesis 2: Lack of Efficacy
Another possibility is that RU58841 was not effective for hair regrowth in humans.
It is actually quite common for companies to bury disappointing studies while only publishing favorable ones. However, when it comes to RU58841, this reason is highly unlikely, mainly due to the consistent anecdotal reports across forums describing visible regrowth and stabilization with RU58841.
While anecdotes cannot replace clinical trials, the volume and consistency of regrowth reports make it unlikely that RU58841 was completely ineffective in humans. If efficacy were truly absent, the enthusiasm around this research chemical would almost certainly not have persisted throughout the past two decades.
Hypothesis 3: Safety Concerns
This leaves the most plausible explanation, something in the human data made RU58841 commercially or medically unattractive to advance.
While this hypothesis is speculative, it is supported by several warning signals. Follow-up research on RU58841 reported that at least one of its metabolites possessed strong antiandrogenic activity, undermining the original premise that the drug would only act locally in the scalp.[19]Cousty-Berlin, D., Bergaud, B., Bruyant, M.C., Battmann, T., Branche, C., Philibert, D. (1994). Preliminary Pharmacokinetics And Metabolism Of Novel Non-Steroidal Antiandrogens In The Rat: Relation … Continue reading If systemically active metabolites were forming in humans, this would fundamentally change the drug’s risk profile.
Preclinical work also raised more subtle molecular concerns that are rarely discussed online. Some androgen-receptor antagonists can exhibit something called antagonistic-agonism, where compounds designed to block the receptor can, under certain conditions, partially activate it as well.[20]Miyamoto, H., Yeh, S., Wilding, G., Chang, C. (1998). Promotion Of Agonist Activity Of Antiandrogens By The Androgen Receptor Coactivator, ARA70, In Human Prostate Cancer DU145 Cells. Proceedings of … Continue reading
This appears to involve androgen-receptor co-activators such as ARA70, which amplify androgen signaling once the receptor complex is transported into the nucleus. In experimental systems, RU58841 showed a mild but measurable ability to enhance androgen-receptor co-activation in wild-type receptors in a dose-dependent manner. [21]Miyamoto, H., Yeh, S., Wilding, G., Chang, C. (1998). Promotion Of Agonist Activity Of Antiandrogens By The Androgen Receptor Coactivator, ARA70, In Human Prostate Cancer DU145 Cells. Proceedings of … Continue reading While it remains unknown whether this effect occurs in humans or is clinically meaningful, it represents exactly the type of risk that would make long-term use of a topical antiandrogen far less predictable than originally hoped.
Additionally, it’s worth noting that some people trying RU58841 online have reported chest pain, brain fog, and other issues, such as sexual dysfunction. We have not found credible evidence that these effects are permanent, but their nature is consistent with unintended endocrine or cardiovascular involvement.
Together, the evidence is quite difficult to ignore. RU58841 may well have been effective for hair regrowth, but a combination of systemically active metabolites, loss of localization in the scalp, and early molecular signals suggesting complex androgen-receptor behavior may have made the drug too risky to advance.
If you’re a member and would like to find out more about these molecular safety mechanisms, click the link here for more details in our RU58841 Ultimate Guide
Final Thoughts
So is RU58841 legit for efficacy? Plausibly yes. Given the preclinical data and sheer volume of regrowth anecdotes, RU58841 does likely block androgen signaling in the scalp.
But is RU58841 legit as a recommended treatment? No. Its safety profile is still a clinical mystery. Its human trials were never published, and the drug was abandoned at a stage where most drugs either move forward or fail. The gap in clinical information changes the entire risk calculation.
In our opinion, evidence quality and long-term safety hold more weight than exciting anecdotes.
For individuals who cannot tolerate finasteride, there are topical antiandrogen options with more transparent development histories and human data, including topical fluridil, topical CB-03-03, topical spironolactone, and newer molecules like pyrilutamide that are currently progressing through regulated clinical trials. While none are perfect, they at least exist within a framework of published safety monitoring.
If you’d like to learn more about pyrilutamide, read our article here, which covers all of the publicly available data on pyrilutamide and evaluates its mechanisms of action, efficacy, and safety.
We recommend proceeding with extreme caution when using RU58841, and only use it if you understand and are willing to accept the absence of human safety and efficacy data.
The best practice is to prioritize therapies with published human trials and regulatory oversight. Ultimately, given the lack of safety information and regulatory oversight for RU58841, we recommend erring on the side of caution.
References[+]
References ↑1 Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, Androgenetic Alopecia and Hirsutism. J Steroid Biochem Mol Biol. 48(1). 55-60. Available at: https://doi.org/10.1016/0960-0760(94)90250-x ↑2 Ho, C.H., Sood, T., Zito, P.M. (2024). Androgenetic Alopecia. In: StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430924/ ↑3 Wikipedia, (26 December 2018), RU-58841. Available at: https://en.wikipedia.org/wiki/RU-58841#/media/File:RU-58841_structure.svg (Accessed: 02 January 2026) ↑4, ↑16 ISRCTN Registry, (no date), ISRCTN49873657. Available at: https://www.isrctn.com/ISRCTN49873657 (Accessed: 02 January 2026) ↑5, ↑17 ISRCTN Registry, (no date), ISRCTN71083772. Available at: https://www.isrctn.com/ISRCTN71083772 (Accessed: 02 January 2026) ↑6, ↑7, ↑8, ↑9, ↑12, ↑13 Battmann, T., Bonfils, A., Branche, C., Humbert, J., Goubet, F., Teutsch, G., Philibert, D. (1994). RU 58841, A New Specific Topical Antiandrogen: A Candidate of Choice for the Treatment of Acne, Androgenetic Alopecia and Hirsutism. J Steroid Biochem Mol Biol. 48(1). 55–60. Available at: https://doi.org/10.1016/0960-0760(94)90250-x ↑10 Matias, J.R., Gaillard, M. (1995). Local Inhibition Of Sebaceous Gland Growth By Topically Applied RU 58841. Annals of the New York Academy of Sciences. 761. 56-65. Available at: https://doi.org/10.1111/j.1749-6632.1995.tb31369.x ↑11 Matias, J.R., Gaillard, M. (1995). Local Inhibition Of Sebaceous Gland Growth By Topically Applied RU 58841. Annals of the New York Academy of Sciences. 761. 56-65. Available at: https://doi.org/10.1111/j.1749-6632.1995.tb31369.x ↑14 Obana, N., Chang, C., Uno, H. (1997). Inhibition Of Hair Growth By Testosterone In The Presence Of Dermal Papilla Cells From The Frontal Bald Scalp Of The Postpubertal Stumptailed Macaque. Endocrinology. 138(1). 356-361. Available at: https://doi.org/10.1210/endo.138.1.4890 ↑15 Obana, N., Chang, C., Uno, H. (1997). Inhibition Of Hair Growth By Testosterone In The Presence Of Dermal Papilla Cells From The Frontal Bald Scalp Of The Postpubertal Stumptailed Macaque. Endocrinology. 138(1). 356-361. Available at: https://doi.org/10.1210/endo.138.1.4890 ↑18 Sun, D., Gao, W., Hu, H., Zhou, S. (2022). Why 90% Of Clinical Drug Development Fails And How To Improve It? Acta Pharmaceutica Sinica B. 12(7). 3049-3062. Available at: https://doi.org/10.1016/j.apsb.2022.02.002 ↑19 Cousty-Berlin, D., Bergaud, B., Bruyant, M.C., Battmann, T., Branche, C., Philibert, D. (1994). Preliminary Pharmacokinetics And Metabolism Of Novel Non-Steroidal Antiandrogens In The Rat: Relation Of Their Systemic Activity To The Formation Of A Common Metabolite. Journal of Steroid Biochemistry and Molecular Biology. 51(1-2). 47-55. Available at: https://doi.org/10.1016/0960-0760(94)90114-7 ↑20, ↑21 Miyamoto, H., Yeh, S., Wilding, G., Chang, C. (1998). Promotion Of Agonist Activity Of Antiandrogens By The Androgen Receptor Coactivator, ARA70, In Human Prostate Cancer DU145 Cells. Proceedings of the National Academy of Sciences of the United States of America. 95(13). 7379-7384. Available at: https://doi.org/10.1073/pnas.95.13.7379 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sophie Grice, PhD
Sophie completed a BSc in Pharmacology before earning a PhD in Immunopharmacology at the University of Liverpool. Her doctoral research examined drug hypersensitivity reactions in patients treated with immune checkpoint inhibitors. She later pursued postdoctoral research focused on T cell mediated immune responses, with an emphasis on the immunogenicity of gene therapies.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down