- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
7 Best Oils for Hair Growth
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesLow-Dose Topical Dutasteride: Better Than Oral Finasteride? (New Study & Photos)
First Published Dec 3 2025Last Updated Dec 20 2025Pharmaceutical Researched & Written By:Sarah King, PhD
Researched & Written By:Sarah King, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
A new clinical trial claims that low-dose topical dutasteride outperforms oral finasteride for hair regrowth, a result so surprising that it contradicts nearly everything we’ve seen in 5+ years of real-world member data. In this deep dive, we break down what we previously knew about topical dutasteride, what this new study reports, why its findings seem biologically implausible, and the two major methodological flaws that may completely invalidate its results.
Full Article
After years of anticipation, we finally have the first-ever study testing topical dutasteride as a standalone treatment for androgenic alopecia (AGA). No microneedling, no formulations mixed with other ingredients, just topical dutasteride itself.
And the results?
Frankly, they are shocking.
According to the study, low-dose topical dutasteride outperformed oral finasteride for hair regrowth.
On the face of it, this is so surprising that it borders on unbelievable, especially given the 5+ years of real-world data we’ve collected from our members.
So, in this article, we’ll break down:
- What we previously knew about topical dutasteride.
- How real-world user experiences compare to clinical findings.
- What this new study claims.
- And why two major methodological problems force us to seriously question the results.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What We Knew About Low-Dose Topical Dutasteride Before This Study
Until now, only two small clinical studies have evaluated low-dose topical dutasteride. Both used it alongside microneedling and applied it just once every 1-4 weeks.
-
Nada et al. (2018): Microneedling + Topical Dutasteride vs. Microneedling Alone
Nada et al. reported that adding low-dose topical dutasteride to a structured microneedling regimen improved hair density and shaft thickness more than microneedling alone, while only slightly reducing serum DHT, likely without meaningful systemic hormonal impact.[1]Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmaged, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). Available at: … Continue reading
30 men with AGA were randomized to either microneedling plus topical dutasteride 0.02% or microneedling alone for six months. Both groups received 13 microneedling sessions with a 1.5 mm Dermapen (12-needle cartridge) on a staggered schedule: weekly during the first 8 weeks, then gradually reduced to once every 2-4 weeks through month six. In the combination arm, up to 2 mL of 0.02% dutasteride
-
Sanchez-Meza et al. (2022): Microneedling + Topical Dutasteride vs. Microneedling Alone
Sanchez-Meza et al. found that adding very low-dose topical dutasteride to microneedling produced greater clinical and trichoscopic improvement than microneedling with placebo, without reported sexual side effects and with much lower total dutasteride exposure than earlier work.[2]Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-Y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical … Continue reading
34 men with AGA were randomized to microneedling plus 1 mL of 0.01% topical dutasteride (~0.1 mg) or microneedling plus 1 mL of saline as a placebo. Both groups received three treatment sessions spaced 4 weeks apart using a Dr. Pen Ultima A6 device set to a 2.5 mm needle depth, and outcomes were assessed over a 16-20 week period.
While both these studies showed mild hair regrowth, the effect is not from topical dutasteride alone, nor from daily use.
Furthermore, low-dose topical dutasteride showed almost no systemic dihydrotestosterone (DHT) suppression.
Across these studies, bloodwork suggested:
- Little to no measurable DHT suppression
- Meaning minimal systemic absorption
- And therefore lower risk of systemic side effects
This makes sense. Dutasteride is a large, lipophilic molecule. At low concentrations, it tends to stay localized to the scalp unless dosing or penetration enhancers are significant.
What Did Real-World Users Experience?
Because early studies were promising, some of our members tried 0.01-0.02% topical dutasteride in the years that followed.
A dozen users also tracked blood DHT levels before and after treatment.
The results? Strong localization with minimal regrowth.
- No meaningful changes in serum DHT (just normal daily variation of 10-15%).
- Even among users applying up to 2 mL daily.
- Meaning: absorption remained low – as expected.
- But…users also reported very little growth.
Instead, most people saw hair maintenance, not cosmetic improvement.
This aligns with the published studies: low-dose topical dutasteride appears to stabilize hair loss, with minimal systemic impact, but not drive substantial growth.
You can get more information about this and comparisons to topical finasteride here:
- Topical Dutasteride Vs. Topical Dutasteride: The Dose Decides Everything (2025)
- Topical Finasteride: Where Do We Stand Today? (2025 Update)
- Topical Finasteride: Same Results, No Side Effects? (2022)
What Happened in the August 2025 Study?
This new randomized, double-blind, placebo-controlled study appeared to be the gold standard of topical dutasteride research.[3]Panuganti, V.K., Mandala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., Rao, S., Dundigalla. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety … Continue reading
It was the first study to test topical dutasteride:
- By itself (no microneedling).
- With daily use.
- Across multiple doses (0.01%, 0.02%, and 0.05%).
- Against both a placebo and oral finasteride.
On paper, this looked like the definitive study that we have long needed.
Study Design
135 men aged 20-60 with Norwood III vertex, IV, or V AGA were randomized across five treatment arms in a 2:2:2:2:1 ratio:
- 0.01% topical dutasteride (n=30)
- 0.02% topical dutasteride (n=30)
- 0.05% topical dutasteride (n=30)
- Oral finasteride 1 mg + placebo topical (n=30)
- Placebo topical + placebo oral (n=15).
All participants were Asian men, average age of ~38 years. Baseline hair loss severity was balanced across groups.
Each 1 mL dose contained:
- 0.01% = 0.1 mg dutasteride
- 0.02% = 0.2 mg dutasteride
- 0.05% = 0.5 mg dutasteride
Importantly, the solution was nearly 30% dehydrated alcohol, a known penetration enhancer. This choice may have implications for systemic absorption and hair-count interpretation, though the authors claim systemic exposure remained minimal.
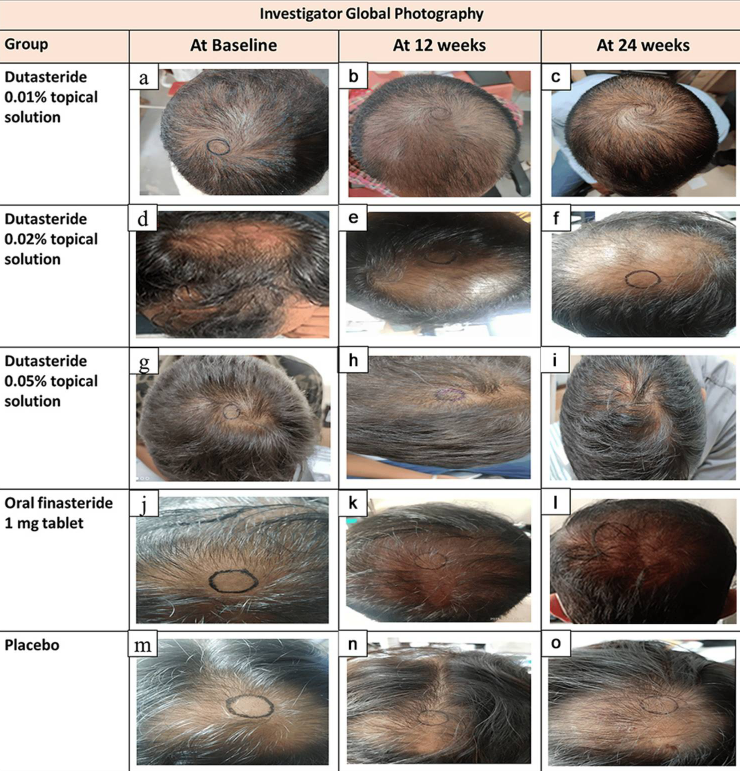
The researchers measured hair counts (total area hair counts) and hair widths (total area hair widths). Using a Dino-Lite microscope, researchers identified a 1.9 cm2 circular region at the vertex, clipped hairs to 0.5 – 1 mm, marked the center of the circle, and captured macrophotographs at baseline, week 12, and week 24.
The authors do not mention tattooing, ink permanence, or the use of a positioning device, which becomes critically important later.
The Study’s Reported Results
Here’s where the shock factor begins.
At 24 weeks, the study reported:
- 0.01% dutasteride: +32.32 hairs/cm2
- 0.02% dutasteride: +27.48 hairs/cm2
- 0.05% dutasteride: +75.52 hairs/cm2
- Oral finasteride: +41.21 hairs/cm2
- Placebo: 0.07 hairs/cm2
The headline claim: “0.05% topical dutasteride significantly outperformed oral finasteride (p=0.0083)”
This is a remarkable result, and one that contradicts every known real-world case we’ve observed at similar dosing.
Hair-Width Improvements
All active groups increased hair thickness, with results very close to oral finasteride:
- 0.01% +6.68 μm.
- 0.02% +9.15 μm.
- 0.05% +11.59 μm.
- Finasteride: +10.68 μm.
- Placebo: +4.00 μm.
Only the 0.05% topical and finasteride groups significantly beat placebo. The 0.05% topical did not significantly outperform finasteride in hair-width metrics.
Global Photography Assessment (Investigator-Rated)
At 24 weeks, the percentage of participants rated as having “moderate improvement” or better (GPA ≥ +2) was:
- 0.05% topical: 68.97%.
- Finasteride: 21.43%.
- Placebo: 13.33%.
These numbers suggest a level of regrowth from 0.5 mg/week of topical dutasteride that we’ve simply never witnessed, not in our community, not in the medical literature, and not among clinicians who routinely prescribe topical dutasteride.
Patient-Reported Outcomes (MHGQ)
By week 24, 96.55% of 0.05% topical users were satisfied with the hair on top of their heads. This is far above the finasteride group (71.43%) and far above the placebo (33.33%).
Again, the magnitude of the difference warrants scrutiny.
Serum Hormone Changes
This is where things do align with real-world experiences of low-dose topical users.
DHT Reductions
- Finasteride: 11% to -27%.
- 0.05% topical dutasteride: around -9% to -11%.
Testosterone Increases
- Finasteride: +20%.
- Topical dutasteride: modest, non-significant changes.
The authors emphasize that topical dutasteride caused minimal systemic effects.
This is consistent with our members’ lab data – but inconsistent with the hair-growth magnitude reported.
Pharmacokinetics (How Much Enters the Bloodstream)
According to the PK data:
- Plasma dutasteride levels were near or below quantification limits.
- A few values spiked as high as 2555 pg/mL, suggesting occasional high absorption events.
- Dutasteride remained detectable at day 168, implying accumulation over time.
The authors interpret this as “low systemic exposure”. But variability this large raises questions.
Safety Findings
Across the entire study, no serious adverse events were reported, no withdrawals due to safety, minimal skin irritation, and all groups showed mild effects like “glazing” at similar rates.
This matches expectations for low-dose topicals.
So, Why Don’t the Results Match Real-World Experience?
For more than five years, we’ve tracked user outcomes from low-dose topical dutasteride at comparable or even higher weekly doses than used in this study.
Not once have we seen:
- Regrowth exceeding oral finasteride.
- Cosmetic transformation from 0.05% topical alone.
- Hair-count improvements anywhere near +75 hairs/cm2.
Even in dermatology clinics across the world, this simply isn’t observed. So, why would this study find such dramatically different results?
It comes down to two major methodological problems – both related to hair counts.
Problem #1: Baseline Hair Counts That Defy Biology
The average baseline hair density reported in the study was 305-330 hairs/cm2.
For context:
- A healthy adult without hair loss typically has 100-250 hairs/cm2.
- Men with Norwood III-V AGA typically have 25-100 hairs/cm2 in the vertex.
- Yet this study reports 300+ hairs/cm2 in balding men.
This would mean that severely balding scalps had triple the hair density of a normal, non-balding scalp, and that hair counts exceeded what is physiologically plausible.
Why might this happen?
Possible Explanations
Vellus hairs were counted.
- The study never defines a diameter cutoff.
- Best practice is to exclude hairs ≤40 µm.
- Counting vellus hairs inflates numbers dramatically.
The measurement area was bigger than stated.
- The paper claims 1 cm2.
- Even a small mismeasurement could triple hair counts.
Manual counting errors.
- The study doesn’t specify software use.
- Manual hair counting is outdated and error-prone.
None of these possibilities inspires confidence in the baseline data.
Problem #2: The Measurement Circle Moves Between Photos
This is the more serious problem, and the one most likely to invalidate the findings entirely.
When examining the study’s published before-and-after images, the measurement circles:
- Change location.
- Change size.
- Change shape.
- Appear hand-drawn.
- Clearly do not track the same exact scalp area over time.
Figure 1: Representative hair growth images.[4]Panuganti, V.K., Mandala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., Rao, S., Dundigalla. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety … Continue reading
Take a close look at these images; the center point marks do not appear to be consistent (at least from these photos). This means that you really can’t compare the improvements over time, as the follow-up hair counts would have been conducted in slightly different areas of the scalp!
This is an enormous methodological flaw.
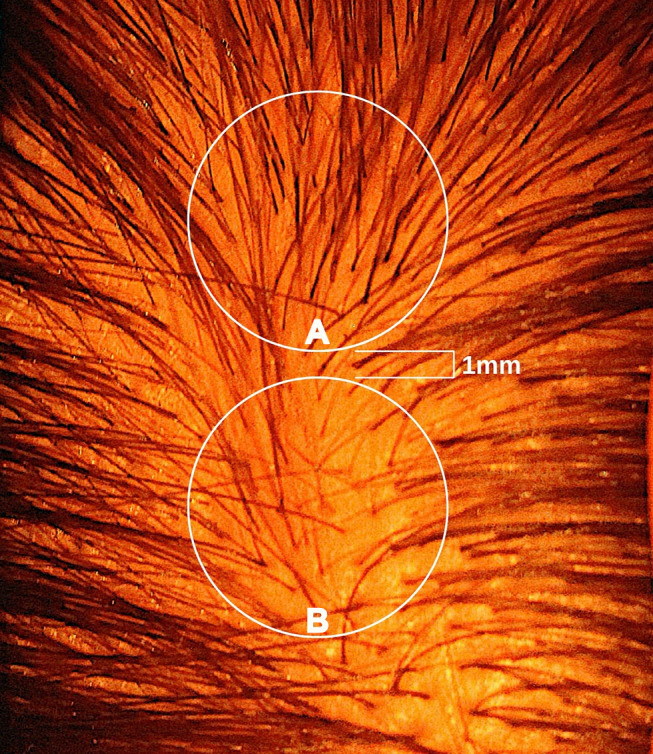
A shift of just 1-2 millimetres in circle placement can change hair counts by 50% or more, something we demonstrated in our 2021 publication.[5]Jnr, R.E., Ruiz, S. (2021). Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle … Continue reading
Fig 2: Circle A has 50% more hair than Circle B.[6]Jnr, R.E., Ruiz, S. (2021). Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle … Continue reading
Yet the improvements reported in this study were around 10-30%.
Meaning: These “improvements” could be fully explained by inconsistent circle placement, not actual regrowth.
This alone is enough to call the validity of the hair-count data into question.
Final Thoughts
This is one situation where we at PHH trust the real-world experiences of our members over a randomized, controlled clinical trial, because while the study appears rigorous on the surface, its hair-counting methods reveal inconsistencies significant enough to undermine its conclusions.
All available evidence still supports the following: low-dose topical dutasteride (0.01-0.05%) localizes well to the scalp, minimally suppresses serum DHT, effectively slows or stops hair loss, and rarely produces meaningful regrowth, whereas high-strength topical dutasteride (≥0.1%) is more likely to leak systemically, carry a greater risk of side effects, and generate visible regrowth approaching the results of oral finasteride.
References[+]
References ↑1 Nada, E.A., El-Dawla, R.E., El-Maged, W.M.A., Elmaged, M.A.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Sohag Medical Journal. 22(1). Available at: https://smj.journals.ekb.eg/article_42083_24b61cbba4be9982db23c318414034c0.pdf (Accessed: December 2025) ↑2 Sanchez-Meza, E., Ocampo-Candiani, J., Gomez-Flores, M., Herz-Ruelas, M.E., Ocampo-Garza, J., Orizaga-Y-Quiroga, T.L., Martinez-Moreno, A., Ocampo-Garza, S.S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. Journal of the European Academy of Dermatology and Venereology. 36(10). E806-e808. Available at: https://doi.org/10.1111/jdv.18285 ↑3 Panuganti, V.K., Mandala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., Rao, S., Dundigalla. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects with Androgenetic Alopecia. Cureus. 17(8). E89309. Available at: https://doi:10.7759/cureus.89309 ↑4 Panuganti, V.K., Mandala, P.K., Grandhi, V.R., Alluri, C.V., Mohammad, J., Rao, S., Dundigalla. (2025). A Randomized, Double-Blind, Placebo and Active Controlled Phase II Study to Evaluate the Safety and Efficacy of Novel Dutasteride Topical Solution (0.01%, 0.02%, and 0.05% w/v) in Male Subjects with Androgenetic Alopecia. Cureus. 17(8). E89309. Available at: https://doi:10.7759/cureus.89309 ↑5 Jnr, R.E., Ruiz, S. (2021). Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle Miniaturization Partly to Blame? Clinical, Cosmetic and Investigative Dermatology. 14. 357-365. Available at: https://doi.org/10.2147/CCID.S306157 ↑6 Jnr, R.E., Ruiz, S. (2021). Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle Miniaturization Partly to Blame? Clinical, Cosmetic and Investigative Dermatology. 14. 357-365. Available at: https://doi.org/10.2147/CCID.S306157 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sarah King, PhD
Dr. Sarah King is a researcher & writer who holds a BSc in Medical Biology, an MSc in Forensic Biology, and a Ph.D. in Molecular and Cellular Biology. While at university, Dr. King’s research focused on cellular aging and senescence through NAD-dependent signaling – along with research into prostaglandins and their role in hair loss. She is a co-author on several upcoming manuscripts with the Perfect Hair Health team.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down