- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesOral vs. Topical Dutasteride: What Studies Show
First Published Dec 17 2025Last Updated Dec 20 2025Pharmaceutical Researched & Written By:Michael Williams, PhD
Researched & Written By:Michael Williams, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Oral dutasteride is one of the most effective treatments for androgenic alopecia, but its systemic hormone suppression raises safety concerns. Topical dutasteride is often promoted as a way to deliver similar scalp-level benefits with fewer side effects, but does the evidence support that claim? In this article, we break down the efficacy, side-effect profiles, formulation quality, and real-world practicality of oral and topical dutasteride.
Full Article
Dutasteride is one of the most powerful pharmacologic treatments for androgenic alopecia (AGA). Initially approved for benign prostatic hyperplasia, dutasteride is now commonly prescribed off-label for hair loss in men and, more recently, has gained attention in topical form to minimize systemic exposure. This has created an important clinical question: how do oral and topical dutasteride compare in terms of effectiveness, safety, and real-world practicality?
This article examines what the current scientific literature reveals about oral versus topical dutasteride, reviewing mechanisms of action, efficacy data, and safety profiles to help you make more informed treatment decisions.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

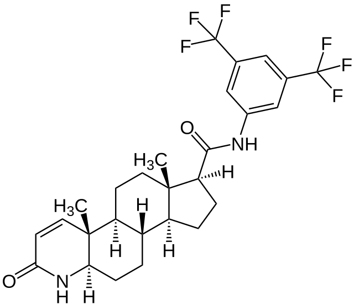
Dutasteride Mechanism of Action and Formulations
AGA is driven by increases in dihydrotestosterone (DHT). DHT binds to androgen receptors in hair follicles, triggering miniaturization that leads to thinner, shorter hairs. Dutasteride works by inhibiting an enzyme called 5α-reductase (5AR), which converts testosterone into DHT. Dutasteride is a dual 5AR inhibitor, meaning it blocks both type I and type II 5AR, leading to decreased DHT levels and ultimately halting the progression of AGA and restoring thicker hair.[1]Gerst, C., Dalko, M., Pichaud, P., Galey, J. B., Buan, B., & Bernard, B. A. (2002). Type-1 steroid 5α-reductase is functionally active in the hair follicle as evidenced by new selective … Continue reading,[2]Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by … Continue reading
Figure 1. Structure of dutasteride, which is a dual 5AR inhibitor.[3]Wikimedia Commons. (n.d.). Dutasterid.svg [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Dutasterid.svg (Accessed: November 2025) Image used under Creative Commons License.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Oral Dutasteride – Mechanism and Formulations
Oral dutasteride is absorbed through the gastrointestinal tract into systemic circulation, leading to significant and sustained reductions in DHT in the blood (serum). This includes reductions in scalp DHT, since less DHT is available systemically to diffuse into hair follicles.[4]Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by … Continue reading,[5]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading
The flip side is that any symptoms tied to systemic DHT suppression, such as sexual side-effects, are driven by this same mechanism. Given the long half-life and accumulation, systemic exposure persists even if doses are missed or therapy is stopped, and any adverse effects may take weeks to resolve.[6]Tsai, T.-F., Choi, G. S., Kim, B. J., Kim, M.-B., Ng, C. F., Kochhar, P., Jasper, S., Brotherton, B., Orban, B., & Lulic, Z. (2018). Prospective randomized study of sexual function in men taking … Continue reading
Topical Dutasteride – Mechanism and Formulations
Topical dutasteride treatment might avoid some of these pitfalls: high exposure at the scalp and low exposure elsewhere can target delivery and avoid systemic issues. It is applied directly to the scalp in formulations such as:
- Simple solutions (often alcohol- or glycol-based)
- Gels or lotions
- Liposomal formulations
- Nanoemulsions and nanoemulgels
- Nanocrystalline and other particulate systems
These vehicles are designed to ensure that dutasteride can cross the stratum corneum (the outermost protective layer of the skin) and, ideally, remain localized within the scalp.
What is the Available Evidence for Oral Dutasteride?
The effect of dutasteride on hair loss is well documented: multiple randomized placebo-controlled studies have been conducted over the last 25 years. Dutasteride was first developed as a treatment for benign prostatic hyperplasia and taken as a tablet. Early studies into dutasteride for hair loss therefore used oral formulations, with topical treatments developed later.
Here, we’ll take a look at the evidence supporting the use of oral dutasteride for hair loss. Many studies compare dutasteride with other treatments, most notably finasteride, or use the drug in combination with other therapies. We’ll focus on data showing the effect of dutasteride alone compared to placebo, but will also touch on comparisons with other popular treatments.
Below is a summary of the findings to date on the safety and efficacy of oral dutasteride:
Study Design and Population Treatments and Concentrations (Daily) Hair Growth Outcomes Systemic Effects Study #1[7]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Randomized, placebo-controlled, double-blinded trial. 415 men with male pattern hair loss aged 21-45 years old; 24 weeks. 0.05, 0.1, 0.5, or 2.5 mg dutasteride; 5 mg finasteride. Dose-dependent increase in total hair count vs placebo. Improved hair count vs finasteride. Serum decrease in DHT level (92% at 0.5 mg). Study #2[8]Stough, D. (2007). Dutasteride improves male pattern hair loss in a randomized study in identical twins. *Journal of Cosmetic Dermatology.* 6(1). 9–13. Available at: … Continue reading Randomized, placebo-controlled, double-blinded trial. 17 pairs of identical twin males with AGA; 12 months.
0.5 mg dutasteride. Significant increase in total hair counts in dutasteride-treated twins. Not assessed. Study #3[9]Eun, H. C., Kwon, O. S., Yeon, J. H., Shin, H. S., Kim, B. Y., Ro, B. I., Cho, H. K., et al. (2010). Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male … Continue reading Randomized, placebo-controlled, double-blinded trial. 153 men with male pattern hair loss aged 18-49; 6 months 0.5 mg dutasteride. Significant increase in total hair count. Sexual dysfunction was reported in 4.1% of the treatment group and 2.7% of placebo (not statistically significant). Study #4[10]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Randomized, placebo-controlled, double-blinded trial. 917 men with AGA aged 20-50; 24 weeks. 0.02, 0.1, or 0.5 mg dutasteride; 1 mg finasteride Increase in terminal hair count and width vs placebo. Significant increase in hair width vs dutasteride. No significant changes in reported adverse events. Study #5[11]Tsunemi, Y., Irisawa, R., Yoshiie, H., Brotherton, B., Ito, H., Tsuboi, R., Kawashima, M., Manyak, M., & ARI114264 Study Group. (2016). Long-term safety and efficacy of dutasteride in the … Continue reading Open-label, prospective study, no control. 120 men with AGA aged 20-50; 52 weeks. 0.5 mg dutasteride. Increase in terminal hair count and hair width compared to baseline. 12% of men experienced libido decrease and impotence, of which 50% and 57%, respectively, were not resolved by the end of the study. Study #6[12]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Open-label, randomized study; no placebo control. 90 men with AGA aged 18-40; 24 weeks. 0.5 mg dutasteride or 1 mg finasteride. Increase in total hair count and width compared to baseline. Increase in total hair count was significantly higher in dutasteride than finasteride group. Erectile dysfunction and loss of libido reported in 3 and 4 patients respectively. Study #7[13]Tsai, T.-F., Choi, G. S., Kim, B. J., Kim, M.-B., Ng, C. F., Kochhar, P., Jasper, S., Brotherton, B., Orban, B., & Lulic, Z. (2018). Prospective randomized study of sexual function in men taking … Continue reading Randomized, placebo-controlled, double-blinded trial. 117 men with AGA aged 23-50; 24 weeks. 0.5 mg dutasteride. Not assessed. Incidence of sexual adverse events was approximately double in the dutasteride group (16% vs 8% in placebo). Study #8[14]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading Retrospective chart review. 600 men over 18 with AGA. 0.5 mg dutasteride or 1 mg finasteride. Greater improvements in BASP classifications of AGA compared to baseline were found in dutasteride vs finasteride. Incidence of adverse events was higher in finasteride group (10.5%) than in dutasteride group (7.6%). What is the Available Evidence for Topical Dutasteride?
While evidence for the efficacy of oral dutasteride is well established, research into topical dutasteride solutions started relatively recently. Below is an overview of the studies into the efficacy of topical dutasteride:
Study Design and Population Treatments and Concentrations (Daily) Hair Growth Outcomes Systemic Effects Study #1[15]Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus … Continue reading Randomized, placebo-controlled, double-blinded trial. 34 men with AGA aged 18-65; 20 weeks. Microneedling with 0.01% dutasteride solution. Placebo was microneedling with saline solution. Significant improvements in hair thickness, hair density, and vellous: terminal hair ratio. Not assessed. Study #2[16]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading Randomized, placebo-controlled, double-blinded trial. 135 adult males aged 20-60 with AGA; 24 weeks. 0.01%, 0.02%, or 0.05% topical dutasteride; 1mg oral dutasteride Dose-dependent increase in total hair count and hair width. 0.05% solution showed significant improvement over finasteride in total hair count. No change in serum DHT levels observed vs placebo. How Does Oral and Topical Dutasteride Stack Up Against Each Other?
When comparing oral and topical dutasteride, the most important point to note is that there are currently no published clinical trials directly comparing the two formulations alone head-to-head for AGA. All comparisons must be made indirectly, using separate studies with different designs, populations, timelines, and formulations.
What About the Quality of the Available Evidence?
Despite this limitation, the evidence available for each formulation allows us to evaluate its strength and predictability. The current evidence quality for each formulation is as follows:
- Oral dutasteride — 59/100
- Topical dutasteride — 55/100
By our metrics, this means that both treatments have similar evidence quality overall. Longer-term data support oral dutasteride, while topical dutasteride remains less validated, largely due to fewer studies and limited long-term follow-up.
We’ll take a closer look at the strength of the evidence supporting both formulations.
Oral Dutasteride Studies
Oral dutasteride can reduce serum DHT levels by up to 90% and scalp DHT levels by 51-79%.[17]Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by … Continue reading,[18]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Randomized controlled trials consistently demonstrate that dutasteride, particularly at the standard 0.5 mg daily dose, suppresses serum and scalp DHT and delivers some of the most robust regrowth outcomes documented for AGA.
A number of the studies outlined above include large sample sizes, often over 100 participants, with the largest including over 900.[19]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Importantly, these cohorts should be large enough to detect changes in the incidence of adverse events, even if they are relatively rare. The replication of results in multiple trials using similar endpoints is a strong indicator of the efficacy of the drug.
Unfortunately, the longest of these RCTs only lasted 6 months, which may mean that lasting impacts on growth are overlooked. Similarly, side effects associated with long-term use over many years may be missed.
We can turn to longer studies using finasteride for some indication of how sustained benefits may be, given the similar mechanism of action of the two treatments. A 3-year trials using 1 mg daily doses of finasteride showed sustained improvement in hair growth over the course of the study, while placebo groups saw decreased hair counts.[20]Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. … Continue reading
A 10-year follow-up study concluded that improvements in hair growth, as determined by expert analysis of hair photographs, from finasteride do not worsen over time, and in 21% of cases, hair growth got even better after 5 years of treatment.[21]Rossi, A., Cantisani, C., Scarnò, M., Trucchia, A., Fortuna, M. C., & Calvieri, S. (2011). Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year … Continue reading
While we can’t draw any firm conclusions about the long-term efficacy of dutasteride from studies using finasteride, these findings can indicate the impact of long-term inhibition of 5AR, which is also seen in dutasteride treatment.
Topical Dutasteride Studies
Evidence for topical dutasteride is promising but remains less mature. Early clinical research indicates that topical formulations can meaningfully improve hair density, hair shaft diameter, and overall investigator-assessed regrowth, with efficacy in trials approaching that of oral therapy.
One small study with only 34 participants showed significant improvements in hair growth and thickness when combined with microneedling, compared to microneedling alone. Another study combined dutasteride formulations with minoxidil and also found that topical dutasteride delivered via microneedling produced hair density and thickness improvements similar to oral dutasteride, with fewer systemic side effects.[22]Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus … Continue reading,[23]Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through … Continue reading
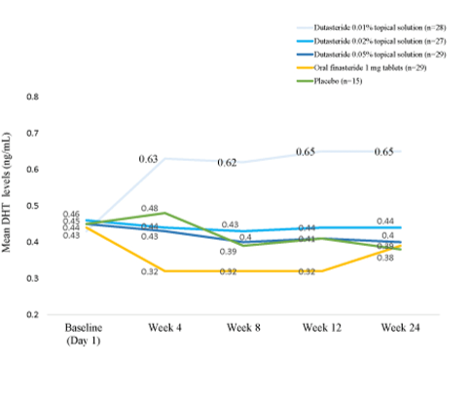
A recently published Phase II trial, the first to test topical dutasteride without microneedling, reported that all concentrations improved hair growth versus placebo, produced minimal changes in serum DHT, and that 1 ml daily of 0.05% appeared to outperform oral finasteride. However, concerns about unusually high baseline hair counts and inconsistencies in the placement and size of the 1 cm² target areas used for hair measurements raise questions about the reliability of these results.[24]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading
Figure 2. Oral dutasteride (yellow line) reduces serum DHT levels more than topical Dutaseride (blue lines). Adapted from Figure 3.[25]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading Image used under Creative Commons License.
Real-world outcomes from low-dose topical dutasteride users (0.01–0.02% applied daily or near-daily) suggest more modest benefits, with good scalp localization and stable serum DHT noted as positives, rather than regrowth exceeding that with oral finasteride.
There have been some concerns raised about the ability of dutasteride to penetrate into the scalp. Dutasteride is a relatively large molecule, with one molecule weighing 528.5 Daltons (Da). There is a commonly cited rule, called the 500 Dalton Rule, that states that molecules should be under 500 Da in order to penetrate the skin. However, dutasteride has been demonstrated to be effective in topical formulations and can measurably lower DHT concentrations. What’s more, a small, unpublished study has also shown that the drug does penetrate into both the scalp and serum, even at low concentrations. As such, in this case, dutasteride does seem to penetrate the skin in the scalp, without the need for microneedling or injection.
How Safe are Dutasteride Formulations?
Because dutasteride produces sustained suppression of DHT, safety considerations, particularly regarding hormonal and sexual side effects, are central to treatment selection. Most clinical trials record adverse events, while some studies are focused specifically on side-effects.[26]Tsai, T.-F., Choi, G. S., Kim, B. J., Kim, M.-B., Ng, C. F., Kochhar, P., Jasper, S., Brotherton, B., Orban, B., & Lulic, Z. (2018). Prospective randomized study of sexual function in men taking … Continue reading
Oral Dutasteride
Across randomized trials and long-term observational studies, the most consistently reported adverse effects of oral dutasteride involve sexual function, including:
- Decreased libido
- Erectile dysfunction
- Ejaculation disorders
- Gynecomastia and breast tenderness (rare)
Trials report overall total adverse event rates between 4 and 12%, with sexual adverse events such as decreased libido as high as 13%, although this was reported in higher doses (2.5mg daily).[27]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading At the standard 0.5mg daily dose, sexual side effects typically occur at a rate of between 1 and 5%. In the context of other popular treatments, comparisons with finasteride show no consistent increase in sexual side effects with dutasteride, despite dutasteride lowering serum DHT by an additional 20-30% beyond finasteride.[28]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
Most controlled studies show that the majority of sexual side effects resolve either during treatment or after discontinuation. However, due to dutasteride’s long half-life (4-5 weeks) and high tissue binding, systemic effects can persist. At standard 0.5mg daily dosing, serum DHT generally returns to 80% of baseline within 6 months of discontinuation.
Topical Dutasteride
Topical dutasteride is generally well tolerated, and reported adverse events are usually mild and localized, including:
- Scalp irritation
- Erythema
- Contact dermatitis
- Pruritus or dryness
These occur inconsistently and are formulation-dependent, influenced by solvent systems, penetration enhancers, and application volume.
The primary safety advantage of topical dutasteride is reduced systemic exposure. In trials, topical regimens in the 0.01–0.05% range produce minimal or no significant changes in serum DHT or testosterone.[29]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading When decreases in serum DHT are observed with topical use, they are generally smaller than those seen with oral dosing.[30]Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through … Continue reading
Adherence and Practical Considerations
Dutasteride, whether oral or topical, is primarily used for AGA in men, though its use for hair loss remains off-label in the United States. Oral dutasteride is formally FDA-approved only for benign prostatic hyperplasia, while topical formulations exist solely through compounding pharmacies.
Adherence and Route of Application
Oral dutasteride offers the simplest adherence burden: one capsule daily (or a modified intermittent schedule). This ease is a major factor behind its consistent outcomes. Topical dutasteride, while potentially safer from a systemic standpoint, requires regular scalp application and attention to dosing volume.
The effectiveness of topical formulations is heavily influenced by factors such as vehicle, scalp contact time, treated surface area, and individual skin permeability. These practical considerations can meaningfully shape both efficacy and systemic absorption.
Advanced vehicles such as nanoemulsions or nanoemulgels can increase scalp penetration by up to 1.5-fold compared to conventional solutions.[31]Ali, M. S., Alam, M. S., Alam, N., & Siddiqui, M. R. (2014). Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. … Continue reading However, this may also increase systemic exposure. Application frequency can be adjusted to balance efficacy and hormonal safety. Because dutasteride binds to 5AR for prolonged periods, daily application is not always necessary.
Time on the scalp also matters. The longer a topical formulation remains in contact with the skin, the more dutasteride can penetrate the follicle. Most protocols recommend allowing at least four hours before washing the hair. Rinsing too soon can reduce efficacy, while prolonged contact increases both potency and the possibility of systemic absorption.
The size of the application area affects systemic exposure. Treating a small region, such as the crown, introduces less drug into circulation than applying the same concentration across the entire scalp. Patients with diffuse thinning often benefit from using lower volumes or concentrations to counterbalance the increased surface area.
As we can see, there is a wide range of factors to consider when making a treatment plan with topical dutasteride, which may make consistent application a challenge.
Use in Women
Off-label use of dutasteride in women is limited and generally reserved for postmenopausal women or women with documented hyperandrogenism or a strong family history of AGA. Due to the systemic androgenic impacts of the drug, it’s typically only used in cases where other therapies (minoxidil, spironolactone) are inadequate.
Clinical Interpretation and Decision-Making
The key trade-off between oral and topical formulations is systemic potency versus localized targeting, balanced against differences in side-effect risk, convenience, and cost. Oral therapy is simpler and less variable; topical therapy requires consistent application but offers greater hormonal safety for risk-averse patients.
Combination therapy, most commonly topical dutasteride with minoxidil, can enhance outcomes through complementary mechanisms. Oral dutasteride may also be paired with topical agents in some cases.
Significant research gaps remain, including the absence of direct head-to-head oral-versus-topical trials, limited long-term safety data for topical dutasteride, and the lack of standardized dosing guidelines for topical formulations.
Final Thoughts
Dutasteride, whether oral or topical, represents one of the most powerful tools currently available for treating AGA. Oral dutasteride offers the most consistent and robust regrowth outcomes, supported by the strongest body of clinical evidence, but with predictable systemic hormonal suppression and long persistence after discontinuation.
Topical dutasteride, while still supported by a smaller and newer evidence base, offers a compelling alternative for patients seeking meaningful scalp-level efficacy with reduced systemic exposure. Its ultimate success depends heavily on formulation quality, dosing strategy, and adherence.
There is no universally “best” option: only the best option for specific circumstances. Treatment selection should be guided by hair-loss severity, prior treatment response, risk tolerance, reproductive plans, and long-term adherence capacity. As research continues to evolve, clinical guidance will become more precise. For now, optimal results come from choosing the formulation that best aligns with your goals and comfort with risk.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

References[+]
References ↑1 Gerst, C., Dalko, M., Pichaud, P., Galey, J. B., Buan, B., & Bernard, B. A. (2002). Type-1 steroid 5α-reductase is functionally active in the hair follicle as evidenced by new selective inhibitors of either type-1 or type-2 human steroid 5α-reductase. *Experimental Dermatology.* 11(1). 52–58. Available at: https://doi.org/10.1034/j.1600-0625.2002.110106.x ↑2, ↑4, ↑17 Clark, R. V., Hermann, D. J., Cunningham, G. R., Wilson, T. H., Morrill, B. B., & Hobbs, S. (2004). Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α-reductase inhibitor. *The Journal of Clinical Endocrinology & Metabolism.* 89(5). 2179–2184. Available at: https://doi.org/10.1210/jc.2003-030330 ↑3 Wikimedia Commons. (n.d.). Dutasterid.svg [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Dutasterid.svg (Accessed: November 2025) ↑5, ↑18 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑6, ↑13, ↑26 Tsai, T.-F., Choi, G. S., Kim, B. J., Kim, M.-B., Ng, C. F., Kochhar, P., Jasper, S., Brotherton, B., Orban, B., & Lulic, Z. (2018). Prospective randomized study of sexual function in men taking dutasteride for the treatment of androgenetic alopecia. *The Journal of Dermatology.* 45(7). 799–804. Available at: https://doi.org/10.1111/1346-8138.14329 ↑7, ↑27 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑8 Stough, D. (2007). Dutasteride improves male pattern hair loss in a randomized study in identical twins. *Journal of Cosmetic Dermatology.* 6(1). 9–13. Available at: https://doi.org/10.1111/j.1473-2165.2007.00297.x ↑9 Eun, H. C., Kwon, O. S., Yeon, J. H., Shin, H. S., Kim, B. Y., Ro, B. I., Cho, H. K., et al. (2010). Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. *Journal of the American Academy of Dermatology.* 63(2). 252–258. Available at: https://doi.org/10.1016/j.jaad.2009.09.018 ↑10, ↑19, ↑28 Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 70(3). 489–498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑11 Tsunemi, Y., Irisawa, R., Yoshiie, H., Brotherton, B., Ito, H., Tsuboi, R., Kawashima, M., Manyak, M., & ARI114264 Study Group. (2016). Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. *The Journal of Dermatology.* 43(9). 1051–1058. Available at: https://doi.org/10.1111/1346-8138.13310 ↑12 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑14 Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. *Annals of Dermatology.* 34(5). 349. Available at: https://doi.org/10.5021/ad.22.027 ↑15, ↑22 Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. *Journal of the European Academy of Dermatology & Venereology.* 36(10).. Available at: https://doi.org/10.1111/jdv.18285 ↑16, ↑25, ↑29 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. *Cureus.* 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑20 Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. *Journal of the American Academy of Dermatology.* 55(1). 71–74. Available at: https://doi.org/10.1016/j.jaad.2005.07.001 ↑21 Rossi, A., Cantisani, C., Scarnò, M., Trucchia, A., Fortuna, M. C., & Calvieri, S. (2011). Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. *Dermatologic Therapy.* 24(4). 455–461. Available at: https://doi.org/10.1111/j.1529-8019.2011.01441.x ↑23, ↑30 Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in treatment of androgenetic alopecia. *QJM: An International Journal of Medicine.* 117(Supplement_2). hcae175–207. Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑24 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. *Cureus.* 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑31 Ali, M. S., Alam, M. S., Alam, N., & Siddiqui, M. R. (2014). Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. *Iranian Journal of Pharmaceutical Research (IJPR).* 13(4). 1125. Available at: https://doi.org/10.3390/pharmaceutics14061152 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Michael Williams, PhD
Michael is a researcher and writer who holds a BSc in Bioscience, an MSc in Regenerative Medicine, and a PhD in Translational Biomedicine. He undertook his PhD research at Houston Methodist Research Institute, Texas, focusing on cell signaling in the ovarian cancer tumor microenvironment. He conducted postdoctoral research at Barts Cancer Institute in London, exploring cellular metabolism in acute myeloid leukemia. He has published work in a range of fields, including oncology, nanomedicine, and cell-based therapeutics.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down