- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesOral vs. Topical Minoxidil: What’s Better for Hair Loss?
First Published Jan 9 2026Last Updated Jan 12 2026Pharmaceutical Researched & Written By:Michael Williams, PhD
Researched & Written By:Michael Williams, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor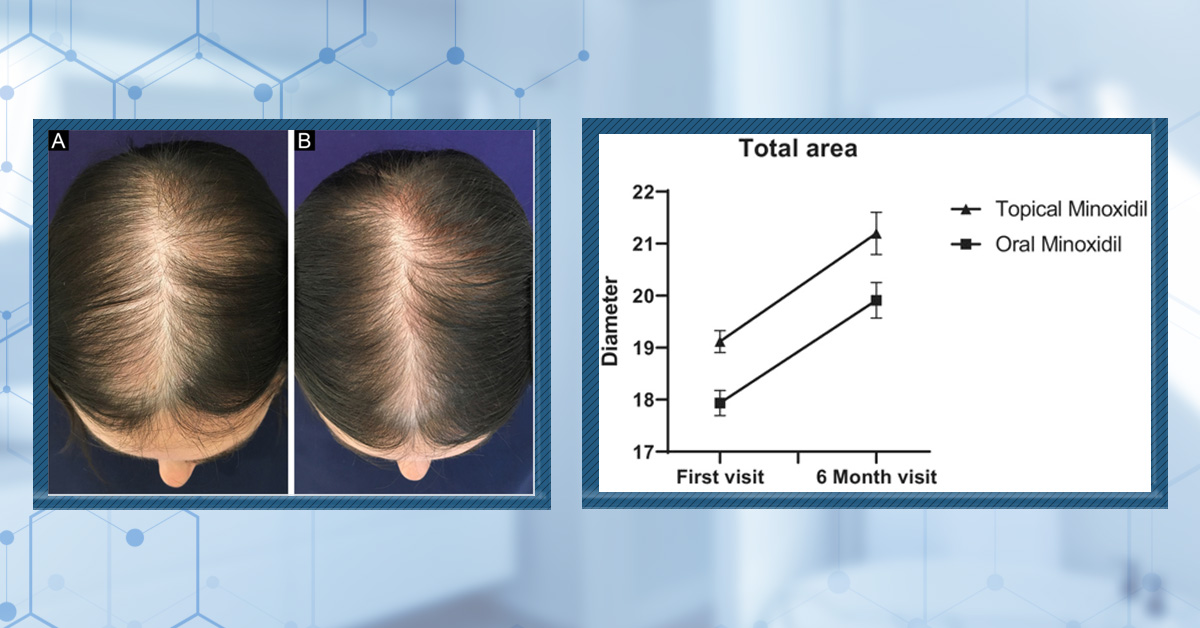
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Oral vs topical minoxidil for hair loss: which works better, and at what cost? We break down the evidence on effectiveness, safety, dosing, and real-world tradeoffs to help you choose the right option.
Full Article
Minoxidil has been a cornerstone of hair-loss treatment for decades, and there are now more options than ever for users. Alongside traditional topical solutions and foams, low-dose oral minoxidil has emerged as a popular off-label alternative.
Which raises the question: which formulation is the best choice for hair loss? And which option comes with the fewest side effects? In this article, we break down how oral and topical minoxidil compare across mechanisms, evidence, side effects, and real-world use, so you can make an informed decision about which approach fits your hair loss pattern, risk tolerance, and lifestyle.
Interested in Topical Minoxidil?
High-strength topical minoxidil available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

How Does Minoxidil Work?
Despite being used to treat hair loss for over 40 years, the mechanisms through which minoxidil promotes hair growth are only partially understood. We know that it alters the hair cycle, shortening the resting (telogen) phase so follicles enter the growth (anagen) phase earlier. This increases the number of growing hairs at any one time. It also increases follicle size, which translates into thicker, longer hair shafts.[1]Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. *British Journal of Dermatology.* 150(2). 186–194. Available at: … Continue reading
Minoxidil can increase blood flow to hair follicles, which increases the flow of oxygen and nutrients to support growth. The drug is also known to activate the Wnt/ꞵ-catenin signaling pathway, which supports the growth of cells in the follicle, and provides cytoprotective effects and regulates local inflammation.
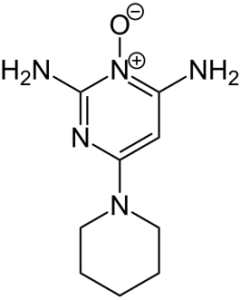
Figure 1. Structure of minoxidil.[2]https://commons.wikimedia.org/wiki/File:Minoxidil_Structural_Formula_V1.svg) Image in the Public Domain.
In contrast to antiandrogenic drugs like finasteride and dutasteride, minoxidil doesn’t impact hormones.
Before we compare topical and oral treatments, we’ll look at the formulations separately to understand the options available for each.
Topical Minoxidil
Topical minoxidil is designed to work primarily at the scalp, not systemically. After you apply it, only a small fraction of the dose actually penetrates intact skin, and an even smaller fraction reaches the follicle structures where it matters most.
Where it does work is at (or near) the follicle, particularly around the outer root sheath and dermal papilla. That’s why topical minoxidil has a strong safety record: most of the drug never leaves the scalp in meaningful quantities.
Why Some People Respond Better to Topical Minoxidil
Minoxidil relies on activating enzymes to work, most notably sulfotransferase SULT1A1. SULT1A1 activity in the hair follicles is closely linked to how well topical minoxidil works, and people with lower levels of activity tend to see less benefit from treatment.[3]Pietrauszka, K., & Bergler-Czop, B. (2022). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: … Continue reading
Because minoxidil needs SULT1A1 to work, people with low levels of the enzyme in the hair follicle might not see much improvement from minoxidil (“enzyme low-responders”).
The effectiveness of topical minoxidil also depends on a range of other factors, including the type of formulation, scalp health, contact time, application technique, and the use of penetration enhancers. This is one reason why topical minoxidil performs reliably in clinical trials, but users find results more variable.
Typical Dosing: How Much and How Often?
Most over-the-counter minoxidil is sold in 2% or 5% formulations. 5% generally produces stronger average results and shows higher satisfaction in many studies. It also comes with a slightly higher irritation risk, especially in solution form. As such, 2% formulations are often used when scalp irritation from higher doses is too disruptive, or for milder cases.[4]Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical … Continue reading
Higher concentrations (7–15%) are also sometimes considered, but they offer diminishing returns for many users and a higher risk of irritation. Because of this, they’re usually reserved for people who don’t respond to standard options and can tolerate the vehicle.
Dosing frequency is about balancing efficacy with adherence: there’s no benefit to be gained from planning a treatment schedule that you can’t stick to.
Twice-daily use is the traditional standard for maximum effect, especially in people using solutions. Once-daily 5% is often good enough for many people, and can dramatically improve adherence, although studies have shown that switching to twice to once daily can slightly worsen outcomes. [5]Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). … Continue reading
Similarly, in women, once-daily 5% foam can perform similarly to twice-daily 2% solution while being easier to sustain. Foam formulations are often recommended for women because foam tends to stay localized on the scalp and drip down less onto the face than solutions. This reduces the chance of unwanted facial hair growing as a result of treatment. Evidence also suggests that foam formulations have lower systemic absorption into the bloodstream.[6]Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., & Garcia Bartels, N. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in … Continue reading,[7]Gogtay, J. A., & Panda, M. (2009). Minoxidil topical foam: a new kid on the block. *International Journal of Trichology.* 1(2). 142. Available at: https://doi.org/10.4103/0974-7753.58560
Limitations of Topical Minoxidil
Topical minoxidil works, but it requires consistency. Results typically require months of consistent use before they become apparent, and many people struggle to maintain the routine, especially with twice-daily application.
Absorption of topical solutions can also be inconsistent, and is impacted by scalp condition, contact time, and the type of vehicle used (propylene glycol-based solutions, foams, propylene glycol-free lotions, nanoemulsions, etc.). As such, even with consistent application, results can vary.
The main side effects associated with topical minoxidil are local rather than systemic. Common issues include itching, dryness, flaking, and contact dermatitis, which are often driven by the formulation rather than the drug itself. Many users also find the product cosmetically inconvenient due to a greasy feel, visible residue, or interference with hairstyling, particularly for those with longer hair.[8]Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). … Continue reading
Evidence for Topical Minoxidil
The use of topical minoxidil is backed up by substantial clinical data showing increased hair counts, density, and coverage in androgenic alopecia (AGA) and other alopecias, with response in a majority but not all patients. We’ll look at evidence from clinical trials involving men and women separately, as they are typically studied in different clinical trials.
Evidence in Men
The earliest significant clinical evidence supporting the use of minoxidil for hair loss is from a 1985 trial, which compared 2% and 3% solutions to placebo in 126 men with early male pattern baldness. They assessed changes in vellus hairs and terminal hairs, an important distinction as vellus hairs are fine, light colored hairs that don’t contribute to the overall appearance of hair thickness.[9]Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). … Continue reading
They found a significant increase in terminal hair counts in 2% and 3% minoxidil-treated participants, with a greater improvement in the 3% group, though this was not statistically significant.
The study methodology switched the placebo group to 3% minoxidil after 4 months and showed that the switch increased hair counts. Unfortunately, this means that it’s not possible to assess actual changes in hair count over the 12-month study. Notably, the placebo group also showed increases in hair count during the first 4 months, which might not be expected in men with pattern baldness or AGA and could cast doubt on the hair counts.
Larger trials have been conducted since. A 48 week study compared 2% and 5% solutions to placebo and found approximately 13% and 19% increases in nonvellus hairs respectively, although hair counts did appear to start to drop after week 16.[10]Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical … Continue reading
Similarly, a Japanese study compared 1% and 5% formulations, and found a 26.4% increase in nonvellus hair count in the 5% topical minoxidil group and 21.2% increase in the 1% group.[11]Tsuboi, R., Arano, O., Nishikawa, T., Yamada, H., & Katsuoka, K. (2009). Randomized clinical trial comparing 5% and 1% topical minoxidil for the treatment of androgenetic alopecia in Japanese … Continue reading
Notably, these trials tend to be relatively short, with many lasting only 16 weeks. Even in these short trials, there is a noticeable drop-off in hair counts after an initial growth boost. Data suggests that topical minoxidil response rates are high in the first 3-6 months, but drop after a year or longer of use. Real-world data show that 86–95% of users discontinue topical minoxidil within one year, most commonly because the results fall short of their expectations for visible cosmetic improvement.[12]Olsen, E. A., Weiner, M. S., Amara, I. A., & DeLong, E. R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. *Journal of the American Academy of … Continue reading,[13]Shadi, Z. (2023). Compliance to topical minoxidil and reasons for discontinuation among patients with androgenetic alopecia. *Dermatology and Therapy.* 13(5). 1157–1169. Available at: … Continue reading
If you’re interested in topical minoxidil for men, check out our article on the best products currently available.
Evidence in Women
Most clinical trials evaluating topical minoxidil in women have traditionally used a 2% solution applied twice daily. A meta-analysis, which combines the results from multiple trials found that 2% minoxidil produced an average increase of about 12 hairs per square centimeter compared with placebo after 24 weeks of treatment.[14]Adil, A., & Godwin, M. (2017). The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. *Journal of the American Academy of Dermatology.* 77(1). … Continue reading
Data also suggest that higher-strength formulations offer similar outcomes: both 5% minoxidil solution used twice daily and 5% minoxidil foam applied once daily have shown no significant difference in efficacy compared with twice-daily 2% solution use.[15]Lucky, A. W., Piacquadio, D. J., Ditre, C. M., Dunlap, F., Kantor, I., Pandya, A. G., Savin, R. C., & Tharp, M. D. (2004). A randomized, placebo-controlled trial of 5% and 2% topical minoxidil … Continue reading
As we’ve already noted, foams are often preferred by women as they can potentially limit off-target hair growth. A large, 24-week trial demonstrated that foam minoxidil formulation resulted in 9.1 hairs more per cm2 more than a placebo (foam without minoxidil), with no significant differences in irritation.[16]Lucky, A. W., Piacquadio, D. J., Ditre, C. M., Dunlap, F., Kantor, I., Pandya, A. G., Savin, R. C., & Tharp, M. D. (2004). A randomized, placebo-controlled trial of 5% and 2% topical minoxidil … Continue reading
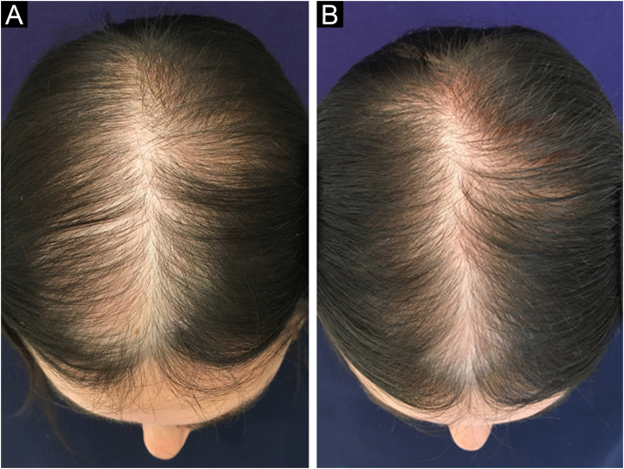
Figure 2. Improvement in hair coverage in a woman with pattern hair loss following 6 months 5% topical minoxidil treatment. Adapted from Figure 1.[17]Ramos, P. M., Melo, D. F., Radwanski, H., Cortez de Almeida, R. F., & Miot, H. A. (2023). Female-pattern hair loss: therapeutic update. *Anais Brasileiros de Dermatologia.* 98. 506–519. … Continue reading Image used under Creative Commons License.
Oral Minoxidil
Unlike topical minoxidil, oral minoxidil is absorbed through the gastrointestinal tract and converted into its active form primarily in the liver, where sulfotransferase activity is abundant. This systemic activation largely bypasses one of the major limitations of topical therapy: variations in follicular enzyme activity. As a result, nearly all ingested minoxidil is activated and made bioavailable, which helps explain why oral formulations can work even in people who do not respond well to topical formulations.
Typical Dosing
Minoxidil was originally developed to treat hypertension, and it is still used for the condition today. Dosage for hair loss is much lower than that for hypertension, and most regimens fall within the low-dose range of 0.25 to 5 mg per day.
Within this range, efficacy appears to be dose-dependent. In men, doses between 2.5 and 5 mg daily are commonly used and tend to produce the most robust regrowth.[18]Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: … Continue reading,[19]Jimenez-Cauhe, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O. M., Fernandez-Nieto, D., & Vaño-Galvan, S. (2019). Effectiveness and safety of low-dose oral … Continue reading
However, lower doses may still be effective for slowing progression or improving density in milder cases. In women, lower doses (0.25 to 1.25 mg daily) are preferred, as they balance efficacy with a lower risk of unwanted body hair growth and fluid retention.[20]Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse … Continue reading
To find out more about minoxidil dosing, check out our article.
Oral Minoxidil Side Effects
Because oral minoxidil is systemically active, its side effects differ from those of topical formulations. Still, at the low doses used for hair loss, oral minoxidil is generally well tolerated, and most adverse effects are reversible with adjustment or discontinuation.
The most commonly reported side effect is unwanted hair growth outside of the scalp, including on the face and arms. While this effect is often mild and reversible, it is often more of an issue for women and becomes more likely as the dose increases.[21]Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair … Continue reading
Another relatively common effect is fluid retention, which may present as mild ankle or lower-leg swelling. This occurs because minoxidil is a vasodilator and can promote sodium and water retention. Some patients also report lightheadedness or dizziness, particularly when starting treatment or increasing the dose.
Less commonly, patients may experience palpitations or changes in heart rate. These symptoms are usually short-lasting and improve with dose reduction. Importantly, serious cardiovascular complications are rare at doses of 0.25-5 mg daily and have been reported primarily in higher-dose antihypertensive use or in individuals with underlying heart disease.[22]Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse … Continue reading
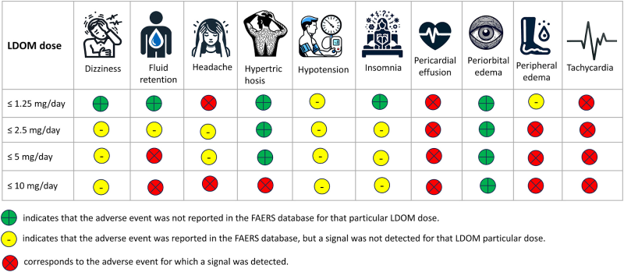
Figure 3. Occurrence of adverse events in individuals taking low-dose oral minoxidil (LDOM), as reported in the FDA Adverse Event Reporting System. Adapted from Figure 1.[23]Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse … Continue reading Image used under Creative Commons License.
If you’re concerned about minoxidil side effects, check out our article on 10 ways to reduce them.
Evidence in Men
Multiple studies in men with AGA show consistent increases in total hair count and visible thickening, with reported response rates ranging from roughly 60% at very low doses (0.25 mg) to 90–100% at doses between 2.5 and 5 mg daily over 6-12 months.
The 60% response at 0.25 mg comes from a 2020 retrospective study of 25 men, looking at the effect of very-low-dose minoxidil. While there was a 60% response rate, there wasn’t any meaningful increase in terminal hair count on average.[24]Pirmez, R., & Salas-Callo, C. I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: a study with quantitative trichoscopic documentation. *Journal of the American Academy of … Continue reading
At higher doses, response rates are higher, and hair growth is more predictable. A 24-week study using 5 mg minoxidil daily found a 19% increase in hair count. An open-label study using 5 mg minoxidil, without a placebo control, found increases in hair count and improvements in photographic assessment at 12 and 24 weeks. They also found a 19% improvement in hair counts compared to baseline.[25]Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: … Continue reading,[26]Panchaprateep, R., & Lueangarun, S. (2020). Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global … Continue reading
A retrospective study of only 41 men found a response rate of 90%, though changes were not well quantified.[27]Jimenez-Cauhe, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O. M., Fernandez-Nieto, D., & Vaño-Galvan, S. (2019). Effectiveness and safety of low-dose oral … Continue reading
In these studies, average gains included double-digit percentage increases in hair count and noticeable improvements on global photographic assessment, indicating that oral minoxidil can produce cosmetically relevant changes rather than purely statistical ones.
Evidence in Women
In female pattern hair loss (FPHL), oral minoxidil at doses between 0.25 and 1.25 mg daily has demonstrated efficacy comparable to 5% topical minoxidil, with the added benefit of avoiding scalp irritation. Combination approaches appear particularly promising: studies pairing low-dose oral minoxidil with spironolactone report earlier reductions in shedding and greater density gains than either agent alone, although direct head-to-head comparisons remain limited.[28]Silva, M. N. E., Ramos, P. M., Silva, M. R., Silva, R. N. E., & Raposo, N. R. B. (2022). Randomized clinical trial of low-dose oral minoxidil for the treatment of female pattern hair loss: 0.25 … Continue reading,[29]Sinclair, R. D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. *International Journal of Dermatology.* 57(1). … Continue reading
Oral Minoxidil in Special Populations
Evidence also supports oral minoxidil’s utility in several special populations. In people with diffuse thinning, including chronic telogen effluvium, low-dose oral minoxidil has been associated with reduced shedding and gradual improvements in density over 6–12 months.[30]Perera, E., & Sinclair, R. (2017). Treatment of chronic telogen effluvium with oral minoxidil: a retrospective study. *F1000Research.* 6. 1650. Available at: … Continue reading
While these studies are largely retrospective and lack placebo controls, the consistency of improvement across patients suggests a real biological effect, especially given minoxidil’s known ability to shorten telogen and promote anagen entry.
In alopecia areata, oral minoxidil alone produces modest response rates, but when added to immunomodulatory agents such as tofacitinib, it appears to substantially enhance regrowth without requiring higher doses of the primary drug.[31]Wambier, C. G., Craiglow, B. G., & King, B. A. (2021). Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. *Journal of the American Academy of Dermatology.* 85(3). … Continue reading
Oral vs. Topical Minoxidil: Direct Comparison
Due to differences in study design and the range of doses and formulations used, it is difficult to compare the results of studies on oral and topical minoxidil. Luckily, a few trials have compared the two treatment routes directly.
Comparison Study #1
The only direct comparison of the efficacy of topical and oral minoxidil in men comes from a 2024 randomized placebo-controlled trial comparing a 5 mg oral dose (once daily) and 5% topical formulation (twice daily) for 24 weeks. They found no significant difference between the two formulations in hair, though there was a 20% increase in hair density in the oral group compared to 7% increase in the topical group. Photographic assessment indicated a significantly greater improvement in the oral group at the crown.[32]Penha, M. A., Miot, H. A., Kasprzak, M., & Ramos, P. M. (2024). Oral minoxidil vs topical minoxidil for male androgenetic alopecia: a randomized clinical trial. *JAMA Dermatology.* 160(6). … Continue reading
There was a higher rate of adverse events in the oral treatment group, though this was largely due to increased hair growth in other parts of the body. Participants taking oral minoxidil experienced significantly more headaches, while the topical group experienced scalp irritation more frequently, as we would expect.
Comparison Study #2
There are more head-to-head comparisons of oral and topical formulations in women. The first direct comparison of oral and topical minoxidil for female-pattern hair loss comes from a 24-week randomized, open comparative study conducted in Brazil. The trial compared low-dose oral minoxidil (1 mg once daily) with topical minoxidil 5% solution applied once daily in women aged 18–65 years.[33]Ramos, P. M., Melo, D. F., Radwanski, H., Cortez de Almeida, R. F., & Miot, H. A. (2023). Female-pattern hair loss: therapeutic update. *Anais Brasileiros de Dermatologia.* 98. 506–519. … Continue reading
After 24 weeks, terminal hair density increased by 12% in the oral minoxidil group and by 7.2% in the topical group. This difference did not reach statistical significance . Secondary outcomes, including hair density, global photographic assessment by dermatologists, quality-of-life scores, and hair shedding, were also evaluated, with no significant between-group differences reported.
Hypertrichosis (hair growth on other parts of the body) was reported in 27% of the oral group, compared to 4% in the topical group. This lower incidence of adverse events in the oral group in this trial compared to the study #1 is likely due to lower doses used.
Comparison Study #3
In a 6-month trial, both treatment groups demonstrated a statistically significant increase in hair diameter. Again, no significant differences between topical and oral minoxidil were reported. Photographic assessment showed significant improvement in hair densityn the topical minoxidil group, whereas no significant improvement was detected in the oral minoxidil group; however, between-group differences were not statistically significant.[34]Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled … Continue reading
Patient satisfaction exceeded 60% in both groups, with no significant difference reported. Both treatments were well tolerated, and no major safety concerns were identified, though two patients in the oral group experienced hypertrichosis on their face and extremities.
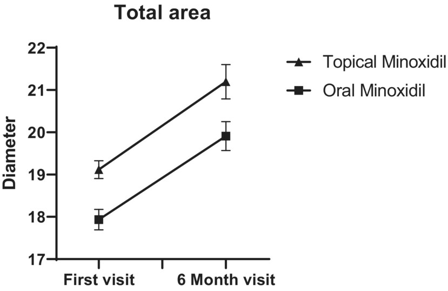
Figure 4. Improvements in average hair diameter were comparable between 5% topical solution or 1 mg/day oral minoxidil over a six-month study period. Adapted from Figure 2.[35]Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled … Continue reading Image used under Creative Commons License.
Comparison Study #4
Our final study again showed no significant difference between the two groups. This study used lower doses than previously seen: 0.25 mg daily with a topical placebo or topical minoxidil 2% solution with an oral placebo for 9 months.[36]Vahabi-Amlashi, S., Layegh, P., Kiafar, B., Hoseininezhad, M., Abbaspour, M., Hajebi Khaniki, S., Forouzanfar, M., & Sabeti, V. (2021). A randomized clinical trial on therapeutic effects of 0.25 … Continue reading
In the oral minoxidil group, average hair density increased from 102/cm² to 115/cm² (1.12% increase), while in the topical minoxidil group, hair density increased from 107/cm² to 113/cm² (1.06%).
These improvements are modest compared to those we have seen previously, likely due to the lower doses used. This is also reflected in the very low incidence of side effects reported.
Meta-Analysis
Meta-analyses compile numerous studies together to see if there are consistent results across different research groups and trials. A recent meta-analysis comparing topical and oral minoxidil found that there was no significant difference between the two approaches.[37]Fazal, F., Malik, B. H., Malik, H. M., Sabir, B., Mustafa, H., Ahmed, M., Abid, A., Adil, M. L., Shafi, U., & Saad, M. (2025). Can oral minoxidil be the game changer in androgenetic alopecia? A … Continue reading
This finding is apparent in the studies we’ve looked at: there are some cases where oral treatment seems to provide better results, but this is not large or consistent enough to be significant when statistical analyses are performed.
Given the trend towards greater improvements in oral formulations, it is possible that larger, longer-term studies might find significant differences. However, on an individual basis, these small, population-scale differences are unlikely to be large enough to influence treatment decisions.
Is Topical Minoxidil Safer?
Both formulations are well tolerated across studies, with the most common reported side effect for topical formulations being scalp irritation. We have seen a higher rate of side effects in groups taking oral minoxidil, though this is most commonly due to increased hypertrichosis, which may be more of a concern for women.
More serious concerns around the use of oral minoxidil are related to cardiovascular events. A large-scale retrospective study assessed the safety of 1404 people taking oral minoxidil at a range of low doses appropriate for hair loss.[38]Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair … Continue reading
Apart from hypertrichosis, adverse events were rare, with lightheadedness reported in 1.7% of participants and tachycardia (fast heart rate) reported in 0.9%. No life‑threatening cardiovascular events were reported.
Therefore, while there is an increased risk of more serious side effects in oral formulations, at the low doses used for hair loss, there is minimal risk of adverse events.
Adherence and Practical Considerations
One of oral minoxidil’s most compelling advantages is its performance in people who do not respond adequately to topical therapy. Because topical minoxidil requires local scalp activation by the sulfotransferase enzyme SULT1A1, and because only a small fraction of applied drug penetrates the skin, many users see limited benefit despite correct use. Oral minoxidil bypasses both of these bottlenecks by being converted to its active sulfate form in the liver and delivered systemically, ensuring uniform exposure to hair follicles across the scalp.
As well as efficacy, there are some other practical considerations that should influence your decisions when comparing oral and topical minoxidil.
Ease of Use
From a day-to-day standpoint, oral and topical minoxidil place very different demands on the user. Oral minoxidil is taken as a pill, usually once daily, or split into morning and evening doses, which makes it largely invisible in daily life. There is no impact on hairstyling, no waiting period before washing, and no concern about residue, odor, or transfer to pillows, clothing, or pets. For many people, this simplicity is the primary reason oral minoxidil feels sustainable.
Topical minoxidil, by contrast, requires direct scalp application once or twice daily, ideally to a dry scalp, with sufficient contact time before washing. Proper use often means parting hair, targeting thinning areas precisely, and tolerating cosmetic downsides such as greasiness, stiffness, or flaking. While none of these steps are difficult in isolation, the cumulative friction can become a barrier over months and years of use.
Cost and Access
Topical minoxidil has a clear accessibility advantage: it is available over the counter in standard 2% and 5% formulations, allowing immediate initiation without a prescription. For many users, this makes topical therapy an easy first-line option. Basic formulations are relatively inexpensive on a monthly basis, especially when purchased in bulk.
Oral minoxidil is prescription-only and used off-label for hair loss. This introduces an additional step and sometimes variability in access depending on region, provider comfort, and insurance coverage. That said, once prescribed, low-dose oral minoxidil is often comparable in cost and, in some cases, less expensive over time than higher-strength or specialty topical formulations.
Who Should Choose What?
Choosing between oral and topical minoxidil isn’t really about which one is “stronger” on paper. Rather, it’s important to consider which one you’re most likely to tolerate and use consistently.
Who Topical Minoxidil Is Best For
Risk-averse individuals – Topical minoxidil remains the best first-line choice for many people because it offers meaningful benefit with minimal systemic exposure. If you want the lowest-risk long-term approach and are comfortable with scalp application, topical is usually the most conservative starting point.
Cardiovascular contraindications (or uncertainty) – Anyone with a history of cardiovascular disease, kidney issues, fluid retention, low blood pressure, or unexplained palpitations should be cautious with oral minoxidil and involve a clinician if considering it. Topical use is generally preferred in these cases because systemic absorption is far lower and systemic side effects are much less common.
Localized thinning – If hair loss is limited to smaller areas (early crown thinning, mild recession, small patches of miniaturization), topical minoxidil can be a good fit. It allows you to treat targeted regions without committing to systemic exposure, and it can work well when the application is straightforward and consistent.
Who Oral Minoxidil Is Best For
Poor topical responders – If you’ve used topical minoxidil correctly for 6-12 months and seen little to no improvement, oral minoxidil may be a logical next step. Topical therapy can fail even with perfect effort due to low follicular sulfotransferase activity or limited penetration through the scalp barrier. Oral minoxidil bypasses both issues by being activated systemically and delivered more uniformly to follicles.
Diffuse or advanced thinning – Oral minoxidil can be especially appealing when thinning is widespread across the scalp (rather than concentrated at the crown or hairline), because systemic delivery doesn’t depend on targeting specific areas. It also tends to be easier to scale for advanced loss, where topical application becomes time-consuming and difficult to do thoroughly.
Convenience-focused patients – If the routine topical treatment has been a major barrier, or if you experience issues with messiness, residue, or styling disruption, oral minoxidil is often more sustainable.
Final Thoughts
There isn’t a universally superior option between oral and topical minoxidil; it’s just a matter of different trade-offs. Oral minoxidil tends to produce more consistent regrowth for many users because it avoids the two biggest bottlenecks of topical therapy: variable absorption and variable enzyme activation. The cost of that consistency is higher systemic exposure, which increases the likelihood of side effects like hypertrichosis, fluid retention, dizziness, or palpitations, even if serious complications remain rare at low doses.
Topical minoxidil offers a safer long-term profile, wide accessibility, and decades of clinical data, but its outcomes are more variable in real life. The same factors that make it safe, local delivery, and limited systemic absorption, also limit predictability, especially when adherence slips or the scalp barrier and follicular activation aren’t favorable.
In the end, the best outcomes come from individualized treatment plans that match the patient’s biology, preferences, and ability to stay consistent. Minoxidil can be a powerful tool either way, and the version you can tolerate and actually use is the one most likely to work.
References[+]
References ↑1 Messenger, A. G., & Rundegren, J. (2004). Minoxidil: mechanisms of action on hair growth. *British Journal of Dermatology.* 150(2). 186–194. Available at: https://doi.org/10.1111/j.1365-2133.2004.05785.x ↑2 https://commons.wikimedia.org/wiki/File:Minoxidil_Structural_Formula_V1.svg) ↑3 Pietrauszka, K., & Bergler-Czop, B. (2022). Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. *Advances in Dermatology and Allergology / Postępy Dermatologii i Alergologii.* 39(3). 472–478. Available at: https://doi.org/10.5114/ada.2020.99947 ↑4, ↑10 Olsen, E. A., Dunlap, F. E., Funicella, T., Koperski, J. A., Swinehart, J. M., Tschen, E. H., & Trancik, R. J. (2002). A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. *Journal of the American Academy of Dermatology.* 47(3). 377–385. Available at: https://doi.org/10.1067/mjd.2002.124088 ↑5 Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). 688–695. Available at: https://doi.org/10.1016/S0190-9622(87)70089-9 ↑6 Blume-Peytavi, U., Hillmann, K., Dietz, E., Canfield, D., & Garcia Bartels, N. (2011). A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. *Journal of the American Academy of Dermatology.* 65(6). 1126–1134. Available at: https://doi.org/10.1016/j.jaad.2010.09.724 ↑7 Gogtay, J. A., & Panda, M. (2009). Minoxidil topical foam: a new kid on the block. *International Journal of Trichology.* 1(2). 142. Available at: https://doi.org/10.4103/0974-7753.58560 ↑8, ↑9 Olsen, E. A., DeLong, E. R., & Weiner, M. S. (1987). Long-term follow-up of men with male pattern baldness treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 16(3). 688–695. Available at: https://doi.org/10.1016/S0190-9622(87)70089-9 ↑11 Tsuboi, R., Arano, O., Nishikawa, T., Yamada, H., & Katsuoka, K. (2009). Randomized clinical trial comparing 5% and 1% topical minoxidil for the treatment of androgenetic alopecia in Japanese men. *The Journal of Dermatology.* 36(8). 437–446. Available at: https://doi.org/10.1111/j.1346-8138.2009.00673.x ↑12 Olsen, E. A., Weiner, M. S., Amara, I. A., & DeLong, E. R. (1990). Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. *Journal of the American Academy of Dermatology.* 22(4). 643–646. Available at: https://doi.org/10.1016/0190-9622(90)70089-Z ↑13 Shadi, Z. (2023). Compliance to topical minoxidil and reasons for discontinuation among patients with androgenetic alopecia. *Dermatology and Therapy.* 13(5). 1157–1169. Available at: https://doi.org/10.1007/s13555-023-00919-x ↑14 Adil, A., & Godwin, M. (2017). The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. *Journal of the American Academy of Dermatology.* 77(1). 136–141. Available at: https://doi.org/10.1016/j.jaad.2017.02.054 ↑15 Lucky, A. W., Piacquadio, D. J., Ditre, C. M., Dunlap, F., Kantor, I., Pandya, A. G., Savin, R. C., & Tharp, M. D. (2004). A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. *Journal of the American Academy of Dermatology.* 50(4). 541–553. Available at: https://doi.org/10.1016/j.jaad.2003.06.014 ↑16 Lucky, A. W., Piacquadio, D. J., Ditre, C. M., Dunlap, F., Kantor, I., Pandya, A. G., Savin, R. C., & Tharp, M. D. (2004). A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. *Journal of the American Academy of Dermatology.* 50(4). 541–553. Available at: https://doi.org/10.1016/j.jaad.2003.06.014 ↑17, ↑33 Ramos, P. M., Melo, D. F., Radwanski, H., Cortez de Almeida, R. F., & Miot, H. A. (2023). Female-pattern hair loss: therapeutic update. *Anais Brasileiros de Dermatologia.* 98. 506–519. Available at: https://doi.org/10.1016/j.abd.2022.09.006 ↑18 Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: https://doi.org/10.1016/j.jaad.2015.02.466 ↑19, ↑27 Jimenez-Cauhe, J., Saceda-Corralo, D., Rodrigues-Barata, R., Hermosa-Gelbard, A., Moreno-Arrones, O. M., Fernandez-Nieto, D., & Vaño-Galvan, S. (2019). Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. *Journal of the American Academy of Dermatology.* 81(2). 648–649. Available at: https://doi.org/10.1016/j.jaad.2019.04.054 ↑20, ↑22 Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse Event Reporting System (FAERS) with a focus on pericardial effusions. *Journal of Cosmetic Dermatology.* 24(1). e16574. Available at: https://doi.org/10.1111/jocd.16574 ↑21, ↑38 Vañó-Galván, S., Pirmez, R., Hermosa-Gelbard, A., Moreno-Arrones, Ó. M., Saceda-Corralo, D., Rodrigues-Barata, R., Jimenez-Cauhe, J., et al. (2021). Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. *Journal of the American Academy of Dermatology.* 84(6). 1644–1651. Available at: https://doi.org/10.1016/j.jaad.2021.02.054 ↑23 Gupta, A. K., Bamimore, M. A., Abdel-Qadir, H., Williams, G., Tosti, A., Piguet, V., & Talukder, M. (2025). Low-dose oral minoxidil and associated adverse events: analyses of the FDA Adverse Event Reporting System (FAERS) with a focus on pericardial effusions. *Journal of Cosmetic Dermatology.* 24(1). e16574. Available at: https://doi.org/10.1111/jocd.16574 ↑24 Pirmez, R., & Salas-Callo, C. I. (2020). Very-low-dose oral minoxidil in male androgenetic alopecia: a study with quantitative trichoscopic documentation. *Journal of the American Academy of Dermatology.* 82(1). e21–e22. Available at: https://doi.org/10.1016/j.jaad.2019.08.084 ↑25 Jaén, P., & Arias-Santiago, S. (n.d.). Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. Available at: https://www.researchgate.net/profile/Suparuj-Lueangarun/publication/319006716_Efficacy_and_safety_of_oral_minoxidil_5_mg_daily_during_24-week_treatment_in_male_androgenetic_alopecia/links/5b46f1690f7e9b4637cde38b/Efficacy-and-safety-of-oral-minoxidil-5-mg-daily-during-24-week-treatment-in-male-androgenetic-alopecia.pdf ↑26 Panchaprateep, R., & Lueangarun, S. (2020). Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. *Dermatology and Therapy.* 10(6). 1345–1357. Available at: https://doi.org/10.1007/s13555-020-00448-x ↑28 Silva, M. N. E., Ramos, P. M., Silva, M. R., Silva, R. N. E., & Raposo, N. R. B. (2022). Randomized clinical trial of low-dose oral minoxidil for the treatment of female pattern hair loss: 0.25 mg versus 1 mg. 396–399. Available at: https://doi.org/10.1016/j.jaad.2022.01.017 ↑29 Sinclair, R. D. (2018). Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. *International Journal of Dermatology.* 57(1). 104–109. Available at: https://doi.org/10.1111/ijd.13838 ↑30 Perera, E., & Sinclair, R. (2017). Treatment of chronic telogen effluvium with oral minoxidil: a retrospective study. *F1000Research.* 6. 1650. Available at: https://doi.org/10.12688/f1000research.11775.1 ↑31 Wambier, C. G., Craiglow, B. G., & King, B. A. (2021). Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. *Journal of the American Academy of Dermatology.* 85(3). 743–745. Available at: https://doi.org/10.1016/j.jaad.2019.08.080 ↑32 Penha, M. A., Miot, H. A., Kasprzak, M., & Ramos, P. M. (2024). Oral minoxidil vs topical minoxidil for male androgenetic alopecia: a randomized clinical trial. *JAMA Dermatology.* 160(6). 600–605. Available at: doi:10.1001/jamadermatol.2024.0284 ↑34, ↑35 Asilian, A., Farmani, A., & Saber, M. (2024). Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: a randomized controlled trial. *Journal of Cosmetic Dermatology.* 23(3). 949–957. Available at: https://doi.org/10.1111/jocd.16086 ↑36 Vahabi-Amlashi, S., Layegh, P., Kiafar, B., Hoseininezhad, M., Abbaspour, M., Hajebi Khaniki, S., Forouzanfar, M., & Sabeti, V. (2021). A randomized clinical trial on therapeutic effects of 0.25 mg oral minoxidil tablets on treatment of female pattern hair loss. *Dermatologic Therapy.* 34(6). e15131. Available at: https://doi.org/10.1111/dth.15131 ↑37 Fazal, F., Malik, B. H., Malik, H. M., Sabir, B., Mustafa, H., Ahmed, M., Abid, A., Adil, M. L., Shafi, U., & Saad, M. (2025). Can oral minoxidil be the game changer in androgenetic alopecia? A comprehensive review and meta-analysis comparing topical and oral minoxidil for treating androgenetic alopecia. *Skin Health and Disease.* vzaf009. Available at: https://doi.org/10.1093/skinhd/vzaf009 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Michael Williams, PhD
Michael is a researcher and writer who holds a BSc in Bioscience, an MSc in Regenerative Medicine, and a PhD in Translational Biomedicine. He undertook his PhD research at Houston Methodist Research Institute, Texas, focusing on cell signaling in the ovarian cancer tumor microenvironment. He conducted postdoctoral research at Barts Cancer Institute in London, exploring cellular metabolism in acute myeloid leukemia. He has published work in a range of fields, including oncology, nanomedicine, and cell-based therapeutics.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down