- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Keeps Review: The Truth About Their Hair Loss Treatments?
-
7 Best Oils for Hair Growth
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
Switching From Finasteride to Dutasteride
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesNew Study: Does Finasteride Cause Birth Defects?
First Published Jan 17 2025Last Updated Sep 19 2025Pharmaceutical Researched & Written By:Sarah King, PhD
Researched & Written By:Sarah King, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
A recent study has raised concerns about paternal finasteride use and cryptorchidism (undescended testicles) in offspring, but does the data actually support a causal link? The study, based on FAERS reports, shows a statistical association—but not proof of risk. Issues like reporting bias, lack of a control group, and extremely small case numbers (only 11 reports over 12 years) make the findings questionable. Additionally, semen finasteride levels are far below doses known to cause fetal harm, and large-scale studies haven’t confirmed a connection with increased birth defect risks. While caution is always advisable, current evidence does not justify major concern for men trying to conceive. Want to know the full story? Read our deep dive into what the science really says.
Full Article
While the medical literature clearly advises women to avoid finasteride before conception during pregnancy, and while breastfeeding, the guidance for men is less definitive. The question of whether men can safely continue using finasteride during the conception period remains a topic of debate among healthcare professionals.
However, a recent large dataset statistical analysis has sent ripples through the hair loss space and regulatory world, as it appears to show further evidence that the use of finasteride in men leads to a congenital anomaly called “cryptorchidism” (undescended testicles). But what does the data actually say? And can we link statistical association to causality? In this article, we will take a deep dive into what the paper shows (or doesn’t show) and whether we think it is a cause of major concern for men.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Let’s first take a look at what the study is all about.
The Study
We’ll begin by summarizing the data from the study and then go into detail about what it all means, how the statistics are analyzed, and whether this is a result to be concerned about.
The researchers analyzed data from the FDA Adverse Event Reporting System (FAERS) from 2010 to 2022 to assess potential safety concerns related to paternal drug exposure on fertility, pregnancy outcomes, and offspring health.[1]Zeng, Y., Lin, W., Zhuang, W. (2024). Safety concerns of paternal drug exposure on fertility, pregnancy, and offspring: an analysis based on the FDA adverse event reporting system. Andrology. 1-12. … Continue reading The FAERS is a computerized database that supports the FDA’s post-marketing safety surveillance program for approved drugs and therapeutic biologic products.[2]US Food and Drug Administration. (no date). FDA Adverse Events Reporting System (FAERS) Public Dashboard. US FDA. Available at: … Continue reading It collects and stores information on adverse events, medication errors, and product quality complaints that may be associated with FDA-approved products.
Reporting to FAERS can be done in two ways:
- Mandatory reporting: Manufacturers are required by law to report adverse events to FAERS within 14 days of becoming aware of them.
- Voluntary reporting: Healthcare professionals (such as physicians, pharmacists, and nurses) and consumers (including patients, family members, and lawyers) can voluntarily submit reports.
Importantly, anyone can submit to FAERS directly; it does not need to be done by a doctor. The FDA provides multiple options for voluntary reporting:
- Online submission through the FDA’s Electronic Submission Gateway (ESG) or Safety Reporting Portal (SRP).
- Using FDA Form 3500, which can be submitted electronically or by mail.[3]US Food and Drug Administration (2024). Reporting Serious Problems to FDA. MedWatch. Available at: … Continue reading
What Were The Study Results?
The researchers conducted a disproportionality analysis, specifically the Reporting Odds Ratio (ROR), to identify drugs disproportionately associated with reproductive-related adverse events.
The study analyzed 16,180,533 total reports; 3,210 cases related to paternal drug exposure were identified, with 7,808 associated adverse events (e.g., spontaneous abortions and small babies). The study found that drugs used to treat rheumatoid arthritis, cancer, infections, and psychotropic conditions were the most frequently implicated.
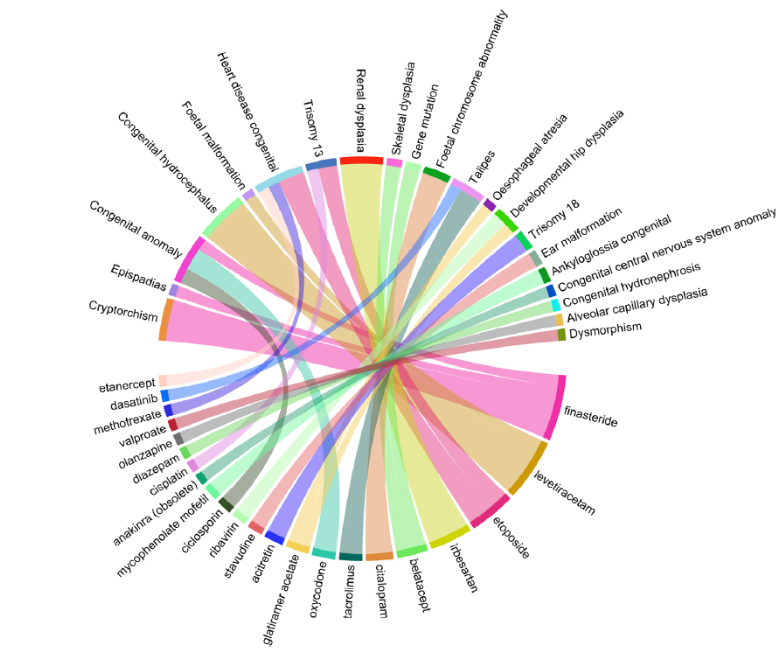
However, one of the strongest links between treatment and adverse health events was between finasteride and cryptorchidism, with an ROR of 891.7 based on 11 reports. This suggests that cryptorchidism was reported for finasteride-exposed fathers at a much higher rate than for most other drugs in the database.
Figure 1: A graph showing the ROR of different drugs and their adverse event pairs. Finasteride and cryptorchidism appear to have a noticeably larger ROR than others.[4]Zeng, Y., Lin, W., Zhuang, W. (2024). Safety concerns of paternal drug exposure on fertility, pregnancy, and offspring: an analysis based on the FDA adverse event reporting system. Andrology. 1-12. … Continue reading
Understanding the Data
Before we go into what the data means, let’s explain some key concepts that are important to the study.
What is a Disproportionality Analysis?
A disproportionality analysis is a tool used in drug safety monitoring. It analyzes large databases of reported side effects from various medications and looks for unusual patterns – like if a particular side effect is reported much more often with one drug than others.[5]Fusaroli, M., Salvo, F., Begaud, B., AlShammari, T.M, Bate, A., Battini, V., Brueckner, A., Candore, G., Carnovale, C., Crisafulli, S., Cutroneo, P.M., Dolladille, C., Drici, M.D., Faillie, J.L., … Continue reading
Imagine a concert where five people in the front row get food poisoning after eating hotdogs from a nearby stall, while only one person in the entire back section reports feeling ill. You might suspect that the front-row hot dogs are bad—but this doesn’t prove the food stand actually caused the sickness. Maybe those five people already had food poisoning before coming. Disproportionality analysis works in a similar way: it flags patterns, i.e., five people sick in the front row, but it doesn’t prove causation.
What is the Reporting Odds Ratio (ROR)?
The ROR is a key statistical measure used in disproportionality analyses. It compares how often a specific side effect is reported for a particular drug versus all other drugs.[6]Rothman, K.J., Lanes, S., Sacks, S.T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiology and Drug Safety. 13(8). 519-523. Available at: … Continue reading If the ROR is high, it means the side effects are being reported more often for that drug than you’d normally expect.
Think about flipping a coin 10 times, and imagine it lands on “heads” 7 times. You might think the coin is biased. But if you had flipped it 1,000 times, the results might have evened out to 50/50. The Reporting Odds Ratio (ROR) works similarly: when there are only a few cases, even small variations make the number seem exaggerated. A few extra reports in a small dataset can make an effect look much larger than it actually is.
Unlike incidence, which measures new cases over time, or prevalence, which measures total cases at a given time, ROR does not provide information about the actual risk of an adverse event occurring. Instead, it indicates whether there’s a disproportionate association between a drug and an adverse event in the reporting database.
The important point to remember is that ROR is primarily a hypothesis-generating tool, helping prioritize which drug-event combinations may need further investigation.
What Do The Study Results Mean?
The ROR calculation was based on reports from the FAERS, which, as we mentioned above, is a system for self-reporting adverse events that anyone can report to. This means that more severe or unusual side effects are more likely to be reported, and as such, FAERS is at risk of reporting bias. This was acknowledged as a limitation in the study.
Furthermore, unlike controlled clinical studies, FAERS does not track how many people take a drug; it only tracks how many people report an issue.
To calculate the true risk of cryptorchidism from finasteride, you need two numbers:
- The number of reported cases (numerator) – FAERS provides this (11 cases).
- The total number of fathers who took finasteride (denominator) – FAERS does not provide this.
Since FAERS does not track drug exposure rates, it is impossible to calculate the incidence or prevalence of an event. This means we can’t determine what percentage of fathers taking finasteride had children with cryptorchidism or whether this percentage is actually higher than background rates in the general population.
Think about it this way: a phone company gets 100 customer complaints in a month. Without knowing how many total customers they have, you can’t tell if that’s a serious problem. If they have 200 customers, that’s bad (50% complaint rate). However, if they have 2 million customers, it’s insignificant (0.005% complaint rate). FAERS has the same issue—it counts events but doesn’t count the total number of people taking the drug.
Furthermore, the disproportionality analysis does not compare finasteride to a control group. Instead, it compares the number of cryptorchidism reports for finasteride (11) to the number of cryptorchidism reports for all other drugs (likely much lower because most drugs do not affect male hormone pathways).
This is problematic for several reasons:
1. This is Not a Matched Control Population
-
- Fathers who took finasteride should be compared to fathers who did not take finasteride while keeping other factors (like age, lifestyle, genetics) constant.
- FAERS instead compares finasteride to all other drugs – but as we just mentioned, most drugs don’t affect male reproductive hormones, making the comparison flawed.
2. Selective Reporting Bias in FAERS
-
- Finasteride users are already aware of post-finasteride syndrome (PFS) and reproductive side effect concerns.
- If a father taking finasteride has a child with any birth defect, they may be more likely to report it than a father taking, say, an antibiotic or a painkiller.
- This could artificially inflate the number of finasteride-related reports, making cryptorchidism appear more common than it is.
3. The Numerator is Small, But the ROR is Huge:
-
- FAERS recorded only 11 cryptorchidism cases for finasteride from 2010 – 2022.
- Cryptorchidism naturally occurs in 2-3% of all full-term male births.[7]Leslie, S.W., Sajjad, H., Villanueva, C.A. (2024). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470270/ … Continue reading
This creates a statistical illusion. If very few cryptorchidism cases are reported for other drugs, the ROR for finasteride skyrockets, even if the actual risk is low.
People fear shark attacks because they’re widely reported in the news, but statistical analyses have shown that the odds of dying from a falling vending machine are higher than being killed by a shark.[8]United States Consumer Product Safety Commission. (1995). CPSC, Soda Vending Machine Industry Labeling Campaign Warns of Deaths and Injuries. US CPSC. Available at: … Continue reading The reason we think sharks are deadlier is that their attacks make headlines while vending machine accidents don’t. Similarly, if only one case of cryptorchidism is reported for another drug, but 11 cases are reported for finasteride, the ROR can look massive—even if the actual risk is tiny.
In 2022, about 2,626,865 men in the United States were estimated to have taken finasteride.[9]ClinCalc. (no date). Finasteride Drug Usage Statistics, United States, 2013-2022. ClinCalc. Available at: https://clincalc.com/drugstats/Drugs/Finasteride (Accessed: January 2025) Even if all 11 reports of cryptorchidism were from distinct individuals and occurred solely in 2022 (instead of over 12 years), this would equate to an incidence rate of 0.00042% (or 4.2 per million users). This is notably below the background risk of cryptorchidism in the general population (2-3%), suggesting that the observed reports in FAERS are likely to be an artifact of a small sample size rather than evidence of a causal relationship.
So, to summarize, this study, while showing a statistical association between men taking finasteride and their children having cryptorchidism, does not show any causal relationship – meaning that it doesn’t actually show that finasteride causes this congenital anomaly.
Why Is This Study Even A Discussion?
Finasteride’s potential impact on reproductive health has been debated for years, largely due to animal studies and regulatory warnings for women. While the FAERS data discussed above suggest a statistical association between finasteride use in men and cryptorchidism in offspring, the underlying question is whether this link is biologically plausible or supported by stronger evidence.
Finasteride is classified as an FDA Pregnancy Category X drug, meaning that it is strictly contraindicated in pregnant women due to teratogenic effects observed in animal studies.[10]Merck & Co. Inc. (no date). Propecia Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf (Accessed: January 2025) These concerns stem from its mechanism of action: blocking the conversion of testosterone into dihydrotestosterone (DHT) by inhibiting the 5ɑ-reductase enzyme. Since DHT is crucial for normal male fetal development, any disruption during pregnancy can result in genital abnormalities.[11]Bormann, C.L., Smith, G.D., Padmanabhan, V., Lee, T.M. (2011). Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reproduction 142(1). Available at: … Continue reading
Studies in rodent and primate models have shown that exposure to high doses of finasteride during pregnancy can lead to:
- Hypospadias – a condition where the urethral opening is misplaced on the underside of the penis instead of at the tip.[12]An, N., Peng, J., He, G., Fan, X., Li, F., Chen, H. (2018). Involvement of activation of mitogen-activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) signaling pathway in … Continue reading
- Cryptorchidism – as mentioned above, is an anomaly in which one or both testicles fail to descend into the scrotum during fetal development, a condition later associated with fertility issues and an increased risk of testicular cancer.
- Reduced anogenital distance (AGD) – a key marker of disrupted androgen signaling during development and is often used as a biomarker for endocrine disruption.[13]Clark, R.L., Anderson, C.A., Prahalada, S., Robertson, R.T., Lochry, E.A., Leonard, Y.M., Stevens, J.L., Hoberman, A.M. (1993). Critical developmental periods for effects on male rat genitalia … Continue reading
One study in rhesus monkeys found that continuous finasteride exposure throughout gestation resulted in severe genital malformations in male offspring.[14]Prahalada, S., Tarantal, A.F., Harris, G.H., Ellsworth, K.P., Clarke, A.P., Skiles, G.L., MacKenzie, K.I., Kruk, L.F., Ablin, D.S., Cukierski, M.A., Peter, C.P., vanZwieten, M.J., Hendrickx, A.G. … Continue reading This was particularly concerning because monkeys have hormonal systems that more closely resemble humans compared to rodents. Due to these findings, the FDA issued strong warnings that pregnant women should never ingest or even handle crushed finasteride tablets, as skin absorption could theoretically lead to fatal exposure.
For more information, see our article diving into the science behind finasteride & conception, and our article about how to use finasteride and minimize its exposure to your partner.
Can These Findings in Animals Be Directly Applied to Humans?
Although these studies raise legitimate concerns, there are important reasons why their findings may not directly apply to human males taking finasteride. For example, the doses used in animal studies are often much higher than those used in human treatment. The studies also involve direct maternal exposure during pregnancy, whereas paternal exposure (via sperm) results in much lower fetal exposure (we’ll discuss this further below). Furthermore, while rodents and monkeys have similar endocrine systems, their sensitivity to 5ɑ-reductase inhibition differs from that of humans.
Therefore, while animal models provide a biological basis for concern, they do not conclusively prove that paternal finasteride exposure affects offspring in humans.
Is It Biologically Plausible That Finasteride Could Cause Issues in Men?
To determine whether paternal finasteride use could biologically contribute to congenital anomalies in humans, we need to look at:
- How much finasteride reaches semen and whether these lead to enough exposure in females to impact fetal development.
- Whether finasteride alters sperm in a way that could lead to birth defects.
How Much Finasteride Reaches Semen?
One way finasteride could hypothetically impact fetal development is through semen exposure during conception or early pregnancy. However, semen levels of finasteride are extremely low.
Clinical data from GlaxoSmithKline (internal studies) measured finasteride concentrations in the semen of men taking a 50 mg dose (which is 10 x higher than the standard dose for hair loss). The result? Only 0.26 ng/mL of finasteride was detected in semen.[15]US Food and Drug Administration. (no date). Propecia (finasteride). US FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020788s017lbl.pdf (Accessed: January 2025) At the standard 1 mg dose used for hair loss, semen concentrations would likely be even lower.
How does this compare to doses known to cause fetal harm?
In the rhesus monkey studies, oral doses of finasteride given directly to pregnant females (at levels over 5,000 x higher than those found in human semen) caused genital abnormalities in male offspring. Therefore, the amount of finasteride that pregnant human women might absorb from semen exposure is likely so low that it falls below the threshold needed to affect fetal development.
Conclusion: There is no compelling evidence to suggest that the trace amounts of finasteride in semen are enough to cause congenital defects in humans.
Can Finasteride Affect Sperm Quality or DNA?
Another possible concern is whether finasteride negatively affects sperm itself, leading to:
- Reduced fertility.
- DNA damage that could increase congenital anomaly risk.
Some studies suggest finasteride can reduce sperm concentration and motility at higher doses (5 mg for prostate treatment), but these effects are reversible after stopping the medication, and studies in men taking the 1 mg dose for hair loss do not show significant sperm abnormalities.
For example, a 2013 study by Samplaski et al. found that men taking an average of 1.04 mg/day of finasteride experienced an 11.6-fold increase in sperm count after stopping the drug, with no participants experiencing a decrease in sperm count post-discontinuation.[16]Samplaski, M.K., Lo, K., Grober, E., Jarvi, K. (2013). Finasteride use in the male infertility population: effects on semen and hormone parameters. Fertility and Sterility. 100(6). 1542-1546. … Continue reading
Similarly, a 1999 study found that 1 mg/day of finasteride did not significantly affect spermatogenesis or semen production in healthy men.[17]Overstreet, J.W., Fuh, V.L., Gould, J., Howards, S.S., Lieber, M.M., Hellstrom, W., Shapiro, S., Carroll, P., Corfman, R.S., Petrous, S., Lewis, R., Toth, P., Shown, T., Roy, J., Jarow, J.P., … Continue reading
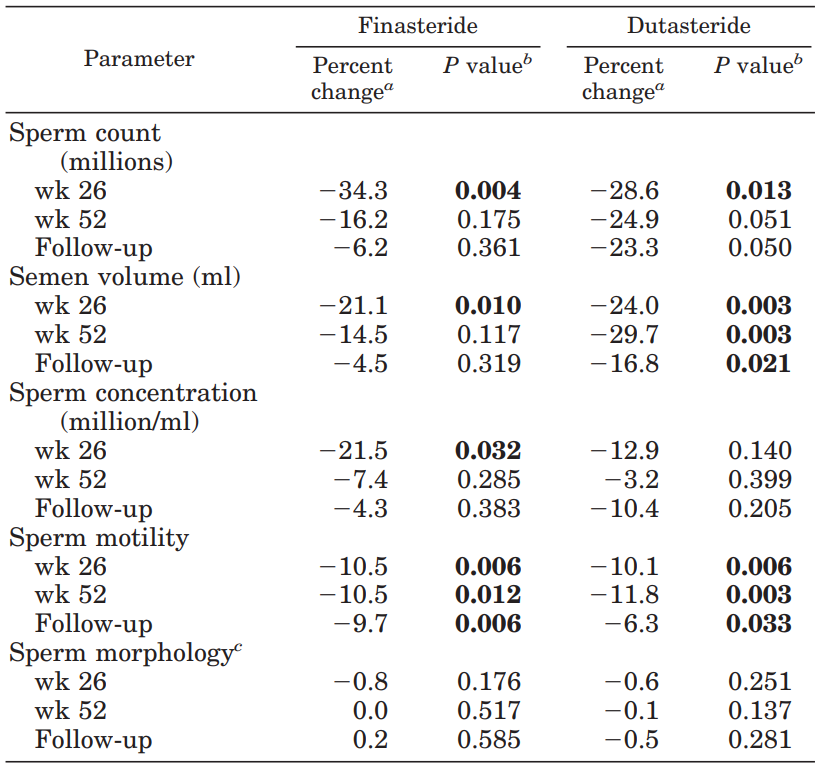
A 2007 study observed that taking 5 mg/day of finasteride exhibited mild reductions in semen volume, sperm concentration, and motility, though these parameters returned just below normal after stopping.[18]J.K., Amory., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J., Clark, R.V. (2007). The effect of 5alpha-reductase inhibition with dutasteride and … Continue reading
Figure 2: Percent changes in sperm count, semen volume, sperm concentration, motility, and morphology at weeks 26 and 52 of treatment with finasteride or dutasteride and at the follow-up after stopping.[19]J.K., Amory., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J., Clark, R.V. (2007). The effect of 5alpha-reductase inhibition with dutasteride and … Continue reading
Could Finasteride Cause Epigenetic Changes to Sperm?
Some scientists have speculated that finasteride could cause epigenetic modifications to sperm, meaning it might alter gene expression without changing the DNA sequence. However, no studies have directly confirmed this in humans. Animal studies suggest some potential for altered gene expression, but these findings haven’t been replicated in human sperm research.[20]Kolasa, A., Roginska, D., Rzeszotek, S., Machalinski, B., Wiszniewska, B. (2021). Paternal finasteride treatment can influence the testicular transcriptome profile of male offspring – … Continue reading
In 2011 and 2012, two separate case reports documented men struggling with fertility issues after long-term finasteride use. While their sperm morphology appeared normal, both had elevated sperm DNA fragmentation-a marker of DNA damage. After stopping finasteride, one saw fragmentation drop from 30% to 16.5% within six months, while the other improved and successfully conceived.[21]Salvarci, A., Istanbulluoglu, O. (2012). Secondary infertility due to use of low-dose finasteride. International urology and nephrology. 45(1). 83-85. Available at: … Continue reading,[22]Tu, H.Y.V., Zini, A. (2011). Finasteride-induced secondary infertility associated with sperm DNA damage. Fertility and sterility. 95(6). e13-4. Available at: … Continue reading
These reports suggest finasteride may contribute to sperm DNA damage, but since they lack controls, other lifestyle changes such as diet, exercise, or better sleep could also explain the improvements. You can read more about our thoughts on these case studies here.
The bottom line is that:
- Finasteride can mildly impact sperm quality, but this effect is reversible and not linked to congenital anomalies.
- No strong evidence exists that finasteride causes long-term DNA changes that could harm offspring.
So, is it biologically plausible that finasteride causes birth defects when taken by men? We think the current evidence for paternal risk is weak.
Is There Any Other Evidence That Finasteride Causes Congenital Anomalies?
To date, no large-scale, well-controlled studies have confirmed an increased risk of congenital anomalies from paternal finasteride use.
Danish Nationwide Study (2017)
Study Type: A large-scale epidemiological study using data from national birth registries.[23]Anderson, J.T., Jenson, T.B., Horwitz, H., Clausen, S.S. (2019). Paternal exposure to finasteride – Before and during pregnancy. Pharmacoepidemiology and Drug Safety. 28(16). Conference … Continue reading
Findings: No increased risk of congenital anomalies in children of men who had taken finasteride before conception. The risk of miscarriage was also not elevated.
Conclusion: No strong evidence linking paternal finasteride use to birth defects.
Case Reports and Small Studies
Some isolated case reports have suggested a potential association between paternal finasteride use and reproductive issues in offspring.[24]Ahn, K.H., Shin, J., Hong, S.C., Han, JY., Lee, E.H., Lee, J.S., Oh, M.J., Kim, H.J. (2015). Pregnancy Outcomes with Paternal Exposure to Finasteride, a Synthetic 5-Alpha-Reductase Inhibitor: A Case … Continue reading However, these reports often lack proper controls and fail to rule out other factors such as genetics, environmental exposures, or maternal health conditions.
Final Thoughts
While the study we have discussed in this article highlights a statistical association between paternal finasteride exposure and cryptorchidism, it does not establish causation. The limitations of FAERS data, including reporting bias and the absence of a control population, make it difficult to draw firm conclusions. Additionally, biological plausibility remains weak, given the extremely low levels of finasteride in semen and the lack of strong evidence linking paternal exposure to congenital anomalies. Ultimately, while more research is warranted, current data does not suggest a major cause for concern. Men using finasteride should, however, discuss any reproductive concerns with their healthcare provider before making any decisions.
References[+]
References ↑1 Zeng, Y., Lin, W., Zhuang, W. (2024). Safety concerns of paternal drug exposure on fertility, pregnancy, and offspring: an analysis based on the FDA adverse event reporting system. Andrology. 1-12. Advance online publication. Available at: https://doi.org/10.1111/andr.13790 ↑2 US Food and Drug Administration. (no date). FDA Adverse Events Reporting System (FAERS) Public Dashboard. US FDA. Available at: https://fis.fda.gov/extensions/FPD-FAQ/FPD-FAQ.html#:~:text=Healthcare%20professionals%2C%20consumers%2C%20and%20manufacturers,members%2C%20lawyers%20and%20others). (Accessed: January 2025) ↑3 US Food and Drug Administration (2024). Reporting Serious Problems to FDA. MedWatch. Available at: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program/reporting-serious-problems-fda (Accessed: January 2025) ↑4 Zeng, Y., Lin, W., Zhuang, W. (2024). Safety concerns of paternal drug exposure on fertility, pregnancy, and offspring: an analysis based on the FDA adverse event reporting system. Andrology. 1-12. Advance online publication. Available at: https://doi.org/10.1111/andr.13790 ↑5 Fusaroli, M., Salvo, F., Begaud, B., AlShammari, T.M, Bate, A., Battini, V., Brueckner, A., Candore, G., Carnovale, C., Crisafulli, S., Cutroneo, P.M., Dolladille, C., Drici, M.D., Faillie, J.L., Goldman, A., Hauben, M., Herdeiro, M.T., Mahaux, O., Manlik, K., Montastruc, F., Noguchi, Y., Noren, G.N., Noseda, R., Onakpoya, I.J., Pariente, A., Poluzzi, E., Salem, M., Sartori, D., Trinh, N.T.H., Tuccori, M., van Hunsel, F., van Puijenbroek., E, Raschi, E., Jhouri, C. (2024). The Reporting of Disproportionality Analysis for Drug Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Development and Statement. Drug Safety. 47(6). 575-584. Available at: https://doi.org/10.1007/s40264-024-01421-9 ↑6 Rothman, K.J., Lanes, S., Sacks, S.T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiology and Drug Safety. 13(8). 519-523. Available at: https://doi.org/10.1002/pds.1001 ↑7 Leslie, S.W., Sajjad, H., Villanueva, C.A. (2024). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470270/ (Accessed: January 2025) ↑8 United States Consumer Product Safety Commission. (1995). CPSC, Soda Vending Machine Industry Labeling Campaign Warns of Deaths and Injuries. US CPSC. Available at: https://www.cpsc.gov/Newsroom/News-Releases/1996/CPSC-Soda-Vending-Machine-Industry-Labeling-Campaign-Warns-Of-Deaths-And-Injuries (Accessed: January 2025) ↑9 ClinCalc. (no date). Finasteride Drug Usage Statistics, United States, 2013-2022. ClinCalc. Available at: https://clincalc.com/drugstats/Drugs/Finasteride (Accessed: January 2025) ↑10 Merck & Co. Inc. (no date). Propecia Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf (Accessed: January 2025) ↑11 Bormann, C.L., Smith, G.D., Padmanabhan, V., Lee, T.M. (2011). Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reproduction 142(1). Available at: https://doi.org/10.1530/REP-10-0210 ↑12 An, N., Peng, J., He, G., Fan, X., Li, F., Chen, H. (2018). Involvement of activation of mitogen-activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) signaling pathway in proliferation of urethral plate fibroblasts in finasteride-induced rat hypospadias. Medical Science Monitor. 24. 8984-8992. Available at: https://doi.org/10.12659/MSM.911271 ↑13 Clark, R.L., Anderson, C.A., Prahalada, S., Robertson, R.T., Lochry, E.A., Leonard, Y.M., Stevens, J.L., Hoberman, A.M. (1993). Critical developmental periods for effects on male rat genitalia induced by finasteride, a 5 alpha-reductase inhibitor. Toxicology and Applied Pharmacology. 119(1). 34-40. Available at: https://doi.org/10.1006/taap.1993.1041 ↑14 Prahalada, S., Tarantal, A.F., Harris, G.H., Ellsworth, K.P., Clarke, A.P., Skiles, G.L., MacKenzie, K.I., Kruk, L.F., Ablin, D.S., Cukierski, M.A., Peter, C.P., vanZwieten, M.J., Hendrickx, A.G. (1997). Effects of finasteride, a type 2 5-alpha reductase inhibitor, on fetal development in the rhesus monkey (Macaca mulatta) 55(2). 119-131. Available at: 10.1002/(SICI)1096-9926(199702)55:2<119::AID-TERA1>3.0.CO;2-Z. ↑15 US Food and Drug Administration. (no date). Propecia (finasteride). US FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020788s017lbl.pdf (Accessed: January 2025) ↑16 Samplaski, M.K., Lo, K., Grober, E., Jarvi, K. (2013). Finasteride use in the male infertility population: effects on semen and hormone parameters. Fertility and Sterility. 100(6). 1542-1546. Available at: https://doi.org/10.1016/j.fertnstert.2013.07.2000 ↑17 Overstreet, J.W., Fuh, V.L., Gould, J., Howards, S.S., Lieber, M.M., Hellstrom, W., Shapiro, S., Carroll, P., Corfman, R.S., Petrous, S., Lewis, R., Toth, P., Shown, T., Roy, J., Jarow, J.P., Bonilla, J., Jacobsen, C.A., Wang, D.Z. Kaufman, K.D. (1999). Chronic treatment with finasteride daily does not affect spermatogenesis or semen production in young men. Journal of Urology. 162(4). 11295-300. Available at: PMID: 10492183 ↑18 J.K., Amory., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J., Clark, R.V. (2007). The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. The Journal of Clinical Endocrinology & Metabolism. 92(5). 1659-1665. Available at: https://doi.org/10.1210/jc.2006-2203 ↑19 J.K., Amory., Wang, C., Swerdloff, R.S., Anawalt, B.D., Matsumoto, A.M., Bremner, W.J., Walker, S.E., Haberer, L.J., Clark, R.V. (2007). The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. The Journal of Clinical Endocrinology & Metabolism. 92(5). 1659-1665. Available at: https://doi.org/10.1210/jc.2006-2203 ↑20 Kolasa, A., Roginska, D., Rzeszotek, S., Machalinski, B., Wiszniewska, B. (2021). Paternal finasteride treatment can influence the testicular transcriptome profile of male offspring – preliminary study. Current issues in molecular biology. 43(2). 868-886. Available at: https://doi.org/10.3390/cimb43020062 ↑21 Salvarci, A., Istanbulluoglu, O. (2012). Secondary infertility due to use of low-dose finasteride. International urology and nephrology. 45(1). 83-85. Available at: https://doi.org/10.1007/s11255-012-0315-9 ↑22 Tu, H.Y.V., Zini, A. (2011). Finasteride-induced secondary infertility associated with sperm DNA damage. Fertility and sterility. 95(6). e13-4. Available at: https://doi.org/10.1016/j.fertnstert.2010.12.061 ↑23 Anderson, J.T., Jenson, T.B., Horwitz, H., Clausen, S.S. (2019). Paternal exposure to finasteride – Before and during pregnancy. Pharmacoepidemiology and Drug Safety. 28(16). Conference Abstract. Available at: https://doi.org/10.1002/pds.4864 ↑24 Ahn, K.H., Shin, J., Hong, S.C., Han, JY., Lee, E.H., Lee, J.S., Oh, M.J., Kim, H.J. (2015). Pregnancy Outcomes with Paternal Exposure to Finasteride, a Synthetic 5-Alpha-Reductase Inhibitor: A Case Series. Journal of Clinical Toxicology. 5(2). Available at: https://doi.org/https://doi.org/10.1002/pds.4864 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sarah King, PhD
Dr. Sarah King is a researcher & writer who holds a BSc in Medical Biology, an MSc in Forensic Biology, and a Ph.D. in Molecular and Cellular Biology. While at university, Dr. King’s research focused on cellular aging and senescence through NAD-dependent signaling – along with research into prostaglandins and their role in hair loss. She is a co-author on several upcoming manuscripts with the Perfect Hair Health team.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down