- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesNatural vs. Pharmaceutical DHT Blockers: Ranked
First Published Nov 25 2025Last Updated Nov 26 2025Natural RemediesPharmaceutical Researched & Written By:Michael Williams, PhD
Researched & Written By:Michael Williams, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Natural and pharmaceutical DHT blockers both aim to slow or reverse hair loss, but their strength, safety, and side effects vary widely. This article ranks the most common DHT inhibitors from weakest to strongest, comparing how natural compounds, such as pumpkin seed oil and EGCG, stack up against prescription options like finasteride and dutasteride.
Full Article
Dihydrotestosterone (DHT) is a potent androgen hormone that can significantly impact hair loss. Its activity, however, isn’t just restricted to the scalp. What’s more, hair loss is a multifactorial condition, with a wide range of causes. As such, the solution to hair loss is much more complicated than simply eliminating DHT.
Many natural or pharmaceutical therapies seek to reduce, or rather, rebalance DHT levels. While lowering DHT can slow or even reverse hair loss, the hormone itself plays vital roles in sexual function, mood regulation, and overall well-being. Therefore, the goal should not be total suppression, but rather modulation that considers the balance between hair health and systemic androgen levels.
In this article, we will rank both natural and pharmaceutical DHT blockers from weakest to strongest, based on research, effectiveness, and risk of side effects.
All-Natural Hair Topical
The top natural ingredients for hair growth, all in one serum.
Take the next step in your hair growth journey with a world-class natural serum. Ingredients, doses, & concentrations built by science.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Understanding DHT: Why Blocking It Helps (and Hurts)
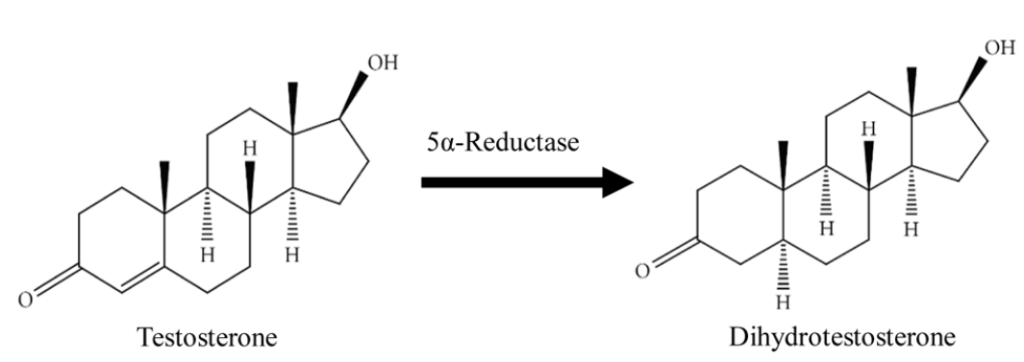
DHT is derived from testosterone through the enzyme 5-alpha-reductase. Once formed, DHT binds to androgen receptors and plays a major role in androgenic alopecia (AGA). In hair follicles genetically sensitive to DHT, such as those on the crown and frontal scalp, the hormone acts as a signal for miniaturization. Over time, the follicle shrinks, producing thinner, shorter hairs until it eventually becomes dormant.[1]York, K., Meah, N., Bhoyrul, B., & Sinclair, R. (2020). A review of the treatment of male pattern hair loss. Expert Opinion on Pharmacotherapy. 21(5). 603-612. Available at: … Continue reading
Figure 1. 5α-reductase converts testosterone into dihydrotestosterone. Finasteride is a 5α-reductase inhibitor, meaning it prevents or reduces this activity. Image adapted from Figure 1.[2]Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti-5-alpha-reductase activity: A prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: … Continue reading Image used under Creative Commons License.
By reducing DHT levels or blocking its receptor binding, therapies can interrupt this miniaturization cascade. The goal is to prevent DHT from triggering the cellular changes that cause follicles to shrink and enter a premature resting (telogen) phase. AGA is influenced not just by DHT levels but also by individual receptor sensitivity, local inflammation, and oxidative stress.
However, because of DHT’s wide-reaching activity, side effects of its reduction can include sexual dysfunction, decreased motivation, and mood changes, especially in individuals who are more hormonally sensitive. As such, careful consideration of different therapies and their potential impacts is essential if you choose to use a DHT-blocking treatment.[3]Traish, A. M., Melcangi, R. C., Bortolato, M., Garcia-Segura, L. M., & Zitzmann, M. (2015). Adverse effects of 5α-reductase inhibitors: what do we know, don’t know, and need to know?. Reviews … Continue reading
Ranking the DHT Blockers: From Mild to Potent
We’ll rank DHT blockers based on their potency, as well as on the quality of evidence supporting their use for hair loss. You can learn more about our treatment metrics, including evidence quality, in our article.
Tier 1 – Mild / Natural DHT Blockers
Natural treatments, typically based on oils and herbs, are generally more gentle than pharmaceutical options. They tend to be less potent, but have far fewer side effects and are not typically subject to the same stringent regulation as pharmaceuticals.
EGCG (Evidence Quality – 6%)
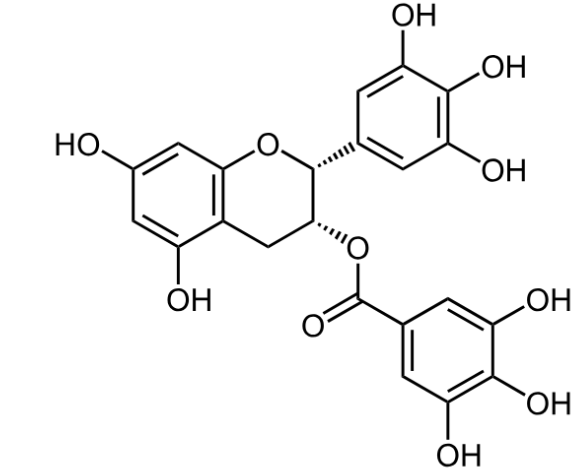
Epigallocatechin gallate (EGCG), a catechin compound found in green tea, is another naturally derived compound that has attracted interest for its potential to influence hair follicle biology. As a potent antioxidant and anti-inflammatory molecule, EGCG helps protect follicular cells from oxidative stress and inflammation, both of which are exacerbated by androgen activity.
Figure 2: Molecular structure of EGCG. Adapted from:[4]Wikipedia. (no date). Epigallocatechin gallate. Available at: https://en.wikipedia.org/wiki/Epigallocatechin_gallate (Accessed: November 2025) Image used under Creative Commons License
EGCG appears to enhance cell-survival pathways such as Akt and Erk, supporting follicular cell proliferation while suppressing apoptotic signals that can trigger hair follicle regression.[5]Kwon, O. S., Han, J. H., Yoo, H. G., Chung, J. H., Cho, K. H., Eun, H. C., & Kim, K. H. (2007). Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). … Continue reading
Laboratory studies also indicate that EGCG can partially inhibit 5AR, reducing the local generation of DHT.[6]Hiipakka, R. A., Zhang, H. Z., Dai, W., Dai, Q., & Liao, S. (2002). Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochemical Pharmacology. 63(6). … Continue reading However, while this study showed that EGCG can inhibit 5AR in cell-free experiments, it did not have the same impact in cells. This suggests that it may not be able to enter cells and would have reduced impacts in humans.
This seems to be the case, and clinical evidence for EGCG in the treatment of hair loss remains limited. A small pilot study of topical EGCG found no visible changes in hair, although this research was conducted in people with no hair loss disorders.[7]Kwon, O. S., Han, J. H., Yoo, H. G., Chung, J. H., Cho, K. H., Eun, H. C., & Kim, K. H. (2007). Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). … Continue reading Despite the lack of large-scale human trials, molecular findings do suggest EGCG contributes meaningfully to the cellular environment that supports hair growth and protection.
Catechins from green tea infusions are generally well tolerated. However, supplements should be used cautiously at high doses, particularly above 800 mg per day, as they have been linked to liver enzyme elevations in sensitive individuals.[8]EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Younes, M., Aggett, P., Aguilar, F., Crebelli, R., Dusemund, B., Filipič, M., Frutos, M. J., Galtier, P., Gott, D., … Continue reading
You can read more about the evidence for using EGCG for hair loss in our article here.
Pumpkin Seed Oil (Evidence Quality – 49%)
Pumpkin seed oil (PSO) is one of the most extensively studied non-pharmaceutical treatments for hair loss. In general, it is mild and well-tolerated. Its effects appear to arise from its profile of phytosterols and essential fatty acids, such as linoleic and oleic acid. These compounds have been shown to exhibit mild 5AR inhibitory activity in laboratory studies, potentially reducing local DHT formation in scalp tissues.[9]Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti 5-alpha-reductase activity: a prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: … Continue reading,[10]Liang, T., & Liao, S. (1992). Inhibition of steroid 5α-reductase by specific aliphatic unsaturated fatty acids. Biochemical Journal. 285(2). 557-562. Available at: … Continue reading
Beyond hormonal modulation, pumpkin seed oil provides antioxidant and anti-inflammatory support, which can help protect hair follicles from oxidative damage and microinflammation – two key contributors to progressive miniaturization.[11]Fahim, A. T., Abd-El Fattah, A. A., Agha, A. M., & Gad, M. Z. (1995). Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacological … Continue reading
A clinical trial carried out in Korea made waves in 2014 when it reported a 40% increase in hair count after 24 weeks. However, the formulation, Octa-Sabal Plus, included multiple additional herbal compounds, alongside pumpkin seed powder, not oil. This means we can’t attribute the growth reported to pumpkin seed oil.[12]Cho, Y. H., Lee, S. Y., Jeong, D. W., Choi, E. J., Kim, Y. J., Lee, J. G., & Cha, H. S. (2014). Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: a randomized, … Continue reading
You can read more about the controversial PSO study here.
Cold-pressed pumpkin seed oil is typically taken orally at doses ranging from 400 to 1000 mg daily, with results accumulating gradually over several months. The compound is generally well tolerated, with gastrointestinal discomfort being rare and mild.[13]Hong, H., Kim, C. S., & Maeng, S. (2009). Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutrition Research and Practice. 3(4). … Continue reading Although PSO alone is unlikely to reverse established AGA, it may serve as a gentle adjunct for individuals seeking natural or preventive strategies to support long-term scalp and hair health.
Saw Palmetto (Evidence Quality – 54%)
Saw palmetto (Serenoa repens) is one of the most widely studied natural 5AR inhibitors and is sometimes called “nature’s finasteride.” It appears to reduce DHT by inhibiting Type II 5AR, reducing the binding of DHT to androgen receptor sites and increasing the conversion of DHT to a weaker metabolite called androstanediol.[14]Suzuki, M., Ito, Y., Fujino, T., Abe, M., Umegaki, K., Onoue, S., Noguchi, H., & Yamada, S. (2009). Pharmacological effects of saw palmetto extract in the lower urinary tract. *Acta … Continue reading
Importantly, saw palmetto appears to reduce DHT selectively, with research showing decreases in DHT in the prostate but not in the blood. As such, it may cause fewer side effects than systemic DHT reducers.[15]Fagelman, E., & Lowe, F. C. (2001). Saw palmetto berry as a treatment for BPH. Reviews in Urology. 3(3). 134-138. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1476047/
Clinical studies have shown modest impacts of saw palmetto on hair loss. In one study, participants using oral extracts over several months showed reductions in hair shedding, increases in hair density, and subjective improvements in scalp coverage and hair quality.{ Importantly, however, this was an open-label study, where participants and researchers both knew what product they were receiving, so is not as strong a form of evidence as a randomzied controlled trial (RCT).[16]Rossi, A., Mari, E., Scarno, M., Garelli, V., Maxia, C., Scali, E., Iorio, A., & Carlesimo, M. (2012). Comparative effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: a … Continue reading
In a recent RCT, a standardized, proprietary saw palmetto extract was given as either a 400mg oral capsule or 5 ml of topical formulation and compared to relevant controls. The study found a significant reduction in hair shedding in both oral and topical groups compared to their controls, alongside an increase in hair density of 5% and 7.5% respectively. However, this study also had significant drawbacks. For example, measurements of hair density were not well defined and may therefore include vellus hairs, which do not contribute to visible hair. Despite this, the study does provide some support for the use of saw palmetto in decreasing hair shedding and increasing hair thickness.[17]Sudeep, H. V., Rashmi, S., Jestin, T. V., Richards, A., Gouthamchandra, K., & Shyamprasad, K. (2023). Oral and topical administration of a standardized saw palmetto oil reduces hair fall and … Continue reading
Evidence from benign prostatic hyperplasia suggests that an oral dose of 320 mg per day is optimal, with increases in dose above that resulting in no further improvements. For topical use, concentrations around 0.5 – 1% applied once or twice daily are common. Both routes are well tolerated, with infrequent reports of mild gastrointestinal discomfort or skin irritation.
You can read about the differences between saw palmetto and finasteride in our article here.
Tier 2 – Over-the-Counter Pharmaceutical Options
Over-the-Counter Pharmaceutical Options bridge the gap between gentle natural therapies and prescription-strength DHT blockers, potentially offering clinically supported benefits with relatively low systemic risk.
Ketoconazole Shampoo (Evidence Quality – 54%)
Originally developed as an antifungal agent, ketoconazole helps normalize the scalp environment by reducing inflammation, fungal overgrowth, and excess sebum, all of which can worsen AGA. Beyond its antifungal activity, laboratory and clinical research suggest that ketoconazole exerts mild local antiandrogenic effects, likely through partial inhibition of 5AR and modulation of DHT activity within the scalp.
Clinical evidence for men is stronger than for women. One 21-month study using 2% ketoconazole shampoo showed progressive improvement in hair shaft diameter and percentage of hairs in the growth (anagen) phase, reversing the slow decline seen in untreated AGA.[18]Piérard-Franchimont, C., De Doncker, P., Cauwenbergh, G., & Piérard, G. E. (1998). Ketoconazole shampoo: effect of long-term use in androgenic alopecia. Dermatology. 196(4). 474-477. Available … Continue reading
Another study, which compared Ketoconazole with other shampoos, found that 1% ketoconazole reduced hair shedding by 17%, improved anagen hair by 4.9%, and lowered scalp sebum levels.[19]Piérard-Franchimont, C., Goffin, V., Henry, F., Uhoda, I., Braham, C., & Piérard, G. E. (2002). Nudging hair shedding by antidandruff shampoos: A comparison of 1% ketoconazole, 1% piroctone … Continue reading Studies also show that ketoconazole performs well as an adjunct therapy, with synergistic effects when combined with finasteride.[20]Perez, B. S. H. (2004). Ketoconazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Medical Hypotheses. 62(1). 112-115. Available at: … Continue reading
Evidence in women is limited, with only one published study investigating ketoconazole solution, rather than shampoo. They compared 2% ketoconazole to 2% minoxidil and reported meaningful regrowth in both groups, with ketoconazole producing improvements by the six-month mark and causing far fewer side effects.[21]El-Garf, A., Mohie, M., & Salah, E. (2019). Trichogenic effect of topical ketoconazole versus minoxidil 2% in female pattern hair loss: a clinical and trichoscopic evaluation. Biomedical … Continue reading
Ketoconazole is generally well tolerated. Mild irritation, dryness, or temporary scalp tightness may occur, especially with 2% formulations or frequent use. Most regimens recommend applying it 2–3 times per week, leaving the shampoo on the scalp for 3–5 minutes before rinsing.
You can read our breakdown of the research into ketoconazole shampoos here.
You can also read about why 2% ketoconazole might be a better choice than 1% here.
Tier 3 – Prescription Pharmaceutical DHT Blockers
Prescription Pharmaceutical DHT Blockers represent the most potent and clinically validated options, offering stronger and more predictable suppression of DHT than natural therapies but with a greater need for careful monitoring of side effects.
Topical Finasteride (Evidence Quality – 69%)
Topical finasteride represents a new, more targeted approach to DHT inhibition, delivering the drug directly to the scalp while aiming to minimize systemic exposure. Like its oral counterpart, it blocks the Type II 5-alpha reductase enzyme. However, by focusing this activity locally within the hair follicle, topical formulations can achieve substantial reductions in scalp DHT while maintaining serum levels that are a fraction of those seen with oral dosing. This distinction is clinically important for individuals concerned about systemic side effects such as decreased libido or hormonal shifts.
Clinical research has consistently shown that topical finasteride can achieve similar improvements in hair density and regrowth as oral therapy. One 24-week study showed a 34.5% decrease in DHT, compared to 55.6% for oral finasteride. However, plasma concentrations were 100 times lower, indicating that systemic exposure is greatly reduced.[22]Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., Galván, J., Tebbs, V., Massana, È., on behalf of the Topical Finasteride Study Group. … Continue reading
Topical finasteride is particularly appealing to men who respond well to oral therapy but wish to lower the risk of systemic adverse effects. When applied once daily at concentrations between 0.1% and 0.25%, it can stabilize shedding within four to six months, with visible improvements generally continuing over the course of a year.[23]Mazzarella, G. F., Loconsole, G. F., Cammisa, G. A., Mastrolonardo, G. M., & Vena, G. A. (1997). Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a … Continue reading For optimal results, it can be combined with topical minoxidil to stimulate regrowth through complementary pathways.[24]Wang, H., Chen, B., & Hong, X. (2020). The efficacy and safety of finasteride combined with topical minoxidil for androgenetic alopecia: A systematic review and meta-analysis. *Journal of … Continue reading
Our members can find the ultimate guide to finasteride here.
Dutasteride (Evidence Quality – 77%)
Dutasteride is the most potent 5AR inhibitor currently available, targeting both Type I and Type II isoenzymes. This dual inhibition results in serum DHT reductions of up to 90 – 95%, creating a profoundly anti-androgenic environment within scalp follicles.[25]Roehrborn, C. G., Marks, L. S., Fenter, T., Freedman, S., Tuttle, J., Gittleman, M., Morrill, B., & Wolford, E. T. (2004). Efficacy and safety of dutasteride in the four-year treatment of men … Continue reading Clinically, this translates into faster and often more pronounced regrowth compared to finasteride, particularly in individuals with advanced hair thinning.
Because dutasteride suppresses DHT more comprehensively, it may pose a slightly higher risk of hormonal side effects such as gynecomastia or reduced libido. However, head-to-head studies have suggested superior efficacy for dutasteride in both short-term and long-term endpoints.
A meta-analysis, which combines results from multiple studies, indicated that dutasteride showed better efficacy in treating AGA.[26]Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., & Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and … Continue reading These results suggest that dutasteride is ~3 times more potent than finasteride in blocking Type II 5α-reductase and up to 100 times more potent against Type I. This dual inhibition reduces scalp DHT levels by over 50%, compared to approximately 41% with finasteride, leading to greater prevention of follicular miniaturization and enhanced regrowth response with similar levels of adverse effects.
Dutasteride has a relatively low evidence quality score compared to finasteride, though this is largely due to there being fewer studies. The studies that do investigate dutasteride have shown higher potency and effectiveness.
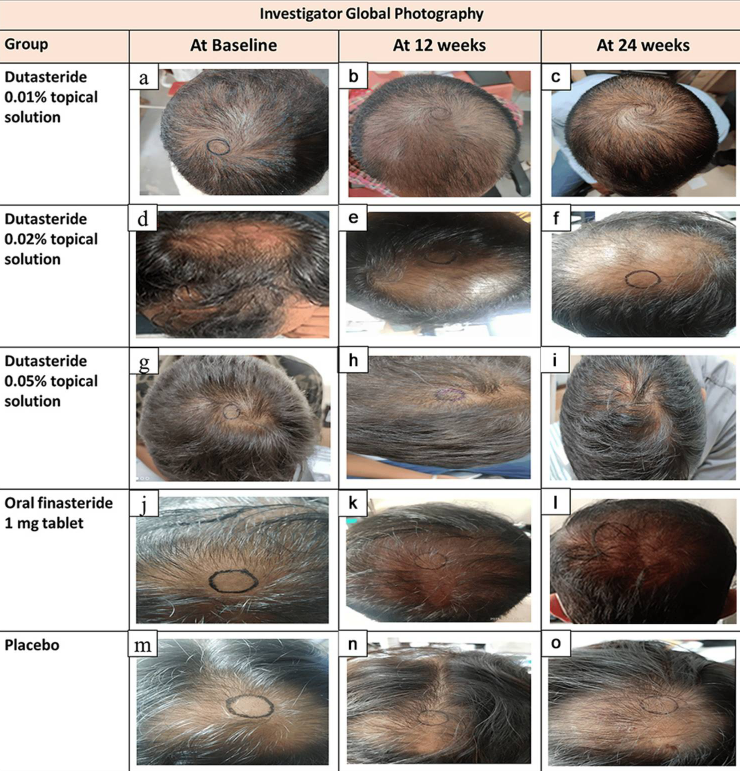
Topical dutasteride formulations are now under active investigation. Concentrations ranging from 0.01% to 0.05% have produced significant gains in target-area hair counts, with studies suggesting that improvements associated with oral formulations can be achieved without drops in serum DHT.[27]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., KSSVV, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo and active controlled Phase II study to … Continue reading
Figure 3. Topical Dutasteride supports hair regrowth. Adapted from Figure 2.[28]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., KSSVV, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo and active controlled Phase II study to … Continue reading Image used under Creative Commons License
A 2018 randomized clinical study further demonstrated that topical dutasteride (0.02%) combined with microneedling significantly improved hair shaft thickness and patient satisfaction compared with microneedling alone, without notable systemic absorption or hormonal side effects. These findings suggest that localized delivery of dutasteride may enhance follicular response while minimizing systemic exposure and related adverse effects.[29]Nada, E.A., El Sharkawy, R.E.E.D., Abd El-Maged, W. M., & Abo Elmagd, M. A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. … Continue reading
Similarly, research published in 2024 looked into the impact of microneedling. However, in this study, topical dutasteride was combined with minoxidil. They found similar improvements in hair density and hair width in participants who had topical dutasteride and minoxidil treatment to those in patients who had oral dutasteride and topical minoxidil.[30]Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through … Continue reading These results suggest that, in combination with other therapies (minoxidil or microneedling), topical dutasteride may be able to produce similar results to oral formulations but with a significantly lower chance of side effects.
A newly published Phase II trial has also drawn significant attention because it is the first study to test topical dutasteride alone, without microneedling. The results appear impressive: all topical doses improved hair parameters versus placebo, showed minimal impact on serum DHT (suggesting good scalp localization), and 1 ml daily of 0.05% topical dutasteride reportedly outperformed oral finasteride in key regrowth metrics.[31]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading
However, the study reports unusually high baseline hair counts, as well as inconsistencies in the placement and size of the 1 cm² target areas used for serial hair counts. These issues can dramatically skew results, creating the illusion of improvement even when none exists. Real-world data from users of low-dose topical dutasteride (0.01–0.02% applied daily or near-daily) also paint a more modest picture: good localization and serum DHT stability, but primarily maintenance rather than regrowth exceeding oral finasteride
Oral Finasteride (Evidence Quality – 99%)
As you can see from our evidence quality score, oral finasteride remains the most clinically validated pharmacological treatment for AGA. It acts as a selective inhibitor of the Type II 5AR enzyme, reducing systemic and scalp DHT levels by approximately 60–70%.[32]Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W. P., Cotterill, P. C., Thiboutot, D. M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S. J., Griffin, E. I., Weiss, D., … Continue reading This substantial suppression slows or halts follicular miniaturization, prolongs the growth (anagen) phase, and enables partial regrowth in many users. The standard 1 mg daily dose for AGA has been extensively studied and represents the optimal balance between efficacy and safety for most men.
Clinical trials have consistently confirmed the effectiveness of finasteride in both the short and long term. A 4-year, randomized study found that 1 mg of finasteride led to increased hair weight (46% vs placebo) and hair count (20% vs. placebo).[33]Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. … Continue reading
You can find an in-depth overview of the clinical studies on finasteride here.
While highly effective, oral finasteride is not without potential drawbacks. A small percentage of users report side effects related to sexual function, including reduced libido, erectile difficulty, and diminished ejaculate volume. Most of these effects are mild and resolve upon discontinuation, although persistent symptoms have been reported in rare cases. Some users also report changes in mood or motivation, likely reflecting individual hormonal sensitivity. For those wishing to minimize systemic exposure, transitioning to topical finasteride or titrating to a lower dose (e.g., 0.25-0.5 mg daily) may be a suitable option.[34]Gupta, A. K., Venkataraman, M., Talukder, M., & Bamimore, M. A. (2022). Finasteride for hair loss: a review. Journal of Dermatological Treatment. 33(4). 1938-1946. Available at: … Continue reading
There have also been reports of a condition that’s been named ‘post-finasteride syndrome’. While not widely accepted by the medical community, similar symptoms have been reported in a number of individuals who are taking finasteride. These symptoms can include sexual dysfunction, such as loss of libido and erectile dysfunction, neuropsychiatric symptoms like depression and anxiety, and physical changes like muscle atrophy and fatigue. Importantly, there have been cases where these symptoms have persisted after users have stopped taking the drug.
While the authors who published the original article on post-finasteride syndrome do highlight the lack of consistent evidence for persistent side effects found in clinical trials, they suggest that finasteride may act to worsen symptoms associated with sexual dysfunction and psychiatric conditions for some users who already have these issues.[35]Pereira, A. F. J. R., & Coelho, T. O. A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001
Overall, oral finasteride remains the gold standard for DHT suppression, providing robust and predictable results when used consistently. It’s also important to consider the potential side effects when choosing to take finasteride, and consult with your doctor if symptoms arise.
Honourable Mention – Pyrilutamide
Pyrilutamide (KX-826)
Pyrilutamide takes a different approach to androgen modulation. Rather than reducing DHT production, it acts as a topical androgen receptor antagonist, blocking DHT and testosterone from binding to their receptors in the follicle. By interfering with receptor activation, pyrilutamide aims to suppress the downstream gene expression that leads to follicular miniaturization.[36]Koralewicz, M. M., & Szatkowska, O. A. (2024, July). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 61-70. Available at: … Continue reading
Clinical data show encouraging signals. Trials conducted in China and the United States suggest that topical formulations applied once or twice daily can produce measurable increases in hair counts over several months. Reported side effects are primarily mild local dermatitis, and systemic exposure appears very low. However, many of these results remain unpublished in peer-reviewed journals and have failed to demonstrate statistical superiority over placebo for some measurements.[37]Kintor Pharmaceuticals. (2022). Developing Novel Drugs and Commercialization Platform. Available at: … Continue reading,[38]Kintor Pharmaceutical Limited. (2023, March 28). Kintor Pharma Announces Completion of Subject Enrollment in Phase III Clinical Trial for KX-826 for Treatment of Male Androgenetic Alopecia in China. … Continue reading Because these reports have not been published in peer-reviewed journals, they can only currently be considered pre-releases, rather than academic papers. The absence of published, peer-reviewed, blinded, controlled data means that we can’t draw firm conclusions on pyrilutamide, even in the wake of the many press releases that Kintor has published.
For now, pyrilutamide should be viewed as an experimental therapy. Its mechanism is compelling and localized, offering a theoretical advantage over systemic antiandrogens. Still, robust, peer-reviewed clinical trials confirming efficacy and long-term safety are needed before it can be considered a mainstream treatment. Those interested in exploring it should do so only under medical supervision or within the context of formal clinical research.
Final Thoughts
DHT blockers span a broad spectrum, ranging from gentle, naturally derived modulators like pumpkin seed oil and EGCG to powerful prescription therapies such as finasteride and dutasteride. Natural options may improve scalp health and have a modest influence on hormonal activity, but their effects are typically gradual and limited. In contrast, pharmacological inhibitors act directly on the enzymatic pathways responsible for DHT synthesis, offering stronger and more predictable outcomes. Topical finasteride has emerged as a compelling middle ground, delivering scalp-level efficacy with minimal systemic impact, while dutasteride represents the upper limit of potency for those requiring more aggressive intervention.
Ultimately, the “best” DHT blocker depends on individual tolerance, goals, and the stage of hair loss. A well-balanced regimen typically combines a DHT inhibitor with a growth stimulant, such as minoxidil, alongside scalp care and lifestyle modifications. With consistent use and proper medical guidance, these approaches can collectively slow, halt, or even reverse the progression of AGA, helping individuals maintain fuller, healthier hair over time.
References[+]
References ↑1 York, K., Meah, N., Bhoyrul, B., & Sinclair, R. (2020). A review of the treatment of male pattern hair loss. Expert Opinion on Pharmacotherapy. 21(5). 603-612. Available at: https://doi.org/10.1080/14656566.2020.1721463 ↑2 Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti-5-alpha-reductase activity: A prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: https://doi.org/10.12688/f1000research.51066.3 ↑3 Traish, A. M., Melcangi, R. C., Bortolato, M., Garcia-Segura, L. M., & Zitzmann, M. (2015). Adverse effects of 5α-reductase inhibitors: what do we know, don’t know, and need to know?. Reviews in Endocrine and Metabolic Disorders. 16(3). 177-198. Available at: https://doi.org/10.1007/s11154-015-9319-y ↑4 Wikipedia. (no date). Epigallocatechin gallate. Available at: https://en.wikipedia.org/wiki/Epigallocatechin_gallate (Accessed: November 2025) ↑5, ↑7 Kwon, O. S., Han, J. H., Yoo, H. G., Chung, J. H., Cho, K. H., Eun, H. C., & Kim, K. H. (2007). Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine. 14(7-8). 551-555. Available at: https://doi.org/10.1016/j.phymed.2006.11.003 ↑6 Hiipakka, R. A., Zhang, H. Z., Dai, W., Dai, Q., & Liao, S. (2002). Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochemical Pharmacology. 63(6). 1165-1176. Available at: https://doi.org/10.1016/S0006-2952(02)00848-1 ↑8 EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Younes, M., Aggett, P., Aguilar, F., Crebelli, R., Dusemund, B., Filipič, M., Frutos, M. J., Galtier, P., Gott, D., Gundert-Remy, U., Kuhnle, G. G., Lambré, C., Leblanc, J.-C., Lillegaard, I. T., Moldeus, P., Mortensen, A., Oskarsson, A., Stankovic, I., Waalkens-Berendsen, I., Woutersen, R. A., Andrade, R. J., Fortes, C., Mosesso, P., Restani, P., Arcella, D., Pizzo, F., Smeraldi, C., & Wright, M. (2018). Scientific opinion on the safety of green tea catechins. *EFSA Journal.* 16(4). e05239. Available at: https://doi.org/10.2903/j.efsa.2018.5239 ↑9 Azizi, A., Mumin, N. H., & Shafqat, N. (2021). Phytochemicals with anti 5-alpha-reductase activity: a prospective for prostate cancer treatment. F1000Research. 10. 221. Available at: https://doi.org/10.12688/f1000research.51066.3 ↑10 Liang, T., & Liao, S. (1992). Inhibition of steroid 5α-reductase by specific aliphatic unsaturated fatty acids. Biochemical Journal. 285(2). 557-562. Available at: https://doi.org/10.1042/bj2850557 ↑11 Fahim, A. T., Abd-El Fattah, A. A., Agha, A. M., & Gad, M. Z. (1995). Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacological Research. 31(1). 73-79. Available at: https://doi.org/10.1016/1043-6618(95)80051-4 ↑12 Cho, Y. H., Lee, S. Y., Jeong, D. W., Choi, E. J., Kim, Y. J., Lee, J. G., & Cha, H. S. (2014). Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: a randomized, double-blind, placebo-controlled trial. *Evidence-Based Complementary and Alternative Medicine.* 2014(1). 549721. Available at: https://doi.org/10.1155/2014/549721 ↑13 Hong, H., Kim, C. S., & Maeng, S. (2009). Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutrition Research and Practice. 3(4). 323-327. Available at: https://doi.org/10.4162/nrp.2009.3.4.323 ↑14 Suzuki, M., Ito, Y., Fujino, T., Abe, M., Umegaki, K., Onoue, S., Noguchi, H., & Yamada, S. (2009). Pharmacological effects of saw palmetto extract in the lower urinary tract. *Acta Pharmacologica Sinica.* 30(3). 271-281. Available at: https://doi.org/10.1038/aps.2009.1 ↑15 Fagelman, E., & Lowe, F. C. (2001). Saw palmetto berry as a treatment for BPH. Reviews in Urology. 3(3). 134-138. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1476047/ ↑16 Rossi, A., Mari, E., Scarno, M., Garelli, V., Maxia, C., Scali, E., Iorio, A., & Carlesimo, M. (2012). Comparative effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: a two-year study. International Journal of Immunopathology and Pharmacology. 25(4). 1167-1173. Available at: https://doi.org/10.1177/039463201202500435 ↑17 Sudeep, H. V., Rashmi, S., Jestin, T. V., Richards, A., Gouthamchandra, K., & Shyamprasad, K. (2023). Oral and topical administration of a standardized saw palmetto oil reduces hair fall and improves hair growth in androgenetic alopecia subjects – a 16-week randomized, placebo-controlled study. Clinical, Cosmetic and Investigational Dermatology. 16. 3251-3266. Available at: https://doi.org/10.2147/CCID.S380214 ↑18 Piérard-Franchimont, C., De Doncker, P., Cauwenbergh, G., & Piérard, G. E. (1998). Ketoconazole shampoo: effect of long-term use in androgenic alopecia. Dermatology. 196(4). 474-477. Available at: https://doi.org/10.1159/000017954 ↑19 Piérard-Franchimont, C., Goffin, V., Henry, F., Uhoda, I., Braham, C., & Piérard, G. E. (2002). Nudging hair shedding by antidandruff shampoos: A comparison of 1% ketoconazole, 1% piroctone olamine and 1% zinc pyrithione formulations. International Journal of Cosmetic Science. 24(5). 249-256. Available at: https://doi.org/10.1046/j.1467-2494.2002.00145.x ↑20 Perez, B. S. H. (2004). Ketoconazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Medical Hypotheses. 62(1). 112-115. Available at: https://doi.org/10.1016/S0306-9877(03)00264-0 ↑21 El-Garf, A., Mohie, M., & Salah, E. (2019). Trichogenic effect of topical ketoconazole versus minoxidil 2% in female pattern hair loss: a clinical and trichoscopic evaluation. Biomedical Dermatology. 3(1). 1-8. Available at: https://doi.org/10.1186/s41702-019-0046-y ↑22 Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., Galván, J., Tebbs, V., Massana, È., on behalf of the Topical Finasteride Study Group. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology. 36(2). 286-294. Available at: https://doi.org/10.1111/jdv.17738 ↑23 Mazzarella, G. F., Loconsole, G. F., Cammisa, G. A., Mastrolonardo, G. M., & Vena, G. A. (1997). Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. Journal of Dermatological Treatment. 8(3). 189-192. Available at: https://doi.org/10.3109/09546639709160517 ↑24 Wang, H., Chen, B., & Hong, X. (2020). The efficacy and safety of finasteride combined with topical minoxidil for androgenetic alopecia: A systematic review and meta-analysis. *Journal of Dermatological Treatment.* Available at: https://doi.org/10.1007/s00403-020-02100-6 ↑25 Roehrborn, C. G., Marks, L. S., Fenter, T., Freedman, S., Tuttle, J., Gittleman, M., Morrill, B., & Wolford, E. T. (2004). Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology. 63(4). 709-715. Available at: https://doi.org/10.1016/j.urology.2004.01.001 ↑26 Zhou, Z., Song, S., Gao, Z., Wu, J., Ma, J., & Cui, Y. (2019). The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis. Clinical Interventions in Aging. 14. 399-406. Available at: https://doi.org/10.2147/CIA.S192435 ↑27 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., KSSVV, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo and active controlled Phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑28 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., KSSVV, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo and active controlled Phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑29 Nada, E.A., El Sharkawy, R.E.E.D., Abd El-Maged, W. M., & Abo Elmagd, M. A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). 387-400. Available at: https://doi.org/10.21608/smj.2018.42083 ↑30 Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between Topical Minoxidil 5% Alone versus Combined with Dutasteride (Topical 0.02% through Microneedling or Oral 0.5 mg) in Treatment of Androgenetic Alopecia. QJM: An International Journal of Medicine. 117(Supplement_2). hcae175-207. Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑31 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. *Cureus.* 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑32 Drake, L., Hordinsky, M., Fiedler, V., Swinehart, J., Unger, W. P., Cotterill, P. C., Thiboutot, D. M., Lowe, N., Jacobson, C., Whiting, D., Stieglitz, S., Kraus, S. J., Griffin, E. I., Weiss, D., Carrington, P., Gencheff, C., Cole, G. W., Pariser, D. M., Epstein, E. S., Tanaka, W. K., Dallob, A. L., Vandormael, K., Geissler, L. A., & Waldstreicher, J. (1999). The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 41(4). 550-554. Available at: https://doi.org/10.1016/S0190-9622(99)80051-6 ↑33 Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. Journal of the American Academy of Dermatology. 55(1). 71-74. Available at: https://doi.org/10.1016/j.jaad.2005.07.001 ↑34 Gupta, A. K., Venkataraman, M., Talukder, M., & Bamimore, M. A. (2022). Finasteride for hair loss: a review. Journal of Dermatological Treatment. 33(4). 1938-1946. Available at: https://doi.org/10.1080/09546634.2021.1959506 ↑35 Pereira, A. F. J. R., & Coelho, T. O. A. (2020). Post-finasteride syndrome. Anais Brasileiros de Dermatologia. 95(3). 271-277. Available at: https://doi.org/10.1016/j.abd.2020.02.001 ↑36 Koralewicz, M. M., & Szatkowska, O. A. (2024, July). Topical solutions for androgenetic alopecia: evaluating efficacy and safety. Forum Dermatologicum. 10(3). 61-70. Available at: https://doi.org/10.5603/fd.101208 ↑37 Kintor Pharmaceuticals. (2022). Developing Novel Drugs and Commercialization Platform. Available at: https://docs.publicnow.com/viewDoc?hash_primary=BFD6444A4669BD4BE0683A8BCC8B5E80F3D7E430 (Accessed: October 2025)
↑38 Kintor Pharmaceutical Limited. (2023, March 28). Kintor Pharma Announces Completion of Subject Enrollment in Phase III Clinical Trial for KX-826 for Treatment of Male Androgenetic Alopecia in China. Available at: https://www.prnewswire.com/news-releases/kintor-pharma-announced-the-primary-endpoint-of-phase-ii-clinical-study-for-kx-826s-treatment-of-female-androgenetic-alopecia-in-china-was-met-301691321.html (Accessed: November 2025)
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Michael Williams, PhD
Michael is a researcher and writer who holds a BSc in Bioscience, an MSc in Regenerative Medicine, and a PhD in Translational Biomedicine. He undertook his PhD research at Houston Methodist Research Institute, Texas, focusing on cell signaling in the ovarian cancer tumor microenvironment. He conducted postdoctoral research at Barts Cancer Institute in London, exploring cellular metabolism in acute myeloid leukemia. He has published work in a range of fields, including oncology, nanomedicine, and cell-based therapeutics.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down