Fluridil: Scientific Deep-Dive
Fluridil, otherwise known as topilutamide (or by the brand name Eucapil) is a topical medication that has been tested as a treatment for both androgenetic hair loss and hirsutism (excess facial hair growth in women). In 2021, the patent for fluridil expired, however, there have been no generic fluridil treatments that have come to the market. Fluridil has been approved for cosmetic use in Europe but does not have FDA or EMA approval as a treatment for androgenetic alopecia. You can however buy this product on shopping websites, such as Amazon, for around $83 (at the time of writing). But is fluridil worth your money?
In this article, we will look at how Fluridil works, what the clinical evidence for it is, and whether it is safe to use.
Key Takeaways
-
- Drug. Fluridil is an anti-androgenic drug that is currently on the market for the treatment of androgenetic alopecia and hirsutism. It is known to reduce the gene expression of androgens. Due to its similar structure to other anti-androgens, it’s thought to also exert its effect by binding to androgen receptors. As it readily breaks down in aqueous solutions, some researchers believe that it is safer to use than other anti-androgens, and can be applied locally without the risk of side effects on the rest of the body.
- Clinical Data. There are only two studies that have been completed for fluridil: one on men and one on women.
- The first study was 9 months long and completed on 43 men with diagnosed androgenetic alopecia (AGA) aged between 20-56, in which 2mls of 2% fluridil was topically applied once nightly. Researchers found that in the first 3 months, there was a significant increase in the number of growing hairs, and a significant decrease in the number of non-growing hair, with no effects on testosterone levels or perceived sexual libido and function. Furthermore, fluridil, or its by-products, was not found to be present in the serum. Researchers also found, however, that the positive effects on the number of growing hairs/non-growing hairs did not last past the 3-month mark, and were instead maintained. A small safety study completed alongside this determined that either 2% or 4% fluridil was ok to use without adverse effects.
- The second study was 9 months long and only completed on 11 women, 2 of which had to drop out of the study due to adverse effects from the isopropanol that the fluridil was diluted in. The participants applied 2mls of 2% fluridil, once nightly. The researchers did not find any significant change in growing or non-growing hairs, however, they did measure an increase in hair thickness. Due to the small sample size, the lack of a placebo, and the fact that every participant had taken another anti-androgenic medication within 3 months of the beginning of the study, it’s not possible to conclude whether fluridil has any effect or not for women with AGA.
- Safety. Unlike oral anti-androgenic drugs, fluridil should only work at the point of application as it should break down once it comes into contact with the blood. Additionally, across the limited parameters of both the studies mentioned above, no serious adverse effects were reported, however as both of the studies were 9 months long, with small sample sizes, we have no real information about what the long-term effects of fluridil usage may be.
- Best Practices. Based on the limited clinical data, if you decide to use fluridil, you could apply 2ml of a 2% fluridil solution in isopropanol once, at night before bed on a dry scalp (it will degrade if your hair or scalp is wet). You might be a candidate for fluridil if you are male with AGA (as the data isn’t sufficient for females with AGA), are comfortable with using a product that has very limited efficacy or safety data, have potentially seen results using other anti-androgens (such as finasteride), and don’t mind limiting hair washing to once or twice a week. If you’re interested in trying fluridil, although it’s not approved by the FDA, you can buy it on shopping sites like Amazon, and it’s possible that you may see limited results, at least over the first 3 months of usage.
What is Fluridil?
As mentioned above, fluridil is a topical treatment that has been used in studies to determine its efficacy and safety in hair loss and excessive hair growth in women (hirsutism).
Fluridil was synthesized alongside four other compounds in a study designed to discover a novel, topical anti-androgenic compound. [1]Seligson, A.L., Campion, B.K., Brown, J.W., Terry, R.C., Kucerova, R., Bienova, M., Hajduch, M., Sovak, M. (2003). Development of Fluridil, a Topical Suppressor of the Androgen Receptor in … Continue reading Androgens are linked to certain types of hair loss such as androgenetic alopecia.
The researchers were also searching for a compound that rapidly degraded (broken down) into non-harmful by-products in the blood so that it does not have effects at the systemic (whole-body) level (but rather limits its activity locally to the area of application). Out of the five total compounds synthesized, the researchers determined that fluridil best fit their criteria, which are described below:
1. Down-regulation of androgen receptors
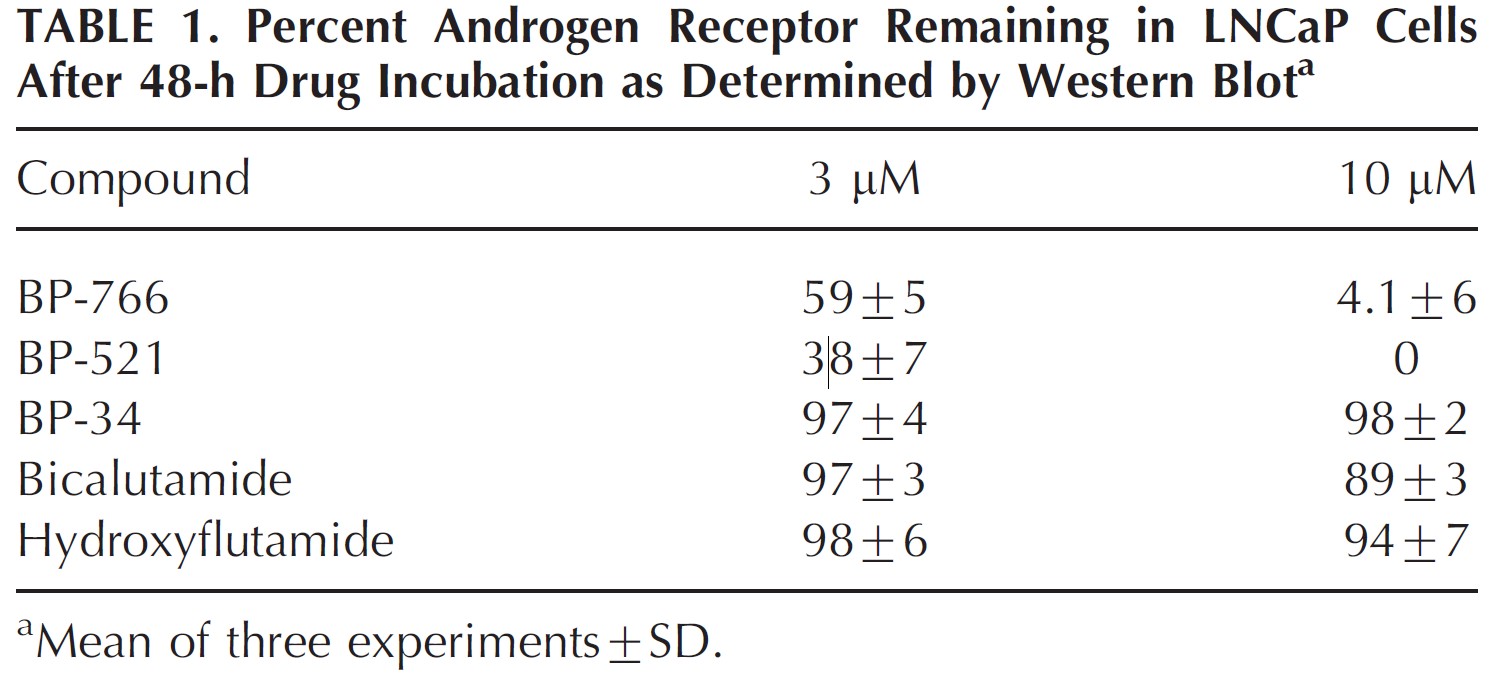
The growth of prostate cancer is often androgen receptor-dependent.[2]Fujita, K., Nonomura, N. (2019). Role of Androgen Receptor in Prostate Cancer: A Review. The World Journal of Men’s Health. 37(3), 288-295. Available at: https://doi.org/10.5534/wjmh.180040Therefore treating prostate cancer cells in a dish can be a useful way of seeing whether drugs have any anti-androgen receptor activity. In the human prostate cancer cell line LNCaP, fluridil reduced protein expression of androgen receptors by 40% at a concentration of 3μM, and by up to 95% at a 10μM concentration (Figure 1). Whilst this was not the most effective result, (BP-521 further reduced expression), it was the combination of this and results shown further down (Figure 2) that led the researchers to choose fluridil. Additionally, these compounds were compared to two other anti-androgen drugs (bicalutamide and hydroxyflutamide) and found that they did not decrease the expression of androgen receptors, indicating an additional mechanism of action of fluridil that other anti-androgen drugs may not have.
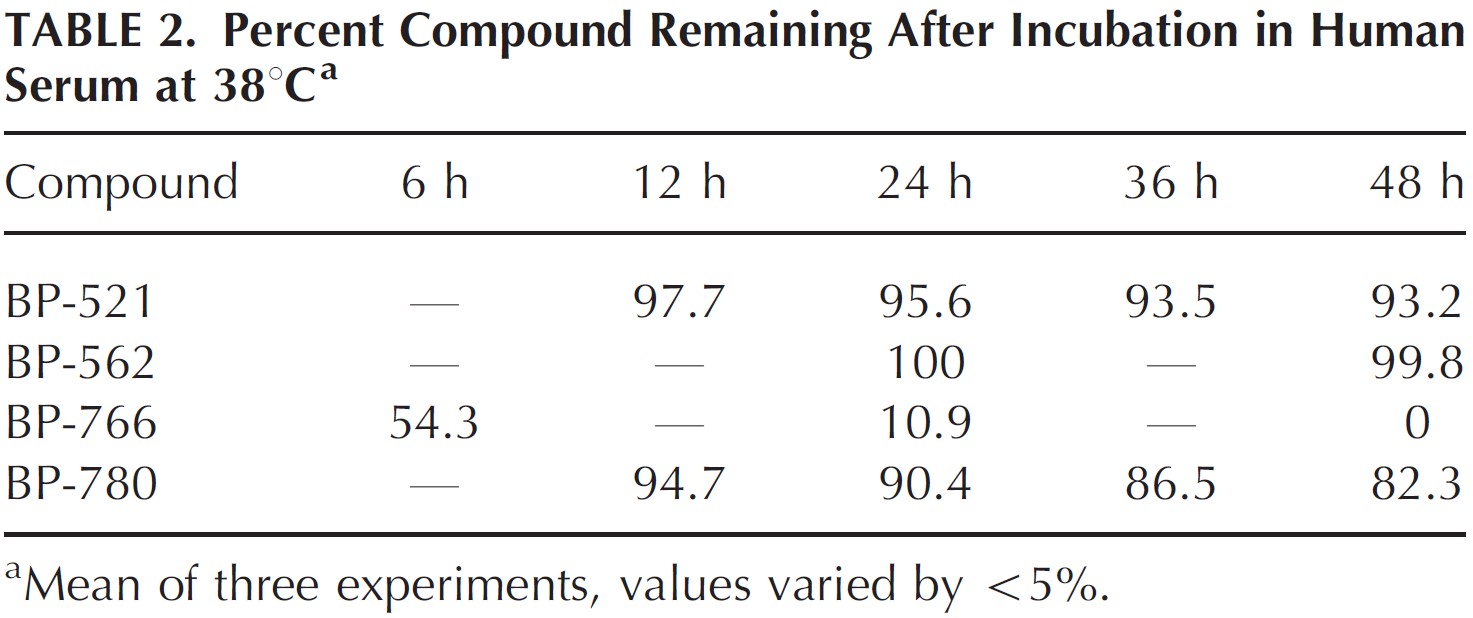
2. Degradation of the compound after incubation in human serum at 38°C
Fluridil was found to be the only compound that fully degraded in human serum by 48h of incubation (Figure 2). By contrast, BP-521, the compound that showed better down-regulation of androgen receptors barely showed any degradation, so did not match the criteria set by the researchers.
Fluridil degrades into BP-34 which – as the researchers show in Figure 1 – doesn’t have a large effect on the expression of androgen receptors. It also degrades into a perfluorocarboxylic acid (PFCA) called trifluoroacetic acid (TFA) which mammals are thought to be insensitive to. [4]Solomon, K., Velders, G., Wilson, S., MAdronich, S., Longstreth, J., Aucamp, P., Bornman, J. (2016). Sources, Fates, Toxicity and Risks of Trifluoroacetic Acid and its Salts: Relevance to Substances … Continue reading
The researchers state that the by-products are non-toxic and that they should be processed rapidly by the kidneys because they are ionic. Ionic substances are attracted to water molecules and undergo dissociation, where they disperse throughout the water, and can therefore be excreted in the urine.[5]Urine pH. (no date). University of Nottingham. Available at: https://www.nottingham.ac.uk/nmp/sonet/rlos/bioproc/kidneydrug/page_six.html (Accessed: 22 January 2022)
The researchers determined that based on their criteria, fluridil was the most promising in terms of its capacity to reduce androgen expression and due to its short half-life in aqueous solutions. This study however did not look at the efficacy of fluridil in the context of hair loss, and also did not provide data that established its safety in humans.
How does Fluridil work?
Androgenetic alopecia (AGA) is a form of hair loss with causes not yet fully understood, however, it is thought to be related to either excess androgens present, or an exaggerated response to androgens such as dihydrotestosterone (DHT). It affects both men and women and is characterized by progressive loss of hair over time in a particular pattern.[7]Ho, C.H., Sood, T., Zito, PM. (2022). Androgenetic Alopecia. StatPearls [Internet]. Treasure Island (FL). Available at: https://www.ncbi.nlm.nih.gov/books/NBK430924/. (Accessed: 22 January 2023)
Fluridil belongs to a class of drugs known as non-steroidal anti-androgens (NSAAs). NSAAs are chemicals that stop androgens (like testosterone and DHT) from binding to their receptors. This means that androgens then can’t exert their effects, as they need to bind to these receptors to initiate the signaling events that can lead to hair follicle miniaturization, amongst other things. NSAAs may also be called androgen receptor antagonists, or androgen receptor blockers. [8]Iverson, P., Melezinek, I., Schmidt, A. (2002). Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function. BJUI … Continue reading [9]Ustuner, E.T. (2013). Cause of Androgenic Alopecia: Crux of the Matter. Plastic and Reconstructive Surgery. Global Open. 1(7), e64. Available at: https://doi.org/10.1097/GOX.0000000000000005
Receptors are specialized proteins in cells that allow specific chemicals to bind to them. In response to the correct chemical binding to the receptor, the receptor can exert downstream effects that have an impact on biology (a process known as signal transduction).[10]Miller, E.J., Lappin, S.L. (2022). Physiology, Cellular Receptor. StatPearls [Internet]. Treasure Island (FL). Available at: https://www.ncbi.nlm.nih.gov/books/NBK554403/#_NBK554403_pubdet_ … Continue reading Other NSAAs include flutamide and bicalutamide.
It’s important to note that there have been no studies to determine if this is really how fluridil works. Researchers have stated that this is because of fluridil’s instability in aqueous environments (meaning it is difficult to store, handle, and therefore study). Researchers hypothesize that because its structure is similar to other NSAAs, it has a similar mechanism of action, in addition to its ability to reduce the expression of androgen receptors. [11]Seligson, A.L., Campion, B.K., Brown, J.W., Terry, R.C., Kucerova, R., Bienova, M., Hajduch, M., Sovak, M. (2003). Development of Fluridil, a Topical Suppressor of the Androgen Receptor in … Continue reading
So, how fluridil actually works has not been fully determined, but is there any information on if and how fluridil affects hair follicles?
How can Fluridil have an impact on hair follicles?
Other than what has been hypothesized above (fluridil may be an androgen receptor inhibitor), it is not possible to know for sure exactly how fluridil might work on the hair follicle. As mentioned, there have been no studies in cells or isolated hair follicles in a dish (which may be due to fluridil being unstable in aqueous environments). Cells and isolated hair follicles require physiological media solutions that contain all the nutrients needed to survive and grow, creating a barrier in experimentation with this drug/to experiment with this drug.
Furthermore, the only work completed on animals was in the initial paper in which the researchers completed skin irritant tests and absorption tests, where they found that fluridil was non-irritant at a concentration of 2% (the concentration at which they sell the product, although they don’t mention whether it’s weight/volume or volume/volume) and that there was no fluridil (or its by-products) detected in rabbit serum after a 1% solution was applied twice daily for 10 days. Samples were collected at 2, 5, 21, and 48 hours after treatment and then once every other day for the next 3 days. [12]Seligson, A.L., Campion, B.K., Brown, J.W., Terry, R.C., Kucerova, R., Bienova, M., Hajduch, M., Sovak, M. (2003). Development of Fluridil, a Topical Suppressor of the Androgen Receptor in … Continue reading
The most important thing however is whether, in humans, fluridil actually works and is safe to use. There have been two studies using fluridil in humans diagnosed with androgenetic alopecia. So let’s see if, regardless of knowing exactly how fluridil works, it is effective at treating hair loss.
Is Fluridil effective at treating hair loss?
There are currently only two studies that look at the efficacy and safety of fluridil in humans.
Study 1
The first study was a randomized, double-blind study to determine the efficacy and safety of a topical 2% fluridil solution on 43 men between 20-56 years of age with diagnosed AGA. [13]Sovak, M., Seligson, A.L., Kucerova, R., Bienova, M., Hajduch, M., Bucek, M. (2002).Fluridil, a Rationally Designed Topical Agent for Androgenetic Alopecia: First Clinical Experience. American … Continue reading Fluridil was dissolved in isopropanol to make the solution, because of its instability in water-based liquids. The participants were randomly assigned into two groups, 23 receiving the fluridil, and 20 receiving a placebo of just isopropanol. Participants were required to apply the fluridil or the placebo solution once nightly before bed, to avoid direct sun exposure and to only wash hair a maximum of twice a week using warm water.
After 3 months, the study then became open-label (all participants knew what they were receiving) and all participants began receiving the 2% fluridil solution with a further follow-up 6 months later (so the study was a total of 9 months long).
Data was collected through:
- Phototrichogram – an area of the hair is shaved (1.1cm2) and then photographed using a digital camera. Individual hairs/follicles are counted at baseline and at specific time points throughout.
- Calculating the ratio of growing (anagen) to non-growing (telogen) hairs.
- Global scalp imaging – photographs are taken of the entire scalp for subjective comparison.
- Blood collection – for measuring serum testosterone, measuring for the presence of serum fluridil and its by-products, and determining other relevant blood chemistry values.
- Self-perception questionnaire – for sexual function and libido
Results at 3 months
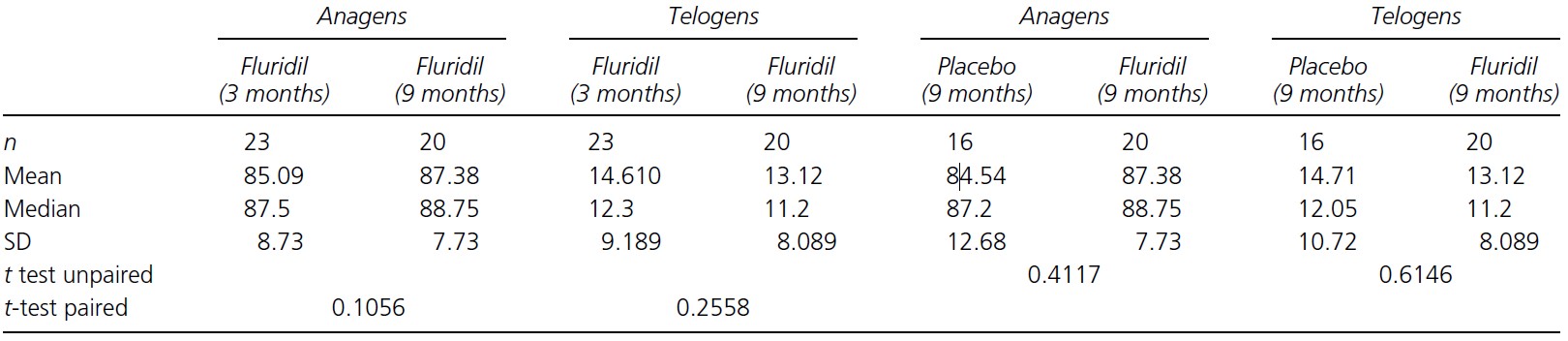
After 3 months, the number of growing to non-growing hairs was compared between the placebo and fluridil groups (Figure 3). The researchers found that the percentage of anagen hairs significantly increased from the baseline in the fluridil-treated group (75.68% at 0 months – 85.09% at 3 months) and the percentage of telogen hairs significantly decreased (25.41% at 0 months -14.61% at 3 months). The placebo group, however, also showed a significant decrease in the percentage of telogen hairs, however (29.51% at 0 months – 21.87 at 3 months) as well.
When comparing the fluridil-treated group to the placebo group after 3 months of treatment, the percentage of anagen hairs was higher in the fluridil (85.09% and 77.25% respectively). Furthermore, there were fewer telogen hairs in the fluridil-treated group than in the placebo group (14.61% and 21.87% respectively).
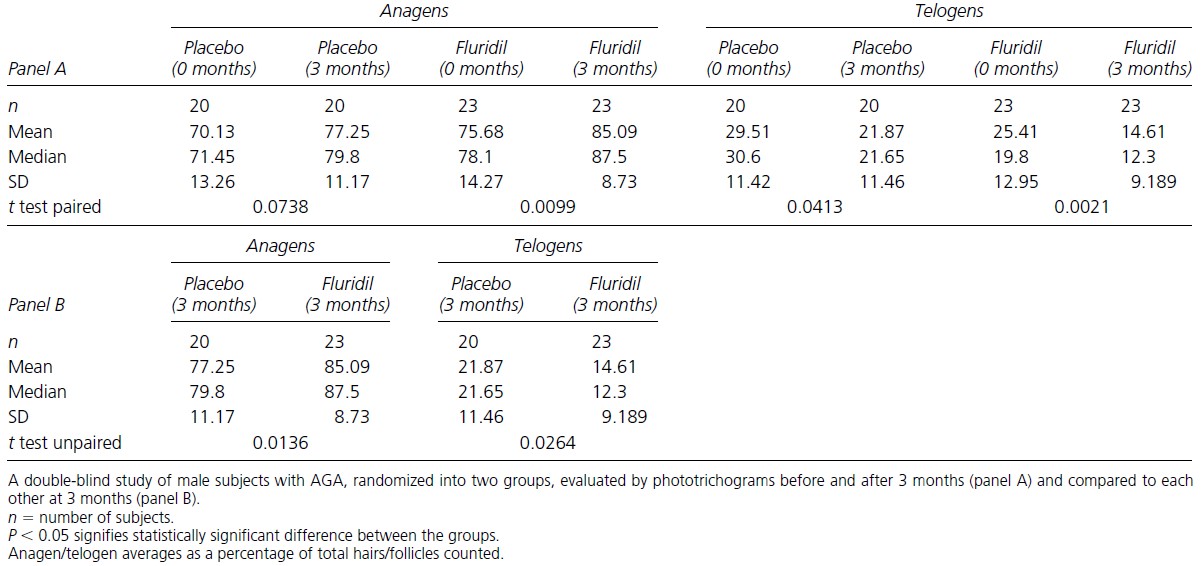
At this point, the researchers decided that the effects of fluridil on hair growth were significant enough to change all participants to the treated group. It would however have been useful for the researchers to have stated what percentage of participants did see a positive effect.
Results at 9 months
To keep us all on track, by the end of the 9 months, the fluridil-treated group will have been using fluridil for 9 months, and the placebo group will have used the placebo for 3 months and then fluridil for 6 months. We’ll call the placebo group the ‘former placebo’ group now.
After counting growing and non-growing hairs, the researchers found that all groups now experienced significant increases in anagen hairs, and significant reductions in telogen hairs (Figure 4).
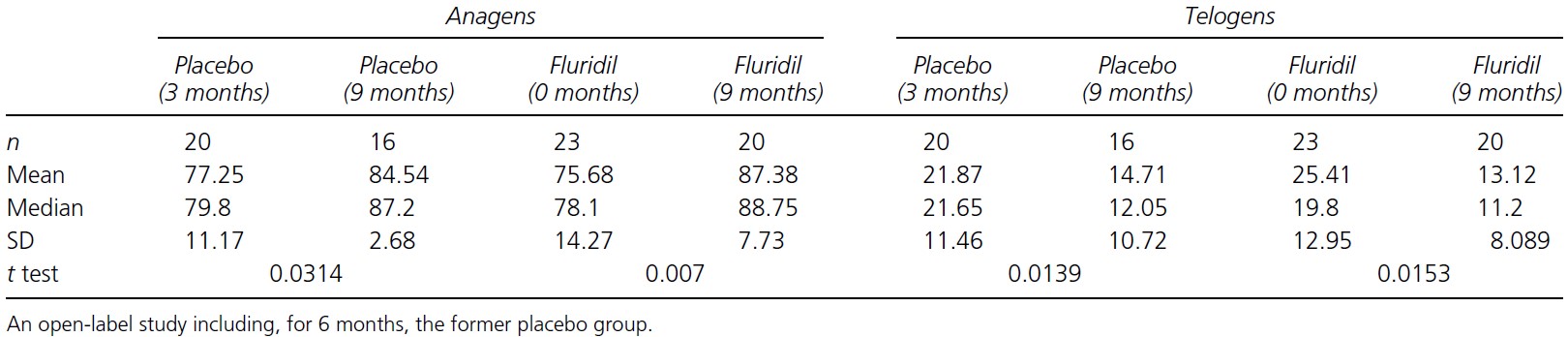
The researchers then compared the changes in growing and non-growing hairs after 3 months and 9 months for the fluridil-treated group and found that there were no significant differences, which indicates that there may be a limit to the effect that fluridil can have on hair growth, and that this limit might be achieved only within the first 3 months. Fluridil however did appear to maintain the number of growing/non-growing hairs after that.
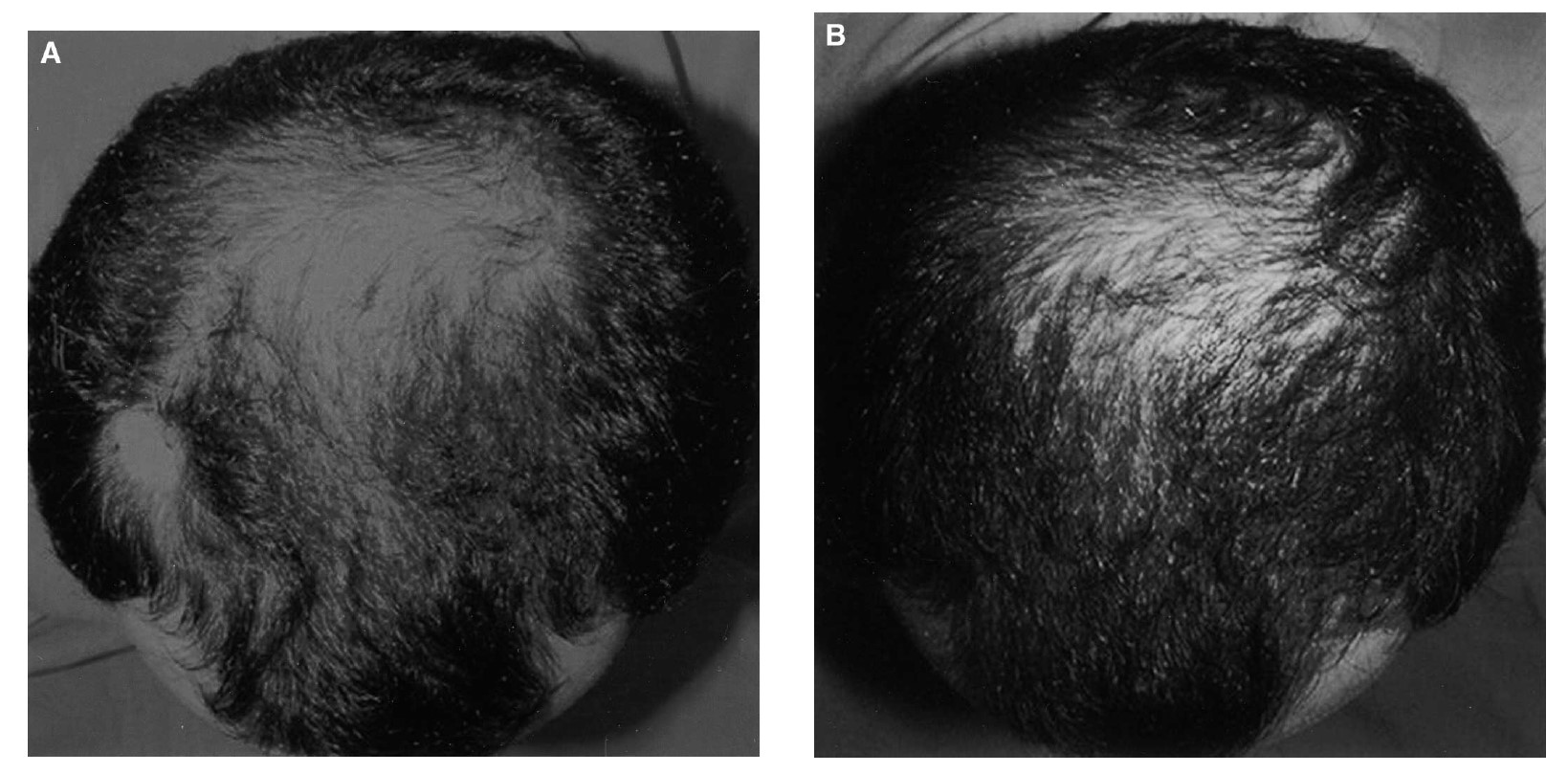
Let’s have a look at before-and-after images to see if this improvement shown in the data actually correlates to a visual improvement. Unfortunately, there is only one image shown (Figure 6), and we are left to wonder if this was the best case and if the reason that the others weren’t shown was that it was more difficult to see the improvements. Furthermore, there are no photos shown of anyone from the placebo group, so we cannot compare between groups.
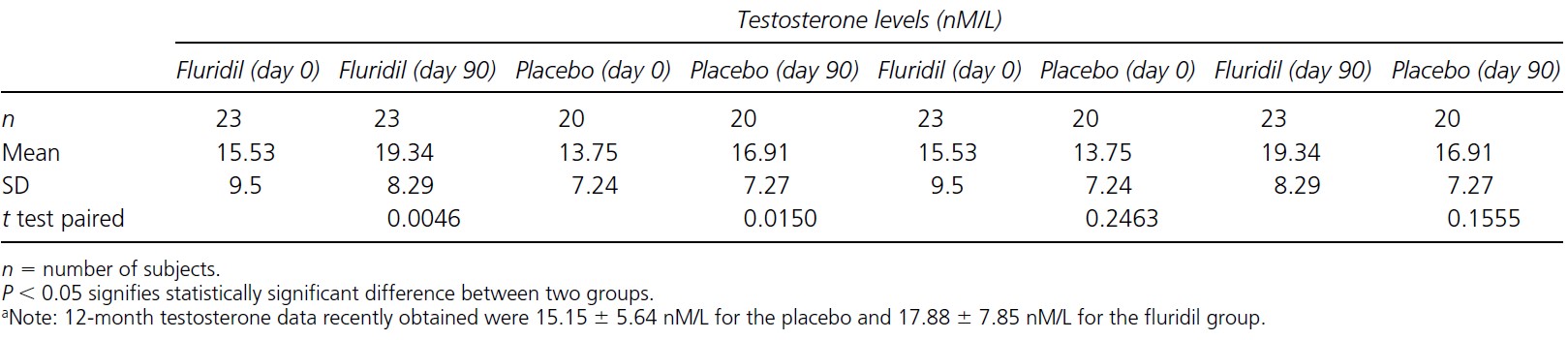
Other than measuring the number of growing/non-growing hairs, serum testosterone levels were also measured. Whilst variation between participants was observed, testosterone levels remained between the physiological range of 8-35 nm/L (Figure 7). Questionnaires also determined that participants did not experience any changes to libido or sexual performance either, however, it is not stated at what time points this data was collected. This is an indication that fluridil can only affect the hair follicle without leading to the systemic side effects that other anti-androgen drugs can cause. Additionally, the researchers did not detect fluridil or its by-product BP-34 in the serum of participants, further lending credence to this hypothesis.
A separate study within the same paper was completed with 20 male participants between the ages of 18-60. Patches containing 2,4, or 6% fluridil in either isopropanol or Vaseline Intensive Care Lotion were applied once a day for 21 days (excluding weekends) to determine if fluridil caused any irritancy to the skin. Isopropanol was also given alone as a control. 2% and 4% fluridil were found to be non-irritant and non-sensitizing. 6% fluridil however caused a slight degree of macular erythema (a rash with flat discolored areas of skin and small raised bumps).
So, to summarize, the data indicates that fluridil treatment leads to more growing hairs and fewer non-growing hairs. This effect however plateaus after 3 months, with no improvement or deterioration occurring after. Fluridil doesn’t appear to affect testosterone levels (which other anti-androgen medications can), and, holding up to the claim of hydrolyzation neither fluridil nor its by-product BP-34 were found to be present in the serum of participants throughout the study. There is only one comparison photo however, and while it does look like there is an improvement, it’s not possible to know if this was just the very best case or whether other participants also experienced a similar level of improvement. Of course, the long-term effects of fluridil (beyond 9-months) are unknown.
Study 2
The second study completed by the same research group was an open study. An open study is a study in which both participants and researchers are aware of what they are using. It sought to determine the efficacy and safety of a 2% fluridil solution in isopropanol on 11 female participants, aged 22-45, with diagnosed AGA. [19]Kucerova, R., Bienova, M., Novotny, R., Fiuraskova, M., Hajduch, M., Sovak, M. (2006). Current Therapies of Female Androgenetic Alopecia and Use of Fluridil, a Novel Topical Antiandrogen, Scripta … Continue reading
Participants were required to massage 2 ml of the fluridil solution into their scalps once a day, in the evening. 2 women discontinued the study due to itching and reddening that developed at the site after treatment. It was determined with an allergy study that this was due to the isopropanol causing irritation, rather than a reaction to the fluridil itself.
Participants were advised to only apply the solution to a dry scalp and to limit hair washes to a maximum of 2 times a week.
In this study, all 11 participants were treated with fluridil, so there was no placebo or a no-treatment group. A placebo group is useful in clinical trials as it provides a group to compare throughout the study. The participants are also aware that they are receiving the actual treatment without a placebo, which may influence the participant’s perception of the effect of the treatment.
Several types of data were collected, including the following methods:
- Phototrichogram
- Global scalp imaging
- Hair Diameter measurement – an area of hair shaved and 3 weeks later ~50 new hairs collected and measured using a microscope.
- Changes in hair surface – five hairs from each patient were collected and prepared for scanning electron microscopy (SEM*).
- Evaluation by a physician (using a scoring system)
- Self-evaluation by participants (using a scoring system) to determine self-perception of hair growth and quality as well as effects on libido and sexual function.
- Blood tests – to measure serum testosterone, sex hormone binding globulin, other relevant blood chemistry, and serum fluridil or its by-products.
*(SEM is a special type of microscope that produces images of a sample by scanning the surface with electrons. These interact with the atoms that make up the sample, creating a much higher resolution image than might be obtained from a light-based camera). [20]Scanning Electron Microscopy. (no date). Nanoscience Instruments. Available at: https://www.nanoscience.com/techniques/scanning-electron-microscopy/ (Accessed: 22 January 2023)
Results
The authors of this study stated that they only included statistically significant data and what they considered to be “otherwise remarkable observations” in their results section.
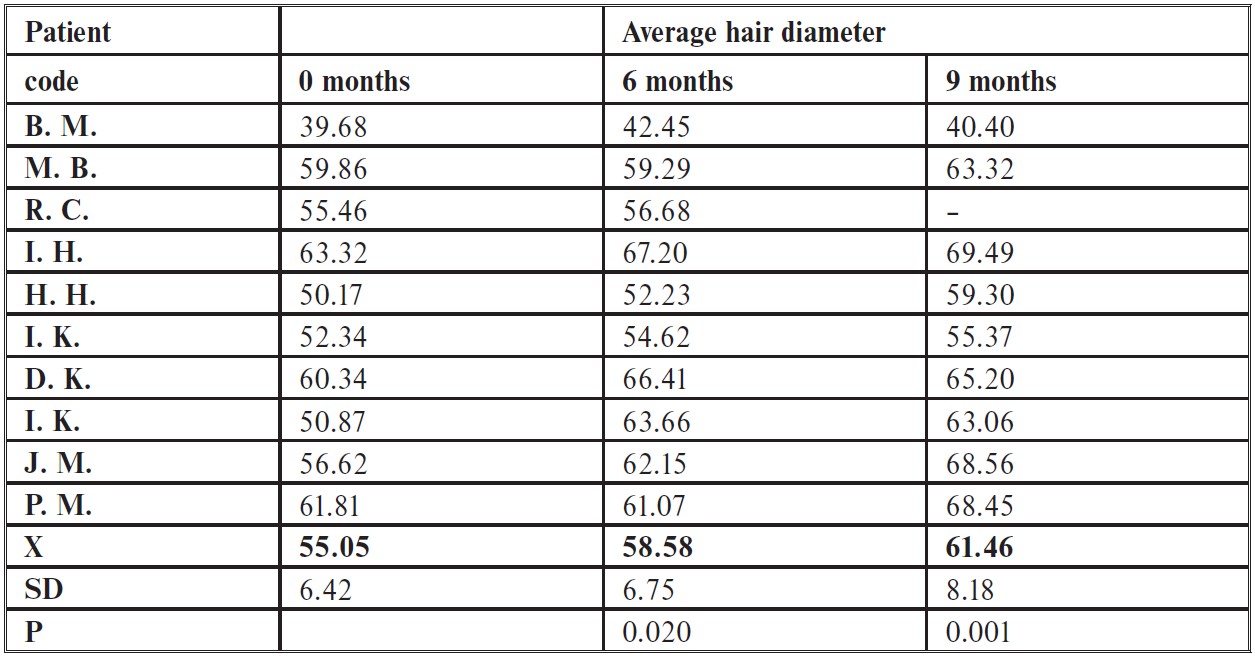
The researchers found that fluridil treatment did not significantly increase the number of growing hairs or decrease the number of non-growing hairs over the whole study period (9 months), however, there was a significant improvement in hair diameter when it was measured at the 6 months and 9-month marks compared to the baseline. Whilst there are no images included in this paper, it is mentioned that the SEM images showed no changes in the overall morphology of the hair and that after evaluating global scalp images (photos taken from the top of the head) hair appeared to be thicker.
5 out of 11 participants reported that they perceived themselves to have reduced hair loss after 6 months of treatment. 1 out of 11 participants reported that they perceived their hair to have improved quality after 6 months, with another one reporting the same after 9 months. One person observed thickening of the hair (after 9 months), one reported new undergrowth (after 6 months) and one person reported accelerated hair growth (after 3 months). Undergrowth is the growth of short, fine hairs underneath longer hair.
So, not very convincing. We have measurements of hair diameter thickening but no data showing any increase in growing hairs or reduction in non-growing hairs. Moreover, the reported ‘positive’ data is almost entirely based on subjective patient self-reporting.
The safety data however does generally follow the same results as with the previous study on men, with no patients reporting adverse side effects.
The blood chemistry data was within normal parameters throughout the study (measured at 3, 6, and 9 months) and there was no presence of fluridil or its by-products in the serum of any of the participants.
With regards to testosterone levels and sexual hormone binding globulin (SHBG), the researchers found no statistically significant differences between measurements taken at baseline and 9 months of treatment. There were however patients with higher than the physiological range of SHBG, which has been attributed to the fact that 8 out of 9 of the final patients were taking the combined oral contraceptive pill, which is known to increase SHBG. [22]Amiri, M., Tehrani, F.R., Nahidi, F., Kabir, A., Azizi, F. (2018). Comparing the Effects of Combined Oral Contraceptives Containing Progestins with Low Androgenic and Antiandrogenic Activities on the … Continue reading
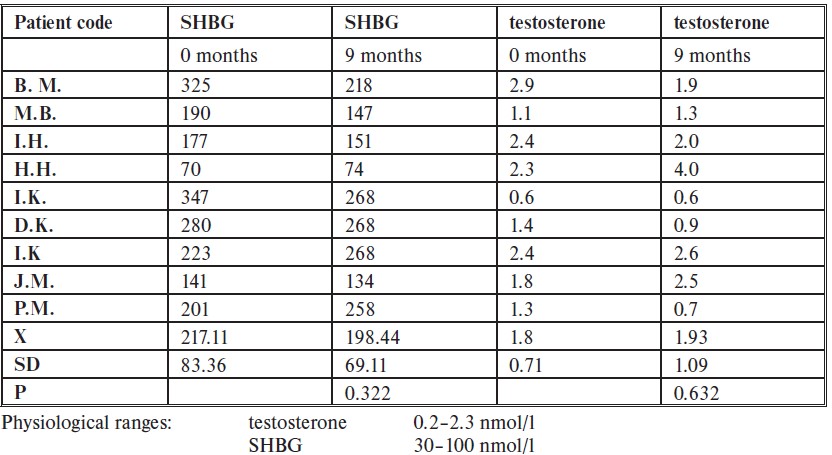
So, why didn’t fluridil work in the same way for women as it did for men? Well, the researchers attribute this to the fact that the participants were taking cyproterone acetate (aka Diane 35) another anti-androgen, and (in some cases) contraceptive at least 3 months previous to the study. They determined that because they were taking Diane 35, it can be assumed that there was a sufficient anti-androgenic effect being exerted and so fluridil could not provide a further effect.
But this is just one of several other problems with this study, such as:
- Small participant number
- No placebo
- Participants had been taking medication that may interfere with the efficacy of fluridil
Overall, we cannot conclude that fluridil has any real beneficial effect for women with AGA.
Is Fluridil safe?
Within the limited parameters of the published studies, fluridil doesn’t appear to have any treatment-related side effects. Furthermore, unlike taking oral anti-androgenic medications, fluridil should work only at the point of application and not have effects anywhere else in the body, due to its ability to degrade when it comes into contact with water.
However, fluridil has not been approved by the FDA or the European Medicines Agency as an anti-androgenic medication and is only approved for use in Europe as a cosmetic. The long-term effects of using fluridil are completely unknown.
Is Fluridil for me?
You may want to try fluridil if you:
- Are male with AGA.
- Want to either maintain the current status of hair loss or see short-term improvement and then a plateau.
- Are comfortable with using a product that has no FDA or EMA approval.
- Have no issues using a product that has very little recent (or otherwise) efficacy or safety data.
- Are ok with reducing hair washing to around once or twice a week.
- Have perhaps seen positive results with other anti-androgens (such as finasteride)
Whilst the previously mentioned points (easily degradable and topical application) may prove appealing, we cannot currently recommend using fluridil until more data becomes available. If you want to take the risk, however, it is possible that you may see some limited benefits.

Rob English is a researcher, medical editor, and the founder of perfecthairhealth.com. He acts as a peer reviewer for scholarly journals and has published five peer-reviewed papers on androgenic alopecia. He writes regularly about the science behind hair loss (and hair growth). Feel free to browse his long-form articles and publications throughout this site.
References
| ↑1 | Seligson, A.L., Campion, B.K., Brown, J.W., Terry, R.C., Kucerova, R., Bienova, M., Hajduch, M., Sovak, M. (2003). Development of Fluridil, a Topical Suppressor of the Androgen Receptor in Androgenetic Alopecia. Drug Development Research. 59, 292-306. Available at: https://doi.org.10.1002/ddr.10166 |
|---|---|
| ↑2 | Fujita, K., Nonomura, N. (2019). Role of Androgen Receptor in Prostate Cancer: A Review. The World Journal of Men’s Health. 37(3), 288-295. Available at: https://doi.org/10.5534/wjmh.180040 |
| ↑3, ↑6, ↑11, ↑12 | Seligson, A.L., Campion, B.K., Brown, J.W., Terry, R.C., Kucerova, R., Bienova, M., Hajduch, M., Sovak, M. (2003). Development of Fluridil, a Topical Suppressor of the Androgen Receptor in Androgenetic Alopecia. Drug Development Research. 59, 292-306. Available at: https://doi.org.10.1002/ddr.10166 |
| ↑4 | Solomon, K., Velders, G., Wilson, S., MAdronich, S., Longstreth, J., Aucamp, P., Bornman, J. (2016). Sources, Fates, Toxicity and Risks of Trifluoroacetic Acid and its Salts: Relevance to Substances Regulated Under the Montreal and Kyoto Protocols. Journal of Toxicology and Environmental Health. 43(15-16), 597-603. Available at: https://doi.org/10.1080/10937404.2016.1175981 |
| ↑5 | Urine pH. (no date). University of Nottingham. Available at: https://www.nottingham.ac.uk/nmp/sonet/rlos/bioproc/kidneydrug/page_six.html (Accessed: 22 January 2022) |
| ↑7 | Ho, C.H., Sood, T., Zito, PM. (2022). Androgenetic Alopecia. StatPearls [Internet]. Treasure Island (FL). Available at: https://www.ncbi.nlm.nih.gov/books/NBK430924/. (Accessed: 22 January 2023) |
| ↑8 | Iverson, P., Melezinek, I., Schmidt, A. (2002). Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function. BJUI International. 87(1), 47-56. Available at: https://doi.org/10.1046/j.1464-410x.2001.00988.x |
| ↑9 | Ustuner, E.T. (2013). Cause of Androgenic Alopecia: Crux of the Matter. Plastic and Reconstructive Surgery. Global Open. 1(7), e64. Available at: https://doi.org/10.1097/GOX.0000000000000005 |
| ↑10 | Miller, E.J., Lappin, S.L. (2022). Physiology, Cellular Receptor. StatPearls [Internet]. Treasure Island (FL). Available at: https://www.ncbi.nlm.nih.gov/books/NBK554403/#_NBK554403_pubdet_ (Accessed: 22 January 2023) |
| ↑13, ↑14, ↑15, ↑16, ↑17, ↑18 | Sovak, M., Seligson, A.L., Kucerova, R., Bienova, M., Hajduch, M., Bucek, M. (2002).Fluridil, a Rationally Designed Topical Agent for Androgenetic Alopecia: First Clinical Experience. American Society for Dermatologic Surgery. 28, 678-685. https://doi.org/10.1046/j.1524-4725.2002.02017.x |
| ↑19 | Kucerova, R., Bienova, M., Novotny, R., Fiuraskova, M., Hajduch, M., Sovak, M. (2006). Current Therapies of Female Androgenetic Alopecia and Use of Fluridil, a Novel Topical Antiandrogen, Scripta Medica (Brno). 79(1), 35-48 |
| ↑20 | Scanning Electron Microscopy. (no date). Nanoscience Instruments. Available at: https://www.nanoscience.com/techniques/scanning-electron-microscopy/ (Accessed: 22 January 2023) |
| ↑21, ↑23 | Kucerova, R., Bienova, M., Novotny, R., Fiuraskova, M., Hajduch, M., Sovak, M. (2006). Current Therapies of Female Androgenetic Alopecia and Use of Fluridil, a Novel Topical Antiandrogen, Scripta Medica (Brno). 79(1), 35-48 |
| ↑22 | Amiri, M., Tehrani, F.R., Nahidi, F., Kabir, A., Azizi, F. (2018). Comparing the Effects of Combined Oral Contraceptives Containing Progestins with Low Androgenic and Antiandrogenic Activities on the Hypothalamic-Pituitary-Gonadal Axis in Patients with Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. JMIR Research Protocols, 7(4), e113. https://doi.org/10.2196/resprot.9024 |
