- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
Scroll Down
Popular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Free CalculatorTopical Finasteride Calculator
- Free Interactive GuideInteractive Guide: What Causes Hair Loss?
- Free ResourceFree Guide: Standardized Scalp Massages
- Free Course7-Day Hair Loss Email Course
- Free DatabaseIngredients Database
- Free Interactive GuideInteractive Guide: Hair Loss Disorders
- Free DatabaseTreatment Guides
- Free Lab TestsProduct Lab Tests: Purity & Potency
- Free Video & Write-upEvidence Quality Masterclass
- Free Interactive GuideDermatology Appointment Guide
More
Articles100+ free articles.
-
7 Best Oils for Hair Growth
-
Hims Hair Growth Reviews: The Pros, Cons, and Real Results
-
Topical Finasteride Before and After: Real Case Studies
-
How to Reduce the Risk of Finasteride Side Effects
-
10 Best DHT-Blocking Shampoos
-
Best Minoxidil for Men: Top Picks for 2026
-
Switching From Finasteride to Dutasteride
-
Best Minoxidil for Women: Top 6 Brands of 2026
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
Before-Afters- Transformation Photos
Our library of before-after photos.
- Client Testimonials
Read the experiences of members
Before-Afters/ Client Testimonials- Popular Treatments
-
ArticlesFinasteride vs. Dutasteride: The Ultimate Guide
First Published Dec 31 2025Last Updated Jan 1 2026Pharmaceutical Researched & Written By:Michael Williams, PhD
Researched & Written By:Michael Williams, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor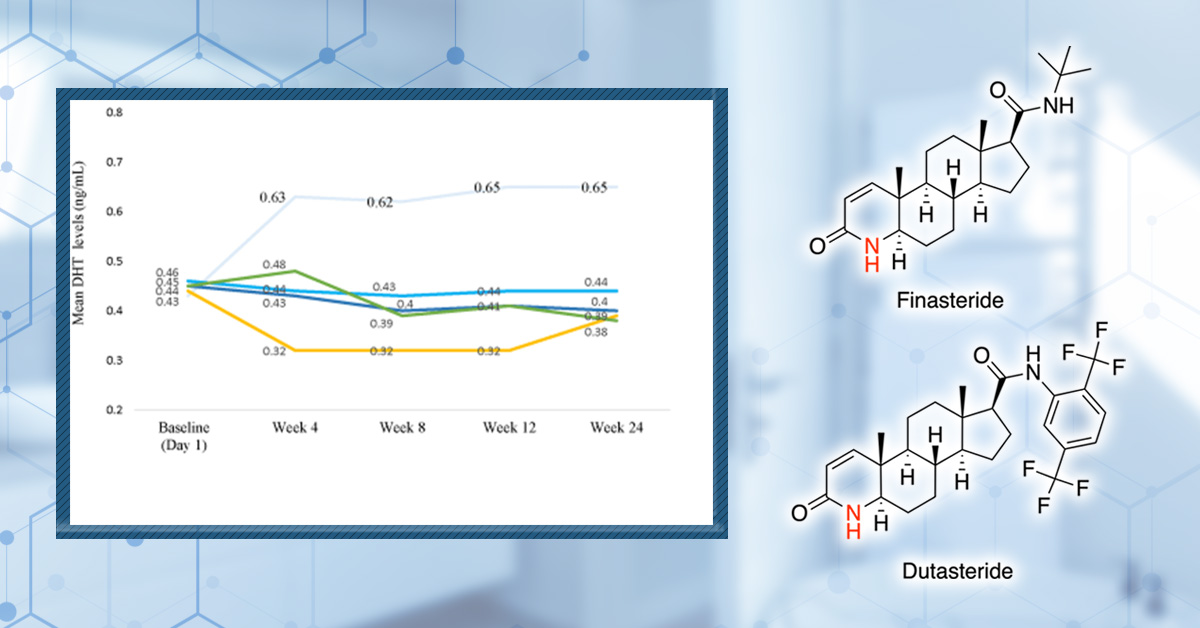
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Finasteride and dutasteride are the two most powerful medications available for androgenetic alopecia, but choosing between them isn’t straightforward. We break down how these drugs truly compare across efficacy, side effects, oral versus topical use, and real-world decision-making, so you can determine which option best fits your pattern of hair loss, risk tolerance, and long-term treatment goals.
Full Article
Finasteride and dutasteride are prescription medications that treat androgenic alopecia (AGA) by targeting the hormone pathway that drives follicle miniaturization. Because they share the same core mechanism and have similar effects on hair loss, understanding which treatment is the best choice for you is not straightforward.
In this article, we tackle the questions that come up most often when choosing between finasteride and dutasteride. We’ll break down how they differ biologically, what head-to-head studies really show about regrowth and side effects, and how oral versus topical use changes the equation, so you can make a more informed decision about which option makes sense for your hair loss and your goals.
Interested in Topical Finasteride?
Low-dose & full-strength finasteride available, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

What do Finasteride and Dutasteride Actually Do?
AGA is not a sudden shedding event but a slow remodeling of the hair follicle driven by androgen sensitivity. Across repeated hair cycles, susceptible follicles undergo progressive miniaturization: the growth phase (anagen) shortens with each cycle, while the rest phase (telogen) remains the same or lengthens.
Dihydrotestosterone (DHT) is the key androgen driving follicular miniaturization. An enzyme called 5α‑reductase (5AR) in the hair follicle converts testosterone to DHT. Because DHT is known to drive hair loss progression, many treatments for AGA target 5AR to reduce scalp DHT levels.[1]Asfour, L., Cranwell, W., & Sinclair, R. (2023). Male androgenetic alopecia. *Endotext [Internet].* Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/
Both finasteride and dutasteride can decrease levels of DHT in the body. They were originally created to treat a condition called benign prostate hyperplasia (BPH), but have been repurposed for AGA since their impact on hair growth was noticed. While finasteride is approved by the FDA for treating both BPH, dutasteride is approved only for BPH and is therefore used off-label for hair loss.
Type I vs Type II 5α-reductase
Finasteride is an inhibitor of Type II 5AR, which is highly expressed in hair follicles in androgen‑sensitive regions such as the scalp and prostate tissue. Type II is responsible for the conversion of testosterone to DHT inside miniaturizing scalp follicles, directly driving AGA‑related changes.[2]Makridakis, N., & Reichardt, J. K. V. (2005). Pharmacogenetic analysis of human steroid 5α-reductase type II: comparison of finasteride and dutasteride. *Journal of Molecular Endocrinology.* … Continue reading
Dutasteride is a dual 5AR inhibitor, which means that it blocks Type I 5AR as well as Type II. Type I 5AR plays a more supportive role and is mainly found in sebaceous glands and skin, so it has a less direct impact on scalp follicular miniaturization.[3]Frye, S. V. (2006). Discovery and clinical development of dutasteride, a potent dual 5α-reductase inhibitor. *Current Topics in Medicinal Chemistry.* 6(5). 405–421. Available at: … Continue reading
Because of this dual action, dutasteride may decrease levels of DHT to a greater extent than finasteride. In fact, studies have shown that dutasteride may decrease DHT by 50% more than finasteride.
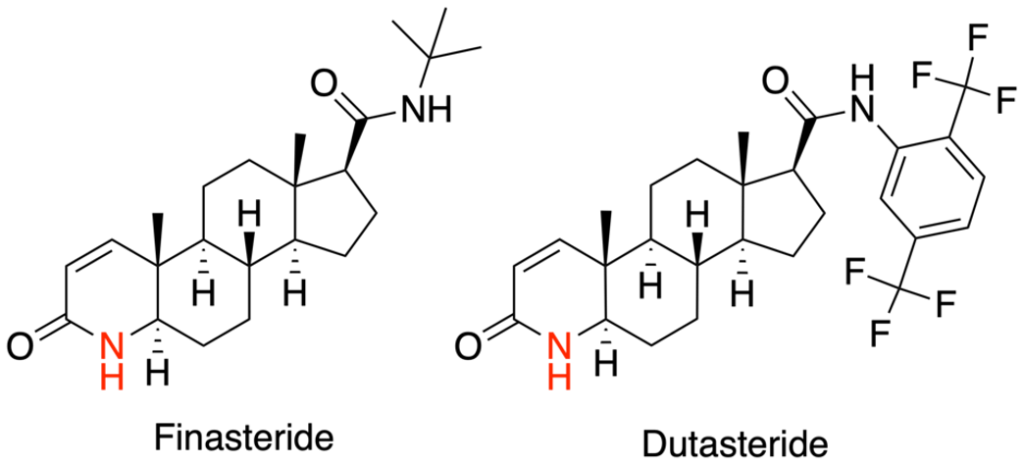
Figure 1. The structures of finasteride and dutasteride.[4]Wikimedia Commons. (n.d.). Azasteroid Pharmaceuticals [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Azasteroid_Pharmaceuticals.png (Accessed: November 2025) Image in the Public Domain.
Does that mean that dutasteride is the better option? Not necessarily. Lower DHT doesn’t necessarily translate into improved hair growth. What’s more, side effects associated with decreased DHT, particularly sexual dysfunction, can affect other aspects of health and well-being.
To understand the potential benefits and side effects of finasteride and dutasteride, we’ll dive into the science and studies that can help us make choices about treatment options.
Formulations: Topical or Oral?
It’s important to recognize that there are many different ways both finasteride and dutasteride can be taken. This includes the drug’s concentration, the frequency of administration, and the formulation used.
Oral finasteride and later oral dutasteride were designed to suppress DHT throughout the body, and they remain the most thoroughly studied options for AGA. Oral finasteride is most commonly prescribed at 1mg daily for AGA, while oral dutasteride is typically taken at 0.5mg daily. Some studies have tested a wider range of concentrations to investigate if there are any changes in benefit or risk.
Topical formulations were introduced later to change the risk profile without affecting the mechanism. Instead of lowering DHT systemically by default, topical finasteride was designed to concentrate drug activity in the scalp while limiting how much reaches the bloodstream.
Topical dutasteride represents the newest and least mature category, but evidence is promising. Because dutasteride is more potent than finasteride, even very low topical concentrations (around 0.01-0.05%) can meaningfully suppress scalp DHT. Importantly, both finasteride and dutasteride can and do enter systemic circulation when applied to the scalp, so safety concerns still need to be addressed.
Which Improves Hair Growth Most?
Because there is a range of treatment options for both finasteride and dutasteride, there is also a web of comparisons we can make when trying to compare the two. First, we’ll compare oral formulations. These are best researched, and there are a number of head-to-head comparison studies we can use to assess their effectiveness.
Oral Finasteride vs Oral Dutasteride
Here is a summary of the clinical trials that compare oral finasteride and dutasteride treatment directly:
Study Design and Population Treatments Impact on DHT Hair Growth Comparison Study #1[5]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Randomized, placebo-controlled, double-blinded trial. 415 men with male pattern hair loss aged 21-45 years old; 24 weeks. 5 mg finasteride; 0.05, 0.1, 0.5, or 2.5 mg dutasteride; placebo. Serum DHT was significantly lower at 0.5 and 2.5 mg dutasteride than at 5 mg finasteride. 5mg finasteride decreased scalp DHT by 41%, while 0.5 and 2.5 mg dutasteride decreased it by 51% and 79% respectively. The proportion of participants with at least a 10% increase in hair counts was 41% for finasteride, and
48%, and 56% for 0.5 and 2.5 mg
dutasteride, respectively.
Study #2[6]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Randomized, placebo-controlled, double-blinded trial. 917 men with AGA aged 20-50; 24 weeks. 1 mg finasteride; 0.02, 0.1, or 0.5 mg dutasteride; placebo Not assessed. 0.5 mg dutasteride significantly improved hair growth compared to 1 mg finasteride. Study #3[7]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Open-label, randomized study; no placebo control. 90 men with AGA aged 18-40; 24 weeks. 1 mg finasteride; 0.5 mg dutasteride. Not assessed. Increase in total hair count was significantly higher in dutasteride compared to finasteride. Study #4[8]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading Retrospective chart review. 600 men over 18 with AGA. 1 mg finasteride; 0.5 mg dutasteride. Not assessed. Dutasteride improved BASP basic M classification of AGA significantly better than finasteride. These RCTs directly comparing finasteride and dutasteride provide the best insight into their comparative efficacy. We’ll focus on 1 mg finasteride vs. 0.5 mg as these are the most commonly used doses. While these studies differ in design and duration, their findings are consistent across several clinically meaningful outcomes.
Key Takeaways from Trials
- Across head-to-head trials lasting 24 weeks, dutasteride consistently outperforms finasteride in increasing hair counts within a predefined target scalp area.[9]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading[10]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading
- Dutasteride also increases average hair shaft thickness more than finasteride 1 mg over 24 weeks.
- In trials that included investigator global photo assessment (GPA) or similar global assessments, dutasteride-treated participants were more likely to be rated as “improved” or “markedly improved” compared with those receiving finasteride.[11]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading
An important limitation to consider when interpreting these trials is the length of the studies. Most head-to-head RCTs run for 24 weeks, which may favor dutasteride if it is faster acting. Finasteride, by contrast, is known from longer studies to continue producing gains for 48 months, with hair counts typically plateauing only after around 2 years.[12]Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. … Continue reading
However, long-term data, though mostly retrospective rather than randomized, still suggest sustained superiority of dutasteride on global classification scales.[13]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading
How Big is the Difference in Reality?
On paper, dutasteride often looks clearly superior to finasteride. But translating statistical superiority from randomized trials into real-world expectations requires more nuance. The practical difference between these drugs depends less on averages and more on what kind of hair loss someone has, how fast it’s progressing, and how much regrowth is biologically possible.
Safety & Side Effects: Is Dutasteride Actually Riskier?
Blocking 5AR interferes with hormone activity and is known to cause some side effects. The most frequently discussed effects include:
- Sexual side effects – These include decreased libido, erectile dysfunction, and ejaculation disorders. Across randomized trials, these effects occur in a minority of users (generally in the single-digit percentages), appear most often early in treatment, and frequently resolve spontaneously, even while continuing therapy.[14]Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic … Continue reading
- Breast-related effects – Breast tenderness or enlargement has been reported, but rates are low across both drugs and typically comparable between finasteride and dutasteride.
- Other reported effects: Fatigue, mood changes, or nonspecific symptoms are occasionally reported, but these occur inconsistently, lack clear dose–response relationships, and are difficult to separate from background rates and expectation effects.
As we’ve already noted, the dual action of dutasteride could mean that it poses an increased risk of side effects. To see whether the evidence backs this up, we’ll take a look at what randomized trials and retrospective studies tell us about the comparative risks of each treatment. Below is a summary of the head-to-head studies to date.
Average incidence of side effects Decreased libido Ejaculation disorders Erectile dysfunction Breast enlargement and tenderness Study #1[15]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading Finasteride 2.6% vs dutasteride 4.6% Finasteride – 5 mg: 4%. Dutasteride – 0.1 mg: 3%; 0.5 mg: 1%; 2.5 mg: 13%. Dutasteride – 0.1 mg: 4%; 0.5 mg: 1%; 2.5 mg: 13%. Finasteride – 5 mg: 1% Dutasteride – 0.1 mg: 0%; 0.5 mg: 0%; 2.5 mg: 0%. Finasteride – 5 mg: 1% Not assessed. Study #2[16]Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study … Continue reading Finasteride 10.0% vs dutasteride 10.0% Not assessed. Not assessed. Not assessed. Not assessed. Study #3[17]Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled … Continue reading Finasteride 13.4% vs dutasteride 11.5% Finasteride – 1 mg: 6.7%. Dutasteride – 0.02 mg: 8.1%; 0.1 mg: 6.9%; 0.5 mg: 3.3%. Finasteride – 1 mg: 3.9%. Dutasteride – 0.02 mg: 2.2%; 0.1 mg: 4.8%; 0.5 mg: 3.9%. Finasteride – 1 mg: 5.6%. Dutasteride – 0.02 mg: 4.3%; 0.1 mg: 3.7%; 0.5 mg: 5.6%. Finasteride – 1 mg: 0.6%. Dutasteride – 0.02 mg: 0.5%; 0.1 mg: 0.5%; 0.5 mg: 0.6%. Study #4[18]Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male … Continue reading Finasteride 10.5% vs dutasteride 7.6% Finasteride 0.7% vs dutasteride 1.2% Not assessed Finasteride 0% vs dutasteride 0.4% Not assessed As we can see, across randomized and controlled studies comparing dutasteride and finasteride, the overall incidence of side effects is similar between the two drugs. When averaged across trials, total reported side effects occur at nearly identical rates (approximately 8-9% for both drugs).
In some studies, finasteride shows equal or higher rates of certain sexual side effects despite producing less DHT suppression. Importantly, these findings hold even when dutasteride reduces serum DHT by an additional 19-23% beyond finasteride and scalp DHT by significantly greater margins. The most closely scrutinized adverse effects, such as reduced libido, erectile dysfunction, and ejaculation disorders, do not follow a clear dose-response pattern with dutasteride.
Why Don’t We See More Side Effects With Dutasteride?
Given that dutasteride suppresses systemic and scalp DHT substantially more than finasteride, the lack of a clear increase in side effects may seem counterintuitive.
DHT reduction alone does not appear to be a reliable predictor of side-effect risk. This suggests that once DHT is reduced beyond a certain threshold, further suppression may not meaningfully increase the likelihood of side effects, particularly if compensatory mechanisms come into play. For example, lowering of DHT below a certain level might activate hormonal mechanisms to redress the balance. Long-term studies further support this idea, showing that side-effect incidence with dutasteride tends to decrease over time rather than accumulate.
Dutasteride’s size and tissue accessibility might also play a role. It has a much higher molecular weight (around 528 daltons) than finasteride (372 daltons), which likely limits its ability to cross the blood–brain barrier. Finasteride, by contrast, is small enough to enter brain tissue more readily. Because androgen metabolism in the brain is implicated in libido and sexual function, a drug that primarily exerts its effects in peripheral tissues may suppress DHT more aggressively without proportionally increasing neurological side effects.
Why do Incidence Estimates Vary?
Reported rates of side effects with finasteride and dutasteride vary widely across studies largely because incidence is highly dependent on study design. The makeup of the participant groups can impact how likely they are to experience side effects. How side effects are defined and recorded can also influence estimates. Some studies rely on spontaneous self-reporting, while others use targeted questionnaires or direct investigator questioning. These different approaches produce different reporting rates.
Psychological and selection factors further complicate interpretation. Participants who expect problems are more likely to notice and report them, even in placebo groups (this is called the nocebo effect). This means the information that participants receive can impact side effects.
How Long-Lasting are Side Effects?
Most randomized controlled trials are short (e.g., 24 weeks), enroll relatively healthy participants, and use structured adverse-event reporting. This means they tend to capture early, transient side effects, while missing longer-term patterns such as spontaneous resolution or adaptation. In contrast, observational and retrospective studies follow broader populations over longer periods but rely on less standardized reporting, which can inflate or obscure true incidence.
As a result, short-term trials may overestimate persistent risk, while long-term datasets may underrepresent early intolerance.
Where trials discuss the resolution of side effects, one trial reported that around half of affected participants taking dutasteride reported spontaneous resolution of symptoms while continuing treatment.[19]Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase … Continue reading By contrast, finasteride-associated side effects in these trials were less likely to resolve during ongoing treatment, even though many resolved after stopping the drug.
Interested in Oral Dutasteride?
Oral Dutasteride Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Post-Finasteride Syndrome: What to Know
Post-Finasteride Syndrome (PFS) is a term used to describe persistent sexual, cognitive, or mood-related symptoms that continue for months after stopping finasteride. Its existence and prevalence remain highly debated among dermatologists, endocrinologists, and hair loss researchers.
While some clinicians argue that PFS represents a real, drug-induced condition affecting a very small subset of users, others contend that the available evidence is low quality, heavily confounded by psychological factors, media influence, and the high background rates of sexual dysfunction in adult men.[20]Traish, A. M. (2020). Post-finasteride syndrome: a surmountable challenge for clinicians. *Fertility and Sterility.* 113(1). 21–50. Available at: https://doi.org/10.1016/j.fertnstert.2019.11.030,[21]Trüeb, R. M., Régnier, A., Rezende, H. D., & Dias, M. F. R. G. (2019). Post-finasteride syndrome: an induced delusional disorder with the potential of a mass psychogenic illness?. *Skin … Continue reading
Critically, high-quality clinical trials of finasteride did not report persistent side effects after discontinuation, with symptoms resolving within three months in all affected participants.[22]Shapiro, J., & Kaufman, K. D. (2003). Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). *Journal of Investigative Dermatology Symposium Proceedings.* … Continue reading Claims of PFS largely arise from case reports, small observational studies, and self-reported data, all of which suffer from major limitations such as lack of controls and small sample sizes.
One important exception is finasteride-induced gynecomastia (enlarged breast tissue in males), which is a well-documented side effect and can persist in a minority of cases even after stopping the drug, demonstrating that long-lasting tissue changes are possible, albeit rare.[23]Kaplan, S. A., Chung, D. E., Lee, R. K., Scofield, S., & Te, A. E. (2012). A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has … Continue reading
If PFS is real, it is likely extremely rare, plausibly affecting fewer than 1 in 5,000 – 10,000 users. For users, the key takeaway is one of risk context: while persistent side effects cannot be ruled out entirely, their estimated risk appears very low when weighed against the well-established benefits of finasteride for AGA, especially when dosing strategies are used to minimize overall exposure.
Topical Formulations
After oral formulations, the next logical question is whether you can get the same DHT-blocking benefits where it matters most. That’s the core promise of topical finasteride and dutasteride formulations: to localize 5AR inhibition to the scalp and reduce the likelihood of systemic side effects.
The potential for using topical formulations opens up a host of new options for treating AGA. Like oral formulations, topicals can also come in a range of concentrations, but can also be delivered in a range of formulations and solutions, including:
- Solutions and Sprays – Alcohol‑ or hydroalcoholic‑based liquids applied with a dropper, spray, or pipette, sometimes combined with propylene glycol or similar penetration enhancers.
- Gels and Lotions – Semi‑solid preparations designed to increase contact time on the scalp and modulate absorption.
- Nanoparticle and Liposomal Carriers: Research formulations load finasteride or dutasteride into lipid or polymeric nanoparticles or liposomes to target follicular delivery and reduce systemic exposure.
Formulations can also be combined with physical methods, such as microneedling, or with other common pharmaceuticals, like minoxidil. This also means that the decisions we need to make when choosing the right hair loss treatment are more complicated. Again, we’ll focus on head-to-head trials that compare different topical and oral formulations, as well as what clinical studies can tell us about side effects.
Does Topical Finasteride Work?
Based on trials comparing topical dutasteride to other AGA treatments, the answer is a resounding yes. However, the challenge is that finasteride has a highly sensitive, logarithmic dose-response curve. In other words, you don’t need much finasteride in the bloodstream to create a big systemic DHT change. That’s why topical finasteride can still “leak” systemically, and why even small systemic absorption can defeat the original purpose of going topical.
To take a closer look, we’ll first see how topical formulations compare to oral.
Oral vs Topical Finasteride
Several trials directly comparing topical to oral finasteride show that topical treatment can improve target-area hair counts and other hair parameters similarly to oral dosing, while often producing less suppression of serum DHT and far lower measured systemic drug exposure.
In one Phase III trial, topical finasteride produced hair-count improvements comparable to oral finasteride. What’s more, the oral formulation reduced mean serum DHT by 55.6%, whereas topical finasteride reduced it by 34.5%, and sexual side effects were more associated with oral treatment.[24]Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic … Continue reading
Other randomized comparisons similarly report meaningful regrowth with topical finasteride, with most adverse events being local (irritation) and relatively few systemic complaints.[25]Hajheydari, Z., Akbari, J., Saeedi, M., & Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. *Indian Journal of … Continue reading
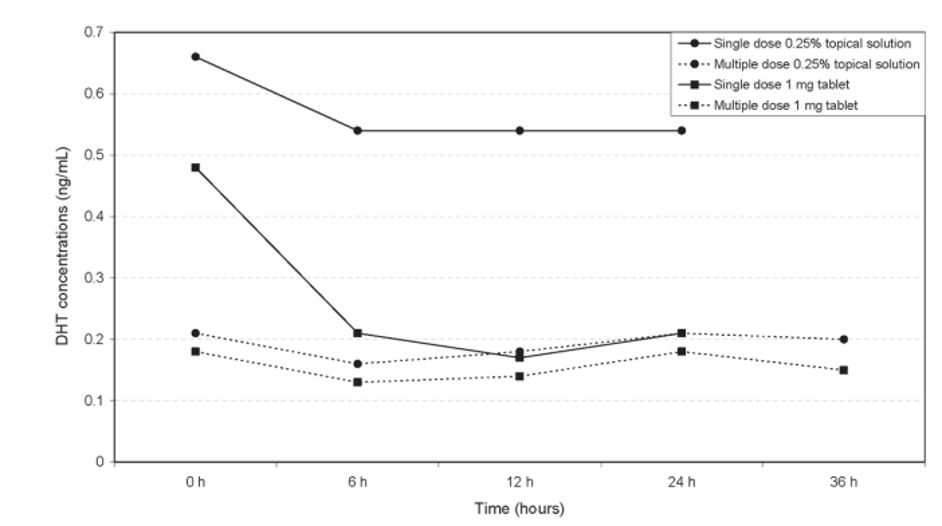
However, even at lower concentrations, topical finasteride can reduce serum DHT depending on how much you apply and how often you apply it. For example, one versus two daily applications of the same topical concentration can produce dramatically different serum DHT suppression, illustrating that frequency and volume can matter as much as concentration.[26]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
Figure 2. Multiple daily doses of topical finasteride can reduce serum DHT more than a single dose. Adapted from Figure 3.[27]Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen … Continue reading
What Determines Systemic Absorption?
There are a few key factors that impact absorption:
- Vehicle/carrier – Alcohol- and propylene glycol–based carriers tend to enhance penetration. Stronger penetration can improve scalp delivery, but it can also increase systemic leakage.
- Amount applied per use – This is often the most overlooked variable. Two people using the same concentration can have totally different systemic exposure if one applies 0.5 mL and the other applies 2 mL.
- Frequency of application – Twice-daily use can more than double systemic effects compared to once-daily use in certain protocols.
Does Topical Dutasteride Work?
Topical dutasteride research is newer and still developing, but early findings are encouraging. The current literature suggests that topical dutasteride can meaningfully improve hair density and shaft caliber while producing minimal changes in serum DHT.
A randomized, placebo-controlled trial in 34 men found that microneedling plus 0.01% topical dutasteride produced significant improvements in hair thickness, density, and the vellus-to-terminal ratio, compared with microneedling plus saline.[28]Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus … Continue reading
This is only a proof-of-concept study with a small sample size. However, it does demonstrate that topical dutasteride can drive clinically meaningful changes when follicular delivery is boostedby microneedling. But it also limits generalizability: microneedling itself is an active intervention, and it may not reflect what happens with topical dutasteride alone.
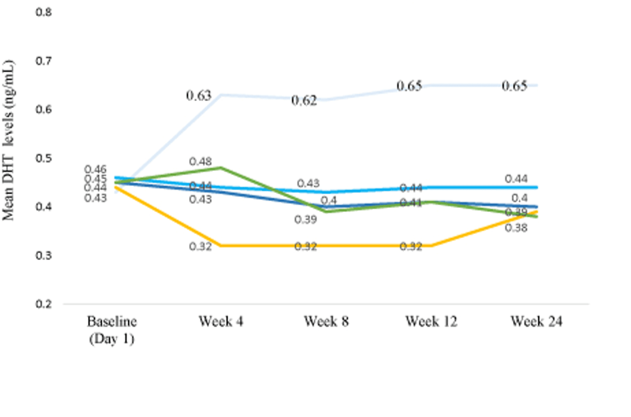
A newer Phase II trial tested 0.01%, 0.02%, and 0.05% topical dutasteride and reported dose-dependent improvements in total hair count and hair width versus placebo.[29]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading
The authors also reported that 0.05% appeared to outperform oral finasteride in total hair count. That’s an attention-grabbing claim, but it should be interpreted cautiously. Unusually high baseline counts and possible inconsistencies in target-area placement, which could inflate apparent differences, raise concerns about the methodology used.
Figure 3. Oral finasteride (yellow line) reduces serum DHT levels more than topical Dutaseride (blue lines). Adapted from Figure 3.[30]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading Image used under Creative Commons License
A common objection is that dutasteride is too large to penetrate the skin well. At around 528.5 Daltons, it sits slightly above the widely cited 500 Dalton rule, which suggests that larger molecules struggle to cross the stratum corneum. In practice, dutasteride appears capable of penetrating the scalp, as it can suppress DHT and promote hair growth.
Combination strategies: where topical regimens often perform best
Topical regimens tend to perform best when they’re used alongside other treatments, rather than as a single agent. DHT drives miniaturization, but follicular stimulation, blood-flow signaling, and wound-healing pathways all influence whether a follicle can thicken and re-enter robust growth.
Microneedling consistently appears in the literature as an accelerator of topical outcomes. Controlled micro-injury activates wound-healing signals that can independently improve density and shaft diameter, while also enhancing follicular penetration and local drug exposure.
Combination with other treatments, primarily minoxidil, can also improve outcomes. The underlying logic is straightforward: minoxidil boosts growth, while dual 5AR blockade increases the probability that follicles remain protected from hormonal changes.
One study showed that adding microneedling to topical finasteride therapy alongside minoxidil can increase hair density and shaft diameter more than minoxidil alone.[31]Chang, Y., Zhang, W., Zhou, J., Lv, L., He, Q., Chen, Y., Wang, P., & Zhai, Q. (2025). Clinical efficacy of microneedle combined with 5% minoxidil solution and finasteride in the treatment of … Continue reading
Similarly, combining dutasteride with microneedling and minoxidil has also demonstrated promising results. In a small study, all patients used topical minoxidil 5%, while adding dutasteride, either systemically or locally, significantly improved hair density and shaft caliber compared with minoxidil alone. The strongest overall results were seen with topical dutasteride 0.02% delivered via monthly microneedling, which outperformed oral dutasteride plus minoxidil on objective measures while maintaining a more favorable safety profile.[32]Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through … Continue reading
Overall, the evidence suggests that topical regimens may be most effective when they combine anti-androgen suppression, follicular stimulation, and delivery-enhancing strategies.
Interested in Topical Dutasteride?
Hair gains bigger than finasteride? Dutasteride makes this possible, if prescribed*
Take the next step in your hair regrowth journey. Get started today with a provider who can prescribe a topical solution tailored for you.
*Only available in the U.S. Prescriptions not guaranteed. Restrictions apply. Off-label products are not endorsed by the FDA.

Dosing
Choosing the right dose depends on both the medication and the clinical context. Finasteride, whether oral or topical, is generally the best starting point for most men beginning pharmacologic treatment for androgenetic alopecia. Its pharmacokinetics are more forgiving, dose adjustments are easier, and when side effects occur, they are often manageable with modest reductions in dose or changes in dosing frequency.
Dutasteride, in contrast, is better suited for men with aggressive, fast-progressing AGA or for those who have not responded well to finasteride. Its greater potency comes with less flexibility, largely due to its long half-life and sustained enzyme binding. As a result, dosing with dutasteride should be conservative and deliberate, with careful consideration of long-term exposure and tolerability.
Oral Finasteride
The standard dose of oral finasteride for androgenetic alopecia is 1 mg once daily. This regimen has been used in the majority of randomized controlled trials and has consistently demonstrated improvements in key hair growth outcomes, including hair counts, hair shaft caliber, and investigator global photographic assessments.
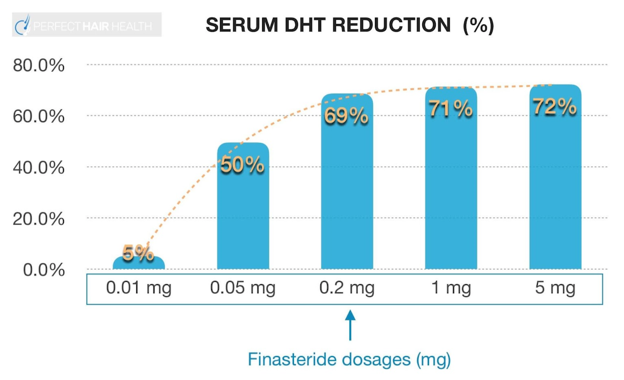
A key pharmacologic feature of finasteride is its logarithmic dose–response curve. Most of the drug’s DHT-lowering effect occurs at relatively low doses, and by the time a daily dose of 1 mg is reached, inhibition of type II 5-alpha reductase is already close to maximal.
This explains why lower doses, such as 0.2–0.5 mg daily, can still meaningfully suppress both serum and scalp DHT, and why increasing the dose beyond 1 mg does not lead to proportionally greater hair regrowth. Instead, higher doses primarily increase systemic exposure and may raise the risk of side effects without offering additional cosmetic benefit.
Figure 4. The dose response of finasteride demonstrates that increasing the concentration of treatment doesn’t translate to increased DHT reduction.
From a practical standpoint, this dose–response relationship allows for flexibility in real-world use. Many individuals are able to maintain hair stabilization and regrowth with reduced dosing or alternate-day schedules, particularly after an initial period of stabilization.
When side effects do occur, they also tend to be dose-sensitive rather than all-or-nothing, meaning that lowering the dose or adjusting the dosing schedule is often sufficient to improve tolerability while preserving therapeutic benefit.
Oral Dutasteride
The standard oral dose of dutasteride for androgenetic alopecia is 0.5 mg once daily. This is the dose most consistently studied in clinical trials and the dose used in nearly all head-to-head comparisons with finasteride.
The reason dutasteride is prescribed at a lower milligram dose than finasteride comes down to its greater pharmacologic potency. Dutasteride’s pharmacokinetics are also different. The drug has a longer half-life (roughly 4–5 weeks), which means it accumulates in tissues over time.[33]Arif, T., Dorjay, K., Adil, M., & Sami, M. (2017). Dutasteride in androgenetic alopecia: an update. *Current Clinical Pharmacology.* 12(1). 31–35. Available at: … Continue reading
Because of this, missed doses tend to matter less for maintaining efficacy. However, the flip side is that if adverse effects occur, they may take weeks to months to fully resolve after discontinuation, as the drug slowly clears from the body.
These properties have important practical implications for dosing. Daily dosing is not always necessary to maintain clinical benefit, and some clinicians employ intermittent schedules, such as 0.5 mg taken two to three times per week, in an effort to balance efficacy with tolerability.
Topical Finasteride
Topical finasteride dosing is often misunderstood because the percentage concentration listed on the label is not the most important variable. What ultimately determines both efficacy and systemic risk is total daily finasteride exposure, measured in milligrams per day.
This total exposure is shaped by several interacting factors: the concentration of finasteride in the solution, the volume applied per use, how often it is applied (once versus twice daily), and the vehicle or carrier used, such as alcohol or propylene glycol, which influences skin penetration.
The key principle underlying topical finasteride dosing is that finasteride is extremely potent systemically. Only a very small fraction of the drug needs to enter circulation to meaningfully suppress serum DHT. As a result, topical formulations can unintentionally behave like oral finasteride if dosing is too aggressive, even when the product is intended to act locally on the scalp.
An evidence-informed dosing range is 1-2 mL of a 0.005%-0.02% topical finasteride solution applied once daily. This corresponds to roughly 0.1-0.2 mg of finasteride applied to the scalp per day, with estimated systemic exposure in common carriers of approximately 0.01-0.03 mg per day.
Generally, high-concentration formulations (≥0.25-1%) should be avoided, especially when combined with large application volumes or twice-daily use. These regimens can suppress serum DHT to a degree similar to 1 mg of oral finasteride, effectively defeating the purpose of choosing a topical formulation in the first place.
Topical Dutasteride
Topical dutasteride dosing is less standardized than topical finasteride, largely because the evidence base is newer and consists of fewer clinical trials. That said, several consistent patterns are beginning to emerge from the available data.
Most studies have evaluated topical dutasteride in a concentration range of 0.01% to 0.05%, typically applied once daily. In many protocols, topical dutasteride has been paired with microneedling to enhance follicular delivery, although some studies suggest that meaningful efficacy can be achieved even without microneedling.[34]Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study … Continue reading
The practical takeaway is that low-concentration, low-volume topical dutasteride protocols appear to offer the best balance between efficacy and safety. Given the variability in formulations, vehicles, and individual skin permeability, optimal dosing is likely individualized, and careful titration remains essential as the clinical evidence continues to evolve.
Monitoring & Risk Management
Monitoring and risk management with finasteride or dutasteride are about using the minimum effective exposure while staying alert to tolerability over time. Ideally, treatment begins with a clear diagnosis of androgenetic alopecia, baseline photos, and, especially for topical or risk-averse users, optional baseline serum DHT testing to provide context for future changes.
Side effects, if they occur, should be addressed early with dose reduction, spacing doses, or switching delivery routes rather than abrupt discontinuation, since most are dose-sensitive and reversible. For topical users, repeat serum DHT testing after ~30 days can help confirm that systemic exposure remains low.
Ongoing monitoring should focus on objective progress via standardized photos every 3–6 months and general symptom awareness rather than day-to-day fluctuations.
Extra caution is warranted with dutasteride due to its long half-life and accumulation, making conservative dosing and deliberate adjustments especially important. When approached with planned checkpoints and proportional responses, both drugs can be used safely, predictably, and effectively over the long term.
Who Should Use Finasteride or Dutasteride?
Finasteride and dutasteride are among the most effective pharmacologic options for AGA, but they are not one-size-fits-all treatments. Choosing which drug to use depends on hair-loss pattern, rate of progression, prior treatment response, side-effect tolerance, and long-term goals.
Both drugs require consistent, long-term use to maintain results. Candidates should be comfortable with ongoing therapy and realistic timelines, as visible improvements often take 6-12 months, with maximal benefits closer to two years.
Consider Finasteride If…
You’re starting treatment, or your AGA is early-to-moderate. Oral finasteride is the usual baseline because it’s effective for most men and has a long track record. It’s also more forgiving: if you need to adjust dose, frequency, or route due to side effects, finasteride is generally easier to fine-tune.
If you’re risk-averse or simply want the most studied on-label option for hair loss, finasteride is usually the logical starting point, especially if you can commit to consistent use and track progress with photos over months.
Consider Dutasteride If…
Your hair loss is aggressive, or you’re losing ground despite finasteride. Dutasteride’s broader 5AR inhibition often translates into greater average gains in hair counts and thickness in shorter head-to-head trials. Clinically, it’s most often considered when progression is rapid, miniaturization is extensive, or finasteride hasn’t provided adequate stabilization after a consistent trial.
Who Should be Cautious (or Avoid)?
Men without androgenetic alopecia – Finasteride and dutasteride are ineffective for conditions such as alopecia areata, telogen effluvium, traction alopecia, or scarring alopecias. Using them in these contexts adds risk without meaningful benefit.
Those highly risk-averse to hormonal manipulation – While most users tolerate these medications well, anyone uncomfortable with altering systemic hormone levels, even at low risk, may prefer non-hormonal options or topical-only approaches with careful dosing.
Men who experienced significant adverse effects previously – Dutasteride may be more appropriate for men with rapid progression, extensive miniaturization, or those who continue to lose ground despite consistent finasteride use. Its stronger and broader DHT suppression can provide an additional margin of control in these cases.
Men trying to conceive in the near term – Although evidence of fertility harm at hair-loss doses is limited, many clinicians advise avoiding systemic 5AR inhibitors when actively trying to conceive, particularly dutasteride due to its long half-life. This decision should be individualized and discussed with a clinician.
Those unable to maintain consistency – Oral therapies require daily (or structured intermittent) dosing, while topical therapies demand regular application and attention to volume and frequency. If adherence is unlikely, expected benefits drop substantially.
Final Thoughts and Takeaways
Finasteride and dutasteride, in their different formulations, are not competing “good vs bad” options. Instead, they are different tools for different stages and severities of androgenetic alopecia.
The real decision isn’t which drug suppresses more DHT on paper, but how much suppression you need to control your hair loss, and how much systemic exposure you’re comfortable carrying long term.
Finasteride has the longest safety record, strong long-term efficacy, and a forgiving dose–response curve that allows real-world flexibility. For early-to-moderate AGA, or for men prioritizing reversibility and adjustability, finasteride, either oral or carefully dosed topical, covers the majority of clinical needs.
When hair loss is aggressive, rapidly progressive, or insufficiently controlled on finasteride, dutasteride’s broader 5AR inhibition offers a higher ceiling of protection. Head-to-head trials consistently show greater average gains, and it does not show a clearly higher overall incidence of side effects in controlled studies despite stronger systemic and scalp DHT reduction.
Topical vs oral delivery, total daily exposure, application volume, and frequency often have a larger impact on outcomes and tolerability than the finasteride-vs-dutasteride label alone. Hair loss therapy is a long game. The optimal choice is not the strongest drug you can tolerate for a few months, but the strategy you can follow consistently for years with acceptable trade-offs.
References[+]
References ↑1 Asfour, L., Cranwell, W., & Sinclair, R. (2023). Male androgenetic alopecia. *Endotext [Internet].* Available at: https://www.ncbi.nlm.nih.gov/books/NBK278957/ ↑2 Makridakis, N., & Reichardt, J. K. V. (2005). Pharmacogenetic analysis of human steroid 5α-reductase type II: comparison of finasteride and dutasteride. *Journal of Molecular Endocrinology.* 34(3). 617–623. Available at: https://doi.org/10.1677/jme.1.01725 ↑3 Frye, S. V. (2006). Discovery and clinical development of dutasteride, a potent dual 5α-reductase inhibitor. *Current Topics in Medicinal Chemistry.* 6(5). 405–421. Available at: https://doi.org/10.2174/156802606776743101 ↑4 Wikimedia Commons. (n.d.). Azasteroid Pharmaceuticals [Image]. *Wikimedia Commons.* Available at: https://commons.wikimedia.org/wiki/File:Azasteroid_Pharmaceuticals.png (Accessed: November 2025) ↑5, ↑15, ↑19 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑6, ↑10, ↑16 Harcha, W. G., Barboza Martínez, J., Tsai, T.-F., Katsuoka, K., Kawashima, M., Tsuboi, R., Barnes, A., Ferron-Brady, G., & Chetty, D. (2014). A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. *Journal of the American Academy of Dermatology.* 70(3). 489–498. Available at: https://doi.org/10.1016/j.jaad.2013.10.049 ↑7, ↑17 Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. *Indian Journal of Dermatology, Venereology and Leprology.* 83. 47. Available at: https://doi.org/10.4103/0378-6323.188652 ↑8, ↑11, ↑13, ↑18 Choi, G.-S., Sim, W.-Y., Kang, H., Huh, C. H., Lee, Y. W., Shantakumar, S., Ho, Y.-F., et al. (2022). Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. *Annals of Dermatology.* 34(5). 349. Available at: https://doi.org/10.5021/ad.22.027 ↑9 Olsen, E. A., Hordinsky, M., Whiting, D., Stough, D., Hobbs, S., Ellis, M. L., Wilson, T., Rittmaster, R. S., & Dutasteride Alopecia Research Team. (2006). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. *Journal of the American Academy of Dermatology.* 55(6). 1014–1023. Available at: https://doi.org/10.1016/j.jaad.2006.05.007 ↑12 Price, V. H., Menefee, E., Sanchez, M., & Kaufman, K. D. (2006). Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. *Journal of the American Academy of Dermatology.* 55(1). 71–74. Available at: https://doi.org/10.1016/j.jaad.2005.07.001 ↑14 Hirshburg, J. M., Kelsey, P. A., Therrien, C. A., Gavino, A. C., & Reichenberg, J. S. (2016). Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. *The Journal of Clinical and Aesthetic Dermatology.* 9(7). 56–62. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5023004/ ↑20 Traish, A. M. (2020). Post-finasteride syndrome: a surmountable challenge for clinicians. *Fertility and Sterility.* 113(1). 21–50. Available at: https://doi.org/10.1016/j.fertnstert.2019.11.030 ↑21 Trüeb, R. M., Régnier, A., Rezende, H. D., & Dias, M. F. R. G. (2019). Post-finasteride syndrome: an induced delusional disorder with the potential of a mass psychogenic illness?. *Skin Appendage Disorders.* 5(5). 320–326. Available at: https://doi.org/10.1159/000497362 ↑22 Shapiro, J., & Kaufman, K. D. (2003). Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). *Journal of Investigative Dermatology Symposium Proceedings.* 8(1). 20–23. Available at: https://doi.org/10.1046/j.1523-1747.2003.12167.x ↑23 Kaplan, S. A., Chung, D. E., Lee, R. K., Scofield, S., & Te, A. E. (2012). A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. *International Journal of Clinical Practice.* 66(11). 1052–1055. Available at: https://doi.org/10.1111/j.1742-1241.2012.03010.x ↑24 Piraccini, B. M., Blume-Peytavi, U., Scarci, F., Jansat, J. M., Falqués, M., Otero, R., Tamarit, M. L., et al. (2022). Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. *Journal of the European Academy of Dermatology and Venereology.* 36(2). 286–294. Available at: https://doi.org/10.1111/jdv.17738 ↑25 Hajheydari, Z., Akbari, J., Saeedi, M., & Shokoohi, L. (2009). Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. *Indian Journal of Dermatology, Venereology and Leprology.* 75. 47. Available at: https://doi.org/10.4103/0378-6323.45220 ↑26 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑27 Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. *International Journal of Clinical Pharmacology and Therapeutics.* 52(10). 842–849. Available at: https://doi.org/10.5414/CP202119 ↑28 Sánchez-Meza, E., Ocampo-Candiani, J., Gómez-Flores, M., Herz-Ruelas, M. E., Ocampo-Garza, J., Orizaga-y-Quiroga, T. L., Martínez-Moreno, A., & Ocampo-Garza, S. S. (2022). Microneedling plus topical dutasteride solution for androgenetic alopecia: a randomized placebo-controlled study. Journal of the European Academy of Dermatology & Venereology. 36(10). Available at: https://doi.org/10.1111/jdv.18285 ↑29 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑30 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. *Cureus.* 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 ↑31 Chang, Y., Zhang, W., Zhou, J., Lv, L., He, Q., Chen, Y., Wang, P., & Zhai, Q. (2025). Clinical efficacy of microneedle combined with 5% minoxidil solution and finasteride in the treatment of androgenetic alopecia in males. *Archives of Dermatological Research.* 317(1). 428. Available at: https://doi.org/10.1007/s00403-025-03891-y ↑32 Abu Obeid, M. N., Abdel Fattah, N. S., Elfangary, M. M., & Al Husseni, R. M. (2024). Comparison between topical minoxidil 5% alone versus combined with dutasteride (topical 0.02% through microneedling or oral 0.5 mg) in treatment of androgenetic alopecia. *QJM: An International Journal of Medicine.* 117(Supplement_2). hcae175–207. Available at: https://doi.org/10.1093/qjmed/hcae175.207 ↑33 Arif, T., Dorjay, K., Adil, M., & Sami, M. (2017). Dutasteride in androgenetic alopecia: an update. *Current Clinical Pharmacology.* 12(1). 31–35. Available at: https://doi.org/10.2174/1574884712666170310111125 ↑34 Panuganti, V. K., Madala, P. K., Grandhi, V. R., Alluri, C. V., Mohammad, J., Kssvv, S. R., & Dundigalla, M. R. (2025). A randomized, double-blind, placebo- and active-controlled phase II study to evaluate the safety and efficacy of novel dutasteride topical solution (0.01%, 0.02%, and 0.05% w/v) in male subjects with androgenetic alopecia. Cureus. 17(8). e89309. Available at: https://doi.org/10.7759/cureus.89309 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Michael Williams, PhD
Michael is a researcher and writer who holds a BSc in Bioscience, an MSc in Regenerative Medicine, and a PhD in Translational Biomedicine. He undertook his PhD research at Houston Methodist Research Institute, Texas, focusing on cell signaling in the ovarian cancer tumor microenvironment. He conducted postdoctoral research at Barts Cancer Institute in London, exploring cellular metabolism in acute myeloid leukemia. He has published work in a range of fields, including oncology, nanomedicine, and cell-based therapeutics.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
 Scroll Down
Scroll Down